Simple Summary
Antimicrobial resistance is a phenomenon spreading through animals and humans, often with food as a vector. In this study, the presence of extended-spectrum β-lactamase producing E. coli isolates was examined in raw poultry carcasses from Greece. Among the samples, 64% were positive. One hundred and twenty strains were isolated, among which 71.67% were classified as true ESBL, 20.00% as AmpC, and 8.33% as “of unknown etiology”. The genetic background of the isolates for ESBL production featured the presence of variants of the blaCTX-M and the blaTEM genes, with non-gene harboring strains also isolated. The results demonstrate the existence of E. coli isolates producing extended-spectrum β-lactamases in raw poultry meat from Greece, posing a risk for antibiotic resistance transfer to humans. Further studies are needed to access microbial resistance trends, elucidate possible transmission routes, and further strengthen public health surveillance.
Abstract
Antimicrobial resistance is considered a topic of utmost interest under the concept of “One Health”, having severe implications in both human and veterinary medicine. Among the antibiotic-resistant bacteria, gram-negative bacteria, especially those belonging to the order of Enterobacterales (such as Escherichia coli), hold a prominent position in terms of both virulence and possessing/disseminating antimicrobial resistance (AMR) traits. The aim of this study was to examine the presence of extended-spectrum β-lactamase producing E. coli isolates in raw poultry carcasses collected from a university club. Five hundred raw poultry skin samples were collected from the Aristotle University of Thessaloniki (AUTh) club in Thessaloniki, Greece. A total of 64% of the samples were positive for the presence of extended-spectrum β-lactamase (ESBL)-producing E. coli. The isolates were further examined for their susceptibility to selected antibiotics by the disc diffusion method and were characterized as true ESBL, as producing class C cephalosporinases (AmpC) or “of unknown etiology” by the combination disc test. The 86 of the 120 isolates (71.67%) were classified as true ESBL, 24 (20.00%) as AmpC, and 10 (8.33%) as “of unknown etiology”. The isolates were screened for the occurrence of β-lactamase genes (blaTEM, blaCTX-M, blaSHV, and blaOXA). Thirty-six isolates (32 ESBL- and 4 AmpC-phenotype) harbored both blaTEM and blaCTX-M genes, twenty-two isolates (among which 19 ESBL-phenotype and 2 AmpC-phenotype) harbored blaCTX-M only, whereas twenty-six (14 ESBL- and 12 AmpC-phenotype) isolates harbored blaTEM alone. No isolate harboring blaSHV or blaOXA was detected. The results demonstrate the existence of E. coli isolates producing extended-spectrum β-lactamases in poultry carcasses from Greece, pausing a risk for antibiotic resistance transfer to humans.
1. Introduction
The emergence of antimicrobial-resistant (AMR) bacteria is one of the major emerging health threats to human populations worldwide [1,2], as it has been linked to increased morbidity and mortality, as well as to rising healthcare costs [2,3]. This phenomenon is not new; the occurrence of resistance has risen along with the spread of antibiotics in both pathogenic and commensal bacteria [4]. More specifically, Enterobacterales are often considered microorganisms associated with antibiotic resistance due to several factors, including their role as both commensals and pathogens [5,6]. Among the resistance factors, the production of β-lactamases has been given much attention since they can exert resistance to β-lactam antibiotics, such as penicillins, cephalosporins, cephamycins, monobactams, and carbapenems [6]. Their importance is such that WHO has set as critical the priority of development of new antibiotics against carbapenem- and 3rd/4th generation cephalosporin-resistant Enterobacterales [7].
Acquired antibiotic resistance can be a consequence of the use and abuse of antibiotics [3]. Antibiotic use in human medicine has been mainly implicated in the emergence of resistance in nosocomial settings, being notorious for the occurrence of almost untreatable bacteria. Still, farm animals and their products are considered a significant pool of resistance [8]. In the era where antibiotics were used as growth promoters or for mere prevention purposes (i.e., without treatment claims), the emergence of resistant bacteria was quite common [4]. Although the use of antibiotics as growth promoters has been banned in the European Union and elsewhere, the situation is uneven in different parts of the world [9]. Among farm animals, poultry and their products are considered the most frequent carriers of ESBL- and AmpC-(collectively referred to as “extended-spectrum cephalosporinases”—ESCs) producing Escherichia coli [10]. Poultry farming is the most intensive form of animal production, making poultry prone to immune deficiency and disease. In addition, the large population densities facilitate the spread of bacteria within the flocks. Moreover, ESBL- and AmpC-producing Enterobacterales originating from poultry have been documented to cause infections in humans [11,12].
Many Mediterranean countries have been associated with the emergence of several new ESBL or AmpC genes [6]. The circulating antibiotic resistance factors in Greek patients follow the same trend. Nevertheless, little information is available concerning the occurrence of ESBL- and AmpC-producing bacteria in Greek poultry products. Therefore, the scope of this study was to evaluate the occurrence of ESC-producing E. coli in poultry products and to estimate the risk of food-borne antibiotic resistance in humans.
2. Materials and Methods
2.1. Sample Collection
An overall of 500 whole poultry carcasses were sampled, by collecting neck skin specimens, according to the Commission Regulation (EC) 2073/2005 on microbiological criteria for foodstuffs. The samples were procured from the university club of Aristotle University of Thessaloniki (AUTh-UC), Greece, according to its daily routine. AUTh-UC is able to provide up to 15,000 meals per day served primarily in two main refectories, which seat 1000 and 500 students, respectively. The collection of the neck skins was performed aseptically from individually packed chicken carcasses with the use of sterile utensils. All chicken carcasses originated from Greek farms and were collected randomly throughout the year on the premises of AUTh-UC. The samples were transported to the Laboratory of Animal Food Products Hygiene and Veterinary Public Health into portable coolers and processed within 4 h after collection. Samples were pooled in groups of five, all originating from the same producer and production date, resulting in one hundred pooled samples further used for examination.
2.2. Microbiological Examination
The isolation procedure was aimed at selecting only resistant bacteria from the samples examined. For the isolation of resistant E. coli, the methods of Agersø et al. [13] and Egervärn et al. [14] were used, with modifications. The samples were rinsed with McConkey broth (Oxoid, Basingstoke, UK) supplemented with 1 mg/L cefotaxime (cefotaxime sodium salt, Sigma-Aldrich, Saint Louis, MO, USA), which also served as a pre-enrichment medium. The rinsates were incubated at 42 °C for 18 h. After enrichment, plates of Violet Red Bile Glucose agar (Oxoid, Basingstoke, UK) with 1 mg/L cefotaxime (Sigma Aldrich, Saint Louis, USA) and Chromocult TBX agar (VRBG, Merck GmbH, Darmstadt, Germany) with 1 mg/L cefotaxime were inoculated and were further incubated at 42 °C for 24 h. E. coli form blue to green colonies in Chromocult TBX agar and purple to pink colonies with or without halos in VRBG agar. A maximum of five characteristic colonies of each plate were picked and pure-cultured in appropriate media. An initial indole test was performed (Kovac’s reagent, Liofilchem, Roseto degli Abruzzi, Italy) followed by standard biochemical tests for isolate typing to the species level, including typing of the isolates with the use of the automated microbial identification system VITEK® 2 Compact (bioMérieux, Marcy-l’Étoile, France) and the appropriate VITEK® 2 GN cards (bioMérieux, Marcy-l’Étoile, France) for the biochemical identification of gram-negative strains.
2.3. Determination of the Susceptibility of Isolates to Antibiotics
The disc diffusion method was used for the determination of the susceptibility of isolated strains to selected antibiotics, according to the recommendations of the Clinical and Laboratory Standards Institute [15]. One or two pure colonies were picked from an overnight culture and were suspended in 10 mL of sterile normal saline. The turbidity of the suspension was adjusted to 0.5 McFarland scale with the use of a nephelometer (Densitomat, bioMérieux, France), corresponding to approximately 108 CFU/mL, and an aliquot was streaked subsequently over Mueller Hinton agar plates with a sterile swab (bioMérieux, Marcy-l’Étoile, France). The antibiotic discs utilized contained penicillins [ampicillin (AMP 10 μg) and the amoxicillin-clavulanic acid combination (AMC 20/10 μg)], cephalosporins [cefotaxime (CTX 5 μg), and ceftazidime (CAZ 10 μg)], carbapenems [meropenem (MEM 10µg)], fluoroquinolones [ciprofloxacin (CIP 5 μg)], aminoglycosides [tobramycin (TOB 10µg), amikacin (AK 30µg), and gentamicin (CN 10 μg)], sulfonamides [trimethoprim-sulfamethoxazole combination (SXT 1.25/23.75 μg)], phenicols [chloramphenicol (CAF 30 μg)], and tetracyclines [tigecycline (TGC 15 μg)]. All antibiotic discs were supplied by Oxoid (Basingstoke, UK). The E. coli ATCC 25922 type culture was used for quality control. Inhibition zones were measured to the nearest millimeter (mm) using a digital caliper (Powerfix, Model Z22855, London, UK) and were characterized according to the EUCAST clinical resistance breakpoints [16], as mentioned in the European Commission Decision 652/2013.
2.4. Combination Disk Test
For the phenotypic characterization of the isolates, the combination disk test was utilized according to CLSI [15] and EUCAST [17] guidelines. In brief, an initial inoculum was prepared as already described, and Mueller Hinton agar plates (bioMérieux, France) were inoculated. Antibiotic discs (Himedia, Mumbai, India) containing cefepime (FEP 30 μg), cefepime/clavulanic acid (FEP 30 μg), ceftazidime (CAZ 30 μg), ceftazidime/clavulanic acid (CAC 30/10 μg) cefotaxime (CTX 30 μg), cefotaxime/clavulanic acid (CEC 30/10 μg), and cefoxitin (FOX 30 μg) were used. An increase of ≥5 mm in the zone diameter of a combination of the antibiotic with clavulanic acid against the zone diameter of the antibiotic alone was reported as corresponding to an ESBL phenotype. The isolates were characterized as AmpC if resistance to cefotaxime and ceftazidime was recorded without induction by clavulanic acid and exhibited a ≥5 mm increase in the inhibition zone. For quality control, the type cultures E. coli ATCC 25922 and K. pneumoniae ATCC 700603 were used.
2.5. PCR Screening of Isolates
For the determination of the genotype coding the ESBL or AmpC phenotypes, a multiplex PCR method was employed, as described by Fang et al. [18]. The method is suitable for detecting the presence of the blaTEM, blaSHV, blaCTX-M, and blaOXA genes in one multiplex PCR reaction. In brief, pure cultures of the isolates were subjected to DNA extraction, according to Tsiouris et al. [19]. One loopful of cells was dispersed in 100 μL dispersal buffer (50 mM Tris-HCl, 50 mM ethylenediaminetetraacetate (EDTA), 1% v/v Triton X-100, pH 7.5), in which 100 μL of lysis buffer I [50 mM Tris-HCl, 50 mM EDTA, 4 M guanidine hydrochloride (GuHCl), 10 mM CaCl2, 1% v/v Triton X-100, 2% N-Lauroyl-Sarcosine, pH 7.5], and 25 μL of proteinase K solution (22.4 mg/mL) were added, followed by incubation at 56 °C for 1 h. Consequently, 250 μL of lysis buffer II (50 mM Tris-HCl, 25 mM EDTA, 8 M GuHCl, 3% v/v Triton X-100, 3% N-Lauroyl-Sarcosine, pH 6.3) were added, and the mixture was incubated at 70 °C for 10 min. All reagents used for DNA extraction were manufactured by AppliChem GmbH (Darmstadt, Germany). After incubation, 250 μL of absolute ethanol was added, and the mixture was applied to silica columns (Qiagen, Venlo, The Netherlands). The column was then washed twice with wash I buffer [25 mM Tris-HCl, 4 M GuHCl, 50% ethanol (Merck GmbH, Darmstadt, Germany), pH 6.6] and once with wash buffer IΙ (10 mM Tris-HCl, 80% ethanol, 100 mM NaCl, pH 6.6). The DNA was eluted with ultrapure water (AppliChem GmbH, Darmstadt, Germany) and stored at −30 °C until examination. DNA quality and recovery were evaluated with the use of a NanoDrop microvolume spectrophotometer (Nanodrop 2000, Thermo Fisher Scientific, Waltham, MA, USA).
PCR was performed at 25 μL volume containing 5 μL of 5× OneTaq Standard Reaction Buffer (NEB), 0.5 μL of DNTP mix (NEB), 1 U of OneTaq ™ Hot Start DNA Polymerase (New England Biolabs, Hitchin, UK) and 2 μL of the sample. The primers used are shown in Table 1. The reaction volume was made up to 25 μL with the addition of sterile MiliQ grade water. PCR was performed in a thermal cycler (LabCycler gradient, SensoQuest, Göttingen, Germany). Initial denaturation (30 s at 95 °C) was followed by 30 cycles of amplification (denaturation at 94 °C for 30 s, annealing at 62 °C for 90 s, and extension at 72 °C for 60 s), ending with a final extension at 72 °C for 10 min. The PCR products were visualized in 1.5% agarose gels stained with ethidium bromide with the use of a UVP DigiDoc-It® 125 gel imaging system (UVP, Cambridge, UK).

Table 1.
Characteristics of the primers used for the detection of ESBL or AmpC genes.
2.6. Statistical Analysis
Frequencies were compared after the application of contingency tables and the use of χ2 goodness-of-fit tests with the use of the IBM® SPSS® Statistics software (version 25). The level of significance was set at 5% (critical p-value: 0.05).
3. Results
Of the 100 pooled samples tested, 64 (64%) were positive for ESBL isolates. In total, 120 E. coli isolates were recovered. The resistance phenotypes, the β-lactamase production phenotype, and the genetic background of β-lactam resistance are presented in Table 2. The percentages of antibiotic resistance among the isolates ranged from 0% (against MEM or TGC) to 100% (against AMC), as shown in Figure 1. According to the disc diffusion method, all isolates were multi-drug resistant (MDR) since they were resistant to at least three classes of antibiotics (Table 1). The most common resistance phenotype featured resistance to AMP, CTX, CAZ, CIP, TOB, CN, SXT, AMK, and AMC and was shared among 15% (n = 18) of the isolates examined. The resistance phenotype [AMP, CTX, CAZ, CIP, TOB, CN, AMK, AMC] was shared among 10% (n = 12) of the isolates, whereas the resistance phenotypes [AMP, CTX, CAZ, CIP, TOB, CN, SXT, CAF, AMK, AMC] and [AMP, CTX, CAZ, CIP, TOB, CN, SXT, AMC] were both found in 9.17% (n = 11) of the isolates.

Table 2.
Resistance profiles, β-lactam resistance phenotypes, and genetic determinants of the isolates examined for their antimicrobial resistance.
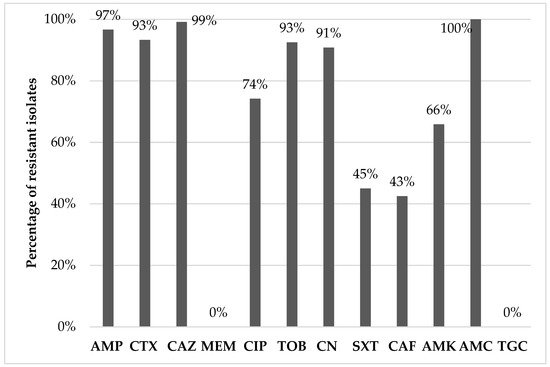
Figure 1.
Percentage of AMR of the Escherichia coli isolates (AK: amikacin; AMC: amoxicillin-clavulanic acid; AMP: ampicillin; CAF: chloramphenicol; CAZ: ceftazidime; CIP: ciprofloxacin; CN: gentamicin; CTX: cefotaxime; MEM: meropenem; SXT: trimethoprim-sulfamethoxazole; TGC: tigecycline. TOB: tobramycin).
Concerning the phenotype of β-lactam resistance, 86 (71.67%) and 24 (20%) of the 120 isolates were classified as true ESBL- or AmpC-producers, respectively, whereas 10 of the isolates (8.33%) were not phenotypically categorized as β-lactamase producers by the classification scheme used [significant difference in the occurrence of phenotypes; χ2(2) = 81.800, p < 0.001]. Concerning the occurrence of the investigated β-lactamase genes (blaTEM, blaSHV, blaCTX-M, and blaOXA), blaTEM was detected in 26 (21.67%) isolates, blaCTX-M was detected in 22 (18.66%) isolates, whereas 36 (30.00%) isolates harbored both blaTEM and blaCTX-M genes (Figure 2). No isolate harboring blaSHV or blaOXA was detected. Among the isolates of this study that displayed an ESBL phenotype and harbored bla determinants, 14 harbored blaTEM genes, 19 harbored blaCTX-M genes, whereas 32 harbored both blaTEM and blaCTX-M genes. The frequency of occurrence of these three categories was not statistically different [χ2(2) = 3.271, p = 0.195]. In contrast, among the AmpC phenotype isolates, the ones harboring both blaTEM and blaCTX-M genes (n = 4) were fewer than those harboring blaCTX-M genes (n = 12) only [χ2(1) = 4.000, p = 0.046]. Overall, a significant interaction between the ESBL or AmpC phenotype and the occurrence of bla genes was observed [χ2(2) = 13.364, p = 0.001] with ESBL-phenotype isolates carrying significantly more frequently the [blaTEM + blaCTX-M] combination than the AmpC-phenotype isolates.
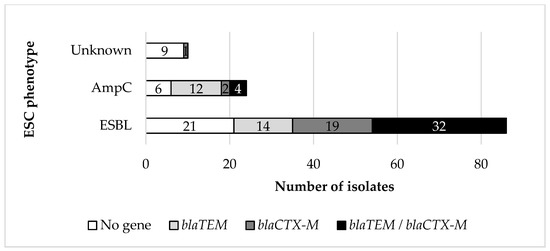
Figure 2.
Detection of ESBL encoding genes related to the ESBL phenotype.
Regarding the ESC phenotype, overall, 36 isolates (30%) harbored both blaTEM and blaCTX-M genes, 26 (21.67%) harbored blaTEM genes, and 22 (18.33%) harbored blaCTX-M genes. In 6 isolates characterized phenotypically as true ESBL producers, no β-lactamase encoding genes were detected. Among the isolates exhibiting an AmpC phenotype, 12 isolates (50%) harbored blaTEM genes, and four (16.67%) both blaTEM and blaCTX-M genes. In six AmpC phenotype isolates (25.00%), no blaTEM, blaSHV, blaCTX-M, or blaOXA genes were detected. The ESBL- or AmpC-phenotype isolates in which no β-lactamase encoding genes were detected most frequently exhibited the [AMP, CTX, CAZ, CIP, TOB, CN, CAF, AMC] (ESBL = 44, 44%, n = 4), [AMP, CTX, CAZ, TOB, CN, AMC] (ESBL = 33.33%, AmpC = 50%, n = 5), [AMP, CAZ, CIP, TOB, CN, CAF, AMC] (ESBL = 100%, n = 2), and [AMP, CAZ, TOB, CN, CAF, AMC] (ESBL = 100%, n = 2) phenotypes.
4. Discussion
Poultry meat (mostly of chicken origin, but also derived from turkey and other avian species) is an easily accessible, low-cost protein source and a widely consumed staple food throughout the world, both in high-income and in low- and middle-income countries. Production, processing, and trade of poultry meat are inherently associated with a significant risk of contamination by bacteria, as well as by AMR determinants [20]. A point of grave concern is the dissemination and acquisition of food-borne bacteria that express AMR (especially multiple-drug resistance, MDR) against critically important antibiotics (i.e., 3rd, 4th, and 5th generation cephalosporins, glycopeptides, macrolides and ketolides, polymyxins, and [fluoro]quinolones) in human medicine [21,22], since food-producing animals and foods of animal origin are well-established reservoirs for AMR bacteria and genes [14,23,24]. Specifically, bacteria resistant to extended-spectrum cephalosporins have been identified as a significant zoonotic hazard, as they are increasingly isolated in animals and humans and are often implicated in infections for which the chemotherapeutic options are limited [23,25,26,27]. Resistance to extended-spectrum cephalosporins in Enterobacterales is typically conferred by ESBL, AmpC, or carbapenemase enzymes [24,28,29]. ESBL-producing Enterobacterales have historically been more prevalent in Europe, whereas AmpC enzymes are also a serious concern, especially in southern America [23,25,30]. Both seem to have been detected in virtually every stage of chicken or turkey meat production (from farm to slaughterhouses and in the final retail), much like the undoubtedly most common Enterobacterales species, i.e., E. coli [21,22], with chicken and turkeys considered as a reservoir of extended-spectrum cephalosporin-resistant Enterobacteriaceae [14,31].
The presence of extended-spectrum cephalosporin-resistant E. coli in the present study was detected in 64% of the pooled poultry skin samples, a percentage that lies well within the wide range reported during the last decade in several studies performed in other European studies. Xexaki et al. [32] have studied the prevalence of antibiotic-resistant E. coli in farmed broilers in Greece, essentially from the same area as the present study; they report a lower prevalence of ESC-producing E. coli with 13.6% of the isolates producing ESBL and 2.7% producing AmpC β-lactamase. The lower percentages can be attributed to the methodology used that did not select ESBL-producing microorganisms or to a further step in poultry processing that contaminates meat along the production chain. Concerning poultry meat, Randall et al. [33] examined chicken meat samples in the United Kingdom collected in 2016 and 2018 and reported a respective 13.6% and 45% detection rate of ESBL and/or AmpC phenotype E. coli. Huizinga et al. [34] reported a 54.3% prevalence of ESBL-producing Enterobacteriaceae (94.4% of which were identified as E. coli) in chicken meat samples analyzed between 2014 and 2015 in the Netherlands. In a study conducted in Bosnia and Herzegovina, Hadžić-Hasanović et al. [35] isolated ESBL-producing E. coli in 29 out of the 100 chicken skin samples collected in 2018–2019, whereas Egervärn et al. [14] reported the isolation of ESBL/pAmpC E. coli in 34 out of 90 broiler meat samples (37.8%) imported into Sweden from across Europe in 2010–2011. On the other side of the spectrum, extended-spectrum cephalosporin-resistant (ESBL- and/or AmpC-phenotype) E. coli detection rates as high as 71.9–93.3% were reported in chicken (and turkey) meat samples in countries such as Spain, Germany, France, etc. [24,25,28,36]. Specifically, Egea et al. [28] report the rise in the prevalence of ESBL-producing E. coli in chicken meat samples from Spain from 62.5% in 2007 to 93.3% in 2010. Casella et al. [25] report that 91.7% of the 48 chicken samples from France tested positive for ESBL-producing E. coli. Kaesbohrer et al. [36] have examined several types of meat for the occurrence of ESBL-producing E. coli; they report that cefotaxime resistance was most common in E. coli isolated from chicken meat (74.9%).
Among the investigated isolates, resistance to aminoglycosides (tobramycin, gentamicin, and amikacin) ranged from 65.8% (79/120 for amikacin) to 92.5% (111/120 for tobramycin). A total of 74.2% (110/120) of the isolates were resistant to ciprofloxacin (used as an indicator fluoroquinolone), whereas 45% (54/120) and 41.7% (50/120) were also resistant to trimethoprim-sulfamethoxazole and chloramphenicol. All isolates were susceptible to meropenem and tigecycline, two of the last-resort antibiotics in human (and occasionally in companion animals) medicine. Interestingly, concurrent resistance yielded an MDR rate of 100% since all 120 isolates were resistant to antibiotics belonging to at least three classes. This finding was barely surprising as extended-spectrum cephalosporin-resistant E. coli isolates have been regularly known to express MDR patterns [20,23,28]. Díaz-Jiménez et al. [24] examined 100 chicken and turkey breast samples in 2016–2017 and found that 136 out of the 137 E. coli isolates were MDR, whereas Egervärn et al. [14], Moawad et al. [29], Nüesch-Inderbinen et al. [26], and Irrgang et al. [30] also reported very high MDR rates in extended-spectrum cephalosporin-resistant E. coli isolated from chicken and turkey samples, as well as from poultry-containing raw meat-based diets. Particularly, resistance to fluoroquinolones, aminoglycosides, and trimethoprim-sulfonamide combinations is very frequent in MDR ESBL/AmpC-producing isolates and raises profound concerns about its implication in the treatment of severe infections in both animals and humans [14,34,35]. In general, AMR in E. coli isolated from poultry is more prominent compared with other food-producing species [14,24,29], with a trend for turkeys to harbor resistant bacteria more frequently than broilers, possibly due to increased antibiotic use and/or prolonged fattening [21,24]; still, the latter finding is controversial, since data on an increased AMR occurrence in chicken compared with turkeys have also been published [36]. Concerning the AMR patterns observed, most of them correlate with the treatments usually followed for the most common poultry diseases. Specifically, the proposed antibiotics used for E. coli infections are aminopenicillins, fluoroquinolones, aminoglycosides, sulfonamides/trimethoprim, or tetracyclines [37]. Unfortunately, no data exist on the use of antibiotics in poultry, specifically in Greece. However, according to the sales of veterinary antimicrobial agents in veterinary medicine in Greece in 2021 [38], the most commonly used antibiotics in food-producing animals are tetracyclines, penicillins, aminoglycosides, sulfonamides, fluoroquinolones, macrolides and amphenicols (sales of veterinary antimicrobial agents in 31 European countries in 2021); the high resistance rate observed for β-lactams and aminoglycosides in the present study could possibly be due to the extensive use of these agents.
The increased prevalence of ESBL compared to the AmpC phenotype observed in this study (86/120 vs. 24/120 isolates) is on par with available data published in Europe. For example, Casella et al. [25] examined 77 non-clonal E. coli isolates from chicken meat sampled in France, of which 74 and three displayed ESBL and AmpC phenotypes, respectively. Moreover, Muller et al. [23] reported ESBL and AmpC phenotypes in 29 and seven E. coli isolates from imported meat in Germany. However, Egervärn et al. [14] reported a similar prevalence (18.9%, 90 samples) of ESBL- and AmpC-producing E. coli in broiler meat samples imported into Sweden (with a remarkable, complete prevalence of AmpC phenotype in the samples of Danish origin). Conversely, Kaesbohrer et al. [36] reported a higher prevalence of the AmpC-encoding blaCMY-2 gene in 138 E. coli isolated from chicken meat samples compared to classic ESBL genes (such as blaTEM, blaSHV, and blaCTX-M) prevalence; still, this involved the genotype of the isolates and not their phenotypic expression.
Rather surprisingly, no blaSHV (or blaOXA) genes were detected, although they are frequently observed in human patients in Greece [39]. Poultry, in general, are a reservoir of blaSHV-12, blaSHV-2, blaSHV-2a, and other variants of this category [30,32,33]. Especially blaSHV-12 has been very widespread in ESBL-producing E. coli isolated from chicken and/or turkey meat samples and has occasionally been found to be the most prominent ESBL gene [24,28,33]. However, it seems that blaCTX-M genes (mainly blaCTX-M-1, blaCTX-M-14, and related variants) have lately gained momentum, and, in many cases, their occurrence is now considered to have surpassed the one of blaSHV not only in poultry but in livestock in general [14,22,26]. Indeed, blaCTX-M-1 has been shown to constitute the principal ESBL enzyme in several studies on extended-spectrum cephalosporin-resistant E. coli of chicken and turkey origin, with other variants, such as blaCTX-M-15 (most prevalent in humans), blaCTX-M-2, blaCTX-M-8, blaCTX-M-9, blaCTX-M-12, blaCTX-M-14/17, blaCTX-M-32, blaCTX-M-79, or blaCTX-M-104 also being seldomly detected [25,26,31,33,34,35]. Concerning blaTEM genes (detected in more than half of the isolates in this study), they seem to represent the third most prevalent category in ESBL-producing E. coli isolates of poultry origin. Overall, the blaTEM-52, blaTEM-52B/C, blaTEM-104, and blaTEM-135 variants have been found to be responsible for the production of extended-spectrum cephalosporin-inactivating enzymes, at least in studies performed in Europe [14,24,25,29,34]. Finally, blaOXA genes are rather rarely detected in E. coli of avian origin [29], and their absence in the isolates of the present study does not likely pose any new concerns.
To our knowledge, the present study is the first to report the presence of extended-spectrum cephalosporin-resistant E. coli isolates in retail poultry in Greece, also exploring their genetic context. This information can elucidate the current situation and provide a starting point for the evaluation of time trends to assess the need for the implementation of intervention measures. Further investigation is required to establish a sound connection between the presence of ESC-producing E. coli in food and their impact on public health.
5. Conclusions
The increased prevalence of extended-spectrum cephalosporin-resistant E. coli in poultry (especially chicken meat) raises both public health and healthcare cost-associated concerns. AMR bacteria, in general, and extended-spectrum cephalosporin-resistant E. coli likely rise from overuse/misuse of antibiotics in livestock or from contamination along the production/storage chain. Increased prevalence of isolates with an ESBL and/or an AmpC-phenotype, mostly multi-drug resistant, was observed in the present study, therefore indicating that poultry marketed in Greece can be a vector for transmission of resistance factors to humans. Still, their occurrence was within the range reported in other European countries. The genetic background of the isolates for ESBL production featured the presence of variants of the blaCTX-M and the blaTEM genes, although non-gene harboring isolates were also isolated; no blaSHV or blaOXA genes were detected. Further studies are needed in order to access microbial resistance trends, elucidate possible transmission routes and further strengthen public health surveillance.
Author Contributions
Conceptualization, V.E. and P.G.; methodology, V.E., D.S., E.C. and P.G.; validation, D.S., P.G. and G.D.; investigation, V.E., D.S., A.T., T.M., E.C. and N.K.; data curation, V.E. and G.D.; writing—original draft preparation, V.E. and G.D.; writing—review and editing, V.E., G.D., P.G. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the non-involvement of humans or live animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization (WHO). Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015. Available online: https://apps.who.int/iris/rest/bitstreams/864486/retrieve (accessed on 1 June 2023).
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Graham, J.; Price, L.B. Industrial food animal production, antimicrobial resistance, and human health. Annu. Rev. Public Health 2008, 29, 151–169. [Google Scholar] [CrossRef]
- Iredell, J.; Brown, J.; Tagg, K. Antibiotic resistance in Enterobacteriaceae: Mechanisms and clinical implications. BMJ 2016, 352, h6420. [Google Scholar] [CrossRef]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC-AMR 2021, 3, dlab092. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017. Available online: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 1 June 2023).
- Lee, S.; Mir, R.A.; Park, S.H.; Kim, D.; Kim, H.; Boughton, R.K.; Morris, J.G.; Jeong, K.C. Prevalence of extended-spectrum β-lactamases in the local farm environment and livestock: Challenges to mitigate antimicrobial resistance. Crit. Rev. Microbiol. 2020, 46, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Van Boeckel, T.; Frost, I.; Kariuki, S.; Khan, E.A.; Limmathurotsakul, D.; Larsson, D.G.J.; Levy-Hara, G.; Mendelson, M.; Outterson, K.; et al. The Lancet infectious diseases commission on antimicrobial resistance: 6 years later. Lancet Infect. Dis. 2020, 20, e51–e60. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.; Projahn, M.; Burow, E.; Käsbohrer, A. Are there effective intervention measures in broiler production against the ESBL/AmpC producer Escherichia coli? Pathogens 2021, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Saliu, E.M.; Ren, H.; Boroojeni, F.G.; Zentek, J.; Vahjen, W. The impact of direct-fed microbials and phytogenic feed additives on prevalence and transfer of extended-spectrum beta-lactamase genes in broiler chicken. Microorganisms 2020, 8, 322. [Google Scholar] [CrossRef]
- van Hoek, A.H.A.M.; Dierikx, C.; Bosch, T.; Schouls, L.; van Duijkeren, E.; Visser, M. Transmission of ESBL-producing Escherichia coli between broilers and humans on broiler farms. J. Antimicrob. Chemother. 2020, 75, 543–549. [Google Scholar] [CrossRef]
- Agersø, Y.; Aarestrup, F.M.; Pedersen, K.; Seyfarth, A.M.; Struve, T.; Hasman, H. Prevalence of extended-spectrum cephalosporinase (ESC)-producing Escherichia coli in Danish slaughter pigs and retail meat identified by selective enrichment and association with cephalosporin usage. J. Antimicrob. Chemother. 2012, 67, 582–588. [Google Scholar] [CrossRef]
- Egervärn, M.; Börjesson, S.; Byfors, S.; Finn, M.; Kaipe, C.; Englund, S.; Lindblad, M. Escherichia coli with extended-spectrum beta-lactamases or transferable AmpC beta-lactamases and Salmonella on meat imported into Sweden. Int. J. Food Microbiol. 2014, 171, 8–14. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests, M02, 13th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; ISBN 1-56238-835-5. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. 2022. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf (accessed on 1 June 2023).
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. Version 2.0. 2017. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf (accessed on 1 June 2023).
- Fang, H.; Ataker, F.; Hedin, G.; Dornbusch, K. Molecular epidemiology of extended-spectrum beta-lactamases among Escherichia coli isolates collected in a Swedish hospital and its associated health care facilities from 2001 to 2006. J. Clin. Microbiol. 2008, 46, 707–712. [Google Scholar] [CrossRef]
- Tsiouris, V.; Economou, V.; Lazou, T.; Georgopoulou, I.; Sossidou, E. The role of whey on the performance and campylobacteriosis in broiler chicks. Poult. Sci. 2019, 98, 236–243. [Google Scholar] [CrossRef]
- Alonso, C.A.; Zarazaga, M.; Ben Sallen, R.; Jouini, A.; Ben Slama, K.; Torres, C. Antibiotic resistance in Escherichia coli in husbandry animals: The African perspective. Lett. Appl. Microbiol. 2017, 64, 318–334. [Google Scholar] [CrossRef]
- Davis, G.S.; Waits, K.; Nordstrom, L.; Grande, H.; Weaver, B.; Papp, K.; Horwinski, J.; Koch, B.; Hungate, B.A.; Liu, C.M.; et al. Antibiotic-resistant Escherichia coli from retail poultry meat with different antibiotic use claims. BMC Microbiol. 2018, 18, 174. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.A.; Seo, Y.H.; Lee, H.; Lee, K. Prevalence and molecular epidemiology of extended-spectrum-β-lactamase (ESBL)-producing Escherichia coli from multiple sectors of poultry industry in Korea. Antibiotics 2021, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Jansen, W.; Grabowski, N.T.; Monecke, S.; Ehricht, R.; Kehrenberg, C. ESBL- and AmpC-producing Escherichia coli from legally and illegally imported meat: Characterization of isolates brought into the EU from third countries. Int. J. Food Microbiol. 2018, 283, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Jiménez, D.; García-Meniño, I.; Fernández, J.; García, V.; Mora, A. Chicken and turkey meat: Consumer exposure to multidrug-resistant Enterobacteriaceae including mcr-carriers, uropathogenic E. coli and high-risk lineages such as ST131. Int. J. Food Microbiol. 2020, 331, 108750. [Google Scholar] [CrossRef] [PubMed]
- Casella, T.; Nogueira, M.C.L.; Saras, E.; Haenni, M.; Madec, J.-Y. High prevalence of ESBLs in retail chicken meat despite reduced use of antimicrobials in chicken production, France. Int. J. Food Microbiol. 2017, 257, 257–275. [Google Scholar] [CrossRef]
- Nüesch-Inderbinen, M.; Treier, A.; Zurfluh, K.; Stephan, R. Raw met-based diets for companion animals: A potential source of transmission of pathogenic and antimicrobial-resistant Enterobacteriaceae. R. Soc. Open Sci. 2019, 6, 191170. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Laidlaw, A.; Li, L.; Young, K.; Tamber, S. Isolation of third generation cephalosporin resistant Enterobacteriaceae from retail meats and detection of extended spectrum beta-lactamase activity. J. Microbiol. Methods 2021, 189, 106314. [Google Scholar] [CrossRef]
- Egea, P.; López-Cerero, L.; Torres, E.; del Carmen Gómez-Sánchez, M.; Serrano, L.; Sánchez-Ortiz, M.D.N.; Rodriguez-Baño, J.; Pascual, A. Increased raw poultry meat colonization by extended spectrum beta-lactamase-producing Escherichia coli in the south of Spain. Int. J. Food Microbiol. 2012, 159, 69–73. [Google Scholar] [CrossRef]
- Moawad, A.A.; Htzel, H.; Awad, O.; Tomaso, H.; Neubauer, H.; Hafez, H.M.; El-Adawy, H. Occurrence of Salmonella enterica and Escherichia coli in raw chicken and beef meat in northern Egypt and dissemination of their antibiotic resistance markers. Gut Pathol. 2017, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, A.; Zhao, G.; Juraschek, K.; Kaesbohrer, A.; Hammerl, J.A. Characterization of E. coli isolates producing extended spectrum beta-lactamase SHV-variants from the food chain in Germany. Microorganisms 2021, 9, 1926. [Google Scholar] [CrossRef]
- Wei, B.; Shang, K.; Cha, S.-Y.; Zhang, J.-F.; Jang, H.-K.; Kang, M. Conjugative plasmid-mediated extended spectrum cephalosporin resistance in genetically diverse Escherichia coli from a chicken slaughterhouse. Animals 2021, 11, 2491. [Google Scholar] [CrossRef] [PubMed]
- Xexaki, A.; Papadopoulos, D.K.; Alvanou, M.V.; Giantsis, I.A.; Papageorgiou, K.V.; Delis, G.A.; Economou, V.; Kritas, S.K.; Sossidou, E.N.; Petridou, E. Prevalence of antibiotic resistant E. coli strains isolated from farmed broilers and hens in greece, based on phenotypic and molecular analyses. Sustainability 2023, 15, 9421. [Google Scholar] [CrossRef]
- Randall, L.P.; Horton, R.H.; Chanter, J.J.; Lemma, F.; Evans, S.J. A decline in the occurrence to extended-spectrum β-lactamase-producing Escherichia coli in retail chicken meat in the UK between 2013 and 2018. J. Appl. Microbiol. 2021, 130, 247–527. [Google Scholar] [CrossRef]
- Huizinga, P.; Kluytmans-van den Bergh, M.; Rossen, J.W.; Willemsen, I.; Verhulst, C.; Savelkoul, P.H.M.; Friedrich, A.W.; García-Cobos, S.; Kluytmans, J. Decreasing prevalence of contamination with extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-E) in retail chicken meat in the Netherlands. PLoS ONE 2019, 14, e0226828. [Google Scholar] [CrossRef]
- Hadžić-Hasanović, V.; Jerkocić-Mujkić, A.; Hasanović, E.; Bačić, A.; Hukić, M. Phenotypic and genotypic detection of ESBL-producing E. coli isolates from chicken skin in Bosnia and Herzegovina. Med. Glas. 2020, 17, 308–315. [Google Scholar] [CrossRef]
- Kaesbohrer, A.; Bakran-Lebl, K.; Irrgang, A.; Fischer, J.; Kämpf, P.; Schiffmann, A.; Werckenthin, C.; Busch, M.; Kreienbrock, L.; Hille, K. Diversity in prevalence and characteristics of ESBL/pAmpC producing E. coli in food in Germany. Vet. Microbiol. 2019, 233, 52–60. [Google Scholar] [CrossRef] [PubMed]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare); Nielsen, S.S.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortazar Schmidt, C.; Herskin, M.; et al. Scientific Opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: Poultry. EFSA J. 2021, 19, 47. [Google Scholar] [CrossRef]
- European Medicines Agency. European Surveillance of Veterinary Antimicrobial Consumption. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2021. Trends from 2010 to 2021. Twelfth ESVAC Report. 18 November 2022. Available online: https://www.ema.europa.eu/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2021-trends-2010-2021-twelfth-esvac_en.pdf (accessed on 1 July 2023).
- Bush, K.; Bradford, P.A. Epidemiology of β-Lactamase-Producing Pathogens. Clin. Microbiol. Rev. 2020, 26, e00047-19. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).