Abstract
Adapting low-viscosity liquids for individuals with dysphagia presents persistent challenges in both texture modification and patient compliance. This exploratory study assessed the flow characteristics and nutritional contributions of 30 beverages (22 plant-based and 8 dairy-based) across different thickener concentrations and syringe models, following the International Dysphagia Diet Standardization Initiative (IDDSI) flow test. Flow measurements were obtained using four available 10 mL syringes that differed from the IDDSI-specified model, intending to evaluate their potential impact on results and inform strategies for situations where standard syringes are unavailable. Findings show that flow performance and IDDSI classification are strongly influenced by syringe design, thickener type, and beverage composition. The use of alternative syringes introduced variability in consistency measurements, highlighting the importance of equipment standardization. Interpretation of flow levels was further complicated by transitional IDDSI thresholds and subjective assessments. Nutritionally, the study reinforces the role of hydration in dysphagia management and explores the potential of plant-based beverages to enhance both fluid intake and fiber contribution. Several samples provided meaningful contributions to daily fiber and micronutrient requirements. Importantly, the study found that half the manufacturer’s recommended thickener dose was often sufficient to achieve IDDSI compliance. These findings support the practical use of non-standard syringes in IDDSI testing, inform more efficient thickening strategies, and highlight plant-based beverages as promising alternatives to dairy in dysphagia diets. Together, they offer actionable insights for improving consistency control and nutritional quality in dysphagia care.
1. Introduction
Swallowing disorders pose significant clinical challenges and often result in adverse outcomes such as choking and aspiration, particularly among individuals with dysphagia. The term dysphagia, derived from Greek roots meaning “difficulty in eating,” refers to impaired safety or efficiency in the swallowing process. It is commonly associated with various conditions, including stroke, Parkinson’s disease, and muscular dystrophies, and may manifest as oral, esophageal, or oropharyngeal dysphagia. Notably, oropharyngeal dysphagia is the most prevalent subtype, accounting for approximately 80% of cases [1,2,3].
Elderly individuals are especially susceptible due to age-related physiological changes in oral and pharyngeal function, such as age-related dysphagia (presbyphagia) and sarcopenic dysphagia [4,5]. These impairments may lead to malnutrition, dehydration, and respiratory complications, significantly diminishing quality of life [2,6].
Developing appropriate beverages for dysphagia patients is essential to prevent such complications. Texture-modified foods and fluids are often necessary to ensure safe nutrient intake; however, only specific liquid consistencies are considered safe within a dysphagia diet, raising concerns about the safety of fluid consumption [3,7,8]. While thicker fluids enhance swallowing safety, they are often unpalatable, with thickened water frequently reported as unsatisfactory for quenching thirst. The type and concentration of thickening agent greatly influence beverage texture and patient acceptability, and carbonated beverages present additional challenges for hydration [9]. Exploring alternative, nutritionally enriched beverages is therefore a key objective. For instance, Cartagena et al. [7] developed protein- and fiber-fortified strawberry-based desserts using hydrocolloids tailored to dysphagia-specific needs, showing how these approaches can be useful.
Changes in rheological properties due to altered food consistency can directly impact swallowing safety. However, accurate and consistent modification techniques are often lacking, resulting in unsuitable diets for dysphagia patients [10]. Compounding this issue is the limited availability of specialized equipment and trained personnel to measure viscosity in clinical environments, posing barriers to implementation of standardized practices [11].
To address these issues, the International Dysphagia Diet Standardization Initiative (IDDSI) introduced a globally harmonized framework for food texture and fluid thickness modification, developed through international collaboration and research [12]. This system comprises eight classification levels (0–7), with levels 0–4 corresponding to beverages and the remaining levels covering solid foods. It should be noted that in Figure 1, only the beverage levels (0–4) are presented, in order to emphasize this category. The framework employs universal descriptors and color codes to facilitate rapid, consistent assessments, aiding in safe and individualized diet planning for dysphagia patients [5,13].
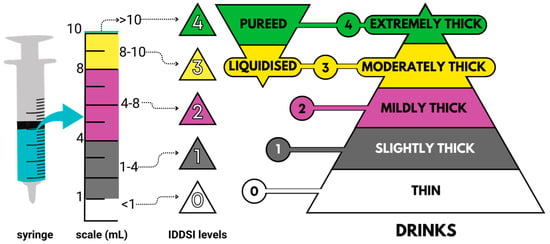
Figure 1.
Schematic diagram depicting the classification of beverages according to their rheological behavior, utilizing the syringe-based IDDSI flow test. Source: Adapted from IDDSI [14].
The IDDSI framework has been widely adopted for evaluating various fluid types. Previous studies have applied it to assess commercial beverages—including flavored carbonated water, isotonic drinks, and powdered sodas—thickened with xanthan gum-based agents [15], as well as to 3D-printed gels and starch-based dysphagia-friendly formulations [16,17]. Furthermore, the increasing popularity of plant-based beverages, driven by nutritional benefits and consumer preference, presents opportunities to expand safe dietary options for individuals with swallowing disorders [18,19].
Despite these advances, significant challenges remain. Even with IDDSI’s proposal of a standard testing funnel [20], healthcare providers often face difficulties due to the unavailability of standardized syringes and the variability introduced by different thickening agents and concentrations. Accordingly, this study aimed to investigate how thickener type, thickener concentration, and the use of non-standard syringes influence the IDDSI flow test classification of commercially available plant-based and dairy-based beverages. By systematically comparing flow performance, consistency classification, and related nutritional attributes across these variables, the research seeks to provide evidence-based guidance for clinical practice and home-based dysphagia management.
2. Materials and Methods
2.1. Materials
A total of 30 beverage samples were selected for the primary test, consisting of 22 plant-based beverages and 8 animal milk samples (Table 1). The plant-based beverages were selected to represent a wide variety of ingredients and formulations, while the milk samples provided a comparative baseline. To simulate thickened beverages for dysphagia patients, two commercially available thickeners were used: Nutilis Clear® (T1) and Resource ThickenUp Clear® (T2). Both thickeners are formulated with maltodextrin and xanthan gum, specifically designed to modify the viscosity of liquids for individuals with swallowing difficulties. The beverages were thickened to various consistency levels, including nectar, honey, and pudding, experimentally determined using the IDDSI flow test and described according to the IDDSI nomenclature (thin, slightly thick, mildly thick, moderately thick and extremely thick), taking into account the manufacturer’s recommendations and adapted testing criteria.

Table 1.
Coding of plant-based beverages, milks, and commercial thickeners, including brand, manufacturer, production sites, and their respective ingredients.
Table 1 provides a detailed list of the plant-based beverages, animal milks, and commercial thickeners used in the study, along with their specific ingredients. The plant-based beverages included a diverse range of options such as oat, almond, hazelnut, rice, soy, coconut, and other non-dairy formulations. The composition of these beverages indicates that they were fortified with various stabilizers, vitamins, and flavorings, some from organic farming sources, to enhance their nutritional profile. Additionally, several of the beverages were fortified with calcium, phosphorus, and other mineral salts to mimic the nutritional content of animal milks. For animal milks, samples included whole, semi-skimmed, and skimmed versions of cow’s, goat’s, and sheep’s milk, including lactose-free variants.
Table 2 lists the specifications of the four syringes used in this study for the viscosity testing. The syringes—BD Emerald™ and BD Discardit™ II from Becton Dickinson (Madrid, Spain), ICO (plus3®) from ICO (Novico Medica, Barcelona, Spain), and Aposán® (Madrid, Spain)–are commonly available on the market in Madrid, Spain, and were chosen for their reliability and ease of use. The measured volumes were slightly adjusted from the specified volumes, and the actual volumes of liquid dispensed were recorded for accuracy. The use of non-standard syringes reflects the specific constraints of data collection in 2020 and does not imply equivalence with the IDDSI-recommended device.

Table 2.
Syringe specifications according to manufacturers and supplementary data obtained in the laboratory, and mass (g) of thickener for beverages prepared at different consistency levels according to the manufacturer’s recommendation (A) and the proposed adaptation (B).
To evaluate the effect of the thickeners, the amount of thickener added to each beverage was set to achieve different consistency levels (nectar, honey, and pudding). After preliminary tests, we selected only the “nectar” consistency, as the other levels were too thick to allow for robust comparisons. The mass of thickener used was based on the manufacturer’s recommendations (denoted as A), as well as a proposed adaptation (denoted as B), corresponding to half of the manufacturer’s dose. This adjustment was made with a methodological intent, as preliminary tests showed that the full recommended amounts tended to concentrate most beverages within Levels 2 and 3, limiting the ability to distinguish the influence of syringe design, beverage matrix, and thickener formulation. By applying half the dose, the results were distributed more evenly across Levels 1 to 3, enhancing the sensitivity of the comparative analysis. The detailed mass values required for different consistency levels are provided in Table 2.
2.2. IDDSI Flow Test
The rheological behavior of the thickened beverages was assessed based on their gravity flow using a 10 mL syringe. The volume of liquid remaining after a 10 s flow was used to determine the thickness classification of the fluids [14], following the detailed IDDSI descriptors illustrated in Figure 1. For thickened sample preparation, 200 mL of beverage was used, to which the thickener was added according to the protocol as described in the notes of Table 2. Prior to the tests, the syringes were calibrated with water. All experiments (including syringe calibration and the consistency assessment of thickened plant-based and animal milk samples using the IDDSI Flow Test) were conducted under the same protocol, selected for its cost-effectiveness, ease of execution, and applicability in both hospital and domestic environments.
Before adding thickening agents, all beverages were checked at room temperature (24 ± 1 °C) and classified as level 0 (thin). For all beverages, the flow test was first performed in the non-thickened base form (Level 0) to determine the baseline ‘time to exhaustion’, followed by the thickened samples. Sample preparation involved homogenizing the thickener with a portable mixer (IKEA, Shanghai, China) for 1 min and allowing it to stand for 10 min until complete solubilization. Although the manufacturer did not specify the required resting time after adding the thickener for complete solubilization, a duration of 10 min was deemed suitable for both hospital and domestic environments (refer to Table 2).
To conduct the test, the syringe body was filled with fluid samples using another syringe up to the 10 mL mark, and the syringe tip was sealed with a gloved finger to prevent fluid leakage. The syringe was then positioned vertically, and the finger was released. After a 10 s flow, the syringe tip was sealed again with a gloved finger, and the remaining fluid volume in the syringe was measured and classified accordingly: no content left indicated level 0; remaining fluid from 1 to 4 mL represented level 1; remaining fluid from 4 to 8 mL indicated level 2; and remaining fluid from 8 to 10 mL denoted level 3. Following the hygiene protocol specified by IDDSI, the syringes were sanitized and reused using warm, soapy water, followed by three pump actions, and then rinsed twice with clean, warm water. Syringes were only discarded if their scales were compromised, potentially affecting the accuracy of readings.
2.3. Nutritional Information
Nutritional information was gathered from product labels of both the beverages and thickeners, supplemented by reference values from the Recommended Dietary Allowance (RDA) for the elderly population, as provided by the Institute of Medicine—IOM [21]. Given that the evaluated beverages are intended as options for lactose-free diets or as plant-based alternatives to milk, their nutritional profiles play a crucial role in dietary considerations.
2.4. Statistical Analysis
The triplicate measurement data were presented as mean ± standard deviation (SD). Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA) based on similarities were conducted to explore potential correlations among the samples, using Statistica 10.0 (StatSoft Inc., Tulsa, OK, USA). Origin 8.6 (OriginLab Corporation, Northampton, MA, USA) was used to produce line charts. The dataset comprised 30 beverages and water (n = 31), with 16 responses derived from the IDDSI flow test (mL). The data underwent standardization, and PCA was performed with all variables that contributed >60% towards total variability. The clustering of both variables and samples within HCA was based on Euclidean distances, using Ward’s method.
3. Results
3.1. Nutritional Profile Context
To contextualize the IDDSI flow test outcomes, we first report the beverages’ nutritional composition (Table S1). Plant-based beverages exhibited a broader nutritional range, with EV09, EV15 and EV16 displaying high carbohydrate content and EV18 showing elevated dietary fiber. In contrast, dairy-based beverages offered more uniform macronutrient content and higher protein concentrations. Understanding these profiles is essential, as viscosity and nutritional composition may influence both flow behavior and the clinical suitability of thickened beverages. Energy (in kJ per 100 mL), carbohydrates and sugars varied widely across plant-based and dairy matrices, whereas protein and fiber displayed more product-specific patterns. These parameters are important because they affect apparent viscosity and retained volume in the IDDSI test, particularly when combined with different thickener types and dosages. In general, beverages with higher total solids (e.g., sugars and non-fat solids) tend to yield greater residual volume after 10 s, an effect that can be magnified by thickeners with high hydration capacity. Therefore, comparisons across syringes S1–S4 should be interpreted against this compositional background, in order to avoid anecdotal attributions to syringe brand or dose alone.
3.2. Time to Exhaustion and Syringe Variability
The results of the IDDSI flow test for 30 distinct beverages, including water supplemented with commercial thickeners, are detailed in Table S2. Initially, the data were analyzed based on the time taken for the exhaustion of 10 mL of fluid, as depicted in Figure 2.
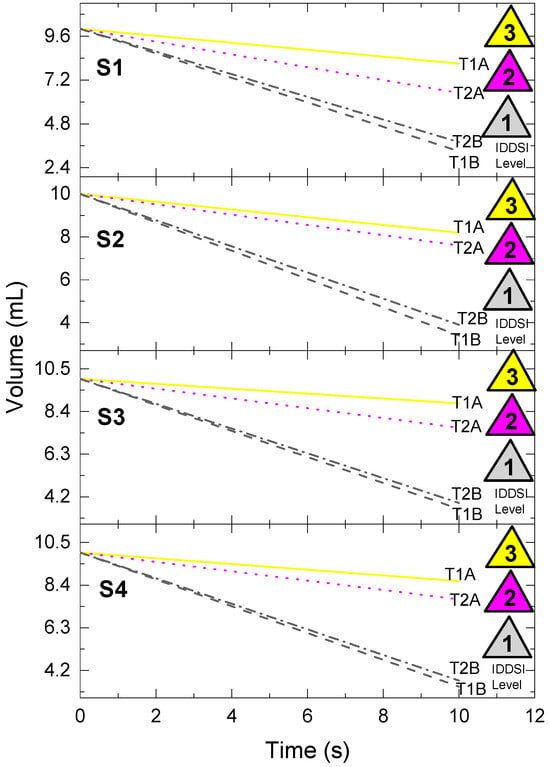
Figure 2.
Volume trends obtained in various water-based solutions containing different concentrations of two thickeners, measured using the IDDSI flow testing for 10 s with different syringes (S1–S4). T1A = Thickener 1, manufacturer’s recommended concentration; T1B = Thickener 1, adapted concentration; T2A = Thickener 2, manufacturer’s recommended concentration; T2B = Thickener 2, adapted concentration.
The IDDSI flow test, conducted at room temperature with a standardized volume of 10 mL in all analyses, revealed that the time to exhaustion varied across beverage types and syringe models (Table S2). While all syringes showed acceptable performance with water (baseline), the time to exhaustion increased substantially for thicker samples, especially with samples EV07 and EV11, which consistently reached the 10.00 s maximum value. Notably, syringes S1 and S2 frequently recorded longer flow times compared to S3 and S4, suggesting that syringe design impacts classification precision even at Level 0.
3.3. Consistency Classification Using Manufacturer-Recommended Preparation (T1A and T2A)
Table 3 highlights the impact of using different syringes to measure fluid flow with three levels of added thickener in 100 mL of water, juxtaposed with data from existing literature. On the other hand, Tables S3 and S4 present the volume remaining in the syringe body after a 10 s flow test of different beverages. Specifically, Table S3 pertains to utilizing the two thickeners in proportions recommended by the manufacturer (measuring spoon),while Table S4 exhibits the outcomes of the flow test on samples using an alternate proposed mass of thickener, thereby indicating variations in the levels specified in the IDDSI categorization.

Table 3.
Retained volume (mL) of T2 thickener at different concentrations in 100 mL of water (mean ± SD) and their corresponding IDDSI levels.
Table 3 demonstrates that the retained volume was greater when using SR syringes compared to BD syringes, at both thickener concentrations (1.2 g and 2.4 g), confirming the influence of syringe design on flow outcomes [22]. As verified in Table S3, when using the manufacturer’s preparation protocols, the IDDSI classification of thickened beverages showed variations depending on both the sample and syringe. Plant-based beverages (e.g., EV01 to EV22) generally retained higher IDDSI levels with both T1A (3.0 g) and T2A (2.4 g) thickeners, showing consistent classification as mildly thick or moderately thick. Dairy-based samples (LA01 to LA08) demonstrated slightly lower flow resistance and higher flow volume across syringes. Interestingly, despite identical thickener concentrations, several beverages shifted IDDSI categories depending on the syringe type used, underlining the importance of standardization in clinical settings.
3.4. Performance of Proposed Modified Preparation (T1B and T2B)
The results from the modified preparation protocol (T1B = 1.5 g and T2B = 1.2 g) revealed a clearer differentiation between samples, particularly at lower thickener dosages (Table S4). It was also observed that syringe type significantly influenced (p < 0.05) the classifications: while S1 and S2 predominantly indicated Level 3 (moderately thick), syringes S3 and S4 yielded higher residual flow volumes, reclassifying several dairy-based beverages as Level 1 (slightly thick, gray) or Level 2 (mildly thick, pink). This underestimation may lead to misclassification and compromise safety for individuals with dysphagia. Differences between thickeners were also noted, with T1B generally resulting in lower residual volumes (higher viscosity) compared to T2B, particularly in dairy-based matrices, suggesting a stronger thickening capacity. In contrast, plant-based beverages such as EV03 and EV05 showed greater sensitivity to thickener adjustments, presenting marked transitions from Levels 1–2 to Level 3, indicating more pronounced shear-thinning behavior. These findings reinforce that syringe design, thickener formulation, and beverage matrix composition directly influence IDDSI flow test classifications.
3.5. Correlations Among Beverage Samples
The relationships among beverage samples were explored using flow test data analyzed through principal component analysis–PCA (Figure 3). The first two principal components (PCs) captured variability in the dataset, effectively grouping variables and samples based on their proximity in a two-dimensional plot. PC1 and PC2 explained 61.59% and 16.26% of the variability, respectively, cumulatively accounting for over 77% of the total variance. Figure 3a depicts the grouping of variables, while Figure 3b illustrates the arrangement of the sample set. All 16 response variables used in the analysis contributed significantly (>0.6) to the principal components, thereby revealing relevant correlations. Figure 3b reveals three distinct sample clusters, with plant-based beverages predominantly grouped together, dairy-based beverages forming a separate cluster, and a small number of plant-based samples (e.g., EV15, EV08) diverging due to greater sensitivity to preparation conditions. These multivariate outcomes emphasize that beverage classification in the IDDSI flow test arises not from thickener concentration alone, but from the combined influence of thickener formulation, syringe design, and beverage matrix composition.
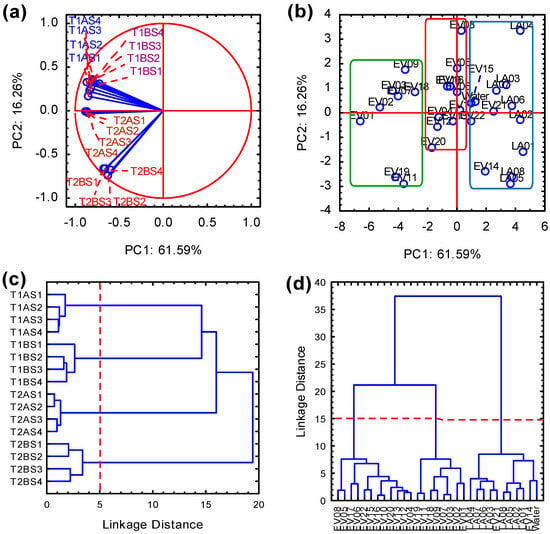
Figure 3.
Principal Component Analysis of plant-based (EV01–EV22) and milk-based (LA01–LA08) beverages with added thickeners (T1 and T2), evaluated using four different syringes (S1–S4), depicting the grouping of variables (a) and samples (b) with their corresponding percentage of data explanation, and Hierarchical Cluster Analysis highlighting three clusters of response variables (c) and samples (d).
To validate the results obtained from PCA, clustering evaluations were conducted, yielding outcomes consistent with those of PCA. Figure 3c demonstrates the grouping of variables, while Figure 3d highlights the formation of three sample clusters. Hierarchical cluster analysis (HCA) reinforced the findings of PCA, as the clustering patterns matched those established earlier. In Figure 3c, clear clusters of compounds emerged, corresponding to different amounts of thickener (T1A, T1B, T2A, and T2B) and the four syringes used (S1, S2, S3, and S4). Utilizing Ward’s method, the linkage distances effectively portrayed data clustering, indicating similarities among most plant-based beverages (20 of them) when combined with thickening agents. In contrast, EV14 and EV21, consisting of whole rice, quinoa, almond, and cashew, exhibited strong correlations with animal milk samples, suggesting similar rheological properties. From a compositional perspective, both EV14 and EV21 contain lecithin-based emulsifiers, which are not expected to drastically influence rheology. However, EV22, with a similar formulation but including cane sugar and added fibers, displayed distinct rheological behavior and was positioned closer to the intermediate cluster rather than with dairy samples.
4. Discussion
4.1. Thickened Beverages Flow Test
As recommended by Cichero et al. [14], accurate calibration of syringes is essential for reliable IDDSI flow test outcomes. In this study, calibration was performed using pure water and water thickened with varying concentrations of commercial thickeners. As detailed in Table S2 and illustrated in Figure 2, fluid exhaustion behavior was monitored over a 10 mL volume. With the exception of samples EV07 and EV11, all exhibited flow within the 10 s limit, with residual volumes below 1 mL—qualifying them as IDDSI Level 0 (thin).
Because the specifically recommended 10 mL slip-tip Becton Dickinson® syringe (61.5 mm barrel) was not available in Spain at the time of data collection in 2020, likely due to the COVID-19 pandemic, which redirected most healthcare resources to hospitals and other healthcare facilities, four alternative syringe models with comparable dimensions were used as a strategy to overcome resource shortage. We acknowledge that this deviation from the IDDSI protocol constitutes a methodological limitation, as small geometric differences between syringes can affect the retained volume and, consequently, flow classification, as reported elsewhere [23]. Rather than implying equivalence with the standard device, this study sought to demonstrate how design variability among commonly available syringes may influence IDDSI outcomes, reflecting practical challenges frequently encountered in clinical and home-based contexts. Nonetheless, this study intentionally examined this limitation to reflect real-world constraints while still maintaining methodological rigor [14].
This methodological limitation is consistent with previous reports. Dantas and Oliveira [22] observed that the BD syringe retained less fluid than other syringe models across several thickener concentrations—an effect also mirrored in our T2-water trials (Table 3). When adjusted to a standardized 1.2 g thickener dose per 100 mL, both methods yielded consistent Level 2 (mildly thick) classifications for water samples, supporting the reliability of the IDDSI categorization framework despite these minor device-related variations. Although this study did not measure rheological properties under shear, our data aligned with published viscosities for common beverages [24], such as oat (52 cP), almond (65 cP), coconut (358 cP), and skimmed milk (12 cP), supporting the flow-based classifications reported. The stability of thickened liquid viscosity is composition-dependent [25]. A limitation of this study is the absence of direct rheological measures (e.g., viscosity curves). Although IDDSI flow test outcomes provide clinically relevant information and have been widely adopted in dysphagia research, they cannot replace full rheological characterization. Our approach prioritized feasibility, given the limited access to laboratory equipment during data collection, focusing instead on standardized flow tests under controlled conditions. Therefore, the interpretation of results relied on IDDSI categories and complementary information from the literature. Future studies should combine flow testing with rheological measurements to enhance the precision of texture characterization.
The transitional levels within the IDDSI framework, such as those between slightly thick (1 to 4 mL), mildly thick (4 to 8 mL), and moderately thick (8 to 10 mL), have the potential to cause confusion and discrepancies in level interpretation. To address this issue, we revised the ranges to slightly thick (1 to 3.9 mL), mildly thick (4 to 7.9 mL), and moderately thick (8 to 10 mL), to minimize uncertainty until clearer evidence regarding the clinical significance of subtle fluid flow variations becomes available [11]. This adjustment aims to bring the results closer to the midpoint of each IDDSI interval, offering a more cautious approach.
Given the fluid’s sensitivity to variation, particular readings may lead to false interpretations of the corresponding level. Despite the prevalent use of thickened fluids in dysphagia treatment, substantial variability in practice can emerge without precise definitions for different thickening degrees. Those purchasing or preparing such liquids rely on product label information or subjective cues such as agitation, liquid handling, observation during pouring, or oral analysis [26]. This reliance on subjective assessments often results in suboptimal quality control over liquid consistency, compromising the accuracy of thickening levels, as evidenced in this study (Tables S2 and S3).
Changes in thickening methods can result in significant variations in consistency, influenced by several factors, including the type and quantity of thickener utilized, adherence to the recipe, mixing technique, temperature during preparation, and the time allowed for the thickened mixture to set [24]. Ensuring consistency in thickening methods is crucial for maintaining the safety and efficacy of dysphagia management protocols. This is particularly important given the observed variability among caregivers and speech therapists in their assessments of the amount of thickener needed to achieve specific consistency ranges [25].
In a study by Barbon and Steele [11], tests were conducted using a xanthan gum-based thickener in lemon-flavored water, similar to the T2 brand used in this study. They tested four concentrations and performed multiple assessments, including the IDDSI flow test. Their findings showed distinct results based on the thickener concentration: 0.65 g (resulting in 1.9 mL retention, classified as slightly thick, level 1), 1.25 g (resulting in 5.2 mL retention, classified as mildly thick, level 2), 2.10 g (resulting in 9.1 mL retention, classified as moderately thick, level 3), and finally, no liquid drainage when 7.5 g of thickener was used, confirming extreme thickness (level 4). In our study, where the thickener proportion was consistent (0.6 g per 100 mL of vegetable beverage), 56.7% of samples exhibited mild thickness viscosity (level 2). This suggests that the fluid’s composition, particularly the type of thickener used, significantly influences consistency changes. Thickeners containing gums are resistant to salivary amylase digestion and maintain stable viscosity, which explains their suitability in this context [27].
Thickeners based on gums work by forming a network that retains water, a characteristic that directly affects their viscosity. Dewar and Joyce [28] reported an increase in viscosity in maltodextrin-based thickeners 30 min after preparation. This finding prompted us to perform viscosity measurements at the 10 min mark, which is ideal for initial consumption during meals [29,30]. Although viscosity remains stable within the 10 to 30 min range, maintaining consistency for longer periods would be more convenient.
4.2. Nutritional Information of Plant-Based and Dairy-Based Beverages
Adequate hydration is critical in managing dysphagia, but patient resistance to thickened water is common [29]. Sensory acceptability, including flavor, texture, and mouthfeel, plays a key role in compliance. Reduced fluid intake is often observed after thickening, especially in institutionalized older adults [13]. While water remains the ideal hydrating fluid, palatable alternatives are essential for maintaining adequate intake [30].
Despite these challenges, offering alternatives is a crucial strategy in addressing dysphagia. Vegetable-based beverages designed as milk substitutes contain over 80% water, as indicated in Table 1. Additionally, these beverages provide valuable nutrients that can help adjust the dietary intake of dysphagia patients, as detailed in Table S1. This approach offers potential benefits in addressing both hydration and nutritional needs for individuals with swallowing difficulties.
Individuals experiencing swallowing impairments often face challenges with oral feeding, which may affect their nutritional status. Thus, broadening diets to include fruits, vegetables, grains, legumes, meats, dairy, and eggs is recommended. Vegetable beverages, serving as milk alternatives, offer an opportunity to diversify liquid intake, enriching diets with additional fiber, plant-based proteins, vitamins, and minerals. Table S1 outlines the nutritional profile of the samples, detailing specific nutrients according to the Institute of Medicine (IOM) recommendations [21].
Considering a 200 mL portion, Soy–EV06 samples exhibit protein levels comparable to most animal milk options, except for Sheep milk–LA02, which is the richest source of this nutrient. Conversely, vegetable beverages are advantageous for their inclusion of dietary fiber—a component absent in animal-derived counterparts. Almond–EV02 and Walnut–EV18 samples, enriched with thickener T1, contribute significantly to a 200 mL portion. For instance, Almond–EV02 contains 3.2 g of dietary fiber with thickener T1 and 3.6 g with T2. Similarly, Walnut–EV18 provides 3.4 g of dietary fiber with T1 and 3 g with T2, aligning with IDDSI’s moderately thick classification.
Converted to the Recommended Dietary Allowance (RDA) for older adults, the Almond–EV02 sample contributes 10.7% for men and 15.2% for women with thickener T1, and 10.0% for men and 14.2% for women with T2. The Walnut–EV18 sample offers 12.0% for men and 17.1% for women with T1, and 11.3% for men and 16.2% for women with T2. These values align with the recommended daily intake of 30 g/day for men and 21 g/day for women for fiber, providing important nutritional support for patients at high nutritional risk, especially during snack times.
Some vegetable beverages and milk alternatives also contain added micronutrients, such as vitamins and minerals. Among the 30 samples evaluated, 13 provided additional nutritional information, of which 6 were plant-based. For calcium, with an RDA of 1200 mg/day for the elderly, a 200 mL portion supplemented with 3 g of thickener T1 contributed 21.3% of the RDA for samples Almond–EV02, Rice–EV04, Soy–EV06, and Coconut–EV07, and 11.3% for Almond & Cashew–EV21 and Almond, Hazelnut, Walnut & Cashew–EV22. For comparison, animal milk samples with available data and their respective calcium contributions include Goat milk–LA01 (21.3%), Sheep milk–LA02 (31.1%), Whole milk–LA03 (27.9%), Semi-skimmed milk–LA04 (19.6%), Semi-skimmed milk–LA06, and Semi-skimmed lactose-free milk–LA07 (47.1%).
Calcium, essential for bone and dental health, along with vitamin D—crucial for calcium absorption—were included in the nutritional data for 9 samples, including 6 plant-based beverages. According to IOM (2011), the recommended intake for vitamin D in the elderly (over 71 years) is 800 IU/day (20 μg/day). The Almond–EV02 sample contributed 20.0% of this requirement in a 200 mL serving, while Rice–EV04, Soy–EV06, and Coconut–EV07 offered 7.5%, and Almond & Cashew–EV21 and Almond, Hazelnut, Walnut & Cashew–EV22 provided 3.8%. Importantly, neither thickener T1 nor T2 included this nutrient in their formulations, despite being added to the beverages.
4.3. Correlations Among Samples
The multivariate analyses performed in this study (PCA and HCA) highlight the complex relationships between beverage formulation, thickener type, and IDDSI flow outcomes. The strong contribution of all response variables (>0.6) confirms the robustness of the dataset. The clustering observed in PCA suggests that beverages thickened with either T1 or T2 segregate based on their compositional similarities and flow performance. This is reinforced by the HCA dendrogram, where syringes and thickener concentrations formed clear and interpretable groups. Together, these methods were able to illustrate the influence of syringe type and thickener dosage on the consistency outcomes, supporting the hypothesis that both factors are critical in IDDSI level classification.
Notably, plant-based beverages such as EV14 and EV21, composed of whole rice, quinoa, almond, and cashew, clustered with animal milk samples. This unexpected grouping suggests that certain plant-based matrices may mimic the rheological behavior of dairy products when thickened. These findings are relevant for clinical practice, particularly when selecting texture-modified beverages for patients requiring dietary variety. The results also underscore the importance of standardizing both the thickening process and the equipment used (e.g., syringes) to reduce variability.
5. Conclusions
This study provides an exploratory assessment of IDDSI flow test outcomes on thickened plant- and dairy-based beverages evaluated with non-standard syringes, highlighting the variability introduced by differences in syringe design. Through systematic evaluation, the findings offer practical guidance for healthcare professionals in selecting and preparing appropriate formulations for dysphagia management, thereby improving patient safety and satisfaction. Notably, under certain conditions, using only half of the manufacturer’s recommended amount of thickener appeared sufficient to achieve target IDDSI levels. However, this outcome was not consistent across all cases, with several samples—particularly those prepared with Thickener 1—remaining in the “slightly thick” (Level 1) category. Therefore, this observation should be regarded as a methodological indication of potential optimization rather than a clinical recommendation. Flow performance also varied with thickener type: samples prepared with Thickener 1 often remained in the “slightly thick” (Level 1) category, while formulations containing other thickeners more reliably reached higher IDDSI levels. Similarly, syringe design influenced flow performance, with narrower barrels (e.g., S1 and S2) yielding longer retention times than wider ones (S3 and S4). These observations should therefore be regarded as methodological indications of potential optimization rather than clinical recommendations. Additionally, multivariate analyses (PCA and HCA) revealed meaningful interrelationships among beverage samples, identifying clusters that reflect similarities in flow characteristics. Interestingly, some plant-based formulations exhibited rheological behaviors comparable to those of animal milk, highlighting their potential as functional alternatives in dysphagia diets. By characterizing how thickener type, concentration, and syringe design influence flow outcomes, this study contributes to the growing body of research supporting evidence-based texture modification. Collectively, these findings contribute to evidence-based texture modification practices and emphasize the need for greater accessibility of standardized IDDSI syringes, continued education for healthcare professionals, and further research aimed at improving reproducibility, safety, and inclusivity in dysphagia management.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/beverages11060159/s1: Table S1: Nutritional composition of thickened plant-based and dairy beverages (per 100 mL serving); Table S2: Time to exhaustion (mean ± SD) during the IDDSI flow test at room temperature (24 ± 1 °C) for non-thickened (Level 0) and thickened beverages; Table S3: IDDSI flow test results (mL) for beverages prepared according to manufacturer’s thickener (T1A and T2A) recommendations (mean ± SD); Table S4: IDDSI flow test results (mL) for beverages prepared with modified thickener dosages (T1B and T2B).
Author Contributions
Conceptualization, H.A.M.; Data curation, G.L.T.; Formal analysis, H.A.M.; Investigation, H.A.M.; Methodology, H.A.M.; Supervision, M.d.C.S.-M. and P.M.; Visualization, G.L.T.; Writing—original draft, H.A.M., G.L.T., M.E., D.P.G., L.G.S. and B.V.d.S.; Writing—review & editing, H.A.M., G.L.T., M.E., D.P.G., L.G.S., B.V.d.S., M.d.C.S.-M. and P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordination for the Improvement of Higher Education Personnel (CAPES), through the Institutional Internationalization Program, notice no 41/2017 (CAPES/PrInt).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available from the Corresponding Author upon reasonable request. A preprint can be found at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4946766. Accessed on 6 September 2025.
Acknowledgments
H. A. Maieves gratefully acknowledges the support of the Coordination for the Improvement of Higher Education Personnel (CAPES), provided through the Institutional Internationalization Program, notice no 41/2017 (CAPES/PrInt).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Clavé, P.; Shaker, R. Dysphagia: Current Reality and Scope of the Problem. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H. Presbyphagia and Sarcopenic Dysphagia: Association between Aging, Sarcopenia, and Deglutition Disorders. J. Frailty Aging 2014, 3, 97–103. [Google Scholar] [CrossRef]
- Atherton, M.; Bellis-Smith, N.; Cichero, J.A.Y.; Suter, M. Texture-Modified Foods and Thickened Fluids as Used for Individuals with Dysphagia: Australian Standardised Labels and Definitions. Nutr. Diet. 2007, 64, S53–S76. [Google Scholar] [CrossRef]
- Baijens, L.W.J.; Clavé, P.; Cras, P.; Ekberg, O.; Forster, A.; Kolb, G.F.; Leners, J.C.; Masiero, S.; Mateos-Nozal, J.; Ortega, O.; et al. European Society for Swallowing Disorders—European Union Geriatric Medicine Society White Paper: Oropharyngeal Dysphagia as a Geriatric Syndrome. Clin. Interv. Aging 2016, 11, 1403–1428. [Google Scholar] [CrossRef]
- Cichero, J.A.Y. Unlocking Opportunities in Food Design for Infants, Children, and the Elderly: Understanding Milestones in Chewing and Swallowing across the Lifespan for New Innovations. J. Texture Stud. 2017, 48, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Christmas, C.; Rogus-Pulia, N. Swallowing Disorders in the Older Population. J. Am. Geriatr. Soc. 2019, 67, 2643–2649. [Google Scholar] [CrossRef]
- Cartagena, M.; Giura, L.; Ansorena, D.; Astiasaran, I. A Texture-Modified Dessert with High Nutritional Value Designed for People with Dysphagia: Effect of Refrigeration and Frozen Storage. Food Sci. Hum. Wellness 2024, 13, 462–471. [Google Scholar] [CrossRef]
- Ihrke, M.; Beck, A.; Mürbe, D.; Voß, L.J. IDDSI-Compliant Recipes Containing Oral Contrast Agents for Radiological Dysphagia Diagnostics. J. Texture Stud. 2024, 55, e12833. [Google Scholar] [CrossRef] [PubMed]
- Baixauli, R.; Dobiašová, A.; Tarrega, A.; Laguna, L. Pairing Physical and Sensory Properties of Dysphagia Thickeners to Understand Disliking. Food Hydrocoll. Health 2023, 3, 100140. [Google Scholar] [CrossRef]
- Cichero, J.A.Y.; Steele, C.; Duivestein, J.; Clavé, P.; Chen, J.; Kayashita, J.; Dantas, R.; Lecko, C.; Speyer, R.; Lam, P.; et al. The Need for International Terminology and Definitions for Texture-Modified Foods and Thickened Liquids Used in Dysphagia Management: Foundations of a Global Initiative. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 280–291. [Google Scholar] [CrossRef]
- Barbon, C.E.A.; Steele, C.M. Thickened Liquids for Dysphagia Management: A Current Review of the Measurement of Liquid Flow. Curr. Phys. Med. Rehabil. Rep. 2018, 6, 220–226. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, M.; Hanson, B.; Moodley, L.; Pillay, M. The Impact of Modification Techniques on the Rheological Properties of Dysphagia Foods and Liquids. J. Texture Stud. 2019, 51, jtxs.12476. [Google Scholar] [CrossRef]
- Cichero, J.A.Y. Adjustment of Food Textural Properties for Elderly Patients. J. Texture Stud. 2016, 47, 277–283. [Google Scholar] [CrossRef]
- Cichero, J.A.Y.; Lam, P.; Steele, C.M.; Hanson, B.; Chen, J.; Dantas, R.O.; Duivestein, J.; Kayashita, J.; Lecko, C.; Murray, J.; et al. Development of International Terminology and Definitions for Texture-Modified Foods and Thickened Fluids Used in Dysphagia Management: The IDDSI Framework. Dysphagia 2017, 32, 293–314. [Google Scholar] [CrossRef]
- Maieves, H.A.; da Silva, B.V.; Ewerling, M.; Comparotto, U.R.; Lessa, F.B.; Lemos, A.N.; Hepp, J.P. The Behavior of Lemon-Based Thickened Fluids Submitted to the IDDSI Flow Test as a Strategy for Dysphagia Treatment. Food Sci. Today 2023, 1, 1–8. [Google Scholar] [CrossRef]
- An, S.; Lee, W.; Yoo, B. Comparison of National Dysphagia Diet and International Dysphasia Diet Standardization Initiative Levels for Thickened Drinks Prepared with a Commercial Xanthan Gum-Based Thickener Used for Patients with Dysphagia. Prev. Nutr. Food Sci. 2023, 28, 83–88. [Google Scholar] [CrossRef]
- Bitencourt, B.S.; Guedes, J.S.; Saliba, A.S.M.C.; Sartori, A.G.O.; Torres, L.C.R.; Amaral, J.E.P.G.; Alencar, S.M.; Maniglia, B.C.; Augusto, P.E.D. Mineral Bioaccessibility in 3D Printed Gels Based on Milk/Starch/ĸ-Carrageenan for Dysphagic People. Food Res. Int. 2023, 170, 113010. [Google Scholar] [CrossRef]
- Lifschitz, C.; Szajewska, H. Cow’s Milk Allergy: Evidence-Based Diagnosis and Management for the Practitioner. Eur. J. Pediatr. 2015, 174, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Pierrepont, C.; Duarte, C.M.; Filipe, A.; Medronho, B.; Sousa, I. Legume Beverages from Chickpea and Lupin, as New Milk Alternatives. Foods 2020, 9, 1458. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.M.; Liu, Q.; MacCallum, H.; Peladeau-Pigeon, M.; Chen, J.; Hanson, B.; Vanderwegen, J.; Lam, P. Validation of the IDDSI Funnel for Liquid Flow Testing. J. Texture Stud. 2024, 55, e12823. [Google Scholar] [CrossRef]
- IOM (Institute of Medicine). Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Dantas, R.O.; Oliveira, L. Influence of the Syringe Model on the Results of the International Dysphagia Diet Standardisation Initiative Flow Test. Rev. CEFAC 2018, 20, 382–387. [Google Scholar] [CrossRef]
- Alves, D.C.; Alves, N.A.; Dantas, R.O. Consistency Stability of Water Thickened with Maltodextrin, Xanthan Gum and Potassium Chloride. J. Texture Stud. 2017, 48, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Cichero, J.A.Y.; Jackson, O.; Halley, P.J.; Murdoch, B.E. How Thick Is Thick? Multicenter Study of the Rheological and Material Property Characteristics of Mealtime Fluids and Videofluoroscopy Fluids. Dysphagia 2000, 15, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Glassburn, D.L.; Deem, J.F. Thickener Viscosity in Dysphagia Management: Variability among Speech-Language Pathologists. Dysphagia 1998, 13, 218–222. [Google Scholar] [CrossRef]
- Steele, C.M.; Van Lieshout, P.H.H.M.; Goff, H.D. The Rheology of Liquids: A Comparison of Clinicians’ Subjective Impressions and Objective Measurement. Dysphagia 2003, 18, 182–195. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Yoon, S.-R.; Yoo, W.; Yoo, B. Effect of Salivary Reaction Time on Flow Properties of Commercial Food Thickeners Used for Dysphagic Patients. Clin. Nutr. Res. 2016, 5, 55. [Google Scholar] [CrossRef]
- Dewar, R.J.; Joyce, M.J. Time-Dependent Rheology of Starch Thickeners and the Clinical Implications for Dysphagia Therapy. Dysphagia 2006, 21, 264–269. [Google Scholar] [CrossRef]
- Shim, J.S.; Oh, B.M.; Han, T.R. Factors Associated with Compliance with Viscosity-Modified Diet among Dysphagic Patients. Ann. Rehabil. Med. 2013, 37, 628–632. [Google Scholar] [CrossRef]
- Nishinari, K.; Turcanu, M.; Nakauma, M.; Fang, Y. Role of Fluid Cohesiveness in Safe Swallowing. NPJ Sci. Food 2019, 3, 5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).