Bioremediation of High-Concentration Heavy Metal-Contaminated Soil by Combined Use of Acidithiobacillus ferrooxidans and Fe3O4–GO Anodes
Highlights
- A Fe3O4–GO-modified anode, A. ferrooxidans, and electric current formed a synergistic system.
- The integrated system achieved high PTE removal (pH ≈ 2.0, ORP ≈ 600 mV).
- Each component was indispensable for adsorption, reduction, and ion migration.
- The electrochemical–biological–nanocatalytic synergy provides a green and efficient approach for remediating heavy-metal-contaminated soils.
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Synthesis and Characterization of Fe3O4–GO Anode
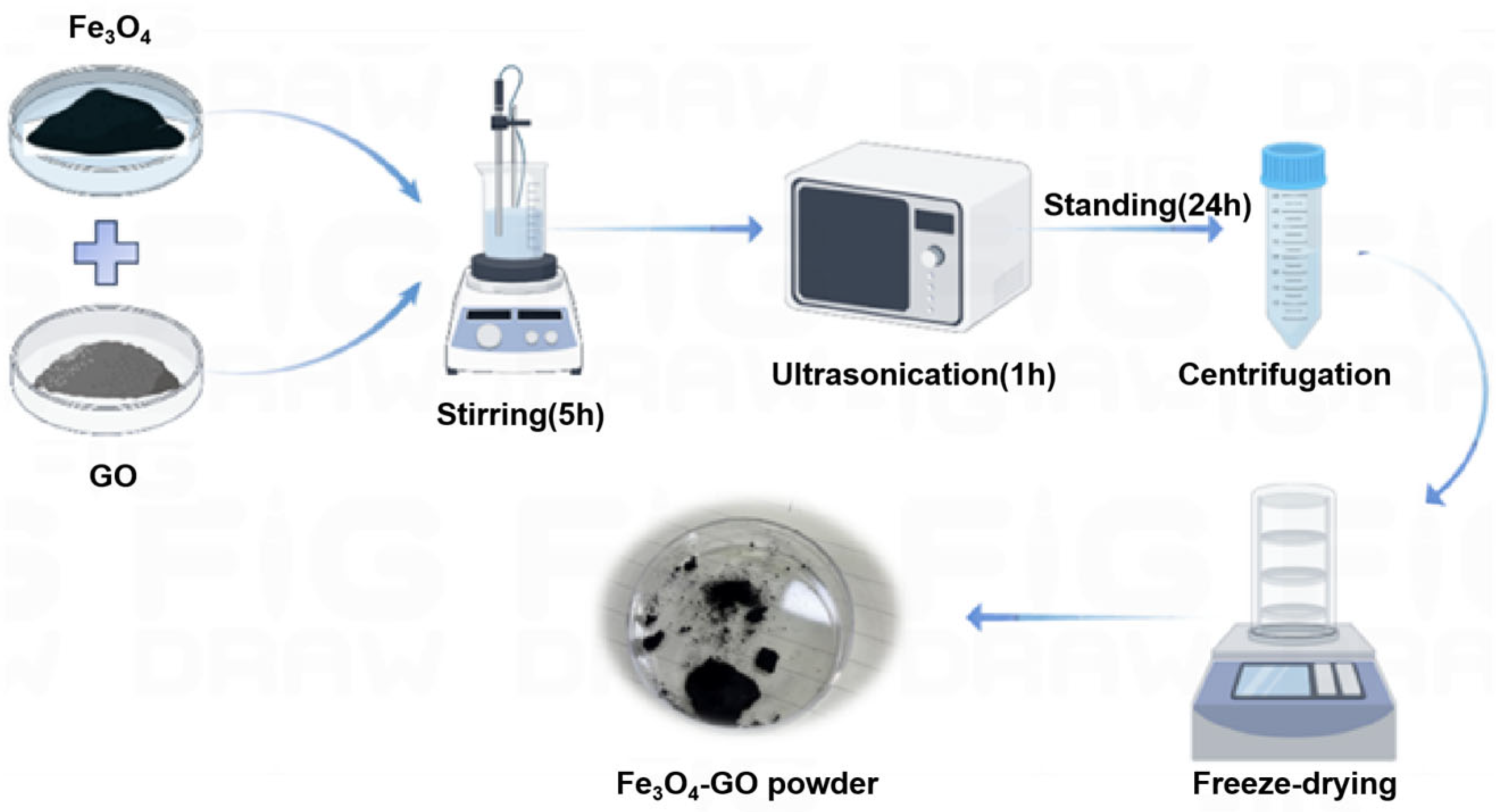
2.2.1. Preparation for Composite Material
2.2.2. Electrode Preparation
2.2.3. Structural Characterization of Fe3O4–GO Composite Material
2.3. Construction of BP-C-MEDC
3. Results and Discussion
3.1. Performance of the Fe3O4–GO Anode
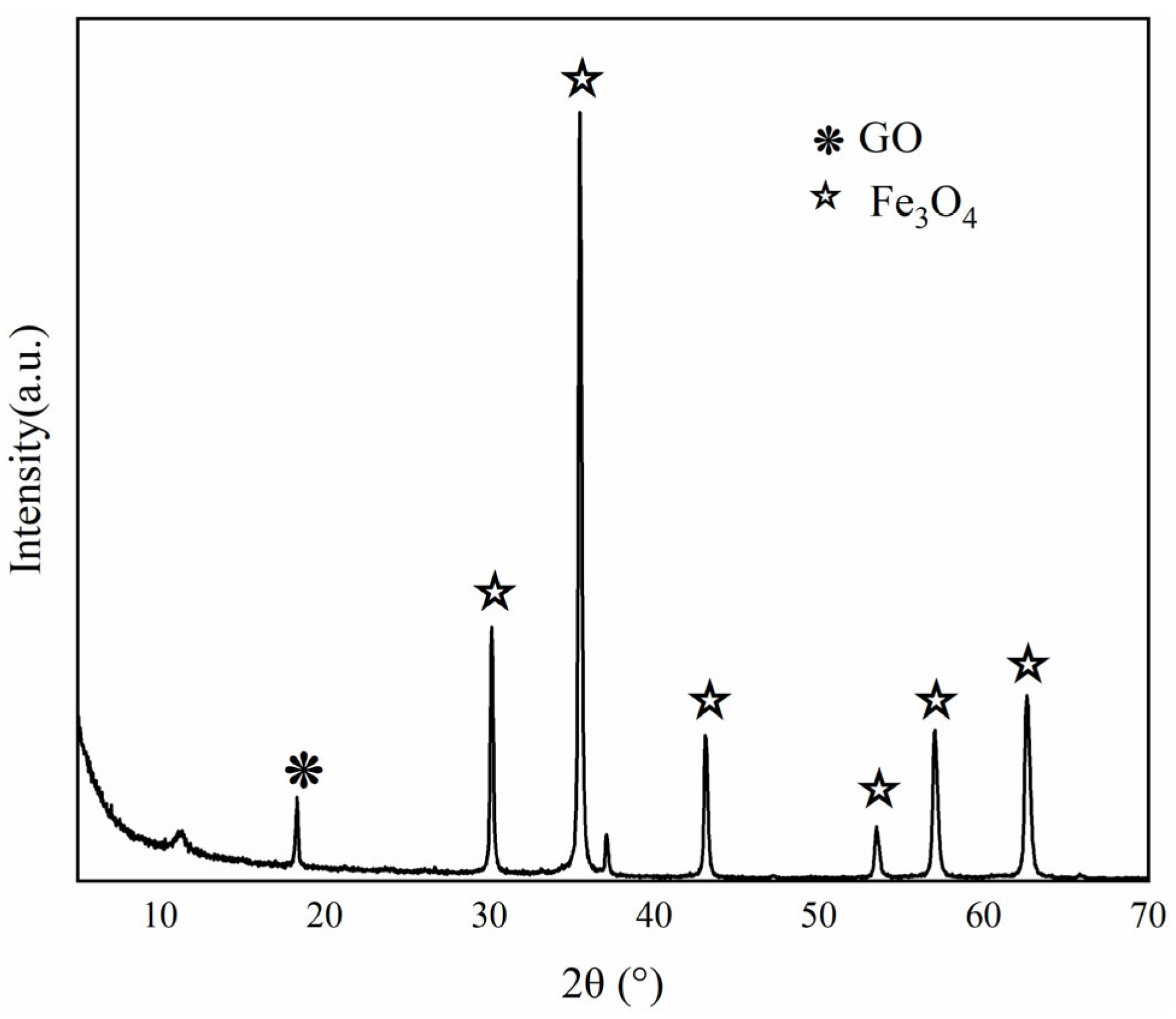
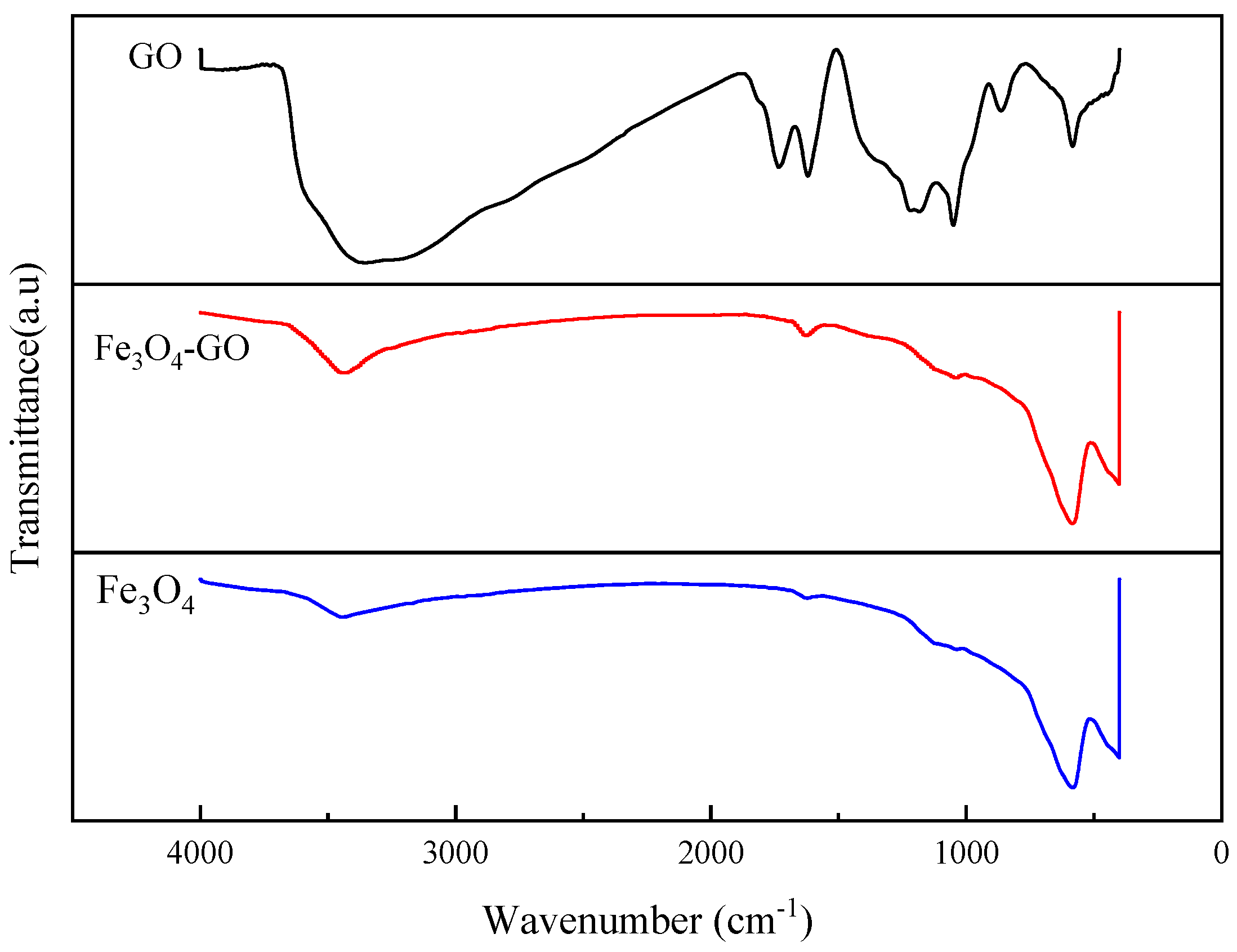
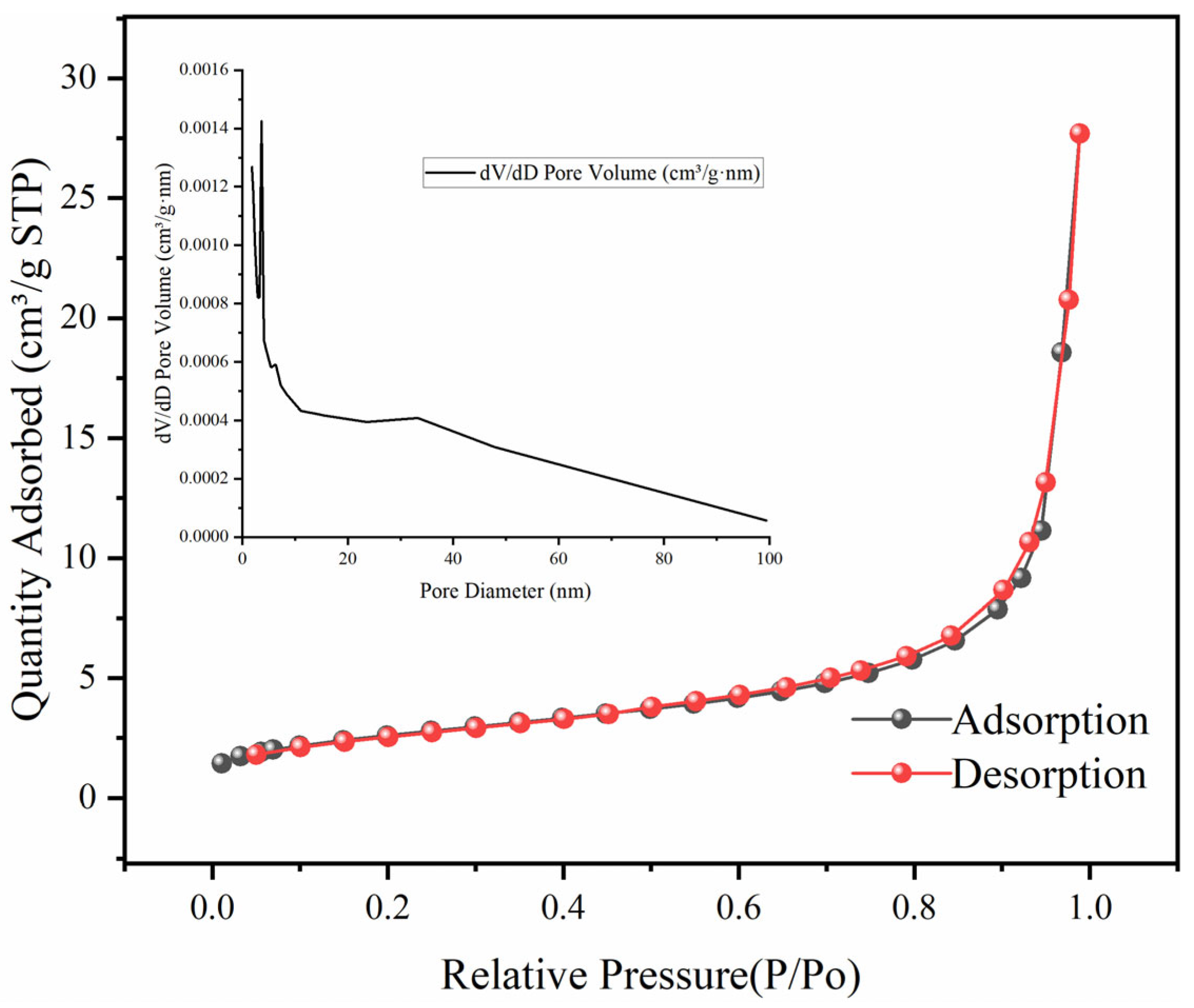
3.1.1. Structural Properties of Fe3O4–GO Composite Material
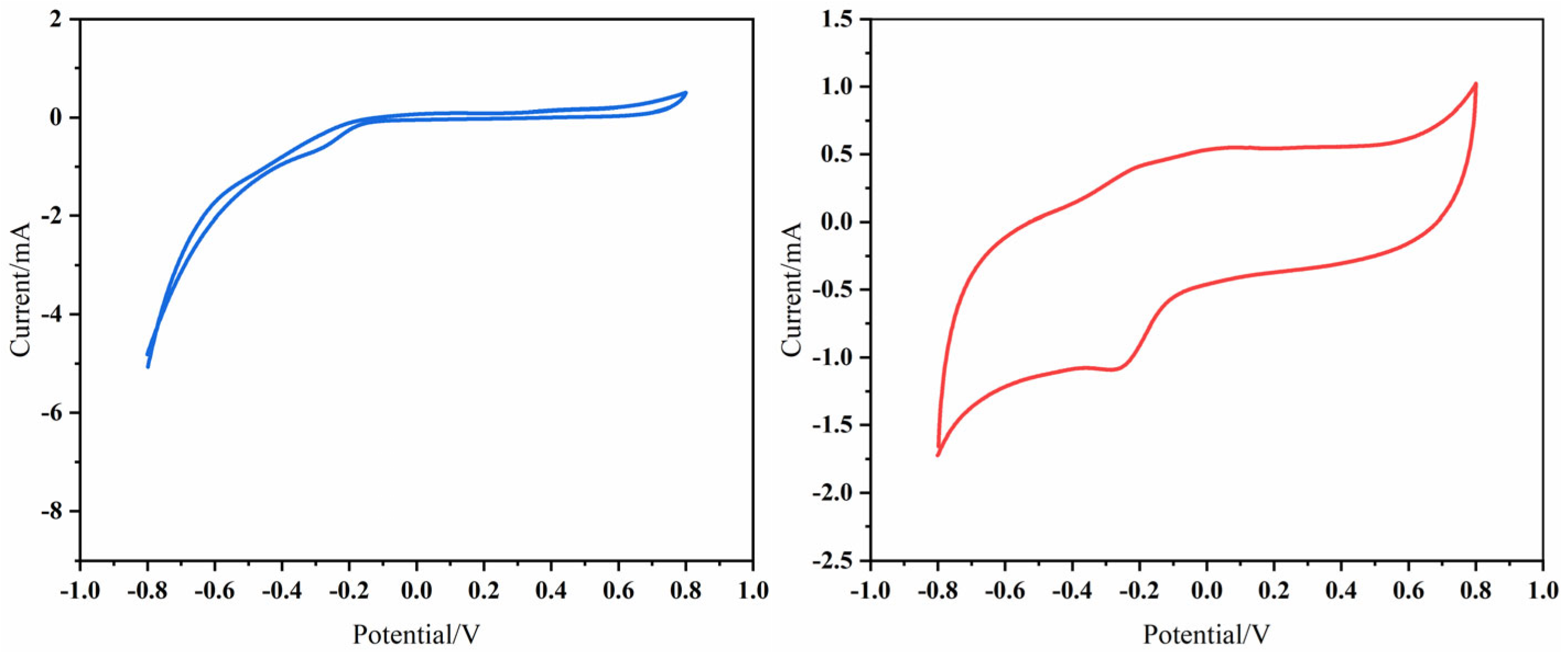
3.1.2. Electrochemical Performance of the Fe3O4–GO Anode
3.2. Removal Efficiency for PTEs in Soil by Acclimated Strains
3.3. System-Level Remediation Efficiency
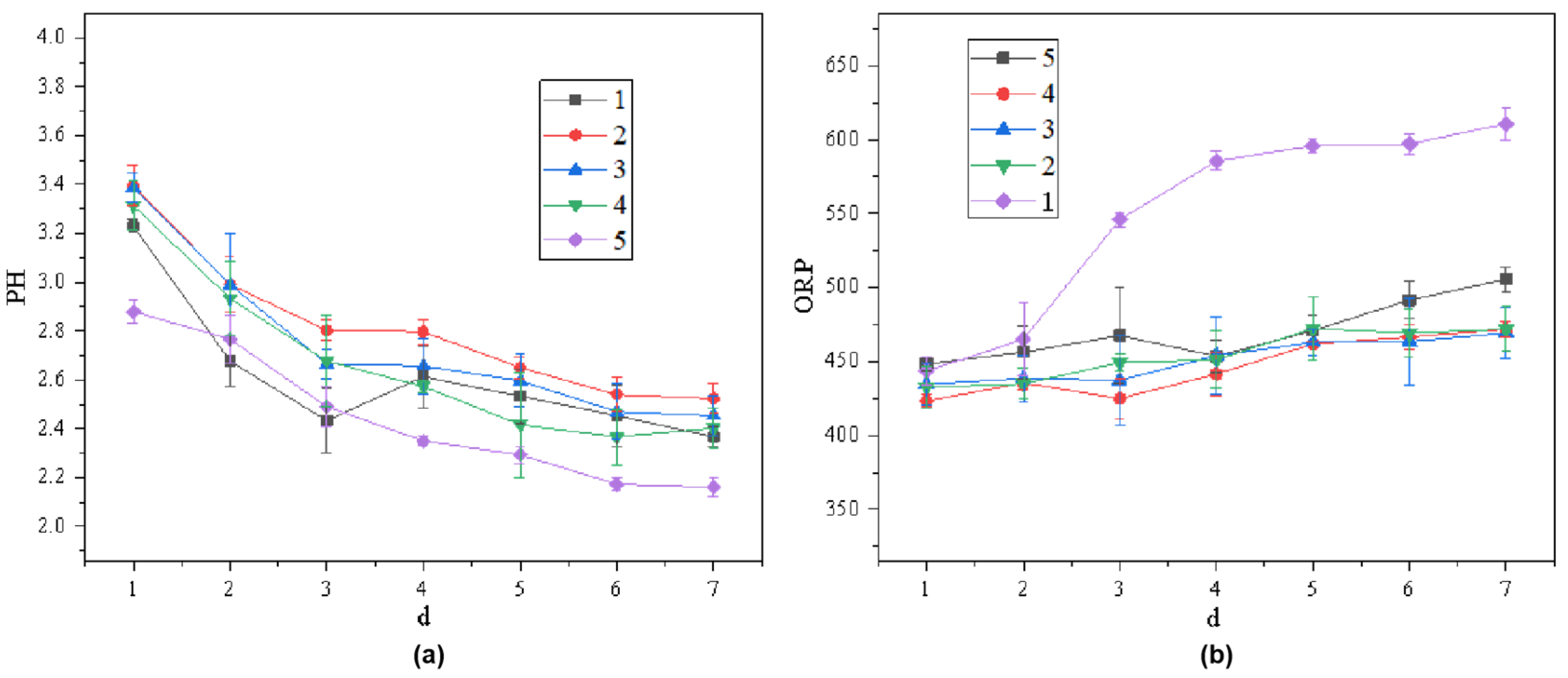
3.3.1. Changes in System pH and ORP
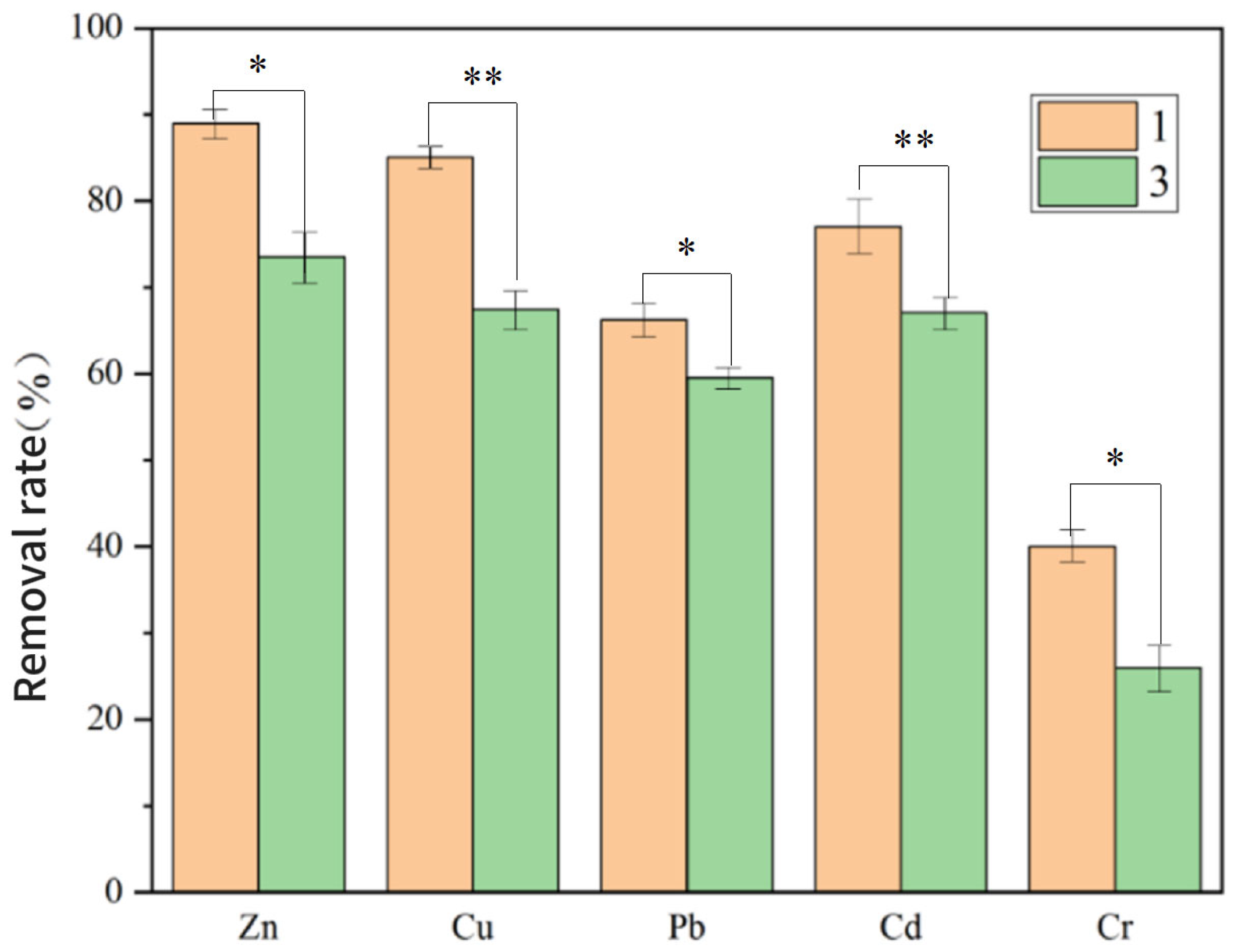
3.3.2. Effect of Fe3O4–GO on PTE Removal Efficiency in the System
3.3.3. Effect of Electric Current on PTE Removal Efficiency in the System
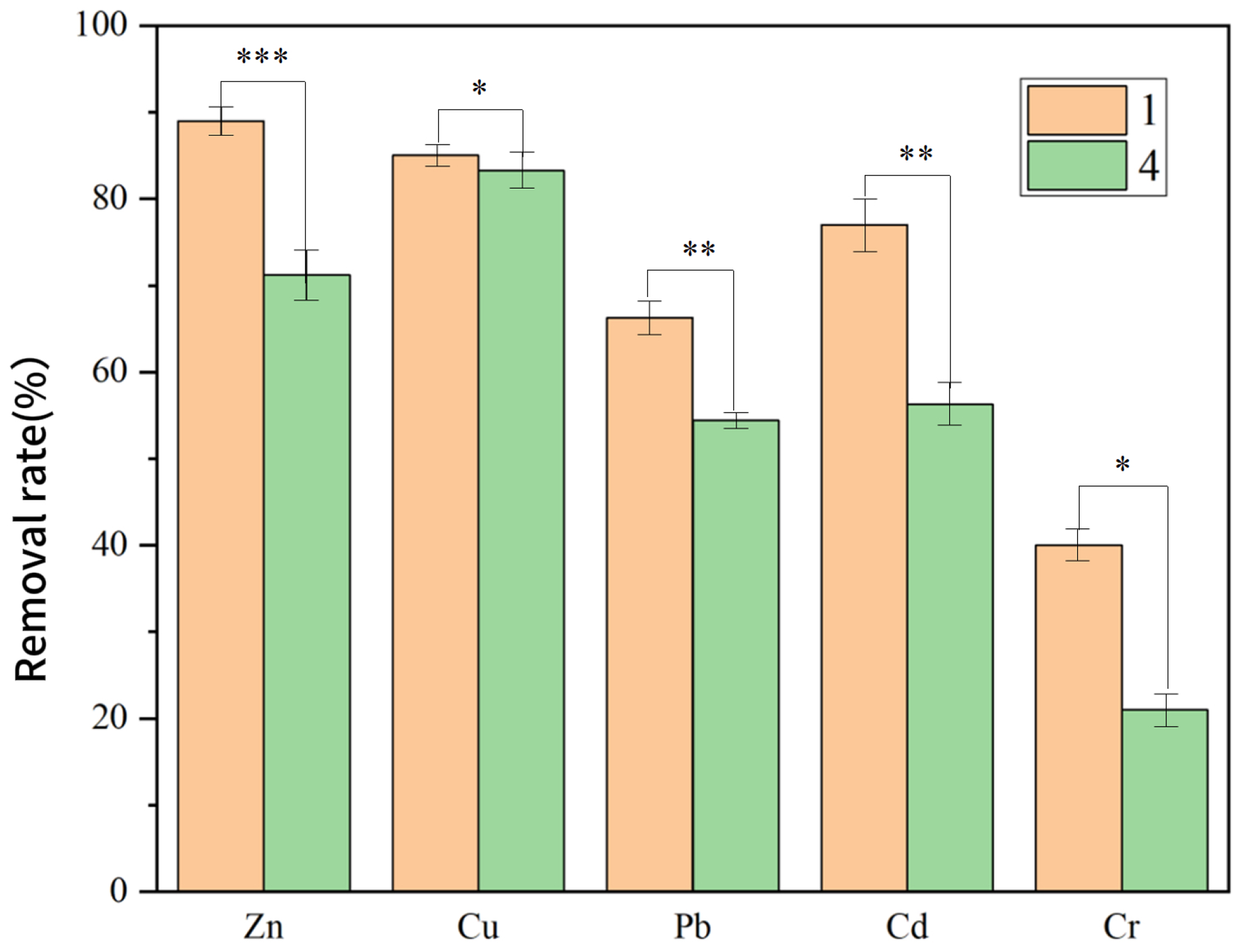
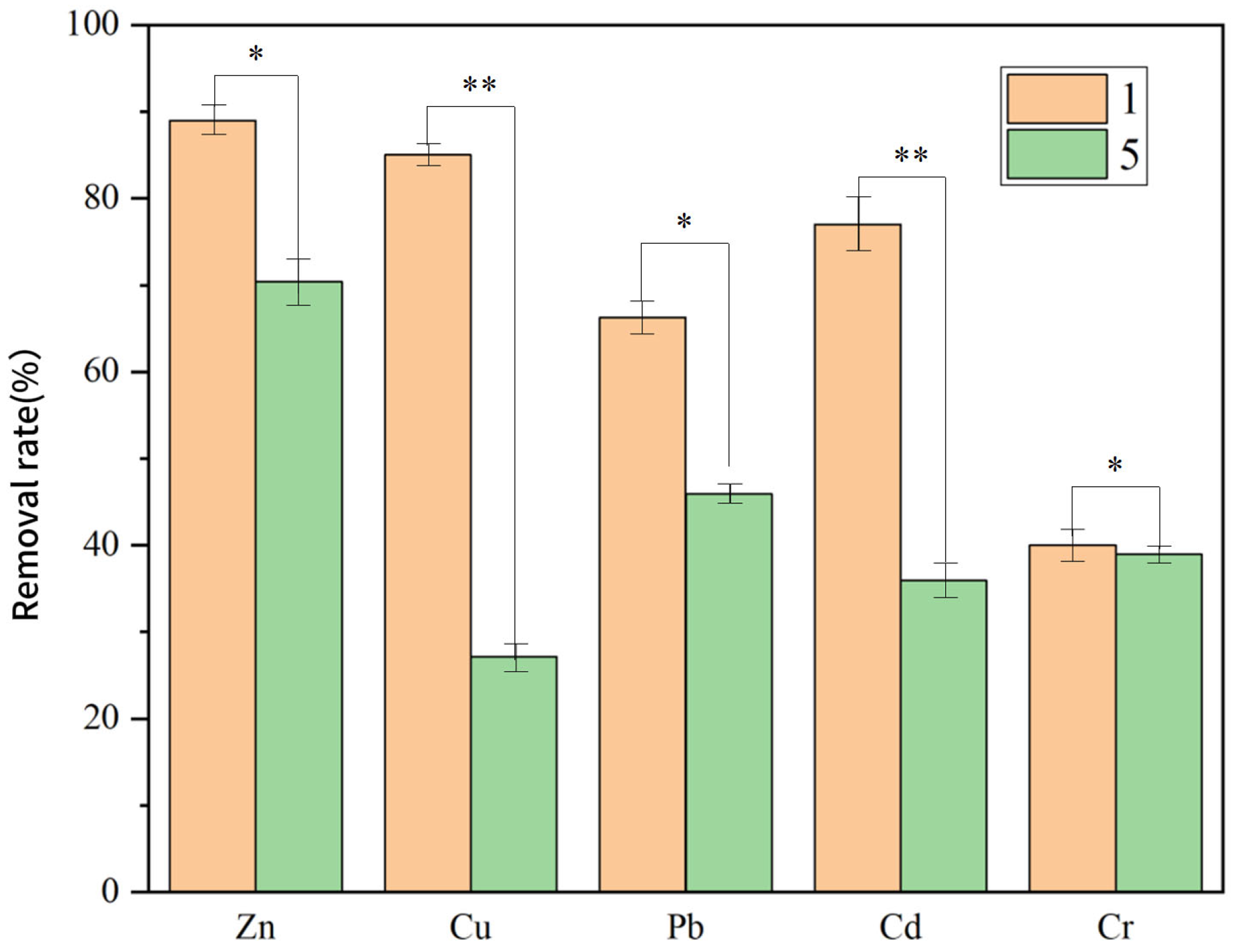
3.3.4. Effect of Modified Electrode on PTE Removal Efficiency in the System
3.3.5. Tripartite Synergistic Mechanism of Fe3O4–GO in Enhancing PTE Removal
Biological Metabolism-Driven Transformation by A. ferrooxidans
- 1.
- Metabolic acidification promotes the dissolution of solid-phase PTEs
- 2.
- Redox cycling mediates H+ production and enhances metal bioavailability
Catalysis and Adsorption Mediated by Fe3O4–GO Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GO | Graphene Oxide |
| BES | Bioelectrochemical System |
| EET | Extracellular electron transfer |
| SMFCs | Sediment microbial fuel cells |
| AAMCC | Aluminum alloy mesh/carbon cloth composite electrode |
| A. ferrooxidans | Acidithiobacillus ferrooxidans |
| PTEs | potentially toxic elements |
| ORP | Oxidation–Reduction potential |
References
- Deep, S.; Kumar, S.P. In situ phytoremediation of heavy metal-contaminated soil and groundwater: A green inventive approach. Environ. Sci. Pollut. Res. 2020, 27, 4104–4124. [Google Scholar]
- Wang, Z.; Wang, H.; Wang, H.; Li, Q.; Li, Y. Effect of soil washing on heavy metal removal and soil quality: A two-sided coin. Ecotoxicol. Environ. Saf. 2020, 203, 110981. [Google Scholar] [CrossRef]
- Yee, E.G.; Szlavecz, K.; Avolio, M.L. Urban weedy plantains (Plantago spp.) do not hyperaccumulate heavy metals nor shelter their soil microarthropod communities from these metals. Urban For. Urban Green. 2025, 104, 128632. [Google Scholar] [CrossRef]
- Shao, G.; Dong, J.; Zhang, W.H.; Sun, S.F.; Li, C.L.; Li, Y. In situ bioelectrochemical remediation of contaminated soil and groundwater: A review. Environ. Pollut. 2025, 374, 126250. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.H.; Tse, E.C.M.; Yates, M.D.; Otero, F.J.; Trammell, S.A.; Stemp, E.D.A.; Barton, J.K.; Tender, L.M.; Newman, D.K. Extracellular DNA promotes efficient extracellular electron transfer by pyocyanin in pseudomonas aeruginosa biofilms. Cell 2020, 182, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Marmanis, D.; Emmanouil, C.; Fantidis, J.G.; Thysiadou, A.; Marmani, K. Description of a Fe/Al Electrocoagulation Method Powered by a Photovoltaic System, for the (Pre-)Treatment of Municipal Wastewater of a Small Community in Northern Greece. Sustainability 2022, 14, 4323. [Google Scholar] [CrossRef]
- Wen, L.M.; Huang, L.Y.; Wang, Y.; Yuan, Y.; Zhou, L.H. Facet-engineered hematite boosts microbial electrogenesis by synergy of promoting electroactive biofilm formation and extracellular electron transfer. Sci. Total Environ. 2022, 819, 153154. [Google Scholar] [CrossRef]
- Zhou, M.H.; Chi, M.L.; Luo, J.M.; He, H.H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Song, T.S.; Tan, W.M.; Wu, X.Y.; Zhou, C.C. Effect of graphite felt and activated carbon fiber felt on performance of freshwater sediment microbial fuel cell. J. Chem. Technol. Biotechnol. 2012, 87, 1436–1440. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wang, C.T.; Yang, Y.C.; Chen, W.J. Application of aluminum-alloy mesh composite carbon cloth for the design of anode/cathode electrodes in Escherichia coli microbial fuel cell. Int. J. Hydrogen Energy 2013, 38, 11131–11137. [Google Scholar] [CrossRef]
- Lowy, D.A.; Tender, L.M.; Zeikus, J.G.; Park, D.H.; Lovley, D.R. Harvesting energy from the marine sediment–water interface II. Biosens. Bioelectron. 2006, 21, 2058–2063. [Google Scholar] [CrossRef]
- Fu, Y.B.; Xu, Q.; Zai, X.R.; Liu, Y.Y.; Lu, Z.K. Low electrical potential anode modified with Fe/ferric oxide and its application in marine benthic microbial fuel cell with higher voltage and power output. Appl. Surf. Sci. 2014, 289, 472–477. [Google Scholar] [CrossRef]
- Bond, D.R.; Lovley, D.R. Electricity production by geocacher sulphureousness attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef]
- Liu, P.P.; Liang, P.; Jiang, Y.; Hao, W.; Miao, B.; Wang, D.L.; Huang, X. Stimulated electron transfer inside electroactive biofilm by magnetite for increased performance microbial fuel cell. Appl. Energy 2018, 216, 382–388. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wang, Z.W.; Wang, Y.J.; Du, F.; Li, Y.H.; Su, Y.Q.; Wang, M.Y.; Ma, M.Y.; Yang, G.R.; Ding, S.J. Graphene supported FeS2 nanoparticles with sandwich structure as a promising anode for high-rate potassium-ion batteries. J. Colloid Interface Sci. 2023, 636, 73–82. [Google Scholar] [CrossRef]
- You, S.; Ma, M.; Wang, W.; Qi, D.; Chen, X.; Qu, J.; Ren, N. 3D Microporous Nitrogen-Enriched graphitic carbon scaffold for efficient bioelectricity generation in microbial fuel cells. Adv. Energy Mater. 2017, 7, 1601364. [Google Scholar] [CrossRef]
- Wang, R.; Yan, M.; Li, H.; Zhang, L.; Peng, B.; Sun, J.; Liu, D.; Liu, S. FeS2 Nanoparticles Decorated Graphene as Microbial-Fuel-Cell Anode Achieving High Power Density. Adv. Mater. 2018, 30, e1800618. [Google Scholar] [CrossRef]
- Wu, X.S.; Qiao, Y.; Shi, Z.Z.; Tang, W.; Li, C.M. Hierarchically porous N-doped carbon nanotubes/reduced graphene oxide composite for promoting flavin-based interfacial electron transfer in microbial fuel cells. ACS Appl. Mater. Interfaces 2018, 10, 11671–11677. [Google Scholar] [CrossRef]
- Habibi, M.F.; Arvand, M.; Sohrabnezhad, S. Boosting bioelectricity generation in microbial fuel cells using metal oxides/nitrogen-doped carbon quantum dots. Energy 2021, 223, 120103. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Al-Raoush, R.I.; Abu-Reesh, I.M.; Pant, D. A microbial fuel cell configured for the remediation of recalcitrant pollutants in soil environment. RSC Adv. 2019, 9, 41409–41418. [Google Scholar] [CrossRef]
- Lovley, D.R.; Stolz, J.F.; Nord, G.L.; Phillips, E.J.P. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature 1987, 330, 252–254. [Google Scholar] [CrossRef]
- Qi, X.Y.; Gao, X.Y.; Wang, X.; Xu, P. Harnessing Pseudomonas Putida in bio electrochemical systems. Trends Biotechnol. 2024, 42, 877–894. [Google Scholar] [CrossRef]
- Xing, D.; Zuo, Y.; Cheng, S.; Regan, J.M.; Logan, B.E. Electricity generation by Rhod pseudomonas palustris DX-1. Environ. Sci. Technol. 2008, 42, 4146–4151. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Zhang, B.C.; Wu, D.G.; Li, F.; Song, H. Research progress in screening method of exoelectrogens. Chin. J. Biotechnol. 2020, 36, 2719–2731. [Google Scholar]
- He, W.; Liu, J.; Wang, H.; Feng, Y. Wastewater treatment process based on microbial electrochemistry: Opportunities and challenges. J. Electrochem. 2017, 23, 283–296. [Google Scholar]
- Li, K.; Yao, J.; Masakorala, K.; Li, X.; Li, S.; Li, X. Innovation and prospects of heavy metalsolidification/stabilization techniques: A comprehensive review on materials, mechanisms, and evaluation systems. Environ. Technol. Innov. 2025, 37, 104040. [Google Scholar] [CrossRef]
- Cui, M.H.; Liu, W.Z.; Tang, Z.E.; Cui, D. Recent advancements in azo dye decolorization in bio-electrochemical systems (BESs): Insights into decolorization mechanisms and practical application. Water Res. 2021, 203, 117512. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Zhang, Y.; Wang, Y. Application of Metal–Organic Frameworks (MOFs) in Heavy Metal Removal from Water. Nanomaterials 2024, 14, 702. [Google Scholar] [CrossRef]
- Shaku, B.; Mofokeng, T.P.; Coville, N.J.; Ozoemena, K.I.; Maubane-Nkadimeng, M.S. Biomass valorisation of marula nutshell waste into nitrogen-doped activated carbon for use in high performance supercapacitors. Electrochem. Acta 2023, 442, 141828. [Google Scholar] [CrossRef]
- Selvaraj, M.; Balamoorthy, E.; Manivasagam, T.G. Biomass derived nitrogen-doped activatedcarbon and novel biocompatible gel electrolytes for solid-state supercapacitor applications. J. Energy Storage 2023, 72, 108543. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Y.N.; Cao, Y.R.; Huang, J.; Chen, X.P.; Pan, X.J. Collaboratively scavenge tetracycline and Cu2+ from their combined system by Fe3+-modified magnetic chitosan: Performance, mechanisms, and dynamic sorption process. Chem. Eng. J. 2024, 484, 149625. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Lin, S.; Liu, Y.; Xie, Y. Soil Pollution Management in China: A Brief Introduction. Sustainability 2019, 11, 556. [Google Scholar] [CrossRef]
- Zlati, M.L.; Georgescu, L.P.; Iticescu, C.; Ionescu, R.V.; Antohi, V.M. Spatio-temporal dynamics of heavy metal water pollution and policy evolution in the European Union. Int. J. Environ. Res. Public Health 2023, 20, 45. [Google Scholar] [CrossRef]
- Hemdan, B.; Garlapati, V.K.; Sharma, S.; Bhadra, S.; Maddirala, S.; Varsha, K.M.; Motru, V.; Goswami, P.; Sevda, S.; Aminabhavi, T.M. Bioelectrochemical systems-based metal recovery: Resource, conservation and recycling of metallic industrial effluents. Environ. Res. 2022, 204, 112346. [Google Scholar] [CrossRef]
- HJ/T 166-2004; Technical Specification for Soil Environmental Monitoring. China Environmental Science Press: Beijing, China, 2004.
- HJ 832-2017; Microwave Digestion Method for Total Metal Elements in Soil and Sediment. China Environmental Science Press: Beijing, China, 2017.
- Yuan, H.R.; Deng, L.F.; Qian, X.; Wang, L.F.; Li, D.N.; Chen, Y.; Yuan, Y. Significant enhancement of electron transfer from Shewanella oneidensis using a porous N-doped carbon cloth in bio electrochemical system. Sci. Total Environ. 2019, 665, 882–889. [Google Scholar] [CrossRef]
- Fan, L.P.; Feng, W.X. Preparation of PANI-SA/CF anode to enhance the remediation and power generation capabilities of plant microbial fuel cells for chromium contaminated soil. Bioprocess Biosyst. Eng. 2024, 47, 509–518. [Google Scholar] [CrossRef]
- Zheng, Y.G.; Shang, M.G.; Tian, J.G.; Wang, Z.G.; Xu, W.H. Characteristics of modified Fe3O4 nanoparticles and application for immobilization of functioning bacterium and denitrification in water. Chem. Ind. Eng. Prog. 2015, 34, 2080–2085+2096. [Google Scholar]
- Nkulu, G.; Gaydardzhiev, S.; Mwema, E.; Compere, P. SEM and EDS observations of Carrol lite bioleaching with a mixed culture of acidophilic bacteria. Miner. Eng. 2015, 75, 70–76. [Google Scholar] [CrossRef]
- Pathak, A.; Dastidar, M.G.; Sree Krishnan, T.R. Bioleaching of heavy metals from sewage sludge by indigenous iron-oxidizing microorganisms using ammonium ferrous sulfate and ferrous sulfate as energy sources: A comparative study. J. Hazard. Mater. 2009, 171, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Liu, H.Q.; Wang, Q.P.; Chen, Z.L. Bioleaching of heavy metals in sewage sludge using Acid thiobacillus ferroxidase. China Environ. Sci. 2014, 34, 2617–2623. [Google Scholar]
- Wang, X.X.; Zhao, Y.; Jin, L.E.; Liu, B. Performance and mechanism of a bio electrochemical system for reduction of heavy metal cadmium ions. RSC Adv. 2024, 14, 5390–5399. [Google Scholar] [CrossRef] [PubMed]
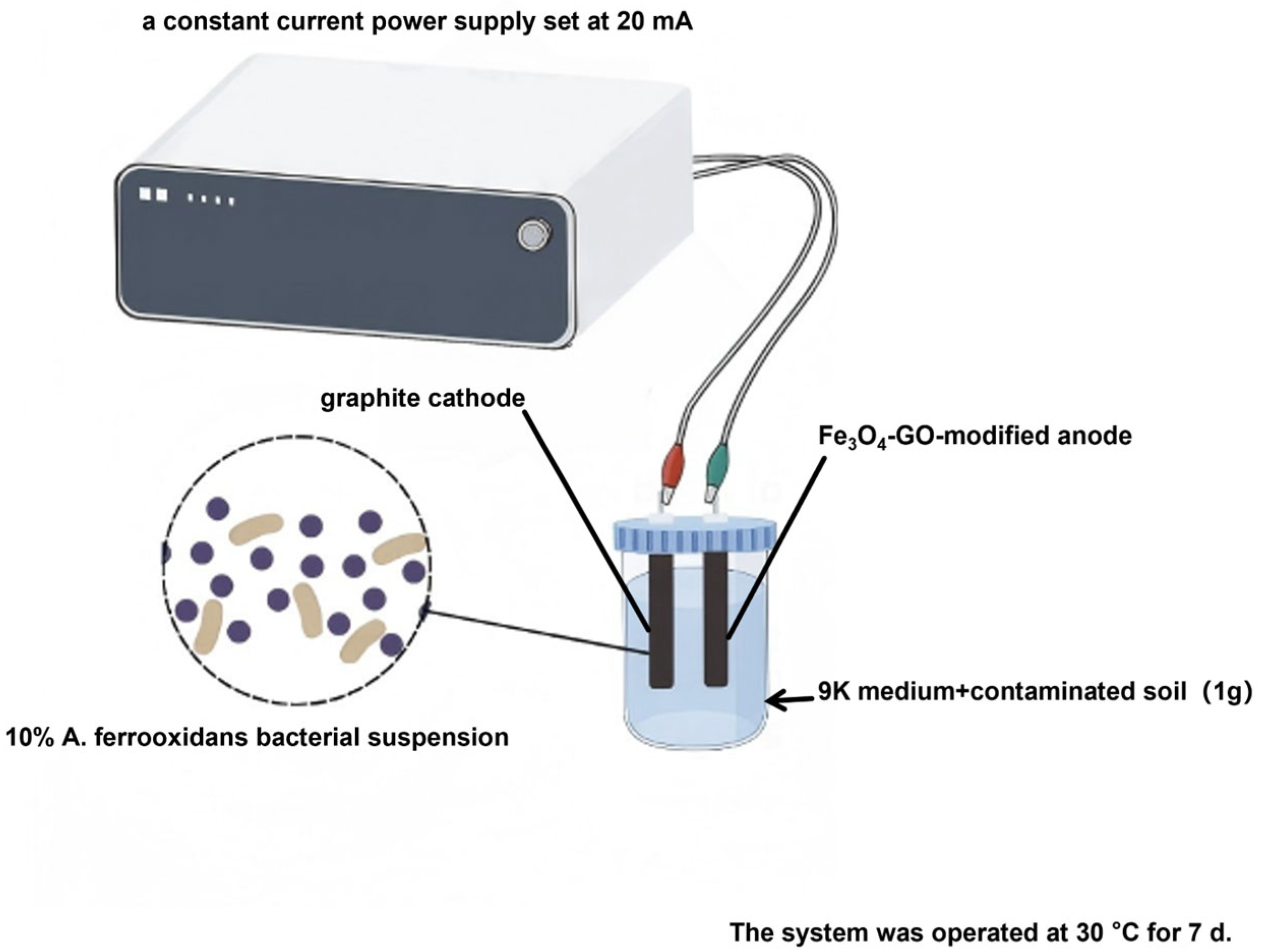
| Serial Number | Electrode (Anode/Cathode) | Inoculation (10%) | Soil (1 g) | Current (20 mA) | Fe3O4–GO |
|---|---|---|---|---|---|
| 1 | Fe3O4–GO/GR | √ | √ | √ | √ |
| 2 | Fe3O4–GO/GR | × | √ | √ | √ |
| 3 | Fe3O4–GO/GR | √ | √ | √ | × |
| 4 | Fe3O4–GO/GR | √ | √ | × | √ |
| 5 | GR/GR | √ | √ | √ | √ |
| Experimental Group | Configuration | Zn Removal (%) | Cu Removal (%) | Pb Removal (%) | Cd Removal (%) | Cr Removal (%) |
|---|---|---|---|---|---|---|
| Group 1 | Full system | 89.0 | 85.9 | 66.3 | 77.9 | 40.6 |
| Group 2 | No Bacteria | 76.6 | 31.6 | 47.6 | 38.9 | 29 |
| Group 3 | No Fe3O4–GO | 73.5 | 67.5 | 59.5 | 67.1 | 26 |
| Group 4 | No Current | 71.2 | 83.3 | 54.4 | 56.2 | 21.7 |
| Group 5 | Unmodified Anode (Graphite) | 70.4 | 27.2 | 45.9 | 35.9 | 38.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilahamu, A.; Wu, X.; Wang, X.; Peng, S.; Gu, W. Bioremediation of High-Concentration Heavy Metal-Contaminated Soil by Combined Use of Acidithiobacillus ferrooxidans and Fe3O4–GO Anodes. Toxics 2025, 13, 959. https://doi.org/10.3390/toxics13110959
Yilahamu A, Wu X, Wang X, Peng S, Gu W. Bioremediation of High-Concentration Heavy Metal-Contaminated Soil by Combined Use of Acidithiobacillus ferrooxidans and Fe3O4–GO Anodes. Toxics. 2025; 13(11):959. https://doi.org/10.3390/toxics13110959
Chicago/Turabian StyleYilahamu, Alifeila, Xuewen Wu, Xiaonuan Wang, Shengjuan Peng, and Weihua Gu. 2025. "Bioremediation of High-Concentration Heavy Metal-Contaminated Soil by Combined Use of Acidithiobacillus ferrooxidans and Fe3O4–GO Anodes" Toxics 13, no. 11: 959. https://doi.org/10.3390/toxics13110959
APA StyleYilahamu, A., Wu, X., Wang, X., Peng, S., & Gu, W. (2025). Bioremediation of High-Concentration Heavy Metal-Contaminated Soil by Combined Use of Acidithiobacillus ferrooxidans and Fe3O4–GO Anodes. Toxics, 13(11), 959. https://doi.org/10.3390/toxics13110959







