Comparison of the Fatty Acid Profiles of Sow and Goat Colostrum
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Colostrum Collection
2.2. Chemical Composition Analyses and Immunoglobulin Quantification
2.3. Sample Processing
2.4. Fatty Acid Analyses
2.5. Statistical Analyses
3. Results
3.1. Chemical Composition of Colostrum
3.2. Fatty Acid Profile of Sow Colostrum
3.3. Fatty Acid Profile of Goat Colostrum
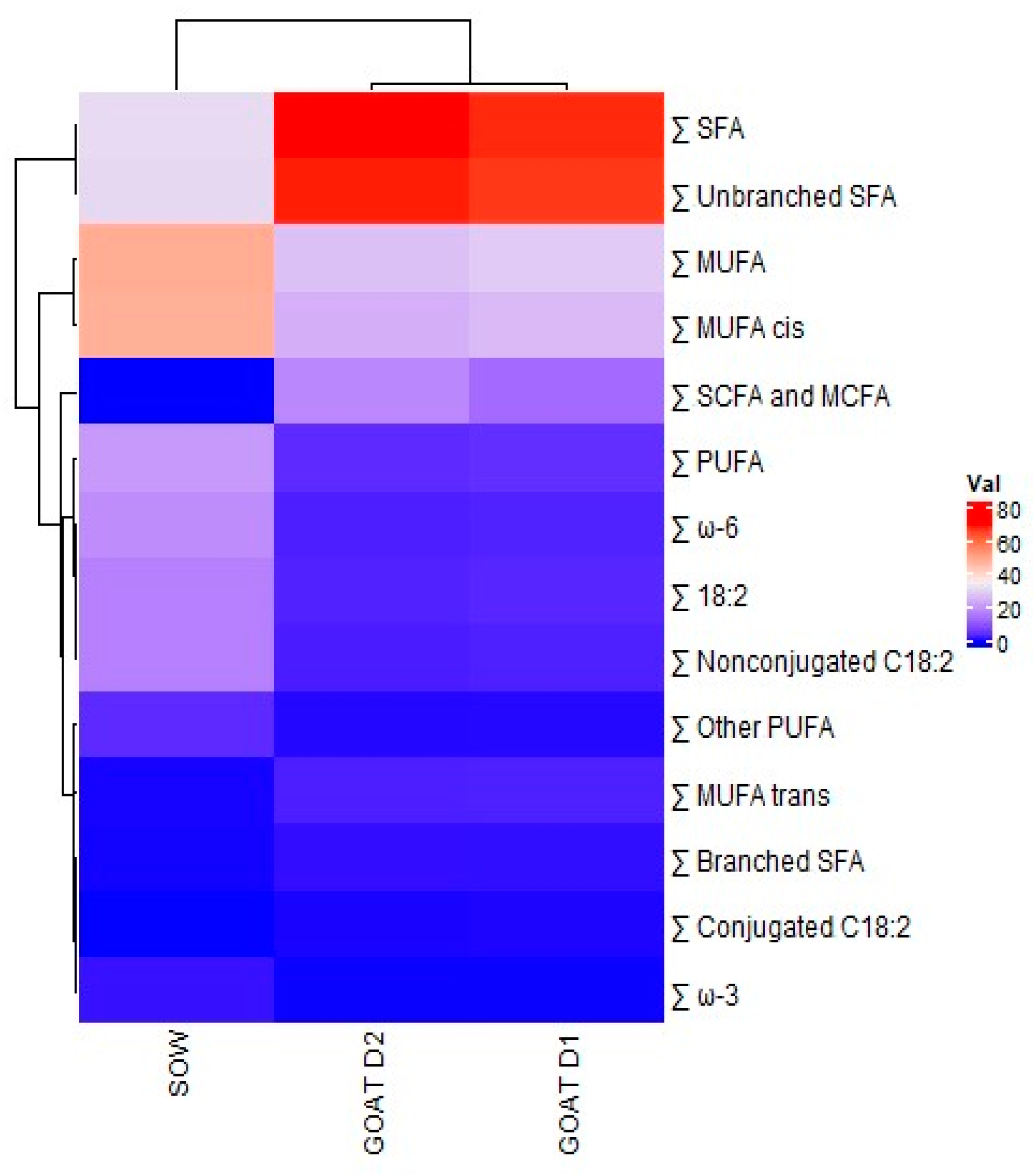
3.4. Comparison of Swine and Goat Colostrum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmitt, O.; Baxter, E.M.; Lawlor, P.G.; Boyle, L.A.; O’Driscoll, K. A single dose of fat-based energy supplement to light birth weight pigs shortly after birth does not increase their survival and growth. Animals 2019, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Antonczyk, C.; Ratert, C.; Schwennen, C.; Kamphues, J.; Abd El-Wahab, A. Chemical Composition of Newborn Piglets with Different Weights at Birth in Sows with a High Reproductive Performance. Animals 2024, 14, 1380. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, C.; Liu, T.; Shi, M.; Wu, G.; Bazer, F.W. Physiological alterations associated with intrauterine growth restriction in fetal pigs: Causes and insights for nutritional optimization. Mol. Reprod. Dev. 2017, 84, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Knap, P.W.; Knol, E.F.; Sørensen, A.C.; Huisman, A.E.; van der Spek, D.; Zak, L.J.; Granados Chapatte, A.; Lewis, C.R.G. Genetic and Phenotypic Time Trends of Litter Size, Piglet Mortality, and Birth Weight in Pigs. Front. Anim. Sci. 2023, 4, 1218175. [Google Scholar] [CrossRef]
- Devillers, N.; Farmer, C.; Le Dividich, J.; Prunier, A. Variability of colostrum yield and colostrum intake in pigs. Animal 2007, 1, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Bortolozzo, F.P.; Zanin, G.P.; Ulguim, R.D.R.; Mellagi, A.P.G. Managing Reproduction in Hyperprolific Sow Herds. Animals 2023, 13, 1842. [Google Scholar] [CrossRef] [PubMed]
- Herpin, P.; Damon, M.; Le Dividich, J. Development of thermoregulation and neonatal survival in pigs. Livest. Prod. Sci. 2002, 78, 25–45. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- Vötterl, J.C.; Schwartz-Zimmermann, H.E.; Lerch, F.; Yosi, F.; Sharma, S.; Aigensberger, M.; Rennhofer, P.M.; Berthiller, F.; Metzler-Zebeli, B.U. Variations in Colostrum Metabolite Profiles in Association with Sow Parity. Transl. Anim. Sci. 2024, 8, txae062. [Google Scholar] [CrossRef]
- Segura, M.; Martínez Miró, S.; López, M.J.; Madrid, J.; Hernández, F. Effect of parity on reproductive performance and composition of sow colostrum during first 24 h postpartum. Animals 2020, 10, 1853. [Google Scholar] [CrossRef]
- Luise, D.; Cardenia, V.; Zappaterra, M.; Motta, V.; Bosi, P.; Rodriguez-Estrada, M.T.; Trevisi, P. Evaluation of breed and parity order effects on the lipid composition of porcine colostrum. J. Agric. Food Chem. 2018, 66, 12911–12920. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Fang, Z.; Lin, Y.; Che, L.; Wu, C.; Xu, S.; Feng, B.; Lian, J.; Wu, D. Influence of dietary fat source on sow and litter performance, colostrum and milk fatty acid profile in late gestation and lactation. Anim. Sci. J. 2017, 88, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Settachaimongkon, S.; Homyog, K.; Mekboonsonglarp, W.; Soonoue, P.; Lerdamnuaylarp, T.; Prayoonpeeraput, P.; Theil, P.K.; Nuntapaitoon, M. Dynamics of Fatty Acid and Non-Volatile Polar Metabolite Profiles in Colostrum and Milk Depending on the Lactation Stage and Parity Number of Sows. Sci. Rep. 2023, 13, 1989. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Orro, T.; Valros, A.; Junnikkala, S.; Peltoniemi, O.; Oliviero, C. Factors affecting sow colostrum yield and composition, and their impact on piglet growth and health. Livest. Sci. 2019, 227, 60–67. [Google Scholar] [CrossRef]
- Van Ginneken, C.; Ayuso, M.; Van Bockstal, L.; Van Cruchten, S. Preweaning Performance in Intrauterine Growth-Restricted Piglets: Characteristics and Interventions. Mol. Reprod. Dev. 2023, 90, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Muns, R.; Silva, C.; Manteca, X.; Gasa, J. Effect of cross-fostering and oral supplementation with colostrums on performance of newborn piglets. J. Anim Sci. 2014, 92, 1193–1199. [Google Scholar] [CrossRef][Green Version]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Effect of oral supplementation with different energy boosters in newborn piglets on pre-weaning mortality, growth and serological levels of IGF-I and IgG. J. Anim. Sci. 2017, 95, 353–360. [Google Scholar] [PubMed]
- Engelsmann, M.N.; Hansen, C.F.; Nielsen, M.N.; Kristensen, A.R.; Amdi, C. Glucose injections at birth, warmth and placing at a nurse sow improve the growth of IUGR piglets. Animals 2019, 9, 519. [Google Scholar] [CrossRef] [PubMed]
- Romero, T.; Beltrán, M.C.; Rodríguez, M.; De Olives, A.M.; Molina, M.P. Goat colostrum quality: Litter size and lactation number effects. J. Dairy Sci. 2013, 96, 7526–7531. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U. The Role of Dietary and Microbial Fatty Acids in the Control of Inflammation in Neonatal Piglets. Animals 2021, 11, 2781. [Google Scholar] [CrossRef]
- Fundación Española para el Desarrollo de la Nutrición Animal (FEDNA). Necesidades Nutricionales para Ganado Porcino Normas FEDNA; Fundación Española para el Desarrollo de la Nutrición Animal: Madrid, Spain, 2013. [Google Scholar]
- Fundación Española para el Desarrollo de la Nutrición Animal (FEDNA). Necesidades Nutricionales para Rumiantes de Leche Normas FEDNA; Fundación Española para el Desarrollo de la Nutrición Animal: Madrid, Spain, 2009. [Google Scholar]
- Luna, P.; Juárez, M.; De La Fuente, M.A. Validation of a Rapid Milk Fat Separation Method to Determine the Fatty Acid Profile by Gas Chromatography. J. Dairy Sci. 2005, 88, 3377–3381. [Google Scholar] [CrossRef] [PubMed]
- ISO 15884-IDF 182:2002; Milk Fat—Preparation of Fatty Acid Methyl Esters. ISO-IDF: Brussels, Belgium, 2002.
- De la Fuente, M.A.; Luna, P.; Juárez, M. Chromatographic techniques to determine conjugated linoleic acid. Trends Anal. Chem. 2006, 25, 917–926. [Google Scholar] [CrossRef]
- De la Fuente, M.A.; Rodríguez-Pino, V.; Juárez, M. Use of an extremely polar 100-m column in combination with a cyanoalkyl polysiloxane column to complement the study of milk fats with different fatty acid profiles. Int. Dairy J. 2015, 47, 52–63. [Google Scholar] [CrossRef][Green Version]
- Marounek, M.; Pavlata, L.; Mišurová, L.; Volek, Z.; Dvořák, R. Changes in the composition of goat colostrum and milk fatty acids during the first month of lactation. Czech J. Anim. Sci. 2012, 57, 28–33. [Google Scholar] [CrossRef]
- Zervas, G.; Tsiplakou, E. Goat Milk. In Milk and Dairy Products in Human Nutrition. Production, Composition and Health; Park, Y.W., Haenlein, G.F.W., Eds.; Wiley Blackwell: Ames, IA, USA, 2013; pp. 498–518. [Google Scholar]
- Ren, C.; Jin, J.; Wang, X.; Zhang, Y.; Jin, Q. Evaluation of fatty acid profile of colostrum and milk fat of different sow breeds. Int. Dairy J. 2022, 126, 105250. [Google Scholar] [CrossRef]
- Laws, J.; Juniper, D.T.; Lean, I.J.; Amusquivar, E.; Herrera, E.; Dodds, P.F.; Clarke, L. Supplementing sow diets with palm oil during late gestation and lactation: Effects on milk production, sow hormonal profiles and growth and development of her offspring. Animal 2018, 12, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Vodolazska, D.; Lauridsen, L. Effects of dietary hemp seed oil to sows on fatty acid profiles, nutritional and immune status of piglets. J. Anim. Sci. Biotechnol. 2020, 11, 28. [Google Scholar]
- Hu, P.; Yang, H.; Lv, B.; Zhao, D.; Wang, J.; Zhu, W. Dynamic changes of fatty acids and minerals in sow milk during lactation. J. Anim. Physiol. Anim. Nutr. 2019, 103, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Aguinaga, M.A.; Haro, A.; Lara, L.; Gómez-Carballar, F.; Nieto, R.; Aguilera, J.F. Utilization of Milk Fatty Acids by the Suckling Iberian Piglets. Animal 2016, 10, 1786–1795. [Google Scholar] [CrossRef]
- Boyd, R.D.; Britton, R.A.; Knoche, H.; Moser, B.D.; Peo, E.R., Jr.; Johnson, R.K. Oxidation rates of major fatty acids in fasting neonatal pigs. J. Anim. Sci. 1982, 55, 95–100. [Google Scholar] [CrossRef]
- Buccioni, A.; Decandia, M.; Minieri, S.; Molle, G.; Cabiddu, A. Lipid Metabolism in the Rumen: New Insights on Lipolysis and Biohydrogenation with an Emphasis on the Role of Endogenous Plant Factors. Anim. Feed Sci. Technol. 2012, 174, 1–25. [Google Scholar] [CrossRef]
- Yakan, A.; Özkan, H.; Çamdeviren, B.; Kaya, U.; Karaaslan, I.; Dalkiran, S. Expression patterns of major genes in fatty acid synthesis, inflammation, oxidative stress pathways from colostrum to milk in Damascus goats. Sci. Rep. 2021, 11, 9448. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Juárez, M.; De la Fuente, M.A. Milk fatty acids and potential health benefits: An updated vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef]
- Hanczakowska, E. The use of medium-chain fatty acids in piglet feeding-a review. Ann. Anim. Sci. 2017, 17, 967–977. [Google Scholar] [CrossRef]
- Mao, S.; Liu, Z.; Tian, Y.; Li, D.; Gao, X.; Wen, Y.; Peng, T.; Shen, W.; Xiao, D.; Wan, F.; et al. Branched-Long-Chain Monomethyl Fatty Acids: Are They Hidden Gems? J. Agric. Food Chem. 2023, 71, 18674–18684. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Ye, A.; Maughan, P.J.; Singh, H. Composition, structure, and digestive dynamics of milk from different species. A Review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef] [PubMed]
- Martínez Miró, S.; Naranjo, S.; Madrid, J.; López, M.J.; Sánchez, C.J.; Segura, M.M.; Hernández, F. Evaluation of Immunoglobulin G Absorption from Goat Colostrum by Newborn Piglets. Animals 2020, 10, 637. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Hurley, W.L.; Theil, P.K. Perspectives on Immunoglobulins in Colostrum and Milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
| Item | Sow | Goat D1 | Goat D2 | SEM | p Value |
|---|---|---|---|---|---|
| Dry matter, % | 26.8 a | 24.7 b | 22.1 c | 0.38 | <0.001 |
| Crude Protein, % | 18.5 a | 10.5 b | 7.4 c | 0.29 | <0.001 |
| Crude Fat, % | 5.5 b | 9.0 a | 9.1 a | 0.30 | <0.001 |
| Lactose, % | 2.8 b | 3.4 a | 3.8 a | 0.07 | <0.001 |
| IgG, mg/mL | 91.2 a | 37.5 b | 16.7 b | 6.27 | <0.001 |
| Fatty Acid | Parity 1 | Parity 2–4 | Parity ≥ 5 | SEM | p-Value |
|---|---|---|---|---|---|
| 10:0 | 0.01 | 0.01 | 0.01 | 0.001 | 0.277 |
| 12:0 | 0.04 | 0.03 | 0.03 | 0.005 | 0.558 |
| 14:0 | 1.52 | 1.49 | 1.43 | 0.089 | 0.898 |
| 15:0 | 0.15 | 0.14 | 0.14 | 0.007 | 0.792 |
| 16:0 | 21.75 | 23.06 | 23.39 | 0.411 | 0.276 |
| 17:0 | 0.33 | 0.32 | 0.33 | 0.017 | 0.993 |
| 18.0 | 5.51 | 6.02 | 5.32 | 0.190 | 0.334 |
| 20:0 | 0.10 | 0.11 | 0.10 | 0.005 | 0.473 |
| 21:0 | 0.03 | 0.02 | 0.02 | 0.001 | 0.297 |
| 22:0 | 0.10 | 0.12 | 0.10 | 0.005 | 0.469 |
| 23:0 | 0.03 a | 0.02 b | 0.02 b | 0.001 | 0.017 |
| 24:0 | 0.07 | 0.08 | 0.09 | 0.005 | 0.301 |
| Total non-branched SFA | 29.61 | 31.41 | 30.94 | 0.537 | 0.421 |
| iso 15:0 | 0.08 | 0.07 | 0.07 | 0.003 | 0.382 |
| anteiso 15:0 | 0.05 | 0.05 | 0.05 | 0.002 | 0.992 |
| iso 16:0 | 0.03 | 0.03 | 0.03 | 0.002 | 0.743 |
| iso 17:0 | 0.05 | 0.05 | 0.04 | 0.003 | 0.299 |
| anteiso 17:0 | 0.09 | 0.04 | 0.08 | 0.022 | 0.565 |
| iso 18:0 | 0.16 | 0.16 | 0.14 | 0.011 | 0.752 |
| Total branched SFA | 0.46 | 0.39 | 0.42 | 0.031 | 0.676 |
| TOTAL SFA | 30.10 | 31.83 | 31.38 | 0.552 | 0.465 |
| cis-9 14:1 | 0.05 | 0.04 | 0.04 | 0.005 | 0.538 |
| cis-7 16:1 | 1.53 | 1.58 | 1.60 | 0.051 | 0.829 |
| cis-9 16:1 | 3.93 | 3.17 | 3.91 | 0.220 | 0.325 |
| cis-9 17:1 | 0.31 | 0.26 | 0.28 | 0.010 | 0.163 |
| cis-9 18:1 | 36.6 | 35.6 | 35.9 | 0.612 | 0.810 |
| cis-11 18:1 | 4.96 | 4.68 | 5.18 | 0.132 | 0.332 |
| cis-13 18:1 | 0.23 | 0.22 | 0.25 | 0.007 | 0.306 |
| Other cis-20:1 | 0.05 | 0.05 | 0.05 | 0.004 | 0.769 |
| cis-11 20:1 | 0.25 | 0.23 | 0.24 | 0.017 | 0.838 |
| cis-13 22:1 | 0.05 | 0.05 | 0.05 | 0.002 | 0.986 |
| Total cis MUFA | 47.9 | 45.8 | 47.5 | 0.725 | 0.509 |
| trans-16:1 | 0.07 | 0.07 | 0.06 | 0.003 | 0.674 |
| trans-18:1 | 0.46 | 0.45 | 0.41 | 0.022 | 0.669 |
| Total trans MUFA | 0.52 | 0.52 | 0.47 | 0.024 | 0.645 |
| TOTAL MUFA | 48.44 | 46.35 | 47.93 | 0.706 | 0.496 |
| Other trans-trans | 0.04 | 0.04 | 0.04 | 0.004 | 0.980 |
| trans-9 trans-12 | 0.02 | 0.02 | 0.02 | 0.001 | 0.873 |
| cis-9 trans-13 + trans-8 cis-12 | 0.04 | 0.03 | 0.03 | 0.003 | 0.249 |
| trans-8 cis-13 + cis-9 trans-12 | 0.09 | 0.08 | 0.09 | 0.005 | 0.622 |
| trans-11 cis-15 + trans-10 cis-15 | 0.04 | 0.03 | 0.04 | 0.004 | 0.511 |
| cis-9 cis-12 | 16.39 | 16.61 | 15.80 | 0.431 | 0.724 |
| Total non-conjugated 18:2 | 16.63 | 16.80 | 16.01 | 0.439 | 0.731 |
| cis-9 trans-11 | 0.06 a | 0.06 a | 0.05 b | 0.002 | 0.006 |
| trans-9 cis-11 | 0.01 | 0.01 | 0.01 | 0.001 | 0.970 |
| trans-11 trans-13 | 0.02 | 0.02 | 0.02 | 0.002 | 0.501 |
| trans-8 trans-10 + trans-9 trans-trans-11+ trans-10 cis-12 | 0.04 | 0.03 | 0.03 | 0.002 | 0.080 |
| Total conjugated 18:2 | 0.14 a | 0.12 b | 0.11 c | 0.003 | 0.010 |
| TOTAL 18:2 | 16.76 | 16.92 | 16.12 | 0.440 | 0.724 |
| 18:3 n-6 | 0.36 | 0.29 | 0.29 | 0.028 | 0.582 |
| 18:3 n-3 | 0.92 | 0.93 | 0.87 | 0.031 | 0.678 |
| 20:2 n-6 | 0.40 | 0.40 | 0.38 | 0.013 | 0.746 |
| 20:3 n-6 | 0.27 | 0.31 | 0.28 | 0.011 | 0.355 |
| 20:3 n-3 | 0.08 | 0.08 | 0.08 | 0.003 | 0.597 |
| 20:4 n-6 | 1.13 | 1.26 | 1.13 | 0.046 | 0.462 |
| 22:2 n-6 | 0.04 | 0.04 | 0.04 | 0.001 | 0.810 |
| 20:5 n-3 EPA | 0.08 | 0.08 | 0.07 | 0.005 | 0.667 |
| 22:4 n-6 | 0.24 | 0.25 | 0.21 | 0.013 | 0.472 |
| 22:5 n-6 | 0.03 | 0.04 | 0.03 | 0.003 | 0.440 |
| 22:5 n-3 DPA | 0.35 | 0.40 | 0.34 | 0.024 | 0.610 |
| 22:6 n-3 DHA | 0.12 | 0.14 | 0.12 | 0.008 | 0.663 |
| Total other PUFA | 4.03 | 4.21 | 3.84 | 0.128 | 0.503 |
| Total omega-3 | 1.57 | 1.63 | 1.48 | 0.053 | 0.522 |
| Total omega-6 | 18.85 | 19.19 | 18.16 | 0.472 | 0.657 |
| TOTAL PUFA | 20.80 | 21.13 | 19.96 | 0.531 | 0.644 |
| omega-6/omega-3 | 12.07 | 11.81 | 12.33 | 0.195 | 0.569 |
| Collection Day | ||||
|---|---|---|---|---|
| Fatty Acid | D1 | D2 | SEM | p-Value |
| 4:0 | 2.00 | 2.66 | 0.097 | 0.012 |
| 5:0 | 0.01 | 0.02 | 0.001 | 0.038 |
| 6:0 | 1.72 | 2.44 | 0.085 | 0.004 |
| 7:0 | 0.02 | 0.02 | 0.001 | 0.013 |
| 8:0 | 1.62 | 2.42 | 0.082 | 0.002 |
| 9:0 | 0.02 | 0.03 | 0.001 | 0.005 |
| 10:0 | 4.95 | 7.02 | 0.169 | 0.000 |
| 11:0 | 0.03 | 0.03 | 0.002 | 0.079 |
| 12:0 | 2.67 | 3.24 | 0.045 | 0.000 |
| 13:0 | 0.04 | 0.04 | 0.002 | 0.553 |
| 14:0 | 11.57 | 11.75 | 0.112 | 0.456 |
| 15:0 | 0.51 | 0.49 | 0.011 | 0.531 |
| 16:0 | 31.56 | 29.11 | 0.362 | 0.012 |
| 17:0 | 0.59 | 0.57 | 0.007 | 0.178 |
| 18.0 | 7.77 | 7.41 | 0.156 | 0.292 |
| 20:0 | 0.17 | 0.15 | 0.007 | 0.213 |
| 22:0 | 0.06 | 0.05 | 0.002 | 0.029 |
| 23:0 | 0.01 | 0.01 | 0.001 | 0.173 |
| 24:0 | 0.02 | 0.01 | 0.002 | 0.301 |
| Total non-branched SFA | 65.32 | 67.47 | 0.275 | 0.006 |
| iso 13:0 | 0.01 | 0.01 | 0.000 | 0.263 |
| anteiso 13:0 | 0.02 | 0.02 | 0.000 | 0.020 |
| iso 14:0 | 0.03 | 0.03 | 0.001 | 0.909 |
| iso 15:0 | 0.15 | 0.15 | 0.004 | 0.846 |
| anteiso 15:0 | 0.13 | 0.12 | 0.003 | 0.335 |
| iso 16:0 | 0.17 | 0.16 | 0.006 | 0.117 |
| iso 17:0 | 0.43 | 0.41 | 0.006 | 0.113 |
| anteiso 17:0 | 0.35 | 0.36 | 0.001 | 0.586 |
| iso 18:0 | 0.06 | 0.07 | 0.020 | 0.035 |
| Total branched SFA | 1.34 | 1.32 | 0.020 | 0.593 |
| TOTAL SFA | 66.66 | 68.79 | 0.290 | 0.008 |
| Collection Day | ||||
|---|---|---|---|---|
| Fatty Acid | D1 | D2 | SEM | p-Value |
| 10:1 | 0.10 | 0.14 | 0.006 | 0.005 |
| cis-9 12:1 | 0.004 | 0.01 | 0.000 | 0.490 |
| cis-11 12:1 | 0.03 | 0.04 | 0.001 | 0.010 |
| cis-9 14:1 | 0.13 | 0.15 | 0.004 | 0.202 |
| cis-7 16:1 | 0.44 | 0.41 | 0.009 | 0.066 |
| cis-9 16:1 | 1.06 | 1.08 | 0.021 | 0.598 |
| cis-9 17:1 | 0.33 | 0.35 | 0.012 | 0.615 |
| cis-9 18:1 | 22.00 | 20.40 | 0.270 | 0.021 |
| cis-11 18:1 | 1.08 | 1.05 | 0.025 | 0.479 |
| cis-12 18:1 | 0.25 | 0.24 | 0.005 | 0.345 |
| cis-13 18:1 | 0.11 | 0.10 | 0.003 | 0.137 |
| cis-14 18:1 | 0.09 | 0.09 | 0.002 | 0.246 |
| cis-15 18:1 | 0.05 | 0.04 | 0.001 | 0.028 |
| cis-16 18:1 | 0.03 | 0.02 | 0.002 | 0.056 |
| cis-11 20:1 | 0.09 | 0.07 | 0.003 | 0.068 |
| cis-13 22:1 | 0.03 | 0.02 | 0.001 | 0.019 |
| Total cis MUFA | 25.83 | 24.20 | 0.283 | 0.023 |
| trans-15:1 | 0.03 | 0.03 | 0.001 | 0.468 |
| Other trans-16:1 | 0.30 | 0.30 | 0.007 | 0.652 |
| trans-6+7+8 16:1 | 0.08 | 0.08 | 0.002 | 0.223 |
| trans-9 16:1 | 0.06 | 0.05 | 0.006 | 0.257 |
| trans-4 18:1 | 0.02 | 0.02 | 0.002 | 0.149 |
| trans-5 18:1 | 0.02 | 0.02 | 0.001 | 0.882 |
| trans-6+7+8 18:1 | 0.19 | 0.16 | 0.004 | 0.011 |
| trans-9 18:1 | 0.23 | 0.20 | 0.007 | 0.047 |
| trans-10 18:1 | 0.28 | 0.36 | 0.018 | 0.067 |
| trans-11 18:1 | 1.36 | 1.22 | 0.034 | 0.064 |
| trans-12 18:1 | 0.31 | 0.29 | 0.007 | 0.312 |
| Total trans MUFA | 2.88 | 2.73 | 0.047 | 0.152 |
| TOTAL MUFA | 28.71 | 26.92 | 0.267 | 0.012 |
| Other trans-trans | 0.10 | 0.10 | 0.003 | 0.313 |
| cis-9 trans-12 + trans-8 cis-12 | 0.14 | 0.14 | 0.002 | 0.102 |
| trans-8 cis-13 + cis-9 trans-12 | 0.08 | 0.06 | 0.002 | 0.029 |
| trans-9 cis-12 | 0.01 | 0.01 | 0.001 | 0.053 |
| trans-11 cis-15 + trans-10 cis-15 | 0.02 | 0.01 | 0.001 | 0.013 |
| cis-9 cis-12 | 2.47 | 2.22 | 0.031 | 0.005 |
| cis-12 cis-15 | 0.05 | 0.03 | 0.001 | 0.001 |
| Total non-conjugated 18:2 | 2.87 | 2.56 | 0.038 | 0.005 |
| cis-9 trans-11 | 0.58 | 0.51 | 0.021 | 0.130 |
| trans-9 cis-11 | 0.03 | 0.02 | 0.001 | 0.241 |
| trans-11 cis-13 | 0.02 | 0.02 | 0.002 | 0.147 |
| trans-8 trans-10 + trans-9 trans-11+ trans-10 trans-12 | 0.02 | 0.02 | 0.001 | 0.178 |
| Total conjugated 18:2 | 0.65 | 0.57 | 0.023 | 0.123 |
| TOTAL 18:2 | 3.52 | 3.13 | 0.056 | 0.010 |
| 18:3 n-6 | 0.03 | 0.03 | 0.001 | 0.169 |
| 18:3 n-3 | 0.10 | 0.10 | 0.003 | 0.604 |
| 20:2 n-6 | 0.03 | 0.03 | 0.002 | 0.409 |
| cis-9 trans-11 cis-15 18:3 | 0.04 | 0.04 | 0.004 | 0.582 |
| 20:3 n-6 | 0.04 | 0.03 | 0.003 | 0.226 |
| 20:4 n-6 | 0.40 | 0.40 | 0.014 | 0.458 |
| 20:5 n-3 EPA | 0.03 | 0.03 | 0.001 | 0.558 |
| 22:4 n-6 | 0.10 | 0.10 | 0.004 | 0.747 |
| 22:5 n-6 | 0.02 | 0.02 | 0.001 | 0.153 |
| 22:5 n-3 DPA | 0.11 | 0.10 | 0.005 | 0.310 |
| 22:6 n-3 DHA | 0.02 | 0.02 | 0.002 | 0.618 |
| TOTAL Other PUFA | 0.90 | 0.86 | 0.032 | 0.573 |
| Total omega-3 | 0.30 | 0.28 | 0.012 | 0.538 |
| Total omega-6 | 3.07 | 2.80 | 0.050 | 0.029 |
| TOTAL PUFA | 4.43 | 4.00 | 0.081 | 0.033 |
| omega-6/omega-3 | 10.45 | 9.95 | 0.284 | 0.409 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala, L.; Gómez-Cortés, P.; Hernández, F.; Madrid, J.; Martínez-Miró, S.; de la Fuente, M.A. Comparison of the Fatty Acid Profiles of Sow and Goat Colostrum. Vet. Sci. 2024, 11, 341. https://doi.org/10.3390/vetsci11080341
Ayala L, Gómez-Cortés P, Hernández F, Madrid J, Martínez-Miró S, de la Fuente MA. Comparison of the Fatty Acid Profiles of Sow and Goat Colostrum. Veterinary Sciences. 2024; 11(8):341. https://doi.org/10.3390/vetsci11080341
Chicago/Turabian StyleAyala, Lucía, Pilar Gómez-Cortés, Fuensanta Hernández, Josefa Madrid, Silvia Martínez-Miró, and Miguel Angel de la Fuente. 2024. "Comparison of the Fatty Acid Profiles of Sow and Goat Colostrum" Veterinary Sciences 11, no. 8: 341. https://doi.org/10.3390/vetsci11080341
APA StyleAyala, L., Gómez-Cortés, P., Hernández, F., Madrid, J., Martínez-Miró, S., & de la Fuente, M. A. (2024). Comparison of the Fatty Acid Profiles of Sow and Goat Colostrum. Veterinary Sciences, 11(8), 341. https://doi.org/10.3390/vetsci11080341





