Abstract
This resource contains a checklist of the benthic macroinvertebrate community sampled biannually from 1999 to 2010 in eight natural lakes from the middle Rio Doce Valley lake system and eight river segments in the Piracicaba River basin (sub-basin of Doce river), Minas Gerais State, Brazil. Three of the lakes are located inside a protected state park and are surrounded by preserved vegetation (Atlantic Forest). The other five lakes are in private properties, surrounded by Eucalyptus plantations. The seven stretches of rivers have a distinct degree of anthropogenic impacts. Samples were collected with a kick net and fixed with formaldehyde solution. Four phyla were represented: Mollusca, Annelida, Arthropoda, and Platyhelminthes. For Insecta, 76 families were identified, one family was identified for Crustacea, and nine families were identified for Mollusca. This subproject belongs to the International Long-Term Ecological Research Project (ILTER—Programa de Pesquisas Ecológicas de Longa Duração—PELD) site 4.
Dataset:https://doi.org/10.15468/cev8wb. Readers can also find the data directly from https://www.gbif.org/dataset/32ba1b9a-b06d-417e-98ea-eb0cfb67466a or https://ipt.sibbr.gov.br/peld/resource?r=diversidade_de_macroinvertebrados_bentonicos_peld.
Data Set License: CC-BY-NC 4.0 License
1. Introduction
In environmental evaluation practices, the use of biological variables represents a significant advantage over using exclusively physical and chemical parameters [1]. This approach enables not only the study of a momentary situation but also the influence of past modifications on environmental quality that still affects the aquatic biota [2]. In this perspective, the use of benthic macroinvertebrates is a powerful tool for biomonitoring programs due to the clear influence of habitat modifications over their community structure and taxa distribution, which makes this community a useful environmental bioindicator [3,4,5].
The benthic macroinvertebrate community is composed of organisms from several taxonomic groups and trophic guilds. During at least part of their life cycles, they live associated with the substrate of water bodies (sediments, wood debris, rocks, aquatic macrophytes, filamentous algae, etc.).
The most common groups are insects, annelids, mollusks, and crustaceans, among other smaller groups [6]. These organisms occupy a variety of niches and play fundamental roles in the ecological processes of aquatic ecosystems, in both the detritivore and the secondary production chain. They can be considered a linkage between mass and energy fluxes along the aquatic food web, taking part in the biogeochemical cycles [7].
In addition to the traditional taxonomic approach, the use of functional types represents an important complement to this kind of work [8]. This approach evaluates the community organization patterns through an ecosystem services perspective, allowing a complementary point-of-view on the relationship between the abiotic environment and community responses [9,10].
The Doce River basin is one of the most unique areas of the Brazilian landscape, and is the home of two of the country’s most threatened biomes, the Atlantic Forest and the Cerrado, which are considered hotspots of biodiversity [11,12] and under great anthropic impact [13,14,15]. The mid-catchment zone of the Doce River basin has a high value for Brazilian biodiversity, since in this region lies one of the biggest continuous remnants of the Atlantic Forest, the Rio Doce State Park (RDSP), which is 35,970 hectares in size and was recognized in 2010 as a RAMSAR site for the conservation of wetlands [16].
The Doce River basin covers a total of 230 municipalities and has a population of over 3.5 million inhabitants. In addition to the impacts of human occupation, the basin has the largest steel complex in Latin America, where several steelmaking and mining companies are settled [17]. Many anthropogenic environmental impacts have already been identified in both rivers and lakes, such as extensive Eucalyptus plantations, pasturelands for cattle raising, unplanned urbanization with disposal of untreated sewage, illegal hunting and fishing inside the RDSP, and the intentional and/or accidental introductions of exotic species (e.g., mollusks, fish, plants, and primates).
2. Data Description
This project aimed to unify the data and information obtained during the sampling period of the International Long-Term Ecological Research Project (ILTER—Programa de Pesquisas Ecológicas de Longa Duração—PELD) site 4, from 1999 to 2010, evaluating the zoobenthic community in lotic and lentic systems in the mid-catchment zone of the Doce River basin. The objective of the project was to evaluate both the spatial and the temporal variations in the community structure, including the effects of distinct degrees of anthropogenic impacts. Data on species occurrences is available on https://doi.org/10.15468/cev8wb. Readers can also find the data directly from the Global Biodiversity Information Facility (GBIF) site, https://www.gbif.org/dataset/32ba1b9a-b06d-417e-98ea-eb0cfb67466a, or from Sistema de Informação sobre a Biodiversidade Brasileira (SIBBr) site, https://ipt.sibbr.gov.br/peld/resource?r=diversidade_de_macroinvertebrados_bentonicos_peld.
3. Geographic Coverage
Bounding Coordinates South-West [−20, −42.9], North East [−19.22, −42.2]
This project was developed in the mid-catchment zone of Doce River basin, located in the southeast portion of the Minas Gerais state, Brazil (Figure 1). In this region, there is the Middle Rio Doce lake system, which is the third-largest lake system in the Brazilian territory, with more than 300 identified water bodies [18]. Around 50 lakes are located inside the limits of the RDSP. The predominant climate is mesothermic, with two well-defined seasons: a dry winter from April to September and a rainy summer from October to March (Figure 2).
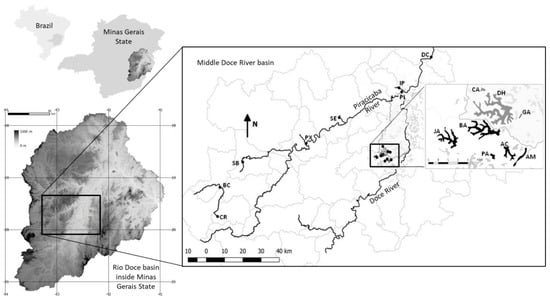
Figure 1.
The mid-catchment of the Doce River basin showing the Piracicaba River with the eight samples points (CR—Caraça, BC—Barão de Cocais, SB—Santa Bárbara, PX—Peixe, SE—Severo, IP—Ipatinga, PI—Piranga, DC—Doce) and eight lakes (JA—Jacaré, BA—Barra, PA-Palmeirinha, AC—Águas Claras, AM—Amarela, CA—Carioca, DH—Dom Helvécio, GA—Gambazinho). In light gray are the municipality’s limits. The whole river network is not shown.
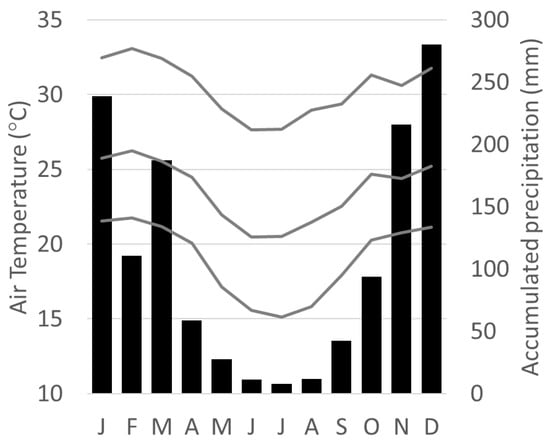
Figure 2.
Seasonal climate pattern of the mean, maximum, and minimum air temperature (gray lines), and the accumulated precipitation (bars) measured by the meteorological stations located in Ipatinga City between 1998 and 2013.
4. Temporal Coverage
The database includes benthic macroinvertebrates at the family, genus, or species level reported for different river and lake segments in the freshwater basin during the period 1999–2010. Eight river segments (Caraça, Barão de Cocais, Santa Bárbara, Peixe, Severo, Piracicaba, Ipanema, Doce) and eight lakes (Dom Helvécio, Gambazinho, Carioca, Amarela, Águas Claras, Barra, Jacaré and Palmeirinha) were sampled once in the dry and rainy seasons of each year (Table 1).

Table 1.
An overview of number of samples collected in each period in each site. The sampling date is in the first line (m/yyyy), “D” stands for dry period and “R” for rainy period. The number in the cells represents the number of samples collected in that period.
The lakes are oligo-mesotrophic, and the Caraça River (CR) is the most preserved of all the river segments sampled because it is inside a private natural heritage reserve (Table 2). The other river segments are subject to high impact due to the growing urbanization. Percentage of organic matter and granulometry showed an expressive variation within the sites and we provided an overview (Table 3).

Table 2.
Mean ± standard deviation of total phosphorus, total nitrogen, and chlorophyll a in samples collected during rainy and dry periods during 1999–2010.

Table 3.
Percentage of organic matter (OM) and granulometry (sand/silt + clay) of the sampled sites’ substrate.
5. Taxonomic Coverage
Specimens are identified at the lowest possible taxonomic level as possible, mostly at the family level (Table 4). Some taxa were identified until the genus or the species level. Four phyla were represented: Mollusca, Annelida, Arthropoda, and Platyhelminthes. Seven classes were identified: Insecta, Bivalvia, Gastropoda, Clitellata, Malacostraca, Ostracoda, and Arachnida. For Insecta, 76 families were identified, one family was identified for Crustacea, and nine families were identified for Mollusca.

Table 4.
Checklist (1 for presence and 0 for absence) of families and phylum/subphylum identified in each site within the whole sampled period.
In major lines, it is possible to observe differences in macroinvertebrate community structure from rivers and lakes (Figure 3). The phylum Annelida and the subphylum Hexapoda were present in all lake and river sites sampled. The frequency of Arthropoda in lakes inside the RDSP was higher than lakes outside the RDSP (except for lake BA), especially for Trichoptera (e.g., Leptoceridae and Polycentropodidae), Ephemeroptera (e.g., Caenidae and Leptophlebidae), and Heteroptera (e.g., Notonectidae, Belostomatidae, and Corixidae). The river segment CR had a higher frequency of Trichoptera (e.g., Helicopsychidae, Limnephilidae, and Hydroptilidae), and Heteroptera (e.g., Naucoridae). The crustaceans had a higher frequency in lakes when compared to rivers. However, the Carioca (CA) and the Águas Claras (AC) lakes showed no crustaceans sampled within their shores. The Mollusca phylum showed a higher frequency in the Dom Helvécio (DH), Jacaré (JH), and Barra (BA) lakes, and in the Santa Bárbara (SB) and Doce (DC) rivers. This high frequency is due to the presence of the invasive species Melanoides tuberculatus and the high frequency of the genus Pomaceae (Ampullaridae) in the Jacaré (JA) lake.
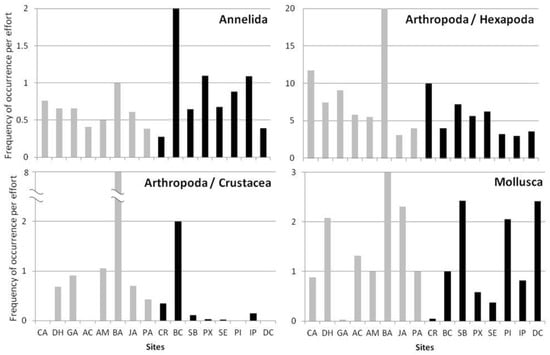
Figure 3.
Mean absolute frequency (y-axis) of occurrence per sample of the four main groups found in the samples. Gray bars are lakes (the first three lakes are inside Rio Doce State Park) and black bars are rivers (ordered from upstream to downstream).
6. Methods
Method step description: For each lake, one or more sampling stations were determined in the littoral region. In the rivers, a single sampling station was determined in the left margin of each environment. Samples were collected with a kick net; then, they were packed in plastic bags, fixed with 10 mL of 40% formaldehyde solution, labeled, and stored in polystyrene boxes. In the laboratory, the collected material was washed, and the organisms were retained in descending mesh screens (meshes of 2, 1, 0.5, and 0.250 mm). The organisms were screened using a stereomicroscope. Taxonomic identifications were made, whenever possible, up to the level of family, genus, and/or species, based on the following literatures: [6,19,20,21,22,23,24].
Author Contributions
G.E.N.A. (data manager, data publisher, metadata provider), I.M.M. (data manager, data publisher, metadata provider), D.G.F.P. (data manager, data publisher, curator), L.G.C.S. (data collector, collection identifier), N.M.d.L.D. (data collector, collection identifier), M.M.M. (data collector, collection identifier, subproject coordinator), P.M.M.-B. (data collector, project coordinator), F.A.R.B. (data collector, project coordinator).
Funding
This research was funded by PELD/MCT-CNPq grant number 520031/98-9.
Acknowledgments
The authors thank the National Council for Scientific and Technological Development (CNPq-MCT) for financing the Long-Term Ecological Research Program—Site 4, The Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES), Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG), the Forestry Institute of Minas Gerais (IEF-MG), colleagues from the Laboratório of Limnologia, Ecotoxicologia and Ecologia Aquática of ICB—UFMG (LIMNEA), and all the staff of Rio Doce State Park.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Callisto, M.; Moreno, P.; Barbosa, F.A.R. Habitat diversity and benthic functional trophic groups at Serra do Cipó, Southeast Brazil. Braz. J. Biol. 2001, 61, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Marvan, P. Alga assays—An introduction into the problem. In Algal Assays and Monitoring Eutrophicatio; Marvan, N., Lhotssky, P., Eds.; E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgard, Germany, 1979; p. 253. [Google Scholar]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Johnson, R.K. The indicator concept in freshwater biomonitoring. In Chironomid: From Gene to Ecosystems; Cranston, P., Ed.; CSIRO: East Melbourne, Australia, 1995; pp. 313–376. [Google Scholar]
- Bonada, N.; Prat, N.; Resh, V.H.; Statzner, B. Developments in aquatic insect biomonitoring: A Comparative Analysis of Recent Approaches. Annu. Rev. Entomol. 2006, 51, 495–523. [Google Scholar] [CrossRef] [PubMed]
- Mccafferty, W.P. Aquatic Entomology: The Fishermen’s and Ecologists Illustrated Guide to Insects and Their Relatives; Jones and Bartlett Publishers Inc.: Boston, MA, USA, 1981; p. 448. [Google Scholar]
- Rosenberg, D.M.; Resh, V.H. (Eds.) Freshwater Biomonitoring and Benthic Macroinvertebrates; Chapman & Hall: New York, NY, USA, 1993. [Google Scholar]
- Leslie, A.W.; Lamp, W.O. Taxonomic and functional group composition of macroinvertebrate assemblages in agricultural drainage ditches. Hydrobiologia 2016, 1, 99–110. [Google Scholar] [CrossRef]
- Bêche, L.A.; Mcelravy, E.P.; Resh, V.H. Long-term seasonal variation in the biological traits of benthic-macroinvertebrates in two Mediterranean-climate streams in California, U.S.A. Freshw. Biol. 2006, 51, 56–75. [Google Scholar] [CrossRef]
- Podgaiski, L.R.; Mendonça, M.S.; Pillar, V.D. O uso de Atributos Funcionais de Invertebrados terrestres na Ecologia: O que, como e por quê? Oecol. Aust. 2011, 15, 835–853. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Bates, J.M. Biogeographic patterns and conservation in the South American Cerrado: A tropical savanna hotspot. Bioscience 2002, 52, 225–233. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Robles-Gil, P.; Mittermeier, C.G. Hotspots: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; CEMEX/Agrupaión Seirra Madre: Mexico City, Mexico, 1999. [Google Scholar]
- Klink, A.C.; Machado, B.R. A Conservação do Cerrado Brasileiro; Departamento de Ecologia, Instituto de Biologia, Universidade de Brasilia (UnB): Brasilia, Brasil, 2005; Volume 1. [Google Scholar]
- Jepson, W.A. A disappearing biome? Reconsidering land-cover change in the Brazilian savanna. Geogr. J. 2005, 171, 99–111. [Google Scholar] [CrossRef]
- RAMSAR. The RAMSAR List of Wetlands of International Importance. 2010. Available online: http://www.ramsar.org/pdf/sitelist_order.pdf (accessed on 21 May 2018).
- PIRH–Doce. Plano Integrado de Recursos Hídricos da Bacia Hidrográfica do Rio Doce e Planos de Ações Para as Unidades de Planejamento e Gestão de Recursos Hídricos no Âmbito da Bacia do Rio Doce; Contrato N° 043/2008–IGAM. CHB-Doce, 2010. Available online: http://www.cbhdoce.org.br/wp-content/uploads/2014/10/PIRH_Doce_Volume_I.pdf (accessed on 22 May 2018).
- Maillard, P.; Pivari, M.; Luis, C. Remote Sensing for Mapping and Monitoring Wetlands and Small Lakes in Southeast Brazil. In Remote Sensing of Planet Earth; InTech: Rijeka, Croatia, 2012; pp. 21–44. [Google Scholar]
- Wiggins, G.B. Larvae of the North America Caddisfly Genera (Trichoptera); University Toronto Press: Toronto, ON, Canada, 1977. [Google Scholar]
- Merritt, R.W.; Cummins, K.W. An Introduction to the Aquatic Insects of North America, 2nd ed.; Kendall/Hunt: Dubuque, IA, USA, 1984; p. 722. [Google Scholar]
- Dominguez, P.L. Roots, Tubers, Plantains and Bananas in Animal Feeding; Machin, D., Nyvold, S., Eds.; FAO Animal Production and Health Paper 95; FAO: Roma, Italy, 1992. [Google Scholar]
- Nieser, N.; Melo, A.L. Os Heterópteros Aquáticos de Minas Gerais; UFMG: Belo Horizonte, Brazil, 1997; p. 180. [Google Scholar]
- Costa-Leonardo, A.M. Secretion of salivary glands of the Brazilian termite Serritermes serrifer Hagen & Bates (Isoptera: Serritermitidae). Ann. Soc. Entomol. Fr. 1997, 33, 29–37. [Google Scholar]
- Mugnai, R.; Nessimian, J.L.; Baptista, D.F. Manual de Identificação de Macroinvertebrados Aquáticos do Estado do Rio de Janeiro; Technical BooksLivraria: Rio de Janeiro, Brazil, 2010; p. 176. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).