The Transcript Levels and the Serum Profile of Biomarkers Associated with Clinical Endometritis Susceptibility in Buffalo Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Study Design
2.2. Blood Sampling
2.3. Transcript Levels of Immune and Antioxidant Genes
2.4. Biochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Patterns of Immune and Antioxidant Marker mRNA Levels
3.2. The Biochemical Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perera, B. Reproductive cycles of buffalo. Anim. Reprod. Sci. 2011, 124, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Haimerl, P.; Arlt, S.; Borchardt, S.; Heuwieser, W. Antibiotic treatment of metritis in dairy cows—A meta-analysis. J. Dairy Sci. 2017, 100, 3783–3795. [Google Scholar] [CrossRef] [PubMed]
- Paiano, R.B.; Bonilla, J.; Moreno, A.M.; Baruselli, P.S. Clinical endometritis with Trueperella pyogenes reduces reproductive performance and milk production in dairy cows. Reprod. Domest. Anim. 2021, 56, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.; Vieira-Neto, A.; Snodgrass, J.; De Vries, A.; Santos, J. Economic comparison of systemic antimicrobial therapies for metritis in dairy cows. J. Dairy Sci. 2019, 102, 7345–7358. [Google Scholar] [CrossRef] [PubMed]
- Paiano, R.B.; Birgel, D.B.; Bonilla, J.; Birgel Junior, E.H. Alterations in biochemical profiles and reproduction performance in postpartum dairy cows with metritis. Reprod. Domest. Anim. 2020, 55, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Paiano, R.B.; Bonilla, J.; Pugliesi, G.; Moreno, A.M.; Baruselli, P.S. Assessment of clinical and subclinical endometritis impacts on the reproductive performance and milk production of dairy cows in Brazilian herds. Res. Sq. 2022; preprint. [Google Scholar]
- Moghaddam, A.; Mamoei, M. A survey on some of the reproductive and productive traits of the buffalo in Iran. In Proceedings of the 23rd World Buiatrics, Québec, QC, Canada, 11–16 July 2004; p. 1910. [Google Scholar]
- Melendez, P.; McHale, J.; Bartolome, J.; Archbald, L.; Donovan, G. Uterine involution and fertility of Holstein cows subsequent to early postpartum PGF2α treatment for acute puerperal metritis. J. Dairy Sci. 2004, 87, 3238–3246. [Google Scholar] [CrossRef] [PubMed]
- Földi, J.; Kulcsar, M.; Pecsi, A.; Huyghe, B.; De Sa, C.; Lohuis, J.; Cox, P.; Huszenicza, G. Bacterial complications of postpartum uterine involution in cattle. Anim. Reprod. Sci. 2006, 96, 265–281. [Google Scholar] [CrossRef]
- Bicalho, M.; Santin, T.; Rodrigues, M.; Marques, C.; Lima, S.; Bicalho, R. Dynamics of the microbiota found in the vaginas of dairy cows during the transition period: Associations with uterine diseases and reproductive outcome. J. Dairy Sci. 2017, 100, 3043–3058. [Google Scholar] [CrossRef]
- Bogado Pascottini, O.; Spricigo, J.; Van Schyndel, S.; Mion, B.; Rousseau, J.; Weese, J.; LeBlanc, S. Effects of parity, blood progesterone, and non-steroidal anti-inflammatory treatment on the dynamics of the uterine microbiota of healthy postpartum dairy cows. PLoS ONE 2021, 16, e0233943. [Google Scholar] [CrossRef]
- Giuliodori, M.J.; Magnasco, R.; Becu-Villalobos, D.; Lacau-Mengido, I.M.; Risco, C.; de la Sota, R.L. Clinical endometritis in an Argentinean herd of dairy cows: Risk factors and reproductive efficiency. J. Dairy Sci. 2013, 96, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Várhidi, Z.; Csikó, G.; Bajcsy, Á.C.; Jurkovich, V. Uterine Disease in Dairy Cows: A Comprehensive Review Highlighting New Research Areas. Vet. Sci. 2024, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Owens, S.E. Postpartum uterine infection and endometritis in dairy cattle. Anim. Reprod. (AR) 2018, 14, 622–629. [Google Scholar] [CrossRef]
- Venjakob, P.; Pieper, L.; Heuwieser, W.; Borchardt, S. Association of postpartum hypocalcemia with early-lactation milk yield, reproductive performance, and culling in dairy cows. J. Dairy Sci. 2018, 101, 9396–9405. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.S.d. Recent advances and future directions for uterine diseases diagnosis, pathogenesis, and management in dairy cows. Anim. Reprod. 2020, 17, e20200063. [Google Scholar] [CrossRef]
- Paiano, R.B.; Morrison, E.I.; LeBlanc, S.J. Randomized clinical trial of ketoprofen or ceftiofur for treatment of metritis in dairy cows. J. Dairy Sci. 2024; in press. [Google Scholar] [CrossRef]
- Faye, B.; Bengoumi, M. Camel Clinical Biochemistry and Hematology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Faraz, A.; Younas, M.; Waheed, A.; Yaqoob, M.; Ishaq, K. Growth performance and hair mineral status of Marecha (Camelus dromedarius) calves reared under different management systems. Pak. J. Zool. 2019, 51, 503. [Google Scholar] [CrossRef]
- Abebe, W.; Getinet, A.; Mekonnen, H. Study on live weight, carcass weight and dressing percentage of Issa camels in Ethiopia. Rev. Méd. Vét. 2002, 153, 713–771. [Google Scholar]
- Berry, D.P.; Bermingham, M.L.; Good, M.; More, S.J. Genetics of animal health and disease in cattle. Ir. Vet. J. 2011, 64, 5. [Google Scholar] [CrossRef]
- Sallam, A.M.; Reyer, H.; Wimmers, K.; Bertolini, F.; Aboul-Naga, A.; Braz, C.U.; Rabee, A.E. Genome-wide landscape of runs of homozygosity and differentiation across Egyptian goat breeds. BMC Genom. 2023, 24, 573. [Google Scholar] [CrossRef]
- Adams, L.G.; Schutta, C.J. Natural Resistance Against Brucellosis: A Review. Open Vet. Sci. J. 2010, 4, 61–71. [Google Scholar] [CrossRef]
- Radostits, O.M.; Gay, C.; Hinchcliff, K.W.; Constable, P.D. Veterinary Medicine E-Book: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Pascottini, O.B.; LeBlanc, S.J.; Gnemi, G.; Leroy, J.L.; Opsomer, G. Genesis of clinical and subclinical endometritis in dairy cows. Reproduction 2023, 166, R15–R24. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Arshad, U.; Erdoğan, G.; Ahmad, N. Evaluation of haemodynamic changes of uterine arteries using Doppler ultrasonography during different stages of pregnancy in Bos indicus cows. Reprod. Domest. Anim. 2020, 55, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- PetitClerc, C.; Solberg, H. Approved recommendation (1987) on the theory of reference values. Part 2. Selection of individuals for the production of reference values. Clin. Chim. Acta 1987, 170, S1–S11. [Google Scholar] [CrossRef]
- Mili, B.; Pandita, S.; Kumar, B. Association of blood metabolites with reproductive disorders in postpartum Murrah buffaloes. Buffalo Bull. 2016, 35, 643–651. [Google Scholar]
- Soliman, S.S.; Kandil, O.M.; Abdoon, A.S. Impact of Reproductive Status, Body Condition Score, and Locality on Hormonal, and Some Blood Metabolites in Egyptian Buffaloes. Egypt. J. Vet. Sci. 2024, 55, 1387–1396. [Google Scholar] [CrossRef]
- Perumal, P.; De, A.; Sunder, J.; Bhattacharya, D. Physiological, Heamatological, Biochemical and Endocrinological Profiles Changes in Crossbred Cows Affected With Metritis under Tropical Island Ecosystem. Int. J. Bio-Resour. Stress Manag. 2022, 13, 155–161. [Google Scholar]
- El-Hawary, A.; El-Hady, A. Improving the Immune and Health Status and Blood Constituents of Egyptian Buffaloes and their Offspring in Response to Treating Dams with Selenomethionine and Levamisole. J. Anim. Poult. Prod. 2018, 9, 305–314. [Google Scholar] [CrossRef]
- Amin, Y.; Mohamed, R.; Zakaria, A.; Wehrend, A.; Hussein, H.A. Effects of aflatoxins on some reproductive hormones and composition of buffalo’s milk. Comp. Clin. Pathol. 2019, 28, 1191–1196. [Google Scholar] [CrossRef]
- Elsayed, D.H.; El-Azzazi, F.E.; Mahmoud, Y.K.; Dessouki, S.M.; Ahmed, E.A. Subclinical endometritis and postpartum ovarian resumption in respect to TNF-α, IL-8 and CRP in Egyptian buffaloes. Anim. Reprod. 2020, 17, e20190027. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Kumar, H.; Kumar, S.; Malla, W.; Sharma, R. Comparative biochemical profiles, utero-ovarian function, and fertility of the postpartum buffalo with and without subclinical endometritis. Trop. Anim. Health Prod. 2021, 53, 73. [Google Scholar] [CrossRef]
- Bollwein, H.; Lüttgenau, J.; Herzog, K. Bovine luteal blood flow: Basic mechanism and clinical relevance. Reprod. Fertil. Dev. 2012, 25, 71–79. [Google Scholar] [CrossRef]
- El-Sayed, A.; Refaai, M.; Ateya, A. Doppler ultrasonographic scan, gene expression and serum profile of immune, APPs and antioxidant markers in Egyptian buffalo–cows with clinical endometritis. Sci. Rep. 2024, 14, 5698. [Google Scholar] [CrossRef]
- Taha, D.A.; Mahfouz, E.R.; Bibars, M.A.; Hassan, N.A.; Othman, O.E. Cytokine genes expression in uteri of Bubalus bubalis associated with endometritis infection. Jordan J. Biol. Sci. 2021, 14, 245–251. [Google Scholar]
- Ateya, A.; Al-Sharif, M.; Abdo, M.; Fericean, L.; Essa, B. Individual genomic loci and mRNA levels of immune biomarkers associated with pneumonia susceptibility in baladi goats. Vet. Sci. 2023, 10, 185. [Google Scholar] [CrossRef]
- Al-Sharif, M.; Abdo, M.; Shabrawy, O.E.; El-Naga, E.M.A.; Fericean, L.; Banatean-Dunea, I.; Ateya, A. Investigating Polymorphisms and Expression Profile of Immune, Antioxidant, and Erythritol-Related Genes for Limiting Postparturient Endometritis in Holstein Cattle. Vet. Sci. 2023, 10, 370. [Google Scholar] [CrossRef]
- Darwish, A.; Ebissy, E.; Ateya, A.; El-Sayed, A. Single nucleotide polymorphisms, gene expression and serum profile of immune and antioxidant markers associated with postpartum disorders susceptibility in Barki sheep. Anim. Biotechnol. 2023, 34, 327–339. [Google Scholar] [CrossRef]
- Al-Sharif, M.; Marghani, B.H.; Ateya, A. DNA polymorphisms and expression profile of immune and antioxidant genes as biomarkers for reproductive disorders tolerance/susceptibility in Baladi goat. Anim. Biotechnol. 2023, 34, 2219–2230. [Google Scholar] [CrossRef]
- Essa, B.; Al-Sharif, M.; Abdo, M.; Fericean, L.; Ateya, A. New insights on nucleotide sequence variants and mRNA levels of candidate genes assessing resistance/susceptibility to mastitis in Holstein and montbéliarde dairy cows. Vet. Sci. 2023, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Ateya, A.I.; Ibrahim, S.S.; Al-Sharif, M.M. Single nucleotide polymorphisms, gene expression and economic evaluation of parameters associated with mastitis susceptibility in European Cattle Breeds. Vet. Sci. 2022, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Ateya, A.; Safhi, F.A.; El-Emam, H.; Marawan, M.A.; Fayed, H.; Kadah, A.; Mamdouh, M.; Hizam, M.M.; Al-Ghadi, M.Q.; Abdo, M. Combining Nucleotide Sequence Variants and Transcript Levels of Immune and Antioxidant Markers for Selection and Improvement of Mastitis Resistance in Dromedary Camels. Agriculture 2023, 13, 1909. [Google Scholar] [CrossRef]
- Ebissy, E.; Darwish, A.; Hafez, A.A.; Ateya, A.; El-Sayed, A. Individual genomic loci, transcript level, and biochemical profile of immune and antioxidant markers associated with genetically identified bacterial mastitis in Shami goats in Egypt. Open Vet. J. 2024, 14, 370. [Google Scholar] [CrossRef] [PubMed]
- Ateya, A.; Safhi, F.A.; El-Emam, H.; Al-Ghadi, M.Q.; Abdo, M.; Fericean, L.; Olga, R.; Mihaela, O.; Hizam, M.M.; Mamdouh, M. DNA Polymorphisms and mRNA Levels of Immune Biomarkers as Candidates for Inflammatory Postpartum Disorders Susceptibility in Italian Buffaloes. Vet. Sci. 2023, 10, 573. [Google Scholar] [CrossRef]
- Ateya, A.; Al-Sharif, M.; Faraj, S.H.; Abdo, M.; Fericean, L.; Banatean-Dunea, I.; Hammad, S.J.; Mamdouh, M.; Fayed, H.; Marawan, M.A. Exploring genetic polymorphisms and transcript levels of antioxidant and metabolic markers for prediction and monitoring diarrhea in Holstein dairy calves. Acta Agric. Scand. Sect. A—Anim. Sci. 2024, 1–11. [Google Scholar] [CrossRef]
- Borth, W. α2 “Macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992, 6, 3345–3353. [Google Scholar] [CrossRef]
- Botos, I.; Segal, D.M.; Davies, D.R. The structural biology of Toll-like receptors. Structure 2011, 19, 447–459. [Google Scholar] [CrossRef] [PubMed]
- White, S.N.; Taylor, K.H.; Abbey, C.A.; Gill, C.A.; Womack, J.E. Haplotype variation in bovine Toll-like receptor 4 and computational prediction of a positively selected ligand-binding domain. Proc. Natl. Acad. Sci. USA 2003, 100, 10364–10369. [Google Scholar] [CrossRef]
- Salim, T.; Sershen, C.L.; May, E.E. Investigating the role of TNF-α and IFN-γ activation on the dynamics of iNOS gene expression in LPS stimulated macrophages. PLoS ONE 2016, 11, e0153289. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Ganguly, I.; Singh, R.; Deb, S.M.; Kumar, S.; Sharma, A.; Mitra, A. DNA polymorphism in SLC11A1 gene and its association with brucellosis resistance in Indian zebu (Bos indicus) and crossbred (Bos indicus × Bos taurus) Cattle. Asian-Australas. J. Anim. Sci. 2011, 24, 898–904. [Google Scholar] [CrossRef]
- Ju, Z.; Wang, C.; Wang, X.; Yang, C.; Sun, Y.; Jiang, Q.; Wang, F.; Li, M.; Zhong, J.; Huang, J. Role of an SNP in alternative splicing of bovine NCF4 and mastitis susceptibility. PLoS ONE 2015, 10, e0143705. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Wang, C.; Wang, X.; Yang, C.; Zhang, Y.; Sun, Y.; Jiang, Q.; Li, R.; Li, J.; Zhong, J. The effect of the SNP g. 18475 A> G in the 3′ UTR of NCF4 on mastitis susceptibility in dairy cattle. Cell Stress Chaperones 2018, 23, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Kirby, D.M.; Salemi, R.; Sugiana, C.; Ohtake, A.; Parry, L.; Bell, K.M.; Kirk, E.P.; Boneh, A.; Taylor, R.W.; Dahl, H.-H.M. NDUFS6 mutations are a novel cause of lethal neonatal mitochondrial complex I deficiency. J. Clin. Investig. 2004, 114, 837–845. [Google Scholar] [CrossRef]
- Boussaha, M.; Esquerré, D.; Barbieri, J.; Djari, A.; Pinton, A.; Letaief, R.; Salin, G.; Escudié, F.; Roulet, A.; Fritz, S. Genome-wide study of structural variants in bovine Holstein, Montbéliarde and Normande dairy breeds. PLoS ONE 2015, 10, e0135931. [Google Scholar] [CrossRef] [PubMed]
- Durán Aguilar, M.; Román Ponce, S.; Ruiz López, F.; González Padilla, E.; Vásquez Peláez, C.; Bagnato, A.; Strillacci, M.G. Genome-wide association study for milk somatic cell score in holstein cattle using copy number variation as markers. J. Anim. Breed. Genet. 2017, 134, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Suzuki, T.; Takahashi, Y.; Hishinuma, E.; Saigusa, D.; Yamamoto, M. Geldanamycin-derived HSP90 inhibitors are synthetic lethal with NRF2. Mol. Cell. Biol. 2020, 40, e00377-20. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.-I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- Wadley, A.J.; Aldred, S.; Coles, S.J. An unexplored role for Peroxiredoxin in exercise-induced redox signalling? Redox Biol. 2016, 8, 51–58. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme oxygenase-1 signaling and redox homeostasis in physiopathological conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.K.; Mobasher, M.A.; Ebiya, R.A.; Hassen, M.T.; Hagag, H.M.; El-Sayed, R.; Abdel-Ghany, S.; Said, M.M.; Awad, N.S. Anti-inflammatory, anti-apoptotic, and antioxidant roles of honey, royal jelly, and propolis in suppressing nephrotoxicity induced by doxorubicin in male albino rats. Antioxidants 2022, 11, 1029. [Google Scholar] [CrossRef]
- Trevisan, M.; Browne, R.; Ram, M.; Muti, P.; Freudenheim, J.; Carosella, A.M.; Armstrong, D. Correlates of markers of oxidative status in the general population. Am. J. Epidemiol. 2001, 154, 348–356. [Google Scholar] [CrossRef]
- Ateya, A.; El-Sayed, A.; Mohamed, R. Gene expression and serum profile of antioxidant markers discriminate periparturient period time in dromedary camels. Mammal Res. 2021, 66, 603–613. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Hori, O.; Stern, D.M.; Hartmann, E.; Ogawa, S.; Tohyama, M. Stress-associated endoplasmic reticulum protein 1 (SERP1)/Ribosome-associated membrane protein 4 (RAMP4) stabilizes membrane proteins during stress and facilitates subsequent glycosylation. J. Cell Biol. 1999, 147, 1195–1204. [Google Scholar] [CrossRef]
- Song, Y.; Masison, D.C. Independent regulation of Hsp70 and Hsp90 chaperones by Hsp70/Hsp90-organizing protein Sti1 (Hop1). J. Biol. Chem. 2005, 280, 34178–34185. [Google Scholar] [CrossRef]
- Ellis, R.J.; Van der Vies, S.M. Molecular chaperones. Annu. Rev. Biochem. 1991, 60, 321–347. [Google Scholar] [CrossRef]
- Rinaldi, M.; Moroni, P.; Paape, M.J.; Bannerman, D.D. Evaluation of assays for the measurement of bovine neutrophil reactive oxygen species. Vet. Immunol. Immunopathol. 2007, 115, 107–125. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef]
- Duhig, K.; Chappell, L.C.; Shennan, A.H. Oxidative stress in pregnancy and reproduction. Obstet. Med. 2016, 9, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guan, D.; Huo, J.; Biswas, S.K.; Huang, Y.; Yang, Y.; Xu, S.; Lam, K.-P. IL-10 enhances human natural killer cell effector functions via metabolic reprogramming regulated by mTORC1 signaling. Front. Immunol. 2021, 12, 619195. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Mellado, M.; Leyva, C.; García, J.E.; Véliz, F.G.; Hernández-Bustamante, J. Serum metabolites and body condition score associated with metritis, endometritis, ketosis, and mastitis in Holstein cows. Pesqui. Agropecu. Bras. 2020, 55, e01308. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Tezuka, E.; Sasaki, K.; Takahashi, M.; Yamagishi, N.; Izaike, Y.; Osawa, T. Correlation of blood metabolite concentrations and body condition scores with persistent postpartum uterine bacterial infection in dairy cows. J. Reprod. Dev. 2016, 62, 457–463. [Google Scholar] [CrossRef]
- Kaufmann, T.B.; Drillich, M.; Tenhagen, B.-A.; Heuwieser, W. Correlations between periparturient serum concentrations of non-esterified fatty acids, beta-hydroxybutyric acid, bilirubin, and urea and the occurrence of clinical and subclinical postpartum bovine endometritis. BMC Vet. Res. 2010, 6, 47. [Google Scholar] [CrossRef]
- Blondet, J.J.; Beilman, G.J. Glycemic control and prevention of perioperative infection. Curr. Opin. Crit. Care 2007, 13, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, H.; Watanabe, C.; Kohiruimaki, M.; Ando, T.; Watanabe, D.; Masui, M.; Hayashi, T.; Abe, R.; Koiwa, M.; Sato, S. Comparison of two different nutritive conditions against the changes in peripheral blood mononuclear cells of periparturient dairy cows. J. Vet. Med. Sci. 2006, 68, 1161–1166. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Moyes, K. Nutrition, immune function and health of dairy cattle. Animal 2013, 7, 112–122. [Google Scholar] [CrossRef]
- Cui, L.; Wang, H.; Ding, Y.; Li, J.; Li, J. Changes in the blood routine, biochemical indexes and the pro-inflammatory cytokine expressions of peripheral leukocytes in postpartum dairy cows with metritis. BMC Vet. Res. 2019, 15, 157. [Google Scholar] [CrossRef]
- Wankhade, P.R.; Manimaran, A.; Kumaresan, A.; Jeyakumar, S.; Ramesha, K.; Sejian, V.; Rajendran, D.; Varghese, M.R. Metabolic and immunological changes in transition dairy cows: A review. Vet. World 2017, 10, 1367. [Google Scholar] [CrossRef] [PubMed]
- Amin, Y.A.; Ali, R.A.; Fouad, S.S.; Ibrahim, R.M. The deleterious effect of postpartum pyometra on the reproductive indices, the metabolic profile, and oxidant/antioxidant parameters of dairy cows. Vet. World 2021, 14, 329. [Google Scholar] [CrossRef] [PubMed]
- Paiano, R.B.; Birgel, D.B.; Bonilla, J.; Birgel Junior, E.H. Evaluation of biochemical profile of dairy cows with metabolic diseases in tropical conditions. Reprod. Domest. Anim. 2020, 55, 1219–1228. [Google Scholar] [CrossRef]
- Nischala, S.; Sireesha, G. Serum proteins in endometritis affected buffaloes. J. Livest. Sci. 2018, 9, 81–83. [Google Scholar]
- Elmetwally, M.A.; Elshopakey, G.E.; El-Desouky, A.M.; Eldomany, W.B.; Bazer, F.W. Serum biochemical profile in buffalo endometritis and impact of treatment with PGF2α and intrauterine gentamicin infusion on postpartum reproductive performance. Trop. Anim. Health Prod. 2020, 52, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J. Dairy Sci. 2008, 91, 3300–3310. [Google Scholar] [CrossRef] [PubMed]
- Van Saun, R.J. Metabolic profiling and health risk in transition cows. In Proceedings of the American Association of Bovine Practitioners Conference Proceedings, Fort Worth, TX, USA, 23–25 September 2004; pp. 212–213. [Google Scholar]
- Benedet, A.; Manuelian, C.L.; Zidi, A.; Penasa, M.; De Marchi, M. Invited review: β-hydroxybutyrate concentration in blood and milk and its associations with cow performance. Animal 2019, 13, 1676–1689. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.; Mansfeld, R. Herd health monitoring in dairy farms–discover metabolic diseases. An overview. Tierärztl. Prax. Ausg. G Großtiere/Nutztiere 2019, 47, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.H.; Filho, O.G.S.; Absalón-Medina, V.A.; Pelton, S.H.; Butler, W.R.; Gilbert, R.O. Metabolic and endocrine differences between dairy cows that do or do not ovulate first postpartum dominant follicles. Biol. Reprod. 2016, 94, 18. [Google Scholar] [CrossRef]
- Tian, H.; Wang, W.; Zheng, N.; Cheng, J.; Li, S.; Zhang, Y.; Wang, J. Identification of diagnostic biomarkers and metabolic pathway shifts of heat-stressed lactating dairy cows. J. Proteom. 2015, 125, 17–28. [Google Scholar] [CrossRef]
- Perumal, P.; Chaurasia, D.; De, A.; Bhattacharya, D.; Sunder, J.; Bhowmick, S.; Kundu, A.; Mishra, P. Effect of clinical endometritis on physiological, hematological, biochemical and endocrinological profiles in crossbred cows under tropical island ecosystem. Indian J. Anim. Sci. 2020, 90, 1296–1299. [Google Scholar] [CrossRef]
- Sheldon, I.; Noakes, D.; Rycroft, A.; Pfeiffer, D.; Dobson, H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 2002, 123, 837–845. [Google Scholar] [CrossRef]
- Karsch, F.J.; Battaglia, D.F.; Breen, K.M.; Debus, N.; Harris, T.G. Mechanisms for ovarian cycle disruption by immune/inflammatory stress. Stress 2002, 5, 101–112. [Google Scholar] [CrossRef]
- Williams, C.Y.; Harris, T.G.; Battaglia, D.F.; Viguié, C.; Karsch, F.J. Endotoxin inhibits pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology 2001, 142, 1915–1922. [Google Scholar] [CrossRef]
- Herath, S.; Lilly, S.T.; Fischer, D.P.; Williams, E.J.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Bacterial lipopolysaccharide induces an endocrine switch from prostaglandin F2α to prostaglandin E2 in bovine endometrium. Endocrinology 2009, 150, 1912–1920. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; Noakes, D.E.; England, G.C.; Rycroft, A.; Dobson, H.; Sheldon, I.M. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 2007, 68, 549–559. [Google Scholar] [CrossRef]
- Hanafi, E.M.; Ahmed, W.; Abd El Moez, S.I.; El Khadrawy, H.; Abd El Hameed, A.R. Effect of clinical endometritis on ovarian activity and oxidative stress status in Egyptian buffalo-cows. Am.-Eurasian J. Agric. Environ. Sci. 2008, 4, 530–536. [Google Scholar]
- Macfarlane, M.; Breen, K.; Sakurai, H.; Adams, B.; Adams, T. Effect of duration of infusion of stress-like concentrations of cortisol on follicular development and the preovulatory surge of LH in sheep. Anim. Reprod. Sci. 2000, 63, 167–175. [Google Scholar] [CrossRef]
- Hazeldine, J.; Arlt, W.; Lord, J.M. Dehydroepiandrosterone as a regulator of immune cell function. J. Steroid Biochem. Mol. Biol. 2010, 120, 127–136. [Google Scholar] [CrossRef]
- Gautam, G.; Nakao, T.; Yusuf, M.; Koike, K. Prevalence of endometritis during the postpartum period and its impact on subsequent reproductive performance in two Japanese dairy herds. Anim. Reprod. Sci. 2009, 116, 175–187. [Google Scholar] [CrossRef]
- Arthur, H.; Noakes, D.; Parkinson, T.; England, G. Veterinary Reproduction and Obstetrics, 8th ed.; WB Sounders Company: Philadelphia, PA, USA, 2001; pp. 131–132. [Google Scholar]
- Adnane, M.; Kaidi, R.; Hanzen, C.; England, G.C. Risk factors of clinical and subclinical endometritis in cattle: A review. Turk. J. Vet. Anim. Sci. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Bicalho, M.; Lima, F.; Ganda, E.; Foditsch, C.; Meira, E., Jr.; Machado, V.; Teixeira, A.; Oikonomou, G.; Gilbert, R.; Bicalho, R. Effect of trace mineral supplementation on selected minerals, energy metabolites, oxidative stress, and immune parameters and its association with uterine diseases in dairy cattle. J. Dairy Sci. 2014, 97, 4281–4295. [Google Scholar] [CrossRef] [PubMed]
- Heppelmann, M.; Krach, K.; Krueger, L.; Benz, P.; Herzog, K.; Piechotta, M.; Hoedemaker, M.; Bollwein, H. The effect of metritis and subclinical hypocalcemia on uterine involution in dairy cows evaluated by sonomicrometry. J. Reprod. Dev. 2015, 61, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet. J. 2008, 176, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Badinand, F. Les métrites chez la vache: Influence des facteurs hormonaux et nutritionnels. Cah. Méd. Vet. 1975, 44, 205–221. [Google Scholar]
- Heidari, M.; Kafi, M.; Mirzaei, A.; Asaadi, A.; Mokhtari, A. Effects of follicular fluid of preovulatory follicles of repeat breeder dairy cows with subclinical endometritis on oocyte developmental competence. Anim. Reprod. Sci. 2019, 205, 62–69. [Google Scholar] [CrossRef]
- Brodzki, P.; Brodzki, A.; Kurek, Ł.; Szpetnar, M.; Bochniarz, M. Effect of uterine inflammatory status as well as calcium and magnesium concentrations on the uterine involution process in dairy cows. Ann. Anim. Sci. 2016, 16, 759–768. [Google Scholar] [CrossRef]
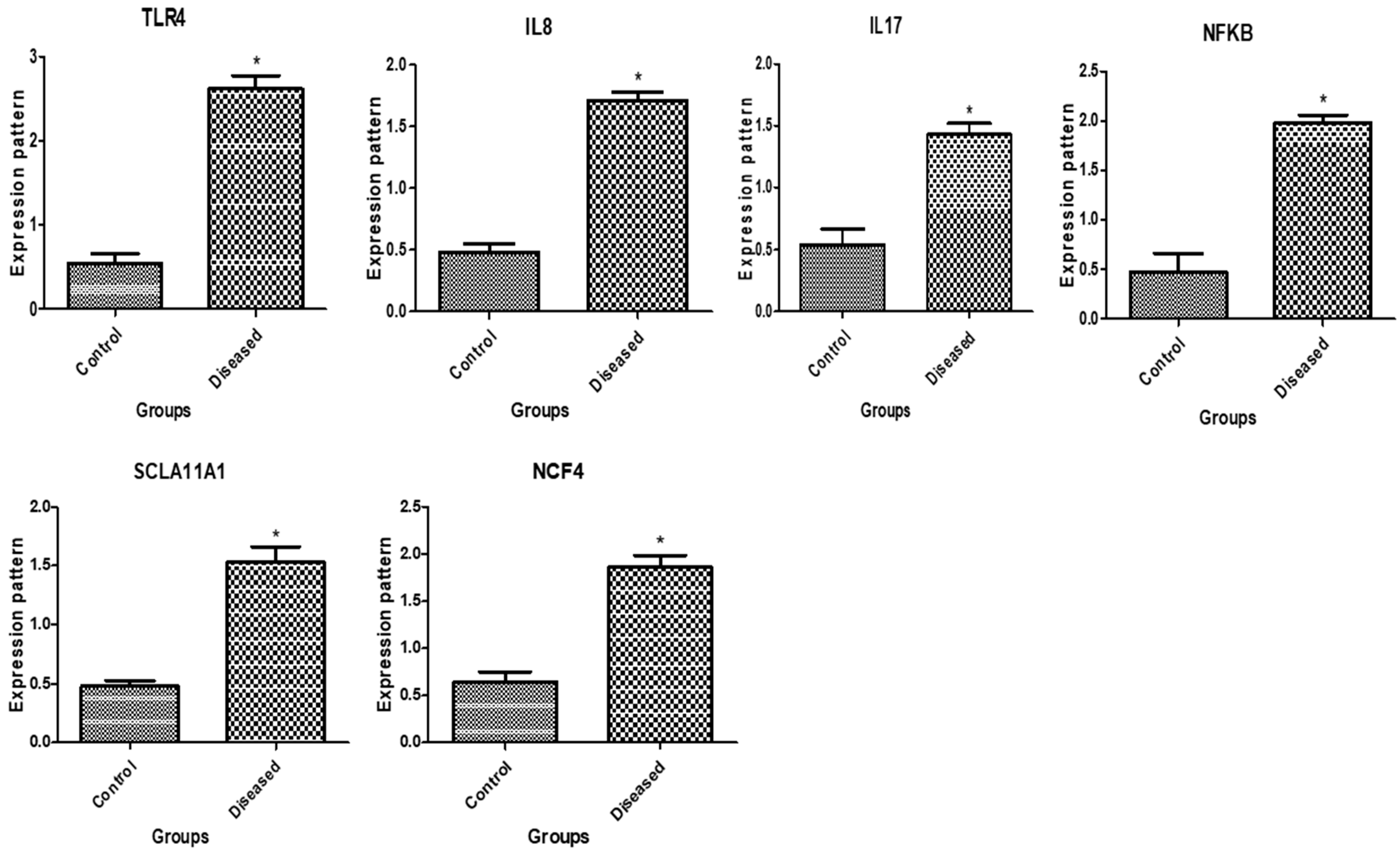
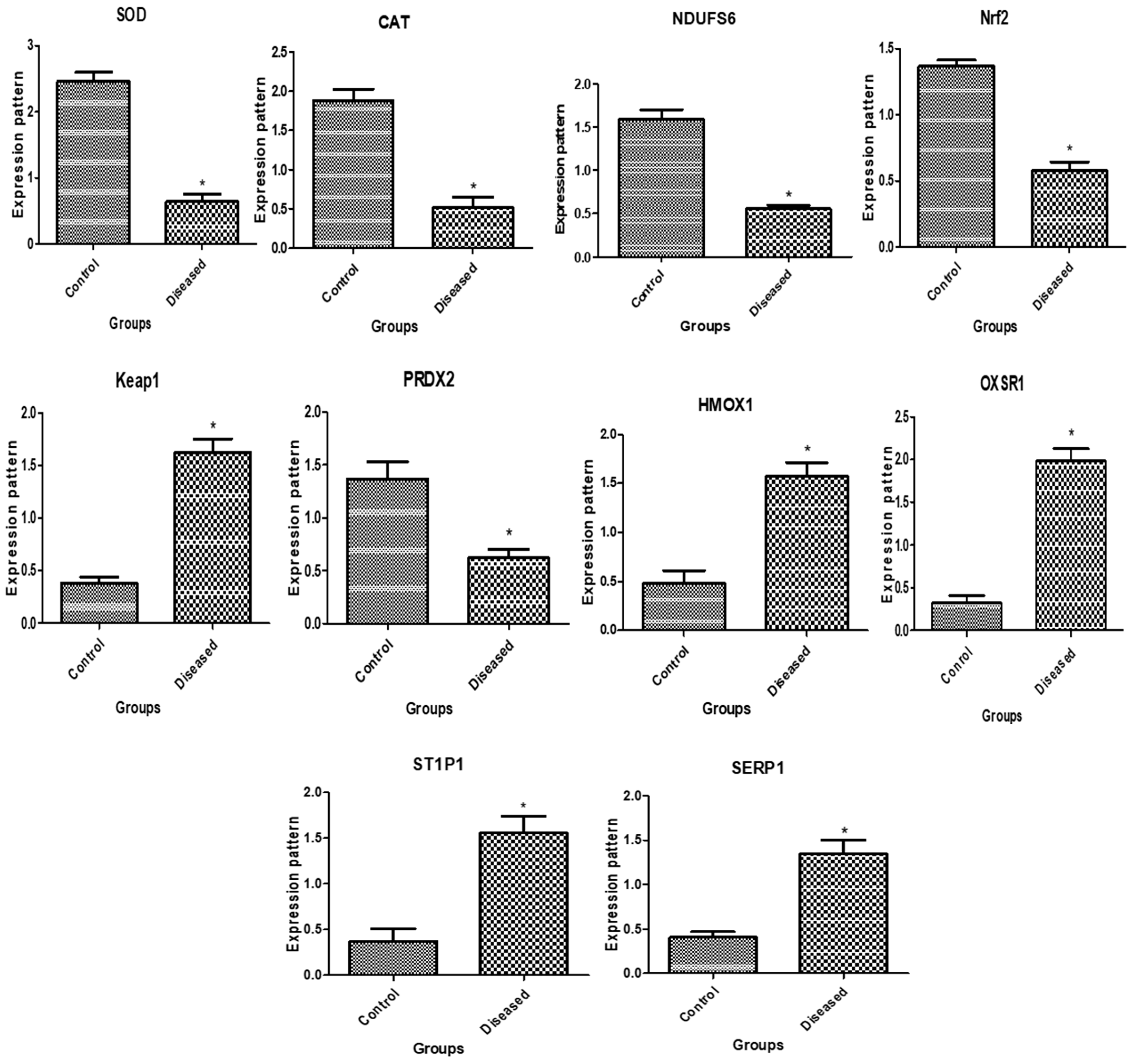
| Investigated Marker | Primer | Product Size (bp) | Annealing Temperature (°C) | GenBank Isolate | Origin |
|---|---|---|---|---|---|
| TLR4 | F5′-CCTGCATTGAAGCTCAGTTCTC-3 R5′-GGTTTTCTAGTTGATTTCCGCC-3′ | 244 | 58 | MT424002.1 | Present Research |
| IL-8 | F5′-TCTCTGCAGCTCTGTGTGAA-3 R5′-GGGTGGAAAGGTGTGGAATGT-3′ | 94 | 60 | NM_001290920.1 | |
| IL-17 | F5′-GGACTCTCCACCGCAATGAG-3′ R5′-CCTAAGCCAAATGGCGGACA-3′ | 249 | 58 | OQ730437.1 | |
| NFKB | F5′-CGAAAGCGAATCTCTCCTGGT-3′ R5′-TGACTGGGCCTAAGGAATGG-3′ | 182 | 58 | XM_006046119.4 | |
| SLC11A1 | F5′-TCATGTCAGGTGACACAGGC-3′ R5′-CCAGCCTGAAGATCCGACTC-3′ | 247 | 58 | XM_006046401.3 | |
| NCF4 | F5′-TCAGCCAACATCGCTGACAT-3′ R5′-TCCAGCTTGCTCTGTAAGGC-3′ | 143 | 60 | XM_006056976.4 | |
| SOD | F5′-GTCCCAGGTGCTCGACTCT-3′ R5′-ATCTCCTGCCAGATCTCCGT-3′ | 160 | 60 | XM_006041479.4 | |
| CAT | F5′-CTGAGTGGCGGAGTCTGAAG-3′ R5′-CTGGATTACCGCCTCCAGTG-3′ | 200 | 60 | XM_044929272.2 | |
| NDUFS6 | F5′-GGGAGTCGGGTGATATCGTG-3′ R5′-GTCCCCGTCTTCGTTTCCTT-3′ | 92 | 60 | XM_006051698.4 | |
| Nrf2 | F5′-GTCAGGGAGAAGCGAGTTCC-3′ R5′-TACCTCTCGACTTACCCCGA-3′ | 241 | 60 | XM_006051425.4 | |
| Keap1 | F5′-AATCACGACTTCTTCCCCGC-3′ R5′-CTCCCGCCTAACTTTCGCTA-3′ | 196 | 60 | XM_006068351.4 | |
| PRDX2 | F5′-ATGAGCATGGGGAAGTCTGC-3′ R5′-GAGCAGGTCTGGCATTTCCT-3′ | 193 | 60 | XM_006041572.4 | |
| HMOX1 | F5′-CAAGCGCTATGTTCAGCGAC-3′ R5′-GCTTGAACTTGGTGGCACTG-3′ | 206 | 58 | XM_045165381.1 | |
| OXSR1 | F5′-GATGAGCTGTGGCTCGTCAT-3′ R5′-GTGGTTGGTGTTAGCAAGGC-3′ | 125 | 60 | XM_025272781.3 | |
| ST1P1 | F5′-AGCTGGAGCCAACCTTCATC-3′ R5′-CATCATGCAGCGCTGGTAAC-3′ | 155 | 58 | XM_006050858.3 | |
| SERP1 | F5′-TATGGCCAACGAGAAGCACA-3′ R5′-GGTCCTACAGACGCCTTCTC-3′ | 147 | 58 | XM_006048532.4 | |
| ß. actin | F5′-GGAATCCTGCGGTATTCACGA-3′ R5′-CCGCCAATCCACACAGAGTA-3′ | 222 | 58 | NM_001290932.1 |
| Parameters | Unit | Control | Endometritis | p-Value | Reference Interval |
|---|---|---|---|---|---|
| Glucose | (mg/dL) | 57.3 ± 0.8 | 46 ± 0.5 | 0.004 | 22.3–97.4 [29] |
| NEFA | (mmol/L) | 0.1 ± 0.01 | 0.3 ± 0.008 | 0.001 | 0.2–0.29 [30] |
| BHBA | (mmol/L) | 0.5 ± 0.05 | 1.4 ± 0.05 | 0. 001 | 0.35–0.67 [30] |
| Cholesterol | (mg/dL) | 77.6 ± 9.2 | 56.6 ± 3.5 | 0.02 | 80–120 [29] |
| Triglycerides | (mg/dL) | 43 ± 2.6 | 67 ± 4.6 | 0.01 | 10.3–59.3 [29] |
| Total protein | (g/dL) | 5.7 ± 0.08 | 4.5 ± 0.2 | 0.005 | 5.4–9.3 [29] |
| Albumin | (g/dL) | 4.4 ± 0.05 | 3.3 ± 0.05 | 0.001 | 2.2–4.6 [29] |
| Globulin | (g/dL) | 0.4 ± 0.02 | 0.7 ± 0.05 | 0.004 | 3.9–4.1 [31] |
| Blood urea nitrogen | (mg/dL) | 68.6 ± 0.1.4 | 56 ±.1.1 | 0.002 | 13.2–64.1 [29] |
| Creatinine | (mg/dL) | 0.1 ± 0.04 | 0. 5 ± 0.02 | 0.002 | 1.07–2.52 [29] |
| Estrogen | (pg/mL) | 90 ± 1.1 | 69 ± 3.5 | 0. 005 | 50–90 [32] |
| Progesterone | (ng/mL) | 1 ± 0.06 | 0.5 ± 0.04 | 0. 004 | 0.9–1.3 [32] |
| FSH | (mU/mL) | 5.6 ± 0.08 | 3.7 ± 0.01 | 0.001 | 3.5–6 [32] |
| LH | (mU/mL) | 3.8 ± 0.06 | 2.7 ± 0.1 | 0.001 | 3.2–5 [32] |
| T4 | (ng/mL) | 8.8 ± 0. 5 | 4.7 ± 0.3 | 0.004 | 8.6–9.2 [33] |
| Cortisol | (ng/mL) | 18 ± 0.5 | 27 ± 1.1 | 0.002 | 17.5–20 [34] |
| PGF2α | (pg/mL) | 51 ± 1.5 | 34 ± 1.1 | 0.001 | 53.67 ± 2.4 [35] |
| Calcium | (mg/dL) | 6.6 ± 0.08 | 5.4 ± 0.1 | 0.001 | 6.4–12.8 [29] |
| Phosphorus | (mg/dL) | 3.5 ± 0.04 | 3.7 ± 0.05 | 0.06 | 3.2–3.9 [36] |
| Magnesium | (mg/dL) | 2.4 ± 0.05 | 2.3 ± 0.02 | 0.3 | 2.20–3.93 [29] |
| Iron | (Ug/dL) | 132.6 ± 1.4 | 115 ± 0.5 | 0.001 | 60.1–187.4 [29] |
| Selenium | (Ug/dL) | 3.9 ± 0.08 | 2.8 ± 0.05 | 0.001 | 3.6–4.1 [33] |
| Copper | (Ug/dL) | 84.6 ± 1.4 | 84.6 ± 2 | 1 | 51–151.1 [29] |
| Zinc | (Ug/dL) | 67 ± 1.1 | 67.6 ± 0.8 | 0.6 | 52.2–130.9 [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, A.; Faraj, S.H.; Marghani, B.H.; Safhi, F.A.; Abdo, M.; Fericean, L.; Banatean-Dunea, I.; Alexandru, C.-C.; Alhimaidi, A.R.; Ammari, A.A.; et al. The Transcript Levels and the Serum Profile of Biomarkers Associated with Clinical Endometritis Susceptibility in Buffalo Cows. Vet. Sci. 2024, 11, 340. https://doi.org/10.3390/vetsci11080340
El-Sayed A, Faraj SH, Marghani BH, Safhi FA, Abdo M, Fericean L, Banatean-Dunea I, Alexandru C-C, Alhimaidi AR, Ammari AA, et al. The Transcript Levels and the Serum Profile of Biomarkers Associated with Clinical Endometritis Susceptibility in Buffalo Cows. Veterinary Sciences. 2024; 11(8):340. https://doi.org/10.3390/vetsci11080340
Chicago/Turabian StyleEl-Sayed, Ahmed, Salah H. Faraj, Basma H. Marghani, Fatmah A. Safhi, Mohamed Abdo, Liana Fericean, Ioan Banatean-Dunea, Cucui-Cozma Alexandru, Ahmad R. Alhimaidi, Aiman A. Ammari, and et al. 2024. "The Transcript Levels and the Serum Profile of Biomarkers Associated with Clinical Endometritis Susceptibility in Buffalo Cows" Veterinary Sciences 11, no. 8: 340. https://doi.org/10.3390/vetsci11080340
APA StyleEl-Sayed, A., Faraj, S. H., Marghani, B. H., Safhi, F. A., Abdo, M., Fericean, L., Banatean-Dunea, I., Alexandru, C.-C., Alhimaidi, A. R., Ammari, A. A., Eissa, A., & Ateya, A. (2024). The Transcript Levels and the Serum Profile of Biomarkers Associated with Clinical Endometritis Susceptibility in Buffalo Cows. Veterinary Sciences, 11(8), 340. https://doi.org/10.3390/vetsci11080340







