Physiological and Behavioral Evaluation of Shelter Dogs During Veterinary Routine Health Checks
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Setting and Procedure
2.3. Blood Collection, Storage and Analysis
2.4. Statistical Analysis
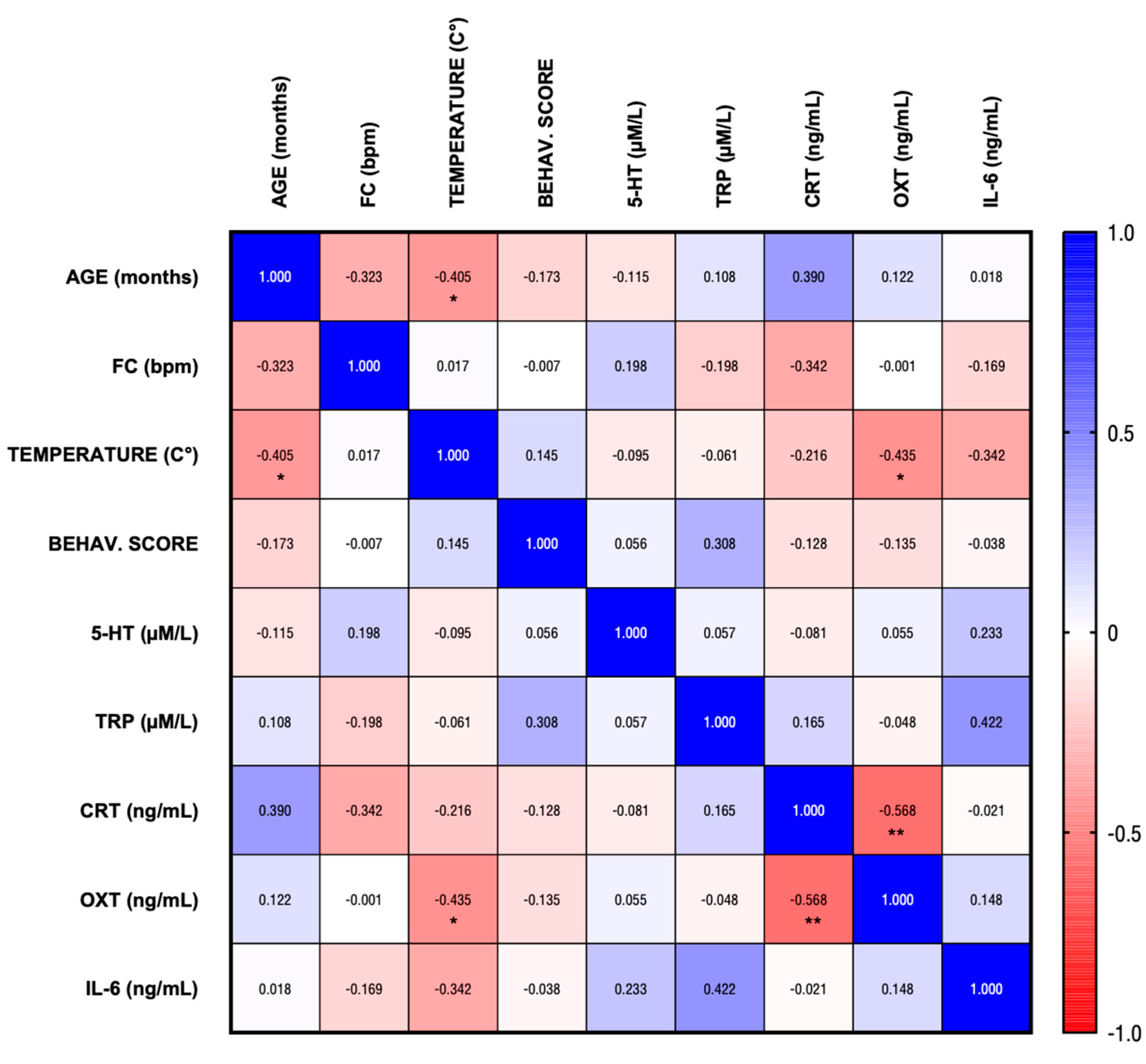
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariti, C.; Ricci, E.; Zilocchi, M.; Gazzano, A. Owners as a Secure Base for Their Dogs. Behaviour 2013, 150, 1275–1294. [Google Scholar] [CrossRef]
- Carlone, B.; Sighieri, C.; Gazzano, A.; Mariti, C. The Dog (Canis Familiaris) as Part of the Family: A Pilot Study on the Analysis of Dog Bond to All the Owners. Dog Behav. 2019, 5, 1–14. [Google Scholar] [CrossRef]
- Cafazzo, S.; Maragliano, L.; Bonanni, R.; Scholl, F.; Guarducci, M.; Scarcella, R.; Di Paolo, M.; Pontier, D.; Lai, O.; Carlevaro, F.; et al. Behavioural and Physiological Indicators of Shelter Dogs’ Welfare: Reflections on the No-Kill Policy on Free-Ranging Dogs in Italy Revisited on the Basis of 15 years of Implementation. Physiol. Behav. 2014, 133, 223–229. [Google Scholar] [CrossRef]
- Cozzi, A.; Mariti, C.; Ogi, A.; Sighieri, C.; Gazzano, A. Behavioral Modification in Sheltered Dogs. Dog Behav. 2016, 2, 1–12. [Google Scholar] [CrossRef]
- Bollen, K.S.; Horowitz, J. Behavioral Evaluation and Demographic Information in the Assessment of Aggressiveness in Shelter Dogs. Appl. Anim. Behav. Sci. 2008, 112, 120–135. [Google Scholar] [CrossRef]
- Richard, O.; Pagnon, R.; Rouch-Buck, P.; Mugnier, A.; Chastant, S. Educational Difficulties and Behavioural Problems in Dogs: Prevalence and Risk Factors. Dog Behav. 2018, 4, 9–20. Available online: https://dogbehavior.it/dogbehavior/article/view/187 (accessed on 1 February 2025).
- Gazzano, V.; Ogi, A. Canine Phobia. Dog Behav. 2020, 6, 31–39. [Google Scholar] [CrossRef]
- Lepper, M.; Kass, P.H.; Hart, L.A. Prediction of Adoption versus Euthanasia among Dogs and Cats in a California Animal Shelter. J. Appl. Anim. Welf. Sci. 2002, 5, 29–42. [Google Scholar] [CrossRef]
- Iacopini, L.; Gazzano, V. Analysis of the Management of a Shelter Dog Kennel. Dog Behav. 2023, 9, 41–45. [Google Scholar] [CrossRef]
- Ciurli, L.; Casini, L.; Cecchi, F.; Baragli, P.; Macchioni, F.; Curadi, C.; Gazzano, V.; Capsoni, S.; Gazzano, A. The Canine Cognitive Dysfunction Syndrome: Epidemiology, Pathophysiology and Diagnosis. Dog Behav. 2023, 9, 1–8. [Google Scholar] [CrossRef]
- Ciurli, L.; Casini, L.; Cecchi, F.; Baragli, P.; Macchioni, F.; Curadi, M.C.; Gazzano, V.; Capsoni, S.; Gazzano, A. The Canine Cognitive Dysfunction Syndrome: Rating Scales. Dog Behav. 2023, 9, 23–35. [Google Scholar]
- Lind, A.K.; Hydbring-Sandberg, E.; Forkman, B.; Keeling, L.J. Assessing Stress in Dogs during a Visit to the Veterinary Clinic: Correlations between Dog Behavior in Standardized Tests and Assessments by Veterinary Staff and Owners. J. Vet. Behav. Clin. Appl. Res. 2017, 17, 24–31. [Google Scholar] [CrossRef]
- Mandese, W.W.; Griffin, F.C.; Reynolds, P.S.; Blew, A.C.; Deriberprey, A.S.; Estrada, A.H. Stress in Client-Owned Dogs Related to Clinical Exam Location: A Randomised Crossover Trial. J. Small Anim. Pract. 2021, 62, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Csoltova, E.; Martineau, M.; Boissy, A.; Gilbert, C. Behavioral and Physiological Reactions in Dogs to a Veterinary Examination: Owner-Dog Interactions Improve Canine Well-Being. Physiol. Behav. 2017, 177, 270–281. [Google Scholar] [CrossRef]
- Edwards, P.T.; Smith, B.P.; McArthur, M.L.; Hazel, S.J. Fearful Fido: Investigating Dog Experience in the Veterinary Context in an Effort to Reduce Distress. Appl. Anim. Behav. Sci. 2019, 213, 14–25. [Google Scholar] [CrossRef]
- Squair, C.; Proudfoot, K.; Montelpare, W.; Overall, K.L. Effects of Changing Veterinary Handling Techniques on Canine Behaviour and Physiology Part 1: Physiological Measurements. Animals 2023, 13, 1253. [Google Scholar] [CrossRef]
- Barrera, G.; Dzik, V.; Cavalli, C.; Bentosela, M. Effect of Intranasal Oxytocin Administration on Human-Directed Social Behaviors in Shelter and Pet Dogs. Front. Psychol. 2018, 9, 2227. [Google Scholar] [CrossRef]
- Moberg, G.P. Animal Stress, 1st ed.; American Physiological Society: Baltimore, MD, USA, 1985. [Google Scholar]
- Polgár, Z.; Blackwell, E.J.; Rooney, N.J. Assessing the Welfare of Kennelled Dogs—A Review of Animal-Based Measures. Appl. Anim. Behav. Sci. 2019, 213, 1–13. [Google Scholar] [CrossRef]
- Jankord, R.; Herman, J.P. Limbic Regulation of Hypothalamo-Pituitary-Adrenocortical Function during Acute and Chronic Stress. Ann. N. Y. Acad. Sci. 2008, 1148, 64–73. [Google Scholar] [CrossRef]
- Hennessy, M.B. Using Hypothalamic-Pituitary-Adrenal Measures for Assessing and Reducing the Stress of Dogs in Shelters: A Review. Appl. Anim. Behav. Sci. 2013, 149, 1–12. [Google Scholar] [CrossRef]
- Lemay, L.; Vander, A.J.; Kluger, M.J.; L~may, L.G.; Vander, A.J.; Kluger, M.J. The Effects of Psychological Stress on Plasma Interleukin-6 Activity in Rats. Physiol. Behav. 1990, 47, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Schüttler, J.; Neumann, S. Interleukin-6 as a Prognostic Marker in Dogs in an Intensive Care Unit. Vet. Clin. Pathol. 2015, 44, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Mariti, C.; Diverio, S.; Gutierrez, J.; Baragli, P.; Gazzano, A. Partial Analytic Validation of Determination of Cortisol in Dog Hair Using a Commercial EIA Kit. Dog Behav. 2020, 6, 1–15. [Google Scholar] [CrossRef]
- Haverbeke, A.; Diederich, C.; Depiereux, E.; Giffroy, J.M. Cortisol and Behavioral Responses of Working Dogs to Environmental Challenges. Physiol. Behav. 2008, 93, 59–67. [Google Scholar] [CrossRef]
- Hill, E.E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A.C. Exercise and Circulating Cortisol Levels: The Intensity Threshold Effect. J. Endocrinol. Investig. 2008, 31, 587. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Onaka, T. Roles of Oxytocin in Stress Responses, Allostasis and Resilience. Int. J. Mol. Sci. 2022, 23, 150. [Google Scholar] [CrossRef]
- Ogi, A.; Mariti, C.; Baragli, P.; Sergi, V.; Gazzano, A. Effects of Stroking on Salivary Oxytocin and Cortisol in Guide Dogs: Preliminary Results. Animals 2020, 10, 708. [Google Scholar] [CrossRef]
- Ogi, A.; Mariti, C.; Pirrone, F.; Baragli, P.; Gazzano, A. The Influence of Oxytocin on Maternal Care in Lactating Dogs. Animals 2021, 11, 1130. [Google Scholar] [CrossRef]
- Petersson, M.; Uvnäs-Moberg, K.; Nilsson, A.; Gustafson, L.L.; Hydbring-Sandberg, E.; Handlin, L. Oxytocin and Cortisol Levels in Dog Owners and Their Dogs Are Associated with Behavioral Patterns: An Exploratory Study. Front. Psychol. 2017, 8, 1796. [Google Scholar] [CrossRef]
- Handlin, L.; Hydbring-Sandberg, E.; Nilsson, A.; Ejdebäck, M.; Jansson, A.; Uvnäs-Moberg, K. Short-Term Interaction between Dogs and Their Owners: Effects on Oxytocin, Cortisol, Insulin and Heart Rate-an Exploratory Study. Anthrozoos 2011, 24, 301–315. [Google Scholar] [CrossRef]
- Mills, D.S.; Ramos, D.; Estelles, M.G.; Hargrave, C. A Triple Blind Placebo-Controlled Investigation into the Assessment of the Effect of Dog Appeasing Pheromone (DAP) on Anxiety Related Behaviour of Problem Dogs in the Veterinary Clinic. Appl. Anim. Behav. Sci. 2006, 98, 114–126. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Riggio, G.; Mariti, C.; Sergi, V.; Diverio, S.; Gazzano, A. Serotonin and Tryptophan Serum Concentrations in Shelter Dogs Showing Different Behavioural Responses to a Potentially Stressful Procedure. Vet. Sci. 2020, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Gazzano, A.; Casini, L.; Macchioni, F.; Mariti, C.; Baragli, P.; Preziuso, G.; Curadi, M.C.; Giuliotti, L.; Riggio, G.; Ogi, A. L-Tryptophan Supplementation Increases Serotonin Blood Levels in Dogs Fed a Dissociated Carbohydrate-Based Diet. Dog Behav. 2021, 7, 43–50. [Google Scholar] [CrossRef]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A Review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef]
- Rosado, B.; García-Belenguer, S.; León, M.; Chacón, G.; Villegas, A.; Palacio, J. Blood Concentrations of Serotonin, Cortisol and Dehydroepiandrosterone in Aggressive Dogs. Appl. Anim. Behav. Sci. 2010, 123, 124–130. [Google Scholar] [CrossRef]
- León, M.; Rosado, B.; García-Belenguer, S.; Chacón, G.; Villegas, A.; Palacio, J. Assessment of Serotonin in Serum, Plasma, and Platelets of Aggressive Dogs. J. Vet. Behav. Clin. Appl. Res. 2012, 7, 348–352. [Google Scholar] [CrossRef]
- Riva, J.; Bondiolotti, G.; Michelazzi, M.; Verga, M.; Carenzi, C. Anxiety Related Behavioural Disorders and Neurotransmitters in Dogs. Appl. Anim. Behav. Sci. 2008, 114, 168–181. [Google Scholar] [CrossRef]
- Mariti, C.; Lenzini, L.; Carlone, B.; Zilocchi, M.; Ogi, A.; Gazzano, A. Does Attachment to Man Already Exist in 2 Months Old Normally Raised Dog Puppies? A Pilot Study. Dog Behav. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Mariti, C.; Raspanti, E.; Zilocchi, M.; Carlone, B.; Gazzano, A. The Assessment of Dog Welfare in the Waiting Room of a Veterinary Clinic. Anim. Welf. 2015, 24, 299–305. [Google Scholar] [CrossRef]
- Mariti, C.; Gazzano, A.; Moore, J.L.; Baragli, P.; Chelli, L.; Sighieri, C. Perception of Dogs’ Stress by Their Owners. J. Vet. Behav. Clin. Appl. Res. 2012, 7, 213–219. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, B.H.; Van Hooff, J.A.R.A.M.; De Vries, H.W.; Mol, J.A. Behavioural, Saliva Cortisol and Heart Rate Responses to Different Types of Stimuli in Dogs. Appl. Anim. Behav. Sci. 1998, 58, 365–381. [Google Scholar] [CrossRef]
- Bradshaw, J.W.S.; Blackwell, E.J.; Casey, R.A. Dominance in Domestic Dogs-Useful Construct or Bad Habit? J. Vet. Behav. Clin. Appl. Res. 2009, 4, 135–144. [Google Scholar] [CrossRef]
- Fatjó, J.; Feddersen-Petersen, D.; Ruiz de la Torre, J.L.; Amat, M.; Mets, M.; Braus, B.; Manteca, X. Ambivalent Signals during Agonistic Interactions in a Captive Wolf Pack. Appl. Anim. Behav. Sci. 2007, 105, 274–283. [Google Scholar] [CrossRef]
- Scott, J.P. The social behavior of dogs and wolves: An illus-tration of sociobiological systematics. Ann. N. Y. Acad. Sci. 1950, 51, 1009–1021. [Google Scholar] [CrossRef]
- Fox, M.W. The anatomy of aggression and its ritualization in canidae: A developmental and comparative study. Behavior 1969, 35, 242–258. [Google Scholar] [CrossRef]
- Chance, M.R.A. An Interpretation of Some Agonistic Postures: The Role of Cut-off Acts and Postures. In Evolutionary Aspects of Animal Communication; Cambridge University Press: Cambridge, UK, 1962; pp. 71–89. [Google Scholar]
- Rugaas, T. On Talking Terms with Dogs: Calming Signals, 2nd ed.; Dogwise Publishing: Wenatchee, WA, USA, 2006; ISBN 9781929242368. [Google Scholar]
- Landsberg, G.M.; Hunthausen, W.L.; Ackerman, L.J. Behavior Problems of the Dog and Cat, 3rd ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2013; ISBN 9780702043352. [Google Scholar]
- Kuhne, F.; Hößler, J.C.; Struwe, R. Emotions in Dogs Being Petted by a Familiar or Unfamiliar Person: Validating Behavioural Indicators of Emotional States Using Heart Rate Variability. Appl. Anim. Behav. Sci. 2014, 161, 113–120. [Google Scholar] [CrossRef]
- Shepherd, K. Behavioural Medicine as an Integral Part of Veterinary Practice. In BSAVA Manual of Canine and Feline Behavioural Medicine; Horwitz, D.F., Mills, D.S., Heath, S., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2009; pp. 10–23. ISBN 978-1-905319-87-9. [Google Scholar]
- Cohen, H.-Y. Calming Signals. Vet. Nurs. J. 2007, 22, 26–28. [Google Scholar] [CrossRef]
- Mariti, C.; Falaschi, C.; Zilocchi, M.; Fatjó, J.; Sighieri, C.; Ogi, A.; Gazzano, A. Analysis of the Intraspecific Visual Communication in the Domestic Dog (Canis Familiaris): A Pilot Study on the Case of Calming Signals. J. Vet. Behav. 2017, 18, 49–55. [Google Scholar] [CrossRef]
- Lürzel, S.; Bückendorf, L.; Waiblinger, S.; Rault, J.L. Salivary Oxytocin in Pigs, Cattle, and Goats during Positive Human-Animal Interactions. Psychoneuroendocrinology 2020, 115, 104636. [Google Scholar] [CrossRef]
- Onaka, T.; Takayanagi, Y. Role of Oxytocin in the Control of Stress and Food Intake. J. Neuroendocr. 2019, 31, e12700. [Google Scholar] [CrossRef] [PubMed]
- Konorski, J. A New Method of Physiological Investigation of Recent Memory in animals. Bull Acad. Pol. Sci. 1959, 7, 115–117. [Google Scholar]
- Travain, T.; Colombo, E.S.; Heinzl, E.; Bellucci, D.; Prato Previde, E.; Valsecchi, P. Hot Dogs: Thermography in the Assessment of Stress in Dogs (Canis Familiaris)-A Pilot Study. J. Vet. Behav. Clin. Appl. Res. 2015, 10, 17–23. [Google Scholar] [CrossRef]
- Blatteis, C.M. Age-Dependent Changes in Temperature Regulation—A Mini Review. Gerontology 2012, 58, 289–295. [Google Scholar] [CrossRef]
- Zanghi, B.M.; Gardner, C.; Araujo, J.; Milgram, N.W. Diurnal Changes in Core Body Temperature, Day/Night Locomotor Activity Patterns, and Actigraphy-Generated Behavioral Sleep in Aged Canines with Varying Levels of Cognitive Dysfunction. Neurobiol. Sleep Circadian Rhythms 2016, 1, 8–18. [Google Scholar] [CrossRef]
- Terrien, J.; Perret, M.; Aujard, F. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1428–1444. [Google Scholar] [CrossRef]
- Petraszek, M.H.; Takada, Y.; Yan, D.; Urano, T.; Senzawa, K.; Takada, A. Relationship between serotonergic measures in periphery and the brain of mouse. Life Sci. 1992, 51, 75–82. [Google Scholar] [CrossRef]
- Walz, J.C.; Stertz, L.; Fijtman, A.; dos Santos, B.T.M.Q.; de Almeida, R.M.M. Tryptophan Diet Reduces Aggressive Behavior in Male Mice. Psychol. Neurosci. 2013, 6, 397–401. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Rayment, D.J.; Peters, R.A.; Marston, L.C.; De Groef, B. Relationships between Serum Serotonin, Plasma Cortisol, and Behavioral Factors in a Mixed-Breed, -Sex, and -Age Group of Pet Dogs. J. Vet. Behav. 2020, 38, 96–102. [Google Scholar] [CrossRef]
- Çakiroǧlu, D.; Meral, Y.; Sancak, A.A.; Cifti, G. Relationship between the Serum Concentrations of Serotonin and Lipids and Aggression in Dogs. Vet. Rec. 2007, 161, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Young, S.N. The Effect of Raising and Lowering Tryptophan Levels on Human Mood and Social Behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20110375. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N.; Aringer, M.; Boentert, M. Role of Interleukin-6 in Stress, Sleep, and Fatigue. Ann. N. Y. Acad. Sci. 2012, 1261, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Navarra, P.; Tsagarakis, S.; Faria, M.S.; Rees, L.H.; Michael Besser, G.; Grossman, A.B. Interleukins-1 and-6 Stimulate the Release of Corticotropin-Releasing Hormone-41 from Rat Hypothalamus in Vitro via the Eicosanoid Cyclooxygenase Pathway. Endocrinology 1991, 128, 37–44. [Google Scholar] [CrossRef]
- Spinedi, E.; Hadid, R.; Daneva, T.; Gaillard, R.C. Cytokines Stimulate the CRH but Not the Vasopressin Neuronal System: Evidence for a Median Eminence Site of Lnterleukin-6 Action. Neuroendocrinology 1992, 56, 46–53. [Google Scholar] [CrossRef]
- Žarković, M.; Ignjatović, S.; Dajak, M.; Ćirić, J.; Beleslin, B.; Savić, S.; Stojković, M.; Bulat, P.; Trbojević, B. Cortisol Response to ACTH Stimulation Correlates with Blood Interleukin 6 Concentration in Healthy Humans. Eur. J. Endocrinol. 2008, 159, 649–652. [Google Scholar] [CrossRef]
- Szeto, A.; Nation, D.A.; Mendez, A.J.; Dominguez-Bendala, J.; Brooks, L.G.; Schneiderman, N.; Mccabe, P.M. Oxytocin Attenuates NADPH-Dependent Superoxide Activity and IL-6 Secretion in Macrophages and Vascular Cells. Am. J. Physiol. Endocrinol. Metab. 2008, 295, 1495–1501. [Google Scholar] [CrossRef]
| Dog ID | Sex | Age (Months) | Heart Rate (BPM) | Temp. (C°) | Behav. Score | 5-HT (µM/L) | TRP (µM/L) | CRT (ng/mL) | OXT (ng/mL) | IL-6 (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 72 | 78 | 39.0 | 1 | 0.48 | 29.1 | 47.41 | 51.99 | 0.010 |
| 2 | M | 120 | 90 | 38.2 | 1 | 1.09 | 16.8 | 49.3 | 99.43 | 0.45 |
| 3 | M | 180 | 96 | 38.5 | 1 | 0.73 | 19.1 | 16.29 | 92.4 | 0.42 |
| 4 | M | 48 | 118 | 39.6 | 1 | 0.36 | 11.5 | 38.83 | 42.06 | 0.14 |
| 5 | M | 36 | 90 | 38.3 | 1 | 0.82 | 11.1 | 48.1 | 34.83 | 0.38 |
| 6 | F | 72 | 150 | 37.9 | 1 | n.d. | n.d. | n.d. | 61.35 | n.d. |
| 7 | M | 24 | 144 | 39.1 | 1 | 1.28 | 12.4 | 8.53 | 55.9 | 0.09 |
| 8 | F | 120 | 84 | 38.6 | 1 | 0.91 | 21.7 | 49.81 | 22.69 | 0.78 |
| 9 | M | 108 | 138 | 38.6 | 1 | 1.03 | 8.7 | 71.36 | 21.82 | 0.011 |
| 10 | F | 24 | 132 | 38.6 | 1 | 0.47 | 10.1 | 0.85 | 225.77 | 0.21 |
| 11 | F | 96 | 72 | 38.5 | 1 | 0.77 | 14.3 | 10.12 | 303.64 | 0.67 |
| 12 | M | 6 | 90 | 39.5 | 2 | 1.46 | 13.5 | n.d. | 14.01 | 0.061 |
| 13 | M | 96 | 84 | 39.0 | 2 | 0.63 | 18.6 | 41.08 | 39.2 | 0.42 |
| 14 | M | 96 | 78 | 38.7 | 2 | 0.33 | 9.1 | 37.28 | 81.66 | 0.012 |
| 15 | M | 60 | 101 | 38.0 | 2 | 0.96 | 24.5 | 74.56 | 82.97 | n.d. |
| 16 | M | 84 | 100 | 39.0 | 3 | 1.28 | 12.4 | 0.87 | 107.96 | 0.06 |
| 17 | F | 36 | 98 | 39.5 | 3 | 0.21 | 20.0 | 31.94 | 14.04 | n.d. |
| 18 | M | 84 | 102 | 39.1 | 3 | 0.63 | 18.6 | 71.23 | 14.03 | n.d. |
| 19 | F | 24 | 118 | 38.3 | 3 | 1.04 | 23.9 | 0.88 | 90.83 | 3.06 |
| 20 | M | 132 | 104 | 37.4 | 3 | 0.52 | 15.7 | 24.67 | 71.72 | 0.009 |
| 21 | M | 132 | 78 | 38.2 | 3 | 0.69 | 17.7 | 79.87 | 40.63 | 0.22 |
| 22 | F | 36 | 84 | 38.5 | 4 | n.d. | n.d. | n.d. | 71.09 | n.d. |
| 23 | M | 36 | 96 | 39.2 | 4 | 0.52 | 15.7 | n.d. | n.d. | n.d. |
| 24 | M | 60 | 102 | 38.2 | 5 | 0.89 | 15.0 | 35.5 | 45.01 | 0.45 |
| 25 | M | 60 | 96 | 39.1 | 5 | 0.91 | 21.7 | 43.39 | n.d. | n.d. |
| 26 | M | 60 | 108 | 39.4 | 6 | 1.17 | 20.1 | 0.89 | 42.56 | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazzano, V.; Curadi, M.C.; Baragli, P.; Mariti, C.; Cecchi, F.; Cavallo, S.; Sacchettino, L.; Gazzano, A. Physiological and Behavioral Evaluation of Shelter Dogs During Veterinary Routine Health Checks. Vet. Sci. 2025, 12, 583. https://doi.org/10.3390/vetsci12060583
Gazzano V, Curadi MC, Baragli P, Mariti C, Cecchi F, Cavallo S, Sacchettino L, Gazzano A. Physiological and Behavioral Evaluation of Shelter Dogs During Veterinary Routine Health Checks. Veterinary Sciences. 2025; 12(6):583. https://doi.org/10.3390/vetsci12060583
Chicago/Turabian StyleGazzano, Valentina, Maria Claudia Curadi, Paolo Baragli, Chiara Mariti, Francesca Cecchi, Stefano Cavallo, Luigi Sacchettino, and Angelo Gazzano. 2025. "Physiological and Behavioral Evaluation of Shelter Dogs During Veterinary Routine Health Checks" Veterinary Sciences 12, no. 6: 583. https://doi.org/10.3390/vetsci12060583
APA StyleGazzano, V., Curadi, M. C., Baragli, P., Mariti, C., Cecchi, F., Cavallo, S., Sacchettino, L., & Gazzano, A. (2025). Physiological and Behavioral Evaluation of Shelter Dogs During Veterinary Routine Health Checks. Veterinary Sciences, 12(6), 583. https://doi.org/10.3390/vetsci12060583









