The Individual Variations in Sperm Quality of High-Fertility Boars Impact the Offspring Production and Early Physiological Functions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Boars and Sows
2.3. Semen Collection
2.4. AI Doses Preparation
2.5. Spermatozoa Analysis
2.5.1. Sperm Motility
2.5.2. Sperm Viability
2.5.3. Sperm Acrosome Integrity
2.5.4. Sperm Mitochondrial Activity
2.5.5. Sperm DNA Fragmentation
2.6. Artificial Insemination (AI) and Pregnancy Diagnosis
2.7. Farrowing, Litter Performance, and Offspring Growth
2.8. Blood Collection and Analysis
2.9. Experimental Design
2.10. Statistical Analysis
3. Results
3.1. Descriptive Statistics and Within and Between Variable Set Correlation
3.2. Multivariate Linear Regression of Sperm Variables for Explaining Litter and Blood Parameters
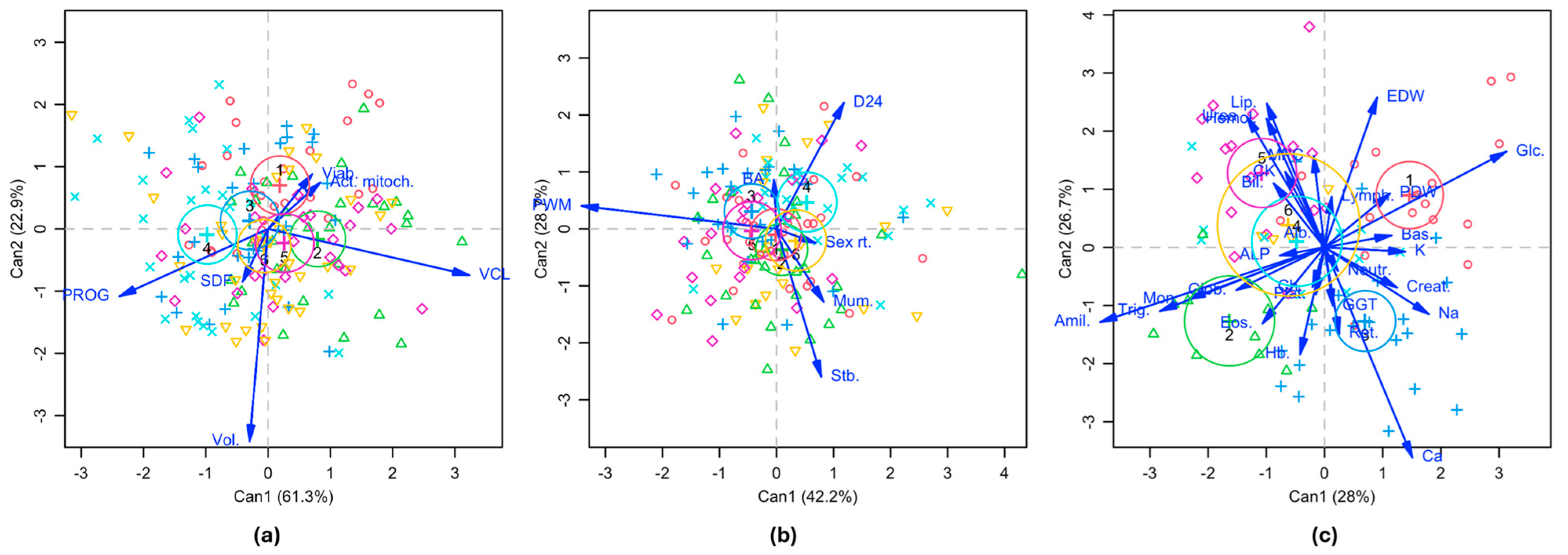
3.3. Canonical Correlation Analysis (CCA) of the Sperm Variable Set with the Litter and Blood Variables Sets
3.4. Canonical Discriminant Analysis (CDA) of the Sperm, Litter, and Blood Variables Sets for the Boar Factor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riesenbeck, A. Review on International Trade with Boar Semen. Reprod. Domest. Anim. 2011, 46, 1–3. [Google Scholar] [CrossRef] [PubMed]
- López Rodriguez, A.; Van Soom, A.; Arsenakis, I.; Maes, D. Boar Management and Semen Handling Factors Affect the Quality of Boar Extended Semen. Porc. Health Manag. 2017, 3, 15. [Google Scholar] [CrossRef]
- Koketsu, Y.; Tani, S.; Iida, R. Factors for Improving Reproductive Performance of Sows and Herd Productivity in Commercial Breeding Herds. Porc. Health Manag. 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of Lifetime Health around the Time of Conception: Causes and Consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Costa-Júnior, J.M.; Ferreira, S.M.; Kurauti, M.A.; Bernstein, D.L.; Ruano, E.G.; Kameswaran, V.; Schug, J.; Freitas-Dias, R.; Zoppi, C.C.; Boschero, A.C.; et al. Paternal Exercise Improves the Metabolic Health of Offspring via Epigenetic Modulation of the Germline. Int. J. Mol. Sci. 2022, 23, 1. [Google Scholar] [CrossRef]
- Bromfield, J.J.; Schjenken, J.E.; Chin, P.Y.; Care, A.S.; Jasper, M.J.; Robertson, S.A. Maternal Tract Factors Contribute to Paternal Seminal Fluid Impact on Metabolic Phenotype in Offspring. Proc. Natl. Acad. Sci. USA 2014, 111, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Gadea, J.; García-Vazquez, F.; Matás, C.; Gardón, J.C.; Cánovas, S.; Gumbao, D. Cooling and Freezing of Boar Spermatozoa: Supplementation of the Freezing Media with Reduced Glutathione Preserves Sperm Function. J. Androl. 2005, 26, 396–404. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.4.1; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Robinson, J.A.B.; Buhr, M.M. Impact of Genetic Selection on Management of Boar Replacement. Theriogenology 2005, 63, 668–678. [Google Scholar] [CrossRef]
- Evans, J.P.; Wilson, A.J.; Pilastro, A.; Garcia-Gonzalez, F. Ejaculate-Mediated Paternal Effects: Evidence, Mechanisms and Evolutionary Implications. Reproduction 2019, 157, R109–R126. [Google Scholar] [CrossRef]
- Merlot, E.; Pastorelli, H.; Prunier, A.; Père, M.C.; Louveau, I.; Lefaucheur, L.; Perruchot, M.H.; Meunier-Salaün, M.C.; Gardan-Salmon, D.; Gondret, F.; et al. Sow Environment during Gestation: Part I. Influence on Maternal Physiology and Lacteal Secretions in Relation with Neonatal Survival. Animal 2019, 13, 1432–1439. [Google Scholar] [CrossRef]
- Luongo, C.; Llamas-López, P.J.; Hernández-Caravaca, I.; Matás, C.; García-Vázquez, F.A. Should All Fractions of the Boar Ejaculate Be Prepared for Insemination Rather Than Using the Sperm Rich Only? Biology 2022, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Guardiola, S.M.; Párraga-Ros, E.; Seva, J.; Luongo, C.; García-Vázquez, F.A.; Soriano-Úbeda, C.; Matás, C. Artificial Insemination of All Ejaculated Sperm Fractions Accelerates Embryo Development and Increases the Uterine Vascularity in the Pig. Theriogenology 2024, 219, 32–38. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Muro, B.B.D.; Poor, A.P.; Leal, D.F.; Carnevale, R.F.; Shiroma, M.P.; Almond, G.W.; Garbossa, C.A.P.; Moreno, A.M.; Viana, C.H.C. Effects of Farrowing Induction with Prostaglandins on Farrowing Traits and Piglet Performance: A Systematic Review and Meta-Analysis. Theriogenology 2022, 180, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Edwards, S.A. Review: Improving the Performance of Neonatal Piglets. Animal 2022, 16, 100350. [Google Scholar] [CrossRef] [PubMed]
- Foxcroft, G.R.; Dixon, W.T.; Novak, S.; Putman, C.T.; Town, S.C.; Vinsky, M.D.A. The Biological Basis for Prenatal Programming of Postnatal Performance in Pigs. J. Anim. Sci. 2006, 84, E105–E112. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M. Role of Sperm Chromatin Abnormalities and DNA Damage in Male Infertility. Hum. Reprod. Update 2003, 9, 331–345. [Google Scholar] [CrossRef]
- Simon, L.; Murphy, K.; Shamsi, M.B.; Liu, L.; Emery, B.; Aston, K.I.; Hotaling, J.; Carrell, D.T. Paternal Influence of Sperm DNA Integrity on Early Embryonic Development. Hum. Reprod. 2014, 29, 2402–2412. [Google Scholar] [CrossRef]
- Colaco, S.; Sakkas, D. Paternal Factors Contributing to Embryo Quality. J. Assist. Reprod. Genet. 2018, 35, 1953–1968. [Google Scholar] [CrossRef]
- Mateo-Otero, Y.; Madrid-Gambin, F.; Llavanera, M.; Gomez-Gomez, A.; Haro, N.; Pozo, O.J.; Yeste, M. Sperm Physiology and in Vitro Fertilising Ability Rely on Basal Metabolic Activity: Insights from the Pig Model. Commun. Biol. 2023, 6, 344. [Google Scholar] [CrossRef]
- Morgan, H.L.; Watkins, A.J. The Influence of Seminal Plasma on Offspring Development and Health. Semin. Cell Dev. Biol. 2020, 97, 131–137. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H. Role of the Oviduct in Sperm Capacitation. Theriogenology 2007, 68, S138–S146. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, P.; Garriga, J.; Casas, I.; Yeste, M.; Bartumeus, F. Predicting Fertility from Sperm Motility Landscapes. Commun. Biol. 2022, 5, 1027. [Google Scholar] [CrossRef]
- Moraes, C.R.; Meyers, S. The Sperm Mitochondrion: Organelle of Many Functions. Anim. Reprod. Sci. 2018, 194, 71–80. [Google Scholar] [CrossRef]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria Functionality and Sperm Quality. Reproduction 2013, 146, 163–174. [Google Scholar] [CrossRef]
- Mateo-Otero, Y.; Llavanera, M.; Torres-Garrido, M.; Yeste, M. Embryo Development Is Impaired by Sperm Mitochondrial-Derived ROS. Biol. Res. 2024, 57, 5. [Google Scholar] [CrossRef]
- Satake, N.; Elliott, R.M.A.; Watson, P.F.; Holt, W.V. Sperm Selection and Competition in Pigs May Be Mediated by the Differential Motility Activation and Suppression of Sperm Subpopulations within the Oviduct. J. Exp. Biol. 2006, 209, 1560–1572. [Google Scholar] [CrossRef]
- Mcpherson, F.J.; Nielsen, S.G.; Chenoweth, P.J. Seminal Factors Influencing Return to Estrus in Female Pigs Following Artificial Insemination. Anim. Reprod. 2014, 11, 24–31. [Google Scholar]
- Bhadsavle, S.S.; Golding, M.C. Paternal Epigenetic Influences on Placental Health and Their Impacts on Offspring Development and Disease. Front. Genet. 2022, 13, 1068408. [Google Scholar] [CrossRef] [PubMed]
- Schjenken, J.E.; Robertson, S.A. The Female Response to Seminal Fluid. Physiol. Rev. 2020, 100, 1077–1117. [Google Scholar] [CrossRef]
- Berger, T.; Guerrero, V.; Boeldt, R.; Legacki, E.; Roberts, M.; Conley, A.J. Development of Porcine Accessory Sex Glands. Animals 2024, 14, 462. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Martinez, E.A.; Calvete, J.J.; Peña Vega, F.J.; Roca, J. Seminal Plasma: Relevant for Fertility? Int. J. Mol. Sci. 2021, 22, 4368. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Otero, Y.; Llavanera, M.; Recuero, S.; Delgado-Bermúdez, A.; Barranco, I.; Ribas-Maynou, J.; Yeste, M. Sperm DNA Damage Compromises Embryo Development, but Not Oocyte Fertilisation in Pigs. Biol. Res. 2022, 55, 15. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-López, M.J.; Espeso, G.; Evenson, D.P.; Roldan, E.R.S.; Gomendio, M. Paternal Levels of DNA Damage in Spermatozoa and Maternal Parity Influence Offspring Mortality in an Endangered Ungulate. Proc. R. Soc. B Biol. Sci. 2010, 277, 2541–2546. [Google Scholar] [CrossRef]
- Li, F.; Duan, X.; Li, M.; Ma, X. Sperm DNA Fragmentation Index Affect Pregnancy Outcomes and Offspring Safety in Assisted Reproductive Technology. Sci. Rep. 2024, 14, 356. [Google Scholar] [CrossRef]
- Opuwari, C.S.; Henkel, R.R. An Update on Oxidative Damage to Spermatozoa and Oocytes. BioMed Res. Int. 2016, 2016, 9540142. [Google Scholar] [CrossRef]
- Peña, S.T.; Stone, F.; Gummow, B.; Parker, A.J.; Paris, D.B.B.P. Tropical Summer Induces DNA Fragmentation in Boar Spermatozoa: Implications for Evaluating Seasonal Infertility. Reprod. Fertil. Dev. 2019, 31, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Czubaszek, M.; Andraszek, K.; Banaszewska, D. Influence of the Age of the Individual on the Stability of Boar Sperm Genetic Material. Theriogenology 2020, 147, 176–182. [Google Scholar] [CrossRef]
- Ausejo, R.; Martínez, J.M.; Soler-llorens, P.; Bolarín, A.; Tejedor, T.; Falceto, M.V. Seasonal Changes of Nuclear DNA Fragmentation in Boar Spermatozoa in Spain. Animals 2021, 11, 465. [Google Scholar] [CrossRef]
- Okada, Y.; Kamatani, Y. Common Genetic Factors for Hematological Traits in Humans. J. Hum. Genet. 2012, 57, 161–169. [Google Scholar] [CrossRef]
- McClellan, K.; Lindemann, M.; Levesque, C. Assessment of Hemoglobin Concentration in Sows and Their Offspring over Consecutive Reproductive Cycles. J. Swine Health Prod. 2024, 32, 248–257. [Google Scholar] [CrossRef]
- Mayr, A.; Pajk, W.; Hasibeder, W. Oxygen Supply and Consumption in Tissues. In Sepsis and Organ Dysfunction; Bauer, A.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–2. [Google Scholar]
- Pittman, R.N. Regulation of Tissue Oxygenation; Morgan & Claypool: San Rafael, CA, USA, 2016; ISBN 9781615041770. [Google Scholar]
- Álvarez-Rodríguez, M.; Martínez, C.; Wright, D.; Barranco, I.; Roca, J.; Rodríguez-Martínez, H. The Transcriptome of Pig Spermatozoa, and Its Role in Fertility. Int. J. Mol. Sci. 2020, 21, 1572. [Google Scholar] [CrossRef] [PubMed]
- Bischoft, R.J.; Lee, C.-S.; Brandon, M.R.; Meeusen, E. Inflammatory Response in the Pig Uterus Induced by Seminal Plasma. J. Reprod. Immunol. 1994, 26, 131–146. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.; Jasper, M.J.; Warnes, G.M.; Armstrong, D.T.; Robertson, S.A. Seminal Plasma Regulates Endometrial Cytokine Expression, Leukocyte Recruitment and Embryo Development in the Pig. Reproduction 2004, 128, 237–247. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Robertson, S.A. Seminal Fluid and Immune Adaptation for Pregnancy—Comparative Biology in Mammalian Species. Reprod. Domest. Anim. 2014, 49, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Waberski, D.; Schäfer, J.; Bölling, A.; Scheld, M.; Henning, H.; Hambruch, N.; Schuberth, H.J.; Pfarrer, C.; Wrenzycki, C.; Hunter, R.H.F. Seminal Plasma Modulates the Immune-Cytokine Network in the Porcine Uterine Tissue and Pre-Ovulatory Follicles. PLoS ONE 2018, 13, e0202654. [Google Scholar] [CrossRef]
- Pliszczak-Król, A.; Rząsa, A.; Gemra, M.; Król, J.; Łuczak, G.; Zyzak, A.; Zalewski, D.; Iwaszko-Simonik, A.; Graczyk, S. Age-Related Changes of Platelet and Plasma Coagulation Parameters in Young Pigs. J. Vet. Diagn. Investig. 2016, 28, 561–567. [Google Scholar] [CrossRef]
- Faustini, M.; Bronzo, V.; Maffeo, G.; Russo, V.; Munari, E.; Vigo, D. Reference Intervals and Age-Related Changes for Platelet Count, Mean Platelet Volume and Plateletcrit in Healthy Pre-Weaning Piglets in Italy. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2003, 50, 466–469. [Google Scholar] [CrossRef]
- Vahedi Raad, M.; Firouzabadi, A.M.; Tofighi Niaki, M.; Henkel, R.; Fesahat, F. The Impact of Mitochondrial Impairments on Sperm Function and Male Fertility: A Systematic Review. Reprod. Biol. Endocrinol. 2024, 22, 83. [Google Scholar] [CrossRef]

| Litter Parameter | Model Components (Sperm Analysis Variables) | Coefficient Estimate | Coefficient Standard Error | p-Value | Model Adjusted R2 | Model p-Value |
|---|---|---|---|---|---|---|
| Total Born | Intercept | 0.115 | 0.130 | 0.380 | 0.05 | 0.085 |
| Ejaculate Volume | −0.734 | 0.415 | 0.085 | |||
| Stillborn | Intercept | −0.077 | 0.145 | 0.600 | 0.07 | 0.043 |
| Viability | 2.275 | 1.091 | 0.043 | |||
| Females | Intercept | −0.015 | 0.148 | 0.9193 | 0.09 | 0.093 |
| Ejaculate Volume | −1.047 | 0.456 | 0.0271 | |||
| Progressive Motility | 2.343 | 1.112 | 0.0416 | |||
| VCL | 1.041 | 0.609 | 0.0950 | |||
| DNA Fragmentation | −3.451 | 2.473 | 0.1706 | |||
| Piglet Weight 24 h | Intercept | 0.107 | 0.158 | 0.501 | 0.139 | 0.029 |
| Progressive Motility | 2.484 | 1.176 | 0.041 | |||
| VCL | 1.340 | 0.647 | 0.045 | |||
| DNA Fragmentation | −7.745 | 2.634 | 0.005 | |||
| Litter Weight 24 h | Intercept | −0.009 | 0.164 | 0.958 | 0.12 | 0.065 |
| Progressive Motility | 2.118 | 1.223 | 0.091 | |||
| VCL | 1.353 | 0.778 | 0.090 | |||
| Viability | −2.214 | 1.560 | 0.164 | |||
| DNA Fragmentation | −7.778 | 2.800 | 0.008 |
| Blood Parameter | Model Components (Sperm Analysis Variables) | Coefficient Estimate | Coefficient Standard Error | p-Value | Model Adjusted R2 | Model p-Value |
|---|---|---|---|---|---|---|
| Hemolysis | Intercept | 0.207 | 0.140 | 0.146 | 0.15 | 0.013 |
| Ejaculate Volume | −0.916 | 0.449 | 0.048 | |||
| DNA Fragmentation | 3.810 | 1.317 | 0.006 | |||
| Globulins | Intercept | −0.059 | 0.133 | 0.659 | 0.12 | 0.025 |
| Ejaculate Volume | 1.020 | 0.427 | 0.022 | |||
| DNA Fragmentation | −2.780 | 1.253 | 0.032 | |||
| Urea | Intercept | −0.008 | 0.123 | 0.948 | 0.06 | 0.065 |
| Viability | 1.756 | 0.926 | 0.065 | |||
| Glucose | Intercept | −0.108 | 0.121 | 0.377 | 0.22 | 0.002 |
| Ejaculate Volume | −1.865 | 0.504 | <0.001 | |||
| Viability | −3.236 | 1.176 | 0.009 | |||
| Triglycerides | Intercept | 0.072 | 0.115 | 0.537 | 0.05 | 0.079 |
| Ejaculate volume | 0.664 | 0.368 | 0.079 | |||
| Lipase | Intercept | −0.018 | 0.121 | 0.881 | 0.16 | 0.011 |
| Progressive motility | 1.414 | 0.446 | 0.003 | |||
| Viability | 2.244 | 1.086 | 0.045 | |||
| Creatin Kinase (CK) | Intercept | −0.038 | 0.140 | 0.789 | 0.05 | 0.083 |
| Viability | 1.877 | 1.056 | 0.083 | |||
| Alanine Aminotransaminase (ALT) | Intercept | 0.019 | 0.143 | 0.893 | 0.06 | 0.058 |
| Viability | 2.093 | 1.075 | 0.058 | |||
| Calcium | Intercept | 0.107 | 0.158 | 0.501 | 0.139 | 0.029 |
| Progressive Motility | 2.484 | 1.176 | 0.041 | |||
| VCL | 1.340 | 0.647 | 0.045 | |||
| Potassium | Intercept | −0.191 | 0.168 | 0.263 | 0.08 | 0.071 |
| VCL | 0.580 | 0.380 | 0.134 | |||
| DNA Fragmentation | −2.420 | 1.500 | 0.114 | |||
| Sodium | Intercept | −0.197 | 0.150 | 0.196 | 0.06 | 0.063 |
| DNA Fragmentation | −2.561 | 1.340 | 0.063 | |||
| Chlorine | Intercept | −0.198 | 0.147 | 0.185 | 0.07 | 0.043 |
| DNA Fragmentation | −2.738 | 1.312 | 0.043 | |||
| Erythrocytes Concentration | Intercept | 0.000 | 0.141 | 0.999 | 0.06 | 0.064 |
| DNA fragmentation | −2.400 | 1.262 | 0.064 | |||
| Cell Hemoglobin Concentration Mean (CHCM) | Intercept | 1.116 | 0.138 | 0.999 | 0.06 | 0.053 |
| DNA Fragmentation | 2.464 | 1.235 | 0.064 | |||
| Hemoglobin Distribution Width (HDW) | Intercept | −0.011 | 0.123 | 0.929 | 0.18 | 0.007 |
| VCL | 1.075 | 0.388 | 0.008 | |||
| Viability | −3.968 | 1.220 | 0.002 | |||
| Leukocytes Concentration | Intercept | 0.031 | 0.126 | 0.805 | 0.14 | 0.016 |
| Progressive Motility | −1.327 | 0.528 | 0.016 | |||
| VCL | −1.207 | 0.408 | 0.005 | |||
| Lymphocytes Concentration | Intercept | −0.022 | 0.126 | 0.864 | 0.08 | 0.067 |
| Ejaculate Volume | −0.996 | 0.527 | 0.066 | |||
| Viability | −2.894 | 1.229 | 0.023 | |||
| Monocytes (%) | Intercept | 0.134 | 0.103 | 0.203 | 0.10 | 0.048 |
| Ejaculate Volume | 0.566 | 0.348 | 0.112 | |||
| Progressive Motility | −0.798 | 0.334 | 0.022 | |||
| Monocytes Concentration | Intercept | 0.141 | 0.116 | 0.231 | 0.07 | 0.090 |
| Progressive Motility | −0.953 | 0.428 | 0.031 | |||
| Viability | −1.593 | 1.040 | 0.133 | |||
| Eosinophils (%) | Intercept | 0.144 | 0.113 | 0.212 | 0.05 | 0.080 |
| Ejaculate Volume | 0.651 | 0.363 | 0.080 | |||
| Eosinophils Concentration | Intercept | 0.107 | 0.115 | 0.358 | 0.04 | 0.096 |
| VCL | −0.470 | 0.276 | 0.096 | |||
| Basophils (%) | Intercept | 0.081 | 0.130 | 0.533 | 0.13 | 0.046 |
| Ejaculate Volume | −0.928 | 0.515 | 0.079 | |||
| Progressive Motility | −1.784 | 0.619 | 0.006 | |||
| Viability | −2.026 | 1.379 | 0.150 | |||
| DNA Fragmentation | 3.604 | 1.671 | 0.037 | |||
| Basophils Concentration | Intercept | 0.030 | 0.113 | 0.793 | 0.23 | 0.004 |
| Ejaculate Volume | −1.285 | 0.470 | 0.009 | |||
| Progressive Motility | −1.250 | 0.414 | 0.004 | |||
| Viability | −3.761 | 1.246 | 0.004 | |||
| Platelets Concentration | Intercept | 0.028 | 0.115 | 0.812 | 0.09 | 0.052 |
| Ejaculate Volume | 0.528 | 0.367 | 0.158 | |||
| VCL | −0.516 | 0.272 | 0.065 | |||
| Plateletcrit | Intercept | −0.023 | 0.111 | 0.834 | 0.05 | 0.071 |
| VCL | −0.493 | 0.266 | 0.071 | |||
| Platelet Distribution Width (PDW) | Intercept | 0.001 | 0.104 | 0.990 | 0.06 | 0.069 |
| Ejaculate Volume | −0.622 | 0.333 | 0.069 | |||
| Platelet Component Distribution Width (PCDW) | Intercept | −0.048 | 0.115 | 0.681 | 0.08 | 0.033 |
| Progressive Motility | −0.788 | 0.358 | 0.033 | |||
| Platelet Mass Distribution Width (PMDW) | Intercept | 0.040 | 0.127 | 0.756 | 0.09 | 0.059 |
| Ejaculate Volume | −0.961 | 0.408 | 0.023 | |||
| DNA Fragmentation | 1.714 | 1.196 | 0.159 | |||
| Reticulocytes Concentration | Intercept | −0.086 | 0.142 | 0.548 | 0.09 | 0.081 |
| Progressive Motility | −0.972 | 0.589 | 0.107 | |||
| VCL | −1.125 | 0.518 | 0.036 | |||
| Viability | 2.594 | 1.419 | 0.075 |
| Variate | Canonical R | Canonical R2 | Eigenvalues | Percent | Cumulative Percent | Wilks Lambda | d.f. | p-Value |
|---|---|---|---|---|---|---|---|---|
| X1Y1 | 0.396 | 0.157 | 0.186 | 44.5 | 44.5 | 0.676 | 36 | 0.020 |
| X Variate | X1 | Y Variate | Y1 |
|---|---|---|---|
| Ejaculate Volume | 0.27 | Born Alive | 0.16 |
| Progressive Motility | 0.86 | Stillborn | −0.69 |
| VCL | −0.89 | Mummified | −0.26 |
| Viability | −0.59 | Dead 24 h | 0.63 |
| Mitochondrial Activity | −0.71 | Sex Ratio | 0.03 |
| DNA Fragmentation | 0.03 | Piglet Weight 24 h | 0.07 |
| Variable Set | Variate | Canonical R | Canonical R2 | Eigenvalues | Percent | Cumulative Percent | Wilks Lambda | d.f. | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Sperm parameters | Can1 | 0.482 | 0.233 | 0.303 | 61.3 | 61.3 | 0.639 | 30 | <0.001 |
| Can2 | 0.319 | 0.102 | 0.113 | 22.9 | 84.2 | 0.833 | 20 | 0.069 | |
| Litter parameters | Can1 | 0.334 | 0.112 | 0.126 | 42.2 | 42.2 | 0.752 | 30 | 0.090 |
| Can2 | 0.281 | 0.079 | 0.086 | 28.7 | 70.9 | 0.846 | 20 | 0.247 | |
| Blood parameters | Can1 | 0.755 | 0.570 | 1.325 | 28.0 | 28.0 | 0.040 | 140 | <0.001 |
| Can2 | 0.747 | 0.557 | 1.260 | 26.7 | 54.7 | 0.092 | 108 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo-Guardiola, S.M.; Luongo, C.; Martínez-Pastor, F.; Soriano-Úbeda, C.; Matás, C. The Individual Variations in Sperm Quality of High-Fertility Boars Impact the Offspring Production and Early Physiological Functions. Vet. Sci. 2025, 12, 582. https://doi.org/10.3390/vetsci12060582
Toledo-Guardiola SM, Luongo C, Martínez-Pastor F, Soriano-Úbeda C, Matás C. The Individual Variations in Sperm Quality of High-Fertility Boars Impact the Offspring Production and Early Physiological Functions. Veterinary Sciences. 2025; 12(6):582. https://doi.org/10.3390/vetsci12060582
Chicago/Turabian StyleToledo-Guardiola, Santa María, Chiara Luongo, Felipe Martínez-Pastor, Cristina Soriano-Úbeda, and Carmen Matás. 2025. "The Individual Variations in Sperm Quality of High-Fertility Boars Impact the Offspring Production and Early Physiological Functions" Veterinary Sciences 12, no. 6: 582. https://doi.org/10.3390/vetsci12060582
APA StyleToledo-Guardiola, S. M., Luongo, C., Martínez-Pastor, F., Soriano-Úbeda, C., & Matás, C. (2025). The Individual Variations in Sperm Quality of High-Fertility Boars Impact the Offspring Production and Early Physiological Functions. Veterinary Sciences, 12(6), 582. https://doi.org/10.3390/vetsci12060582







