Functional Carbon-Based Materials for Blood Purification: Recent Advances Toward Improved Treatment of Renal Failure and Patient Quality of Life
Abstract
1. Introduction
2. Overview of Blood Purification Methods in Kidney Disease Management
2.1. Hemodialysis (HD): Diffusion-Based Toxin Removal with Emerging Carbon Integration
2.2. Hemoperfusion (HP): Direct Blood Contact with Activated Carbon for Toxin Adsorption
2.3. Oral Carbon Adsorbents: Gastrointestinal Toxin Removal Using Carbon-Based Therapies
3. Current Kidney Replacement Therapies and Carbon-Based Adsorbents
3.1. Role of Activated Carbons in Enhancing Dialysis Efficiency
3.2. Graphene Oxide Nanomaterials for Renal Replacement and Bioartificial Systems
4. Synthesis and Processing of Activated Carbon Materials
4.1. Carbonaceous Precursors for Activated Carbon Production
4.2. Carbonization and Activation Techniques
4.3. Role of Activating Agents in Surface Development
4.4. Synthesis and Structural Features of Graphene-Based Materials for Blood Purification Applications
5. Toxins and Solute Clearance
5.1. Organic Solutes and Ionic Species
- i.
- Water-Soluble, Low-Molecular-Weight Compounds (<0.5 kDa)
- ii.
- Middle-Molecular-Weight Compounds (0.5–60 kDa)
- iii.
- Protein-Bound Uremic Toxins (PBUTs)
5.2. Urea
5.3. Other Uremic Toxins
5.4. Bilirubin
6. Mechanism of Adsorption by Carbon-Based Materials
7. Therapeutic Applications of Carbon-Based Materials for Blood Purification
7.1. Activated Carbon: Hemocompatible Adsorbents for Renal Support
7.1.1. Integration of Activated Carbon in Hemodialysis Membranes and Dialysate
7.1.2. Hemoperfusion with Modified Activated Carbon Adsorbents
7.1.3. Oral Carbon Adsorbents for Toxin Removal in CKD
7.2. Graphene-Based Nanocomposites for Blood Purification
7.2.1. Functional Properties of Graphene Materials in Blood Purification
7.2.2. Hemodialysis Systems Using Graphene-Based Materials
7.2.3. Hemoperfusion Using Graphene Composites
7.2.4. Commercial Integration of Graphene in Dialysis Devices
8. Conclusions and Future Prospects
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACs | Activated Carbons |
| CKD | Chronic Kidney Disease |
| PCS | P-Cresyl Sulfate |
| OSCA | Oral Spherical Carbon Adsorbent |
| IL-6 & IL-8 | Serum and Urine Interleukin-6 and Interleukin-8 Levels |
| ESRD | End Stage Renal Disease |
| SWCNTs | Single Wall Carbon Nanotubes |
| MWCNTs | Multi Wall Carbon Nanotubes |
| HD | Hemodialysis |
| REDY | Regenerative/Recirculating Dialysate |
| PAC | Powdered Activated Carbon |
| GAC | Granular Activated Carbon |
| EAC | Extruded Activated Carbon |
| BAC | Bead Activated Carbon |
| ICC | Impregnated Coated Carbon |
| PCC | Polymer Coated Carbon |
| PFAC | Phenol Formaldehyde Activated Carbon |
| GO | Graphene Oxide |
| EUTox | Uremic Toxins |
| PBUTs | Protein-Bound Uremic Toxins |
| WAK | Wearable Artificial Kidneys |
| HSA | Human Serum Albumin |
| CS/GO | Chitosan/Graphene Oxide |
| Ch/GO | Chitin/Graphene Oxide |
| UF | Ultrafiltration |
| ΔG | Gibbs Free Energy Change value |
| ΔH | Enthalpy Change value |
| R2 | Correlation Coefficient |
| 3D-pGR | Three-Dimensional Porous Graphene |
| BSA | Bovine Serum Albumin |
| HP | Hemoperfusion |
| PQ | paraquat |
| HMCSs | Hollow Mesoporous Carbon Spheres |
| MMMAs | Mixed Matrix Membrane Adsorbers |
| PAH | Para-Amino Hippuric Acid |
| MMM | Mixed-Matrix Membrane |
| LDL | Low-Density Lipoprotein |
| DHP | Direct Hemoperfusion |
| PHEMA | Polyhydroxy ethyl Methacrylate |
| CNTs/P-ACSs | Carbon Nanotubes/Phenolic-Resin-Derived Activated Carbon Spheres |
| P-ACSs | Phenolic-Derived Activated Carbon Spheres |
| MDAC | Multiple-dose Activated Charcoal |
| CH | Charcoal Hemoperfusion |
| ACAC | Albumin-Cellulose nitrate-coated Activated Charcoal |
| TNFC | Thin-film Nanofibrous Composite |
| rGO | reduced GO |
| TRGO | Thermally Reduced Graphene Oxide |
| UPAS-MGO | Urease-Immobilized Magnetic Graphene Oxide |
References
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; Group, E.U.T.W. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. JASN 2012, 23, 1258. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Ise, M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J. Lab. Clin. Med. 1994, 124, 96–104. [Google Scholar] [PubMed]
- Ash, S.R. Innovation in the treatment of uremia: Proceedings from the Cleveland Clinic workshop: Sorbents in treatment of uremia: A short history and a great future. Semin. Dial. 2009, 22, 615–622. [Google Scholar] [CrossRef]
- Maeda, K.; Saito, A.; Kawaguchi, S.; Sezaki, R.; Niwa, T.; Naotsuka, M.; Kobayashi, K.; Asada, H.; Yamamoto, Y.; Ohta, K. Problems with activated charcoal and alumina as sorbents for medical use. Artif. Organs 1979, 3, 336–340. [Google Scholar] [CrossRef]
- Gashti, M.P.; Bourquin, M.; Stir, M.; Hulliger, J. Glutamic acid inducing kidney stone biomimicry by a brushite/gelatin composite. J. Mater. Chem. B 2013, 1, 1501–1508. [Google Scholar] [CrossRef]
- Gashti, M.P.; Stir, M.; Bourquin, M.; Hulliger, J.R. Mineralization of calcium phosphate crystals in starch template inducing a brushite kidney stone biomimetic composite. Cryst. Growth Des. 2013, 13, 2166–2173. [Google Scholar] [CrossRef]
- Gashti, M.P.; Stir, M.; Hulliger, J. Synthesis of bone-like micro-porous calcium phosphate/iota-carrageenan composites by gel diffusion. Colloids Surf. B Biointerfaces 2013, 110, 426–433. [Google Scholar] [CrossRef]
- Gashti, M.P.; Stir, M.; Burgener, M.; Hulliger, J.; Choobar, B.G.; Nooralian, Z.; Moghaddam, M.R. Hydroxypropyl methylcellulose-controlled in vitro calcium phosphate biomineralization. New J. Chem. 2022, 46, 20082–20091. [Google Scholar] [CrossRef]
- Lindholm, B.; Heimbürger, O.; Stenvinkel, P.; Bergström, J. Uremic toxicity. Nutr. Manag. Ren. Dis. 2004, 2, 63–98. [Google Scholar]
- Vanholder, R.; Massy, Z.; Argiles, A.; Spasovski, G.; Verbeke, F.; Lameire, N. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol. Dial. Transplant. 2005, 20, 1048–1056. [Google Scholar] [CrossRef]
- Kent, S.K. Adsorbent Selection; Adsorption Research Inc.: Dublin, OH, USA, 2003; pp. 1–23. [Google Scholar]
- Ju, J.; Liang, F.; Zhang, X.; Sun, R.; Pan, X.; Guan, X.; Cui, G.; He, X.; Li, M. Advancement in separation materials for blood purification therapy. Chin. J. Chem. Eng. 2019, 27, 1383–1390. [Google Scholar] [CrossRef]
- Müller, B.R. Effect of particle size and surface area on the adsorption of albumin-bonded bilirubin on activated carbon. Carbon 2010, 48, 3607–3615. [Google Scholar] [CrossRef]
- Nic, M.; Hovorka, L.; Jirat, J.; Kosata, B.; Znamenacek, J. IUPAC compendium of chemical terminology. In The Gold Book; International Union of Pure and Applied Chemistry: Zürich, Switzerland, 2005. [Google Scholar]
- Bekchanov, D.; Mukhamediev, M.; Yarmanov, S.; Lieberzeit, P.; Mujahid, A. Functionalizing natural polymers to develop green adsorbents for wastewater treatment applications. Carbohydr. Polym. 2024, 323, 121397. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, S.; Tonelli, M.; Unsworth, L.D. Adsorption-based strategies for removing uremic toxins from blood. Microporous Mesoporous Mater. 2021, 319, 111035. [Google Scholar] [CrossRef]
- Pereao, O.; Bode-Aluko, C.; Laatikainen, K.; Nechaev, A.; Petrik, L. Morphology, modification and characterisation of electrospun polymer nanofiber adsorbent material used in metal ion removal. J. Polym. Environ. 2019, 27, 1843–1860. [Google Scholar] [CrossRef]
- Shinke, K.; Ando, K.; Koyama, T.; Takai, T.; Nakaji, S.; Ogino, T. Properties of various carbon nanomaterial surfaces in bilirubin adsorption. Colloids Surf. B Biointerfaces 2010, 77, 18–21. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, L.; Zhang, J.; Zhou, J.; He, Q.; Zeng, S.; Cui, X.; Shi, J. Hollow mesoporous carbon spheres—An excellent bilirubin adsorbent. Chem. Commun. 2009, 40, 6071–6073. [Google Scholar] [CrossRef]
- Ando, K.; Shinke, K.; Yamada, S.; Koyama, T.; Takai, T.; Nakaji, S.; Ogino, T. Fabrication of carbon nanotube sheets and their bilirubin adsorption capacity. Colloids Surf. B Biointerfaces 2009, 71, 255–259. [Google Scholar] [CrossRef]
- Rezaee, A.; Ghanizadeh, G.; Behzadiyannejad, G.; Yazdanbakhsh, A.; Siyadat, S. Adsorption of endotoxin from aqueous solution using bone char. Bull. Environ. Contam. Toxicol. 2009, 82, 732–737. [Google Scholar] [CrossRef]
- Gao, Q.; Han, B. Three-dimensionally porous graphene: A high-performance adsorbent for removal of albumin-bonded bilirubin. Colloids Surf. B Biointerfaces 2017, 149, 146–153. [Google Scholar]
- Rahman, M.H.; Haqqie, S.S.; McGoldrick, M.D. Acute hemolysis with acute renal failure in a patient with valproic acid poisoning treated with charcoal hemoperfusion. Hemodial. Int. 2006, 10, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.; Kramer, M.; Raja, R. Amberlite hemoperfusion in the treatment of acute drug intoxication. Int. J. Artif. Organs 1979, 2, 316–318. [Google Scholar] [PubMed]
- Arduino, M.J. Dialysis-Associated. In Bennett & Brachman’s Hospital Infections; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; p. 341. [Google Scholar]
- Cheung, A.K. Hemodialysis; hemofiltration. Kidney Dis. 2009, 5, 446–471. [Google Scholar]
- Collins, A.J.; Hao, W.; Xia, H.; Ebben, J.P.; Everson, S.E.; Constantini, E.G.; Ma, J.Z. Mortality risks of peritoneal dialysis and hemodialysis. Am. J. Kidney Dis. 1999, 34, 1065–1074. [Google Scholar] [CrossRef]
- Azar, A.T.; Canaud, B. Hemodialysis System, Modelling and Control of Dialysis Systems: Volume 1: Modeling Techniques of Hemodialysis Systems; Springer: Berlin/Heidelberg, Germany, 2013; pp. 99–166. [Google Scholar]
- Roberts, M. The regenerative dialysis (REDY) sorbent system. Nephrology 1998, 4, 275–278. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work Group (EUTox). Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. CJASN 2009, 4, 1551. [Google Scholar] [CrossRef]
- Agar, J.W. Understanding sorbent dialysis systems. Nephrology 2010, 15, 406–411. [Google Scholar] [CrossRef]
- Mikhalovsky, S.; Nikoleav, V. Activated carbons as medical adsorbents. In Activated Carbon Surfaces in Environmental Remediation; Bandosz, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 7. [Google Scholar]
- Mikhalovsky, S.V. Emerging technologies in extracorporeal treatment: Focus on adsorption. Perfusion 2003, 18 (Suppl. S1), 47–54. [Google Scholar] [CrossRef]
- Sandeman, S.R.; Howell, C.A.; Phillips, G.J.; Lloyd, A.W.; Davies, J.G.; Mikhalovsky, S.V.; Tennison, S.R.; Rawlinson, A.P.; Kozynchenko, O.P.; Owen, H.L. Assessing the in vitro biocompatibility of a novel carbon device for the treatment of sepsis. Biomaterials 2005, 26, 7124–7131. [Google Scholar] [CrossRef]
- Mamdani, B.; Dunea, G.; Siemsen, A.W. Long-term hemoperfusion with coated activated charcoal. Clin. Toxicol. 1980, 17, 543–546. [Google Scholar] [CrossRef]
- Eloot, S.; Ledebo, I.; Ward, R.A. Extracorporeal removal of uremic toxins: Can we still do better? Semin. Nephrol. 2014, 34, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Winchester, J.F.; Silberzweig, J.; Ronco, C.; Kuntsevich, V.; Levine, D.; Parker, T.; Kellum, J.A.; Salsberg, J.A.; Quartararo, P.; Levin, N.W. Sorbents in acute renal failure and end-stage renal disease: Middle molecule and cytokine removal. Blood Purif. 2004, 22, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Odaka, M.; Hiraswa, H.; Kobayashi, H.; Ohkawa, M.; Soeda, K.; Tabita, Y.; Soma, M.; Sato, H. Clinical and Fundamental-Studies of Cellulose-Coated Bead-Shaped Charcoal Hemoperfusion in Chronic-Renal-Failure, Artificial Organs; Blackwell Science Inc.: Malden, MA, USA, 1979; p. 397. [Google Scholar]
- Zellner, T.; Prasa, D.; Färber, E.; Hoffmann-Walbeck, P.; Genser, D.; Eyer, F. The use of activated charcoal to treat intoxications. Dtsch. Aerzteblatt Int. 2019, 116, 311. [Google Scholar] [CrossRef] [PubMed]
- Seger, D. Single dose activated charcoal. J. Med. Toxicol. 2008, 4, 65. [Google Scholar] [CrossRef][Green Version]
- Hu, X.; Wang, T.; Li, F.; Mao, X. Surface modifications of biomaterials in different applied fields. RSC Adv. 2023, 13, 20495–20511. [Google Scholar] [CrossRef]
- Shi, Y.; Xue, Z.; Li, P.; Yang, S.; Zhang, D.; Zhou, S.; Guan, Z.; Li, Y.; Wang, L.-N. Surface modification on biodegradable zinc alloys. J. Mater. Res. Technol. 2023, 25, 3670–3687. [Google Scholar] [CrossRef]
- Gao, C.; Guo, M.; Liu, Y.; Zhang, D.; Gao, F.; Sun, L.; Li, J.; Chen, X.; Terrones, M.; Wang, Y. Surface modification methods and mechanisms in carbon nanotubes dispersion. Carbon 2023, 212, 118133. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface nano-functionalization of biomaterials. Mater. Sci. Eng. R Rep. 2010, 70, 275–302. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, K.; Zhang, L. Visible light photocatalysis of BiOI and its photocatalytic activity enhancement by in situ ionic liquid modification. J. Phys. Chem. C 2011, 115, 14300–14308. [Google Scholar] [CrossRef]
- Tang, M.; Kalim, S. Novel Approaches for the Removal of Uremic Solutes; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2022; pp. 1113–1115. [Google Scholar]
- Dou, W.; Wang, J.; Yao, Z.; Xiao, W.; Huang, M.; Zhang, L. A critical review of hemoperfusion adsorbents: Materials, functionalization and matrix structure selection. Mater. Adv. 2022, 3, 918–930. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ito, T.; Sato, M.; Goto, S.; Kazama, J.J.; Gejyo, F.; Narita, I. Adsorption of protein-bound uremic toxins using activated carbon through direct hemoperfusion in vitro. Blood Purif. 2019, 48, 215–222. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R. Hemoperfusion: Technical aspects and state of the art. Crit. Care 2022, 26, 135. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Cowgill, L.D.; Auñon, J.D. Activated Carbon Hemoperfusion and Plasma Adsorption: Rediscovery and Veterinary Applications of These Abandoned Therapies. Adv. Small Anim. Care 2021, 2, 131–142. [Google Scholar] [CrossRef]
- Cupisti, A.; Gallieni, M.; Avesani, C.M.; D’Alessandro, C.; Carrero, J.J.; Piccoli, G.B. Medical nutritional therapy for patients with chronic kidney disease not on dialysis: The low protein diet as a medication. J. Clin. Med. 2020, 9, 3644. [Google Scholar] [CrossRef] [PubMed]
- Mekaj, Y.H.; Mekaj, A.Y.; Duci, S.B.; Miftari, E.I. New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther. Clin. Risk Manag. 2015, 11, 967–977. [Google Scholar] [CrossRef]
- Al-Shazly, A.A.; Mohamed, F.S.; Abdel-Aziz, E.M. Role of Oral Activated Charcoal in decreasing Blood Urea, Creatinine and Phosphorous in Chronic Kidney Disease. Egypt. J. Hosp. Med. 2022, 89, 7211–7216. [Google Scholar] [CrossRef]
- Ramada, D.L.; de Vries, J.; Vollenbroek, J.; Noor, N.; Beek, O.T.; Mihăilă, S.M.; Wieringa, F.; Masereeuw, R.; Gerritsen, K.; Stamatialis, D. Portable, wearable and implantable artificial kidney systems: Needs, opportunities and challenges. Nat. Rev. Nephrol. 2023, 19, 481–490. [Google Scholar] [CrossRef]
- Kumar, A. Preparation and Characterization of Novel Activated Carbons for Adsorption and Adsorption Assisted Biodegradation of Organic and Inorganic Water Pollutants. Ph.D. Thesis, National Institute of Technology, Rourkela, India, 2017. [Google Scholar]
- Thajudeen, B.; Issa, D.; Roy-Chaudhury, P. Advances in hemodialysis therapy. Fac. Rev. 2023, 12, 12. [Google Scholar] [CrossRef]
- Lawson, J.H.; Niklason, L.E.; Roy-Chaudhury, P. Challenges and novel therapies for vascular access in haemodialysis. Nat. Rev. Nephrol. 2020, 16, 586–602. [Google Scholar] [CrossRef]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A comprehensive review on the chemical regeneration of biochar adsorbent for sustainable wastewater treatment. NPJ Clean Water 2022, 5, 29. [Google Scholar] [CrossRef]
- Lu, W.; Jiang, G.; Group, S.H.-H.C. Hemoperfusion in maintenance hemodialysis patients. Blood Purif. 2022, 51, 803–811. [Google Scholar] [CrossRef]
- Damianaki, A.; Stambolliu, E.; Alexakou, Z.; Petras, D. Expanding the potential therapeutic options of hemoperfusion in the era of improved sorbent biocompatibility. Kidney Res. Clin. Pract. 2023, 42, 298. [Google Scholar] [CrossRef]
- Cheng, W.; Luo, Y.; Wang, H.; Qin, X.; Liu, X.; Fu, Y.; Ronco, C. Survival outcomes of hemoperfusion and hemodialysis versus hemodialysis in patients with end-stage renal disease: A systematic review and meta-analysis. Blood Purif. 2022, 51, 213–225. [Google Scholar] [CrossRef]
- Ronco, C. Combined hemoperfusion-hemodialysis in end-stage renal disease patients. Contrib. Nephrol. 2023, 200, 118–122. [Google Scholar]
- Denti, E.; Luboz, M.P.; Tessore, V.; Castino, F.; Gaglia, P.F. Adsorbents in hemoperfusion. Kidney Int. Suppl. 1975, 3, 401–405. [Google Scholar]
- Sternkopf, M.; Thoröe-Boveleth, S.; Beck, T.; Oleschko, K.; Erlenkötter, A.; Tschulena, U.; Steppan, S.; Speer, T.; Goettsch, C.; Jankowski, V.; et al. A bifunctional adsorber particle for the removal of hydrophobic uremic toxins from whole blood of renal failure patients. Toxins 2019, 11, 389. [Google Scholar] [CrossRef]
- Pavlenko, D.; Giasafaki, D.; Charalambopoulou, G.; van Geffen, E.; Gerritsen, K.G.F.; Steriotis, T.; Stamatialis, D. Carbon Adsorbents with Dual Porosity for Efficient Removal of Uremic Toxins and Cytokines from Human Plasma. Sci. Rep. 2017, 7, 14914. [Google Scholar] [CrossRef]
- Chen, J.; Ma, Y.; Wang, L.; Han, W.; Chai, Y.; Wang, T.; Li, J.; Ou, L. Preparation of chitosan/SiO2-loaded graphene composite beads for efficient removal of bilirubin. Carbon 2019, 143, 352–361. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Li, K.; Liu, Y.; Xie, D.; Shan, S.; He, L.; Mei, Y. Adsorption of uremic toxins using biochar for dialysate regeneration. Biomass Convers. Biorefinery 2021, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Horikoshi, K.; Kumagai, S.; Saito, Y.; Yoshioka, T. Adsorption of urea; creatinine, and uric acid from three solution types using spherical activated carbon and its recyclability. Chin. J. Chem. Eng. 2020, 28, 2993–3001. [Google Scholar] [CrossRef]
- Kameda, T.; Ito, S.; Yoshioka, T. Kinetic and equilibrium studies of urea adsorption onto activated carbon: Adsorption mechanism. J. Dispers. Sci. Technol. 2017, 38, 1063–1066. [Google Scholar] [CrossRef]
- Kameda, T.; Horikoshi, K.; Kumagai, S.; Saito, Y.; Yoshioka, T. Adsorption of urea; creatinine, and uric acid onto spherical activated carbon. Sep. Purif. Technol. 2020, 237, 116367. [Google Scholar] [CrossRef]
- Botella, J.; Ghezzi, P.M.; Sanz-Moreno, C. Adsorption in hemodialysis. Kidney Int. 2000, 58, S60–S65. [Google Scholar] [CrossRef][Green Version]
- Magnani, S.; Atti, M. Uremic toxins and blood purification: A review of current evidence and future perspectives. Toxins 2021, 13, 246. [Google Scholar] [CrossRef]
- Chang, T. Microencapsulated adsorbent hemoperfusion for uremia, intoxication and hepatic failure. Kidney Int. Suppl. 1975, 3, 387–392. [Google Scholar][Green Version]
- Chen, W.; Wang, B.; Liang, S.; Wang, M.; Zheng, L.; Xu, S.; Wang, J.; Fang, H.; Yang, P.; Feng, W. Renal clearance of graphene oxide: Glomerular filtration or tubular secretion and selective kidney injury association with its lateral dimension. J. Nanobiotechnol. 2023, 21, 51. [Google Scholar] [CrossRef]
- Rode, R.P.; Chung, H.H.; Miller, H.N.; Gaborski, T.R.; Moghaddam, S. Trilayer Interlinked Graphene Oxide Membrane for Wearable Hemodialyzer. Adv. Mater. Interfaces 2021, 8, 2001985. [Google Scholar] [CrossRef]
- Modi, A.; Verma, S.K.; Bellare, J. Surface-Functionalized Poly(Ether Sulfone) Composite Hollow Fiber Membranes with Improved Biocompatibility and Uremic Toxins Clearance for Bioartificial Kidney Application. ACS Appl. Bio Mater. 2020, 3, 1589–1597. [Google Scholar] [CrossRef]
- Xiaojian, Z.; Zhansheng, W.; Xiasheng, G. Simple combination of biodegradation and carbon adsorption—The mechanism of the biological activated carbon process. Water Res. 1991, 25, 165–172. [Google Scholar] [CrossRef]
- Razumiene, J.; Gureviciene, V.; Sakinyte, I.; Rimsevicius, L.; Laurinavicius, V. The synergy of thermally reduced graphene oxide in amperometric urea biosensor: Application for medical technologies. Sensors 2020, 20, 4496. [Google Scholar] [CrossRef]
- Jin, Y.; Ding, S.; Li, P.; Wang, X. Coordination of thin-film nanofibrous composite dialysis membrane and reduced graphene oxide aerogel adsorbents for elimination of indoxyl sulfate. Chin. J. Chem. Eng. 2022, 49, 111–121. [Google Scholar] [CrossRef]
- Li, Z.; Yan, X.; Wu, K.; Jiao, Y.; Zhou, C.; Yang, J. Surface modification of reduced graphene oxide beads: Integrating efficient endotoxin adsorption and improved blood compatibility. ACS Appl. Bio Mater. 2021, 4, 4896–4906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, B.; He, C.; Hao, Y.; Sun, S.; Zhao, W.; Zhao, C. Urease-immobilized magnetic graphene oxide as a safe and effective urea removal recyclable nanocatalyst for blood purification. Ind. Eng. Chem. Res. 2020, 59, 8955–8964. [Google Scholar] [CrossRef]
- Gejyo, F.; Amano, I.; Ando, T.; Ishida, M.; Obayashi, S.; Ogawa, H.; Ono, T.; Kanno, Y.; Kitaoka, T.; Kukita, K. Survey of the effects of a column for adsorption of β2-microglobulin in patients with dialysis-related amyloidosis in Japan. Ther. Apher. Dial. 2013, 17, 40–47. [Google Scholar] [CrossRef]
- Ronco, C.; Brendolan, A.; Winchester, J.F.; Golds, E.; Clemmer, J.; Polaschegg, H.D.; Muller, T.E.; La Greca, G.; Levin, N.W. First clinical experience with an adjunctive hemoperfusion device designed specifically to remove β2-microglobulin in hemodialysis. Blood Purif. 2001, 19, 260–263. [Google Scholar] [CrossRef]
- Malchesky, P.S.; Zborowski, M.; Hou, K.C. Extracorporeal techniques of endotoxin removal: A review of the art and science. Adv. Ren. Replace. Ther. 1995, 2, 60–69. [Google Scholar] [CrossRef]
- Schneider, K.M. Plasmapheresis and immunoadsorption: Different techniques and their current role in medical therapy. Kidney Int. Suppl. 1998, 64, S61–S65. [Google Scholar]
- Bosch, T. State of the art of lipid apheresis. Artif. Organs 1996, 20, 292–295. [Google Scholar] [CrossRef]
- Mitzner, S.R.; Stange, J.; Klammt, S.; Peszynski, P.; Schmidt, R.; NO, G. Extracorporeal detoxification using the molecular adsorbent recirculating system for critically ill patients with liver failure. J. Am. Soc. Nephrol. 2001, 12 (Suppl. S1), S75–S82. [Google Scholar] [CrossRef]
- Allen, S.; Whitten, L.; McKay, G. The production and characterisation of activated carbons: A review. Dev. Chem. Eng. Miner. Process. 1998, 6, 231–261. [Google Scholar] [CrossRef]
- Parra, J.; Sousa, J.D.; Bansal, R.C.; Pis, J.; Pajares, J. Characterization of activated carbons by the BET equation—An alternative approach. Adsorpt. Sci. Technol. 1995, 12, 51–66. [Google Scholar] [CrossRef]
- Mahamallik, A. Synthesis and Characterization of Porous Activated Carbon from a New Precursor (Karanja Oil Cake). Bachelor’s Thesis, National Institute of Technology, Rourkela, India, 2013. [Google Scholar]
- Karthik, V. Preparation and Characterization of Activated Carbon from Jackfruit Peel. Master’s Thesis, National Institute of Technology, Rourkela, India, 2016. [Google Scholar]
- Natrayan, L.; Kumar, P.A.; Dhanraj, J.A.; Kaliappan, S.; Sivakumar, N.; Patil, P.P.; Sekar, S.; Paramasivam, P. Synthesis and analysis of impregnation on activated carbon in multiwalled carbon nanotube for Cu adsorption from wastewater. Bioinorg. Chem. Appl. 2022, 2022, 7470263. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. High surface area microporous activated carbons prepared from Fox nut (Euryale ferox) shell by zinc chloride activation. Appl. Surf. Sci. 2015, 356, 753–761. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Plaza, M.; González, A.; Pis, J.; Rubiera, F.; Pevida, C. Production of microporous biochars by single-step oxidation: Effect of activation conditions on CO2 capture. Appl. Energy 2014, 114, 551–562. [Google Scholar] [CrossRef]
- Copelli, S.; Lorenzin, A.; Ronco, C. Chemical-Physical Mechanisms of Adsorption for Blood Purification. Contrib. Nephrol. 2023, 200, 8–16. [Google Scholar]
- Lee, L.Z.; Zaini, M.A.A. Metal chloride salts in the preparation of activated carbon and their hazardous outlook. Desalination Water Treat. 2016, 57, 16078–16085. [Google Scholar] [CrossRef]
- Tkachenko, O.; Nikolaichuk, A.; Fihurka, N.; Backhaus, A.; Zimmerman, J.B.; Strømme, M.; Budnyak, T.M. Kraft Lignin-Derived Microporous Nitrogen-Doped Carbon Adsorbent for Air and Water Purification. ACS Appl. Mater. Interfaces 2024, 16, 3427–3441. [Google Scholar] [CrossRef]
- Macías-García, A.; Carrasco-Amador, J.P.; Encinas-Sánchez, V.; Díaz-Díez, M.A.; Torrejón-Martín, D. Preparation of activated carbon from kenaf by activation with H3PO4. Kinetic study of the adsorption/electroadsorption using a system of supports designed in 3D, for environmental applications. J. Environ. Chem. Eng. 2019, 7, 103196. [Google Scholar] [CrossRef]
- Yang, Z.; Gleisner, R.; Mann, D.H.; Xu, J.; Jiang, J.; Zhu, J.Y. Lignin Based Activated Carbon Using H3PO4 Activation. Polymers 2020, 12, 2829. [Google Scholar] [CrossRef]
- Alfatah, T.; Mistar, E.M.; Supardan, M.D. Porous structure and adsorptive properties of activated carbon derived from Bambusa vulgaris striata by two-stage KOH/NaOH mixture activation for Hg2+ removal. J. Water Process Eng. 2021, 43, 102294. [Google Scholar] [CrossRef]
- Yue, Z.; Economy, J.; Bordson, G. Preparation and characterization of NaOH-activated carbons from phenolic resin. J. Mater. Chem. 2006, 16, 1456–1461. [Google Scholar] [CrossRef]
- Eftekhari, A.; Dizaj, S.M.; Ahmadian, E.; Przekora, A.; Khatibi, S.M.H.; Ardalan, M.; Vahed, S.Z.; Valiyeva, M.; Mehraliyeva, S.; Khalilov, R.; et al. Application of Advanced Nanomaterials for Kidney Failure Treatment and Regeneration. Materials 2021, 14, 2939. [Google Scholar] [CrossRef] [PubMed]
- Groth, T.; Stegmayr, B.G.; Ash, S.R.; Kuchinka, J.; Wieringa, F.P.; Fissell, W.H.; Roy, S. Wearable and implantable artificial kidney devices for end-stage kidney disease treatment: Current status and review. Artif. Organs 2023, 47, 649–666. [Google Scholar] [CrossRef]
- van Gelder, M.K.; Jong, J.A.; Folkertsma, L.; Guo, Y.; Blüchel, C.; Verhaar, M.C.; Odijk, M.; Van Nostrum, C.F.; Hennink, W.E.; Gerritsen, K.G. Urea removal strategies for dialysate regeneration in a wearable artificial kidney. Biomaterials 2020, 234, 119735. [Google Scholar] [CrossRef]
- Locatelli, F.; La Milia, V.; Violo, L.; Del Vecchio, L.; Di Filippo, S. Optimizing haemodialysate composition. Clin. Kidney J. 2015, 8, 580–589. [Google Scholar] [CrossRef]
- Mudraya, I.; Nikolaev, V.; Galinskaya, V.; Aleinikov, V.; Medvedev, S. Physiological assessment of autohemoperfusion through activated charcoal. Bull. Exp. Biol. Med. 1977, 84, 1684–1687. [Google Scholar] [CrossRef]
- Baseri, J.R.; Palanisamy, P.; Sivakumar, P. Preparation and characterization of activated carbon from Thevetia peruviana for the removal of dyes from textile waste water. Adv. Appl. Sci. Res. 2012, 3, 377–383. [Google Scholar]
- Bernal, J.D. The structure of graphite, Proceedings of the Royal Society of London. Series A. Contain. Pap. A Math. Phys. Character 1924, 106, 749–773. [Google Scholar]
- Kaleekkal, N.J.; Thanigaivelan, A.; Durga, M.; Girish, R.; Rana, D.; Soundararajan, P.; Mohan, D. Graphene oxide nanocomposite incorporated poly(ether imide) mixed matrix membranes for in vitro evaluation of its efficacy in blood purification applications. Ind. Eng. Chem. Res. 2015, 54, 7899–7913. [Google Scholar] [CrossRef]
- Abdel-Motagaly, A.T.; El Rouby, W.M.; El-Dek, S.; El-Sherbiny, I.M.; Farghali, A. Fast technique for the purification of as-prepared graphene oxide suspension. Diam. Relat. Mater. 2018, 86, 20–28. [Google Scholar] [CrossRef]
- Rabb, H.; Lee, K.; Parikh, C.R. Beyond kidney dialysis and transplantation: What’s on the horizon? J. Clin. Investig. 2022, 132, e159308. [Google Scholar] [CrossRef] [PubMed]
- Tölle, F.J.; Gamp, K.; Mülhaupt, R. Scale-up and purification of graphite oxide as intermediate for functionalized graphene. Carbon 2014, 75, 432–442. [Google Scholar] [CrossRef]
- Liu, R.-L.; Ji, W.-J.; He, T.; Zhang, Z.-Q.; Zhang, J.; Dang, F.-Q. Fabrication of nitrogen-doped hierarchically porous carbons through a hybrid dual-template route for CO2 capture and haemoperfusion. Carbon 2014, 76, 84–95. [Google Scholar] [CrossRef]
- Kidambi, P.R.; Jang, D.; Idrobo, J.C.; Boutilier, M.S.; Wang, L.; Kong, J.; Karnik, R. Nanoporous atomically thin graphene membranes for desalting and dialysis applications. Adv. Mater. 2017, 29, 1700277. [Google Scholar] [CrossRef]
- Modi, A.; Verma, S.K.; Bellare, J. Graphene oxide-doping improves the biocompatibility and separation performance of polyethersulfone hollow fiber membranes for bioartificial kidney application. J. Colloid Interface Sci. 2018, 514, 750–759. [Google Scholar] [CrossRef]
- Pandey, N.; Bellare, J. Titanium dioxide/graphene oxide blending into polyethersulfone hollow fiber membranes improves biocompatibility and middle molecular weight separation for bioartificial kidney and hemodialysis applications. J. Mater. Chem. B 2025, 13, 9392–9406. [Google Scholar] [CrossRef]
- Asai, M.; Kumakura, S.; Kikuchi, M. Review of the efficacy of AST-120 (KREMEZIN®) on renal function in chronic kidney disease patients. Ren. Fail. 2019, 41, 47–56. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T. Uraemic syndrome of chronic kidney disease: Altered remote sensing and signalling. Nat. Rev. Nephrol. 2019, 15, 301–316. [Google Scholar] [CrossRef]
- Hassan, S.B.; El-demery, A.B.; Ahmed, A.I.; Abukhalil, R.E. Soluble TWEAK and cardiovascular morbidity and mortality in chronic kidney disease patients. Arab J. Nephrol. Transplant. 2012, 5, 27–32. [Google Scholar]
- Stange, J.; Mitzner, S.R.; Risler, T.; Erley, C.M.; Lauchart, W.; Goehl, H.; Klammt, S.; Peszynski, P.; Freytag, J.; Hickstein, H. Molecular adsorbent recycling system (MARS): Clinical results of a new membrane-based blood purification system for bioartificial liver support. Artif. Organs 1999, 23, 319–330. [Google Scholar] [CrossRef]
- Xiong, S.; Lyu, Y.; Davenport, A.; Choy, K.L. Sponge-like chitosan based porous monolith for uraemic toxins sorption. Nanomaterials 2021, 11, 2247. [Google Scholar] [CrossRef] [PubMed]
- Karakuş, A.; Değer, Y.; Yıldırım, S. Protective effect of Silybum marianum and Taraxacum officinale extracts against oxidative kidney injuries induced by carbon tetrachloride in rats. Ren. Fail. 2017, 39, 1–6. [Google Scholar] [CrossRef]
- De Pascale, M.; De Angelis, M.G.; Boi, C. Mixed matrix membranes adsorbers (MMMAs) for the removal of uremic toxins from dialysate. Membranes 2022, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The impact of CKD on uremic toxins and gut microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef]
- Poesen, R.; Viaene, L.; Verbeke, K.; Claes, K.; Bammens, B.; Sprangers, B.; Naesens, M.; Vanrenterghem, Y.; Kuypers, D.; Evenepoel, P. Renal clearance and intestinal generation of p-cresyl sulfate and indoxyl sulfate in CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 1508–1514. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Lekawanvijit, S.; Kompa, A.R.; Wang, B.H.; Kelly, D.J.; Krum, H. Cardiorenal syndrome: The emerging role of protein-bound uremic toxins. Circ. Res. 2012, 111, 1470–1483. [Google Scholar] [CrossRef]
- Carney, E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020, 16, 251. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Zhao, Y.-Y.; Pahl, M.V. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: The nature, mechanisms, consequences and potential treatment. Nephrol. Dial. Transplant. 2016, 31, 737–746. [Google Scholar] [CrossRef]
- Yoshida, F.; Okazaki, M. Treatment of artificial kidney dialysate with cycling adsorber-desorbers. Ann. Biomed. Eng. 1974, 2, 327–334. [Google Scholar] [CrossRef]
- Kaleekkal, N.J. Heparin immobilized graphene oxide in polyetherimide membranes for hemodialysis with enhanced hemocompatibility and removal of uremic toxins. J. Membr. Sci. 2021, 623, 119068. [Google Scholar] [CrossRef]
- McGill, R.L.; Weiner, D.E. Dialysate composition for hemodialysis: Changes and changing risk. Semin. Dial. 2017, 30, 112–120. [Google Scholar] [CrossRef]
- Roumelioti, M.-E.; Trietley, G.; Nolin, T.D.; Ng, Y.-H.; Xu, Z.; Alaini, A.; Figueroa, R.; Unruh, M.L.; Argyropoulos, C.P. Beta-2 microglobulin clearance in high-flux dialysis and convective dialysis modalities: A meta-analysis of published studies. Nephrol. Dial. Transplant. 2018, 33, 1025–1039. [Google Scholar] [CrossRef]
- Vanholder, R.; Van Laecke, S.; Glorieux, G. What is new in uremic toxicity? Pediatr. Nephrol. 2008, 23, 1211–1221. [Google Scholar] [CrossRef]
- Clark, W.R.; Dehghani, N.L.; Narsimhan, V.; Ronco, C. Uremic toxins and their relation to dialysis efficacy. Blood Purif. 2019, 48, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Glorieux, G.; De Smet, R.; Lameire, N. New insights in uremic toxins. Kidney Int. 2003, 63, S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Recht, N.S.; Hostetter, T.H.; Meyer, T.W. Removal of P-cresol sulfate by hemodialysis. J. Am. Soc. Nephrol. 2005, 16, 3430–3436. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.; Warwick, G.; Venturi, M.; Streat, M.; Hellgardt, K.; Hoenich, N.; Dale, J. Preparation of novel mesoporous carbons for the adsorption of an inflammatory cytokine (IL-1β). Biomaterials 2004, 25, 2933–2940. [Google Scholar] [CrossRef]
- Malik, D.; Warwick, G.; Mathieson, I.; Hoenich, N.; Streat, M. Structured carbon haemoadsorbents for the removal of middle molecular weight toxins. Carbon 2005, 43, 2317–2329. [Google Scholar] [CrossRef]
- Dhondt, A.; Vanholder, R.; Van Biesen, W.; Lameire, N. The removal of uremic toxins. Kidney Int. 2000, 58, S47–S59. [Google Scholar] [CrossRef]
- Daneshamouz, S.; Eduok, U.; Abdelrasoul, A.; Shoker, A. Protein-bound uremic toxins (PBUTs) in chronic kidney disease (CKD) patients: Production pathway, challenges and recent advances in renal PBUTs clearance. NanoImpact 2021, 21, 100299. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Alornyo, K.K.; Luke, P.P.W.; Sener, A. Application of carbon monoxide in kidney and heart transplantation: A novel pharmacological strategy for a broader use of suboptimal renal and cardiac grafts. Pharmacol. Res. 2021, 173, 105883. [Google Scholar] [CrossRef]
- Huang, X.; Ma, Y.; Li, Y.; Han, F.; Lin, W. Targeted Drug Delivery Systems for Kidney Diseases. Front. Bioeng. Biotechnol. 2021, 9, 683247. [Google Scholar] [CrossRef]
- Abe, T.; Yazawa, K.; Fujino, M.; Imamura, R.; Hatayama, N.; Kakuta, Y.; Tsutahara, K.; Okumi, M.; Ichimaru, N.; Kaimori, J.-Y. High-pressure carbon monoxide preserves rat kidney grafts from apoptosis and inflammation. Lab. Investig. 2017, 97, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, Q.; Yang, Y.; Li, Y.; Lin, W. Recent trends in therapeutic application of engineered blood purification materials for kidney disease. Biomater. Res. 2022, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Adewole, S.; Salako, A.; Doherty, O.; Naicker, T. Effect of melatonin on carbon tetrachloride-induced kidney injury in Wistar rats. Afr. J. Biomed. Res. 2007, 10, 153–164. [Google Scholar] [CrossRef]
- Fu, C.-C.; Hsiao, Y.-S.; Ke, J.-W.; Syu, W.-L.; Liu, T.-Y.; Liu, S.-H.; Juang, R.-S. Adsorptive removal of p-cresol and creatinine from simulated serum using porous polyethersulfone mixed-matrix membranes. Sep. Purif. Technol. 2020, 245, 116884. [Google Scholar] [CrossRef]
- Brettschneider, F.; Tölle, M.; von der Giet, M.; Passlick-Deetjen, J.; Steppan, S.; Peter, M.; Jankowski, V.; Krause, A.; Kühne, S.; Zidek, W. Removal of protein-bound, hydrophobic uremic toxins by a combined fractionated plasma separation and adsorption technique. Artif. Organs 2013, 37, 409–416. [Google Scholar] [CrossRef]
- Kato, S.; Otake, K.-I.; Chen, H.; Akpinar, I.; Buru, C.T.; Islamoglu, T.; Snurr, R.Q.; Farha, O.K. Zirconium-based metal–organic frameworks for the removal of protein-bound uremic toxin from human serum albumin. J. Am. Chem. Soc. 2019, 141, 2568–2576. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, L.; You, X.; Wang, X.; Wu, J.; Zheng, Z. Low dimensional nanomaterials for treating acute kidney injury. J. Nanobiotechnology 2022, 20, 505. [Google Scholar] [CrossRef]
- Krebs, H.; Hems, R.; Weidemann, M.; Speake, R. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem. J. 1966, 101, 242. [Google Scholar] [CrossRef]
- Wollborn, J.; Schlueter, B.; Steiger, C.; Hermann, C.; Wunder, C.; Schmidt, J.; Diel, P.; Meinel, L.; Buerkle, H.; Goebel, U. Extracorporeal resuscitation with carbon monoxide improves renal function by targeting inflammatory pathways in cardiac arrest in pigs. Am. J. Physiol.-Ren. Physiol. 2019, 317, F1572–F1581. [Google Scholar] [CrossRef] [PubMed]
- Rognstad, R.; Katz, J. Gluconeogenesis in the kidney cortex: Quantitative estimation of carbon flow. J. Biol. Chem. 1972, 247, 6047–6054. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Li, Y.; Chen, J.; Lin, W. Recent advances in engineered nanomaterials for acute kidney injury theranostics. Nano Res. 2021, 14, 920–933. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Eftekhari, A.; Mammadova, S.; Ahmadian, E.; Ardalan, M.; Davaran, S.; Nasibova, A.; Khalilov, R.; Valiyeva, M.; Mehraliyeva, S.; et al. Nanomaterials for Chronic Kidney Disease Detection. Appl. Sci. 2021, 11, 9656. [Google Scholar] [CrossRef]
- Wernert, V.; Schäf, O.; Faure, V.; Brunet, P.; Dou, L.; Berland, Y.; Boulet, P.; Kuchta, B.; Denoyel, R. Adsorption of the uremic toxin p-cresol onto hemodialysis membranes and microporous adsorbent zeolite silicalite. J. Biotechnol. 2006, 123, 164–173. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Zhan, H.; Chu, Y.; Sun, B. Mechanistic Understanding of the Engineered Nanomaterial-Induced Toxicity on Kidney. J. Nanomater. 2019, 2019, 2954853. [Google Scholar] [CrossRef]
- Wu, S.; Yue, P.; Ma, Y.; Zou, Y.; Liang, W.; Ye, Q. Hemoperfusion adsorbents for removal of common toxins in liver and kidney failure: Recent progress, challenges, and prospects. Adv. Mater. 2023, 37, 2305152. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, R.; Zhao, W.; Zhao, C. Bilirubin removal by polymeric adsorbents for hyperbilirubinemia therapy. Macromol. Biosci. 2023, 23, 2200567. [Google Scholar] [CrossRef]
- Qiao, D.; Zhai, T.; Wang, J.; Liu, J.-M.; Deng, Q.; Wang, S. Highly selective and sustainable hemoperfusion adsorbents for effectively online cleansing bilirubin from plasma. Chem. Eng. J. 2024, 497, 154939. [Google Scholar] [CrossRef]
- Wu, K.; Liu, X.; Li, Z.; Jiao, Y.; Zhou, C. Fabrication of chitosan/graphene oxide composite aerogel microspheres with high bilirubin removal performance. Mater. Sci. Eng. C 2020, 106, 110162. [Google Scholar] [CrossRef]
- Li, Z.; Song, X.; Cui, S.; Jiao, Y.; Zhou, C. Fabrication of macroporous reduced graphene oxide composite aerogels reinforced with chitosan for high bilirubin adsorption. RSC Adv. 2018, 8, 8338–8348. [Google Scholar] [CrossRef]
- Song, X.; Huang, X.; Li, Z.; Li, Z.; Wu, K.; Jiao, Y.; Zhou, C. Construction of blood compatible chitin/graphene oxide composite aerogel beads for the adsorption of bilirubin. Carbohydr. Polym. 2019, 207, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Yu, Y.; Yang, C.; Fan, Q.; Shang, L.; Ye, F. Microfluidic electrospray generation of porous magnetic Janus reduced graphene oxide/carbon composite microspheres for versatile adsorption. J. Colloid Interface Sci. 2022, 624, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, N.R. Functional Blood Chemistry Analysis; CreateSpace Independent Publishing Platform: Charleston, SC, USA, 2013; ISBN 9781492327042/1492327042. [Google Scholar]
- Tienda-Vázquez, M.A.; Morreeuw, Z.P.; Sosa-Hernández, J.E.; Cardador-Martínez, A.; Sabath, E.; Melchor-Martínez, E.M.; Iqbal, H.M.; Parra-Saldívar, R. Nephroprotective plants: A review on the use in pre-renal and post-renal diseases. Plants 2022, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Maduell, F.; Arias-Guillen, M.; Fontseré, N.; Ojeda, R.; Rico, N.; Vera, M.; Elena, M.; Bedini, J.; Wieneke, P.; Campistol, J. Elimination of large uremic toxins by a dialyzer specifically designed for high-volume convective therapies. Blood Purif. 2014, 37, 125–130. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Byvsheva, S.M.; Sozarukova, M.M.; Kottsov, S.Y.; Proskurnina, E.V.; Proskurnin, M.A. High-Throughput Preparation of Uncontaminated Graphene-Oxide Aqueous Dispersions with Antioxidant Properties by Semi-Automated Diffusion Dialysis. Nanomaterials 2022, 12, 4159. [Google Scholar] [CrossRef]
- Fahmi, M.Z.; Wathoniyyah, M.; Khasanah, M.; Rahardjo, Y.; Wafiroh, S. Incorporation of graphene oxide in polyethersulfone mixed matrix membranes to enhance hemodialysis membrane performance. RSC Adv. 2018, 8, 931–937. [Google Scholar] [CrossRef]
- Zareh, M.M.; Ahmed, R.M.; Saleem, N.O.; Abd-ElSattar, A. Graphene oxide versus activated charcoal for La-electrochemical sensor. Mater. Sci. Eng. B 2023, 292, 116389. [Google Scholar] [CrossRef]
- Folaranmi, G.; Bechelany, M.; Sistat, P.; Cretin, M.; Zaviska, F. Activated Carbon Blended with Reduced Graphene Oxide Nanoflakes for Capacitive Deionization. Nanomaterials 2021, 11, 1090. [Google Scholar] [CrossRef]
- Abidin, M.N.Z.; Goh, P.S.; Said, N.; Ismail, A.F.; Othman, M.H.D.; Hasbullah, H.; Abdullah, M.S.; Ng, B.C.; Kadir, S.H.S.A.; Kamal, F. Co-adsorptive removal of creatinine and urea by a three-component dual-layer hollow fiber membrane. ACS Appl. Mater. Interfaces 2020, 12, 33276–33287. [Google Scholar] [CrossRef] [PubMed]
- Kragović, M.; Daković, A.; Marković, M.; Krstić, J.; Gatta, G.D.; Rotiroti, N. Characterization of lead sorption by the natural and Fe (III)-modified zeolite. Appl. Surf. Sci. 2013, 283, 764–774. [Google Scholar] [CrossRef]
- Aksu, Z. Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel (II) ions onto Chlorella vulgaris. Process Biochem. 2002, 38, 89–99. [Google Scholar] [CrossRef]
- Zou, W.; Han, R.; Chen, Z.; Jinghua, Z.; Shi, J. Kinetic study of adsorption of Cu (II) and Pb (II) from aqueous solutions using manganese oxide coated zeolite in batch mode. Colloids Surf. A Physicochem. Eng. Asp. 2006, 279, 238–246. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, X.; Hu, Z.; Yu, M.; Fu, J.; Huang, Y. Fabrication of a novel nitrogen-containing porous carbon adsorbent for protein-bound uremic toxins removal. Mater. Sci. Eng. C 2021, 121, 111879. [Google Scholar] [CrossRef]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Yang, X.; Chen, Q.; Zheng, W.; Shen, J.-W.; Guo, Y. A review: Current urea sorbents for the development of a wearable artificial kidney. J. Mater. Sci. 2024, 59, 11669–11686. [Google Scholar] [CrossRef]
- Moreno-Castilla, C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 2004, 42, 83–94. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Caggiano, G.; Pesce, F.; Colabufo, N.A.; Colangiulo, S.; Amodio, L.; Gesualdo, L. MO571: Novel Insights Into Uremic Toxins in CKD: Rapid Detection of Microbiota-Derived Indoxyl Sulfate in CKD Patients. Nephrol. Dial. Transplant. 2022, 37 (Suppl. S3), gfac074.016. [Google Scholar] [CrossRef]
- Falconi, C.A.; Junho, C.V.D.C.; Fogaça-Ruiz, F.; Vernier, I.C.S.; Da Cunha, R.S.; Stinghen, A.E.M.; Carneiro-Ramos, M.S. Uremic toxins: An alarming danger concerning the cardiovascular system. Front. Physiol. 2021, 12, 686249. [Google Scholar] [CrossRef]
- Wojtaszek, E.; Oldakowska-Jedynak, U.; Kwiatkowska, M.; Glogowski, T.; Malyszko, J. Uremic toxins; oxidative stress, atherosclerosis in chronic kidney disease, and kidney transplantation. Oxidative Med. Cell. Longev. 2021, 2021, 6651367. [Google Scholar] [CrossRef] [PubMed]
- Frąk, W.; Dąbek, B.; Balcerczyk-Lis, M.; Motor, J.; Radzioch, E.; Młynarska, E.; Rysz, J.; Franczyk, B. Role of Uremic Toxins, Oxidative Stress, and Renal Fibrosis in Chronic Kidney Disease. Antioxidants 2024, 13, 687. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, D.; Bansal, B.; Padhye, L.P.; Rachmani, A.; Wright, L.J.; Roberts, G.S.; Baroutian, S. A critical review on current urea removal technologies from water: An approach for pollution prevention and resource recovery. Sep. Purif. Technol. 2023, 314, 123652. [Google Scholar] [CrossRef]
- Asano, T.; Tsuru, K.; Hayakawa, S.; Osaka, A. Bilirubin adsorption property of sol-gel-derived titania particles for blood purification therapy. Acta Biomater. 2008, 4, 1067–1072. [Google Scholar] [CrossRef]
- Liu, J.; Lu, X.; Shu, G.; Ni, F.; Li, K.; Kong, X.; Zheng, S.; Ma, R.; Li, T.; Liu, H. Structure design and performance study on filtration-adsorption bifunctional blood purification membrane. J. Membr. Sci. 2021, 636, 119535. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, Y.; Wei, H.; Jia, L.; Xu, L.; Xie, J. Bilirubin adsorption properties of water-soluble adsorbents with different cyclodextrin cavities in plasma dialysis system. Colloids Surf. B Biointerfaces 2012, 90, 248–253. [Google Scholar] [CrossRef]
- Cai, Q.; Huang, Z.-H.; Kang, F.; Yang, J.-B. Preparation of activated carbon microspheres from phenolic-resin by supercritical water activation. Carbon 2004, 42, 775–783. [Google Scholar] [CrossRef]
- Kobya, M.; Ciftci, C.; Bayramoglu, M.; Sensoy, M. Study on the treatment of waste metal cutting fluids using electrocoagulation. Sep. Purif. Technol. 2008, 60, 285–291. [Google Scholar] [CrossRef]
- Howell, C.A.; Sandeman, S.R.; Phillips, G.J.; Lloyd, A.W.; Davies, J.G.; Mikhalovsky, S.V.; Tennison, S.R.; Rawlinson, A.P.; Kozynchenko, O.P.; Owen, H.L. The in vitro adsorption of cytokines by polymer-pyrolysed carbon. Biomaterials 2006, 27, 5286–5291. [Google Scholar] [CrossRef]
- Niazi, N.S.; Nassar, T.I.; Stewart, I.J.; Honore, P.M.; Sharma, K.; Chung, K.K. A review of extracorporeal blood purification techniques for the treatment of critically ill coronavirus disease 2019 patients. Asaio J. 2022, 68, 1219–1227. [Google Scholar] [CrossRef]
- Li, A.; Li, W.; Hao, F.; Wang, H. Early stage blood purification for paraquat poisoning: A multicenter retrospective study. Blood Purif. 2016, 42, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tijink, M.S.; Wester, M.; Sun, J.; Saris, A.; Bolhuis-Versteeg, L.A.; Saiful, S.; Joles, J.A.; Borneman, Z.; Wessling, M.; Stamatialis, D.F. A novel approach for blood purification: Mixed-matrix membranes combining diffusion and adsorption in one step. Acta Biomater. 2012, 8, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Zhao, W.; Zhao, C. Simple emulsion template method towards self-anticoagulant and high-efficiency carboxymethyl chitosan-based adsorbent for low-density lipoprotein from whole blood. J. Colloid Interface Sci. 2023, 631, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Tijink, M.; Kooman, J.; Wester, M.; Sun, J.; Saiful, S.; Joles, J.; Borneman, Z.; Wessling, M.; Stamatialis, D. Mixed matrix membranes: A new asset for blood purification therapies. Blood Purif. 2014, 37, 1–3. [Google Scholar] [CrossRef]
- Rosiński, S.; Lewińska, D.; Piątkiewicz, W. Application of mass transfer coefficient approach for ranking of active carbons designed for hemoperfusion. Carbon 2004, 42, 2139–2146. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Schulman, G.; Vanholder, R.; Niwa, T. AST-120 for the management of progression of chronic kidney disease. Int. J. Nephrol. Renov. Dis. 2014, 7, 49–56. [Google Scholar] [CrossRef]
- Niwa, T. The role of carbon adsorbent in the conservative management of chronic kidney disease. Panminerva Medica 2016, 59, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kee, Y.K.; Han, S.Y.; Kang, D.-H.; Noh, J.W.; Jeong, K.H.; Kim, G.-H.; Kim, Y.W.; Kim, B.S. Comparison of different types of oral adsorbent therapy in patients with chronic kidney disease: A multicenter, randomized, phase IV clinical trial. Yonsei Med. J. 2021, 62, 41. [Google Scholar] [CrossRef]
- Katona, B.G.; Siegel, E.G.; Cluxton, R.J., Jr. The new black magic: Activated charcoal and new therapeutic uses. J. Emerg. Med. 1987, 5, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yan, X.; Sun, L.; Yang, T.; Hu, X.; He, Z.; Liu, F.; Liu, X. Research progress on extraction, biological activities and delivery systems of natural astaxanthin. Trends Food Sci. Technol. 2019, 91, 354–361. [Google Scholar] [CrossRef]
- Chang, T.M. Clinical experience with ACAC coated charcoal hemoperfusion in acute intoxication. Clin. Toxicol. 1980, 17, 529–542. [Google Scholar] [CrossRef]
- Easom, J.M.; Lovejoy, F.H., Jr. Efficacy and safety of gastrointestinal decontamination in the treatment of oral poisoning. Pediatr. Clin. North Am. 1979, 26, 827–836. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]

| Various Blood Perfusion Treatment | Description | Substances Removed | Advantages | Limitations/Disadvantages | References |
|---|---|---|---|---|---|
| Hemodialysis | Purifying blood indirectly using a device that filters out waste products. | - Small Molecular Weight - Water-soluble substances - Low removal of protein-bound solutes | - Widely available, well-established treatment that can be performed in hospital or at home (flexible). - Effective at removing small, water-soluble uremic toxins and excess fluid. | - Limited in removing larger protein-bound toxins - Associated with high morbidity and mortality rates. - Requires frequent sessions, impacting quality of life. - Time-consuming (3–5 h per session, multiple times a week). - May not replace all kidney functions Potential for adverse reactions and complications. | [13,55,56,57,58] |
| Hemoperfusion | Using direct carbon contact with blood to remove toxins via an extracorporeal circuit. | - Middle molecular weight & protein-bound uremic toxins - Substances adsorbed on activated carbon | - Enhances removal of medium to large uremic toxins, improving patient outcomes. - When combined with hemodialysis (HP + HD), adds adsorption to diffusive/convective clearance for broader toxin coverage. - Reduces complications associated with long-term dialysis. | - Limited availability and higher costs. - Short-term procedure, often requiring repeated treatments. | [17,57,59,60,61,62,63,64] |
| Oral treatment | Removing toxins from the digestive system | Hepatically metabolized substances | - Non-invasive and convenient for patients. - Can slow disease progression and address complications like hyperkalemia or anemia with specific drugs. | - Effectiveness depends on the stage of kidney disease. - Limited to managing symptoms and slowing progression, not a replacement for kidney function. | [13,52,53,57] |
| Various Carbon | Advantages | Limitations/Disadvantages | References |
|---|---|---|---|
| Activated Carbon | - Good removal efficiency - High specific area - low cost - Surface activity - Can be easily modified | - Limited hemocompatibility - Difficult to remove after dosing - Temperature sensitive | [48,68,69,71,78] |
| Graphene Oxide (GO) | - Low cost - Easy to modify surface activity - Water dispensability - Polar functionalization | - Surface random functionalization - Limited hemocompatibility - Lower electrical and thermal conductivity - Poor control on post-preparation functionalization | [23,79,80,81,82] |
| Activated Carbon Type | Physical Characteristics | Typical Applications | References |
|---|---|---|---|
| Powdered-activated carbon(PAC) | Particle size <1.0 mm, typically between 0.15 and 0.25 mm | Rapid adsorption due to high surface area | [56,91] |
| Granular Activated Carbon (GAC) | Large particle size than PAC; lower external surface | Adsorption in liquid and vapor phases | [56,92] |
| Extruded activated carbon (EAC) | cylindrical shape, sizes ranging from 0.8 to 45 mm | Primarily used in gas-stage applications | [56,91,92] |
| Bead activated carbon (BAC) | spherical shape; smaller size than EAC | Fluidized bed applications | [92] |
| Impregnated coated carbon(ICC) | permeable structure | Air pollution control | [56,91] |
| Polymer coated carbon (PCC) | Biocompatible polymer coating, smooth and porous without blocking pores | Medical applications, e.g., hemoperfusion | [56,92] |
| Definition of Activating Agents | Compounds Included in the Precursor Formulation for Producing Activated Carbon (AC) | References |
|---|---|---|
| Common Activating Agents | KOH, ZnCl2, H3PO4, NaOH, Ca (OH)2, K2CO3, FeCl3. | [56,92,108,109] |
| Functions of Activating Agents | - Promote pore formation in AC. - Serve as dehydrating agents to capture moisture. - Stabilize the final product by facilitating intermolecular force arrangement. - Elevate activation temperatures and micropore volumes. | [56,92,108,109] |
| Chemical Activation Method | Single-step preparation involving soaking the carbonaceous precursor in a dehydrating agent followed by activation at high temperatures under an inert atmosphere. | [56,92,108,109] |
| Alternative Activating Agents | Alkali metal carbonates (e.g., K2CO3) and alkali earth metal salts (e.g., FeCl3, ZnCl2) can replace alkali metal hydroxides due to their corrosive nature. | [56,92,108,109] |
| Limitations of Certain Agents | - ZnCl2: Not suitable for pharmaceutical and food industries due to contamination risks. - H2SO4 and H3PO4: Toxicity and high costs limit their use. | [56,92,108,109] |
| Effect of Alkali Metal Carbonates | Different alkali metal carbonates (Li2CO3, Na2CO3, K2CO3, Rb2CO3, Cs2CO3) show a direct relationship between the agent used and the surface area of resulting AC. | [56,92,108,109] |
| Advantages of Chemical Activation | - Lower activation temperature. - Single-step operation. - Shorter drying treatment. - Higher carbon content. | [56,92,108,109] |
| Disadvantages of Chemical Activation | - High cost of activating agents. - Requires additional washing steps. | [56,92,108,109] |
| Physical Characteristics of Activated Carbon | - Smooth, non-homogenous granules. - Average size: 0.5–1.0 mm. - Large surface area. | [56,92,108,109] |
| Phenol Formaldehyde Activated Carbon (PFAC) | - Coarse-mesh charcoal. - Cinder content < 0.05%. - Synthesized by carbonization of phenol formaldehyde resin. | [56,92,108,109] |
| Topics | Details | Challenges & Solutions | References |
|---|---|---|---|
| Toxins in CKD | - Healthy kidneys remove metabolic by-products. - Toxins categorized into small, middle, and protein-bound uremic toxins. | - Accumulation of toxins in CKD leads to systemic complications. | [73,179,183,184,185,186,187] |
| Toxins in Dialysis Urea | - Easily removed in conventional single-pass hemodialysis. | - Difficult to remove in closed-loop WAK systems. - Not representative of all uremic solutes. | [106,136,138,174,188] |
| Uremic | - Creatinine is a major uremic toxin. - Leads to endothelial and immune dysfunction. | - Conventional dialysis inadequate for all uremic toxins. | [1,3,10,17,47,49,125] |
| Bilirubin | - Excessive bilirubin leads to multiorgan dysfunction. | - Various adsorbents developed for removal. - Chitosan/Graphene oxide aerogels show promise. | [67,163,165,189,190,191] |
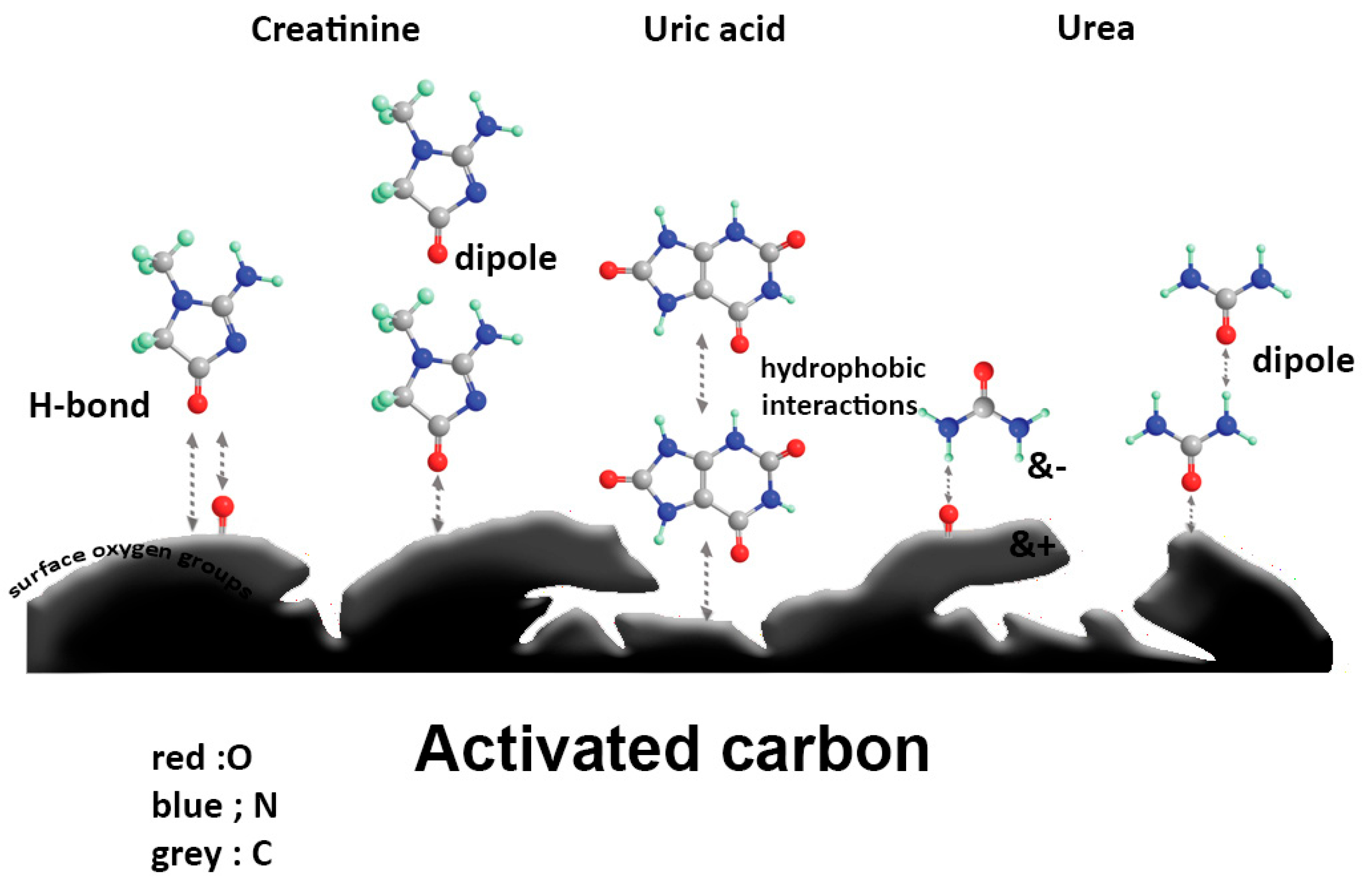
| Molecule | Primary Interaction | Additional Interactions | Activation Energy (kJ/mol) | Multilayer Adsorption | Time to Reach Equilibrium | References |
|---|---|---|---|---|---|---|
| Urea | Dipole–dipole | H-bonding, surface oxygen groups | from –50.6 to –70.1 | Yes | Not specified | [69,70,71] |
| Creatinine | Van der Waals | H-bonding, dipole-induced dipole, surface oxygen groups | −4.9 | Yes | Not specified | [70,71] |
| Bilirubin | π-π, electrostatic | H-bonding, hydrophobic | 17.73 | Not specified | <120 min | [13,69,162] |
| Uric Acid | Hydrophobic | Van der Waals | 14.2 | Yes | Not specified | [70,181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozaffari, A.; Alimohammadi, F.; Parvinzadeh Gashti, M. Functional Carbon-Based Materials for Blood Purification: Recent Advances Toward Improved Treatment of Renal Failure and Patient Quality of Life. Bioengineering 2025, 12, 893. https://doi.org/10.3390/bioengineering12080893
Mozaffari A, Alimohammadi F, Parvinzadeh Gashti M. Functional Carbon-Based Materials for Blood Purification: Recent Advances Toward Improved Treatment of Renal Failure and Patient Quality of Life. Bioengineering. 2025; 12(8):893. https://doi.org/10.3390/bioengineering12080893
Chicago/Turabian StyleMozaffari, Abolfazl, Farbod Alimohammadi, and Mazeyar Parvinzadeh Gashti. 2025. "Functional Carbon-Based Materials for Blood Purification: Recent Advances Toward Improved Treatment of Renal Failure and Patient Quality of Life" Bioengineering 12, no. 8: 893. https://doi.org/10.3390/bioengineering12080893
APA StyleMozaffari, A., Alimohammadi, F., & Parvinzadeh Gashti, M. (2025). Functional Carbon-Based Materials for Blood Purification: Recent Advances Toward Improved Treatment of Renal Failure and Patient Quality of Life. Bioengineering, 12(8), 893. https://doi.org/10.3390/bioengineering12080893






