Abstract
Steady-shear rheology and surface activity of surfactant–polymer solutions were investigated experimentally. Four different polymers were studied as follows: cationic hydroxyethyl cellulose, nonionic hydroxyethyl cellulose, nonionic guar gum, and anionic xanthan gum. The influence of the following four surfactants on each of the polymers was determined: nonionic alcohol ethoxylate, anionic sodium lauryl sulfate, cationic hexadecyltrimethylammonium bromide, and zwitterionic cetyl betaine. The interaction between cationic hydroxyethyl cellulose and anionic sodium lauryl sulfate was extraordinarily strong, resulting in dramatic changes in rheological and surface-active properties. The consistency increased initially, reached a maximum value, and then fell off with the further addition of surfactant. The surface tension of surfactant–polymer solution dropped substantially and exhibited a minimum value. Thus, the surfactant–polymer solutions were much more surface-active compared with pure surfactant solutions. The interaction between anionic xanthan gum and cationic hexadecyltrimethylammonium bromide was also strong, resulting in a substantial decrease in consistency. The surfactant–polymer solution became less surface-active compared with pure surfactant solution due to the migration of surfactant from solution to polymer. The interactions between other polymers and surfactants were weak to moderate, resulting in small to modest changes in rheological and surface-active properties. Surface activity of surfactant–polymer solutions often increased due to the formation of complexes more surface-active than pure surfactant molecules.
1. Introduction
The interactions between polymers and surfactants have been exploited by researchers to accomplish desirable properties of the solution in many applications including drug delivery, enhanced oil recovery, hydraulic fracturing and drilling, cosmetics, foods, chemical processing, and many more [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. In general, surfactants are introduced to a polymeric system or vice versa to control and manipulate rheology, improve the stability of the system, and to control the adsorption of surfactants. The presence of a polymer can help in adsorption or desorption of a surfactant from a surface. It can also speed up the micellization process of surfactants, resulting in a decrease in the free surfactant concentration which is particularly beneficial in skin formulations where free surfactant molecules are harmful for the skin, often causing irritation. The interactions between polymers and surfactants are also exploited to intensify frictional drag reduction in the turbulent flow of liquids [28,29].
The interactions between polymer and surfactant depend on the types of polymers and surfactants involved in addition to the solution conditions such as temperature [1,21,24]. The interactions can be broadly classified into two groups: (a) electrostatic interactions and (b) hydrophobic interactions.
Electrostatic interactions occur between ionic polymers (negative or positive charge) and charged ionic surfactants [1,21,24]. Ionic surfactants may bind to oppositely charged groups on a polymer, resulting in the contraction of polymer chains. Neutralization of electric charge of polymer macromolecules could also cause entanglements and the formation of a network of polymer macromolecules. The electrostatic repulsion between like charges of polymer and surfactant molecules could also lead to stretching of the polymer macromolecules.
Hydrophobic interactions occur between nonionic polymers and nonionic surfactants [1,21,24] by association of the hydrophobic portions of nonionic surfactants with hydrophobic portions of polymer molecules. It is possible for hydrophobic tails of surfactant molecules to aggregate onto less polar regions of the polymer molecules to form micelles.
The interaction between polymer and surfactant molecules usually begins at a certain surfactant concentration called “critical aggregation concentration (CAC).” According to the published literature, the CAC is usually lower than the CMC (critical micelle concentration) of the pure surfactant solution [24]. CAC is generally found to be much lower than the CMC of the surfactant when an ionic polymer interacts with oppositely charged ionic surfactants. However, CAC is close to CMC when the interaction occurs between a nonionic polymer and an ionic surfactant. There is another critical surfactant concentration that is relevant to surfactant–polymer mixed systems. This second critical concentration of surfactant is referred to as PSP (polymer saturation point) where the polymer macromolecules become saturated with bound surfactant molecules or micelles. Critical concentrations of CAC and PSP are usually identified by break points in the slopes of the surface tension, electrical conductivity, and viscosity plots as functions of surfactant concentration.
The interactions between the surfactant and the polymer molecules result in the formation of surfactant–polymer aggregates. Surfactant–polymer interactions can also cause the elongation of polymer chains. The surfactant molecules can form micelles at favorable sites of the polymer chains and open and extend the coiled macromolecule. According to the “necklace model” of polymer–surfactant interaction suggested by Nagarajan [21], the polymer chain warps around the surfactant micelles. Thus, surfactant–polymer interactions can have a strong influence on the rheological behavior of solutions, due to the extension, shrinking, warping, and bridging of polymer macromolecules.
Despite several studies published on surfactant–polymer interactions, the complex behavior of a combination of surfactant and polymer additives in solutions is far from being well understood; therefore, further studies are needed to improve our understanding of the interactions between surfactants and polymers and their effects on properties. For example, the interaction between cationic–anionic systems is intuitively expected to be the strongest. However, this is not always true. For example, Lu and Pal [1] found only weak interactions between an anionic–cationic system (anionic polymer–cationic surfactant). The interactions between the same charge anionic–anionic system (anionic polymer and anionic surfactant) were also weak. Jia and Pal [24], on the other hand, found strong interactions between the same charge anionic–anionic system (anionic polymer and anionic surfactant).
The broad objective of this work was to investigate the steady shear rheology and surface activity of a variety of polymer–surfactant mixtures experimentally. Four different polymers were investigated as follows: cationic hydroxyethyl cellulose, nonionic guar gum, nonionic hydroxyethyl cellulose, and anionic xanthan gum. The influence of the following four surfactants on the rheology and surface activity of each polymer was studied: anionic sodium lauryl sulfate, cationic hexadecyltrimethylammonium bromide, zwitterionic cetyl betaine, and nonionic alcohol ethoxylate. To our knowledge, the interactions between the specified polymers and surfactants and their effect on rheology and surface activity have not been studied to any significant extent.
2. Materials and Methods
2.1. Materials
The cationic hydroxyethyl cellulose (herein referred to as CHEC) used in this work was the UCARE Extreme Polymer, a cationic quaternary ammonium salt of hydroxyethyl cellulose, manufactured by Dow Chemical, New Milford, CT, USA. The chemical structure of CHEC is given in Figure 1. It is a bio-derived and biodegradable water-soluble cationic cellulosic polymer that is used as a principal conditioning agent in conditioners, leave-on products, and shampoos.
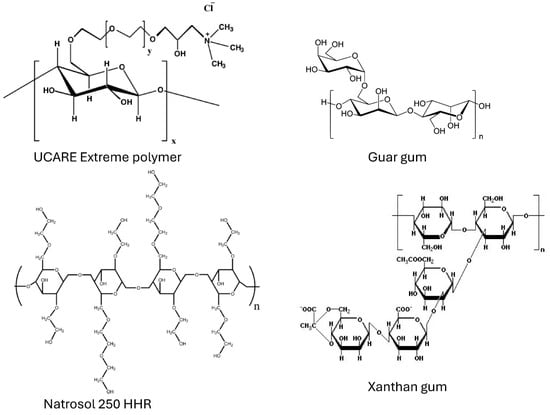
Figure 1.
Chemical structures of the polymers used.
Guar gum, that is, galactomannan polysaccharide, used in this work was a nonionic water-soluble polymer supplied by Sigma-Aldrich, Oakville, ON, Canada. Its chemical structure is shown in Figure 1. Due to its gel-forming property, guar gum is used in the production of many food and non-food products. Examples of products where guar gum is used are cottage cheese, curd, yogurt, sauce, soups, frozen desserts, and many more baked goods.
The nonionic hydroxyethyl cellulose (herein referred to as NHEC) used in this work was Natrosol 250 HHR, manufactured by Ashland Specialty Ingredients, Hopewell, VA, USA. The chemical structure of the polymer is given in Figure 1. It is a water-soluble polymer used extensively in personal care and cosmetic applications. Some of the common applications are hair conditioner, shave gels and foams, toothpaste, makeup/mascara, and lubricant gels.
Xanthan gum, used in this study, is an anionic water-soluble polysaccharide, manufactured by CP Kelco, Atlanta, GA, USA under trade name Kelzan. Its chemical structure is shown in Figure 1. It is a commonly used food additive. It is also used as a thickening agent in toothpaste and improves texture and consistency in ice cream, salad dressings, and baked goods.
The approximate molecular weights of the polymers used in this study are as follows: UCARE extreme polymer: ; Guar gum: ; Nonionic hydroxyethyl cellulose: ; Xanthan gum: .
The influence of four surfactants with different headgroup charge types (anionic, cationic, nonionic, and zwitterionic) was investigated on each polymer.
The anionic surfactant was sodium lauryl sulfate supplied as a dry white powder under the trade name of Stepwet DF-95 by Stepan Company, Northfield, IL, USA. The chemical structure of sodium lauryl sulfate is CH3(CH2)10CH2OSO3Na. This surfactant is used extensively as a foaming agent and mouth dispersant in dentifrices. Other applications where this surfactant is used are hand cleaner, powdered bath, liquid hand soaps, and shampoos.
The cationic surfactant used was hexadecyltrimethylammonium bromide (HTAB). HTAB was supplied in a dry white powder by Sigma-Aldrich, Oakville, ON, Canada. The chemical structure of HTAB is CH3(CH2)15N(Br) (CH3)3. It has many applications including cosmetics fungicide, softener, emulsifier, and antistatic agent.
The nonionic surfactant was C12–14 Alcohol Ethoxylate with 3 EO Units (Alfonic 1412-3), manufactured by Sasol Chemicals, Sandton, South Africa. The chemical structure of the surfactant is CH3(CH2)xCH2(OCH2CH2)3OH where ‘x’ varies between 10 and 12. Some of the applications where alcohol ethoxylates are used include household cleaners, industrial cleaning formulations, agrochemical formulations, wetting agent, emulsifier, and degreaser.
The zwitterionic surfactant used was cetyl betaine (Amphosol CDB), supplied by Stepan Company, Northfield, IL, USA, in aqueous liquid form with 30% active component (cetyl betaine). The chemical formula of cetyl betaine is . It contains both quaternary ammonium and carboxylate groups. It is widely used in cosmetics and personal care products.
Figure 2 shows the chemical structures of surfactants used.
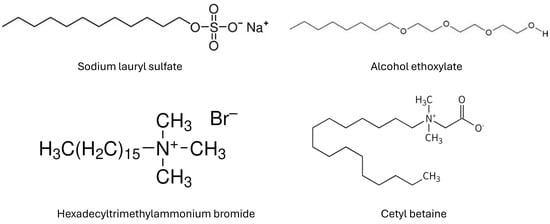
Figure 2.
Chemical structures of the surfactants used.
2.2. Preparation of Polymer and Surfactant–Polymer Solutions
Polymer solution was prepared at room temperature (22 ± 1 °C) in a batch of 1 kg. A known amount of polymer was added to a known amount of deionized water. A variable-speed Gifford-Wood homogenizer (Model 1 L) (Federal Equipment Company, Cleveland, OH, USA) was used to mix the polymer. The speed of the homogenizer was controlled by adjusting the voltage supplied to the homogenizer with the help of a variable autotransformer (variac) (Staco Energy Products Co., Miamisburg, OH, USA). The mixing was carried out for 1 h at a fixed speed (variac setting of 30%) to ensure complete dissolution of polymer. The surfactant–polymer mixture was prepared by adding the known amount of surfactant to the polymer solution while maintaining the mixing by the homogenizer for 1 h at 30% variac setting. For surfactant–polymer mixtures, the polymer concentration was fixed at 2000 ppm by weight, and the surfactant concentration varied from 0 to 500 ppm by weight. In the case of zwitterionic surfactant Amphosol, the concentration (0 to 500 ppm) was based on an active component. All surfactant–polymer mixtures were prepared at room temperature. Care was taken to avoid entrapment of air bubbles.
2.3. Measurement of Rheology
Rheology of the fluids was measured at room temperature (≈22 °C) using Fann and Haake co-axial cylinder viscometers. Fann viscometer (Fann Instrument Co., Houston, TX, USA) was used for lower viscosity fluids and Haake viscometer (Thermo Fisher Scientific, Waltham, MA, USA) was used for high viscosity solutions. Table 1 gives the important dimensions of the viscometers used. The ranges of the rotational speeds of the viscometers were 0.9 to 600 rpm for Fann and 0.01 to 512 rpm for Haake. To calibrate the devices, viscosity standards of known viscosities were used.

Table 1.
Important dimensions of viscometers used.
2.4. Measurement of Surface Tension
The measurement of the surface tension of solutions was carried out at room temperature using the pendant drop tensiometer (Droplet Lab, Markham, ON, Canada). A high-resolution image of the pendant droplet was taken using a smartphone camera and the image was analyzed using a specialized software supplied by the company. The surface tension was obtained by fitting the droplet profile with the Young–Laplace equation [30]. The measurement for each solution was performed multiple times, and the average value was determined.
2.5. Measurement of Electrical Conductivity
The electrical conductivity of polymer and surfactant–polymer mixtures was measured at room temperature with the help of a Thermo Orion 3 Star conductivity meter supplied by Thermo Fisher Scientific, Waltham, MA, USA.
3. Results and Discussion
3.1. Rheology of Pure Polymer Solutions
Figure 3 shows the rheological behavior of pure polymer solutions. Viscosity is plotted as a function of shear rate for different polymer solutions at different concentrations. From the figure, it is clear all polymer solutions are non-Newtonian shear-thinning. Furthermore, the data can be described adequately using the power law model:
where is shear stress, is shear rate, is consistency index, and is flow behavior index. The flow behavior index of the shear-thinning polymer solutions is less than unity [31].
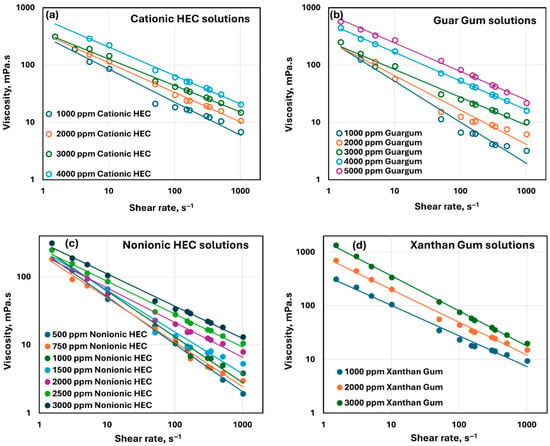
Figure 3.
Rheological behavior of polymer solutions. (a) Cationic HEC solutions, (b) Guar gum solutions, (c) Nonionic HEC solutions, and (d) Xanthan gum solutions.
Figure 4 compares the consistency index and flow behavior index of different polymer solutions, summarized in Table 2. The consistency of xanthan solutions is much larger than that of other polymer solutions at the same ppm concentration. The flow behavior index does not show any clear trend. The values of for different polymer solutions are in the following ranges: cationic HEC (0.423–0.538), guar gum (0.285–0.514), nonionic HEC (0.30–0.527), and xanthan gum (0.345–0.431). Nevertheless, 1 for all polymer solutions indicates that the polymer solutions are highly shear-thinning. The unusual behavior of xanthan gum solutions is due to its unique molecular structure. It has a cellulose backbone with trisaccharide side chains (see Figure 1). The presence of side chains enhances interaction and entanglement of xanthan gum molecules, resulting in the formation of highly viscous solutions. Note that the coefficient of determination R2 given in Table 2 is high (>0.95) for all polymer solutions indicating that the power-law model is a good model to describe the rheological behavior of polymer solutions.
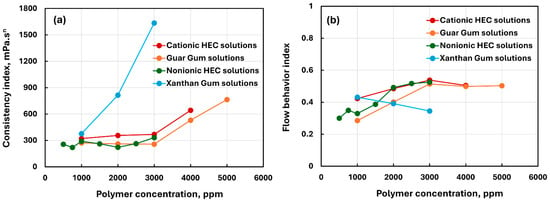
Figure 4.
Comparison of power-law parameters of polymer solutions. (a) Consistency index, (b) flow behavior index.

Table 2.
Summary of power-law parameters of polymer solutions.
3.2. Rheology and Surface Activity of Polymer–Surfactant Solutions
3.2.1. Cationic Polymer (CHEC) + Surfactant Solutions
Figure 5 shows the influence of nonionic surfactant (Alfonic) addition to cationic hydroxyethylcellulose (CHEC) polymer solution. Figure 5a shows the plots of consistency and flow behavior indices and Figure 5b shows the conductivity and surface tension plots as functions of surfactant concentration. Upon addition of surfactant, the consistency index increases whereas the flow behavior index remains nearly constant. The electrical conductivity of polymer–surfactant solution changes only slightly whereas the surface tension decreases substantially with the addition of surfactant to polymer solution.
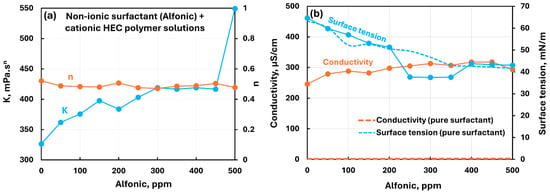
Figure 5.
Influence of nonionic surfactant (Alfonic) addition to cationic hydroxyethylcellulose (CHEC) polymer solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
However, the conductivity of polymer–surfactant solution is much larger than that of pure surfactant solution (see Figure 5b). This is not unexpected as surfactant is nonionic and polymer is ionic. The surface tension versus surfactant concentration plot for polymer–surfactant solution exhibits significant deviation for pure surfactant surface tension plot in the surfactant concentration range of 200 to 400 ppm. The surface tension of polymer–surfactant solution is much lower than that of pure surfactant solution. This indicates that the surfactant forms complexes with polymer which are more surface-active than pure surfactant in the surfactant concentration range of 200 to 400 ppm. The complexes form within 200–400 ppm concentration range probably due to micellization of surfactant molecules on the polymer molecules. Based on the surface tension data, the CMC of the surfactant is 350 ppm.
Figure 6 shows the influence of an anionic surfactant (Stepwet) addition to cationic hydroxyethylcellulose (CHEC) polymer solution. Figure 6a shows dramatic changes in consistency index with the addition of surfactant. The consistency index first increases, reaches a maximum value, and then falls off. The flow behavior index first decreases, reaches a minimum value, and then rises. Clearly the interactions between anionic surfactant and cationic polymer are very strong, resulting in dramatic changes in rheological properties. The electrical conductivity of polymer–surfactant solution decreases up to 200 ppm surfactant concentration and then levels off (see Figure 6b). Also, the conductivity of polymer–surfactant solution is much larger than that of pure surfactant solution. Like consistency index, the surface tension undergoes dramatic changes with the addition of surfactant. The surface tension initially decreases, reaches a minimum value, and then rises with the addition of surfactant. Interestingly, the surface tension of polymer–surfactant solution is much lower than that of pure surfactant solution. This indicates the formation of surfactant–polymer complexes which are more surface-active than the surfactant molecules.
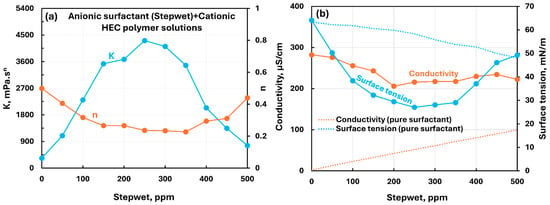
Figure 6.
Influence of anionic surfactant (Stepwet) addition to cationic hydroxyethylcellulose (CHEC) polymer solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
Figure 7 shows the influence of a cationic surfactant (HTAB) addition to cationic hydroxyethylcellulose (CHEC) polymer solution. The consistency index increases modestly in the surfactant concentration range of 50 to 400 ppm. The flow behavior index remains nearly constant. The conductivity increases with the increase in HTAB concentration. Also note that the conductivity of polymer–surfactant solution is much larger than that of pure surfactant solution. The surface tension decreases with the increase in surfactant concentration. Within the surfactant concentration range of 0 to 300 ppm, the surface tension of polymer–surfactant solution is significantly lower than that of pure surfactant solution, indicating the formation of surfactant–polymer complexes which are more surface-active than the surfactant molecules.
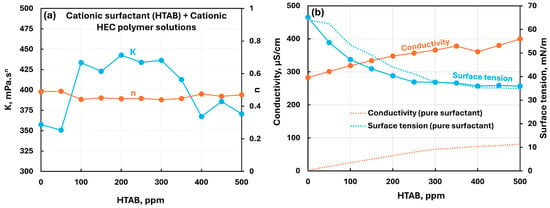
Figure 7.
Influence of cationic surfactant (HTAB) addition to cationic hydroxyethylcellulose (CHEC) polymer solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
The influence of zwitterionic surfactant (Amphosol) addition to cationic hydroxyethylcellulose (CHEC) polymer solution is shown in Figure 8. The consistency index increases initially at low surfactant concentration, reaches a maximum value around 100 ppm surfactant, and then falls off and becomes constant with further addition of surfactant. The flow behavior index remains almost constant. The conductivity increases with the increase in surfactant concentration. Also, the conductivity of polymer–surfactant solution is much larger than that of the pure surfactant solution. The surface tension of polymer–surfactant solution is significantly larger than that of pure surfactant solution over the entire concentration range. This is due to the migration of surfactant molecules from solution to polymer chains.
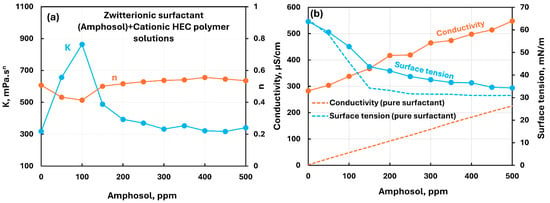
Figure 8.
Influence of zwitterionic surfactant (Amphosol) addition to cationic hydroxyethylcellulose (CHEC) polymer solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
Figure 9 compares the influence of all four surfactants (nonionic Alfonic, anionic Stepwet, cationic HTAB, and zwitterionic Amphosol) on the rheological, conductive, and surface tension behaviors of CHEC polymer solution. The rheological properties (see Figure 9a,b) are strongly affected by the addition of anionic surfactant (Stepwet) indicating a strong interaction between the oppositely charged surfactant and polymer. The interactions between other surfactants and CHEC polymers are much weaker in comparison with the anionic surfactant. The electrical conductivity of the solution decreases (see Figure 9c) with the addition of anionic Stepwet to the polymer solution and increases with the addition of all other surfactants. The surface tension of solutions decreases with the addition of surfactants as expected. However, the surface tension behavior of solution is strongly affected by an anionic surfactant (Stepwet) addition to the polymer solution. A sharp change in the rheological properties and surface activity of the polymer solution upon addition of anionic surfactant and the observed maximum in and minima in and surface tension can be explained as follows: The initial changes in properties upon addition of surfactant are due to the charge neutralization of cationic polymer chains by oppositely charged anionic surfactant (Stepwet). Neutralizing polymer chains probably causes the entanglement of chains and the formation of a three-dimensional network structure of polymer chains. Furthermore, the surfactant–polymer complexes are highly surface-active in nature. With the further increase in surfactant concentration, the excess surfactant likely imparts negative charge to the polymer chains, and they become separated due to electric repulsion between them, destroying the network structure of polymer chains. This causes a sharp drop in K and a rise in surface tension at high surfactant concentrations.
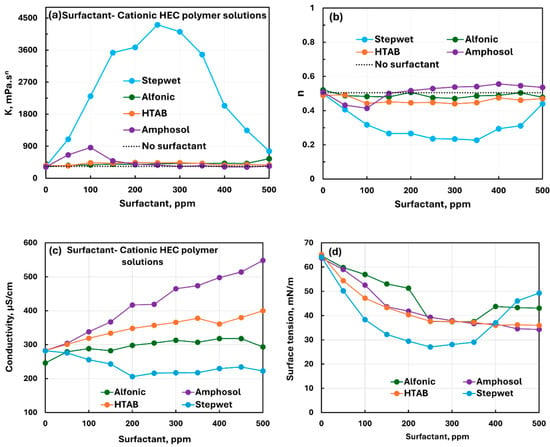
Figure 9.
Comparison of the effects of different surfactants on the properties of surfactant–cationic polymer (CHEC) solutions. (a) Consistency index, (b) flow behavior index, (c) electrical conductivity, and (d) surface tension.
3.2.2. Nonionic Polymer (NHEC) + Surfactant Solutions
Figure 10 shows the influence of the nonionic surfactant (Alfonic) addition to the nonionic hydroxyethylcellulose (NHEC) polymer solution. The consistency index fluctautes with the increase in surfactant concentration, whereas the flow behavior index remains nearly constant. The electrical conductivity of the polymer–surfactant solution remains nearly constant, whereas the surface tension decreases substantially with the addition of surfactant to polymer solution. When surfactant concentration is larger than 150 ppm, the surface tension of polymer–surfactant solution is lower than that of the pure surfactant solution, indicating that the surfactant–polymer complexes formed are more surface-active than the surfactant molecules alone. Note that the polymer NHEC itself is surface-active to some extent (see Figure 10b).
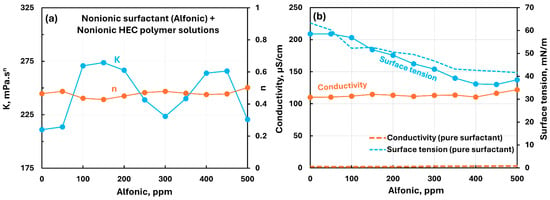
Figure 10.
Influence of nonionic surfactant (Alfonic) addition to nonionic hydroxyethylcellulose (NHEC) polymer solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
The influence of the anionic surfactant (Stepwet) addition to the nonionic hydroxyethylcellulose (NHEC) polymer solution is shown in Figure 11. There are negligible changes in the rheological properties with the addition of a surfactant, indicating weak interactions between the surfactant and the polymer. The electrical conductivity of the polymer–surfactant solution increases linearly with the increase in anionic surfactant, as expected. The conductivity of the surfactant–polymer solution is much larger than that of the pure surfactant solution, due to conductivity of the pure polymer solution. The surface tension plot of surfactant–polymer solution is parallel to the pure surfactant solution and falls below the pure surfactant solution due to surface activity of the pure polymer itself. The surface tension behavior is consistent with rheological behavior, indicating weak interactions between the surfactant and the polymer.
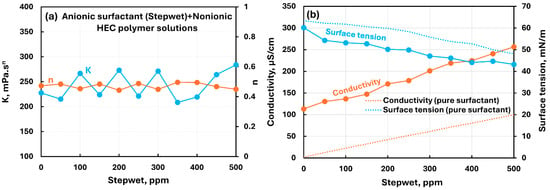
Figure 11.
Influence of anionic surfactant (Stepwet) addition to nonionic hydroxyethylcellulose (NHEC) polymer solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
Figure 12 shows the influence of the cationic surfactant (HTAB) addition to the nonionic hydroxyethylcellulose (NHEC) polymer solution. The consistency index fluctuates with the increases in surfactant concentration with no clear trend. The flow behavior index remains nearly constant. The conductivity increases linearly with the increase in HTAB concentration. The surface tension decreases with the increase in surfactant concentration. The surface tension of polymer–surfactant solution is significantly lower than that of pure surfactant solution due to surface activity of pure polymer solution. Overall, there occur mild interactions between the surfactant and the polymer.
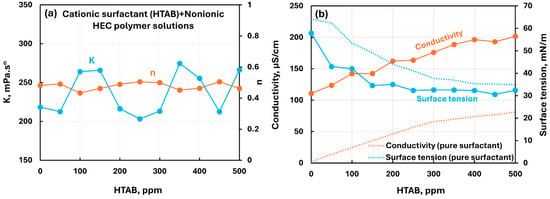
Figure 12.
Influence of cationic surfactant (HTAB) addition to nonionic hydroxyethylcellulose (NHEC) polymer solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
The influence of the zwitterionic surfactant (Amphosol) addition to the nonionic hydroxyethylcellulose (NHEC) polymer solution is shown in Figure 13. The consistency index is affected to only a small extent, without a clear trend, upon the addition of surfactant. The flow behavior index remains almost constant. This reflects weak interactions between surfactant and polymer molecules. The conductivity increases linearly with the increase in surfactant concentration. Also, the conductivity of polymer–surfactant solution is much larger than that of the pure surfactant solution. The effect of surfactant addition on surface tension is also small, indicating weak interactions between surfactant and polymer molecules.
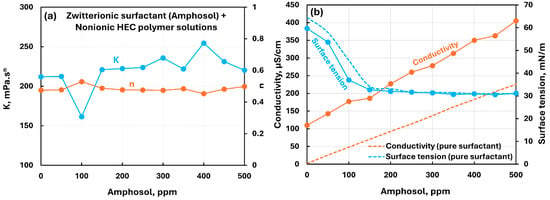
Figure 13.
Influence of zwitterionic surfactant (Amphosol) addition to nonionic hydroxyethylcellulose (NHEC) polymer solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
Figure 14 compares the influence of all four surfactants (nonionic Alfonic, anionic Stepwet, cationic HTAB, and zwitterionic Amphosol) on the rheological, conductive, and surface tension behaviors of NHEC polymer solution. The rheological properties (see Figure 14a,b) vary, to a small extent, upon the addition of surfactants to NHEC polymer, indicating weak to mild interactions between surfactants and nonionic polymer NHEC. The electrical conductivity of solutions increases with the addition of anionic, cationic, and zwitterionic surfactants (see Figure 9c) to NHEC polymer solution. The conductivity of different surfactant–polymer solutions is in the following order: Amphosol > Stepwet > HTAB > Alfonic. The surface tension plots indicate mostly a smooth decrease in surface tension with the addition of different surfactants. The surface tension of different surfactant–polymer solutions is in the following order: Stepwet > Alfonic > HTAB Amphosol. Thus, Amphosol and HTAB solutions are most surface-active and Stepwet solution is the least surface-active in the presence of NHEC polymer.
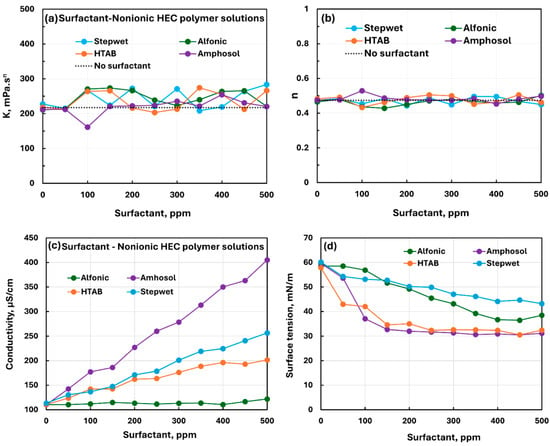
Figure 14.
Comparison of the influence of different surfactants on the properties of surfactant–nonionic polymer (NHEC) solutions. (a) Consistency index, (b) flow behavior index, (c) electrical conductivity, and (d) surface tension.
3.2.3. Nonionic Polymer (Guar Gum) + Surfactant Solutions
Figure 15 shows the influence of the nonionic surfactant (Alfonic) addition to the nonionic guar gum solution. Although the consistency index fluctuates in the surfactant concentration range of 150 to 350 ppm, the overall trend is a modest increase in consistency, upon the addition of surfactant, whereas the flow behavior index remains nearly constant. The electrical conductivity of the polymer–surfactant solution remains constant, whereas the surface tension decreases substantially with the addition of surfactant to polymer solution. The surface tension of surfactant–polymer solution overlaps with the surface tension of pure surfactant solution. Thus, the addition of nonionic surfactant Alfonic to nonionic guar gum solution shows mild interactions between surfactant and polymer.
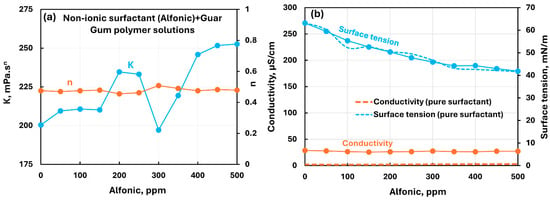
Figure 15.
Influence of nonionic surfactant (Alfonic) addition to nonionic guar gum solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
The influence of the anionic surfactant (Stepwet) addition to the nonionic guar gum solution is shown in Figure 16. There occurs a significant drop in the consistency index with the addition of the anionic surfactant within the surfactant concentration range of 0–250 ppm, indicating modest interactions between the surfactant and the polymer. At higher surfactant concentrations, the original value of the consistency index is recoverd and remains almost constant. The flow behavior index shows negligible variation with the addition of the surfactant. The electrical conductivity of polymer–surfactant solution increases linearly with the increase in anionic surfactant, as expected. The surface tension of surfactant–polymer solution is lower than that of the pure surfactant solution, indicating that the surfactant–polymer complexes are more surface-active than pure surfactant molecules. Thus, modest interaction is observed between anionic surfactant (Stepwet) and nonionic guar gum molecules, especially within the surfactant concentration range of 0–250 ppm.
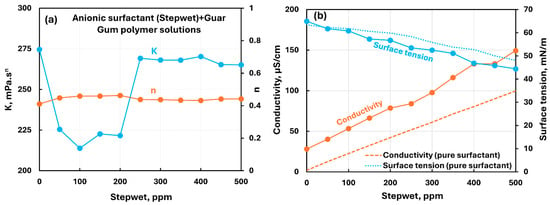
Figure 16.
Influence of anionic surfactant (Stepwet) addition to nonionic guar gum solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
Figure 17 shows the influence of the cationic surfactant (HTAB) addition to the nonionic guar gum solution. The consistency index fluctuates within the surfactant concentration range of 50–250 ppm. Outside this surfactant concentration range, the consistency index is constant. The flow behavior index is nearly constant. The conductivity increases almost linearly with the increase in HTAB concentration. The surface tension decreases with the increase in surfactant concentration. The surface tension of polymer–surfactant solution is slightly lower than that of the pure surfactant solution, indicating an enhanced surface activity of surfactant–polymer compexes. Overall, there occur weak interactions between the cationic surfactant HTAB and nonionic guar gum.
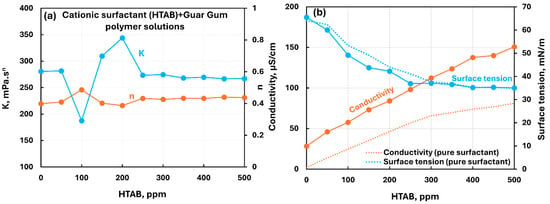
Figure 17.
Influence of cationic surfactant (HTAB) addition to nonionic guar gum solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
Figure 18 shows the influence of the zwitterionic surfactant (Amphosol) addition to the nonionic guar gum solution. The consistency index remains nearly constant up to a surfactant concentration of 250 ppm. It dips at 300 ppm and recovers to original value at 500 ppm. The flow behavior index remains nearly constant. The conductivity increases linearly with the increase in surfactant concentration. The surface tension decreases with the increase in surfactant concentration and it is slightly larger than the pure surfactant solution, indicating mild interactions between surfactant and polymer molecules.
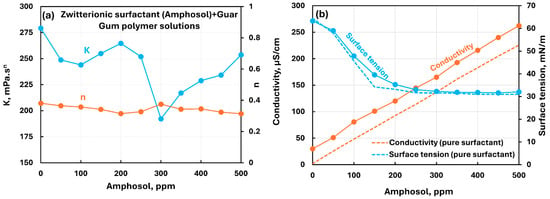
Figure 18.
Influence of zwitterionic surfactant (Amphosol) addition to nonionic guar gum solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
Figure 19 compares the effect of different surfactants (nonionic Alfonic, anionic Stepwet, cationic HTAB, and zwitterionic Amphosol) on the rheological, conductive, and surface tension behaviors of nonionic guar gum solutions. The variation in rheological properties (see Figure 19a,b), upon the addition of surfactants to guar gum solutions, is not large, indicating weak to moderate interactions between surfactants and nonionic polymers. The electrical conductivity of the solution increases with the addition of anionic, cationic, and zwitterionic surfactants (see Figure 9c) to guar gum solutions. The conductivity of different surfactant–polymer solutions is in the following order: Amphosol > HTAB Stepwet > Alfonic. The surface tension of different surfactant–polymer solutions decreases smoothly with the increase in surfactant concentrations. The surface tension of different surfactant–polymer solutions varies in the following order: Stepwet > Alfonic > HTAB Amphosol; that is, Amphosol-guar gum solutions are most surface-active and Stepwet–guar gum solutions are least surface-active.
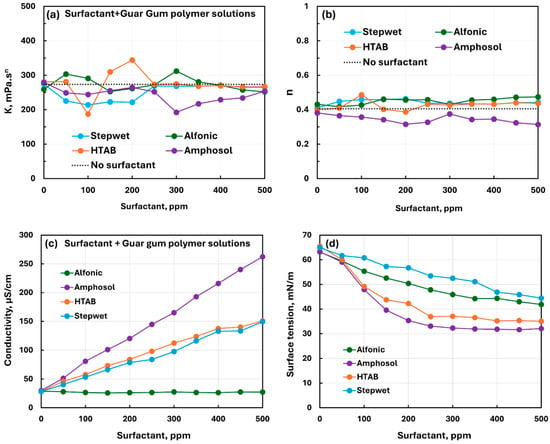
Figure 19.
Comparison of the influence of different surfactants on the properties of surfactant–nonionic polymer (guar gum) solutions. (a) Consistency index, (b) flow behavior index, (c) electrical conductivity, and (d) surface tension.
It should be noted that the rheological, conductive, and surface-active properties of surfactant–polymer mixtures are similar for the nonionic polymers NHEC and guar gum (see Figure 14 and Figure 19). The polymer–surfactant interactions are weak to moderate in these cases. However, the properties of surfactant–polymer mixtures are quite different for cationic polymer CHEC (see Figure 9). Electric charges of the polymer and surfactant molecules are dominant factors in determining the interactions between the surfactant and polymer molecules. The interactions between cationic polymer CHEC and oppositely charged anionic surfactant Stepwet are extraordinarily strong.
3.2.4. Anionic Polymer (Xanthan Gum) + Surfactant Solutions
Figure 20 shows the influence of nonionic surfactant (Alfonic) addition to anionic xanthan gum solution. The consistency index generally increases modestly with the addition of surfactant whereas the flow behavior index remains nearly constant. Fluctuation in consistency and flow behavior indices is observed in the surfactant concentration range of 350–450 ppm. The electrical conductivity of the polymer–surfactant solution increases slightly with the addition of surfactant to polymer solution. However, the conductivity of the polymer–surfactant solution is much larger than that of the pure surfactant solution (see Figure 20b) due to the high conductivity of pure xanthan solution. The surface tension of polymer–surfactant solution decreases with the addition of surfactant. The polymer–surfactant solution has a lower surface tension than that of pure surfactant solution over the surfactant concentration range of 150–500 ppm. Thus, the surfactant interacts with the polymer to form complexes which are slightly more surface-active than the pure surfactant in the surfactant concentration range of 150 to 500 ppm.
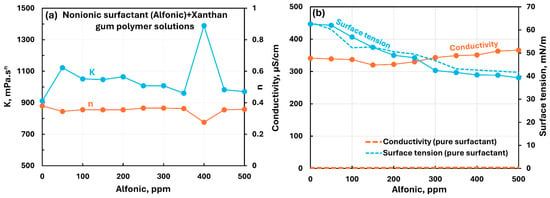
Figure 20.
Influence of nonionic surfactant (Alfonic) addition to anionic xanthan gum solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
Figure 21 shows the influence of the anionic surfactant (Stepwet) addition to the anionic xanthan gum solution. The consistency index rises significantly initially with the increase in surfactant concentration from 0 to 100 ppm. With a further increase in surfactant concentration, the consistency index remains approximately constant. The flow behavior index remains nearly constant with the addition of the surfactant. Clearly, the interaction between surfactant and polymer molecules is significant. The increase in consistency index is likely due to electrostatic repulsion between negatively charged surfactant and polymer molecules. The electrical conductivity of polymer–surfactant solution increases with the addition of ionic surfactant. There occurs a change in the slope of the conductivity plot, at approximately 150 ppm, consistent with the consistency index variation. The surface tension of the polymer–surfactant solution decreases with the addition of surfactant. The surface tension of polymer–surfactant solution is significantly lower than that of the pure surfactant solution when the surfactant concentration is larger than 100 ppm. This indicates the formation of surfactant–polymer complexes which are more surface-active than the surfactant molecules.
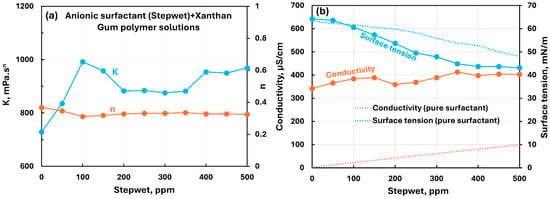
Figure 21.
Influence of anionic surfactant (Stepwet) addition to anionic xanthan gum solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
The influence of the cationic surfactant (HTAB) addition to anionic xanthan gum solution is shown in Figure 22. The consistency index decreases substantially with the addition of the surfactant although the decrease is not smooth with the increase in surfactant concentration. The flow behavior index increases slightly with the addition of the surfactant.
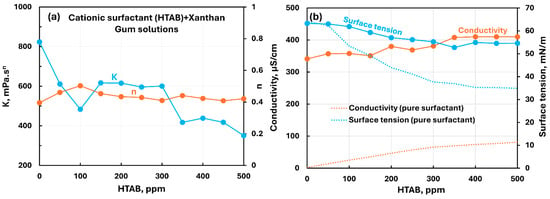
Figure 22.
Influence of cationic surfactant (HTAB) addition to anionic xanthan gum solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
Clearly, there occurs good interaction between the oppositely charged surfactant and polymer molecules. Due to the charge neutralization of the polymer molecules, the extension and interaction of the polymer molecules are reduced with the addition of surfactant, resulting in lower consistency. The conductivity increases almost linearly with the increase in HTAB concentration. The surface tension decreases with the increase in surfactant concentration. However, the surface tension of the polymer–surfactant solution is much larger than that of the pure surfactant solution, indicating the migration of surfactant from solution to polymer molecules. Overall, there are strong interactions between the cationic surfactant HTAB and anionic xanthan gum.
Figure 23 shows the influence of the zwitterionic surfactant (Amphosol) addition to the anionic xanthan gum solution. Except for the spike in consistency index observed at the 50 ppm surfactant, the consistency index increases slightly with the addition of the surfactant. The flow behavior index remains nearly constant with the addition of surfactant to polymer.
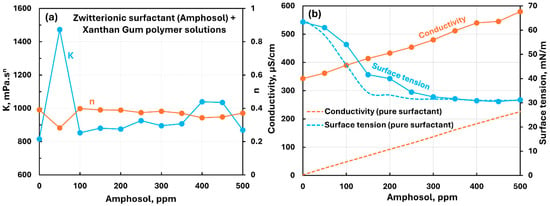
Figure 23.
Influence of zwitterionic surfactant (Amphosol) addition to anionic xanthan gum solution. (a) Consistency and flow behavior indices, (b) conductivity and surface tension.
The conductivity increases linearly with the increase in surfactant concentration. The surface tension decreases with the increase in surfactant concentration and it is significantly larger than the pure surfactant solution, indicating the migration of some surfactant from the solution to the polymer molecules. Overall, there are weak interactions between the surfactant and polymer molecules.
Figure 24 compares the effect of all four surfactants (nonionic Alfonic, anionic Stepwet, cationic HTAB, and zwitterionic Amphosol) on the rheological, conductive, and surface tension behaviors of anionic xanthan gum solutions. The rheological properties (see Figure 24a,b) vary significantly with the addition of surfactants to xanthan gum solutions, indicating weak to strong interactions between surfactants and anionic polymers. The interaction is strong between the oppositely charged cationic surfactant HTAB and anionic xanthan gum molecules.
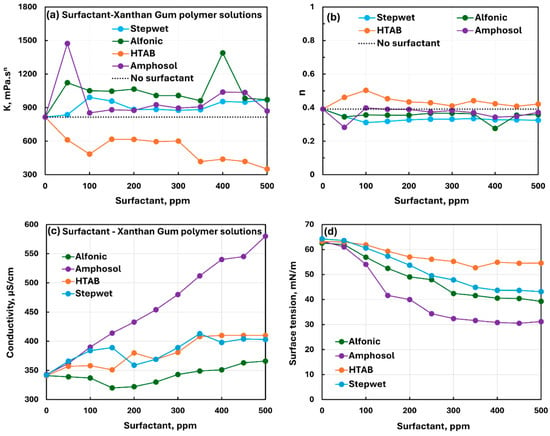
Figure 24.
Comparison of the influence of different surfactants on the properties of surfactant-anionic polymer (xanthan gum) solutions. (a) Consistency index, (b) flow behavior index, (c) electrical conductivity, and (d) surface tension.
The electrical conductivity of solutions increases with the addition of ionic, zwitterionic, or nonionic surfactant to the xanthan gum solution (see Figure 24c). The conductivity of different surfactant–polymer solutions varies in the following order: Amphosol > HTAB Stepwet > Alfonic. The surface tension of different surfactant–polymer solutions decreases smoothly with the increase in surfactant concentration. The surface tension varies with surfactant concentration in the following order: HTAB > Stepwet > Alfonic > Amphosol; that is, Amphosol–xanthan gum solutions are most surface-active and HTAB-xanthan gum solutions are least surface-active.
3.3. Summary of Interactions Between Different Surfactants and Polymers
Table 3 summarizes the interactions between different surfactants and polymers investigated in this work. The symbol “S” denotes surfactant and symbol “P” denotes polymer. The electric charge on the species is 0 for neutral species, − for negatively charged species, + for positively charged species, and +− for zwitterionic species.

Table 3.
Summary of interactions between polymers and surfactants.
4. Conclusions
The influence of surfactants on the rheological and surface-active properties of polymer solutions was investigated. Based on experimental work, the following conclusions can be made:
- The cationic hydroxyethyl cellulose (CHEC) polymer exhibits extraordinarily strong interaction with the anionic surfactant (Stepwet). Dramatic changes occur in the rheological and surface-active properties upon addition of the surfactant to the polymer solution.
- The interactions between CHEC and three other surfactants (nonionic Alfonic, cationic HTAB, zwitterionic Amphosol) are moderate. The consistency generally increases with the addition of surfactants. Except for zwitterionic Amphosol, the surfactant–polymer complexes formed are more surface-active than pure surfactant. Migration of surfactant from solution to polymer occurs, resulting in a decrease in surface activity of solution when zwitterionic Amphosol is added to CHEC.
- The nonionic hydroxyethyl cellulose (NHEC) polymer exhibits weak to mild interactions with the surfactants investigated. The consistency index either varies to a small extent and/or fluctuates with the increase in surfactant concentration. Generally, the surface activity of solutions is higher than that of pure surfactants as the polymer NHEC itself is surface-active.
- The nonionic guar gum exhibits weak to mild interactions with the surfactants investigated. The consistency varies mildly upon the addition of surfactant. The surface activity of surfactant–polymer solution is enhanced compared with pure surfactant solutions in the case of anionic (Stepwet) and cationic (HTAB) surfactants. With nonionic (Alfonic) and zwitterionic (Amphosol) surfactants, the surface activity of surfactant–polymer solutions is unaltered from pure surfactant solutions.
- The anionic xanthan gum exhibits strong interaction with the cationic surfactant (HTAB). The consistency index decreases substantially with the addition of surfactant. The other three surfactants (nonionic, anionic, and zwitterionic) show mild to moderate interactions, resulting in some increase in consistency. In the case of nonionic Alfonic and anionic Stepwet surfactants, the surfactant interacts with the polymer to form complexes which are more surface-active than pure surfactant. Upon addition of cationic (HTAB) and zwitterionic (Amphosol) surfactants, the surfactant–polymer solutions become less surface-active compared with pure surfactant solutions due to the migration of surfactant from solution to polymer.
Author Contributions
Conceptualization, R.P.; methodology, R.P. and C.-C.S.; validation, C.-C.S. and R.P.; formal analysis, R.P.; investigation, C.-C.S. and R.P.; resources, R.P.; data curation, R.P. and C.-C.S.; writing—original draft preparation, R.P.; writing—review and editing, R.P.; visualization, R.P.; supervision, R.P.; project administration, R.P.; funding acquisition, R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Discovery Grant awarded to R.P. by the Natural Sciences and Engineering Research Council of Canada.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lu, Q.; Pal, R. Steady shear rheology and surface activity of polymer-surfactant mixtures. Polymers 2025, 17, 364. [Google Scholar] [CrossRef]
- Gradzielski, M. Polymer–surfactant interaction for controlling the rheological properties of aqueous surfactant solutions. Curr. Opin. Colloid Interface Sci. 2023, 63, 101662. [Google Scholar]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S. A comprehensive review of beneficial applications of viscoelastic surfactants in wellbore hydraulic fracturing fluids. Fuel 2023, 338, 127228. [Google Scholar] [CrossRef]
- Machale, J.; Majumder, S.K.; Ghosh, P.; Sen, T.K. Role of chemical additives and their rheological properties in enhanced oil recovery. Rev. Chem. Eng. 2020, 36, 789–830. [Google Scholar] [CrossRef]
- Raffa, P.; Broekhuis, A.A.; Picchioni, F. Polymeric surfactants for enhanced oil recovery: A review. J. Pet. Sci. Eng. 2016, 145, 723–733. [Google Scholar] [CrossRef]
- Liu, J.; Liu, P.; Du, J.; Wang, Q.; Chen, X.; Zhao, L. Review on High-Temperature-Resistant Viscoelastic Surfactant Fracturing Fluids: State-of-the-Art and Perspectives. Energy Fuels 2023, 37, 9790–9821. [Google Scholar] [CrossRef]
- Goddard, E.D.; Ananthapadmanabhan, K.P. Interactions of Surfactants with Polymers and Proteins; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Somasundaran, P.; Krishnakumar, S. Adsorption of surfactants and polymers at the solid-liquid interface. Colloids Surf. A Physicochem. Eng. Asp. 1997, 123–124, 491–513. [Google Scholar]
- Goddard, E.D.; Gruber, J.V. Principles of Polymer Science and Technology in Cosmetics and Personal Care; Taylor & Francis: Boca Raton, FL, USA, 1999. [Google Scholar]
- Taylor, D.J.F.; Thomas, R.K.; Penfold, J. Polymer/surfactant interactions at the air/water interface. Adv. Colloid Interface Sci. 2007, 132, 69–110. [Google Scholar] [CrossRef]
- Talwar, S.; Scanu, L.F.; Khan, S.A. Hydrophobic interactions in associative polymer/nonionic surfactant systems: Effects of surfactant architecture and system parameters. J. Rheol. 2006, 50, 831–847. [Google Scholar] [CrossRef]
- Petkova, R.; Tcholakova, S.; Denkov, N.D. Foaming and Foam Stability for Mixed Polymer–Surfactant Solutions: Effects of Surfactant Type and Polymer Charge. Langmuir 2012, 28, 4996–5009. [Google Scholar] [CrossRef] [PubMed]
- Kirtil, E.; Oztop, M.H. Mechanism of adsorption for design of role-specific polymeric surfactants. Chem. Pap. 2023, 77, 2343–2361. [Google Scholar] [CrossRef]
- Lazaridis, N.; Alexopoulos, A.H.; Chatzi, E.G.; Kiparissides, C. Steric stabilization in emulsion polymerization using oligomeric nonionic surfactants. Chem. Eng. Sci. 1999, 54, 3251–3261. [Google Scholar] [CrossRef]
- Duro, R.; Souto, C.; Gómez-Amoza, J.L.; Martínez-Pacheco, R.; Concheiro, A. Interfacial Adsorption of Polymers and Surfactants: Implications for the Properties of Disperse Systems of Pharmaceutical Interest. Drug Dev. Ind. Pharm. 1999, 25, 817–829. [Google Scholar] [CrossRef]
- Banerjee, S.; Cazeneuve, C.; Baghdadli, N.; Ringeissen, S.; Leermakers, F.A.M.; Luengo, G.S. Surfactant–polymer interactions: Molecular architecture does matter. Soft Matter 2015, 11, 2504–2511. [Google Scholar] [CrossRef]
- Rapp, M.V.; Donaldson, S.H., Jr.; Gebbie, M.A.; Gizaw, Y.; Koenig, P.; Roiter, Y.; Israelachvili, J.N. Effects of Surfactants and Polyelectrolytes on the Interaction between a Negatively Charged Surface and a Hydrophobic Polymer Surface. Langmuir 2015, 31, 8013–8021. [Google Scholar] [CrossRef] [PubMed]
- Raffa, P.; Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymeric Surfactants: Synthesis, Properties, and Links to Applications. Chem. Rev. 2015, 115, 8504–8563. [Google Scholar] [CrossRef]
- Tam, K.C.; Wyn-Jones, E. Insights on polymer surfactant complex structures during the binding of surfactants to polymers as measured by equilibrium and structural techniques. Chem. Soc. Rev. 2006, 35, 693–709. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, C.; Wang, C.; Wang, J.; Golas, P.; Batteas, J.; Kreeger, L. Phase Behavior of Cationic Hydroxyethyl Cellulose−Sodium Dodecyl Sulfate Mixtures: Effects of Molecular Weight and Ethylene Oxide Side Chain Length of Polymers. Langmuir 2004, 20, 8482–8489. [Google Scholar] [CrossRef]
- Mohsenipour, A.A.; Pal, R. A Review of Polymer-Surfactant Interactions. In Handbook of Surface and Colloid Chemistry, 4th ed.; Birdi, K.S., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 639–684. [Google Scholar]
- Diamant, H.; Andelman, D. Onset of self-assembly in polymer-surfactant systems. Europhys. Lett. 1999, 48, 170. [Google Scholar] [CrossRef][Green Version]
- Hansson, P.; Lindman, B. Surfactant-polymer interactions. Curr. Opin. Colloid Interface Sci. 1996, 1, 604–613. [Google Scholar] [CrossRef]
- Yang, J.; Pal, R. Investigation of surfactant-polymer interactions using rheology ans surface tension measurements. Polymers 2020, 12, 2302. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yan, J.; Miao, J.; Wang, J.; Wang, Z.; Zhang, L. Synthesis and evaluation of Gemini surfactant-polymer copolymers as viscosity reducer for enhancing heavy oil recovery. Chem. Eng. J. 2025, 521, 166868. [Google Scholar] [CrossRef]
- Zeariya, M.; Almarshadi, F.; Elhassan, N.E.; Humaida, M.; Essa, M.; Yousif, A.; El-Shennawy, S.; Abdel-Hameed, R.; Reda, L.M.; Metwally, A.M.; et al. Creating novel green synthesized cationic polymeric surfactants for removing the petroleum films from water surface: Surface-active and biological assessments. J. Mol. Liq. 2025, 435, 128164. [Google Scholar] [CrossRef]
- Chen, X.; Hou, Q.; Liu, Y.; Liu, G.; Zhang, H.; Sun, H.; Zhu, Z.; Liu, W. Experimental Study on Surfactant–Polymer Flooding After Viscosity Reduction for Heavy Oil in Matured Reservoir. Energies 2025, 18, 756. [Google Scholar] [CrossRef]
- Mohsenipour, A.A.; Pal, R. Drag reduction in turbulent pipeline flow of mixed nonionic polymer and cationic surfactant systems. Can. J. Chem. Eng. 2013, 91, 190–201. [Google Scholar] [CrossRef]
- Mohsenipour, A.A.; Pal, R. The role of surfactants in mechanical degradation of drag-reducing polymers. Ind. Eng. Chem. Res. 2013, 52, 1291–1302. [Google Scholar] [CrossRef]
- Chen, H.; Muros-Cobos, J.L.; Holgado-Terriza, J.A.; Amirfazli, A. Surface tension measurement with a smartphone using a pendant drop. Colloids Surf. A 2017, 533, 213–217. [Google Scholar] [CrossRef]
- Pal, R. Rheology of high internal phase ratio emulsions and foams. Adv. Colloid Interface Sci. 2025, 339, 103426. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).