Radiomic Feature Characteristics of Ovine Pulmonary Adenocarcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. CT Acquisition and Analysis
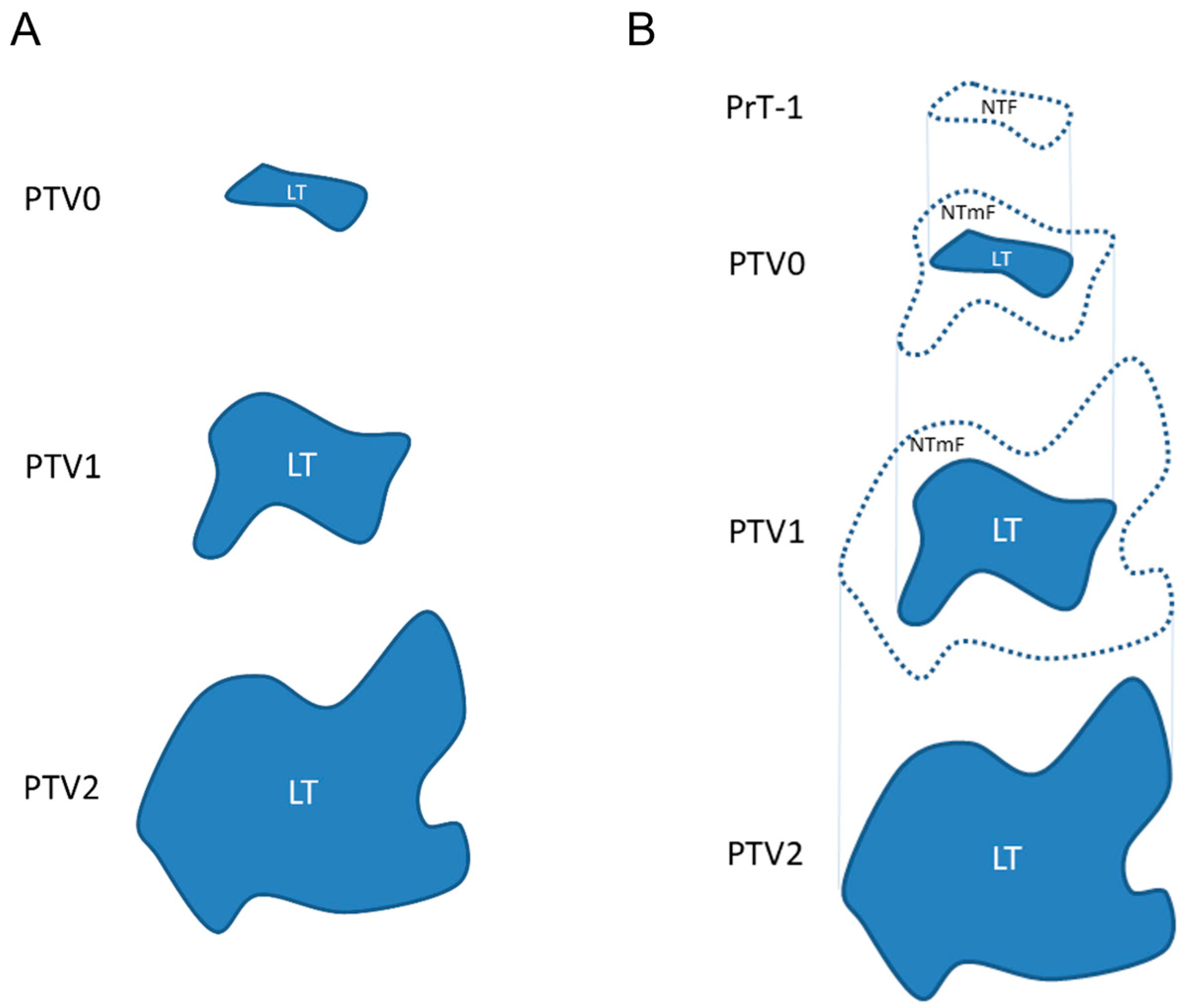
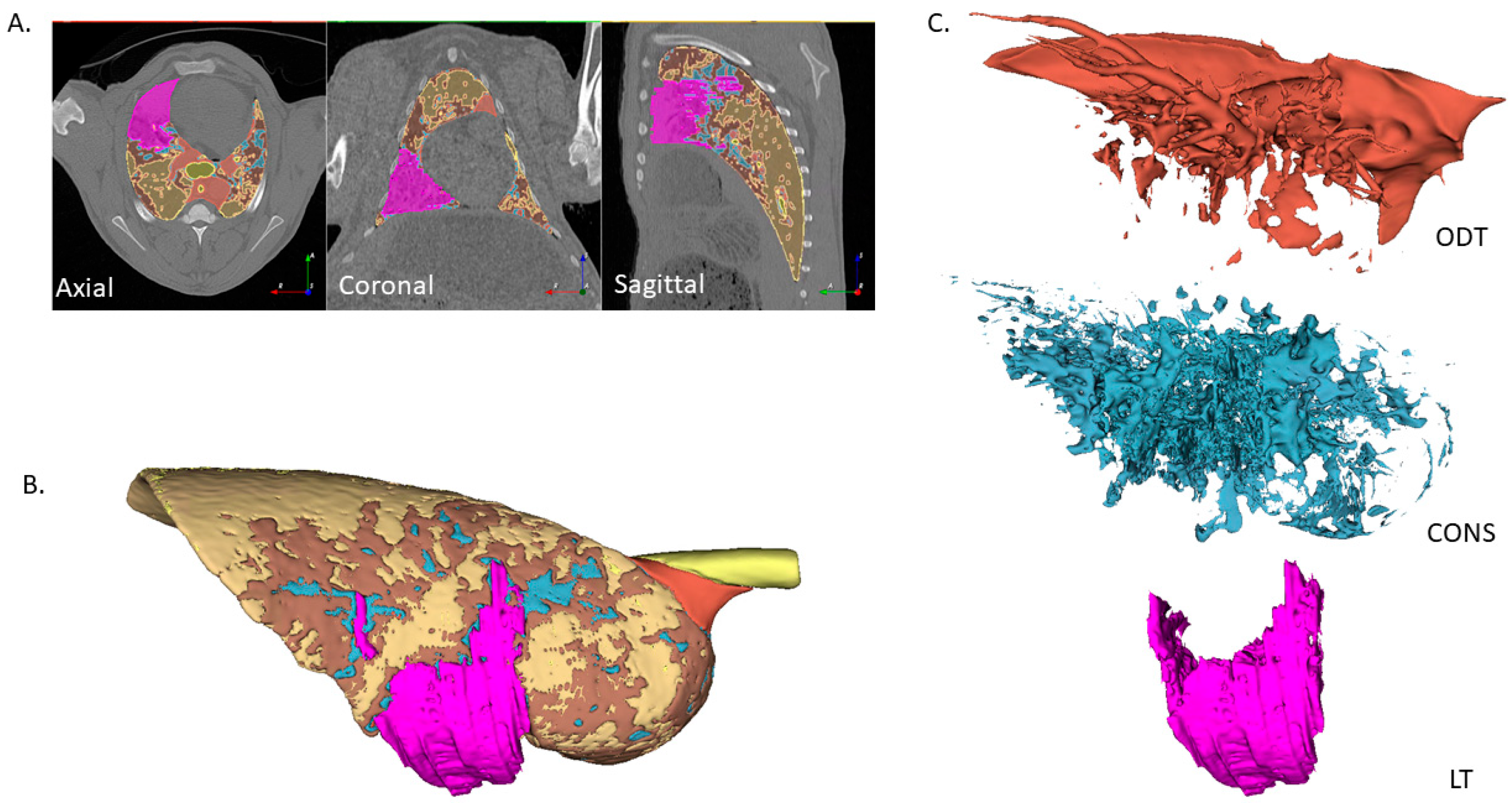
2.3. Image Segmentation
2.4. Image Processing
2.5. Image Registration
2.6. OPA Definitive Diagnosis
2.7. Data Handling, Visualisation, and Statistics
3. Results
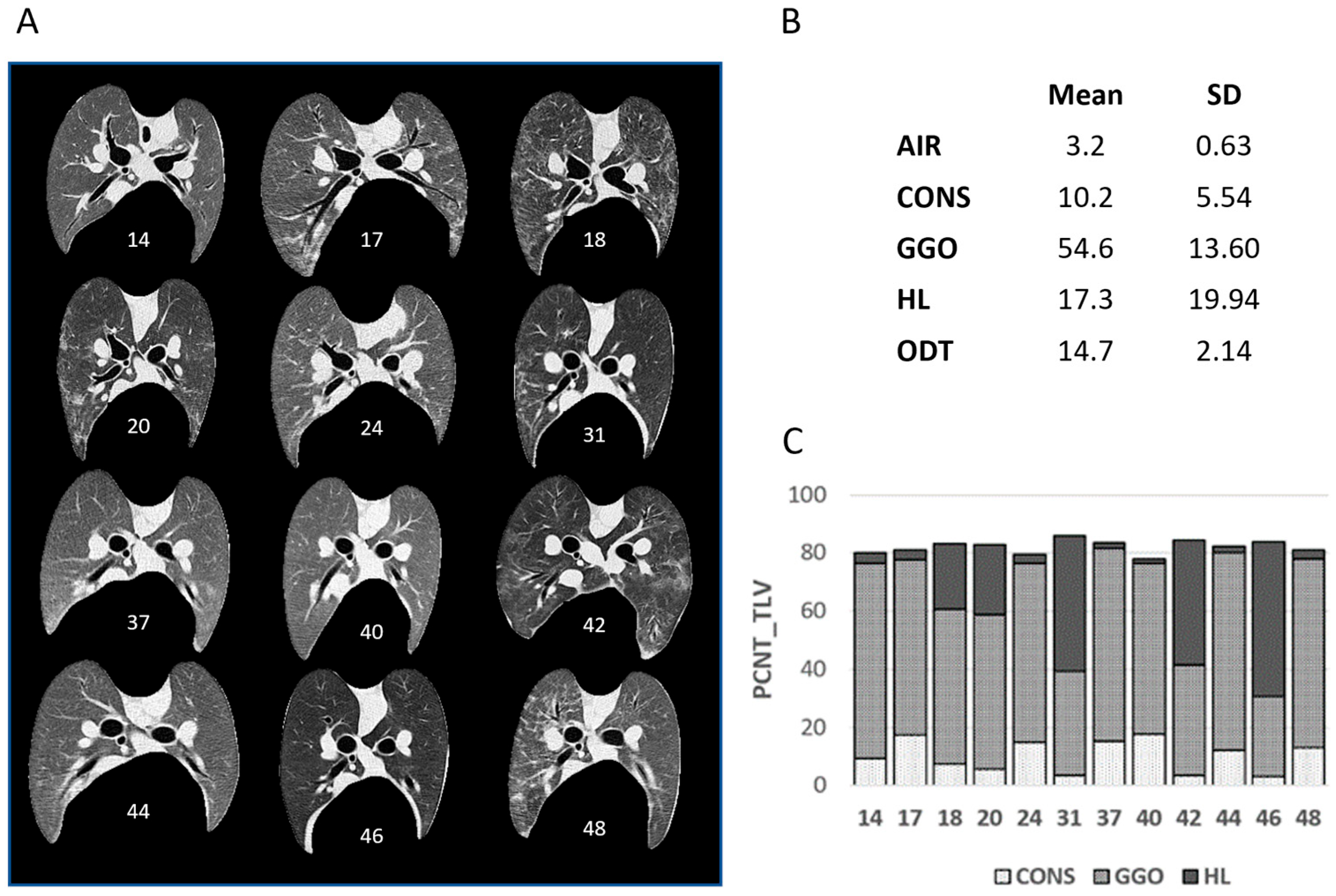
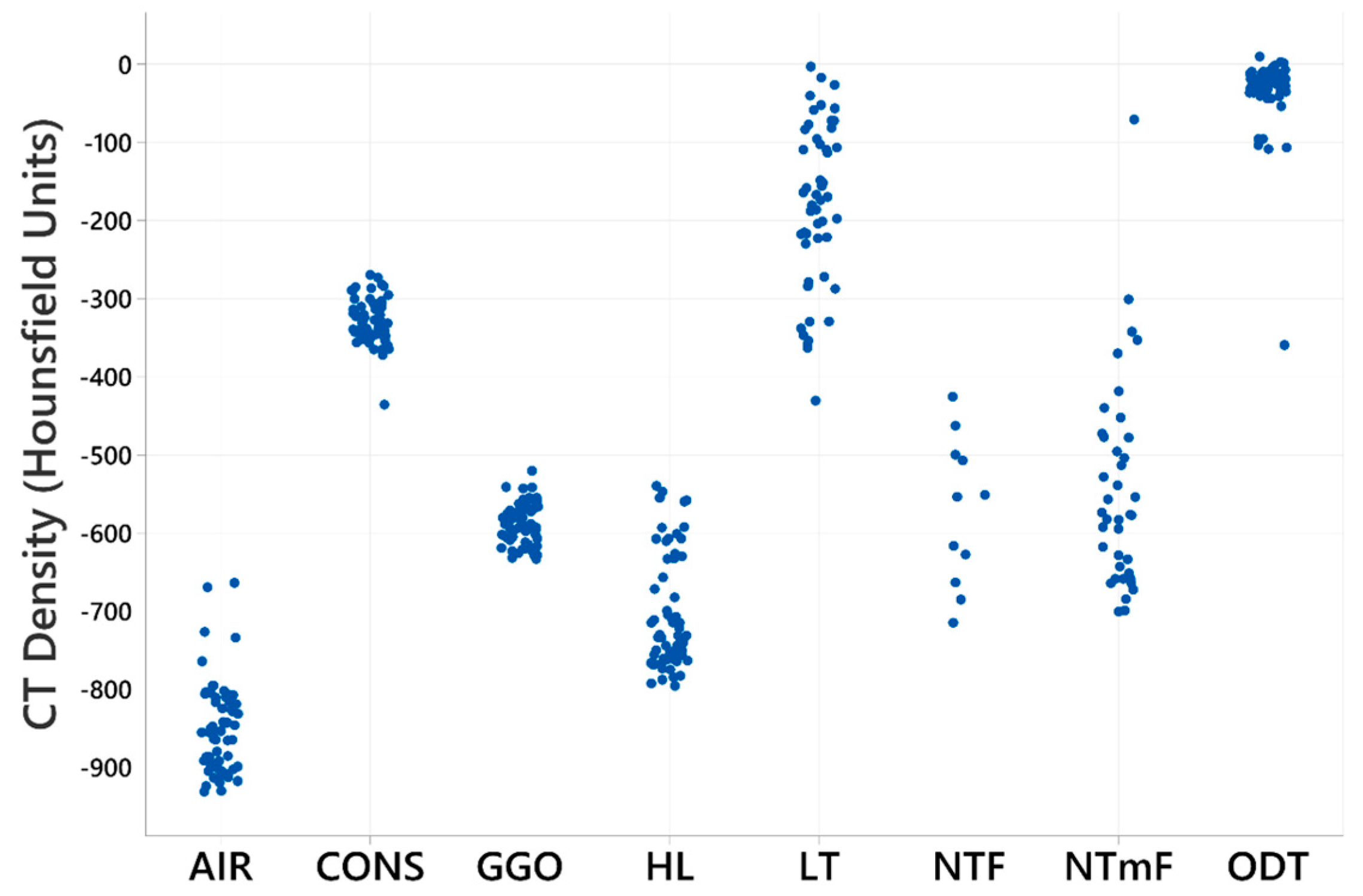
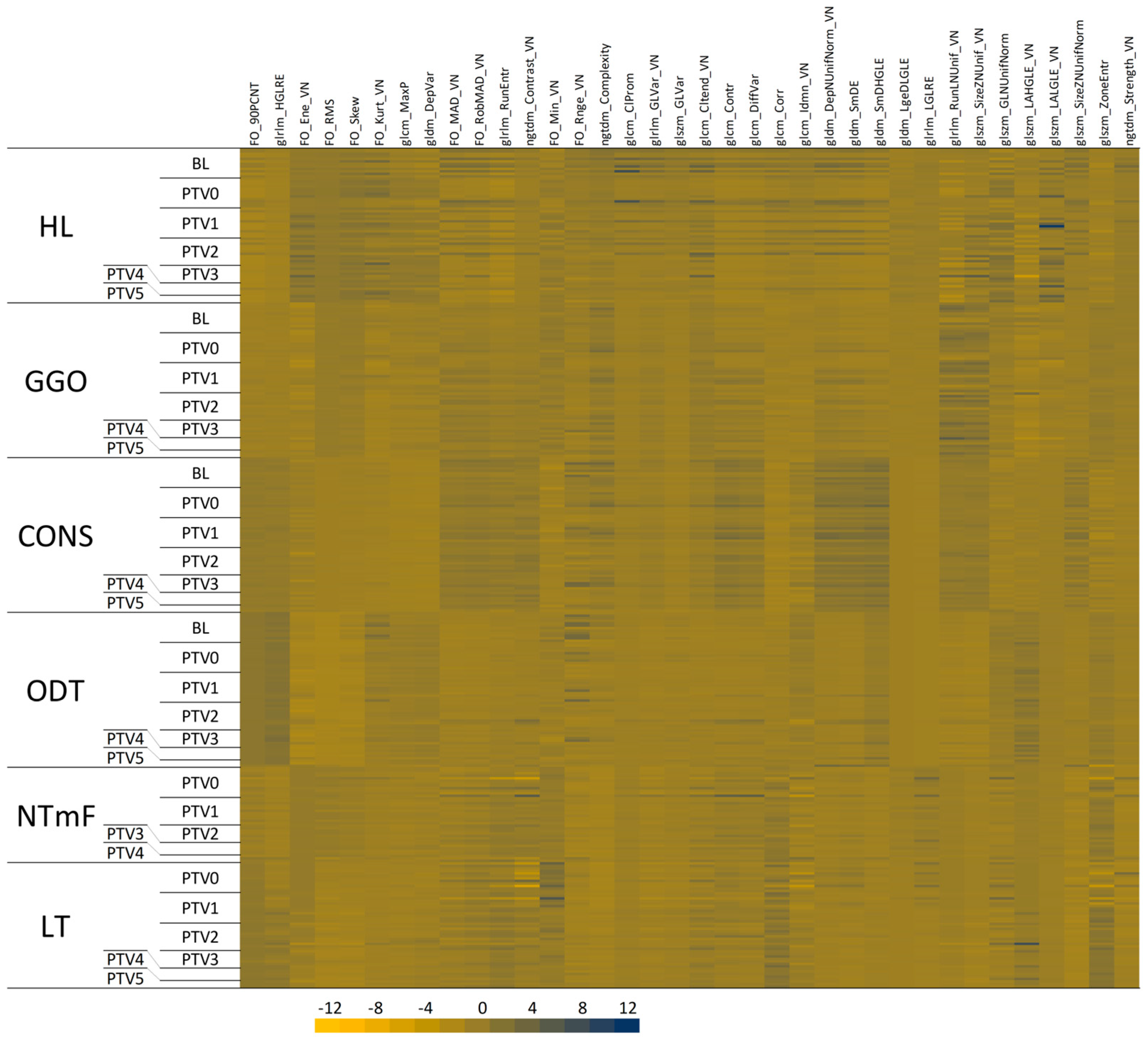
3.1. Density-Based Segmentation of Sheep Lung CT Images
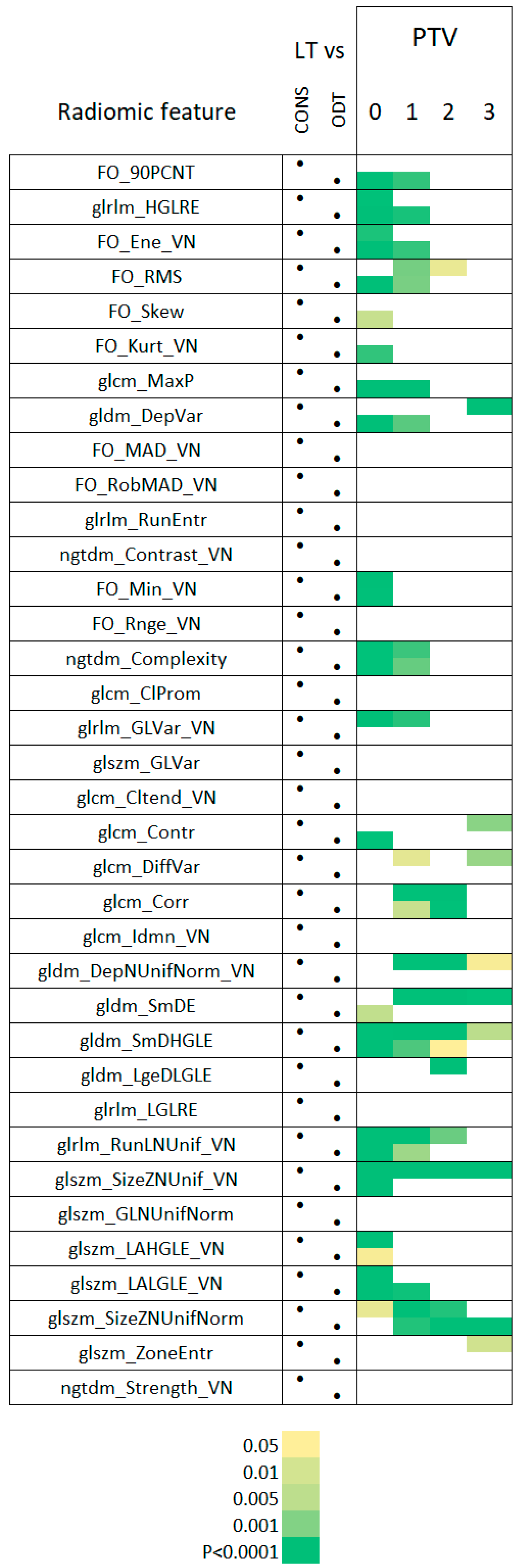
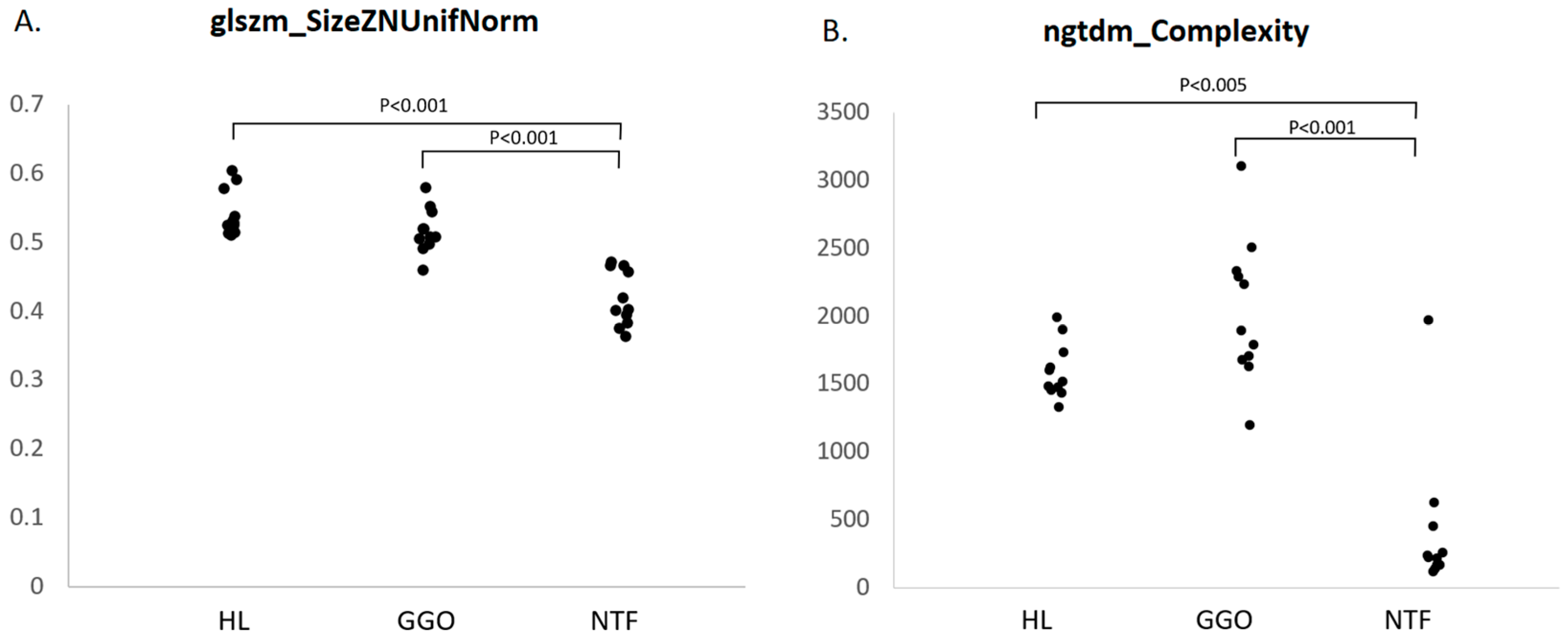
3.2. Radiomic Feature Characteristics of LT Segments
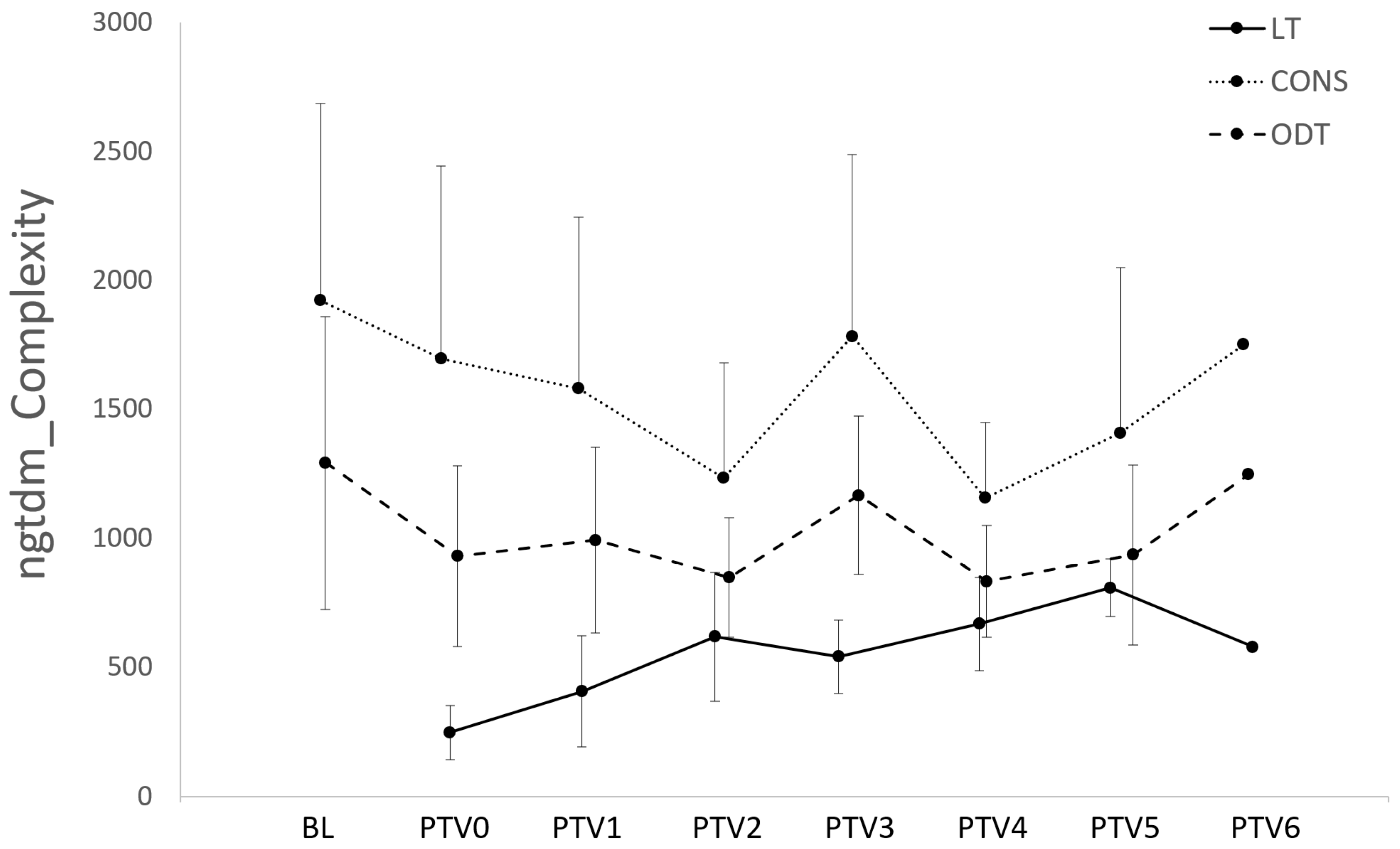
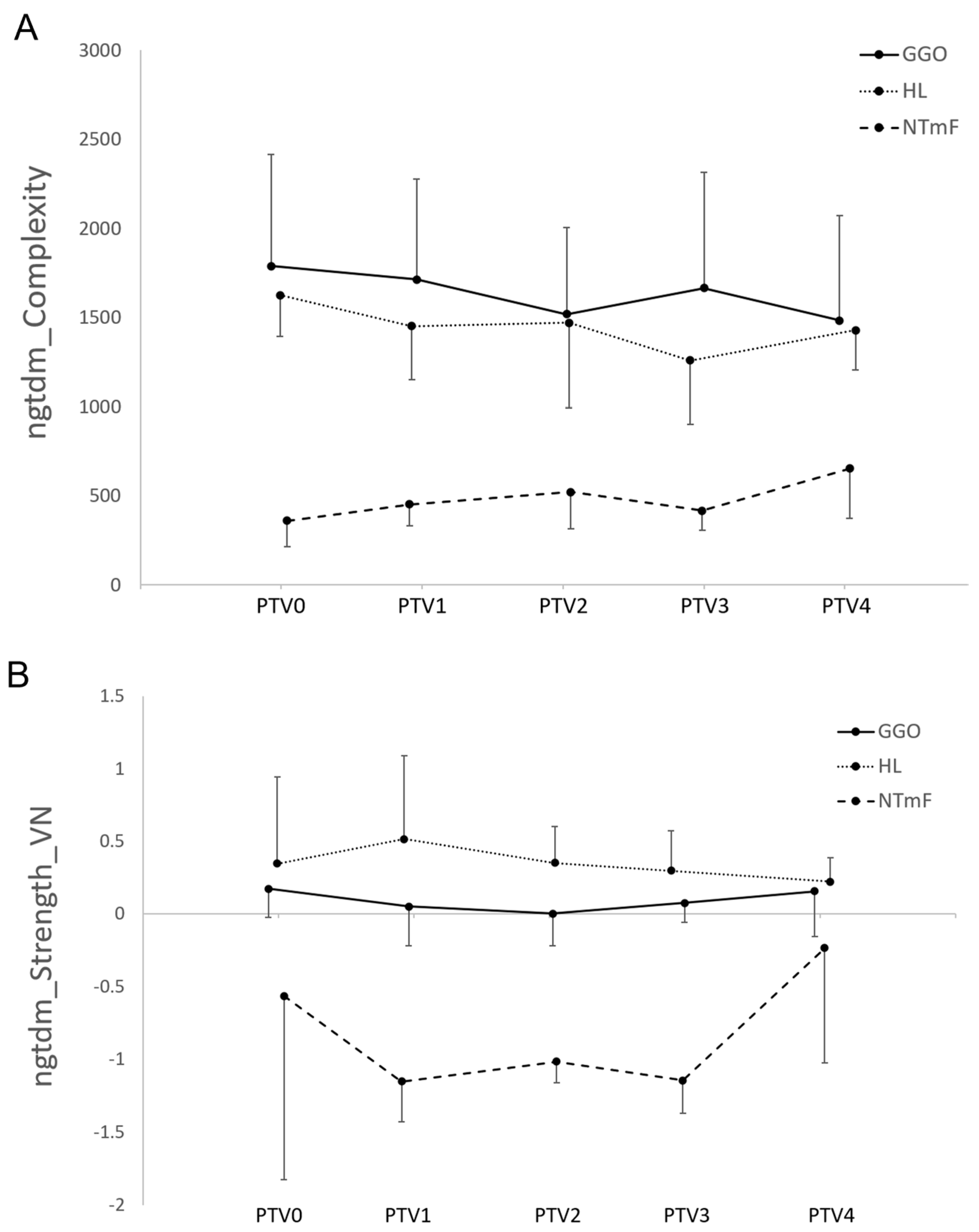
3.3. Radiomic Features of LT Segments Demonstrate Time-Dependent Changes
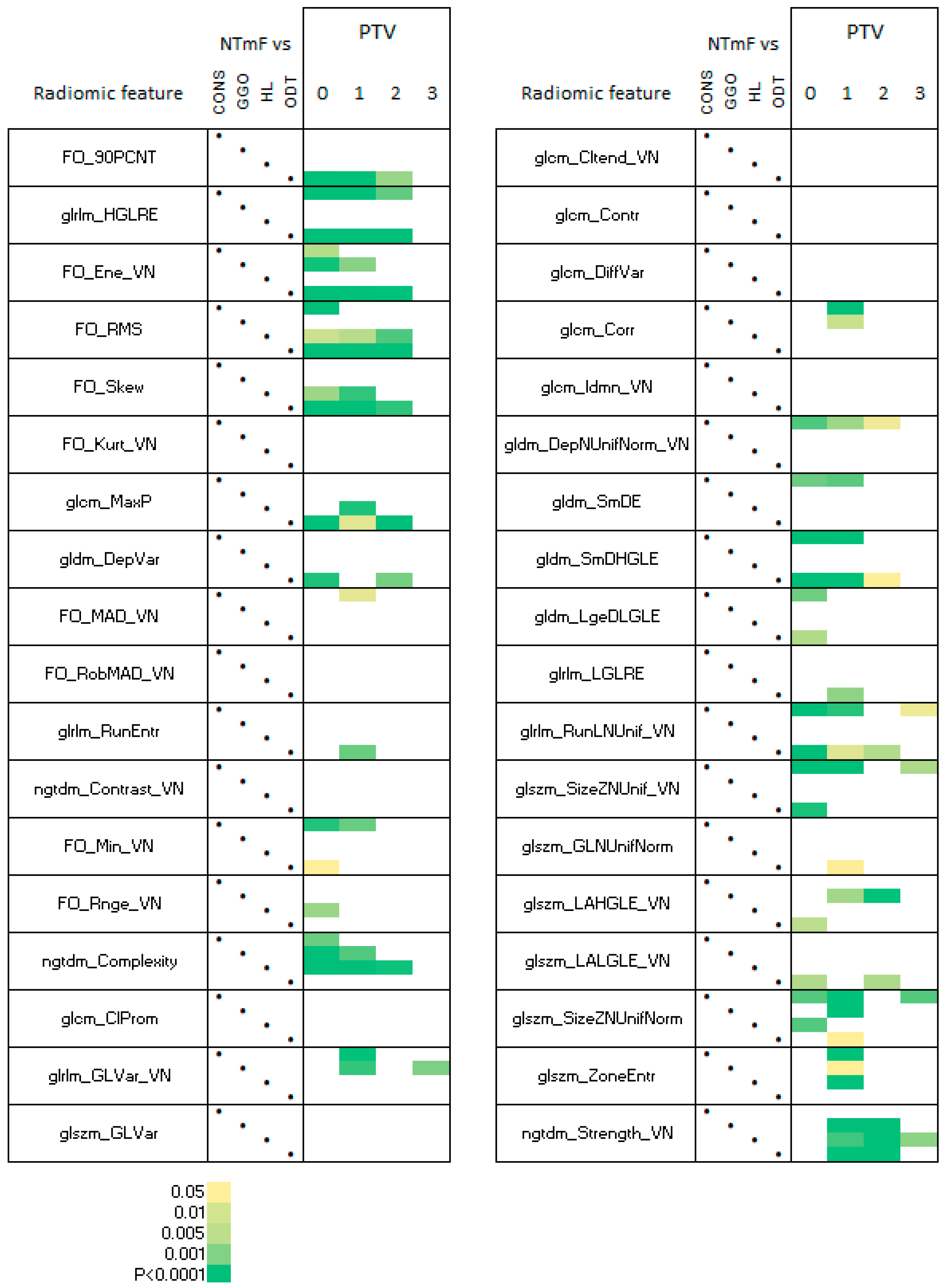
3.4. Radiomic Features of NTF Segments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BL | Baseline |
| ClProm | Cluster Prominence |
| Cltend | Cluster Tendency |
| CONS | Consolidated |
| Contr | Contrast |
| Corr | Correlation |
| CPAP | Continuous positive airway pressure |
| CT | Computed tomography |
| DepNUnifNorm | Dependence Non-Uniformity Normalized |
| DepVar | Dependence Variance |
| DICOM | Digital Imaging and Communications in Medicine |
| DiffVar | Difference Variance |
| Ene | Energy |
| env | JSRV envelope glycoprotein |
| FO | First order |
| GGO | Ground glass opacity |
| glcm | Gray-Level Co-occurrence Matrix |
| GLCM | Graylevel co-occurrence matrix |
| gldm | Gray Level Dependence Matrix |
| GLNUnifNorm | Gray Level Non-Uniformity Normalized |
| glrlm | Gray Level Run Length Matrix |
| glszm | Gray Level Size Zone Matrix |
| GLVar | Gray Level Variance |
| H&E | Haematoxylin and eosin |
| HGLRE | High Gray Level Run Emphasis |
| HL | Healthy lung |
| Idmn | Inverse Difference Moment Normalized |
| IHC | Immunohistochemistry |
| JSRV | Jaagsiekte sheep retrovirus |
| Kurt | Kurtosis |
| LAHGLE | Large Area High Gray Level Emphasis |
| LALGLE | Large Area Low Gray Level Emphasis |
| LgeDLGLE | Large Dependence Low Gray Level Emphasis |
| LGLRE | Low Gray Level Run Emphasis |
| LT | Lung tumour |
| LTR | Long terminal repeat |
| MAD | Mean Absolute Deviation |
| MaxP | Maximum Probability |
| Min | Minimum |
| ngtdm | Neighbouring Gray Tone Difference Matrix |
| NSCLC | Non-small cell lung carcinoma |
| NTF | Nascent tumour field |
| NTmF | Nascent tumour margin field |
| ODT | Other dense tissue |
| OPA | Ovine pulmonary adenocarcinoma |
| PrT-1 | Pre-tumour-1 month |
| PTV | Post-tumour volume |
| RF | Radiomic feature |
| RMS | Root Mean Squared |
| Rnge | Range |
| RobMAD | Robust Mean Absolute Deviation |
| RunEntr | Run Entropy |
| RunLNUnif | Run Length Non-Uniformity |
| SizeZNUnif | Size Zone Non-Uniformity |
| SizeZNUnifNorm | Size Zone Non-Uniformity Normalized |
| Skew | Skewness |
| SmDE | Small Dependence Emphasis |
| SmDHGLE | Small Dependence High Gray Level Emphasis |
| TLV | Total lung volume |
| TmF | Tumour margin field |
| ZoneEntr | Zone Entropy |
| 90PCNT | 90th Percentile |
References
- Geady, C.; Keller, H.; Siddiqui, I.; Bilkey, J.; Dhani, N.C.; Jaffray, D.A. Bridging the gap between micro- and macro-scales in medical imaging with textural analysis—A biological basis for CT radiomics classifiers? Phys. Medica 2020, 72, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, M.R.; Gillies, R.J. The Biological Meaning of Radiomic Features. Radiology 2021, 299, E256. [Google Scholar] [CrossRef] [PubMed]
- Digumarthy, S.R.; Padole, A.M.; Gullo, R.L.; Sequist, L.V.; Kalra, M.K. Can CT radiomic analysis in NSCLC predict histology and EGFR mutation status? Medicine 2019, 98, e13963. [Google Scholar] [CrossRef]
- Digumarthy, S.R.; Padole, A.M.; Lo Gullo, R.; Singh, R.; Shepard, J.O.; Kalra, M.K. CT texture analysis of histologically proven benign and malignant lung lesions. Medicine 2018, 97, e11172. [Google Scholar] [CrossRef]
- Shi, L.; He, Y.; Yuan, Z.; Benedict, S.; Valicenti, R.; Qiu, J.; Rong, Y. Radiomics for Response and Outcome Assessment for Non-Small Cell Lung Cancer. Technol. Cancer Res. Treat. 2018, 17, 1533033818782788. [Google Scholar] [CrossRef]
- Cavouras, D.; Prassopoulos, P.; Pantelidis, N. Image analysis methods for solitary pulmonary nodule characterization by computed tomography. Eur. J. Radiol. 1992, 14, 169–172. [Google Scholar] [CrossRef] [PubMed]
- McNitt-Gray, M.F.; Hart, E.M.; Wyckoff, N.; Sayre, J.W.; Goldin, J.G.; Aberle, D.R. A pattern classification approach to characterizing solitary pulmonary nodules imaged on high resolution CT: Preliminary results. Med. Phys. 1999, 26, 880–888. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.-H.; Jia, Z.-W.; Li, H.-K.; Liang, Z.-G.; Li, K.-C.; He, Q. Multilevel binomial logistic prediction model for malignant pulmonary nodules based on texture features of CT image. Eur. J. Radiol. 2010, 74, 124–129. [Google Scholar] [CrossRef]
- Dennie, C.; Thornhill, R.; Sethi-Virmani, V.; Souza, C.A.; Bayanati, H.; Gupta, A.; Maziak, D. Role of quantitative computed tomography texture analysis in the differentiation of primary lung cancer and granulomatous nodules. Quant. Imaging Med. Surg. 2016, 6, 6–15. [Google Scholar] [CrossRef]
- Able, H.; Wolf-Ringwall, A.; Rendahl, A.; Ober, C.P.; Seelig, D.M.; Wilke, C.T.; Lawrence, J. Computed tomography radiomic features hold prognostic utility for canine lung tumors: An analytical study. PLoS ONE 2021, 16, e0256139. [Google Scholar] [CrossRef]
- Yoon, Y.; Hwang, T.; Lee, H. Prediction of radiographic abnormalities by the use of bag-of-features and convolutional neural networks. Vet. J. 2018, 237, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Boissady, E.; de La Comble, A.; Zhu, X.; Hespel, A.M. Artificial intelligence evaluating primary thoracic lesions has an overall lower error rate compared to veterinarians or veterinarians in conjunction with the artificial intelligence. Vet. Radiol. Ultrasound 2020, 61, 619–627. [Google Scholar] [CrossRef]
- Bouhali, O.; Bensmail, H.; Sheharyar, A.; David, F.; Johnson, J.P. A Review of Radiomics and Artificial Intelligence and Their Application in Veterinary Diagnostic Imaging. Vet. Sci. 2022, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.J.; Martineau, H.M.; Cousens, C. Pathology and pathogenesis of ovine pulmonary adenocarcinoma. J. Comp. Pathol. 2010, 142, 260–283. [Google Scholar] [CrossRef]
- Cousens, C.; Meehan, J.; Collie, D.; Wright, S.; Chang, Z.; Todd, H.; Moore, J.; Grant, L.; Daniel, C.R.; Tennant, P.; et al. Tracking Ovine Pulmonary Adenocarcinoma Development Using an Experimental Jaagsiekte Sheep Retrovirus Infection Model. Genes 2024, 15, 1019. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yin, Y.; Wang, H.; Chang, Z.; Liu, Z.; Cui, L. A review of original articles published in the emerging field of radiomics. Eur. J. Radiol. 2020, 127, 108991. [Google Scholar] [CrossRef]
- Wu, G.; Jochems, A.; Refaee, T.; Ibrahim, A.; Yan, C.; Sanduleanu, S.; Woodruff, H.C.; Lambin, P. Structural and functional radiomics for lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3961–3974. [Google Scholar] [CrossRef]
- Zaffino, P.; Marzullo, A.; Moccia, S.; Calimeri, F.; De Momi, E.; Bertucci, B.; Arcuri, P.P.; Spadea, M.F. An Open-Source COVID-19 CT Dataset with Automatic Lung Tissue Classification for Radiomics. Bioengineering 2021, 8, 26. [Google Scholar] [CrossRef]
- Collie, D.; Cousens, C.; Wright, S.; Chang, Z.; Meehan, J.; Brown, H.; Gray, C.D.; MacGillivray, T.J.; Griffiths, D.J.; Eckert, C.E.; et al. Spatial encoding and growth-related change of sheep lung radiomic features. Front. Vet. Sci. 2025, 12, 1495278. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix. (Version 0.95). 2024. Available online: https://github.com/taiyun/corrplot (accessed on 1 January 2021).
- Welch, M.L.; McIntosh, C.; Haibe-Kains, B.; Milosevic, M.F.; Wee, L.; Dekker, A.; Huang, S.H.; Purdie, T.G.; O’Sullivan, B.; Aerts, H.; et al. Vulnerabilities of radiomic signature development: The need for safeguards. Radiother. Oncol. 2019, 130, 2–9. [Google Scholar] [CrossRef]
- Palmarini, M.; Datta, S.; Omid, R.; Murgia, C.; Fan, H. The long terminal repeat of Jaagsiekte sheep retrovirus is preferentially active in differentiated epithelial cells of the lungs. J. Virol. 2000, 74, 5776–5787. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Miller, A.D. Oncogenic transformation by the jaagsiekte sheep retrovirus envelope protein. Oncogene 2007, 26, 789–801. [Google Scholar] [CrossRef]
- Scott, P.R.; Dagleish, M.P.; Cousens, C. Development of superficial lung lesions monitored on farm by serial ultrasonographic examination in sheep with lesions confirmed as ovine pulmonary adenocarcinoma at necropsy. Ir. Vet. J. 2018, 71, 23. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Jiang, Y.; Tian, D.; Zhang, J.; Guo, Y.; Li, G.; Qin, D.; Li, Z. Radiomics for identifying lung adenocarcinomas with predominant lepidic growth manifesting as large pure ground-glass nodules on CT images. PLoS ONE 2022, 17, e0269356. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.E.; Meehan, J.; Sullivan, P.; Marland, J.R.K.; Greenhalgh, S.N.; Gregson, R.; Clutton, R.E.; Ward, C.; Cousens, C.; Griffiths, D.J.; et al. Ovine Pulmonary Adenocarcinoma: A Unique Model to Improve Lung Cancer Research. Front. Oncol. 2019, 9, 335. [Google Scholar] [CrossRef]
- Wu, L.; Lou, X.; Kong, N.; Xu, M.; Gao, C. Can quantitative peritumoral CT radiomics features predict the prognosis of patients with non-small cell lung cancer? A systematic review. Eur. Radiol. 2023, 33, 2105–2117. [Google Scholar] [CrossRef]
- Liao, G.; Huang, L.; Wu, S.; Zhang, P.; Xie, D.; Yao, L.; Zhang, Z.; Yao, S.; Shanshan, L.; Wang, S.; et al. Preoperative CT-based peritumoral and tumoral radiomic features prediction for tumor spread through air spaces in clinical stage I lung adenocarcinoma. Lung Cancer 2022, 163, 87–95. [Google Scholar] [CrossRef]
- Dou, T.H.; Coroller, T.P.; van Griethuysen, J.J.M.; Mak, R.H.; Aerts, H. Peritumoral radiomics features predict distant metastasis in locally advanced NSCLC. PLoS ONE 2018, 13, e0206108. [Google Scholar] [CrossRef]
- Wang, T.; She, Y.; Yang, Y.; Liu, X.; Chen, S.; Zhong, Y.; Deng, J.; Zhao, M.; Sun, X.; Xie, D.; et al. Radiomics for Survival Risk Stratification of Clinical and Pathologic Stage IA Pure-Solid Non-Small Cell Lung Cancer. Radiology 2022, 302, 425–434. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, M.; Xiong, W.; Zhu, F.; Li, Q.; Zhao, L.; Zhao, Z. The value of intratumoral and peritumoral radiomics features in differentiating early-stage lung invasive adenocarcinoma (≤3 cm) subtypes. Transl. Cancer Res. 2024, 13, 202–216. [Google Scholar] [CrossRef]
| Sheep ID | Age (Months) | Sex | Body Weight (kg) |
|---|---|---|---|
| 14 | 5 | MN | 41 |
| 17 | 5 | F | 47 |
| 18 | 5 | F | 35 |
| 20 | 5 | F | 37 |
| 24 | 5 | MN | 38 |
| 31 | 4 | F | 20 |
| 37 | 4 | F | 25 |
| 40 | 4 | MN | 22 |
| 42 | 4 | MN | 30 |
| 44 | 4 | MN | 25 |
| 46 | 4 | MN | 20 |
| 48 | 4 | MN | 30 |
| Week | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sheep | 0 | 4 | 8 | 12 | 16 | 20 | 24 | 28 | 32 | 36 | 40 |
| 14 | PTV0 | PTV1 | PTV2 | ||||||||
| 17 | PTV0 | PTV1 | PTV2 | PTV3 | |||||||
| 18 | PTV0 | PTV1 | PTV2 | PTV3 | PTV4 | ||||||
| 20 | PTV0 | PTV1 | PTV2 | PTV3 | |||||||
| 24 | PTV0 | PTV1 | PTV2 | PTV3 | PTV4 | PTV5 | |||||
| 31 | PTV0 | PTV1 | PTV2 | PTV3 | PTV4 | PTV5 | PTV6 | ||||
| 37 | PTV0 | PTV1 | PTV2 | ||||||||
| 40 | PTV0 | PTV1 | PTV2 | ||||||||
| 42 | PTV0 | PTV1 | PTV2 | ||||||||
| 44 | PTV0 | PTV1 | PTV2 | PTV3 | PTV4 | PTV5 | |||||
| 46 | PTV0 | PTV1 | PTV2 | PTV3 | PTV4 | ||||||
| 48 | PTV0 | PTV1 | |||||||||
| Feature Class | Features |
|---|---|
| First Order | 90th Percentile (FO_90PCNT), Energy (FO_Ene_VN), Kurtosis (FO_Kurt_VN), Mean Absolute Deviation (FO_MAD_VN), Minimum (FO_Min_VN), Root Mean Squared (FO_RMS), Range (FO_Rnge_VN), Robust Mean Absolute Deviation (FO_RobMAD_VN), Skewness (FO_Skew) |
| GLCM Gray Level Co-occurrence Matrix | Cluster Prominence (glcm_ClProm), Cluster Tendency (glcm_Cltend_VN), Contrast (glcm_Contr), Correlation (glcm_Corr), Difference Variance (glcm_DiffVar), Inverse Difference Moment Normalized (glcm_Idmn_VN), MaximumProbability (glcm_MaxP) |
| GLDM Gray Level Dependence Matrix | Dependence Non-Uniformity Normalized (gldm_DepNUnifNorm_VN), Dependence Variance (gldm_DepVar), Large Dependence Low Gray Level Emphasis (gldm_LgeDLGLE), Small Dependence Emphasis (gldm_SmDE), Small Dependence High Gray Level Emphasis (gldm_SmDHGLE) |
| GLRLM Gray Level Run Length Matrix | Gray Level Variance (glrlm_GLVar_VN), High Gray Level Run Emphasis (glrlm_HGLRE), Low Gray Level Run Emphasis (glrlm_LGLRE), Run Entropy (glrlm_RunEntr), Run Length Non-Uniformity (glrlm_RunLNUnif_VN) |
| GLSZM Gray Level Size Zone Matrix | Gray Level Non-Uniformity Normalized (glszm_GLNUnifNorm), Gray Level Variance (glszm_GLVar), Large Area High Gray Level Emphasis (glszm_LAHGLE_VN), Large Area Low Gray Level Emphasis (glszm_LALGLE_VN), Size Zone Non-Uniformity (glszm_SizeZNUnif_VN), Size Zone Non-Uniformity Normalized (glszm_SizeZNUnifNorm), Zone Entropy (glszm_ZoneEntr) |
| NGTDM Neighbouring Gray Tone Difference Matrix | Complexity (ngtdm_Complexity), Contrast (ngtdm_Contrast_VN), Strength (ngtdm_Strength_VN) |
| AIR | HL | GGO | CONS | LT | NTmF | ODT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| glrlm_HGLRE | ** | ↑ | |||||||||||||
| FO_Ene_VN | ** | ↓ | |||||||||||||
| FO_Skew | ** | ↓ | ** | ↓ | |||||||||||
| gldm_DepVar | ** | ↑ | |||||||||||||
| ngtdm_Complexity | ** | ↓ | ** | ↑ | |||||||||||
| glcm_Idmn_VN | * | ↑ | |||||||||||||
| gldm_SmDE | ** | ↓ | |||||||||||||
| glrlm_LGLRE | * | ↓ | |||||||||||||
| glszm_SizeZNUnif_VN | ** | ↓ | |||||||||||||
| glszm_LALGLE_VN | ** | ↑ | |||||||||||||
| glszm_ZoneEntr | ** | ↑ | |||||||||||||
| ngtdm_Strength_VN | * | ↓ | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collie, D.; Chang, Z.; Meehan, J.; Wright, S.H.; Cousens, C.; Moore, J.; Todd, H.; Savage, J.; Brown, H.; Gray, C.D.; et al. Radiomic Feature Characteristics of Ovine Pulmonary Adenocarcinoma. Vet. Sci. 2025, 12, 400. https://doi.org/10.3390/vetsci12050400
Collie D, Chang Z, Meehan J, Wright SH, Cousens C, Moore J, Todd H, Savage J, Brown H, Gray CD, et al. Radiomic Feature Characteristics of Ovine Pulmonary Adenocarcinoma. Veterinary Sciences. 2025; 12(5):400. https://doi.org/10.3390/vetsci12050400
Chicago/Turabian StyleCollie, David, Ziyuan Chang, James Meehan, Steven H. Wright, Chris Cousens, Jo Moore, Helen Todd, Jennifer Savage, Helen Brown, Calum D. Gray, and et al. 2025. "Radiomic Feature Characteristics of Ovine Pulmonary Adenocarcinoma" Veterinary Sciences 12, no. 5: 400. https://doi.org/10.3390/vetsci12050400
APA StyleCollie, D., Chang, Z., Meehan, J., Wright, S. H., Cousens, C., Moore, J., Todd, H., Savage, J., Brown, H., Gray, C. D., MacGillivray, T. J., Griffiths, D. J., Eckert, C. E., Storer, N., & Gray, M. (2025). Radiomic Feature Characteristics of Ovine Pulmonary Adenocarcinoma. Veterinary Sciences, 12(5), 400. https://doi.org/10.3390/vetsci12050400








