Improved Assessment and Prediction of Groundwater Drinking Quality Integrating Game Theory and Machine Learning in the Nyangchu River Basin, Southwestern Qinghai–Tibet Plateau
Abstract
1. Introduction
2. Materials and Methods
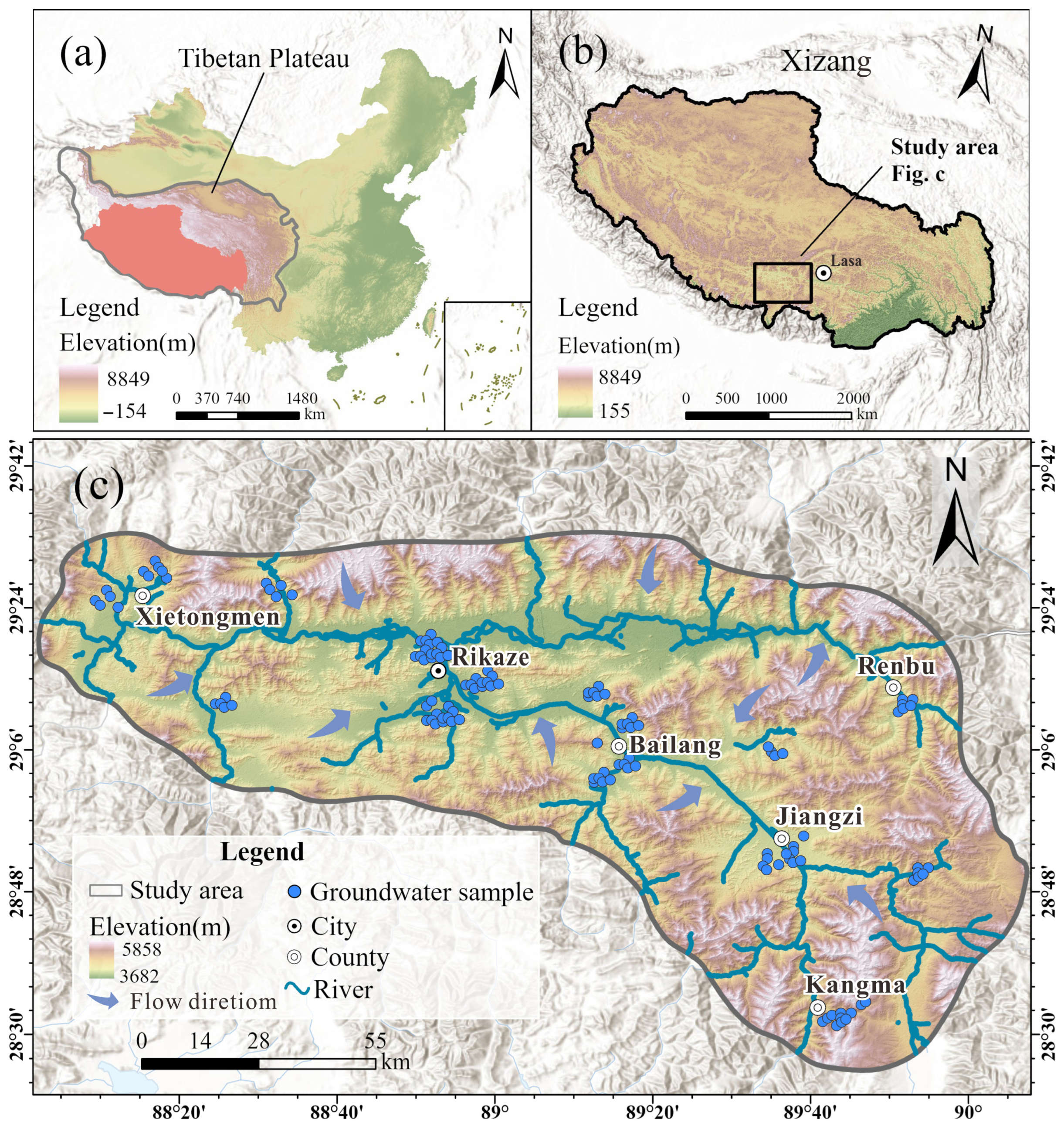
2.1. Study Area
2.2. Groundwater Sampling and Experimental Testing
2.3. Improved Drinking Water Quality Evaluation Approach Based on Game Theory
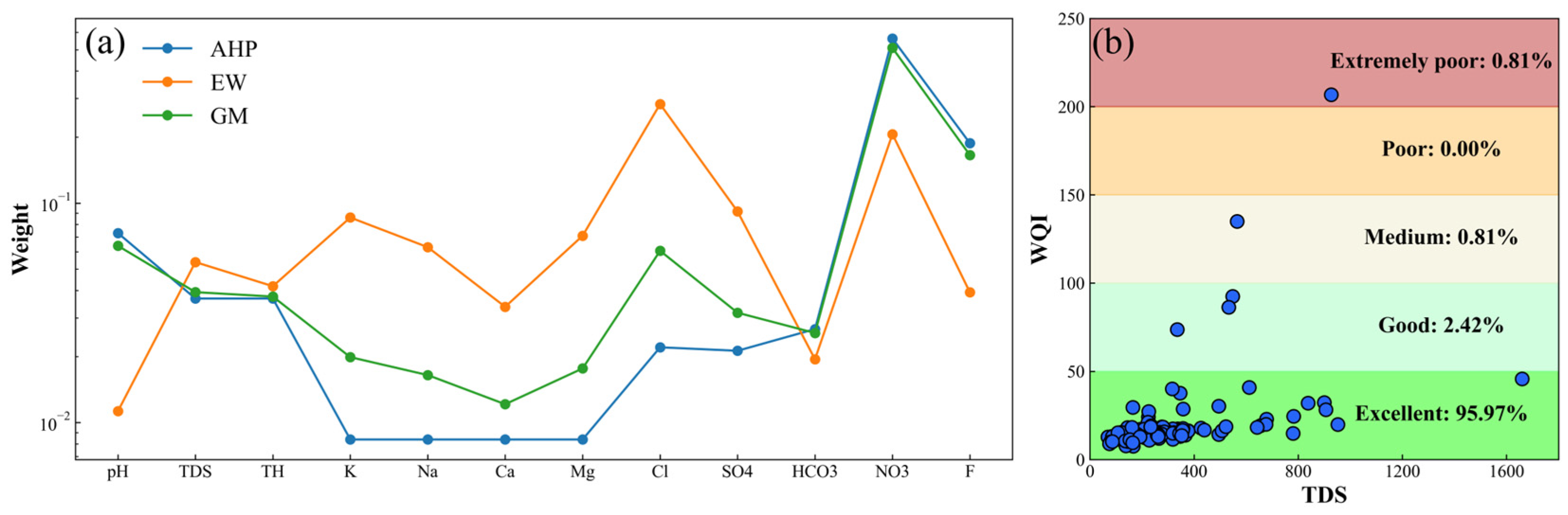
2.3.1. Analytic Hierarchy Process
2.3.2. Entropy–Weighted Method
2.4. Machine Learning Algorithms for Water Quality Prediction
3. Results
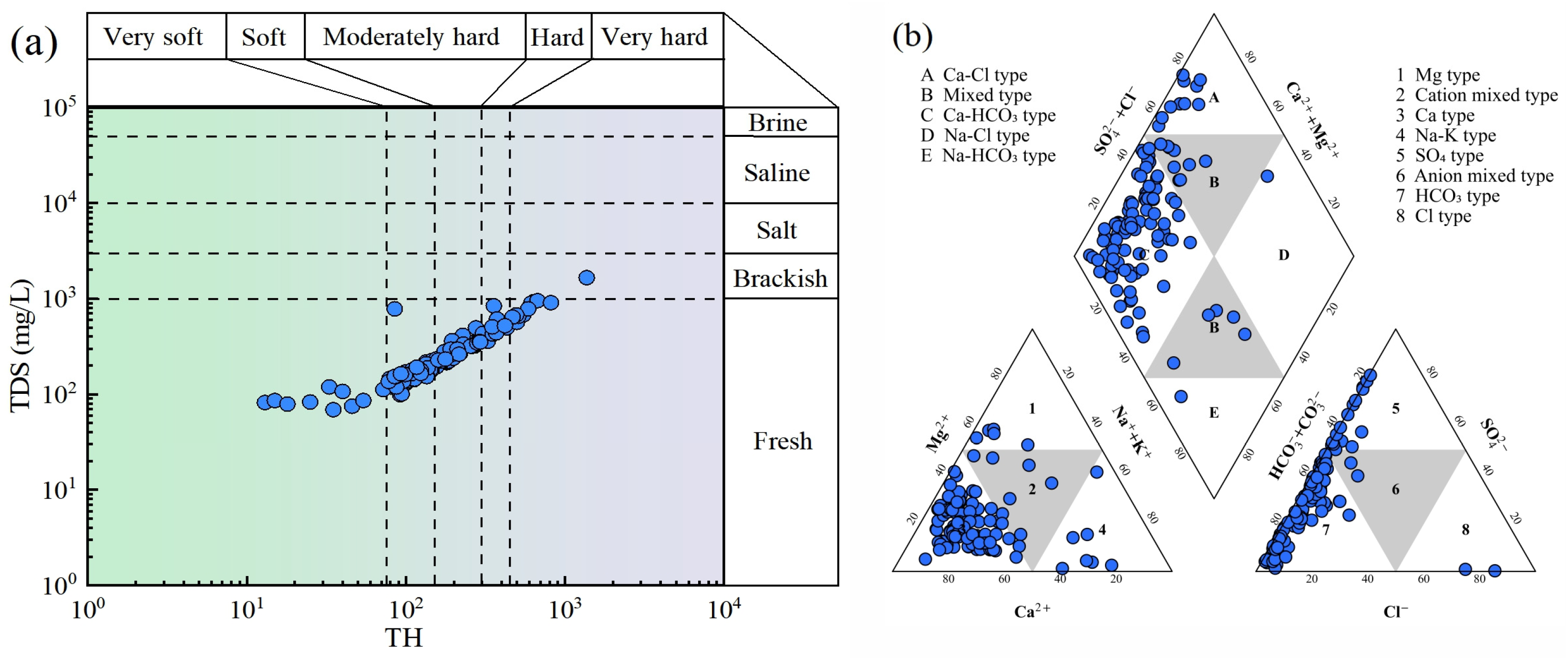
3.1. Hydrochemical Characteristics of Groundwater
3.2. Improved Assessment of Groundwater Drinking Quality
4. Discussion
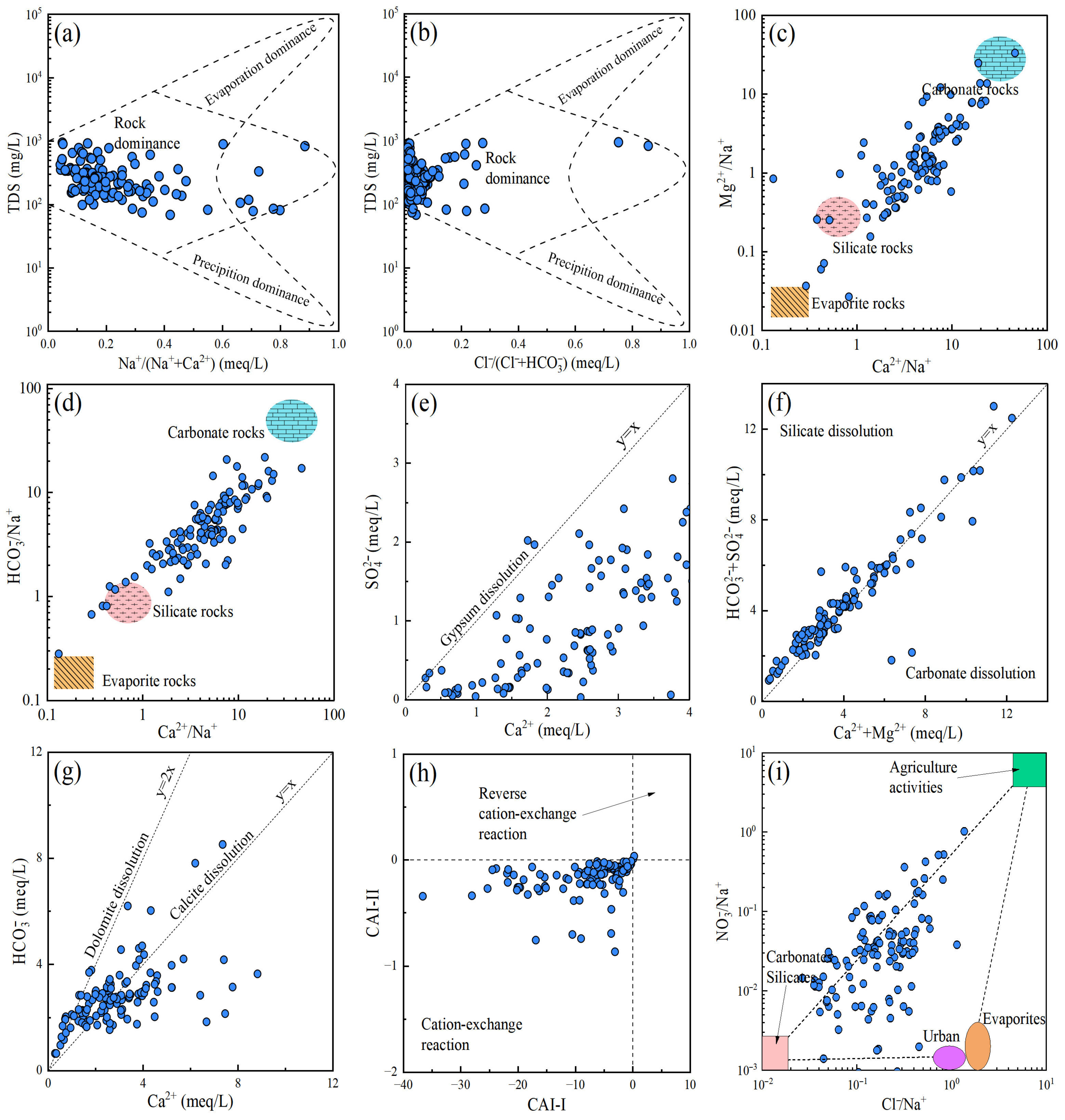
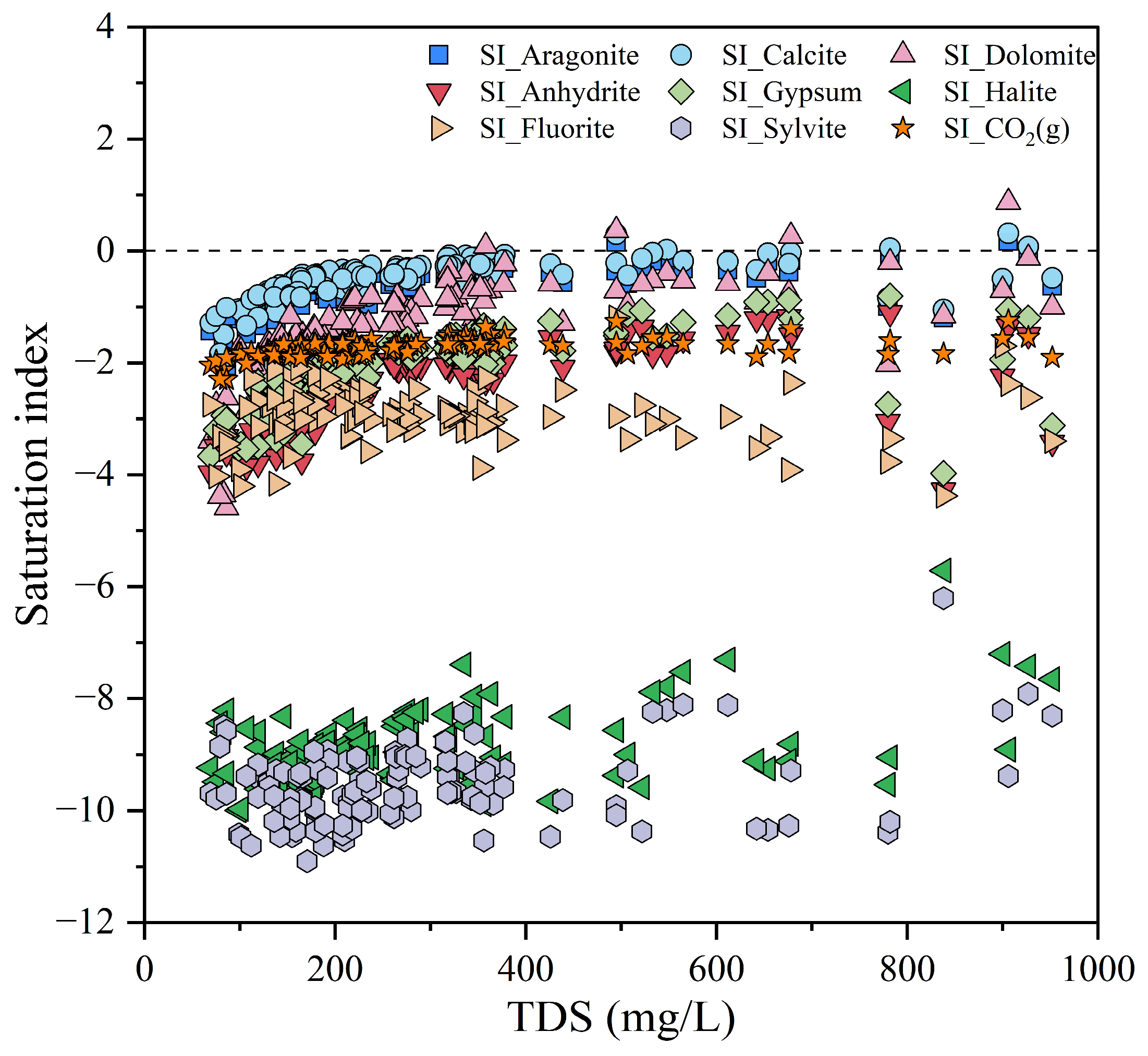
4.1. Hydrochemical Process and Controlling Factors
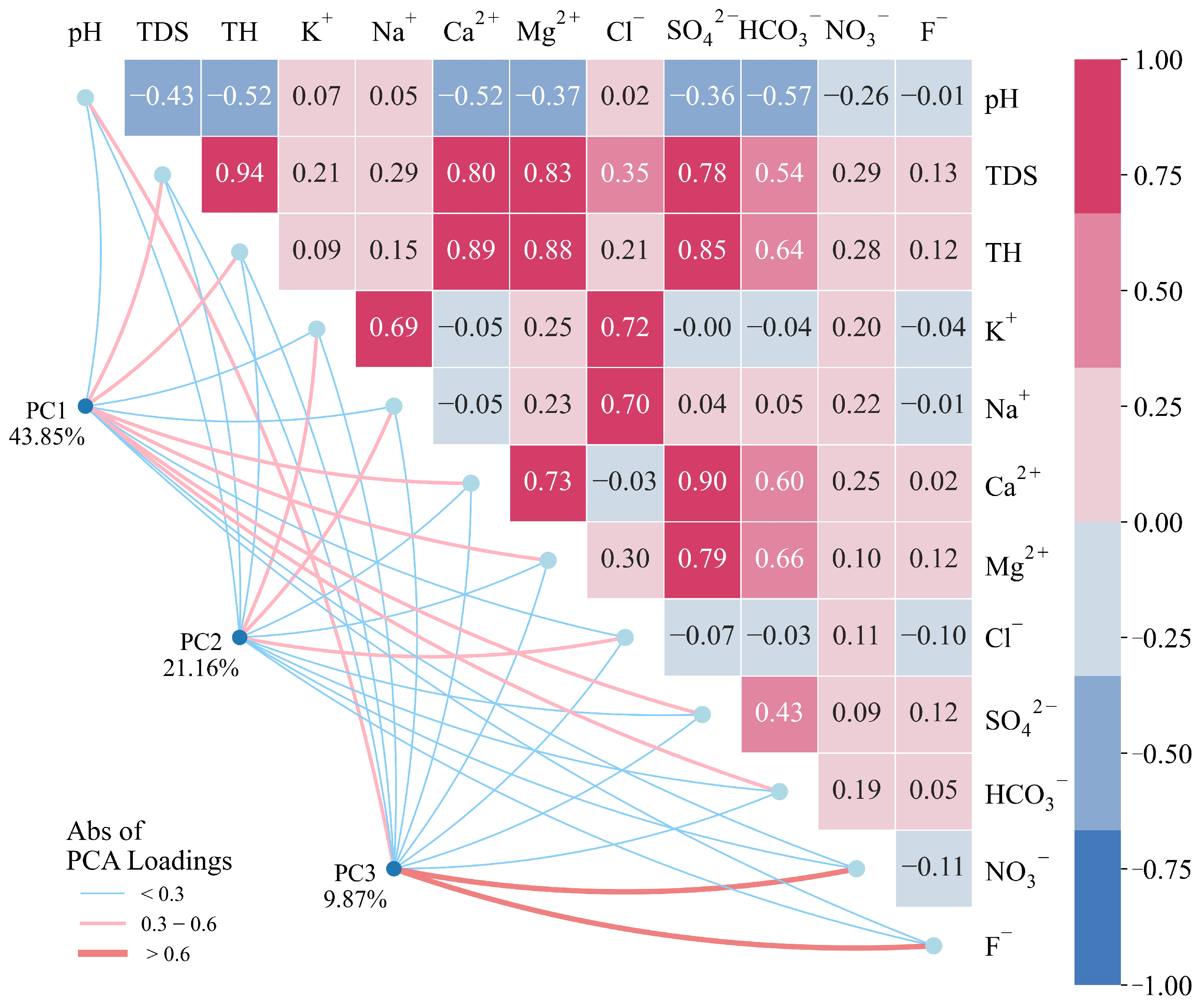
4.1.1. Multivariate Statistical Analysis
4.1.2. Ion Source Analysis
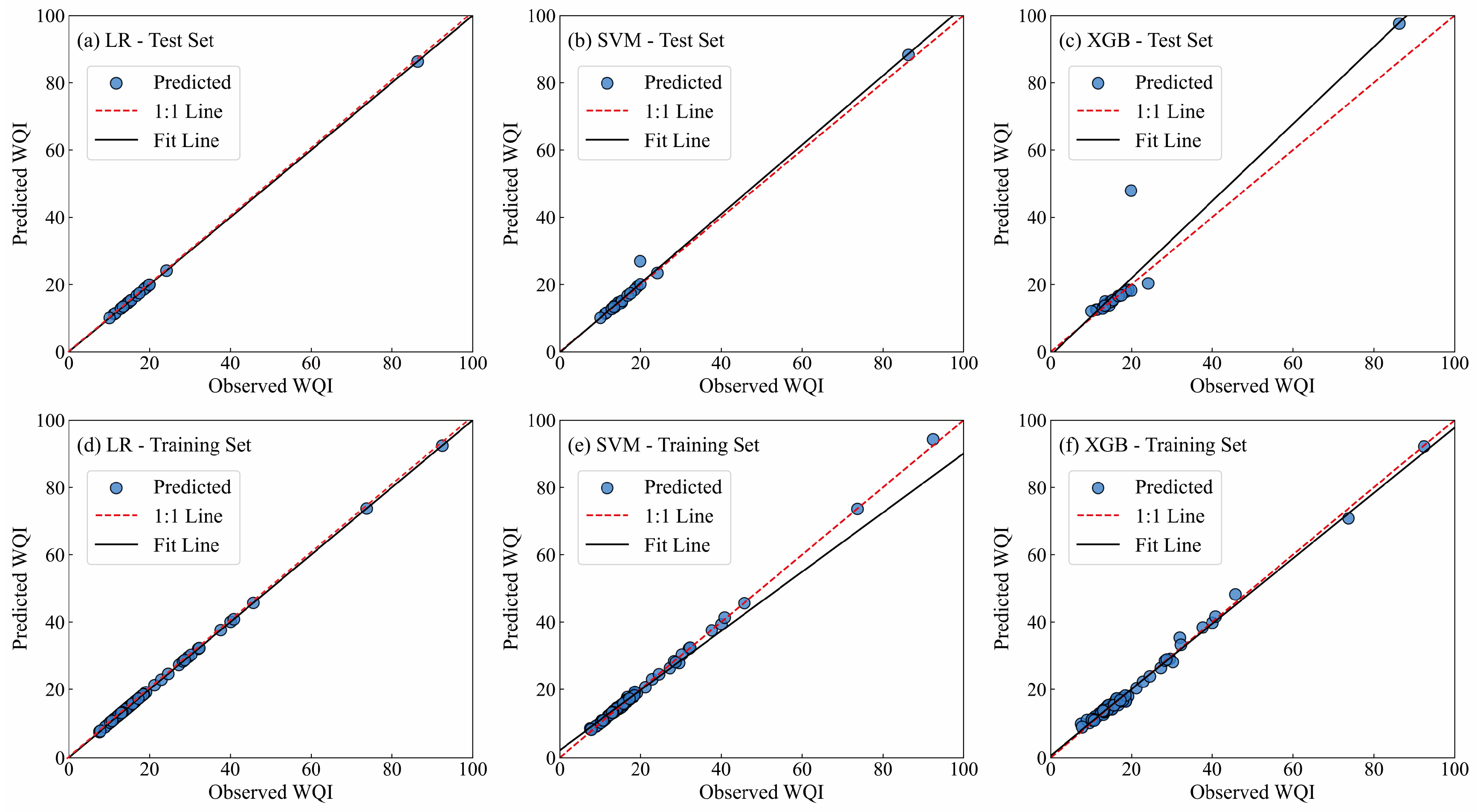
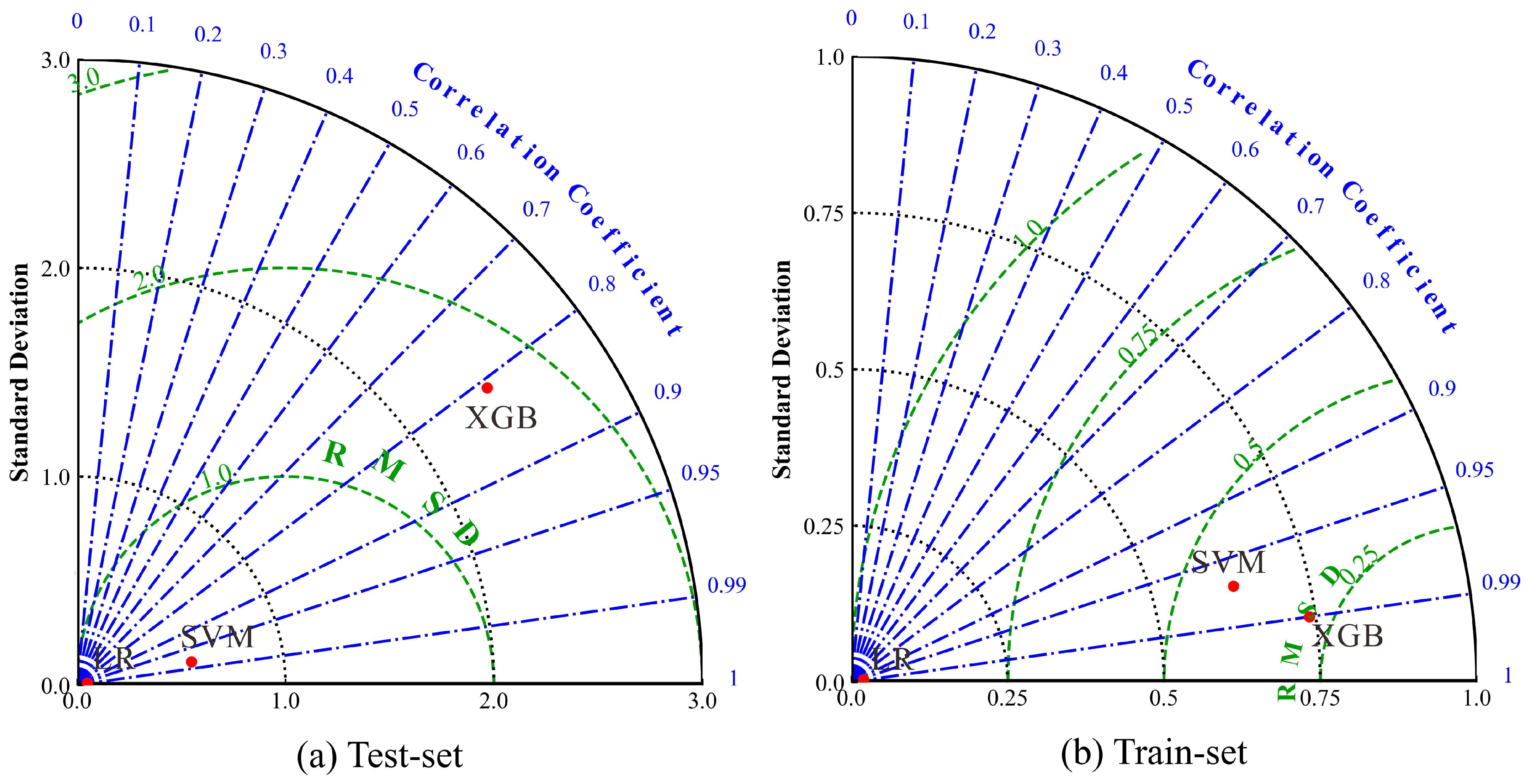
4.2. Water Quality Prediction Based on Machine Learning Approaches
5. Conclusions
- (1)
- The groundwater in the study area is weakly alkaline fresh water, with hardness ranging from soft to medium. The anions and cations with the highest contents are HCO3− and Ca2+, respectively, and the hydrochemical type is dominated by the Ca-HCO3 type.
- (2)
- Water-rock interaction is the dominant factor influencing the hydrochemical characteristics of groundwater. The formation of hydrochemistry is jointly affected by the weathering and dissolution of carbonate rocks and silicate rocks, accompanied by cation exchange and adsorption. In addition, groundwater is also subject to disturbances from certain human activities, such as agricultural practices and landfill disposal.
- (3)
- More than 95% of groundwater samples are considered as excellent water quality, suggesting that the groundwater quality is generally good and suitable for drinking in the study area. The extremely poor water quality may indicate local pollution.
- (4)
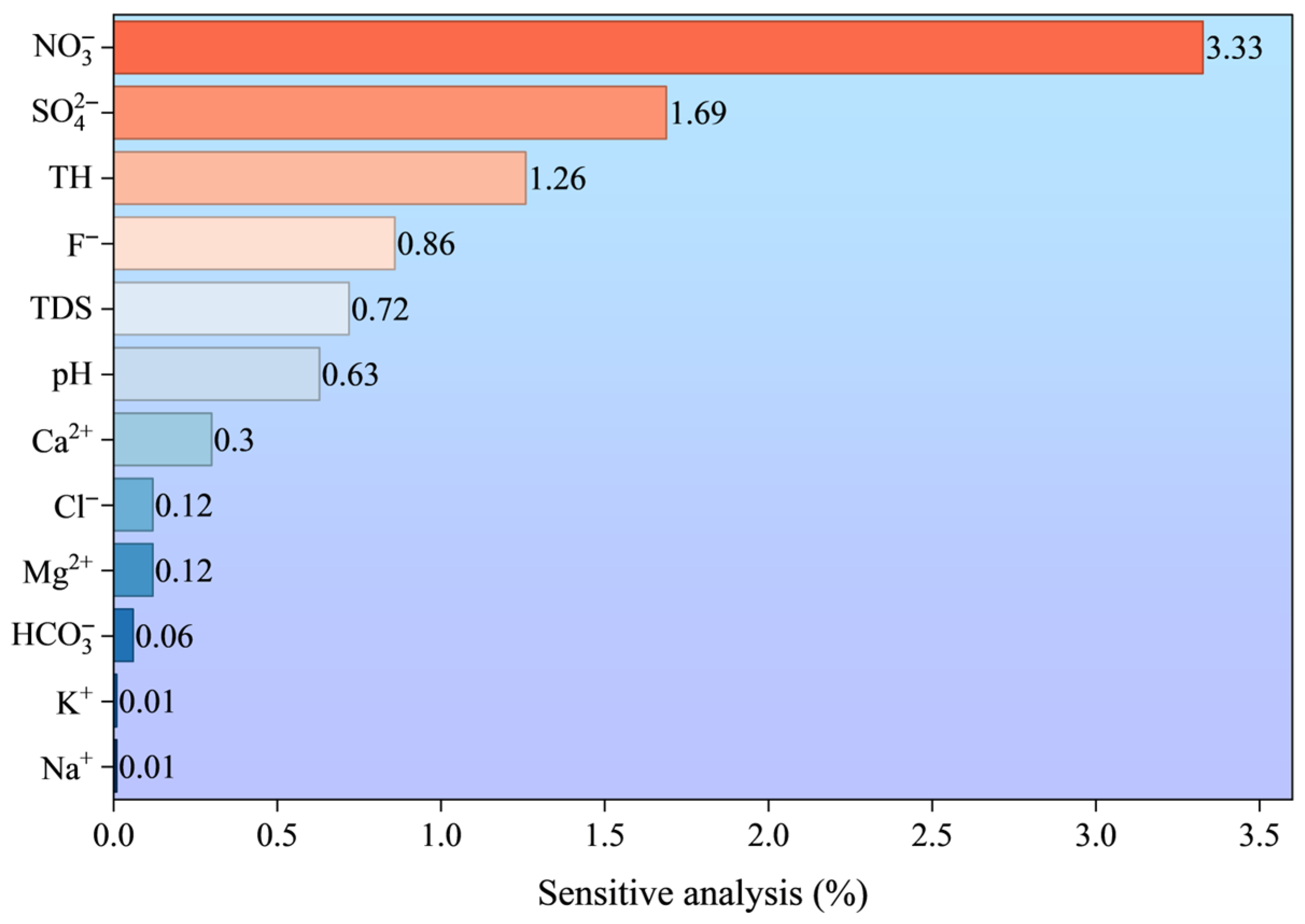
- Among the three machine learning models (LR, SVM, XGB) constructed for groundwater quality prediction, the LR model exhibits the optimal performance, with the highest prediction accuracy and strongest stability, followed by the SVM model. The XGB model, however, shows obvious overfitting and lower generalization ability. The LR model can provide reliable technical support for the dynamic monitoring and early warning of groundwater quality in the study area. The results of the sensitivity analysis indicated that NO3− was the most influential variable affecting the performance of the LR model.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Appukuttan, A.; Aju, C.D.; Reghunath, R.; Srinivas, R.; Krishnan, K.A.; Arya, S. Exploring hydrochemical drivers of drinking water quality in a tropical river basin using self-organizing maps and explainable AI. Water Res. 2025, 284, 123884. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.; Tian, H.; Huang, X.; Zhang, Z.; Liu, Y.; Xiao, Y.; Li, R. Hydrochemistry appraisal, quality assessment and health risk evaluation of shallow groundwater in the Mianyang area of Sichuan Basin, southwestern China. Environ. Earth Sci. 2021, 80, 576. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Y.; Wang, Y.; Huang, X.; Xiao, Y.; Pei, Q. Hydrochemistry, quality and potential health risk appraisal of nitrate enriched groundwater in the Nanchong area, southwestern China. Sci. Total Environ. 2021, 784, 147186. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, H.; Yang, Q. Hydrochemical interpretation of groundwater in Yinchuan basin using self-organizing maps and hierarchical clustering. Chemosphere 2022, 309, 136787. [Google Scholar] [CrossRef] [PubMed]
- El Yousfi, Y.; Himi, M.; El Ouarghi, H.; Aqnouy, M.; Benyoussef, S.; Gueddari, H.; Ait Hmeid, H.; Alitane, A.; Chaibi, M.; Zahid, M.; et al. Assessment and prediction of the water quality index for the groundwater of the Ghiss-Nekkor (Al Hoceima, Northeastern Morocco). Sustainability 2023, 15, 402. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, C.; Liang, X.; Yang, W.; Zhang, L.; Dai, R.; Li, W.; Ni, H. A bayesian maximum entropy fusion model for enhanced prediction and risk assessment of fluoride and arsenic contamination in groundwater. J. Contam. Hydrol. 2025, 274, 104664. [Google Scholar] [CrossRef]
- Yao, R.; Zhang, Y.; Sun, Z.; Zhao, X.; Zhang, H.; Zhang, X.; Luo, M.; Uddin, M.G.; Wang, Y.; Wang, Y. A novel grid-based technique for quantifying groundwater quality under land use/land cover changes to support improved groundwater management. J. Hydrol. 2025, 662, 133955. [Google Scholar] [CrossRef]
- Patel, N.; Bhatt, D. Insights of ground water quality assessment methods—A review. Mater. Today Proc. 2024. [Google Scholar] [CrossRef]
- Asma, B.; Şener, Ş. Appraisal of groundwater suitability and hydrochemical characteristics by using various water quality indices and statistical analyses in the Wadi Righ area, Algeria. Water Supply 2024, 24, 1938–1957. [Google Scholar] [CrossRef]
- Ali, S.; Verma, S.; Agarwal, M.B.; Islam, R.; Mehrotra, M.; Deolia, R.K.; Kumar, J.; Singh, S.; Mohammadi, A.A.; Raj, D.; et al. Groundwater quality assessment using water quality index and principal component analysis in the Achnera block, Agra district, Uttar Pradesh, northern India. Sci. Rep. 2024, 14, 5381. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Wang, X.; Wan, L.; Shi, Z.; Wanke, H.; Uugulu, S.; Uahengo, C.-I. Groundwater quality and associated hydrogeochemical processes in Northwest Namibia. J. Geochem. Explor. 2018, 186, 202–214. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, R.; Wu, J.; Wang, H.; Luo, Z. Uncertain in WQI-based groundwater quality assessment methods: A case study in east of Beijing, China. Environ. Earth Sci. 2022, 81, 202. [Google Scholar] [CrossRef]
- Şener, E.; Şener, Ş.; Varol, S. Appraisal of groundwater quality with WQI and human health risk assessment in Karamık wetland and surroundings (Afyonkarahisar/Turkey). Environ. Geochem. Health 2023, 45, 1499–1523. [Google Scholar] [CrossRef]
- Swain, P.K.; Biswal, T. Assessment of groundwater quality in terms of water quality index (WQI) and fluoride contamination of Nuapada district, Odisha, India. Appl. Water Sci. 2023, 13, 218. [Google Scholar] [CrossRef]
- Hamma, B.; Alodah, A.; Bouaicha, F.; Bekkouche, M.F.; Barkat, A.; Hussein, E.E. Hydrochemical assessment of groundwater using multivariate statistical methods and water quality indices (WQIs). Appl. Water Sci. 2024, 14, 33. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, D.; Xie, Z.; Li, H.; Yang, S.; Luo, M.; Vesković, J.; Onjia, A.; Wang, Y.; Wang, Y.; et al. Spatial source-oriented analysis and probabilistic health risk assessment of potentially toxic elements in soils integrating the geo-detector, APCS-MLR, and monte-carlo models. J. Environ. Chem. Eng. 2025, 13, 117983. [Google Scholar] [CrossRef]
- Sutradhar, S.; Mondal, P. Groundwater suitability assessment based on water quality index and hydrochemical characterization of suri sadar sub-division, west bengal. Ecol. Inform. 2021, 64, 101335. [Google Scholar] [CrossRef]
- Dashora, M.; Kumar, A.; Kumar, S.; Kumar, P.; Kumar, A.; Singh, C.K. Geochemical assessment of groundwater in a desertic region of India using chemometric analysis and entropy water quality index (EWQI). Nat. Hazards 2022, 112, 747–782. [Google Scholar] [CrossRef]
- Raheja, H.; Goel, A.; Pal, M. Assessment of groundwater quality and its vulnerability for safe drinking purpose. J. Hydroinform. 2024, 26, 2302–2324. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; Elumalai, V.; Ning, J.; Xu, F.; Mu, D. Groundwater quality assessment using EWQI with updated water quality classification criteria: A case study in and around Zhouzhi county, Guanzhong basin (China). Expo. Health 2023, 15, 825–840. [Google Scholar] [CrossRef]
- Ding, F.; Chen, L.; Sun, C.; Zhang, W.; Yue, H.; Na, S. An upgraded groundwater quality evaluation based on hasse diagram technique & game theory. Ecol. Indic. 2022, 140, 109024. [Google Scholar] [CrossRef]
- Tian, R.; Wu, J. Groundwater quality appraisal by improved set pair analysis with game theory weightage and health risk estimation of contaminants for xuecha drinking water source in a loess area in northwest China. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 132–157. [Google Scholar] [CrossRef]
- Niazkar, M.; Piraei, R.; Goodarzi, M.R.; Abedi, M.J. Comparative assessment of machine learning models for groundwater quality prediction using various parameters. Environ. Process. 2025, 12, 10. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Z.; Liu, W.; Huang, J.; Chen, S.; Zhang, X.; Yang, C.; Li, J.; Kang, W.; Wang, Y. Source identification and health risk assessment of urban groundwater nitrate contamination in Chongqing, southwestern China. J. Hydrol. Reg. Stud. 2025, 62, 102792. [Google Scholar] [CrossRef]
- Raheja, H.; Goel, A.; Pal, M. Groundwater quality prediction using proximal hyperspectral sensing, GIS, and machine learning algorithms. Water Air Soil Pollut. 2025, 236, 375. [Google Scholar] [CrossRef]
- Thanh, N.N.; Chotpantarat, S.; Ngu, N.H.; Thunyawatcharakul, P.; Kaewdum, N. Integrating machine learning models with cross-validation and bootstrapping for evaluating groundwater quality in Kanchanaburi province, Thailand. Environ. Res. 2024, 252, 118952. [Google Scholar] [CrossRef] [PubMed]
- Moeinzadeh, H.; Yong, K.-T.; Withana, A. A critical analysis of parameter choices in water quality assessment. Water Res. 2024, 258, 121777. [Google Scholar] [CrossRef]
- Deb, S. Enhancing groundwater quality index prediction in data-scarce regions: Application of advanced artificial intelligence models in Nagaland, India. Dyn. Atmos. Ocean. 2025, 111, 101579. [Google Scholar] [CrossRef]
- Banaei, S.M.A.; Javid, A.H.; Hassani, A.H. Numerical simulation of groundwater contaminant transport in porous media. Int. J. Environ. Sci. Technol. 2021, 18, 151–162. [Google Scholar] [CrossRef]
- Chen, C.; He, W.; Zhou, H.; Xue, Y.; Zhu, M. A comparative study among machine learning and numerical models for simulating groundwater dynamics in the heihe river basin, northwestern China. Sci. Rep. 2020, 10, 3904. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, C.; Yang, W.; Liang, X.; Zhang, L.; Wang, X.; Dai, R. Improving prediction of groundwater quality in situations of limited monitoring data based on virtual sample generation and gaussian process regression. Water Res. 2024, 267, 122498. [Google Scholar] [CrossRef]
- Bui, D.T.; Khosravi, K.; Tiefenbacher, J.; Nguyen, H.; Kazakis, N. Improving prediction of water quality indices using novel hybrid machine-learning algorithms. Sci. Total Environ. 2020, 721, 137612. [Google Scholar] [CrossRef]
- Chan Kujiek, D.; Sahile, Z.A. Water quality assessment of Elgo river in Ethiopia using CCME, WQI and IWQI for domestic and agricultural usage. Heliyon 2024, 10, e23234. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, C.; Wang, Y.; Lyu, G.; Lin, S.; Liu, W.; Niu, H.; Hu, Q. Application of machine learning models in groundwater quality assessment and prediction: Progress and challenges. Front. Environ. Sci. Eng. 2023, 18, 29. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Y.; Yang, S.; Wei, D.; Zhang, J.; Li, Q.; Yao, R.; Wu, X.; Wang, Y. Optimized groundwater quality evaluation using unsupervised machine learning, game theory and monte-carlo simulation. J. Environ. Manag. 2024, 371, 122902. [Google Scholar] [CrossRef]
- Khiavi, A.N.; Tavoosi, M.; Kuriqi, A. Conjunct application of machine learning and game theory in groundwater quality mapping. Environ. Earth Sci. 2023, 82, 395. [Google Scholar] [CrossRef]
- Xu, Z.; Kong, F.; Cao, C.; Zhang, Z. Prediction and analysis of tunnel water inrush disasters in Chinese karst area based on variable weight-weighted bayesian network model. Carbonates Evaporites 2024, 40, 2. [Google Scholar] [CrossRef]
- GB/T 14848-2017; Natural Resources and Territory Spatial Planning (SAC/TC 93). State General Administration of the People’s Republic of China for Quality Supervision and Inspection and Quarantine (AQSIQ). Standardization Administration of the People’s Republic of China: Beijing, China, 2017.
- Gao, Y.; Qian, H.; Ren, W.; Wang, H.; Liu, F.; Yang, F. Hydrogeochemical characterization and quality assessment of groundwater based on integrated-weight water quality index in a concentrated urban area. J. Clean. Prod. 2020, 260, 121006. [Google Scholar] [CrossRef]
- Kumar, A.; Tripathi, M.P.; Khalkho, D.; Dewangan, R.; Baghel, S.; Kuriqi, A. Groundwater quality evaluation for drinking and irrigation using analytical hierarchy process with GIS in semi critical block of Chhattisgarh, India. Environ. Earth Sci. 2024, 83, 334. [Google Scholar] [CrossRef]
- Hosseininia, M.; Hassanzadeh, R. Groundwater quality assessment for domestic and agricultural purposes using GIS, hydrochemical facies and water quality indices: Case study of Rafsanjan plain, Kerman province, iran. Appl. Water Sci. 2023, 13, 84. [Google Scholar] [CrossRef]
- Nayak, A.; Matta, G.; Uniyal, D.P. Hydrochemical characterization of groundwater quality using chemometric analysis and water quality indices in the foothills of Himalayas. Environ. Dev. Sustain. 2023, 25, 14229–14260. [Google Scholar] [CrossRef] [PubMed]
- Masteali, S.H.; Bayat, M.; Bettinger, P.; Ghorbanpour, M. Uncertainty analysis of linear and non-linear regression models in the modeling of water quality in the Caspian sea basin: Application of monte-carlo method. Ecol. Indic. 2025, 170, 112979. [Google Scholar] [CrossRef]
- Rastgou, M.; He, Y.; Lou, R.; Jiang, Q. A comparison of metaheuristic optimizations with automated hyperparameter tuning methods in support vector machines algorithm for predicting soil water characteristic curve. Eng. Geol. 2025, 353, 108121. [Google Scholar] [CrossRef]
- Seo, J.; Kim, Y. Assessing the likelihood of drought impact occurrence with extreme gradient boosting: A case study on the public water supply in South Korea. J. Hydroinform. 2023, 25, 191–207. [Google Scholar] [CrossRef]
- Eid, M.H.; Mikita, V.; Eissa, M.; Ramadan, H.S.; Mohamed, E.A.; Abukhadra, M.R.; El-Sherbeeny, A.M.; Kovács, A.; Szűcs, P. An advanced approach for drinking water quality indexing and health risk assessment supported by machine learning modelling in Siwa oasis, Egypt. J. Hydrol. Reg. Stud. 2024, 56, 101967. [Google Scholar] [CrossRef]
- Prasun, A.; Singh, A. Assessment of groundwater contamination in Aurangabad, Bihar using WQI and geostatistical modeling. Stoch. Environ. Res. Risk Assess. 2025, 39, 789–811. [Google Scholar] [CrossRef]
- Sunitha, V.; Reddy, B.M. Geochemical characterization, deciphering groundwater quality using pollution index of groundwater (PIG), water quality index (WQI) and geographical information system (GIS) in hard rock aquifer, south India. Appl. Water Sci. 2022, 12, 41. [Google Scholar] [CrossRef]
- Stevenazzi, S.; Voudouris, K.; Ducci, D. Combination of hydrochemical graphical methods and multivariate statistical analysis to delineate groundwater bodies. J. Environ. Manag. 2025, 381, 125266. [Google Scholar] [CrossRef]
- Traore, A.; Mao, X.; Traore, A.; Yakubu, Y.; Sidibe, A.M. Multivariate statistical analysis of dominating groundwater mineralization and hydrochemical evolution in gao, northern mali. J. Earth Sci. 2024, 35, 1692–1703. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Huang, J.; Zhang, J.; Chen, P.; Wang, Y.; Wang, Y.; Li, Q.; Pu, W.; Yuan, X. Multi−isotopes (H, O, Sr, and S) and element geochemistry driving the genesis of geothermal waters in a sedimentary basin, southwestern China. Geothermics 2025, 133, 103480. [Google Scholar] [CrossRef]
- Nagarajan, R.; Rajmohan, N.; Mahendran, U.; Senthamilkumar, S. Evaluation of groundwater quality and its suitability for drinking and agricultural use in Thanjavur city, Tamil Nadu, India. Environ. Monit. Assess. 2010, 171, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Yang, J.; Wang, J.; Zhang, L.; Tan, Y.; Xiang, D. Hydrochemical characteristics and genetic analysis of groundwater in zhanjiang city, guangdong province, south China. Water 2025, 17, 698. [Google Scholar] [CrossRef]
- Hussein, H.; El Maghraby, M.M.M.S.; Abu Salem, H.S. Application of water quality index and statistical-hydrochemical techniques in groundwater assessment of the quaternary aquifer, southwest nile delta of egypt. Appl. Water Sci. 2024, 14, 143. [Google Scholar] [CrossRef]
- Omonona, O.V.; Okogbue, C.O. Hydrochemical evolution, geospatial groundwater quality and potential health risks associated with intake of nitrate via drinking water: Case of Gboko agricultural district, central Nigeria. Environ. Earth Sci. 2021, 80, 126. [Google Scholar] [CrossRef]
- Tanwer, N.; Deswal, M.; Khyalia, P.; Laura, J.S.; Khosla, B. Assessment of groundwater potability and health risk due to fluoride and nitrate in groundwater of Churu District of Rajasthan, India. Environ. Geochem. Health 2023, 45, 4219–4241. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, Y.; Yuan, X.; Wang, Y.; Lv, G.; Li, X. Genesis of high-sulfate geothermal water in the Namcha Barwa syntaxis, eastern Xizang. Hydrogeol. Eng. Geol. 2025, 52, 173–189. [Google Scholar] [CrossRef]
- Qu, S.; Liao, F.; Wang, G.; Wang, X.; Shi, Z.; Liang, X.; Duan, L.; Liu, T. Hydrochemical evolution of groundwater in overburden aquifers under the influence of mining activity: Combining hydrochemistry and groundwater dynamics analysis. Environ. Earth Sci. 2023, 82, 135. [Google Scholar] [CrossRef]
- Subba Rao, N.; Das, R.; Sahoo, H.K.; Gugulothu, S. Hydrochemical characterization and water quality perspectives for groundwater management for urban development. Groundw. Sustain. Dev. 2024, 24, 101071. [Google Scholar] [CrossRef]
- Singh, A.; Raju, A.; Chandniha, S.K.; Singh, L.; Tyagi, I.; Karri, R.R.; Kumar, A. Hydrogeochemical characterization of groundwater and their associated potential health risks. Environ. Sci. Pollut. Res. 2022, 30, 14993–15008. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, S.; Hu, C.; Zhao, Z.; Gao, Z.; Liu, J. Hydrochemical assessment of groundwater utilizing statistical analysis, integrated geochemical methods, and EWQI: A case study of Laiwu region, north China. Environ. Monit. Assess. 2024, 196, 1222. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, L.; Wang, C.; Zou, Y. Hydrochemical evolution and nitrate contamination sources in laiwu groundwater: Insights from hydrochemistry and dual-isotope analysis. Phys. Chem. Earth Parts A/B/C 2025, 141, 104083. [Google Scholar] [CrossRef]
- Sakthi Priya, R.; Antony Ravindran, A.; Richard Abishek, S. Spatial assessment of submarine groundwater discharge influence on aquifer water quality in the coastal region of Chettikulam to Kolachel, southern India: Using SMI and HFE-D techniques. Environ. Geochem. Health 2025, 47, 112. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, Y.; Sun, Z.; Xie, Z.; Yao, R.; Chen, S.; Uddin, M.G.; Pu, Y.; Yang, C.; Wang, Y.; et al. Using unsupervised machine learning and positive matrix factorization models to drive groundwater chemistry and associated health risks in a coal—Mining rural region. J. Hydrol. 2025, 661, 133691. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Y.; Yao, R.; Wei, D.; Huang, X.; Luo, M.; Wei, C.; Chen, S.; Yang, C. Natural background levels, source apportionment and health risks of potentially toxic elements in groundwater of highly urbanized area. Sci. Total Environ. 2024, 935, 173276. [Google Scholar] [CrossRef]
- Dimple; Singh, P.K.; Rajput, J.; Kumar, D.; Gaddikeri, V.; Elbeltagi, A. Combination of discretization regression with data-driven algorithms for modeling irrigation water quality indices. Ecol. Inform. 2023, 75, 102093. [Google Scholar] [CrossRef]
- Gholami, V.; Khaleghi, M.R.; Salimi, E.T. Comparison of extreme gradient boosting, deep learning, and self-organizing map methods in predicting groundwater depth. Environ. Earth Sci. 2025, 84, 179. [Google Scholar] [CrossRef]
- Nafouanti, M.B.; Li, J.; Mustapha, N.A.; Uwamungu, P.; AL-Alimi, D. Prediction on the fluoride contamination in groundwater at the datong basin, northern China: Comparison of random forest, logistic regression and artificial neural network. Appl. Geochem. 2021, 132, 105054. [Google Scholar] [CrossRef]
- Tsuchihara, T.; Yoshimoto, S.; Shirahata, K.; Nakazato, H.; Ishida, S. Analysis of groundwater-level fluctuation and linear regression modeling for prediction of initial groundwater level during irrigation of rice paddies in the Nasunogahara alluvial fan, central Japan. Environ. Earth Sci. 2023, 82, 473. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Liu, T.; Wei, W.; Zhang, F.; Huang, M.; Liu, H. Enhancing water quality prediction with advanced machine learning techniques: An extreme gradient boosting model based on long short-term memory and autoencoder. J. Hydrol. 2024, 644, 132115. [Google Scholar] [CrossRef]
| n | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RI | 0 | 0 | 0.52 | 0.89 | 1.12 | 1.26 | 1.36 | 1.41 | 1.46 | 1.49 | 1.52 | 1.54 | 1.56 | 1.58 | 1.59 |
| Parameters | pH | TDS | TH | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3− | F− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 7.00 | 69.00 | 15.00 | 0.15 | 2.24 | 5.63 | 0.20 | 1.01 | 1.45 | 39.90 | 0.02 | 0.03 |
| Median | 8.00 | 226.00 | 173.00 | 1.08 | 12.21 | 52.13 | 9.46 | 2.87 | 50.49 | 165.90 | 0.98 | 0.13 |
| Mean | 8.08 | 302.58 | 218.91 | 1.52 | 17.08 | 60.19 | 15.68 | 10.46 | 82.29 | 170.43 | 2.95 | 0.16 |
| Max | 9.10 | 1660.00 | 1370.00 | 20.30 | 172.90 | 330.75 | 144.13 | 443.70 | 951.66 | 519.75 | 73.50 | 0.79 |
| CV | 0.05 | 0.77 | 0.82 | 1.45 | 1.12 | 0.68 | 1.26 | 4.12 | 1.42 | 0.42 | 2.97 | 0.70 |
| Limit | 6.5–8.5 | 450 | 1000 | 200 | 200 | 200 | 150 | 250 | 250 | 450 | 20 | 1 |
| Exceedance | 12.90% | 16.13% | 0.81% | 0.00% | 0.00% | 0.81% | 0.00% | 0.81% | 0.81% | 1.61% | 4.03% | 0.00% |
| Models | LR | SVM | XGB |
|---|---|---|---|
| Hyperparameters | Default | kernel = ‘rbf’ C = 100 gamma = 0.01 epsilon = 0.1 | n_estimators = 150 max_depth = 3 learning_rate = 0.05 subsample = 0.8 colsample_bytree = 1 |
| Parameters | Model | R2 | RMSE | MAE | ΔR2 | ΔRMSE | ΔMAE |
|---|---|---|---|---|---|---|---|
| Test set | LR | 0.99 | 0.00 | 0.00 | - | - | - |
| SVM | 0.98 | 1.55 | 0.56 | - | - | - | |
| XGB | 0.81 | 6.41 | 2.43 | - | - | - | |
| Training set | LR | 0.99 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SVM | 0.97 | 4.01 | 0.63 | 0.02 | 0.59 | 0.94 | |
| XGB | 0.99 | 1.22 | 0.74 | 0.20 | 7.22 | 2.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Wu, X.; Liu, W.; Wei, D.; Wang, Y.; Wu, H.; Wang, Y.; Zhu, B.; Hu, Q.; Zhang, Y.; et al. Improved Assessment and Prediction of Groundwater Drinking Quality Integrating Game Theory and Machine Learning in the Nyangchu River Basin, Southwestern Qinghai–Tibet Plateau. Toxics 2025, 13, 985. https://doi.org/10.3390/toxics13110985
Huang X, Wu X, Liu W, Wei D, Wang Y, Wu H, Wang Y, Zhu B, Hu Q, Zhang Y, et al. Improved Assessment and Prediction of Groundwater Drinking Quality Integrating Game Theory and Machine Learning in the Nyangchu River Basin, Southwestern Qinghai–Tibet Plateau. Toxics. 2025; 13(11):985. https://doi.org/10.3390/toxics13110985
Chicago/Turabian StyleHuang, Xun, Xiyong Wu, Weiting Liu, Denghui Wei, Ying Wang, Hua Wu, Yangshuang Wang, Boyi Zhu, Qili Hu, Yunhui Zhang, and et al. 2025. "Improved Assessment and Prediction of Groundwater Drinking Quality Integrating Game Theory and Machine Learning in the Nyangchu River Basin, Southwestern Qinghai–Tibet Plateau" Toxics 13, no. 11: 985. https://doi.org/10.3390/toxics13110985
APA StyleHuang, X., Wu, X., Liu, W., Wei, D., Wang, Y., Wu, H., Wang, Y., Zhu, B., Hu, Q., Zhang, Y., & Wang, W. (2025). Improved Assessment and Prediction of Groundwater Drinking Quality Integrating Game Theory and Machine Learning in the Nyangchu River Basin, Southwestern Qinghai–Tibet Plateau. Toxics, 13(11), 985. https://doi.org/10.3390/toxics13110985







