First Molecular Insights into the Presence of Canine Kobuvirus in Ecuadorian Dogs Through the Standardization of a Sensitive SYBR Green RT-qPCR Assay
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Nucleic Acid Extraction
2.3. Primer Design
2.4. Standard Curve Construction
2.5. RT-qPCR Assay
2.6. Analytic Specificity of the RT-qPCR Assay
2.7. Analytic Repeatability of the RT-qPCR Assay
2.8. Sequencing and Phylogenetic Analysis
2.9. Diagnostic Performance of the RT-qPCR Assay
2.10. Statistical Analysis
2.11. GenBank Accession Numbers
3. Results
3.1. Analytical and Diagnostic Validation Parameters
3.1.1. Standard Curve and Sensitivity
3.1.2. Analysis of Analytic Specificity
3.1.3. Diagnostic Performance of the Assay
3.1.4. Analysis of Repeatability
3.2. Detection of CaKoV
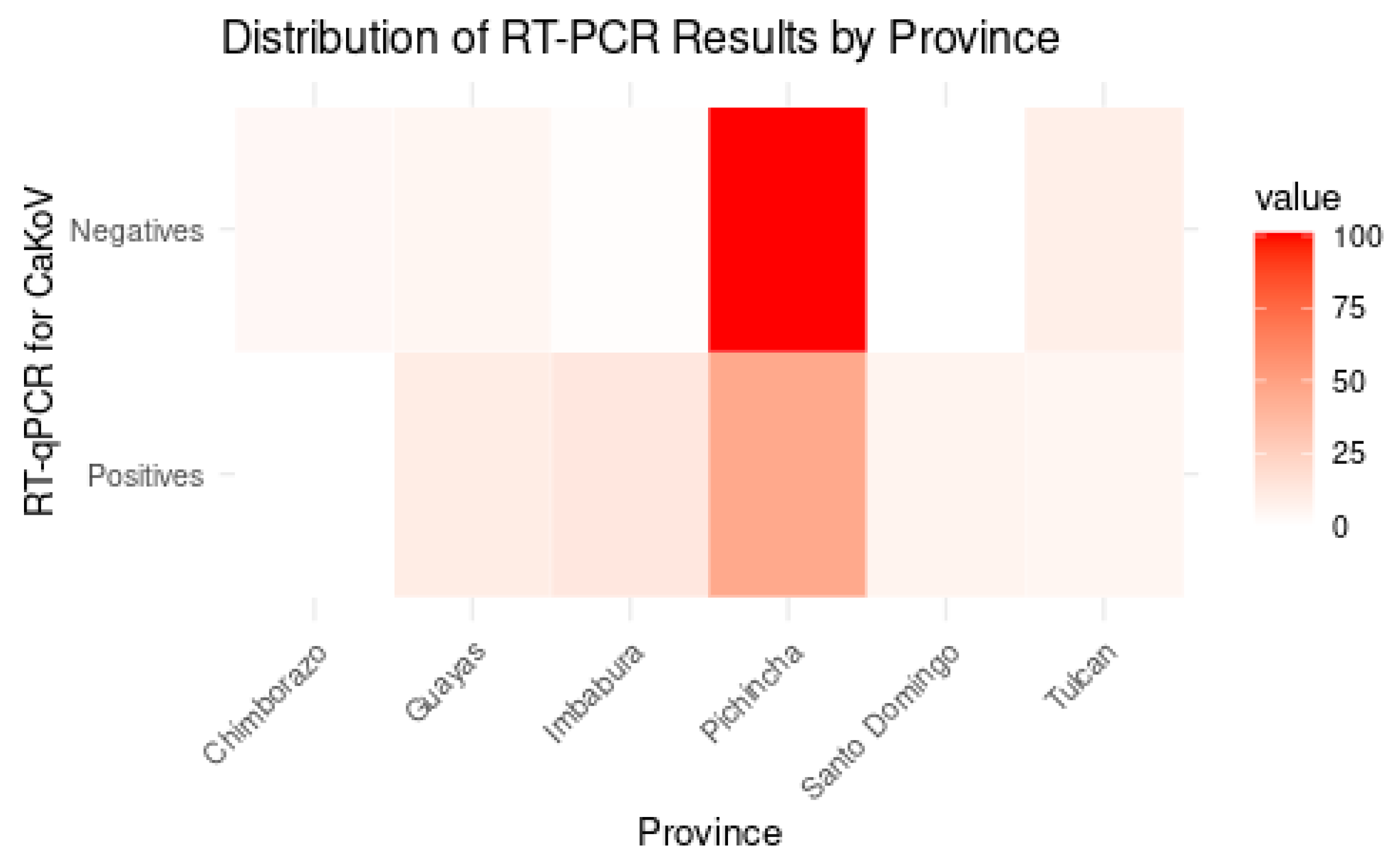
3.2.1. CaKoV Distribution in Dogs by Location
3.2.2. CaKoV Distribution in Dogs by Age
3.2.3. Co-Infections
3.2.4. Statistics
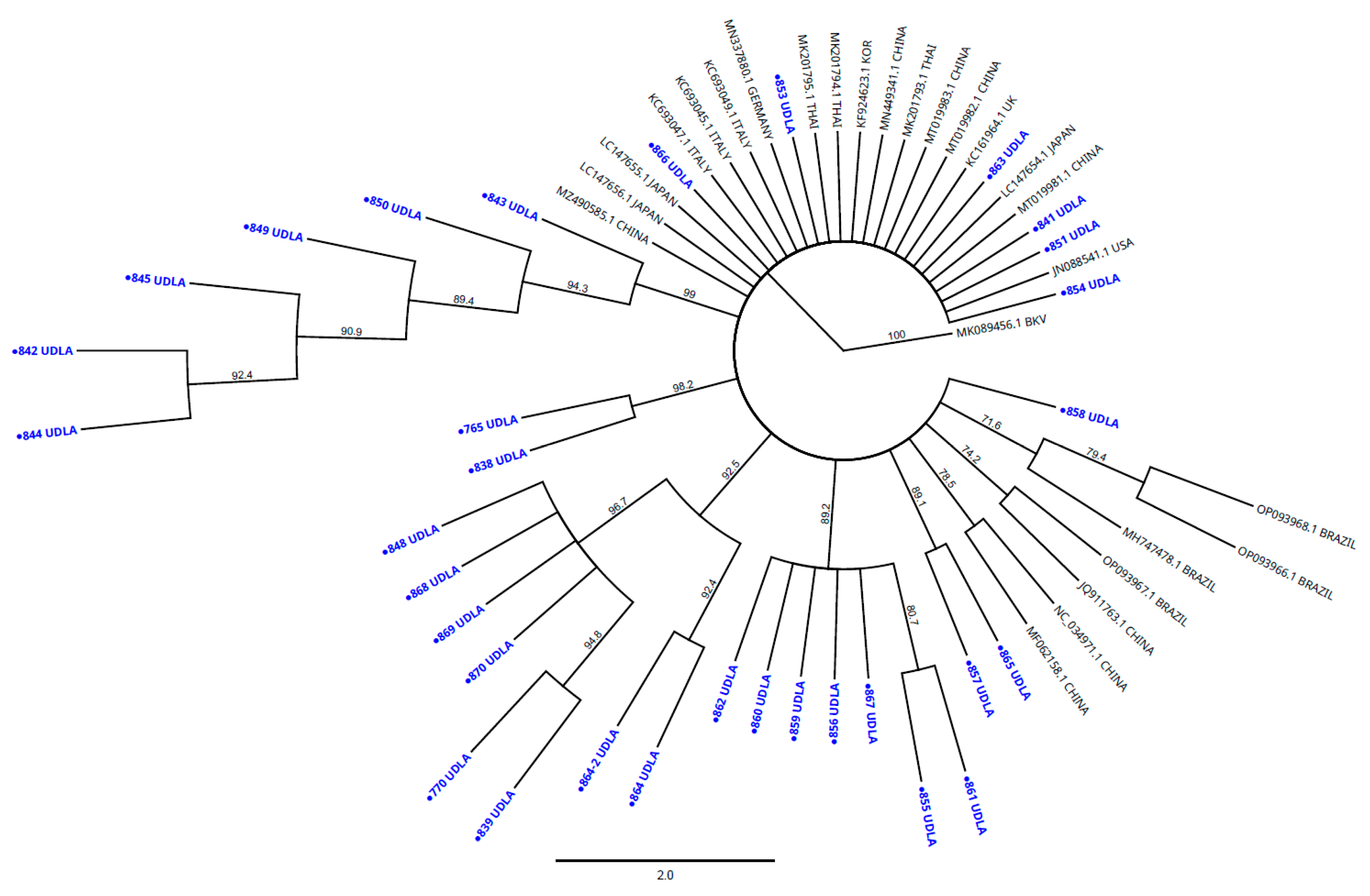
3.3. Sequencing and Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CaKoV | Canine Kobuvirus |
| PCR | Polymerase Chain Reaction |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| UDLA | Universidad de las Americas |
| CPV | Canine Parvovirus |
| CaAstV | Canine Astrovirus |
| CDV | Canine Distemper Virus |
| ORF | Open Reading Frame |
| VP | Virulence Protein |
| NTC | Non-Template Control |
| LoD | Limit of Detection |
| LoQ | Limit of Quantification |
| NT | Nucleotides |
| N° | Number |
| CV | Coefficient of Variation |
| Cq | Cycle of Quantification |
References
- Hao, X.; Liu, R.; He, Y.; Xiao, X.; Xiao, W.; Zheng, Q.; Lin, X.; Tao, P.; Zhou, P.; Li, S. Multiplex PCR Methods for Detection of Several Viruses Associated with Canine Respiratory and Enteric Diseases. PLoS ONE 2019, 14, e0213295. [Google Scholar] [CrossRef]
- Sattasathuchana, P.; Steiner, J.M. Canine Eosinophilic Gastrointestinal Disorders. Anim. Health Res. Rev. 2014, 15, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Enany, M.; Wahdan, A.; El-Metwaly, M.; Hassan, W.; Abo Hashem, M. Bacterial Causes of Hemorrhagic Gastroenteritis in Dogs and Cats with Detection of Some Virulence and β-Lactamase Resistance Genes in Escherichia Coli and Salmonella by Multiplex PCR. Suez Canal Vet. Med. J. SCVMJ 2021, 26, 39–59. [Google Scholar] [CrossRef]
- Li, L.; Pesavento, P.A.; Shan, T.; Leutenegger, C.M.; Wang, C.; Delwart, E. Viruses in Diarrhoeic Dogs Include Novel Kobuviruses and Sapoviruses. J. Gen. Virol. 2011, 92, 2534–2541. [Google Scholar] [CrossRef] [PubMed]
- Thomson, G.W.; Gagnon, A.N. Canine Gastroenteritis Associated with a Parvovirus-like Agent. Can. Vet. J. 1978, 19, 346. [Google Scholar]
- Williams, F.P. Astrovirus-like, Coronavirus-like, and Parvovirus-like Particles Detected in the Diarrheal Stools of Beagle Pups. Arch. Virol. 1980, 66, 215–226. [Google Scholar] [CrossRef]
- Keenan, K.P.; Jervis, H.R.; Marchwicki, R.H.; Binn, L.N. Intestinal Infection of Neonatal Dogs with Canine Coronavirus 1-71: Studies by Virologic, Histologic, Histochemical, and Immunofluorescent Techniques. Am. J. Vet. Res. 1976, 37, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Machida, N.; Kiryu, K.; Oh-ishi, K.; Kanda, E.; Izumisawa, N.; Nakamura, T. Pathology and Epidemiology of Canine Distemper in Raccoon Dogs (Nyctereutes procyonoides). J. Comp. Pathol. 1993, 108, 383–392. [Google Scholar] [CrossRef]
- Liu, D.; Liu, F.; Guo, D.; Hu, X.; Li, Z.; Li, Z.; Ma, J.; Liu, C. One-Step Triplex PCR/RT-PCR to Detect Canine Distemper Virus, Canine Parvovirus and Canine Kobuvirus. J. Vet. Med. Sci. 2019, 81, 1040–1042. [Google Scholar] [CrossRef]
- Kaiser, F.K.; van Dyck, L.; Jo, W.K.; Schreiner, T.; Pfankuche, V.M.; Wohlsein, P.; Baumann, I.; Peters, M.; Baumgärtner, W.; Osterhaus, A.D.M.E.; et al. Detection of Systemic Canine Kobuvirus Infection in Peripheral Tissues and the Central Nervous System of a Fox Infected with Canine Distemper Virus. Microorganisms 2021, 9, 2521. [Google Scholar] [CrossRef]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus Taxonomy: The Database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef]
- Yamashita, T.; Sakae, K.; Tsuzuki, H.; Suzuki, Y.; Ishikawa, N.; Takeda, N.; Miyamura, T.; Yamazaki, S. Complete Nucleotide Sequence and Genetic Organization of Aichi Virus, a Distinct Member of the Picornaviridae Associated with Acute Gastroenteritis in Humans. J. Virol. 1998, 72, 8408–8412. [Google Scholar] [CrossRef] [PubMed]
- Barros, B.d.C.V.; Castro, C.M.O.; Pereira, D.; Ribeiro, L.G.; Júnior, J.W.B.D.; Casseb, S.M.M.; Holanda, G.M.; Cruz, A.C.R.; Júnior, E.C.S.; Mascarenhas, J.D.P. Proposed New Strain of Canine Kobuvirus from Fecal Samples of Brazilian Domestic Dogs. Microbiol. Resour. Announc. 2019, 8, e01292-18. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Li, Y.; Wang, X.; Yang, K.; Zhang, D.; Zhao, L.; Bai, C.; Jiang, S.; Li, Y. Identification and Full-Genome Sequencing of Canine Kobuvirus in Canine Fecal Samples Collected from Anhui Province, Eastern China. Arch. Virol. 2020, 165, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Song, Y.; Li, L.; Zhou, Y.; Zhu, C.; Zhang, W.; Feng, D. Detection and Genetic Characterization of Canine Kobuvirus from Stray Dogs in Shanghai, China. Arch. Virol. 2023, 168, 112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Cui, Y.; Jiang, S.; Liu, H.; Wang, J.; Li, Y. Duplex SYBR Green I-Based Real-Time PCR Assay for the Rapid Detection of Canine Kobuvirus and Canine Astrovirus. J. Virol. Methods 2021, 290, 114066. [Google Scholar] [CrossRef]
- Olarte-Castillo, X.A.; Heeger, F.; Mazzoni, C.J.; Greenwood, A.D.; Fyumagwa, R.; Moehlman, P.D.; Hofer, H.; East, M.L. Molecular Characterization of Canine Kobuvirus in Wild Carnivores and the Domestic Dog in Africa. Virology 2015, 477, 89–97. [Google Scholar] [CrossRef]
- Ribeiro, J.; Headley, S.A.; Diniz, J.A.; Pereira, A.H.T.; Lorenzetti, E.; Alfieri, A.A.; Alfieri, A.F. Extra-Intestinal Detection of Canine Kobuvirus in a Puppy from Southern Brazil. Arch. Virol. 2017, 162, 867–872. [Google Scholar] [CrossRef]
- Oem, J.-K.; Choi, J.-W.; Lee, M.-H.; Lee, K.-K.; Choi, K.-S. Canine Kobuvirus Infections in Korean Dogs. Arch. Virol. 2014, 159, 2751–2755. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Li, Y.; Zhang, D.; Sun, J.; Guo, X.; Liu, G.; Jiang, S.; Li, Y. Development of an SYBR Green I-Based Real-Time PCR Assay for the Rapid Detection of Canine Kobuvirus. J. Virol. Methods 2020, 285, 113944. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, W.; Ye, R.; Pan, Z.; Li, G.; Su, S. One-Step Multiplex TaqMan Probe-Based Method for Real-Time PCR Detection of Four Canine Diarrhea Viruses. Mol. Cell Probes 2020, 53, 101618. [Google Scholar] [CrossRef]
- Wang, H.; Li, G.; Zhao, J.; Li, Y.; Ai, Y. An Overview of Nucleic Acid Testing for the Novel Coronavirus SARS-CoV-2. Front. Med. 2021, 7, 571709. [Google Scholar] [CrossRef]
- Kwit, E.; Rzeżutka, A. Molecular Methods in Detection and Epidemiologic Studies of Rabbit and Hare Viruses: A Review. J. Vet. Diagn. Investig. 2019, 31, 497–508. [Google Scholar] [CrossRef]
- Peeling, R.W.; Heymann, D.L.; Teo, Y.-Y.; Garcia, P.J. Diagnostics for COVID-19: Moving from Pandemic Response to Control. Lancet 2022, 399, 757–768. [Google Scholar] [CrossRef]
- Kong, N.; Zuo, Y.; Wang, Z.; Yu, H.; Zhou, E.; Shan, T.; Tong, G. Molecular Characterization of New Described Kobuvirus in Dogs with Diarrhea in China. SpringerPlus 2016, 5, 2047. [Google Scholar] [CrossRef]
- Charoenkul, K.; Janetanakit, T.; Chaiyawong, S.; Bunpapong, N.; Boonyapisitsopa, S.; Tangwangvivat, R.; Amonsin, A. First Detection and Genetic Characterization of Canine Kobuvirus in Domestic Dogs in Thailand. BMC Vet. Res. 2019, 15, 254. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Kasantikul, T.; Piewbang, C.; Techangamsuwan, S. Evolutionary Dynamics of Canine Kobuvirus in Vietnam and Thailand Reveal the Evidence of Viral Ability to Evade Host Immunity. Sci. Rep. 2024, 14, 12037. [Google Scholar] [CrossRef] [PubMed]
- Timurkan, M.Ö.; Aydin, H.; Polat, E. Detection and Molecular Characterization of Kobuviruses: An Agent of Canine Viral Diarrhea. Curr. Microbiol. 2024, 81, 309. [Google Scholar] [CrossRef]
- Aftab, G.; Arfaee, F.; Akhtardanesh, B.; Nikbakht Brojeni, G. Molecular Characterization of Canine and Feline Kobuvirus Infections in Iran. Vet. Res. Forum 2022, 13, 447–450. [Google Scholar] [PubMed]
- Di Martino, B.; Di Felice, E.; Ceci, C.; Di Profio, F.; Marsilio, F. Canine Kobuviruses in Diarrhoeic Dogs in Italy. Vet. Microbiol. 2013, 166, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Vicente, N.; Buesa, J.; Brown, P.A.; Merga, J.Y.; Darby, A.C.; Stavisky, J.; Sadler, L.; Gaskell, R.M.; Dawson, S.; Radford, A.D. Phylogeny and Prevalence of Kobuviruses in Dogs and Cats in the UK. Vet. Microbiol. 2013, 164, 246–252. [Google Scholar] [CrossRef]
- Soma, T.; Matsubayashi, M.; Sasai, K. Detection of Kobuvirus RNA in Japanese Domestic Dogs. J. Vet. Med. Sci. 2016, 78, 1731–1735. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Isolation of High-Molecular-Weight DNA Using Organic Solvents. Cold Spring Harb. Protoc. 2017, 2017, 356–359. [Google Scholar] [CrossRef]
- Conte, J.; Potoczniak, M.J.; Tobe, S.S. Using Synthetic Oligonucleotides as Standards in Probe-Based QPCR. Biotechniques 2018, 64, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Ruijter, J.M.; van den Hoff, M.J.B.; Kubista, M.; Pfaffl, M.W.; Shipley, G.L.; Tran, N.; Rödiger, S.; Untergasser, A.; Mueller, R.; et al. MIQE 2.0: Revision of the Minimum Information for Publication of Quantitative Real-Time PCR Experiments Guidelines. Clin. Chem. 2025, 71, 634–651. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health Principles and Methods of Validation of Diagnostic Assays for Infectious Diseases. In Manual of Diagnostic Test and Vaccines for Terrestrial Animals; World Organisation for Animal Health (WOAH): Paris, France, 2023; pp. 1–25.
- Harvey, N.D. How Old Is My Dog? Identification of Rational Age Groupings in Pet Dogs Based Upon Normative Age-Linked Processes. Front. Vet. Sci. 2021, 8, 643085. [Google Scholar] [CrossRef]
- Dema, A.; Tallapally, M.R.; Ganji, V.K.; Buddala, B.; Kodi, H.; Ramidi, A.; Yella, N.R.; Putty, K. A Comprehensive Molecular Survey of Viral Pathogens Associated with Canine Gastroenteritis. Arch. Virol. 2023, 168, 36. [Google Scholar] [CrossRef]
- Sarkar, S.L.; Alam, A.S.M.R.U.; Das, P.K.; Pramanik, M.H.A.; Al-Emran, H.M.; Jahid, I.K.; Hossain, M.A. Development and Validation of Cost-Effective One-Step Multiplex RT-PCR Assay for Detecting the SARS-CoV-2 Infection Using SYBR Green Melting Curve Analysis. Sci. Rep. 2022, 12, 6501. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Jatav, M.; Tiwari, A.; Verma, Y. Diagnostic Techniques of Infectious Diseases of Dogs and Cats. In Introduction to Diseases, Diagnosis, and Management of Dogs and Cats; Elsevier: Amsterdam, The Netherlands, 2024; pp. 581–586. [Google Scholar]
- Mundy, P.; da Silva, E.C.; Ledbetter, E.C. Effects of Cyclophosphamide Myelosuppression in Adult Dogs with Latent Canine Herpesvirus-1 Infection. Vet. Microbiol. 2012, 159, 230–235. [Google Scholar] [CrossRef]
- Li, M.; Yan, N.; Wang, M.; Zhang, B.; Yue, H.; Tang, C. Prevalence and Genomic Characteristics of Canine Kobuvirus in Southwest China. Arch. Virol. 2018, 163, 459–466. [Google Scholar] [CrossRef]
- Miyabe, F.M.; Ribeiro, J.; Alfieri, A.F.; Alfieri, A.A. Detection of Canine Kobuvirus RNA in Diarrheic Fecal Samples of Dogs with Parvoviruses. Braz. J. Microbiol. 2019, 50, 871–874. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, Y.; Shan, H.; Han, X. Clinical Detection of Four Emerging Canine Diarrhea-Associated Viruses and Evolutionary Analysis of Canine Kobuvirus. Arch. Virol. 2024, 169, 242. [Google Scholar] [CrossRef] [PubMed]
- Saltık, H.S. Concomitant Virus-Induced Gastrointestinal Infection in Dogs. Pol. J. Vet. Sci. 2023, 26, 203–209. [Google Scholar] [CrossRef]
- Unterer, S.; Busch, K. Acute Hemorrhagic Diarrhea Syndrome in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 79–92. [Google Scholar] [CrossRef]
- Dharmarajan, S.; Lee, J.; Izem, R. Sample Size Estimation for Case-crossover Studies. Stat. Med. 2019, 38, 956–968. [Google Scholar] [CrossRef]
- Chastant, S.; Mila, H. Passive Immune Transfer in Puppies. Anim. Reprod. Sci. 2019, 207, 162–170. [Google Scholar] [CrossRef]
- Pearce, J.; Spibey, N.; Sutton, D.; Tarpey, I. Development of a Novel Canine Parvovirus Vaccine Capable of Stimulating Protective Immunity in Four-Week-Old Puppies in the Face of High Levels of Maternal Antibodies. Vaccines 2023, 11, 1499. [Google Scholar] [CrossRef] [PubMed]
- Caddy, S.L. New Viruses Associated with Canine Gastroenteritis. Vet. J. 2018, 232, 57–64. [Google Scholar] [CrossRef] [PubMed]
- He, H.-J.; Zhang, W.; Liang, J.; Lu, M.; Wang, R.; Li, G.; He, J.-W.; Chen, J.; Chen, J.; Xing, G.; et al. Etiology and Genetic Evolution of Canine Coronavirus Circulating in Five Provinces of China, during 2018–2019. Microb. Pathog. 2020, 145, 104209. [Google Scholar] [CrossRef]
- DiGangi, B.A.; Dingman, P.A.; Grijalva, C.J.; Belyeu, M.; Tucker, S.; Isaza, R. Prevalence and Risk Factors for the Presence of Serum Antibodies against Canine Distemper, Canine Parvovirus, and Canine Adenovirus in Communities in Mainland Ecuador. Vet. Immunol. Immunopathol. 2019, 218, 109933. [Google Scholar] [CrossRef]
- Mylonakis, M.; Kalli, I.; Rallis, T. Canine Parvoviral Enteritis: An Update on the Clinical Diagnosis, Treatment, and Prevention. Vet. Med. Res. Rep. 2016, 7, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Boros, Á.; Albert, M.; Urbán, P.; Herczeg, R.; Gáspár, G.; Balázs, B.; Cságola, A.; Pankovics, P.; Gyenesei, A.; Reuter, G. Unusual “Asian-Origin” 2c to 2b Point Mutant Canine Parvovirus (Parvoviridae) and Canine Astrovirus (Astroviridae) Co-Infection Detected in Vaccinated Dogs with an Outbreak of Severe Haemorrhagic Gastroenteritis with High Mortality Rate in Hungary. Vet. Res. Commun. 2022, 46, 1355–1361. [Google Scholar] [CrossRef]
- Loor-Giler, A.; Santander-Parra, S.; Castillo-Reyes, S.; Campos, M.; Mena-Perez, R.; Prado-Chiriboga, S.; Nunez, L. Molecular Characterization and Lineage Analysis of Canine Astrovirus Strains from Dogs with Gastrointestinal Disease in Ecuador Based on ORF-2 Gene Analysis. Front. Vet. Sci. 2025, 11, 1505903. [Google Scholar] [CrossRef]
- Loor-Giler, A.; Castillo-Reyes, S.; Santander-Parra, S.; Campos, M.; Mena-Pérez, R.; Prado-Chiriboga, S.; Nuñez, L. First Report on the Molecular Detection of Canine Astrovirus (CaAstV) in Dogs with Gastrointestinal Disease in Ecuador Using a Fast and Sensitive RT-QPCR Assay Based on SYBR Green®. Vet. Sci. 2024, 11, 303. [Google Scholar] [CrossRef]
- Loor-Giler, A.; Castillo-Reyes, S.; Santander-Parra, S.; Campos, M.; Mena-Pérez, R.; Prado-Chiriboga, S.; Nuñez, L. Molecular Detection and Quantification of Canine Parvovirus 2 Using a Fast and Sensitive SYBR® Green-Based Quantitative Polymerase Chain Reaction Assay in Dogs Affected with Gastroenteritis. Vet. World 2024, 17, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Loor-Giler, A.; Santander-Parra, S.; Castillo-Reyes, S.; Campos, M.; Mena-Pérez, R.; Prado-Chiriboga, S.; Nuñez, L. Characterization, Quantification, and Molecular Identification of Co-Infection of Canine Parvovirus (CPV-2) Variants in Dogs Affected by Gastroenteritis in Ecuador During 2022–2023. Vet Sci 2025, 12, 46. [Google Scholar] [CrossRef] [PubMed]
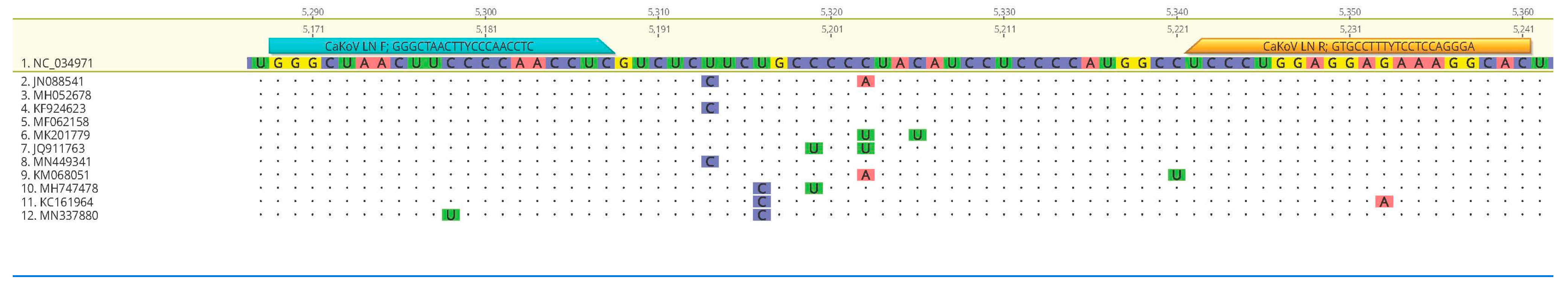

| Primers | Target | Sequences | Assay | Length | Reference |
|---|---|---|---|---|---|
| CaKoV LN F | 2B gene | 5′GGGCTAACTTYCCCAACCTC 3′ | RT-qPCR | 73 bp | This Study |
| CaKoV LN R | 5′GTGCCTTTYTCCTCCAGGGA 3′ | ||||
| CaKoV-3D-F CaKoV-3D-R | 3D gene | 5′CCCTGGAACACCCAAGGCCGCT 3′ 5′TCTGGTTGCCATAGATGTGGTG 3′ | End point PCR | 504 bp | [14] |
| Copy Number | Inter-Assay | Intra-Assay | ||
|---|---|---|---|---|
| Cq Mean | Cq Std Dev | Cq Mean | Cq Std Dev | |
| 108 | 9.79 | 0.876 | 10.19 | 0.948 |
| 107 | 15.32 | 0.413 | 14.83 | 0.550 |
| 106 | 17.99 | 0.485 | 17.72 | 0.360 |
| 105 | 21.11 | 0.324 | 20.88 | 0.412 |
| 104 | 24.54 | 0.305 | 24.47 | 0.335 |
| Distribution of CaKoV in Gastroenteritis Dogs by Age | ||||
|---|---|---|---|---|
| Age Group | * Age Range (months) | Average Number of Gene Copies | Positives | Negatives |
| Puppies | 1–12 | 3.40 × 105 | 64 (32%) | 76 (38%) |
| Adults | 13–84 | 3.82 × 105 | 16 (8%) | 37(18.5%) |
| Seniors | 85+ | 0 | 0 (0%)) | 7(3.5%) |
| CaKoV Co-Infections in Dogs with Gastroenteritis | |||||
|---|---|---|---|---|---|
| Combination N° | CaKoV | CaAstV | CPV-2 | CCoV | Total |
| 1 | x | x | 20 (25%) | ||
| 2 | x | x | 8 (10%) | ||
| 3 | x | x | 0 (0%) | ||
| 4 | x | x | x | 39 (48.75%) | |
| 5 | x | x | x | 0 (0%) | |
| 6 | x | x | x | 2 (2.5%) | |
| 7 | x | x | x | x | 2 (2.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Castro, C.; Loor-Giler, A.; Santander-Parra, S.; Campos, M.; Mena-Pérez, R.; Prado-Chiriboga, S.; Nuñez, L. First Molecular Insights into the Presence of Canine Kobuvirus in Ecuadorian Dogs Through the Standardization of a Sensitive SYBR Green RT-qPCR Assay. Vet. Sci. 2025, 12, 1076. https://doi.org/10.3390/vetsci12111076
Sanchez-Castro C, Loor-Giler A, Santander-Parra S, Campos M, Mena-Pérez R, Prado-Chiriboga S, Nuñez L. First Molecular Insights into the Presence of Canine Kobuvirus in Ecuadorian Dogs Through the Standardization of a Sensitive SYBR Green RT-qPCR Assay. Veterinary Sciences. 2025; 12(11):1076. https://doi.org/10.3390/vetsci12111076
Chicago/Turabian StyleSanchez-Castro, Camila, Anthony Loor-Giler, Silvana Santander-Parra, Martín Campos, Renán Mena-Pérez, Santiago Prado-Chiriboga, and Luis Nuñez. 2025. "First Molecular Insights into the Presence of Canine Kobuvirus in Ecuadorian Dogs Through the Standardization of a Sensitive SYBR Green RT-qPCR Assay" Veterinary Sciences 12, no. 11: 1076. https://doi.org/10.3390/vetsci12111076
APA StyleSanchez-Castro, C., Loor-Giler, A., Santander-Parra, S., Campos, M., Mena-Pérez, R., Prado-Chiriboga, S., & Nuñez, L. (2025). First Molecular Insights into the Presence of Canine Kobuvirus in Ecuadorian Dogs Through the Standardization of a Sensitive SYBR Green RT-qPCR Assay. Veterinary Sciences, 12(11), 1076. https://doi.org/10.3390/vetsci12111076






