Abstract
Muscle stem cells (MuSCs) are essential for skeletal muscle regeneration, influenced by a complex interplay of mechanical, biochemical, and molecular cues. Properties of the extracellular matrix (ECM) such as stiffness and alignment guide stem cell fate through mechanosensitive pathways, where forces like shear stress translate into biochemical signals, affecting cell behavior. Aging introduces senescence which disrupts the MuSC niche, leading to reduced regenerative capacity via epigenetic alterations and metabolic shifts. Transplantation further challenges MuSC viability, often resulting in fibrosis driven by dysregulated fibro-adipogenic progenitors (FAPs). Addressing these issues, scaffold designs integrated with pharmacotherapy emulate ECM environments, providing cues that enhance graft functionality and endurance. These scaffolds facilitate the synergy between mechanotransduction and intracellular signaling, optimizing MuSC proliferation and differentiation. Innovations utilizing human pluripotent stem cell-derived myogenic progenitors and exosome-mediated delivery exploit bioactive properties for targeted repair. Additionally, 3D-printed and electrospun scaffolds with adjustable biomechanical traits tackle scalability in treating volumetric muscle loss. Advanced techniques like single-cell RNA sequencing and high-resolution imaging unravel muscle repair mechanisms, offering precise mapping of cellular interactions. Collectively, this interdisciplinary approach fortifies tissue graft durability and MuSC maintenance, propelling therapeutic strategies for muscle injuries and degenerative diseases.
1. Introduction
Muscle stem cells (MuSCs) are central to skeletal muscle regeneration, playing an indispensable role in maintaining the tissue’s structural integrity and function. These cells are responsive to an intricate network of mechanical, biochemical, and molecular signals [1,2]. The regenerative capacity of skeletal muscle hinges on MuSCs’ ability to sense and adapt to their microenvironment, ensuring efficient repair and sustained functionality [3].
Biomaterial scaffolds have emerged as pivotal tools in enhancing MuSC function and muscle regeneration. These scaffolds not only provide structural support but also mimic the native extracellular matrix (ECM), facilitating cell adhesion, proliferation, and differentiation [4]. Various biomaterials, such as natural polymers like collagen and synthetic polymers like polylactic acid (PLA), have been utilized to create scaffolds that promote MuSC attachment and growth [5]. Moreover, these scaffolds can be functionalized with bioactive molecules to enhance myogenic differentiation and tissue regeneration [6].
Mechanosensitive pathways are critical in regulating MuSC behavior, where mechanical forces like shear stress, tensile stress, and cyclic stretching are converted into biochemical signals that influence key cellular processes such as migration, differentiation, and ECM organization [7,8]. These mechanical cues are integrated by the cytoskeleton, which plays a vital role in cellular adaptation and functionality. Biomaterial scaffolds can be engineered to present specific mechanical properties and topographical cues that modulate mechanotransduction pathways in MuSCs [9]. For instance, scaffolds with aligned nanofibers have been shown to direct MuSC alignment and promote myogenic differentiation [10]. Additionally, meso-scale topological cues activate mechanotransduction pathways, particularly through focal adhesion kinase (FAK) and cytoskeletal proteins, driving MuSC activation and enhancing ECM production [11].
Epigenetic modifications tightly regulate MuSC states, particularly during quiescence and activation. The hypermethylation of genes involved in cell cycle progression and differentiation maintains MuSC dormancy, while demethylation upon injury triggers their activation [12]. Histone modifications also play a crucial role, with acetylation facilitating the expression of myogenic regulatory factors like MyoD, while deacetylation by HDACs leads to gene repression, maintaining quiescence [13]. The balance of histone methylation marks, such as H3K4me3 (promoting transcription) and H3K27me3 (repressing genes), is crucial for proper MuSC function during regeneration [14]. Moreover, chromatin remodelers like the SWI/SNF complex adjust DNA accessibility, further regulating MuSC activation [15]. Metabolic pathways also influence MuSC behavior, with glutamine playing a key role in supporting the TCA cycle, enhancing both self-renewal and regenerative potential [16].
Aging significantly impairs MuSC function, characterized by a reduction in MuSC numbers and a decline in their self-renewal capacity. This decline severely limits the muscle’s ability to repair and regenerate post-injury [17]. Cellular senescence further exacerbates this problem, reducing the MuSC pool and impairing muscle repair mechanisms [18]. The aged MuSC niche undergoes detrimental changes, including dysfunctional fibro/adipogenic progenitors (FAPs), increased fibrosis, and a rise in adipogenesis [19]. Aged MuSCs also exhibit altered metabolic activity, shifting from glycolysis to a greater reliance on oxidative phosphorylation, which leads to increased reactive oxygen species (ROS) levels and oxidative damage [20]. This accumulation of ROS not only reduces the regenerative capacity of MuSCs but also heightens their susceptibility to apoptosis. Ref. [21] explores how excessive ROS induces apoptosis in fibroblasts by mitochondrial dysfunction, which parallels how ROS accumulation in MuSCs can reduce their regenerative capacity and increase apoptosis susceptibility. Ref. [22] discusses how high glucose levels trigger ROS generation, leading to mitochondrial dysfunction and apoptosis, relevant to the impact of ROS on MuSCs.
To mitigate the detrimental effects of ROS on MuSCs, biomaterials with antioxidant properties have been developed. For example, scaffolds incorporating antioxidant molecules like melatonin can scavenge excess ROS, thereby enhancing MuSC survival and function [23]. Additionally, hydrogels loaded with ROS-scavenging nanoparticles can modulate the oxidative environment, promoting muscle regeneration in aged tissues [24]. Conversely, controlled ROS generation from biomaterials can be utilized to trigger signaling pathways that promote MuSC activation and differentiation [25,26].
Pax7 is a paired box transcription factor that serves as a pivotal regulator of muscle stem cells (MuSCs). Highly expressed in quiescent MuSCs, Pax7 is essential for maintaining the myogenic lineage and self-renewal capacity of these cells [27]. It functions by activating target genes that promote MuSC survival and by repressing differentiation-inducing factors, thus preserving the stem cell pool required for effective muscle regeneration [28]. Research indicates that Pax7 expression levels fluctuate during MuSC activation and differentiation, with elevated levels sustaining quiescence and self-renewal, while downregulation corresponds with commitment to myogenic differentiation [29]. Understanding the regulation of Pax7 is critical for developing strategies to manipulate MuSC fate decisions, offering potential therapeutic avenues for muscle repair and regeneration.
Several signaling pathways are integral to MuSC regulation, controlling their quiescence, activation, and differentiation (Figure 1). The Notch pathway is pivotal in maintaining quiescence by preventing premature differentiation and the expression of myogenic factors like MyoD [30]. Wnt signaling, particularly in its non-canonical form, can inhibit myogenic differentiation, highlighting its complex role in MuSC regulation [31]. The Hedgehog signaling pathway supports MuSC activation and differentiation into myogenic lineages, with disruptions potentially leading to impaired regeneration and increased fibrosis [32]. Furthermore, the PI3K/Akt pathway, activated by insulin-like growth factor 1 (IGF-1), promotes MuSC proliferation and differentiation, with IGF-1 delivery enhancing muscle regeneration and recovery [33]. METTL3, through the regulation of the Notch pathway, further supports stemness and maintains MuSC states [34].
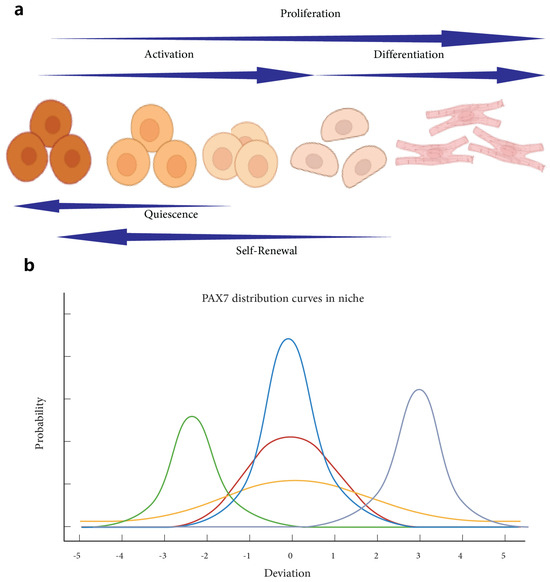
Figure 1.
Understanding muscle stem cell states and PAX7 distribution. Understanding Muscle Stem Cell States: Quiescence, Activation, Proliferation, Differentiation, and Self-Renewal. Panel (a) illustrates the dynamic transition of muscle stem cells through various states, starting from quiescence, progressing through activation and proliferation, leading to differentiation, and the potential for self-renewal [29]. The schematic emphasizes the directional flow and sequential nature of these states, with each phase contributing to muscle regeneration and maintenance. Panel (b) presents PAX7 distribution curves within the niche, highlighting the variability and shifts in PAX7 expression levels during different states. The distribution curves illustrate how PAX7 expression peaks and shifts as muscle stem cells transition between quiescence, activation, and other states, reflecting their changing roles and regulatory mechanisms within the cellular environment [35]. Together, these panels provide a comprehensive overview of muscle stem cell state transitions and the critical role of PAX7 as a marker modulating these processes.
2. Challenges in Muscle Regeneration
2.1. Volumetric Muscle Loss (VML)
Volumetric muscle loss (VML) presents significant challenges in muscle regeneration, primarily due to the extensive damage it causes to both muscle tissue and its microenvironment. Agent-based modeling has underscored the critical role of the MuSC microenvironment in failed regeneration, suggesting that an in-depth understanding of these environmental dynamics is essential for developing effective treatments [36]. VML injuries trigger a complex inflammatory response, which initially aids in recruiting and activating progenitor cells. However, when this inflammation becomes chronic, it can hinder key regenerative processes like myofiber bridging and myotendinous junction formation, thereby limiting overall muscle regeneration [37]. Studies using murine models have shown that VML defects in muscles like the tibialis anterior can lead to significant motoneuron axotomy and a reduction in neuromuscular junction (NMJ) formation, outcomes that are closely linked to the depletion of MuSCs [38]. Current therapeutic strategies for VML involve both cellular and acellular approaches aimed at restoring lost tissue and function, with decellularized ECM scaffolds offering a promising option due to their ability to support host cell infiltration and tissue regeneration, along with advantages like scalability and cost-effectiveness [39,40].
2.2. Fibrosis and Scar Formation
Fibrosis and scar formation are major obstacles in the effective regeneration of muscle tissue following VML injuries. Fibroblast-mediated collagen deposition, while essential for initial wound stabilization, often results in the formation of non-functional scar tissue that impedes muscle regeneration [41]. Collagen I, primarily deposited by fibroblasts, dominates the healing response and hampers the restoration of functional muscle tissue [42]. The challenge lies in balancing fibroblast activity and the inflammatory response to promote functional muscle repair while minimizing fibrosis. Innovative scaffold designs and therapeutic strategies are being explored to achieve this balance, aiming to reduce scar tissue formation and enhance the overall quality of muscle regeneration [43]. However, fibroblast-mediated scar formation remains a significant complication, creating a fibrotic environment that further hinders effective muscle repair [40].
2.3. Immune Response and Inflammation
The immune response plays a dual role in muscle regeneration, initially supporting tissue repair but potentially leading to chronic inflammation if not properly regulated. Chronic inflammation, particularly in the context of aging, disrupts the MuSC niche, resulting in impaired MuSC function and diminished regenerative capacity [44]. During the regeneration process, the immune response must transition from a pro-inflammatory to an anti-inflammatory phase. This involves macrophages shifting from an M1 phenotype, which is pro-inflammatory, to an M2 phenotype, which supports tissue repair. Regulatory T cells (Tregs) also play a crucial role in this phase [45]. Fibro/adipogenic progenitors (FAPs) contribute to muscle regeneration by depositing ECM collagen, which provides the structural support necessary for the alignment and fusion of myogenic cells (Figure 2) [46]. Anti-inflammatory drugs are being explored as a means to promote a pro-regenerative environment in VML injuries by reducing chronic inflammation, which otherwise inhibits critical processes like myofiber bridging [37].
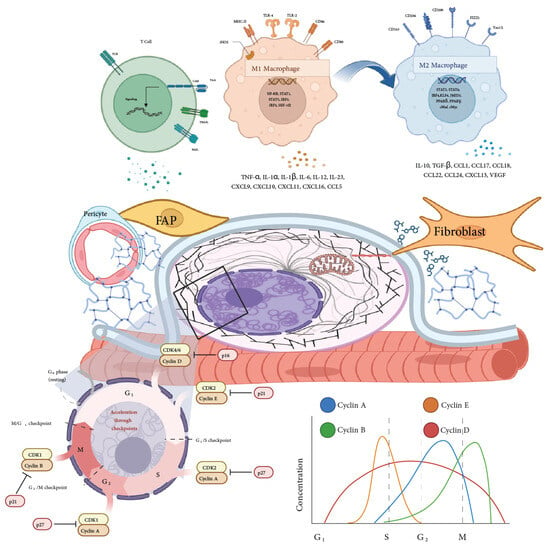
Figure 2.
Cellular interactions and regulatory mechanisms in the muscle stem cell niche. This figure depicts the complex interplay among various cellular components of the muscle stem cell niche, including fibro-adipogenic progenitors (FAPs), pericytes, fibroblasts, and macrophages (M1 and M2 phenotypes). The top section illustrates the role of macrophages, showing the transition from pro-inflammatory M1 macrophages, which secrete cytokines like TNF-α and IL-1β, to anti-inflammatory M2 macrophages involved in tissue repair and regeneration, producing factors such as IL-10 and TGF-β. Adjacent panels highlight interactions with T cells and their influence on the local inflammatory response. The central portion emphasizes cellular and extracellular matrix (ECM) interactions mediated by FAPs, pericytes, and fibroblasts, influencing the structural and regenerative dynamics of the muscle niche. The figure also outlines cell cycle regulation, detailing checkpoints and the role of cyclins (Cyclin A, B, D, E) and cyclin-dependent kinases (CDKs) in governing progression through G1, S, G2, and M phases. The accompanying graph on cyclin and CDK concentration during the cell cycle phases provides a visual representation of how cyclin–CDK complexes fluctuate, coordinating MuSC proliferation and differentiation [47]. Specific cyclin–CDK complexes’ elevated levels promote cell cycle progression, while their inhibition leads to cell cycle arrest, thus tightly regulating MuSC proliferation within the niche [48]. Together, these elements underscore the regulatory pathways and cellular interactions that maintain muscle homeostasis and regeneration within the niche.
2.4. Cellular Senescence and Its Impact
Cellular senescence poses a significant challenge in muscle regeneration, particularly in the context of aging and chronic injury. Senescent cells secrete pro-inflammatory cytokines and matrix-degrading enzymes that exacerbate tissue damage and inhibit regeneration, contributing to a detrimental environment for muscle repair [49]. Therapeutic strategies targeting senescent cells, known as senolytics, show promise in enhancing muscle regeneration. For instance, Dasatinib, a tyrosine kinase inhibitor, selectively induces apoptosis in senescent cells and, when combined with Quercetin, an anti-inflammatory flavonoid, improves muscle function and regeneration in aged mice [50]. Another promising senolytic agent is Navitoclax, which targets BCL-2 family proteins involved in cell survival. However, its clinical use is limited due to potential side effects such as thrombocytopenia [37]. Modulating the immune environment by promoting macrophage polarization towards the M2 phenotype through cytokines like IL-4 and IL-13 is another strategy being investigated, as it can create a more favorable environment for muscle regeneration [46].
3. Microenvironment and Extracellular Matrix (ECM)
The microenvironment, or niche, refers to the local cellular environment surrounding MuSCs, encompassing not only the extracellular matrix (ECM) but also neighboring cells, soluble factors, and physical conditions [51]. The ECM is a vital component of this microenvironment, comprising a complex network of proteins and polysaccharides that provide structural support and biochemical cues to cells [52]. While the ECM focuses on the non-cellular structural components, the microenvironment includes all elements that influence MuSC behavior, such as growth factors, cytokines, cell–cell interactions, and mechanical stimuli [53]. The key difference between the microenvironment and the ECM lies in their scope: the ECM is a structural and biochemical scaffold, whereas the microenvironment is a broader concept that integrates the ECM with other factors affecting stem cell fate (Figure 3).
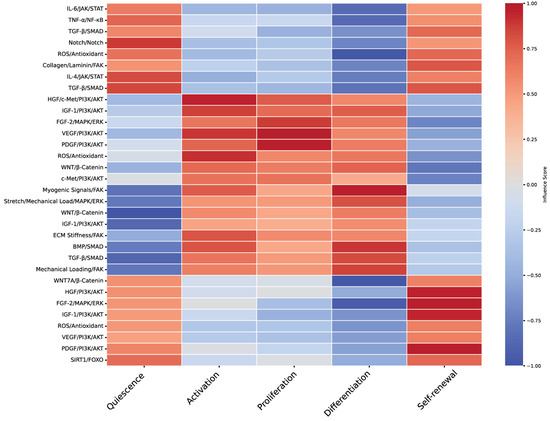
Figure 3.
Heatmap of molecular pathways influencing muscle stem cell fates. This heatmap provides a visualization of the correlation between various molecular pathways and muscle stem cell fates, based on a qualitative exploratory review of relevant literature. The pathways considered include IL-6/JAK/STAT, TNF-α/NF-κB, TGF-β/SMAD, Notch, ROS/antioxidant mechanisms, and others. Muscle stem cell fates—quiescence, activation, proliferation, differentiation, and self-renewal—are represented along the x-axis, while molecular pathways are listed on the y-axis. The color gradient reflects the influence score of each pathway, with red hues indicating a positive correlation and blue hues representing a negative correlation. This visualization highlights how different pathways modulate specific cellular states, providing insight into the complex regulatory networks governing muscle stem cell behavior. The heatmap underscores the critical interplay of signaling cascades that dictate stem cell responses and tissue regeneration, as referenced in Table 1.
3.1. ECM’s Role in Muscle Regeneration
The extracellular matrix (ECM) plays a critical role in muscle regeneration, particularly in the context of volumetric muscle loss (VML) injuries. These injuries severely compromise or destroy the basement membrane, which is essential for guiding regenerative processes [54]. When the ECM is lost in such injuries, the ability of muscle stem cells (MuSCs) to migrate and fuse into myotubes is significantly impaired. This results in MuSCs migrating laterally outside the disrupted basement membrane, which hinders effective muscle repair [55]. The MuSC microenvironment, or niche, is vital for maintaining stemness, with ECM components and cytokines providing the necessary support for MuSC maintenance and activation [56]. Thus, muscle regeneration is a complex process influenced by the interplay between the MuSC microenvironment, signaling pathways, and ECM components [57].
3.2. Mechanical and Biochemical Cues in the ECM
Mechanical and biochemical cues within the ECM are fundamental in regulating stem cell behavior. Mechanical forces are key to tissue patterning, converting physical cues from the environment into biochemical signals that regulate stem cell migration, differentiation, and ECM organization [58]. The ECM not only provides structural support but also delivers biochemical signals that guide stem cell fate. The cytoskeleton integrates these signals, ensuring proper cellular adaptation and functionality [59]. Focal adhesions serve as the connection between the ECM and the cytoskeleton, transmitting mechanical signals that influence gene expression and cellular responses [60]. Additionally, surface features at the microscale and nanoscale can guide cell behavior by affecting cytoskeletal alignment and focal adhesion dynamics, which in turn influence stem cell differentiation and tissue integration [61].
3.3. Influence of ECM Stiffness and Structure
The stiffness and structural composition of the ECM significantly influence MuSC fate and muscle regeneration. Glycoproteins, proteoglycans, and collagens within the ECM provide crucial structural support and regulate the availability of growth factors that impact MuSC behavior [62]. Fibronectin and laminins, for example, are essential for maintaining tissue integrity and repair. Fibronectin, in particular, enhances WNT7a activity, which promotes MuSC proliferation and self-renewal [63]. In 3D culture systems, Type I collagen supports myogenic progression, while Type V and VI collagens interact with specific receptors to maintain MuSC quiescence or delay differentiation [64]. The need for scaffolds that retain the 3D architecture of the native ECM is paramount for mimicking the properties of muscle tissue. However, decellularized ECM scaffolds often present challenges due to the unknown specifics of their composition [65].
3.4. Challenges in ECM Restoration
Restoring the ECM in cases of VML injuries presents considerable challenges. The extensive damage to both muscle tissue and the ECM leads to impaired regenerative processes, making effective restoration difficult [65]. The loss of the basement membrane in these injuries disrupts the essential biophysical and biochemical cues required for proper MuSC behavior, resulting in compromised MuSC migration and myotube fusion [64]. Furthermore, fibroblast-mediated collagen deposition and the formation of scar tissue create a fibrotic environment that complicates muscle regeneration [62]. Although innovative therapeutic approaches, including biocompatible scaffolds and pharmacological agents, are being developed to restore the lost ECM environment, challenges remain in achieving long-term stability, functionality, and scalability (Table 1) [63].

Table 1.
Studies on molecular pathways and mechanisms regulating MuSC states. This table summarizes key studies on molecular pathways and mechanisms regulating MuSC states, highlighting the significant proteins, actions, cellular responses, and outcomes involved in these processes.
Table 1.
Studies on molecular pathways and mechanisms regulating MuSC states. This table summarizes key studies on molecular pathways and mechanisms regulating MuSC states, highlighting the significant proteins, actions, cellular responses, and outcomes involved in these processes.
| Ref. | Key Proteins/Factors | Mechanistic Action | Cellular Response | Key Outcome |
|---|---|---|---|---|
| [66] | NUMB, Template DNA | Asymmetric division and cosegregation of template DNA | Maintenance of stem cell self-renewal | Preservation of stem cell pool |
| [67] | Template DNA | Asymmetric division in cancer stem cells | Maintenance of self-renewal capacity | Stem cell self-renewal |
| [68] | WNT7A | WNT signaling pathway activation | Expansion of self-renewing cells | Enhanced stem cell pool expansion |
| [69] | WNT signaling | Regulation of self-renewal and cardiac stem cells | Maintenance and expansion of cardiac stem cells | Regeneration of infarcted myocardium |
| [70] | JAK/STAT | Promotion of symmetric division | Expansion of self-renewing cell population | Increased stem cell pool |
| [71] | JAK/STAT | Maintenance of stem cell quiescence | Promotion of self-renewal | Preservation of stem cell population |
| [72] | RNAPII | Transcriptional regulation | Maintenance of quiescence | Prevention of premature activation |
| [73] | ATR, Cyclin F-SCF | Regulation of genome integrity and cell cycle factors | Preservation of quiescence | Maintenance of stem cell longevity |
| [74] | Cyclin F-SCF | Degradation of cell cycle proteins | Maintenance of quiescence | Stem cell preservation |
| [75] | PI3K/AKT | Rescues quiescence defects | Rejuvenation of aged MuSCs | Restoration of stem cell balance |
| [76] | Notch, miR-708, KLF7 | Delay of cell cycle reentry | Maintenance of stem cell pool | Prevention of premature depletion |
| [77] | MuSK, BMP | Niche-specific signaling | Regulation of myofiber size | Maintenance of quiescence |
| [78] | N-cadherin, M-cadherin | Niche adhesion | Transition from quiescence to activation | Stem cell activation |
| [79] | Cadherins | Cadherin-dependent adhesion | Niche anchorage | Preservation of stem cell readiness |
| [80] | Collagen, Laminin | Structural support through ECM components | Maintenance of homeostasis | Support for tissue regeneration |
| [81] | ECM components | Structural support for tissue regeneration | Maintenance of tissue integrity | Enhanced regeneration capability |
| [82] | H3K27me3 | Regulation of gene expression during myogenesis | Differentiation-dependent gene activation | Promotion of muscle differentiation |
| [83] | H3K4me2, H3K4me3 | Marking of muscle-relevant genes during myogenesis | Promotion of gene expression | Enhanced muscle differentiation |
| [84] | Smad2, LEF1 | Regulation of histone modifications during differentiation | Control of gene expression | Induction of differentiation |
| [85] | eIF2α | Regulation of gene activation | Maintenance of quiescence | Prevention of premature differentiation |
| [86] | H3K27me3 | Maintenance of repressive chromatin state | Preservation of quiescence | Prevention of premature activation |
| [87] | Notch | Prevention of muscle atrophy | Maintenance of quiescence | Prevention of muscle atrophy |
4. Scaffold Design and Engineering
Scaffold design in muscle tissue engineering is fundamentally guided by the need to replicate the native extracellular matrix (ECM) and provide a conducive environment for muscle regeneration [88]. The basic rationale involves creating scaffolds with physical properties that support MuSC adhesion, proliferation, differentiation, and organization into functional muscle tissue [89]. Key physical properties include mechanical strength, elasticity, porosity, and biodegradability, which must be carefully tuned to match those of native muscle tissue [90].
4.1. Scaffold Properties Mimicking ECM
Scaffolds that closely mimic the properties of the extracellular matrix (ECM) are vital in enhancing muscle regeneration. By enabling crosstalk between physical, biological, material, and pharmacological factors, these scaffolds optimize intracellular signaling pathways and cellular responses, thereby improving regenerative outcomes [91]. Integrating scaffold designs with pharmacotherapy presents a promising strategy to overcome transplantation challenges, offering mechanical and biochemical cues that enhance both the functionality and endurance of grafts [92]. Pharmacotherapy, such as growth factors and senolytic drugs, works synergistically with scaffold properties to modulate MuSC behavior, supporting cell survival, proliferation, and differentiation through engagement with key intracellular pathways [93]. Notably, scaffold alignment has been shown to promote proliferation by activating integrin-mediated signaling via the PI3K/Akt pathway [63].
Physical properties such as stiffness and elasticity are critical, as they influence cell behavior through mechanotransduction pathways [94]. Muscle tissue has a specific range of stiffness, and scaffolds must match this to promote proper MuSC differentiation and function [4]. Additionally, the scaffold’s porosity and pore size are important for nutrient diffusion, vascularization, and waste removal, which are essential for cell survival and tissue integration [95,96]. Biodegradability is another key property, allowing the scaffold to gradually degrade as new tissue forms, reducing the need for surgical removal (Figure 4) [97,98,99].
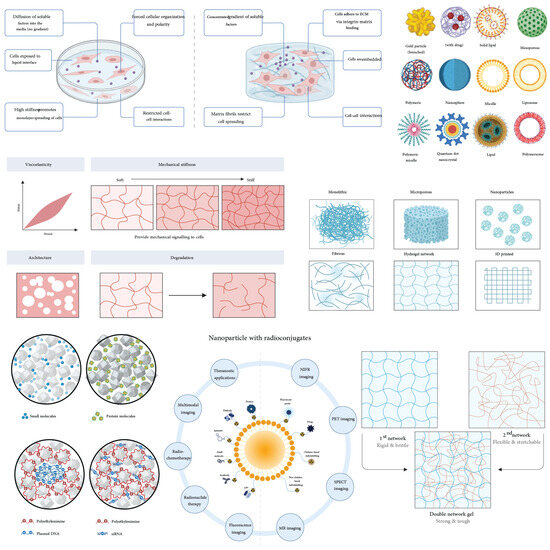
Figure 4.
Scaffold characteristics. The figure provides an overview of critical scaffold properties that influence muscle stem cell function and tissue engineering outcomes. The properties are categorized into structural and functional aspects, including vascularity, architecture, pore size, degradation, mechanical properties, and nano-specific modifications. The upper left panels illustrate how cells interact with scaffolds, emphasizing effects on cellular organization, polarity, and matrix binding, driven by diffusion gradients and cell–extracellular matrix (ECM) interactions. The central portion of the figure demonstrates mechanical properties, ranging from viscoelasticity (depicting a strain-stress curve) to stiffness levels (soft to stiff matrices) that provide mechanical cues to cells, critical for their behavior and differentiation. Adjacent panels depict architectural considerations such as pore size and overall scaffold structure, influencing cell infiltration, tissue integration, and mass transport. Degradation profiles are shown, emphasizing scaffold dissolution kinetics that match tissue regeneration rates. On the right side, nano-specific modifications, including particles (solid lipid, polymeric vesicles, quantum dots, etc.), highlight applications such as drug delivery and enhanced cellular interactions. The bottom panels explore complex architectures, like double-network gels that combine rigid and flexible networks, enhancing mechanical resilience and adaptability in dynamic tissue environments. The integration of nanoparticles with radioconjugates illustrates potential therapeutic and imaging applications in regenerative medicine and diagnostics. The progressive narrative of the figure emphasizes the interplay of scaffold properties with biological, mechanical, and nanoscale features for optimizing tissue regeneration outcomes.
4.2. Integration of Mechanical Cues in Scaffolds
The mechanical forces exerted by scaffold structures play a crucial role in influencing cellular mechanotransduction, which in turn impacts gene expression and protein synthesis—key processes for maintaining MuSC stemness and enhancing the longevity of muscle grafts [100]. The dynamic interplay between mechanical loading and biochemical signaling within the scaffold environment is essential for promoting tissue integration and functional recovery [101]. Conductive nanofibers, for example, have been found to enhance myotube formation during differentiation by activating the MAPK/ERK pathway [102]. Additionally, scaffold stiffness is critical in regulating cell behavior, particularly through the activation of the RhoA/ROCK pathway during differentiation (Figure 5) [103].
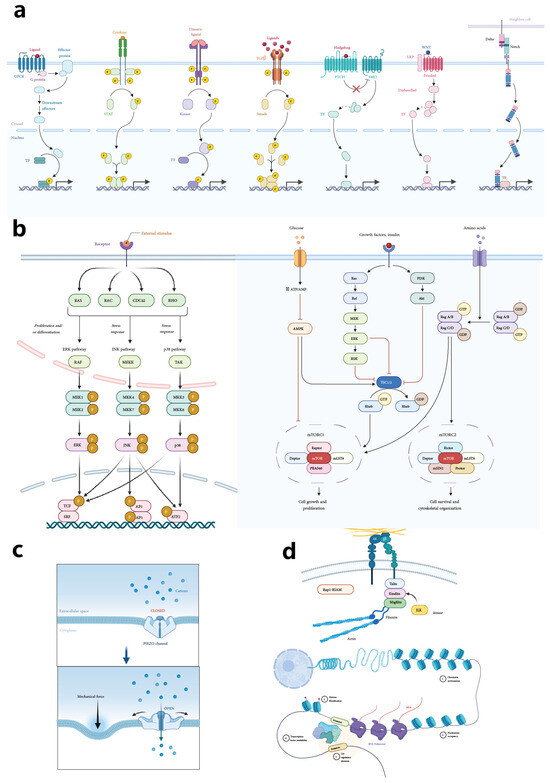
Figure 5.
Mechanisms and pathways in muscle stem cell regulation. This figure encompasses multiple schematic representations that outline critical regulatory mechanisms influencing muscle stem cell behavior and fate. Panel (a) illustrates major signaling pathways, including Wnt, BMP, TGF-β, Notch, and PI3K/AKT pathways, emphasizing the journey from receptor activation at the cell membrane to downstream nuclear transcription factors that modulate gene expression, impacting muscle stem cell states and responses. Panel (b) provides detailed pathway diagrams, showcasing interactions among key regulatory molecules in muscle stem cell signaling. This includes cross-talk between different pathways that guide cell proliferation, differentiation, quiescence, and self-renewal, depicting an integrated regulatory network. Panel (c) highlights the role of mechanical forces, such as shear stress and tensile stress, in triggering cellular mechanotransduction processes, with a specific focus on mechanical receptors and their influence on intracellular pathways. Panel (d) illustrates epigenetic regulatory mechanisms, including DNA methylation, histone modification, and non-coding RNAs, which fine-tune muscle stem cell states by modifying chromatin structure and gene expression, contributing to the maintenance of stem cell quiescence, activation, or differentiation. Together, these panels underscore the complexity and integration of biochemical, mechanical, and epigenetic influences on muscle stem cell regulation.
Materials suitable for muscle tissue engineering include both natural and synthetic polymers. Natural polymers like collagen, gelatin, and fibrin offer excellent biocompatibility and bioactivity, closely resembling the native ECM [104]. However, they may have limited mechanical strength and variable degradation rates. Synthetic polymers such as polylactic acid (PLA), polyglycolic acid (PGA), and polycaprolactone (PCL) provide tunable mechanical properties and degradation rates but may lack cell recognition sites [105]. Combining natural and synthetic materials can leverage the advantages of both, creating composite scaffolds that meet the necessary physical and biological requirements [106].
4.3. Pharmacotherapy Interactions with Scaffolds
Pharmacological interventions aimed at enhancing scaffold durability and preserving muscle youthfulness and stemness are emerging as promising approaches in regenerative medicine [107]. The incorporation of bioactive molecules within scaffolds allows for targeted delivery and sustained release, ensuring the continuous stimulation of MuSCs and bolstering the regenerative capacity of muscle tissues [108]. Furthermore, manipulating immune cells via MSC exosomes within scaffolds can modulate macrophage activity, reducing inflammation and promoting muscle regeneration [109]. MSC-conditioned medium has also been shown to reduce inflammation and fibrosis while promoting the formation of new muscle fibers, particularly in immunocompetent mice [110].
4.4. Advances in Scaffold Manufacturing
Advancements in scaffold manufacturing techniques and material enhancements are critical for overcoming the challenges associated with scaling decellularized ECM scaffolds [111]. Three-dimensional printing technology, in particular, allows for the fabrication of customized scaffolds that can be tailored to match specific injury sites, offering personalized solutions for muscle regeneration [112]. Materials used in 3D printing, such as polylactic acid (PLA), polycaprolactone (PCL), and hydrogels, provide tunable mechanical properties and biocompatibility, making them ideal for muscle scaffold applications [102]. Combining 3D printing with electrospinning techniques can further enhance scaffold design by providing structural support and a microenvironment conducive to effective tissue regeneration [113].
4.5. Novel Materials for Scaffold Design
The development of novel materials for scaffold design focuses on achieving biodegradability and tunable mechanical properties, essential for long-term use in tissue regeneration. Materials such as PLA, PCL, and hydrogels are engineered to possess these qualities, offering the necessary stiffness and elasticity for effective integration [114]. Biomimetic hydrogels, with their high water content and tunable mechanical properties, mimic the physical and biochemical properties of the native ECM, supporting cell growth, proliferation, and differentiation [11]. Dynamic hydrogels, which respond to environmental stimuli like pH or mechanical forces, adapt to the needs of regenerating tissue, thereby enhancing tissue integration [115]. Additionally, hybrid scaffolds that combine hydrogels with electrospun fibers or 3D-printed frameworks provide both mechanical support and biological activity, optimizing the tissue repair process (Table 2) [116].

Table 2.
Summary of scaffold properties in muscle tissue engineering. The table categorizes scaffolds by type, physiological properties, physicochemical properties, and composition.
Table 2.
Summary of scaffold properties in muscle tissue engineering. The table categorizes scaffolds by type, physiological properties, physicochemical properties, and composition.
| Ref. | Scaffold Type | Physiological Properties | Physicochemical Properties | Composition |
|---|---|---|---|---|
| [88] | Poly(glycerol sebacate)-gelatin | Supports cell adhesion and proliferation; promotes muscle regeneration | Biodegradable; tunable mechanical strength and elasticity | Poly(glycerol sebacate), gelatin |
| [117] | Collagen-based scaffold with endothelial cells | Enhances vascularization; supports muscle tissue formation | Biocompatible; mimics native ECM stiffness | Collagen type I, endothelial cells |
| [98] | 3D bioprinted scaffold | Customizable shape for defect site; promotes cell viability | Controlled porosity; mechanical strength suitable for muscle tissue | Polycaprolactone (PCL), bioink with cells |
| [105] | Synthetic polymer scaffold | Supports stem cell differentiation | Adjustable degradation rate; tunable mechanical properties | Polylactic acid (PLA), polyglycolic acid (PGA) |
| [4] | Elastic substrate scaffold | Regulates stem cell self-renewal and differentiation | Elastic modulus matching muscle tissue; biodegradable | Hydrogel substrates of varying stiffness |
| [55,89] | Injectable hydrogel scaffold | Facilitates cell delivery; supports tissue integration | Shear-thinning; self-healing properties | Hyaluronic acid, gelatin methacryloyl |
| [94] | Matrix elasticity-modulated scaffold | Directs stem cell lineage specification | Variable stiffness; elastic properties | Polyacrylamide gel with tunable crosslinking |
| [106] | Composite scaffold | Enhances mechanical strength; supports cell attachment | Biodegradable; controlled porosity | Collagen, hydroxyapatite |
| [95] | Electrospun nanofiber scaffold | Mimics native ECM structure; supports cell infiltration | High surface area; adjustable fiber diameter | Polycaprolactone (PCL) nanofibers |
| [118] | Decellularized ECM scaffold | Provides natural biochemical cues; supports regeneration | Preserved ECM architecture; natural mechanical properties | Decellularized muscle tissue ECM |
5. Emerging Therapies and Technologies
5.1. Pluripotent Stem Cells and Myogenic Progenitors
Human pluripotent stem cells (hPSCs), which can be derived from human embryonic stem cells (hESCs) or induced pluripotent stem cells (iPSCs), hold significant promise for muscle regeneration. These cells can be directed to differentiate into myogenic progenitors using protocols that involve specific small molecules and growth factors, leading to the expression of key markers like Pax7 and MyoD [119]. The differentiation process mirrors embryonic myogenesis, where transcription factors such as Pax3, Pax7, MyoD, and Myf5 guide the cells toward a myogenic fate [102]. Once derived, these myogenic progenitors can be expanded in vitro and transplanted into injured muscle tissue, where they integrate, proliferate, and differentiate into mature muscle fibers [101]. A notable advantage of using iPSC-derived myogenic progenitors is the potential for autologous transplantation, which significantly reduces the risk of immune rejection [93]. However, scalability remains a major challenge, as producing sufficient quantities of myogenic progenitors for clinical applications requires advances in bioreactor technology and scalable culture systems [120].
5.2. Three-Dimensional Printing and Custom Scaffolds
Three-dimensional printing technology has revolutionized scaffold design by enabling precise control over scaffold architecture and the delivery of bioactive cues, making it ideal for creating scaffolds that closely mimic the complex structure of native muscle tissue [121]. By incorporating multiple cell types and bioactive molecules during the 3D printing process, complex tissue constructs that promote cell proliferation and tissue integration can be created [122]. Electrospinning, a complementary technique, produces nanofibrous scaffolds that resemble the ECM of native muscle tissue, offering a high surface area and porosity for cell attachment and nutrient exchange [123]. These electrospun scaffolds can be functionalized with bioactive molecules to enhance cell adhesion and differentiation, and their fiber alignment can effectively guide the regeneration of muscle fibers [124]. Additionally, methods like “Drydux Transduction” have been developed to increase activation efficiency during transduction with viral vectors, further enhancing the functionality of these custom scaffolds [125].
5.3. Nanotechnology and Biomimetic Materials
Nanotechnology is playing an increasingly critical role in developing biomimetic materials that closely replicate the properties of native ECM. Electrospun nanofibrous scaffolds, which mimic the ECM’s structure, offer a high surface area and porosity essential for cell attachment and nutrient exchange, thus supporting muscle tissue regeneration [123]. Biomimetic hydrogels, designed to imitate the physical and biochemical properties of native ECM, provide an optimal environment for cell growth, proliferation, and differentiation due to their high water content and tunable mechanical properties [126]. Dynamic hydrogels, which can respond to environmental stimuli such as pH or mechanical forces, further enhance tissue integration by adapting to the specific needs of regenerating tissues [127]. Additionally, nanotopographical cues delivered via engineered extracellular vesicles (EVs) have shown potential in enhancing proliferation and repairing aged muscle tissue through sequential administration [124]. The combination of 3D printing with electrospinning continues to refine scaffold design, providing both structural support and a conducive microenvironment for effective tissue regeneration [128].
6. Cellular Mechanisms and Metabolic Pathways
6.1. Cytoskeletal Dynamics in Mechanotransduction
The cytoskeleton, a dynamic network composed of actin filaments, microtubules, and intermediate filaments, plays a central role in mechanotransduction—the process by which cells convert mechanical signals into biochemical responses. Focal adhesions serve as crucial connectors between the ECM and the cytoskeleton, transmitting mechanical signals that influence gene expression and cellular behavior [129,130]. The stiffness of the ECM and substrate rigidity initiate signaling pathways such as YAP/TAZ, Rho/ROCK, and FAK, which are key to stem cell mechanotransduction [131]. The cytoskeleton’s unique mechanical properties, including entropic and enthalpic elasticity, allow it to stiffen nonlinearly under shear stress, enhancing cellular resistance to deformation and maintaining structural integrity under mechanical stress [132,133].
6.2. Metabolic Pathways in MuSC Function
Muscle stem cells (MuSCs) rely on distinct metabolic pathways to maintain their function and support muscle regeneration. In their quiescent state, MuSCs primarily utilize oxidative phosphorylation (OXPHOS) to sustain a low metabolic profile. However, upon activation, they shift to glycolysis to meet the increased energy demands required for cell division and biosynthetic activities [134]. Fatty acid oxidation (FAO) is another critical metabolic pathway, providing ATP and acetyl-CoA necessary for energy homeostasis and histone acetylation, with enhanced FAO linked to improved self-renewal and reduced differentiation of MuSCs [135]. Reactive oxygen species (ROS), generated as byproducts of cellular metabolism, serve as signaling molecules that promote MuSC activation at low levels but can cause oxidative stress and impair MuSC function if accumulated excessively [136]. The pentose phosphate pathway (PPP) is vital during MuSC activation, supplying ribose-5-phosphate for nucleotide synthesis and NADPH for reductive biosynthesis and antioxidant defense, supporting anabolic demands and protecting against oxidative stress [137]. Additionally, amino acid metabolism, especially involving branched-chain amino acids (BCAAs), regulates protein synthesis via the mTOR pathway, influencing MuSC growth and differentiation [138].
6.3. Epigenetic Regulation in Muscle Regeneration
Epigenetic mechanisms are crucial in regulating MuSC behavior and muscle regeneration. During quiescence, the hypermethylation of specific genes involved in cell cycle progression and differentiation maintains MuSC dormancy, while demethylation upon muscle injury activates these genes, triggering regeneration [139]. Histone modifications, such as the methylation of H3K4me3 (associated with active transcription) and H3K27me3 (associated with gene repression), play a significant role in regulating gene expression, with the balance between these modifications being crucial for MuSC regeneration [140]. MicroRNAs (miRNAs) like miR-1, miR-206, and miR-133 are also involved, fine-tuning gene expression by regulating mRNA degradation or translational repression, thus influencing MuSC proliferation and differentiation [141]. Long non-coding RNAs (lncRNAs) interact with chromatin-modifying complexes to modulate gene expression, further impacting MuSC function [13]. Chromatin remodelers, such as the SWI/SNF complex, are essential for MuSC activation as they reposition nucleosomes, altering DNA accessibility to transcription factors [142].
6.4. Mechanical Forces and Cellular Adaptation
Mechanical forces exert significant influence on cellular behavior, particularly in muscle development and regeneration. These forces can directly impact gene transcription related to myogenesis, activating transcription factors like MYOD1, which are crucial for processes such as myoblast fusion, myofiber alignment, and overall muscle tissue functionality [133]. Mechanical stimulation also modulates intracellular calcium levels through stretch-activated channels (SACs), further influencing gene transcription and promoting muscle differentiation [143]. In vitro models using materials like acrylamide gels with adjustable stiffness are often employed to study how mechanical and biochemical signals integrate, simulating the mechanical properties of the ECM [140]. Additionally, extrinsic mechanical loading, such as applying force via a four-point bending device, allows researchers to tune substrate stiffness, thereby directly impacting the behavior of cell-seeded substrates and enhancing their regenerative potential [49]. The ECM itself provides essential structural support and biochemical cues that guide stem cell fate, with the cytoskeleton playing a crucial role in integrating these signals to ensure proper cellular adaptation and function [131].
7. Cellular-Based Strategies for Regeneration
7.1. Preloading Scaffolds with Multiple Cell Types
Preloading scaffolds with a variety of cell types, particularly muscle stem cells (MuSCs), has shown significant promise in promoting muscle formation and achieving partial functional restoration in volumetric muscle loss (VML) injuries [144]. These cellular-based strategies typically involve the use of autologous cells to avoid immune rejection, which presents a significant advantage over allogeneic approaches. However, the use of autologous cells necessitates sophisticated manufacturing processes, making cellular-based strategies more complex compared to acellular approaches [90]. Additionally, co-culturing MuSCs with immune cells, such as CD3+ T-cells, has been found to support extensive MuSC expansion, enhancing their therapeutic potential for muscle regeneration [145]. By incorporating a tailored mix of cytokines that reflect the inflammatory milieu, these strategies can sustain MuSC expansion in vitro, further improving their effectiveness. Ref. [76] provides insights into how cytokines like IL-6 and TNF-alpha can be utilized to modulate the inflammatory environment, sustaining MuSC expansion and improving therapeutic outcomes. Ref. [79] highlights the importance of creating a supportive niche for muscle stem cells, which can be enhanced by a cytokine mix.
7.2. Pharmacological Interventions in Muscle Repair
Pharmacological interventions play a crucial role in enhancing muscle repair by modulating the cellular environment and supporting the function of both cellular and acellular therapies. Biomaterial scaffolds, for example, can be engineered to deliver growth factors like IGF-1 and FGF-2 directly to the injury site, stimulating satellite cell proliferation and improving muscle repair outcomes [146]. Additionally, pharmacological agents such as MMP inhibitors are employed to prevent excessive ECM degradation, which is vital for maintaining the structural integrity of biomaterial scaffolds [110]. Another approach involves using MSC-conditioned medium to precondition MuSCs before implantation, thereby improving their regenerative capabilities and enhancing overall muscle repair [147]. These pharmacological strategies are designed to modulate the cellular environment, thereby increasing the effectiveness of therapeutic interventions (Figure 6) [107].
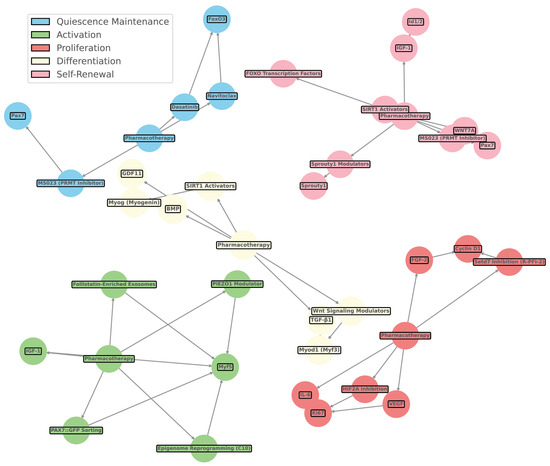
Figure 6.
Network diagram of pharmacotherapy and muscle stem cell pathways. This figure visualizes the integration of pharmacotherapy interventions, such as growth factors, small molecules, and other modulators, with muscle stem cell pathways and markers. Nodes represent key elements of pharmacotherapy and stem cell signaling, while edges illustrate their interactions based on a qualitative exploratory review of the relevant literature. The color-coded nodes indicate distinct muscle stem cell states influenced by pharmacotherapy: blue for quiescence maintenance, green for activation, yellow for proliferation, red for differentiation, and purple for self-renewal. The network emphasizes the complex, multi-faceted approach to muscle regeneration, showcasing how targeted pharmacotherapy influences specific pathways, such as Wnt signaling modulators, SIRT1 activators, BMP signaling, and more. This comprehensive view of pharmacological modulation highlights the interplay between small molecules, growth factors, and muscle stem cell regulatory networks, facilitating targeted therapeutic strategies for muscle repair and regeneration, as referenced in Table 3.
7.3. Integration of Bioactive Molecules
Integrating bioactive molecules into scaffolds is essential for minimizing immune rejection, reducing inflammation, and promoting long-term tissue regeneration. Strategies such as using biocompatible materials, applying surface modifications, and incorporating anti-inflammatory agents into the scaffold help achieve these goals [139]. For instance, seeding decellularized ECM patches with MuSCs or other progenitor cells can enhance muscle repair by improving cell attachment and tissue integration [57]. Additionally, exosomes carrying growth factors like IGF-1 or FGF-2 have been shown to specifically promote MuSC proliferation and differentiation, while exosomes containing anti-inflammatory miRNAs help resolve inflammation, facilitating a conducive environment for tissue repair [99]. The Smad/AKT pathways, activated by follistatin-enriched exosomes from umbilical cord MSCs, also play a critical role in reducing fibrosis and enhancing muscle regeneration [148]. Moreover, modulating Wnt signaling through ASC-exosomes can alter macrophage phenotypes, further enhancing tissue repair through multiple signaling pathways [149].
7.4. Exosome and Immune Modulation
Exosomes derived from mesenchymal stem cells (MSCs) have emerged as a powerful tool for modulating the immune response and promoting MuSC proliferation and differentiation. These exosomes carry a bioactive cargo of proteins, lipids, and RNAs, which contribute to their therapeutic potential [150]. Exosomes that are specifically engineered to carry IGF-1 or FGF-2 have been demonstrated to enhance MuSC proliferation and differentiation, while those loaded with anti-inflammatory miRNAs help mitigate inflammation at the injury site [151]. Compared to traditional cell-based therapies, exosomes offer several advantages, including a lower likelihood of eliciting an immune response, scalability in production, and the ability to deliver targeted therapeutic cargos [152]. However, critical challenges remain, such as ensuring the targeted delivery of exosomes to injury sites, optimizing their therapeutic cargo, understanding their long-term effects, and standardizing production and characterization for clinical use [101]. Additionally, the WDR5-linked network associated with glutamine metabolism plays a role in promoting differentiation and supporting MuSC self-renewal by maintaining mitochondrial function, adding another layer of complexity and potential to exosome-based therapies (Table 3) [148].

Table 3.
Enhancing MuSC youthfulness and proliferative potential: a summary of recent pharmacotherapy advances. This table summarizes recent advances in pharmacological and cellular strategies to enhance the youthfulness and proliferative potential of MuSCs.
Table 3.
Enhancing MuSC youthfulness and proliferative potential: a summary of recent pharmacotherapy advances. This table summarizes recent advances in pharmacological and cellular strategies to enhance the youthfulness and proliferative potential of MuSCs.
| Ref. | Molecular Pathway | Signaling Mechanism | MuSC State Affected | Outcome |
|---|---|---|---|---|
| [153] | PRMT inhibition | Type I PRMT inhibition | Proliferation | Enhances MuSC proliferation and muscle regeneration in Duchenne muscular dystrophy model |
| [154] | CXCL12/CXCR4 axis | MSC homing and early myogenesis | Early myogenesis | Enhances muscle repair through MSC homing, especially with intrarectal administration |
| [155] | MSC-conditioned medium | Anti-inflammatory and anti-fibrotic | New muscle fiber formation | Reduces inflammation and fibrosis, promotes new muscle fiber formation |
| [156] | Notch signaling | DLL1 bioprinting, MuSC maintenance | Maintenance and engraftment | Improves MuSC maintenance and engraftment in dystrophic muscles |
| [157] | Smad/AKT pathways | Follistatin-enriched exosomes | Fibrosis reduction and muscle regeneration | Reduces fibrosis and enhances muscle regeneration |
| [77] | Wnt signaling | ASC exosomes mediated macrophage phenotype modulation | Tissue repair | Enhances tissue repair through Wnt signaling and macrophage phenotype alteration |
| [158] | KDR signaling | Dystrophin complex, asymmetric division | Progenitor generation | Promotes muscle regeneration by enhancing progenitor generation |
| [159] | Histone demethylation | JMJD3-mediated H3K27 demethylation | Muscle repair | Activates MuSCs and enhances muscle repair through demethylation |
| [160] | Dkk3 signaling | Baf60c regulation in myofibers | Paracrine signaling | Supports regulated muscle regeneration through paracrine signaling |
| [8] | Mechanosensitive channels | Piezo1 activation | Morphology and regenerative capacity | Enhances regenerative capacity by influencing MuSC morphology in dystrophic conditions |
| [161] | Dual-specificity phosphatases (DUSP13/27) | MyoD-mediated proliferation | Proliferation and differentiation transition | Regulates MyoD-mediated proliferation and transition to differentiation |
| [162] | HIF2A inhibition | Satellite cell differentiation | Proliferation and differentiation | Boosts proliferation and enhances MuSC regeneration |
| [163] | Setd7 inhibition | (R)-PFI-2-mediated MuSC expansion | Regenerative capabilities post-transplantation | Maintains regenerative capabilities and supports MuSC expansion post-transplantation |
| [164] | Translational modulation | C10-mediated delay in differentiation | Stemness maintenance | Delays differentiation, improving yield of cultured MuSCs and maintaining stemness |
| [165] | PAX7:GFP sorting | Fibrin microfiber bundles, myotube formation | Muscle regeneration | Enhances myotube formation and muscle regeneration from human pluripotent stem cell-derived myogenic progenitors |
| [166] | Bioactive muscle patches | Concurrent administration with proteins and MuSCs | Muscle repair | Improves muscle repair in trauma scenarios |
| [157] | Transcriptome reprogramming | Epigenome changes | Post-transplantation regenerative capacity | Enhances regenerative capacity through transcriptome reprogramming |
| [167] | iPSC correction | Interspecies generation | Functional stem cell production | Produces functional stem cells, addressing therapeutic production and donor shortages |
| [168] | MSC exosome-mediated immune modulation | Macrophage polarization | Inflammation reduction, tissue repair | Reduces inflammation and enhances muscle regeneration through macrophage polarization |
| [169] | MSC exosome-mediated immune modulation | Macrophage polarization | Inflammation reduction, tissue repair | Enhances cartilage repair and reduces inflammation through macrophage polarization |
| [170] | Glutamine metabolism | WDR5-linked network | Differentiation and self-renewal | Promotes differentiation and supports self-renewal by maintaining mitochondrial function |
| [160] | Mitophagy | PINK1/Parkin pathway | Mitochondrial quality, ROS reduction | Improves mitochondrial quality, reduces ROS, and supports MuSC self-renewal |
| [164] | Lipid metabolism | LD turnover | Balanced differentiation and self-renewal | Ensures balanced fate decisions between LDLow and LDHigh MuSC differentiation |
| [161] | Pitx2 regulation | Myogenic precursors | Satellite cell proliferation and differentiation | Enhances satellite cell proliferation and differentiation in specific subpopulations |
| [8] | Primary cilia | Talpid3 (TA3) regulation | Regeneration and self-renewal | Enhances MuSC regeneration and self-renewal via Hedgehog signaling |
| [159] | Epigenetic regulation | SETDB1-mediated genome integrity | Genome integrity, aberrant activation prevention | Preserves genome integrity and prevents aberrant activation |
| [171] | Metabolic regulation | SIRT1 activation and caloric restriction | MuSC activation and function | Enhances MuSC activation and function through histone modifications |
| [172] | Nanotopographical cues | Engineered EVs, sequential administration | Proliferation, aged muscle repair | Enhances proliferation and aged muscle repair through engineered EVs |
8. Advanced Analytical Techniques
8.1. Single-Cell RNA Sequencing (scRNA-Seq)
Single-cell RNA sequencing (scRNA-seq) has revolutionized our ability to analyze the cellular and molecular mechanisms underlying muscle repair. This advanced technique allows for the detailed examination of individual cells within the muscle tissue, providing insights into the dynamic changes occurring in muscle stem cells (MuSCs) and their surrounding niche during regeneration. By revealing distinct cell populations and their functional states, scRNA-seq enables researchers to map out the intricate interactions and regulatory networks that govern muscle repair processes [173]. Furthermore, combining scRNA-seq with spatial transcriptomics links molecular data with histological features, offering an enhanced understanding of tissue organization and the functional roles of different cell types during muscle regeneration [173].
8.2. Advanced Imaging Techniques
Advanced imaging techniques are crucial for providing spatial context to the molecular data obtained through techniques like scRNA-seq. Methods such as confocal microscopy and multiphoton microscopy allow for high-resolution, three-dimensional visualization of cell behavior, enabling real-time tracking of cellular and molecular events within muscle tissue [174]. Intravital microscopy and live cell imaging take this a step further by allowing for the observation of these processes in real time within live animals, offering invaluable insights into the dynamic nature of muscle regeneration [175]. These imaging techniques, when combined with molecular data, allow for a comprehensive understanding of how cells interact and function within the tissue during the repair process [175].
8.3. Combining Molecular Data with Histological Features
The integration of molecular data with histological features is becoming increasingly sophisticated, thanks to advances in single-cell proteomics and spatial profiling technologies. These approaches provide a comprehensive assessment of tissue composition and structure, which is essential for unraveling the complex interactions between different cell types during muscle regeneration [176]. For example, matrix elasticity is known to guide mesenchymal stem cells (MSCs) to differentiate according to the stiffness levels typical of their lineage-specific environments, which in turn affects their morphology, spreading, and the formation of cytoskeletal structures [177]. By combining these advanced molecular and imaging techniques, researchers are able to gain detailed insights into the cellular and molecular mechanisms at play, enabling the precise mapping of cellular interactions and regulatory networks during muscle repair [174].
9. Scaffold Types and Properties
9.1. Decellularized ECM Scaffolds
Decellularized extracellular matrix (ECM) scaffolds are highly regarded for their ability to provide a structural framework that facilitates host cell infiltration and tissue regeneration. These scaffolds are advantageous due to their scalability and cost-effectiveness, making them a popular choice in regenerative medicine [102]. Derived from donor muscle tissue, decellularized ECM scaffolds preserve critical structural and biochemical cues, which are essential for guiding host cell behavior during tissue regeneration [11]. A specific approach involves biologically derived scaffolds created using wet electrospinning, which are composites functionalized with ECM hydrogel. These scaffolds support human mesenchymal stem cells (hMSCs) and exhibit moderate biodegradability, high proangiogenic properties, and moderate sustainability, although they have low electrical properties [178]. Additionally, muscle-specific decellularized ECM scaffolds incorporated with IGF-1 have shown high sustainability and proangiogenic properties, supporting myoblast proliferation despite moderate biodegradability and low electrical properties [179].
9.2. Synthetic and Bioactive Muscle Patches
Synthetic and bioactive muscle patches are designed to provide structural support and deliver bioactive cues that promote muscle repair and integration with host tissue. These patches can be enhanced by seeding with MuSCs or other progenitor cells, which improves muscle repair by facilitating cell attachment and tissue integration [102]. For example, polycaprolactone scaffolds, which are fluorescent and fabricated using 3D printing, are conjugated with near-infrared (NIR) technology to support placental stem cells. These scaffolds are characterized by slow biodegradability and low proangiogenic and electrical properties, but they are highly cost-effective, though less sustainable [178]. Moreover, bioactive muscle patches, when combined with proteins and MuSCs, have demonstrated enhanced muscle repair capabilities, particularly in traumatic injury scenarios [180].
9.3. Composite Scaffolds and Their Functionalization
Composite scaffolds combine various materials to optimize their mechanical and biological properties for tissue engineering applications. For instance, alginate–silk composite scaffolds, created via 3D bioprinting, are designed for dual differentiation and support hMSCs. These scaffolds offer moderate biodegradability, proangiogenic, and electrical properties, along with high cost-effectiveness and moderate sustainability [181]. Another example is collagen/polypyrrole composite scaffolds, which are freeze-dried to achieve alignment and conductivity. These scaffolds, functionalized with polypyrrole, support myoblasts and are known for their slow biodegradability, moderate proangiogenic properties, and high electrical properties, alongside moderate sustainability [182]. Gelatin/chitooligosaccharide/DBM composite scaffolds, produced through lyophilization, are porous and support MSCs. They exhibit moderate biodegradability, proangiogenic properties, and cost-effectiveness, with low electrical properties [183]. Bioactive ceramics/silk fibroin composite scaffolds, created using 3D printing, support hMSCs and offer moderate biodegradability and proangiogenic properties, with low electrical properties and high cost-effectiveness (Figure 7) [184].
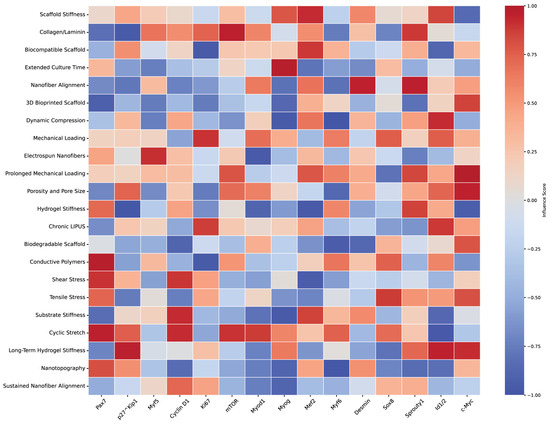
Figure 7.
Heatmap of scaffold characteristics impact on muscle stem cell markers. This heatmap visually represents the estimated impact of various scaffold characteristics on muscle stem cell markers, based on a non-systematic literature review. The scaffold properties examined include stiffness, porosity, mechanical loading, alignment, and others. Muscle stem cell markers (e.g., Pax7, Myf5, Myod1, Myogenin) are displayed along the x-axis, while scaffold characteristics are on the y-axis. Color intensity and direction indicate the correlation strength, with red hues representing positive correlations and blue hues showing negative correlations. These interactions reflect the influence of scaffold design on stem cell behaviors such as quiescence, activation, proliferation, differentiation, and self-renewal. The heatmap provides insights into how scaffold properties can be strategically modulated to optimize muscle regeneration and stem cell function, summarizing findings based on references listed in Table 4.
9.4. Three-Dimensional-Printed and Electrospun Scaffolds
Three-dimensional printing technology allows for the creation of highly customized scaffolds tailored to match specific injury sites, providing personalized solutions for muscle regeneration [181]. This process enables the incorporation of multiple cell types and bioactive molecules, resulting in complex tissue constructs that promote cell proliferation and integration with existing tissue [185]. Electrospinning is another key technique used to create nanofibrous scaffolds that closely mimic the ECM of native muscle tissue, offering a high surface area and porosity for optimal cell attachment and nutrient exchange. Ref. [80] discusses the role of ECM components in tissue regeneration, with electrospun scaffolds providing an ideal environment for muscle cells. Ref. [186] describes how engineered scaffolds, including electrospun ones, mimic the native ECM, supporting cell attachment and function. These electrospun scaffolds can be further functionalized with bioactive molecules to enhance cell adhesion and differentiation, with aligned fibers guiding regenerating muscle fibers effectively [182]. Additionally, innovations such as “Drydux Transduction” have been developed to increase activation efficiency through viral vectors during the transduction process, improving the functionality of these scaffolds in regenerative applications (Figure 8 and Table 4).
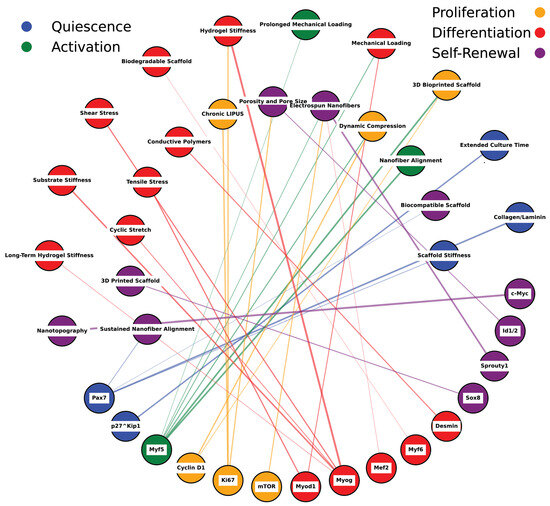
Figure 8.
Network mapping of scaffold properties and muscle stem cell markers. This figure illustrates the estimated relationships between various scaffold properties (e.g., stiffness, alignment, porosity) and muscle stem cell markers, including Pax7, Myf5, Myod1, Myogenin, and others. The network displays interactions based on literature-derived estimates linking scaffold features to different stem cell fates such as quiescence, activation, proliferation, differentiation, and self-renewal. Scaffold properties, represented as nodes, interact through specific pathways with muscle stem cell markers and their regulatory networks. Color-coded nodes indicate stem cell fates influenced by scaffold design: blue represents quiescence, green denotes activation, yellow signifies proliferation, red indicates differentiation, and purple corresponds to self-renewal. The pathways connecting these nodes highlight the mechanistic role of scaffold properties in modulating muscle stem cell behavior and fate, reflecting how physical cues from scaffolds translate into cellular responses. This diagram emphasizes the complexity and interconnectedness of scaffold-induced regulation, illustrating their critical influence on stem cell signaling and tissue regeneration dynamics. The network is informed by selective literature reviews, as referenced in Table 4.

Table 4.
Molecular pathways and signaling in MuSCs. The table categorizes the research by scaffold type, signal transduction mechanisms, cellular responses, and key molecules or proteins involved.
Table 4.
Molecular pathways and signaling in MuSCs. The table categorizes the research by scaffold type, signal transduction mechanisms, cellular responses, and key molecules or proteins involved.
| Ref. | Scaffold Type | Signal Transduction | Cellular Response | Key Molecules/Proteins |
|---|---|---|---|---|
| [187] | Nanotopography | FAK activation | Stem cell differentiation, ECM production | FAK, Cytoskeletal proteins |
| [188] | Meso-scale topological cues | Mechanotransduction | ECM production | FAK, ECM components |
| [189] | Aligned fibrous scaffolds | Integrin–FAK pathway | Muscle progenitor maturation | Integrin, FAK |
| [190] | Fibrous scaffolds | FAK activation | Skeletal regeneration, progenitor cell maturation | FAK |
| [191] | Electrospun fibers | Rho GTPase, FAK pathways | Tenogenic differentiation | Rho GTPase, FAK |
| [192] | Conductive nanofibers | MAPK/ERK pathway | Myotube formation | MAPK, ERK |
| [193] | Conductive nanofibers | MAPK/ERK pathway | Mechanotransduction, myotube formation | MAPK, ERK |
| [194] | Vitronectin, fibronectin, collagen | Integrin-FAK/Src pathway | Smooth muscle cell adhesion and proliferation | Integrin, FAK, Src |
| [195] | Vitronectin, fibronectin | Integrin-mediated mechanotransduction | Vascular smooth muscle cell behavior | Integrin, ECM components |
| [50] | Various ECM components | MAPK/ERK pathway | Myogenic differentiation | MAPK, ERK |
| [196] | Stiff scaffolds | RhoA/ROCK pathway | Cell behavior modulation, differentiation | RhoA, ROCK |
| [197] | Stiff scaffolds | RhoA/ROCK pathway | Response to mechanical cues, differentiation | RhoA, ROCK |
| [157] | Growth factors | JAK/STAT pathway | Satellite cell activation, tissue repair | JAK, STAT |
| [23] | Growth factors | JAK/STAT pathway | Satellite cell activation | JAK, STAT |
| [171] | Biochemical cues | Notch pathway | Satellite cell activation and differentiation | Notch |
| [77] | Biochemical cues | Notch pathway | Satellite cell quiescence, differentiation | Notch |
| [172] | Curved nanofiber networks | Actomyosin filament pathway | Osteogenic differentiation | Actin, Myosin |
| [198] | Graphene oxide substrates | Cytoskeletal protein reorganization | Cell migration, differentiation | Cytoskeletal proteins |
| [199] | Grooved patterns, chiral nematics | Myogenin, MyoD activation | Myotube formation | Myogenin, MyoD |
| [200] | Nanogratings | Extracellular vesicles (EVs) and myogenic proteins | Myogenic differentiation | EVs, Myogenic proteins |
| [201] | 3D topographical features | PGE2, IL-6, MCP-1 | Anti-inflammatory response | PGE2, IL-6, MCP-1 |
| [202] | Mesoporous silica nanoparticles | PI3K/Akt pathway | Vascularization, drug delivery | PI3K, Akt |
| [203] | Shape memory polymers | MyoD, Myogenin signaling | Myogenic differentiation | MyoD, Myogenin |
| [204] | Motor amphiphiles | Membrane potential, calcium signaling | Controlled differentiation | Membrane potential, Calcium signaling |
| [160] | Electrospun PLCL scaffolds | Myogenic markers activation | Myogenesis | MyoD, Myogenin |
| [205] | Drydux scaffolds | Fluid flow optimization | Enhanced viral vector transduction efficiency | Viral vectors |
| [206] | Barium-doped calcium silicate | CaSR, AKT signaling | Osteogenic differentiation | CaSR, AKT |
| [207] | Degradable scaffolds | Wnt/β-catenin pathway | Tissue regeneration | Wnt, β-catenin |
| [97] | Shear stress applied scaffolds | Wnt signaling | Mesoderm differentiation | Wnt |
| [158] | Mechanical stimulation | MAPK pathway | Muscle adaptation | MAPK |
| [208] | Dynamic cellular projections | Rac-Rho GTPase pathway | Injury response, stem cell migration | Rac, Rho GTPase |
| [170] | Glutamine metabolism | WDR5-APC/C interaction | Stem cell differentiation | WDR5, APC/C |
10. Integration of Emerging Technologies
10.1. Role of Biocompatible Materials in Scaffold Design
The selection of biocompatible and biodegradable materials is crucial for the successful clinical translation of scaffolds in muscle regeneration. These materials must ensure not only compatibility with host tissues but also degrade at a rate that supports tissue healing and integration without causing adverse reactions [209]. For instance, bioactive ceramics/silk fibroin composite scaffolds, created through 3D printing, support human mesenchymal stem cells (hMSCs) and offer a balance of moderate biodegradability and proangiogenic properties. These scaffolds are also cost-effective and moderately sustainable, although they possess low electrical properties, which may limit certain applications. Ref. [81] notes that while electrospun scaffolds are cost-effective, they may require modification to enhance electrical properties for specific applications. Ref. [77] emphasizes the need for improving scaffold properties to better support tissue engineering applications, particularly where electrical conductivity is important. Hybrid scaffolds, which combine hydrogels with electrospun fibers or 3D-printed frameworks, offer enhanced mechanical support while maintaining biological activity, making them ideal for optimizing tissue repair processes [121,210]. Similarly, decellularized ECM scaffolds, with their fibrous and porous structure, provide a supportive environment for human adipose-derived stem cells (hASCs) and exhibit high sustainability, despite moderate biodegradability and proangiogenic properties [211].
10.2. Functionalization for Enhanced Regeneration
Functionalization of scaffolds and therapeutic strategies plays a key role in enhancing muscle regeneration. Pharmacological interventions are often employed to modulate the cellular environment, making both cellular and acellular therapies more effective. Ref. [72] illustrates how pharmacological agents can modulate the cellular environment, enhancing the effectiveness of regenerative therapies. Ref. [74] discusses how pharmacological interventions can support tissue repair by modulating the inflammatory response and cellular activity. Exosomes carrying growth factors like IGF-1 or FGF-2 can specifically enhance MuSC proliferation and differentiation, while exosomes containing anti-inflammatory miRNAs help mitigate inflammation, creating a conducive environment for muscle repair [102]. Additionally, histone marks, particularly those influenced by pathways interconnected with MuSC activation, play a significant role in facilitating differentiation and muscle repair. Ref. [78] highlights the role of histone modifications in regulating gene expression during MuSC activation and differentiation, which is crucial for muscle repair. Histone demethylases such as JMJD3 are critical in this context, as they activate MuSCs by demethylating H3K27, thereby improving muscle regeneration outcomes [212]. Scaffold alignment also contributes to enhanced cellular proliferation by activating integrin-mediated signaling via the PI3K/Akt pathway, which is vital for effective tissue regeneration [213].
10.3. Nanotechnology and 3D Topographical Cues
Nanotechnology and 3D topographical cues have become integral to guiding cell behavior and improving tissue integration in regenerative medicine (Table 5). Surface features at the microscale and nanoscale influence cytoskeletal alignment and focal adhesion dynamics, which are essential for stem cell differentiation and effective tissue repair [214]. For example, curved nanofiber networks have been shown to promote osteogenic differentiation by engaging the actomyosin filament pathway, which involves both actin and myosin [213]. Nanogratings, on the other hand, promote myogenic differentiation through the action of extracellular vesicles (EVs) and the expression of myogenic proteins. Ref. [75] explores how nanostructures like nanogratings can influence cell behavior, promoting myogenic differentiation through EV signaling. Ref. [69] examines how extracellular vesicles carry signals that promote myogenic protein expression and differentiation, supported by nanogratings. Furthermore, 3D topographical features are crucial in regulating the crosstalk between mesenchymal stem cells (MSCs) and macrophages, leading to an anti-inflammatory response mediated by factors like PGE2, IL-6, and MCP-1 [214]. Nanotopographical cues, when delivered via engineered EVs, have shown potential in enhancing cell proliferation and aiding in the repair of aged muscle tissue, particularly through sequential administration strategies [215].

Table 5.
Characterization of scaffold innovations for stem cell-based skeletal muscle tissue engineering.
Table 5.
Characterization of scaffold innovations for stem cell-based skeletal muscle tissue engineering.
| Ref. | Scaffold Material | Source | Fabrication Technique | Structural Features | Cell Type Supported | Biodegradability | Proangiogenic Properties | Electrical Properties | Sustainability |
|---|---|---|---|---|---|---|---|---|---|
| [216] | Polycaprolactone (PCL) | Synthetic | 3D Printing | Fluorescent features, NIR conjugation | Placental stem cells | Slow | Low | Low | Low |
| [217] | Polycaprolactone (PCL) | Synthetic | 3D Printing | Structural stability | Placental stem cells | Slow | Low | Low | Low |
| [218] | Decellularized ECM | Biological | Decellularization | Spheroid-derived structures | Endothelial cells | Moderate | High | Low | Moderate |
| [219] | Decellularized ECM | Biological | 3D Bioprinting | Spheroid-derived structures | Endothelial cells | Moderate | High | Low | Moderate |
| [220] | Cellulose | Plant | Decellularization | Grooved structural characteristics | C2C12, HSMCs cells | Slow | Low | Low | High |
| [221] | Cellulose | Plant | Decellularization | Grooved structural characteristics | C2C12, HSMCs cells | Slow | Low | Low | High |
| [222] | Collagen | Biological | Freeze-drying | Fibrillar structures | MSCs | Moderate | Low | Low | Moderate |
| [223] | Collagen | Biological | Freeze-drying | Fibrillar structures | MSCs | Moderate | Low | Low | Moderate |
| [224] | Polycaprolactone (PCL)/Gold | Synthetic | Melt Electrowriting | Hierarchical, anisotropic structure, Gold coating | H9c2 myoblasts | Slow | Moderate | High | Low |
| [225] | Polycaprolactone (PCL)/Polypyrrole | Synthetic | Melt Electrowriting | Hierarchical, anisotropic structure, Gold coating | H9c2 myoblasts | Slow | Moderate | High | Low |
| [226] | Gelatin/Chitooligosaccharide/DBM | Composite | Lyophilization | Porous structure | MSCs | Moderate | Moderate | Low | Moderate |
| [227] | Gelatin/Chitosan | Composite | Salt-leaching/Lyophilization | Porous structure | MSCs | Moderate | Moderate | Low | Moderate |
| [228] | Collagen/Polypyrrole | Composite | Freeze-drying | Aligned, conductive structural features | Myoblasts | Slow | Moderate | High | Moderate |
| [221] | Plant-derived Cellulose | Plant | Decellularization | Striated structures | C2C12 cells | Slow | Low | Low | High |
| [216] | Polycaprolactone (PCL) | Synthetic | 3D Printing | Artificial ECM functionalization | hASCs | Slow | High | Low | Low |
| [229] | Alginate–Gelatin | Composite | 3D Bioprinting | Hierarchical structural features | hMSCs | Moderate | Low | Low | Moderate |
| [230] | Alginate–Gelatin | Composite | 3D Bioprinting | Hierarchical structural features | hMSCs | Moderate | Low | Low | Moderate |
| [179] | Decellularized ECM with IGF-1 | Biological | Decellularization | Muscle-specific structures, IGF-1 incorporation | Myoblasts | Moderate | High | Low | High |
| [231] | Decellularized ECM with topographical cues | Biological | Wet Electrospinning | Composite ECM hydrogel structures | hMSCs | Moderate | High | Low | Moderate |
| [11] | Decellularized ECM | Biological | Wet Electrospinning | Composite ECM hydrogel structures | hMSCs | Moderate | High | Low | Moderate |
| [232] | Decellularized ECM | Biological | Decellularization | Fibrous, porous structures | hASCs | Moderate | Moderate | Low | High |
| [233] | Decellularized ECM | Biological | Infusion Bioreactor | Fibrous, porous structures | hASCs | Moderate | Moderate | Low | High |
| [150] | Bioactive Ceramics/Silk Fibroin | Composite | 3D Printing | Composite structures | hMSCs | Moderate | Moderate | Low | Moderate |
| [228] | Bioactive Ceramics/Silk Fibroin | Composite | 3D Printing | Composite structures | hMSCs | Moderate | Moderate | Low | Moderate |
11. Future Directions
11.1. Hybrid Scaffold Systems
The development of hybrid biomimetic scaffolds that integrate ECM-mimicking hydrogels with nanofibrous architectures through advanced 3D bioprinting and electrospinning techniques represents a significant direction in replicating the anisotropic mechanical properties and biochemical cues of native muscle tissue. Future efforts should focus on engineering scaffolds with dynamic viscoelastic properties, utilizing stimuli-responsive materials that adapt stiffness and degradation rates in response to microenvironmental changes. This approach will ensure synchronized support throughout the various stages of tissue regeneration. Additionally, incorporating multi-modal bioactive molecules, including growth factors, cytokines, and epigenetic modulators, into scaffold matrices will allow for the spatiotemporal control of MuSC behavior, with a particular emphasis on developing controlled release systems that provide localized bioactivity.
11.2. Mechanotransduction Optimization
Investigating the role of mechanosensitive ion channels such as Piezo1 in cytoskeletal reorganization and nuclear mechano-signaling is essential to understanding their downstream effects on YAP/TAZ activation and gene transcription, particularly in the context of myogenesis. Designing substrates with tunable rigidity and topographical features will be critical in modulating focal adhesion dynamics, thereby enhancing the activation of pathways like RhoA/ROCK and FAK. These pathways are instrumental in promoting MuSC alignment, proliferation, and differentiation. Moreover, exploring the crosstalk between mechanotransduction and metabolic reprogramming in MuSCs, especially the impact of actomyosin contractility on glycolysis and oxidative phosphorylation, will provide insights into sustaining cellular energetics during muscle repair.
11.3. Epigenetic Scaffold Integration
The integration of epigenetic modulators within scaffolds presents an innovative strategy for regulating MuSC behavior during muscle regeneration. Future scaffolds should be designed to incorporate histone deacetylase inhibitors (HDACi) or DNA methyltransferase inhibitors (DNMTi) to modulate chromatin accessibility, either maintaining MuSC quiescence or promoting their activation in response to injury. Additionally, incorporating non-coding RNAs such as miR-1 and lncRNAs within scaffold matrices could enhance post-transcriptional gene regulation and chromatin remodeling, improving the precision of MuSC fate determination. Employing CRISPR-dCas9-based epigenetic editing within scaffold environments offers the potential to precisely target myogenic regulatory regions, such as those controlling MyoD and Myf5, to modulate key transcription factors critical for MuSC differentiation and self-renewal.
11.4. Scalable Differentiation Protocols
Optimizing bioreactor-based culture systems is imperative for the large-scale production of iPSC-derived myogenic progenitors, with a focus on maintaining Pax7 expression and minimizing spontaneous differentiation during expansion phases. Integrating Wnt and FGF signaling modulators into differentiation protocols will be essential for enhancing the yield and purity of myogenic progenitors while minimizing the risk of teratoma formation in transplantation settings. Further exploration of 3D spheroid culture systems is needed, as these systems more accurately replicate the in vivo niche of myogenic progenitors, promoting cell–cell interactions and ECM deposition to improve the functional integration of transplanted cells.
11.5. Exosome Engineering for Targeted Delivery
The engineering of exosomes for targeted delivery to injured muscle tissue is a promising avenue for enhancing therapeutic outcomes. Surface modifications, such as PEGylation and antibody conjugation, should be explored to improve tissue-specific homing and reduce off-target effects. Utilizing CRISPR-based gene editing to modify exosome-producing cells will enable the production of exosomes enriched with myogenic miRNAs (e.g., miR-133) and pro-regenerative proteins (e.g., IGF-1, FGF-2). Developing multilayered exosome formulations, incorporating lipid nanoparticles or hydrogel carriers, will be critical for achieving sustained release and enhanced bioavailability at injury sites, with a focus on increasing the in vivo half-life of exosomal cargo.
11.6. Targeted Immune Modulation
Exosome-based therapies specifically engineered to modulate macrophage polarization, particularly the transition from M1 to M2 phenotypes, hold potential for reducing fibrosis and promoting a pro-regenerative microenvironment. These therapies should focus on exosome cargo enriched with anti-inflammatory miRNAs and TGF-β. Additionally, the localized delivery of senolytic agents, such as Dasatinib and Quercetin, within scaffolds offers a targeted approach to selectively eliminate senescent cells in aged muscle, thereby minimizing systemic side effects and enhancing the regenerative potential of MuSCs. The development of biodegradable immunomodulatory scaffolds that release cytokines such as IL-4, IL-10, or Tregs-inducing factors in a controlled manner will be crucial for orchestrating the spatial and temporal aspects of the immune response during muscle regeneration.
11.7. Real-Time Monitoring and Analysis
Future research should emphasize the use of intravital microscopy combined with fluorescent reporter systems to dynamically track MuSC activation, migration, and differentiation in real-time during tissue regeneration. This will provide critical insights into cellular behavior in vivo. Integrating single-cell RNA sequencing (scRNA-seq) with spatial transcriptomics will enable the mapping of molecular heterogeneity within MuSC populations at various stages of muscle repair, focusing on identifying key regulatory networks and cellular interactions. Additionally, applying 3D spatial profiling technologies to analyze ECM composition and cellular architecture within regenerating muscle tissue will be essential for correlating microenvironmental changes with regenerative outcomes and scaffold performance.
12. Conclusions
Muscle stem cells (MuSCs) are central to the process of skeletal muscle regeneration, with their activity intricately regulated by mechanotransduction pathways such as YAP/TAZ, Rho/ROCK, and PI3K/Akt. These signaling cascades, influenced by the biophysical properties of the extracellular matrix (ECM), guide MuSC fate through integrin-mediated focal adhesion dynamics and cytoskeletal reorganization. The strategic incorporation of bioactive molecules within engineered scaffolds offers a powerful tool for modulating these intracellular pathways, with growth factors like IGF-1 and FGF-2 playing a critical role in sustaining MuSC proliferation, differentiation, and myotube formation. This biomimetic approach ensures that scaffolds maintain their bioactivity in alignment with the temporal requirements of muscle tissue regeneration.
The use of pharmacological agents, particularly senolytics such as dasatinib and navitoclax, has shown promise in counteracting the adverse effects of cellular senescence within the aging MuSC niche. By selectively inducing apoptosis in senescent cells, these agents help preserve the regenerative capacity of MuSCs, contributing to tissue homeostasis. When combined with scaffold-based delivery systems, these drugs not only enhance their bioavailability but also maximize their therapeutic efficacy.
Technological advancements, including single-cell RNA sequencing (scRNA-seq) and high-resolution imaging modalities like multiphoton microscopy and spatial transcriptomics, have provided unprecedented insights into the cellular heterogeneity and molecular dynamics of regenerating muscle. These tools have enabled the identification of distinct MuSC subpopulations and the transcriptional networks that govern their activation and differentiation, offering a detailed, single-cell resolution understanding of muscle repair mechanisms.
The integration of these cutting-edge imaging techniques and molecular profiling tools has significantly advanced the field of muscle regeneration research. By unraveling the complex interactions between MuSCs, their niche, and the ECM, researchers have identified key regulatory nodes and epigenetic modifications that control MuSC behavior. The fusion of these insights with bioengineered scaffold technologies has optimized regenerative strategies, enhancing the potential to restore functional muscle architecture and paving the way for more effective tissue engineering approaches in clinical applications.
Author Contributions
Conceptualization, F.Y. and C.Z.; methodology, F.Y. and C.Z.; software, F.Y.; validation, F.Y. and C.Z. formal analysis F.Y. and C.Z.; investigation F.Y., L.A.F., O.A.S. and C.Z.; resources, F.Y. and C.Z.; data curation, F.Y. and C.Z.; writing—original draft preparation, F.Y. writing—review and editing, F.Y., O.A.S., L.A.F. and C.Z.; visualization, F.Y.; supervision, C.Z.; project administration, F.Y. and C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We gratefully acknowledge Ramona L Reisdorf, Aida Sarcon, Gongyin Zhao, Ichiro Tsukamoto, Rou Wan and Elameen Adam for insightful advice and thoughtful feedback.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Madl, C.; Flaig, I.A.; Holbrook, C.; Wang, Y.X.; Blau, H. Biophysical matrix cues from the regenerating niche direct muscle stem cell fate in engineered microenvironments. Biomaterials 2021, 275, 120973. [Google Scholar] [CrossRef] [PubMed]
- Relaix, F.; Bencze, M.; Borok, M.; Der Vartanian, A.; Gattazzo, F.; Mademtzoglou, D.; Pérez-Díaz, S.; Prola, A.; Reyes-Fernandez, P.C.; Rotini, A.; et al. Perspectives on skeletal muscle stem cells. Nat. Commun. 2021, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Boukhatmi, H. Drosophila, an Integrative Model to Study the Features of Muscle Stem Cells in Development and Regeneration. Cells 2021, 10, 2112. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.M.; Havenstrite, K.L.; Magnusson, K.E.; Sacco, A.; Leonardi, N.A.; Kraft, P.; Blau, H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010, 329, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, C. Injectable polyethylene glycol-fibrinogen hydrogel adjuvant improves skeletal muscle regeneration and functional recovery in a volumetric muscle loss model. Cell Death Dis. 2014, 5, e1499. [Google Scholar]
- Quarta, M. Bioengineered constructs combined with exercise enhance stem cell–mediated treatment of volumetric muscle loss. Nat. Commun. 2017, 8, 15613. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, C.S.; Fu, J. Forcing stem cells to behave: A biophysical perspective of the cellular microenvironment. Annu. Rev. Biophys. 2012, 41, 519–542. [Google Scholar] [CrossRef]
- Ma, N.; Chen, D.; Lee, J.-H.; Kuri, P.; Hernandez, E.; Kocan, J.; Mahmood, H.; Tichy, E.; Rompolas, P.; Mourkioti, F. Piezo1 regulates the regenerative capacity of skeletal muscles via orchestration of stem cell morphological states. Sci. Adv. 2022, 8, eabn0485. [Google Scholar] [CrossRef]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bönnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef]
- Bach, A.D.; Arkudas, A.; Tjiawi, J.; Polykandriotis, E.; Kneser, U.; Horch, R.E. A new approach to tissue engineering of vascularized skeletal muscle. J. Cell. Mol. Med. 2006, 10, 716–726. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, X.; Yildirimer, L.; Lang, Q.; Lin, Z.; Zheng, R.; Zhang, Y.; Cui, W.; Annabi, N.; Khademhosseini, A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017, 49, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Rocheteau, P.; Vinet, M.; Chrétien, F. Dormancy and quiescence of skeletal muscle stem cells. Results Probl. Cell Differ. 2015, 56, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Cicciarello, D.; Schaeffer, L.; Scionti, I. Epigenetic Control of Muscle Stem Cells: Focus on Histone Lysine Demethylases. Front. Cell Dev. Biol. 2022, 10, 917771. [Google Scholar] [CrossRef] [PubMed]
- Boonsanay, V.; Zhang, T.; Georgieva, A.; Kostin, S.; Qi, H.; Yuan, X.; Zhou, Y.; Braun, T. Regulation of Skeletal Muscle Stem Cell Quiescence by Suv4-20h1-Dependent Facultative Heterochromatin Formation. Cell Stem Cell 2016, 18, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Gheller, B.J.; Blum, J.E.; Fong, E.H.H.; Malysheva, O.; Cosgrove, B.; Thalacker-Mercer, A. A defined N6-methyladenosine (m6A) profile conferred by METTL3 regulates muscle stem cell/myoblast state transitions. Cell Death Discov. 2020, 6, 95. [Google Scholar] [CrossRef]
- Jacques, E.; Kuang, Y.; Kann, A.P.; Le Grand, F.; Krauss, R.; Gilbert, P. The mini-IDLE 3D biomimetic culture assay enables interrogation of mechanisms governing muscle stem cell quiescence and niche repopulation. eLife 2022, 11, e81738. [Google Scholar] [CrossRef]
- Sato, T.; Yamamoto, T.; Sehara-Fujisawa, A. miR-195/497 induce postnatal quiescence of skeletal muscle stem cells. Nat. Commun. 2014, 5, 4597. [Google Scholar] [CrossRef]
- L’Honoré, A.; Drouin, J.; Buckingham, M.; Montarras, D. Pitx2 and Pitx3 transcription factors: Two key regulators of the redox state in adult skeletal muscle stem cells and muscle regeneration. Free Radic. Biol. Med. 2014, 75 (Suppl. S1), S37. [Google Scholar] [CrossRef]
- Mademtzoglou, D.; Asakura, Y.; Borok, M.; Alonso-Martín, S.; Mourikis, P.; Kodaka, Y.; Mohan, A.; Asakura, A.; Relaix, F. Cellular localization of the cell cycle inhibitor Cdkn1c controls growth arrest of adult skeletal muscle stem cells. eLife 2018, 7, e33337. [Google Scholar] [CrossRef]
- Doria, E.; Buonocore, D.; Focarelli, A.; Marzatico, F. Relationship between human aging muscle and oxidative system pathway. Oxid. Med. Cell. Longev. 2012, 2012, 830257. [Google Scholar] [CrossRef]
- Chowdhury, A.; Ghosh, I.; Datta, K. Excessive reactive oxygen species induces apoptosis in fibroblasts: Role of mitochondrially accumulated hyaluronic acid binding protein 1 (HABP1/p32/gC1qR). Exp. Cell Res. 2008, 314, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, H.; Pal, S.; Sabnam, S.; Pal, A. High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci. 2019, 241, 117148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Noguchi, Y.-t.; Nakayama, H.; Kaji, T.; Tsujikawa, K.; Ikemoto-Uezumi, M.; Uezumi, A.; Okada, Y.; Doi, T.; Watanabe, S.; et al. The CalcR-PKA-Yap1 Axis Is Critical for Maintaining Quiescence in Muscle Stem Cells. Cell Rep. 2019, 29, 2154–2163.e2155. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, J.; Wang, Y.; Shen, S.; Xu, X.; Zhang, L.; Jiang, Q. Bone microenvironment regulative hydrogels with ROS scavenging and prolonged oxygen-generating for enhancing bone repair. Bioact. Mater. 2023, 24, 477–496. [Google Scholar] [CrossRef]
- Patel, K.D.; Keskin-Erdogan, Z.; Sawadkar, P.; Nik Sharifulden, N.S.A.; Shannon, M.R.; Patel, M.; Silva, L.B.; Patel, R.; Chau, D.Y.S.; Knowles, J.C.; et al. Oxidative stress modulating nanomaterials and their biochemical roles in nanomedicine. Nanoscale Horiz. 2024, 9, 1630–1682. [Google Scholar] [CrossRef]
- Shan, H.; Gao, X.; Zhang, M.; Huang, M.; Fang, X.; Chen, H.; Tian, B.; Wang, C.; Zhou, C.; Bai, J.; et al. Injectable ROS-scavenging hydrogel with MSCs promoted the regeneration of damaged skeletal muscle. J. Tissue Eng. 2021, 12, 20417314211031378. [Google Scholar] [CrossRef]
- Seale, P.; Rudnicki, M.A. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev. Biol. 2000, 218, 115–124. [Google Scholar] [CrossRef]
- Buckingham, M.; Relaix, F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu. Rev. Cell Dev. Biol. 2007, 23, 645–673. [Google Scholar] [CrossRef]
- Olguin, H.C.; Olwin, B.B. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: A potential mechanism for self-renewal. Dev. Biol. 2004, 275, 375–388. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Xiao, M.; Balint, K.; Soma, A.; Pinnix, C.C.; Capobianco, A.; Velazquez, O.; Herlyn, M. Inhibition of endothelial cell proliferation by Notch1 signaling is mediated by repressing MAPK and PI3K/Akt pathways and requires MAML1. FASEB J. 2006, 20, 1009–1011. [Google Scholar] [CrossRef]
- Wang, T.; Holt, C.; Xu, C.; Ridley, C.; Jones, R.P.O.; Baron, M.; Trump, D. Notch3 activation modulates cell growth behaviour and cross-talk to Wnt/TCF signalling pathway. Cell. Signal. 2007, 19, 2458–2467. [Google Scholar] [CrossRef] [PubMed]
- Regad, T. Targeting RTK Signaling Pathways in Cancer. Cancers 2015, 7, 1758–1784. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.K.; Phillips, A.M.; Jones, A.; Pisconti, A. SDC3 acts as a timekeeper of myogenic differentiation by regulating the insulin/AKT/mTOR axis in muscle stem cell progeny. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ma, J.; Meng, Y.; Kwiatkowski, D.; Chen, X.; Peng, H.; Sun, Q.; Zha, X.; Wang, F.; Wang, Y.; Jing, Y.; et al. Mammalian target of rapamycin regulates murine and human cell differentiation through STAT3/p63/Jagged/Notch cascade. J. Clin. Investig. 2010, 120, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Gunther, S.; Kim, J.; Kostin, S.; Lepper, C.; Fan, C.M.; Braun, T. Myf5-positive satellite cells contribute to Pax7-dependent long-term maintenance of adult muscle stem cells. Cell Stem Cell 2013, 13, 590–601. [Google Scholar] [CrossRef]
- Westman, A.M.; Peirce, S.; Christ, G.; Blemker, S. Agent-based model provides insight into the mechanisms behind failed regeneration following volumetric muscle loss injury. PLoS Comput. Biol. 2021, 17, e1008937. [Google Scholar] [CrossRef]
- San Emeterio, C.L.; Hymel, L.; Turner, T.; Ogle, M.; Pendleton, E.G.; York, W.Y.; Olingy, C.E.; Liu, A.Y.; Lim, H.S.; Sulchek, T.; et al. Nanofiber-Based Delivery of Bioactive Lipids Promotes Pro-regenerative Inflammation and Enhances Muscle Fiber Growth After Volumetric Muscle Loss. Front. Bioeng. Biotechnol. 2021, 9, 650289. [Google Scholar] [CrossRef]
- Patel, K.H.; Talovic, M.; Dunn, A.J.; Patel, A.; Vendrell, S.; Schwartz, M.A.; Garg, K. Aligned nanofibers of decellularized muscle extracellular matrix for volumetric muscle loss. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2528–2537. [Google Scholar] [CrossRef]
- Greising, S.M.; Rivera, J.; Goldman, S.; Watts, A.; Aguilar, C.; Corona, B. Unwavering Pathobiology of Volumetric Muscle Loss Injury. Sci. Rep. 2018, 7, 13179. [Google Scholar] [CrossRef]
- Corona, B.; Rivera, J.; Greising, S.M. Inflammatory and Physiological Consequences of Debridement of Fibrous Tissue after Volumetric Muscle Loss Injury. Clin. Transl. Sci. 2017, 11, 208–217. [Google Scholar] [CrossRef]
- Morgan, N.; Zhang, H.; Anderson, S.E.; Jang, Y.C. Traumatic muscle injury promotes aberrant fibro-adipogenic progenitor cell population, leading to fibrosis and impaired muscle stem cell function. Physiology 2023, 38, 5734945. [Google Scholar] [CrossRef]
- Larouche, J.A.; Greising, S.M.; Corona, B.; Aguilar, C.A. Robust inflammatory and fibrotic signaling following volumetric muscle loss: A barrier to muscle regeneration. Cell Death Dis. 2018, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.; Greising, S.M.; Watts, A.; Goldman, S.; Peragallo, C.; Zook, C.; Larouche, J.A.; Corona, B. Multiscale analysis of a regenerative therapy for treatment of volumetric muscle loss injury. Cell Death Discov. 2018, 4, 1–11. [Google Scholar] [CrossRef]
- Lukjanenko, L.; Jung, M.J.; Hegde, N.; Perruisseau-Carrier, C.; Migliavacca, E.; Rozo, M.; Karaz, S.; Jacot, G.E.; Schmidt, M.; Li, L.; et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat. Med. 2016, 22, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Deprez, A.; Orfi, Z.; Rieger, L.; Dumont, N.A. Impaired muscle stem cell function and abnormal myogenesis in acquired myopathies. Biosci. Rep. 2022, 43, BSR20220284. [Google Scholar] [CrossRef]
- Lukjanenko, L.; Karaz, S.; Stuelsatz, P.; Gurriarán-Rodríguez, U.; Michaud, J.; Dammone, G.; Sizzano, F.; Mashinchian, O.; Ancel, S.; Migliavacca, E.; et al. Aging Disrupts Muscle Stem Cell Function by Impairing Matricellular WISP1 Secretion from Fibro-Adipogenic Progenitors. Cell Stem Cell 2019, 24, 433–446.e437. [Google Scholar] [CrossRef]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef]
- Baker, S.J.; Poulikakos, P.I.; Irie, H.Y.; Parekh, S.; Reddy, E.P. CDK4: A Master Regulator of the Cell Cycle and Its Role in Cancer. Genes Cancer 2022, 13, 21–45. [Google Scholar] [CrossRef]
- Nakka, K.; Hachmer, S.; Mokhtari, Z.; Kovac, R.; Bandukwala, H.; Bernard, C.; Li, Y.; Xie, G.; Liu, C.; Fallahi, M.; et al. JMJD3 activated hyaluronan synthesis drives muscle regeneration in an inflammatory environment. Science 2022, 377, 666–669. [Google Scholar] [CrossRef]
- Neelakantan, H.; Brightwell, C.R.; Graber, T.G.; Maroto, R.; Wang, H.-Y.L.; McHardy, S.F.; Papaconstantinou, J.; Fry, C.; Watowich, S. Small molecule nicotinamide N-methyltransferase inhibitor activates senescent muscle stem cells and improves regenerative capacity of aged skeletal muscle. Biochem. Pharmacol. 2019, 163, 481–492. [Google Scholar] [CrossRef]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.A.; Lemischka, I.R. Stem cells and their niches. Science 2006, 311, 1880–1885. [Google Scholar] [CrossRef] [PubMed]
- Brazile, B.L.; Lin, S.; Copeland, K.; Butler, J.; Cooley, J.; Brinkman-Ferguson, E.L.; Guan, J.; Liao, J. Ultrastructure and Biomechanics of Skeletal Muscle ECM. In Bio-Instructive Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2017; pp. 139–160. [Google Scholar] [CrossRef]
- Fuoco, C.; Petrilli, L.L.; Cannata, S.; Gargioli, C. Matrix scaffolding for stem cell guidance toward skeletal muscle tissue engineering. J. Orthop. Surg. Res. 2016, 11, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corona, B.; Wu, X.; Ward, C.; McDaniel, J.; Rathbone, C.; Walters, T. The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM. Biomaterials 2013, 34, 3324–3335. [Google Scholar] [CrossRef]
- Grasman, J.; Zayas, M.; Page, R.; Pins, G. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater. 2015, 25, 2–15. [Google Scholar] [CrossRef]
- Sarrafian, T.L.; Bodine, S.; Murphy, B.; Grayson, J.K.; Stover, S. Extracellular matrix scaffolds for treatment of large volume muscle injuries: A review. Vet. Surg. 2018, 47, 524–535. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y. Role of matrix metalloproteinases in skeletal muscle. Cell Adhes. Migr. 2009, 3, 337–341. [Google Scholar] [CrossRef]
- Pauly, R.; Passaniti, A.; Bilato, C.; Monticone, R.; Cheng, L.; Papadopoulos, N.; Gluzband, Y.A.; Smith, L.; Weinstein, C.; Lakatta, E. Migration of cultured vascular smooth muscle cells through a basement membrane barrier requires type IV collagenase activity and is inhibited by cellular differentiation. Circ. Res. 1994, 75, 41–54. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Qian, Y.; Tang, H.; Song, J.; Qu, X.; Yue, B.; Yuan, W. Melatonin-Based and Biomimetic Scaffold as Muscle–ECM Implant for Guiding Myogenic Differentiation of Volumetric Muscle Loss. Adv. Funct. Mater. 2020, 30, 2002378. [Google Scholar] [CrossRef]
- Kim, S.-H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef]
- Hu, L.-Y.; Mileti, C.; Loomis, T.; Brashear, S.E.; Ahmad, S.; Chellakudam, R.R.; Wohlgemuth, R.P.; Gionet-Gonzales, M.A.; Leach, J.K.; Smith, L.R. Skeletal Muscle progenitors are sensitive to collagen architectural features of fibril size and crosslinking. Am. J. Physiol. Cell Physiol. 2021, 321, C330–C342. [Google Scholar] [CrossRef] [PubMed]
- Stearns-Reider, K.M.; D’Amore, A.; Beezhold, K.; Rothrauff, B.B.; Cavalli, L.; Wagner, W.; Vorp, D.; Tsamis, A.; Shinde, S.; Zhang, C.; et al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 2017, 16, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Shinin, V.; Gayraud-Morel, B.; Gomès, D.; Tajbakhsh, S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat. Cell Biol. 2006, 8, 677–682. [Google Scholar] [CrossRef]
- Pine, S.R.; Liu, W. Asymmetric Cell Division and Template DNA Co-Segregation in Cancer Stem Cells. Front. Oncol. 2014, 4, 226. [Google Scholar] [CrossRef]
- Conboy, M.; Karasov, A.; Rando, T. High Incidence of Non-Random Template Strand Segregation and Asymmetric Fate Determination In Dividing Stem Cells and their Progeny. PLoS Biol. 2007, 5, e102. [Google Scholar] [CrossRef]
- Pahnke, A.; Conant, G.; Huyer, L.D.; Zhao, Y.; Feric, N.; Radisic, M. The role of Wnt regulation in heart development, cardiac repair and disease: A tissue engineering perspective. Biochem. Biophys. Res. Commun. 2016, 473, 698–703. [Google Scholar] [CrossRef]
- Siddique, H.R.; Feldman, D.; Chen, C.L.; Punj, V.; Tokumitsu, H.; Machida, K. NUMB phosphorylation destabilizes p53 and promotes self-renewal of tumor-initiating cells by a NANOG-dependent mechanism in liver cancer. Hepatology 2015, 62, 1466–1479. [Google Scholar] [CrossRef]
- Ting, S.; Chagraoui, J.; Sauvageau, G. Differential Expression and Re-Localization of Potential Cell Fate Determinants in Hematopoietic and Leukemia Stem Cells. Blood 2007, 110, 93. [Google Scholar] [CrossRef]
- Shimada, M.; Tsukada, K.; Kagawa, N.; Matsumoto, Y. Reprogramming and differentiation-dependent transcriptional alteration of DNA damage response and apoptosis genes in human induced pluripotent stem cells. J. Radiat. Res. 2019, 60, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Will, B.; Todorova, T.I.; Verma, A.; Steidl, U.; Melnick, A. Regulation of hematopoietic stem cell fate by special at-rich sequence binding protein 1. Exp. Hematol. 2014, 42, S66. [Google Scholar] [CrossRef]
- Rabenhorst, U.; Thalheimer, F.; Gerlach, K.; Kijonka, M.; Böhm, S.; Krause, D.; Vauti, F.; Arnold, H.; Schroeder, T.; Schnütgen, F.; et al. Single-Stranded DNA-Binding Transcriptional Regulator FUBP1 Is Essential for Fetal and Adult Hematopoietic Stem Cell Self-Renewal. Cell Rep. 2015, 11, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Sardone, F.; Lauria, A.; Kleiner, C.; Stott, G.M.; Bosi, A.; Di Palma, S.; Vignoli, A.; Colombo, M.; Ceccherini, I.; Cagnoli, C.; et al. The PI3K/Akt/mTOR pathway regulates mitochondrial activity and bioenergetics of muscle stem cells in the context of a muscular dystrophy. PLoS ONE 2016, 11, e0145523. [Google Scholar] [CrossRef]
- Wu, C.; Gong, Y.; Yu, Z.; Deng, Y.; Wang, X.; Ling, Z. Notch signaling suppresses differentiation and induces stemness in skeletal muscle cells by regulating Wnt/β-catenin signaling. Mol. Cell. Biol. 2017, 37, 7389. [Google Scholar]
- Pawlikowski, J.S.; Morrison, C.D.; Vogel, A.M.; Long, K.M.; Ferrer-Lorente, R.; Braun, T. Mechanisms Regulating Niche Homeostasis in Muscle Stem Cells. Front. Cell Dev. Biol. 2019, 7, 84. [Google Scholar] [CrossRef]
- Goel, A.J.; Rieder, M.; Arnold, H.; Radice, G.; Krauss, R. Niche Cadherins Control the Quiescence-to-Activation Transition in Muscle Stem Cells. Cell Rep. 2017, 21, 2236–2250. [Google Scholar] [CrossRef]
- Hung, M.; Lo, H.F.; Beckmann, A.G.; Demircioglu, D.; Damle, G.; Hasson, D.; Radice, G.L.; Krauss, R.S. Cadherin-dependent adhesion is required for muscle stem cell niche anchorage and maintenance. Development 2024, 151, dev202387. [Google Scholar] [CrossRef]
- Volk, S.; Wang, Y.; Mauldin, E.; Liechty, K.; Adams, S. Diminished Type III Collagen Promotes Myofibroblast Differentiation and Increases Scar Deposition in Cutaneous Wound Healing. Cells Tissues Organs 2011, 194, 25–37. [Google Scholar] [CrossRef]
- Cutroneo, K. Gene therapy for tissue regeneration. J. Cell. Biochem. 2003, 88, 418–425. [Google Scholar] [CrossRef]
- Asp, P.; Blum, R.; Vethantham, V.; Parisi, F.; Micsinai, M.; Cheng, J.; Bowman, C.; Kluger, Y.; Dynlacht, B. Genome-wide remodeling of the epigenetic landscape during myogenic differentiation. Proc. Natl. Acad. Sci. USA 2011, 108, E149–E158. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Bansal, V.; Grunert, M.; Malecova, B.; Dall’Agnese, A.; Latella, L.; Gatto, S.; Ryan, T.; Schulz, K.; Chen, W.; et al. Muscle-relevant genes marked by stable H3K4me2/3 profiles and enriched MyoD binding during myogenic differentiation. PLoS ONE 2017, 12, e0179464. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, X.; Cao, Y.; Li, Z.; Cheng, H.; Zhu, G.; Duan, F.; Na, J.; Han, J.; Chen, Y.-G. Activin/Smad2-induced Histone H3 Lys-27 Trimethylation (H3K27me3) Reduction Is Crucial to Initiate Mesendoderm Differentiation of Human Embryonic Stem Cells. J. Biol. Chem. 2016, 292, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.R.; Murdoch, F.; Fritsch, M. High Histone Acetylation and Decreased Polycomb Repressive Complex 2 Member Levels Regulate Gene Specific Transcriptional Changes During Early Embryonic Stem Cell Differentiation Induced by Retinoic Acid. Stem Cells 2007, 25, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Wang, Z.; Zaromytidou, A.; Zhang, X.; Chow-Tsang, L.-F.; Liu, J.-X.; Kim, H.; Barlas, A.; Manova-Todorova, K.; Kaartinen, V.; et al. A Poised Chromatin Platform for TGF-β Access to Master Regulators. Cell 2011, 147, 1511–1524. [Google Scholar] [CrossRef]
- Cui, K.; Zang, C.; Roh, T.-Y.; Schones, D.E.; Childs, R.; Peng, W.; Zhao, K. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell 2009, 4, 80–93. [Google Scholar] [CrossRef]
- Jana, S.; Levengood, S.K.L. Fabrication and characterization of poly(glycerol sebacate)-gelatin scaffolds for tissue engineering applications. Biomaterials 2015, 67, 285–303. [Google Scholar]
- Mulbauer, G.D.; Matthew, H.W.T. Biomimetic Scaffolds in Skeletal Muscle Regeneration. Discoveries 2019, 7, e90. [Google Scholar] [CrossRef]
- Turner, N.; Adolph, J.Y.; Weber, D.; Irfan, R.Q.; Stolz, D.; Gilbert, T.; Badylak, S. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng. Part A 2010, 16, 3309–3317. [Google Scholar] [CrossRef]
- Robb, K.; Shridhar, A.; Flynn, L. Decellularized Matrices As Cell-Instructive Scaffolds to Guide Tissue-Specific Regeneration. ACS Biomater. Sci. Eng. 2017, 4, 3627–3643. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A. Mimicking growth factors: Role of small molecule scaffold additives in promoting tissue regeneration and repair. RSC Adv. 2019, 9, 18124–18146. [Google Scholar] [CrossRef] [PubMed]
- Schoen, B.; Avrahami, R.; Baruch, L.; Efraim, Y.; Goldfracht, I.; Elul, O.; Davidov, T.; Gepstein, L.; Zussman, E.; Machluf, M. Electrospun Extracellular Matrix: Paving the Way to Tailor-Made Natural Scaffolds for Cardiac Tissue Regeneration. Adv. Funct. Mater. 2017, 27, 1700427. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Sisson, K.; Zhang, C.; Farach-Carson, M.C.; Chase, D.B. Evaluation of cross-linking methods for electrospun gelatin on cell growth and viability. Biomacromolecules 2010, 10, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Sisson, K.; Zhang, C.; Farach-Carson, M.C.; Elsayed-Ali, H.E. Three-dimensional leaching of laser sintered porous polycaprolactone scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 221–230. [Google Scholar]
- Geevarghese, R.; Sajjadi, S.S.; Hudecki, A.; Sajjadi, S.; Jalal, N.R.; Madrakian, T.; Ahmadi, M.; Włodarczyk-Biegun, M.K.; Ghavami, S.; Likus, W.; et al. Biodegradable and Non-Biodegradable Biomaterials and Their Effect on Cell Differentiation. Int. J. Mol. Sci. 2022, 23, 16185. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Kang, M.; Kim, J.-H.; Singh, R.; Jang, J.-H.; Kim, H. Therapeutic-designed electrospun bone scaffolds: Mesoporous bioactive nanocarriers in hollow fiber composites to sequentially deliver dual growth factors. Acta Biomater. 2015, 16, 103–116. [Google Scholar] [CrossRef]
- Magarotto, F.; Sgrò, A.; Henrique Dorigo Hochuli, A.; Andreetta, M.; Grassi, M.; Saggioro, M.; Nogara, L.; Tolomeo, A.; Francescato, R.; Collino, F.; et al. Muscle functional recovery is driven by extracellular vesicles combined with muscle extracellular matrix in a volumetric muscle loss murine model. Biomaterials 2021, 269, 120653. [Google Scholar] [CrossRef]
- Li, W.; Bai, Y.; Cao, J.; Gao, S.; Xu, P.; Feng, G.; Wang, L.; Wang, H.; Kong, D.; Fan, M.; et al. Highly interconnected inverse opal extracellular matrix scaffolds enhance stem cell therapy in limb ischemia. Acta Biomater. 2021, 128, 209–221. [Google Scholar] [CrossRef]
- Zhu, M.; Li, W.; Dong, X.; Yuan, X.; Midgley, A.C.; Chang, H.; Wang, Y.; Wang, H.; Wang, K.; Ma, P.; et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019, 10, 4620. [Google Scholar] [CrossRef] [PubMed]
- Cunniffe, G.; Díaz-Payno, P.; Sheehy, E.J.; Critchley, S.E.; Almeida, H.; Pitacco, P.; Carroll, S.; Mahon, O.R.; Dunne, A.; Levingstone, T.; et al. Tissue-specific extracellular matrix scaffolds for the regeneration of spatially complex musculoskeletal tissues. Biomaterials 2019, 188, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Levenberg, S. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005, 23, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef]
- Sultana, N.; Cole, A.; Strachan, F. Biocomposite Scaffolds for Tissue Engineering: Materials, Fabrication Techniques and Future Directions. Materials 2024, 17, 5577. [Google Scholar] [CrossRef]
- Alcazar, C.; Hu, C.; Rando, T.; Huang, N.; Nakayama, K. Transplantation of insulin-like growth factor-1 laden scaffolds combined with exercise promotes neuroregeneration and angiogenesis in a preclinical muscle injury model. Biomater. Sci. 2020, 8, 5376–5389. [Google Scholar] [CrossRef]
- Kheradmandi, M.; Vasheghani-Farahani, E.; Ghiaseddin, A.; Ganji, F. Skeletal muscle regeneration via engineered tissue culture over electrospun nanofibrous chitosan/PVA scaffold. J. Biomed. Mater. Res. Part A 2016, 104, 1720–1727. [Google Scholar] [CrossRef]
- Sleep, E.; Cosgrove, B.; McClendon, M.T.; Preslar, A.T.; Chen, C.H.; Sangji, M.H.; Pérez, C.R.; Haynes, R.D.; Meade, T.; Blau, H.; et al. Injectable biomimetic liquid crystalline scaffolds enhance muscle stem cell transplantation. Proc. Natl. Acad. Sci. USA 2017, 114, E7919–E7928. [Google Scholar] [CrossRef]
- Wang, L.; Cao, L.; Shansky, J.; Wang, Z.; Mooney, D.; Vandenburgh, H. Minimally invasive approach to the repair of injured skeletal muscle with a shape-memory scaffold. Mol. Ther. 2014, 22, 1441–1449. [Google Scholar] [CrossRef]
- Aurora, A.; Roe, J.; Corona, B.; Walters, T. An acellular biologic scaffold does not regenerate appreciable de novo muscle tissue in rat models of volumetric muscle loss injury. Biomaterials 2015, 67, 393–407. [Google Scholar] [CrossRef]
- Eugenis, I.; Wu, D.; Rando, T. Cells, scaffolds, and bioactive factors: Engineering strategies for improving regeneration following volumetric muscle loss. Biomaterials 2021, 278, 121173. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Li, Q.; Zhao, Y.; Chen, W.; Chen, B.; Xiao, Z.; Lin, H.; Nie, L.; Wang, D.; Dai, J. Stem-cell-capturing collagen scaffold promotes cardiac tissue regeneration. Biomaterials 2011, 32, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, M.; Lee, J.W.; Kim, Y.H.; Kim, S.-H.; Kim, S. Cartilage regeneration with highly-elastic three-dimensional scaffolds prepared from biodegradable poly(L-lactide-co-epsilon-caprolactone). Biomaterials 2008, 29, 4630–4636. [Google Scholar] [CrossRef] [PubMed]
- Atzet, S.K.; Curtin, S.; Trinh, P.; Bryant, S.; Ratner, B. Degradable poly(2-hydroxyethyl methacrylate)-co-polycaprolactone hydrogels for tissue engineering scaffolds. Biomacromolecules 2008, 9, 3370–3377. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.Y.; Jing, X.; Napiwocki, B.; Hagerty, B.S.; Chen, G.; Turng, L. Biocompatible, degradable thermoplastic polyurethane based on polycaprolactone-block-polytetrahydrofuran-block-polycaprolactone copolymers for soft tissue engineering. J. Mater. Chem. B 2017, 5, 4137–4151. [Google Scholar] [CrossRef]
- Levenberg, S.; Golub, J.S.; Amit, M.; Itskovitz-Eldor, J.; Langer, R. Endothelial cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2005, 99, 4391–4396. [Google Scholar] [CrossRef]
- Turner, N.J.; Badylak, S.F. Regeneration of skeletal muscle. Cell Tissue Res. 2012, 347, 759–774. [Google Scholar] [CrossRef]
- Kim, P.; Yuan, A.; Nam, K.; Jiao, A.; Kim, D.H. Fabrication of poly(ethylene glycol): Gelatin methacrylate composite nanostructures with tunable stiffness and degradation for vascular tissue engineering. Biofabrication 2014, 6, 024112. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, J.; Zhang, W.; Yan, S.; Ding, J.; Chen, X.; Cui, L.; Yin, J. In situ formation of hydrophobic clusters to enhance mechanical performance of biodegradable poly(l-glutamic acid)/poly(ε-caprolactone) hydrogel towards meniscus tissue engineering. J. Mater. Chem. B 2018, 6, 7822–7833. [Google Scholar] [CrossRef]
- Park, J.Y.; Gao, G.; Jang, J.; Cho, D. 3D printed structures for delivery of biomolecules and cells: Tissue repair and regeneration. J. Mater. Chem. B 2016, 4, 7521–7539. [Google Scholar] [CrossRef]
- Tarafder, S.; Koch, A.; Jun, Y.; Chou, C.; Awadallah, M.R.; Lee, C.H. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication 2016, 8, 025003. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, M.; Luo, J.; Zhao, H.; Zhou, X.; Gu, Q.-l.; Yang, H.; Zhu, X.; Cui, W.; Shi, Q. Immunopolarization-regulated 3D printed-electrospun fibrous scaffolds for bone regeneration. Biomaterials 2021, 276, 121037. [Google Scholar] [CrossRef] [PubMed]
- El-Habashy, S.E.; El-Kamel, A.; Essawy, M.M.; Abdelfattah, E.Z.A.; Eltaher, H. 3D printed bioinspired scaffolds integrating doxycycline nanoparticles: Customizable implants for in vivo osteoregeneration. Int. J. Pharm. 2021, 607, 121002. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Lee, C.H. 3D printing integrated with controlled delivery for musculoskeletal tissue engineering. J. Pharm. Sci. 2017, 1, 181–189. [Google Scholar] [CrossRef]
- Camacho, P.; Busari, H.; Seims, K.B.; Schwarzenberg, P.; Dailey, H.; Chow, L. 3D printing with peptide-polymer conjugates for single-step fabrication of spatially functionalized scaffolds. Biomater. Sci. 2019, 7, 4237–4247. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, W.; Holmes, B.; Zhang, L.G. Biologically Inspired Smart Release System Based on 3D Bioprinted Perfused Scaffold for Vascularized Tissue Regeneration. Adv. Sci. 2016, 3, 1600058. [Google Scholar] [CrossRef]
- Johnson, B.N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; Engel, E.; Krick, K.; Ju, A.; Meng, F.; et al. 3D Printed Anatomical Nerve Regeneration Pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef]
- Asparuhova, M.; Gelman, L.; Chiquet, M. Role of the actin cytoskeleton in tuning cellular responses to external mechanical stress. Scand. J. Med. Sci. Sports 2009, 19, 490–499. [Google Scholar] [CrossRef]
- Parsons, J.; Martin, K.; Slack, J.K.; Taylor, J.M.; Weed, S. Focal Adhesion Kinase: A regulator of focal adhesion dynamics and cell movement. Oncogene 2000, 19, 5606–5613. [Google Scholar] [CrossRef]
- Kuo, J.-C. Mechanotransduction at focal adhesions: Integrating cytoskeletal mechanics in migrating cells. J. Cell. Mol. Med. 2013, 17, 704–712. [Google Scholar] [CrossRef]
- Kuo, J.-C. Focal adhesions function as a mechanosensor. Prog. Mol. Biol. Transl. Sci. 2014, 126, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Hanson, D.A.; Schlaepfer, D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Ryall, J.G.; Cliff, T.; Dalton, S.; Sartorelli, V. Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell 2015, 17, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Chua, Y.B.; Liu, T.; Liang, K.; Chua, M.J.; Ma, W.; Goh, J.W.; Wang, Y.; Su, J.; Ho, Y.S.; et al. Muscle Injuries Induce a Prostacyclin-PPARγ/PGC1a-FAO Spike That Boosts Regeneration. Adv. Sci. 2023, 10, e2301519. [Google Scholar] [CrossRef]
- Lian, D.; Chen, M.M.; Wu, H.; Deng, S.; Hu, X. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef]
- Nguyen, J.H.; Chung, J.D.; Lynch, G.S.; Ryall, J.G. The Microenvironment Is a Critical Regulator of Muscle Stem Cell Activation and Proliferation. Front. Cell Dev. Biol. 2019, 7, 254. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef]
- Liu, L.; Rando, T. UTX in muscle regeneration--the right dose and the right time. J. Clin. Investig. 2016, 126, 1233–1235. [Google Scholar] [CrossRef]
- Gan, Y.; Zhou, J.; Quan, R.; Hong, L.; Li, Z.; Zheng, E.; Liu, D.; Wu, Z.; Cai, G.; Gu, T. Histone H3K27me3 in the regulation of skeletal muscle development. Yi Chuan Hered. 2019, 41, 285–292. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Tyagi, S. Epigenetic mechanisms underlying cardiac degeneration and regeneration. Int. J. Cardiol. 2014, 173, 1–11. [Google Scholar] [CrossRef][Green Version]
- Tingle, C.F.; Magnuson, B.; Zhao, Y.; Heisel, C.J.; Kish, P.; Kahana, A. Paradoxical Changes Underscore Epigenetic Reprogramming During Adult Zebrafish Extraocular Muscle Regeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4991–4999. [Google Scholar] [CrossRef]
- Chow, M.; Geng, L.; Kong, C.; Keung, W.; Fung, J.; Boheler, K.; Li, R.A. Epigenetic regulation of the electrophysiological phenotype of human embryonic stem cell-derived ventricular cardiomyocytes: Insights for driven maturation and hypertrophic growth. Stem Cells Dev. 2013, 22, 2678–2690. [Google Scholar] [CrossRef]
- Borselli, C.; Christine, A.C.; Dymitri, S.; Vandenburgh, H.; Mooney, D. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials 2011, 32, 8905–8914. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hou, P.; Zhang, W.; Zuo, M.; Liu, Z.; Wang, T.; Zhou, Y.; Chen, W.; Feng, C.; Hu, B.; et al. Species variations in muscle stem cell-mediated immunosuppression on T cells. Sci. Rep. 2024, 14, 23410. [Google Scholar] [CrossRef]
- Sheehan, S.; Allen, R. Skeletal muscle satellite cell proliferation in response to members of the fibroblast growth factor family and hepatocyte growth factor. J. Cell. Physiol. 1999, 181, 499–506. [Google Scholar] [CrossRef]
- Falco, E.E.; Wang, M.O.; Thompson, J.A.; Chetta, J.; Yoon, D.; Li, E.Z.; Kulkami, M.M.; Shah, S.B.; Pandit, A.; Roth, J.; et al. Porous EH and EH-PEG Scaffolds as Gene Delivery Vehicles to Skeletal Muscle. Pharm. Res. 2011, 28, 1306–1316. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef]
- Vardar, E.; Larsson, H.; Engelhardt, E.; Pinnagoda, K.; Briquez, P.; Hubbell, J.; Frey, P. IGF-1-containing multi-layered collagen-fibrin hybrid scaffolds for bladder tissue engineering. Acta Biomater. 2016, 41, 75–85. [Google Scholar] [CrossRef]
- Zhao, G.; Ge, Y.; Zhang, C.; Zhang, L.; Xu, J.; Qi, L.; Li, W. Progress of Mesenchymal Stem Cell-Derived Exosomes in Tissue Repair. Curr. Pharm. Des. 2020, 26, 2022–2037. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef]
- Harrell, C.; Jovicic, N.; Djonov, V.; Volarevic, V. Therapeutic Use of Mesenchymal Stem Cell-Derived Exosomes: From Basic Science to Clinics. Pharmaceutics 2020, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Dominici, C.; Villarreal, O.D.; Dort, J.; Heckel, E.; Wang, Y.C.D.; Ragoussis, I.; Joyal, J.S.; Dumont, N.A.; Richard, S. Inhibition of type I PRMTs reforms muscle stem cell identity enhancing their therapeutic capacity. elife 2023, 12, RP84570. [Google Scholar] [CrossRef] [PubMed]
- Ichiseki, T.; Shimasaki, M.; Ueda, S.; Hirata, H.; Souma, D.; Kawahara, N.; Ueda, Y. Efficacy of Rectal Systemic Administration of Mesenchymal Stem Cells to Injury Sites via the CXCL12/CXCR4 Axis to Promote Regeneration in a Rabbit Skeletal Muscle Injury Model. Cells 2023, 12, 1729. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mittal, A.; Makonchuk, D.Y.; Bhatnagar, S.; Kumar, A. Matrix metalloproteinase-9 inhibition ameliorates pathogenesis and improves skeletal muscle regeneration in muscular dystrophy. Hum. Mol. Genet. 2009, 18, 2584–2598. [Google Scholar] [CrossRef]
- Wang, Y.X.; Feige, P.; Brun, C.E.; Hekmatnejad, B.; Dumont, N.A.; Renaud, J.; Faulkes, S.; Guindon, D.E.; Rudnicki, M.A. EGFR-Aurka Signaling Rescues Polarity and Regeneration Defects in Dystrophin-Deficient Muscle Stem Cells by Increasing Asymmetric Divisions. Cell Stem Cell 2019, 24, 419–432.e416. [Google Scholar] [CrossRef]
- Sassoli, C.; Pierucci, F.; Zecchi-Orlandini, S.; Meacci, E. Transcriptome reprogramming through epigenomic changes enhances regenerative capacity in MuSCs. Int. J. Mol. Sci. 2019, 20, 5545. [Google Scholar] [CrossRef]
- Chang, N.C.; Sincennes, M.C.; Chevalier, F.P.; Brun, C.E.; Lacaria, M.; Segalés, J.; Muñoz-Cánoves, P.; Ming, H.; Rudnicki, M.A. The Dystrophin Glycoprotein Complex Regulates the Epigenetic Activation of Muscle Stem Cell Commitment. Cell Stem Cell 2018, 22, 755–768.e6. [Google Scholar] [CrossRef]
- Kota, S.; Roening, C.; Patel, N.; Baron, R. PRMT5 inhibition promotes osteogenic differentiation of mesenchymal stromal cells and represses basal interferon stimulated gene expression. Bone 2018, 117, 37–46. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Chen, W.; Zhang, Z.; Zhou, Y.; Gou, Y.; Lv, C.A.; Jin, L.; Qiu, X.; Ma, S.; et al. Myofiber Baf60c controls muscle regeneration by modulating Dkk3-mediated paracrine signaling. J. Exp. Med. 2023, 220, e20221123. [Google Scholar] [CrossRef]
- Hayashi, T.; Sadaki, S.; Tsuji, R.; Okada, R.; Fuseya, S.; Kanai, M.; Nakamura, A.; Okamura, Y.; Muratani, M.; Wenchao, G.; et al. Dual-specificity phosphatases 13 and 27 as key switches in muscle stem cell transition from proliferation to differentiation. Stem Cells 2024, 42, 830–847. [Google Scholar] [CrossRef]
- Xie, L.; Yin, A.; Nichenko, A.S.; Beedle, A.M.; Call, J.A.; Yin, H. Transient HIF2A inhibition promotes satellite cell proliferation and muscle regeneration. J. Clin. Investig. 2018, 128, 2339–2355. [Google Scholar] [CrossRef] [PubMed]
- Judson, R.N.; Quarta, M.; Oudhoff, M.J.; Soliman, H.; Yi, L.; Chang, C.K.; Loi, G.; Vander Werff, R.; Cait, A.; Hamer, M.; et al. Inhibition of Methyltransferase Setd7 Allows the In Vitro Expansion of Myogenic Stem Cells with Improved Therapeutic Potential. Cell Stem Cell 2018, 22, 177–190.e7. [Google Scholar] [CrossRef] [PubMed]
- Judson, R.N.; Rossi, F.M.V. Towards stem cell therapies for skeletal muscle repair. NPJ Regen. Med. 2020, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Somers, S.M.; Gilbert-Honick, J.; Choi, I.Y.; Lo, E.K.W.; Lim, H.; Dias, S.; Wagner, K.R.; Mao, H.Q.; Cahan, P.; Lee, G.; et al. Engineering Skeletal Muscle Grafts with PAX7::GFP-Sorted Human Pluripotent Stem Cell-Derived Myogenic Progenitors on Fibrin Microfiber Bundles for Tissue Regeneration. Bioengineering 2022, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Han, W.M.; Mohiuddin, M.; Anderson, S.E.; García, A.J.; Jang, Y.C. Co-delivery of Wnt7a and muscle stem cells using synthetic bioadhesive hydrogel enhances murine muscle regeneration and cell migration during engraftment. Acta Biomater. 2019, 94, 243–252. [Google Scholar] [CrossRef]
- Moradi, S.; Mahdizadeh, H.; Šarić, T.; Kim, J.; Harati, J.; Shahsavarani, H.; Greber, B.; Moore, J.B. 4th. Research and therapy with induced pluripotent stem cells (iPSCs): Social, legal, and ethical considerations. Stem Cell Res. Ther. 2019, 10, 341. [Google Scholar] [CrossRef]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.; Lai, R.C.; Hui, J.; Lim, S.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Xiao, M.; Wu, C.H.; Meek, G.; Kelly, B.; Castillo, D.B.; Young, L.E.A.; Martire, S.; Dhungel, S.; McCauley, E.; Saha, P.; et al. PASK links cellular energy metabolism with a mitotic self-renewal network to establish differentiation competence. eLife 2023, 12, e81717. [Google Scholar] [CrossRef]
- Petersen, P.; Tang, H.; Zou, K.; Zhong, W. The Enigma of the Numb-Notch Relationship during Mammalian Embryogenesis. Dev. Neurosci. 2006, 28, 156–168. [Google Scholar] [CrossRef]
- Wang, K.; Frey, N.; Garcia, A.; Man, K.; Yang, Y.; Gualerzi, A.; Clemens, Z.J.; Bedoni, M.; LeDuc, P.R.; Ambrosio, F. Nanotopographical Cues Tune the Therapeutic Potential of Extracellular Vesicles for the Treatment of Aged Skeletal Muscle Injuries. ACS Nano 2023, 17, 19640–19651. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Qi, Y.; Sun, S.; Tao, Y.; Shi, R. Mesenchymal Stem Cell-derived Exosomes: Hope for Spinal Cord Injury Repair. Stem Cells Dev. 2020, 29, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Girkin, J. Multiphoton microscopy: In depth in vivo sub cellular resolution imaging. Proc. SPIE Int. Soc. Opt. Eng. 2006, 6163, 1–8. [Google Scholar] [CrossRef]
- Peti-Peterdi, J.; Kidokoro, K.; Riquier-Brison, A. Novel in vivo techniques to visualize kidney anatomy and function. Kidney Int. 2015, 88, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Shin, Y.C.; Lee, J.H.; Jun, S.; Kim, C.-S.; Lee, Y.; Park, J.-C.; Lee, S.H.; Park, K.; Han, D. Multiphoton imaging of myogenic differentiation in gelatin-based hydrogels as tissue engineering scaffolds. Biomater. Res. 2016, 20, 1–7. [Google Scholar] [CrossRef]
- Williams, R.M. New Strategies for Cellular-Scale Imaging of Bone and Cartilage. FASEB J. 2015, 29, 12. [Google Scholar] [CrossRef]
- Hogan, K.J.; Smoak, M.; Koons, G.L.; Perez, M.; Bedell, M.L.; Jiang, E.Y.; Young, S.; Mikos, A. Bioinspired electrospun decellularized extracellular matrix scaffolds promote muscle regeneration in a rat skeletal muscle defect model. J. Biomed. Mater. Res. Part A 2022, 110, 1090–1100. [Google Scholar] [CrossRef]
- Lee, H.; Ju, Y.M.; Kim, I.; Elsangeedy, E.; Lee, J.H.; Yoo, J.; Atala, A.; Lee, S.J. A novel decellularized skeletal muscle-derived ECM scaffolding system for in situ muscle regeneration. Methods 2020, 171, 77–85. [Google Scholar] [CrossRef]
- Urciuolo, A.; Urbani, L.; Perin, S.; Maghsoudlou, P.; Scottoni, F.; Gjinovci, A.; Collins-Hooper, H.; Loukogeorgakis, S.; Tyraskis, A.; Torelli, S.; et al. Decellularised skeletal muscles allow functional muscle regeneration by promoting host cell migration. Sci. Rep. 2018, 8, 8398. [Google Scholar] [CrossRef]
- Surendrasingh, Y.S.; Elif, G.E.; Kothapalli, C.; Sikder, P. Extrusion 3D (Bio)Printing of Alginate-Gelatin-Based Composite Scaffolds for Skeletal Muscle Tissue Engineering. Materials 2022, 15, 7945. [Google Scholar] [CrossRef]
- Alireza, M.; Masoumeh Haghbin, N.; Karkhaneh, A. Polypyrrole-chitosan hydrogel reinforced with collagen-grafted PLA sub-micron fibers as an electrically responsive scaffold. Int. J. Polym. Mater. Polym. Biomater. 2020, 71, 302–314. [Google Scholar] [CrossRef]
- Pathum, C.; Seok-Chun, K.; Oh, G.; Seong-Yeong, H.; Nguyen, V.; Jeon, Y.; Bonggi, L.; Jang, C.; Geunhyung, K.; Park, W.; et al. Fish collagen/alginate/chitooligosaccharides integrated scaffold for skin tissue regeneration application. Int. J. Biol. Macromol. 2015, 81, 504–513. [Google Scholar] [CrossRef]
- Tohamy, K.; Mabrouk, M.; Soliman, I.; Beherei, H.; Mohamed, A.A. Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: In vitro cell viability and proliferation of human mesenchymal stem cells. Int. J. Biol. Macromol. 2018, 112, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Pariya, Z.; Mohamad, P.M.; Davachi, S.M.; Pouria, Z.; Yazdian, F.; Simorgh, S.; Hadi, G.; Rashedi, H.; Bagher, Z. Alginate sulfate-based hydrogel/nanofiber composite scaffold with controlled Kartogenin delivery for tissue engineering. Carbohydr. Polym. 2021, 266, 118123. [Google Scholar] [CrossRef]
- Simões, F.C.; Cahill, T.; Kenyon, A.; Gavriouchkina, D.; Vieira, J.; Sun, X.; Pezzolla, D.; Ravaud, C.; Masmanian, E.; Weinberger, M.; et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Teo, B.K.; Wong, S.T.; Lim, C.K.; Kung, T.Y.S.; Yap, C.; Ramagopal, Y.; Romer, L.; Yim, E. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano 2013, 7, 4785–4798. [Google Scholar] [CrossRef]
- D’Amore, A.; Nasello, G.; Luketich, S.K.; Denisenko, D.; Jacobs, D.L.; Hoff, R.; Gibson, G.; Bruno, A.; Raimondi, M.T.; Wagner, W. Meso-scale topological cues influence extracellular matrix production in a large deformation, elastomeric scaffold model. Soft Matter 2018, 14, 8483–8495. [Google Scholar] [CrossRef]
- Wan, S.; Fu, X.; Ji, Y.; Li, M.; Shi, X.; Wang, Y. FAK- and YAP/TAZ dependent mechanotransduction pathways are required for enhanced immunomodulatory properties of adipose-derived mesenchymal stem cells induced by aligned fibrous scaffolds. Biomaterials 2018, 171, 107–117. [Google Scholar] [CrossRef]
- Leucht, P.; Kim, J.B.; Currey, J.A.; Brunski, J.; Helms, J. FAK-Mediated Mechanotransduction in Skeletal Regeneration. PLoS ONE 2007, 2, e390. [Google Scholar] [CrossRef]
- Ye, G.J.; Nesmith, A.P.; Parker, K. The Role of Mechanotransduction on Vascular Smooth Muscle Myocytes Cytoskeleton and Contractile Function. Anat. Rec. 2014, 297, 1758–1769. [Google Scholar] [CrossRef]
- Martineau, L.; Gardiner, P. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J. Appl. Physiol. 2001, 91, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Digabel, J.L.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chakraborty, S.; Heaps, C.; Davis, M.; Meininger, G.; Muthuchamy, M. Fibronectin increases the force production of mouse papillary muscles via α5β1 integrin. J. Mol. Cell. Cardiol. 2011, 50, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Sun, Z.; Hill, M.; Meininger, G. A Calcium Mediated Mechanism Coordinating Vascular Smooth Muscle Cell Adhesion During KCl Activation. Front. Physiol. 2018, 9, 1810. [Google Scholar] [CrossRef]
- Kuo, S.; Lin, H.I.; Ho, J.; Shih, Y.R.V.; Chen, H.; Yen, T.; Lee, O. Regulation of the fate of human mesenchymal stem cells by mechanical and stereo-topographical cues provided by silicon nanowires. Biomaterials 2012, 33, 5013–5022. [Google Scholar] [CrossRef]
- Urciuoli, E.; Peruzzi, B. Involvement of the FAK Network in Pathologies Related to Altered Mechanotransduction. Int. J. Mol. Sci. 2020, 21, 9426. [Google Scholar] [CrossRef]
- Nedjari, S.; Awaja, F.; Guarino, R.; Gugutkov, D.; Altankov, G. Establishing multiple osteogenic differentiation pathways of mesenchymal stem cells through different scaffold configurations. J. Tissue Eng. Regen. Med. 2020, 14, 1428–1437. [Google Scholar] [CrossRef]
- Seo, C.; Jeong, H.; Feng, Y.; Montagne, K.; Ushida, T.; Suzuki, Y.; Furukawa, K. Micropit surfaces designed for accelerating osteogenic differentiation of murine mesenchymal stem cells via enhancing focal adhesion and actin polymerization. Biomaterials 2014, 35, 2245–2252. [Google Scholar] [CrossRef]
- Ozdemir, T.; Xu, L.C.; Siedlecki, C.; Brown, J.L. Substrate curvature sensing through Myosin IIa upregulates early osteogenesis. Integr. Biol. Quant. Biosci. Nano Macro 2013, 5, 1407–1416. [Google Scholar] [CrossRef]
- Pennanen, P.; Alanne, M.; Fazeli, E.; Deguchi, T.; Näreoja, T.; Peltonen, S.; Peltonen, J. Diversity of actin architecture in human osteoclasts: Network of curved and branched actin supporting cell shape and intercellular micrometer-level tubes. Mol. Cell. Biochem. 2017, 432, 131–139. [Google Scholar] [CrossRef]
- Izadpanahi, M.; Seyedjafari, E.; Arefian, E.; Hamta, A.; Hosseinzadeh, S.; Kehtari, M.; Soleimani, M. Nanotopographical cues of electrospun PLLA efficiently modulate non-coding RNA network to osteogenic differentiation of mesenchymal stem cells during BMP signaling pathway. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, X.; Wang, H. Acceleration of osteogenic differentiation of preosteoblastic cells by chitosan containing nanofibrous scaffolds. Biomacromolecules 2009, 10, 2772–2778. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Woo, K.; Tahir, M.; Wu, W.; Elango, J.; Mirza, M.R.; Khan, M.; Shamim, S.; Arany, P.; Rahman, S.U. Enhancing osteoblast differentiation through small molecule-incorporated engineered nanofibrous scaffold. Clin. Oral Investig. 2021, 26, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- VanBlunk, M.; Srikanth, V.; Pandit, S.; Kuznetsov, A.; Brudno, Y. Absorption rate governs cell transduction in dry macroporous scaffolds. Biomater. Sci. 2023, 11, 2372–2382. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.C.; Lin, Y.H.; Chen, Y.W.; Kuo, T.Y.; Shie, M.Y. Additive manufacturing of barium-doped calcium silicate/poly-ε-caprolactone scaffolds to activate CaSR and AKT signalling and osteogenic differentiation of mesenchymal stem cells. J. Mater. Chem. B 2023, 11, 4666–4676. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, J.; Lee, E. Scaffold degradation and tissue regeneration synergy mediated by Wnt/β-catenin pathway. Biomater. Res. 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Lo, C.; Wang, Y.; Lin, F.; Hong, H. Dynamic cellular projections and the Rac-Rho GTPase pathway regulate stem cell niche sensing during injury repair. J. Cell. Physiol. 2012, 227, 3088–3098. [Google Scholar] [CrossRef]
- Nighat, K.-U.; Yan, D.; Michael, S.; Daniela, G.; Jaqueline, A.; Christoph, B.; Brachvogel, B.; Maleki, H. 3D Printing of Antibacterial, Biocompatible, and Biomimetic Hybrid Aerogel-Based Scaffolds with Hierarchical Porosities via Integrating Antibacterial Peptide-Modified Silk Fibroin with Silica Nanostructure. ACS Biomater. Sci. Eng. 2021, 7, 4545–4556. [Google Scholar] [CrossRef]
- Park, H.; Min, K.D.; Min Chae, L.; Kim, S.; Lee, O.; Ju, H.; Bo Mi, M.; Jung Min, L.; Ye Ri, P.; Dong Wook, K.; et al. Fabrication of 3D porous SF/β-TCP hybrid scaffolds for bone tissue reconstruction. J. Biomed. Mater. Res. Part A 2016, 104, 1779–1787. [Google Scholar] [CrossRef]
- Li, J.; Kyungsook, K.; Roohani-Esfahani, S.; Jin, G.; Kaplan, D.; Zreiqat, H. A biphasic scaffold based on silk and bioactive ceramic with stratified properties for osteochondral tissue regeneration. J. Mater. Chem. B 2015, 3, 5361–5376. [Google Scholar] [CrossRef]
- Pandey, B.; Chatterjee, S.; Nimisha, P.; Prashant, A.Y.; Anuya, A.N.; Sayam Sen, G. Silk-Mesoporous Silica-Based Hybrid Macroporous Scaffolds using Ice-Templating Method: Mechanical, Release, and Biological Studies. ACS Appl. Bio Mater. 2018, 1, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Nune, M.; Shivaprasad, M.; Govindaraju, T.; Narayan, K.S. Melanin incorporated electroactive and antioxidant silk fibroin nanofibrous scaffolds for nerve tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ashkan, B.; Amin, S.M.S.; Rafienia, M.; Salamat, M.; Shahram, R.; Raucci, M.; Ambrosio, L. Zn-substituted Mg2SiO4 nanoparticles-incorporated PCL-silk fibroin composite scaffold: A multifunctional platform towards bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112242. [Google Scholar] [CrossRef]
- Jhamak, N.; Fahimeh, R.; Farokhi, M.; Masoumeh Haghbin, N. Silk fibroin/kappa-carrageenan composite scaffolds with enhanced biomimetic mineralization for bone regeneration applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 951–958. [Google Scholar] [CrossRef]
- Jing, L.; Sun, M.; Xu, P.; Yao, K.; Yang, J.; Wang, X.; Liu, H.; Sun, M.; Sun, Y.; Ni, R.; et al. Noninvasive In Vivo Imaging and Monitoring of 3D-Printed Polycaprolactone Scaffolds Labeled with an NIR Region II Fluorescent Dye. ACS Appl. Bio Mater. 2021, 4, 3189–3202. [Google Scholar] [CrossRef]
- Hamid, O.; Eltaher, H.; Sottile, V.; Yang, J. 3D bioprinting of a stem cell-laden, multi-material tubular composite: An approach for spinal cord repair. Mater. Sci. Eng. C 2020, 120, 111707. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Pati, F.; Ha, D.H.; Jang, J.; Han, H.H.; Rhie, J.W.; Cho, D.W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 2014, 62, 164–175. [Google Scholar] [CrossRef]
- Rashad, A.; Mohamed-Ahmed, S.; Ojansivu, M.; Berstad, K.; Yassin, M.; Kivijärvi, T.; Heggset, E.B.; Syverud, K.; Mustafa, K. Coating 3D Printed Polycaprolactone Scaffolds with Nanocellulose Promotes Growth and Differentiation of Mesenchymal Stem Cells. Biomacromolecules 2018, 19, 4307–4319. [Google Scholar] [CrossRef]
- Bhushan, S.; Singh, S.; Maiti, T.K.; Sharma, C.; Dutt, D.; Sharma, S.; Li, C.; Tag Eldin, E.M. Scaffold Fabrication Techniques of Biomaterials for Bone Tissue Engineering: A Critical Review. Bioengineering 2022, 9, 728. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 71, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.W.; Liang, Y.; Rath, R.; Park, A.M.; Maltais, S.; Pintauro, P.; Hofmeister, W.; Lim, C.; Wang, X.; Sung, H. Poly(ε-caprolactone)-carbon nanotube composite scaffolds for enhanced cardiac differentiation of human mesenchymal stem cells. Nanomedicine 2013, 8, 1763–1776. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Mozafari, M.; Bakhtiyari, M.; Moradi, F.; Bagher, Z.; Soleimani, M. Three-dimensional-printed polycaprolactone/polypyrrole conducting scaffolds for differentiation of human olfactory ecto-mesenchymal stem cells into Schwann cell-like phenotypes and promotion of neurite outgrowth. J. Biomed. Mater. Res. Part A 2022, 110, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Thitiset, T.; Damrongsakkul, S.; Yodmuang, S.; Leeanansaksiri, W.; Apinun, J.; Honsawek, S. A novel gelatin/chitooligosaccharide/demineralized bone matrix composite scaffold and periosteum-derived mesenchymal stem cells for bone tissue engineering. Biomater. Res. 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Pezeshki-Modaress, M.; Rajabi-Zeleti, S.; Zandi, M.; Mirzadeh, H.; Sodeifi, N.; Nekookar, A.; Aghdami, N. Cell-loaded gelatin/chitosan scaffolds fabricated by salt-leaching/lyophilization for skin tissue engineering: In vitro and in vivo study. J. Biomed. Mater. Research. Part A 2014, 102, 3908–3917. [Google Scholar] [CrossRef]
- Wang, X.; Molino, B.Z.; Pitkänen, S.; Ojansivu, M.; Xu, C.; Hannula, M.; Hyttinen, J.; Miettinen, S.; Hupa, L.; Wallace, G. 3D Scaffolds of Polycaprolactone/Copper-Doped Bioactive Glass: Architecture Engineering with Additive Manufacturing and Cellular Assessments in a Coculture of Bone Marrow Stem Cells and Endothelial Cells. ACS Biomater. Sci. Eng. 2023, 5, 4496–4510. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.E.; Han, J.; Jeong, S.; Lim, J.; Lee, M.; Son, H.; Kim, H.; Choung, Y.; Seonwoo, H.; et al. 3D-Printed Poly(ε-Caprolactone)/Hydroxyapatite Scaffolds Modified with Alkaline Hydrolysis Enhance Osteogenesis In Vitro. Polymers 2021, 13, 257. [Google Scholar] [CrossRef]
- Lee, H.; Ahn, S.; Bonassar, L.; Kim, G. Cell(MC3T3-E1)-printed poly(ϵ-caprolactone)/alginate hybrid scaffolds for tissue regeneration. Macromol. Rapid Commun. 2013, 34, 142–149. [Google Scholar] [CrossRef]
- Kim, W.; Lee, H.; Lee, J.; Atala, A.; Yoo, J.; Lee, S.; Kim, G.H. Efficient myotube formation in 3D bioprinted tissue construct by biochemical and topographical cues. Biomaterials 2019, 230, 119632. [Google Scholar] [CrossRef]
- Hurd, S.A.; Bhatti, N.; Walker, A.M.; Kasukonis, B.M.; Wolchok, J. Development of a biological scaffold engineered using the extracellular matrix secreted by skeletal muscle cells. Biomaterials 2015, 49, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kasukonis, B.M.; Kim, J.; Washington, T.; Wolchok, J. Development of an infusion bioreactor for the accelerated preparation of decellularized skeletal muscle scaffolds. Biotechnol. Prog. 2016, 32, 745–755. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).