Nephrotoxicity of Phthalates: A Review Based on Epidemiological and Toxicological Evidence
Highlights
- Exposure to phthalates is closely linked to kidney dysfunction and renal tubular abnormalities.
- The main nephrotoxicity mechanisms involve oxidative stress, endoplasmic reticulum stress, and renal fibrosis.
- Emerging phthalates (e.g., DINP, DIDP) may also pose potential renal risks, requiring continuous monitoring.
- Future research should strengthen epidemiological studies and the long-term toxicity monitoring of new alternatives.
- Integrating molecular mechanisms with population-based evidence will refine risk assessment frameworks and provide a scientific basis for future regulatory decisions.
Abstract
1. Introduction
2. Materials and Methods
2.1. Nephrotoxicity of PAEs
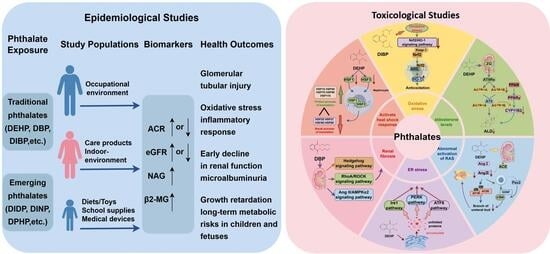
2.1.1. Epidemiological Studies
2.1.2. Adults
2.1.3. Fetal, Neonatal and Adolescent Periods
2.2. In Vivo and In Vitro Experiments
2.2.1. In Vivo Experiment
2.2.2. In Vitro Experiment
2.3. Possible Mechanisms of Phthalates Nephrotoxicity
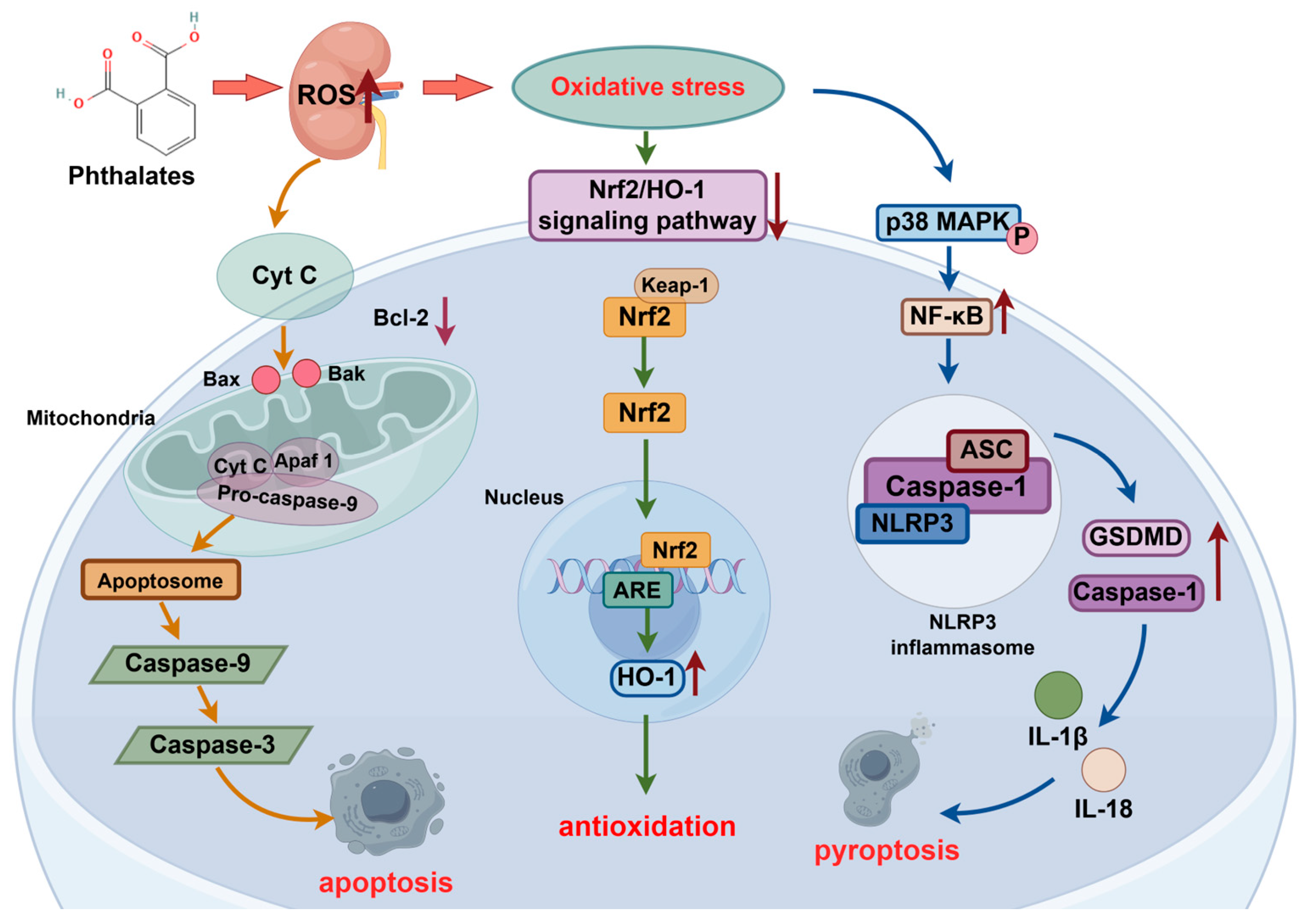
2.4. Oxidative Stress
2.4.1. Nrf2/HO-1 Signaling Pathway
2.4.2. Bax/Caspase-3 Signaling Pathway
2.4.3. NF-κB/Caspase/NLRP3 Signaling Pathway
2.5. Reduction in Aldosterone Levels
2.6. Abnormal Activation of Renin-Angiotensin System
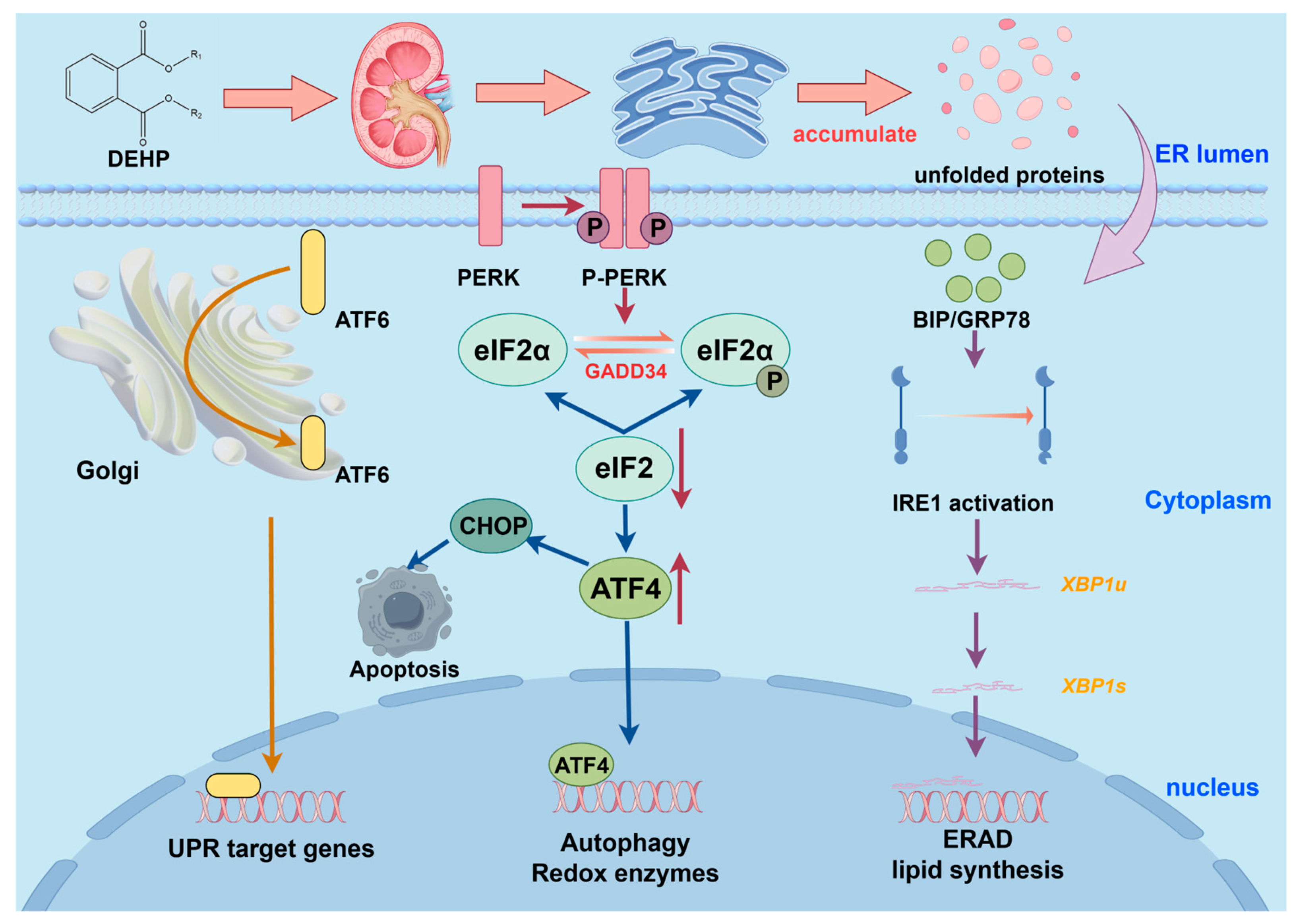
2.7. ER Stress
2.8. Renal Fibrosis
2.9. Sodium and Water Retention
2.10. Activate Heat Shock Response Defense System
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| ACE | Angiotensin-converting enzyme |
| ACR | Albumin/creatinine ratio |
| AMPK | Adenosine 5‘-monophosphate-activated protein kinase |
| ASC | Aptamer protein |
| ATF4/6 | Activating transcription factor 4/6 |
| Bax | Bcl-2 protein family |
| BBP | Butylbenzyl phthalate |
| BBZP | Butyl benzyl phthalate |
| BIP | Binding protein |
| BUN | Blood urea nitrogen |
| CHOP | C/EBP homologous protein |
| CKD | Chronic kidney disease |
| DBP | Dibutyl phthalate |
| DEHP | diethyl phthalate (2-ethylhexyl) |
| DEP | Diethyl phthalate |
| DIBP | Diisobutyl phthalate |
| DIDP | Diisodecyl phthalate |
| DINP | Diisononyl phthalate |
| DMP | Dimethyl phthalate |
| DNOP | Dinoctyl phthalate |
| DOP | Dioctyl phthalate |
| DPHP | Diphenyl phthalate |
| eGFR | Estimated Glomerular Filtration Rate |
| EMT | Epithelial-mesenchymal transition |
| ER | Endoplasmic reticulum |
| ERAD | Endoplasmic reticulum associated degradation |
| GCLC | Glutamate-cysteine ligase |
| GDNF | Glial cell line-derived nerve growth factor |
| GLUT 4 | Glucose transporter 4 |
| GPx1 | Glutathione Peroxidase 1 |
| GSH | Glutathione |
| HO-1 | Heme oxygenase-1 |
| HSR | Heat shock response |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LPS | Lipopolysaccharide |
| MR | Mineralocorticoid receptor |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | Nod-like receptor family P3 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NXR | Nucleoallogenic receptor |
| OPEs | Organic phosphate esters |
| PFASs | Polyfluorinated substances |
| PPARα | Peroxisome proliferator-activated receptor α |
| PS-MPs | Polystyrene microplastics |
| RAAS | renin-angiotensin-aldosterone system |
| RAS | Renin-angiotensin system |
| ROS | Reactive oxygen species |
| SOD | Superoxide Dismutase |
| UB | Ureteral bud |
| UPR | Unfolded protein response |
| XBP1 | X box binding protein 1 |
| ZG | Glomerular zone |
References
- Silva, M.J.; Samandar, E.; Preau, J.J.; Needham, L.L.; Calafat, A.M. Urinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology 2006, 219, 22–32. [Google Scholar] [CrossRef]
- Tao, H.; Wang, Y.; Liang, H.; Zhang, X.; Liu, X.; Li, J. Pollution characteristics of phthalate acid esters in agricultural soil of Yinchuan, northwest China, and health risk assessment. Environ. Geochem. Health 2020, 42, 4313–4326. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Kumar, D.; Singh, J.; Jangra, A. Environmental toxicants and nephrotoxicity: Implications on mechanisms and therapeutic strategies. Toxicology 2024, 504, 153784. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Li, Z.; Wang, H.; Liang, H. An overview of phthalate acid ester pollution in China over the last decade: Environmental occurrence and human exposure. Sci. Total Environ. 2018, 645, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Xie, Z.; Zhao, Z.; Zhong, M.; Mi, W.; Ebinghaus, R.; Tang, J. Occurrence and spatial distribution of phthalate esters in sediments of the Bohai and Yellow seas. Sci. Total Environ. 2019, 653, 792–800. [Google Scholar] [CrossRef]
- Fréry, N.; Santonen, T.; Porras, S.P.; Fucic, A.; Leso, V.; Bousoumah, R.; Duca, R.C.; El Yamani, M.; Kolossa-Gehring, M.; Ndaw, S.; et al. Biomonitoring of occupational exposure to phthalates: A systematic review. Int. J. Hyg. Environ. Health 2020, 229, 113548. [Google Scholar] [CrossRef]
- Wang, J.L.; Ye, Y.C.; Wu, W.Z. Comparison of di-n-methyl phthalate biodegradation by free and immobilized microbial cells. Biomed. Environ. Sci. 2003, 16, 126–132. [Google Scholar]
- Ventrice, P.; Ventrice, D.; Russo, E.; De Sarro, G. Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 88–96. [Google Scholar] [CrossRef]
- Praveena, S.M.; Siok Fong, C.; Amaruddin, A.F. Phthalates in children toys available in Malaysian market: Quantification and potential human health risk. J. Steroid Biochem. 2021, 213, 105955. [Google Scholar] [CrossRef]
- Belmaker, I.; Anca, E.D.; Rubin, L.P.; Magen-Molho, H.; Miodovnik, A.; van der Hal, N. Adverse health effects of exposure to plastic, microplastics and their additives: Environmental, legal and policy implications for Israel. Isr. J. Health Policy 2024, 13, 44. [Google Scholar] [CrossRef]
- Kamrin, M.A. Phthalate Risks, Phthalate Regulation, and Public Health: A Review. J. Toxicol. Environ. Health B 2009, 12, 157–174. [Google Scholar] [CrossRef]
- Benjamin, S.; Pradeep, S.; Josh, M.S.; Kumar, S.; Masai, E. A monograph on the remediation of hazardous phthalates. J. Hazard. Mater. 2015, 298, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Wams, T.J. Diethylhexylphthalate as an environmental contaminant—A review. Sci. Total Environ. 1987, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Determination of Dialkyl Phthalates in High Altitude Atmosphere for Validation of Sampling Method Using a Helicopter. Bull. Environ. Contam. Toxicol. 2001, 66, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Muczynski, V.; Cravedi, J.P.; Lehraiki, A.; Levacher, C.; Moison, D.; Lecureuil, C.; Messiaen, S.; Perdu, E.; Frydman, R.; Habert, R.; et al. Effect of mono-(2-ethylhexyl) phthalate on human and mouse fetal testis: In vitro and in vivo approaches. Toxicol. Appl. Pharmacol. 2012, 261, 97–104. [Google Scholar] [CrossRef]
- Singh, L.K.; Pandey, R.; Siddiqi, N.J.; Sharma, B. Molecular Mechanisms of Phthalate-Induced Hepatic Injury and Amelioration by Plant-Based Principles. Toxics 2025, 13, 32. [Google Scholar] [CrossRef]
- Wallace, M.A. Anatomy and physiology of the kidney. AORN J. 1998, 68, 799–820. [Google Scholar] [CrossRef]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef]
- Chang, W.H.; Herianto, S.; Lee, C.C.; Hung, H.; Chen, H.L. The effects of phthalate ester exposure on human health: A review. Sci. Total Environ. 2021, 786, 147371. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Katsikantami, I.; Kalantzi, O.; Sevim, Ç.; Tsarouhas, K.; Sarigiannis, D.; Tzatzarakis, M.N.; Rizos, A.K. Phthalates: Exposure and Health Effects. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Oxford, UK, 2019; pp. 163–173. [Google Scholar]
- Liu, Y.; Zhang, X.; Yi, R.; Tian, Q.; Xu, J.; Yan, X.; Ma, J.; Wang, S.; Yang, G. Exploring the nephrotoxicity and molecular mechanisms of Di-2-ethylhexyl phthalate: A comprehensive review. Chem.-Biol. Interact. 2025, 405, 111310. [Google Scholar] [CrossRef]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ. Health-Glob. 2014, 13, 43. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Liu, L.; Guo, W.; Yang, H.; Chen, S.; Yu, J.; Li, M.; Fang, Q.; Lai, X.; et al. Urinary phthalate metabolites mixture, serum cytokines and renal function in children: A panel study. J. Hazard. Mater. 2022, 422, 126963. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.T.; Cao, Y.; Dong, J.; Li, C.; Duan, Y.; Li, X.; Qiu, J. Association of urine phthalate metabolites levels with kidney function in 1610 US adolescents. Environ. Sci. Pollut. Res. 2023, 30, 70519–70527. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Park, J.Y.; Kim, S.; An, J.N.; Lee, J.; Park, H.; Jung, S.K.; Kim, S.Y.; Lee, J.P.; Choi, K. Association of exposure to phthalates and environmental phenolics with markers of kidney function: Korean National Environmental Health Survey (KoNEHS) 2015-2017. Environ. Int. 2020, 143, 105877. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Kuo, F.C.; Wu, C.F.; Sun, C.W.; Hsieh, C.J.; Wang, S.L.; Chen, M.L.; Hsieh, H.M.; Chuang, Y.S.; Wu, M.T. Association between two common environmental toxicants (phthalates and melamine) and urinary markers of renal injury in the third trimester of pregnant women: The Taiwan Maternal and Infant Cohort Study (TMICS). Chemosphere 2021, 272, 129925. [Google Scholar] [CrossRef]
- Li, S.S.; Chen, J.J.; Su, M.W.; Lin, C.W.; Chen, C.C.; Wang, Y.H.; Liu, C.C.; Tsai, Y.C.; Hsieh, T.J.; Wu, M.T.; et al. Sex-specific interactive effect of melamine and DEHP on a marker of early kidney damage in Taiwanese adults: A national population-based study from the Taiwan Biobank. Ecotoxicol. Environ. Saf. 2023, 263, 115208. [Google Scholar] [CrossRef]
- Huang, S.; Hsieh, T.; Lee, Y.; Wu, C.; Tsai, Y.; Chen, C.; Li, S.; Geng, J.; Hsu, Y.; Chang, C.; et al. Phthalate exposure increases oxidative stress, early renal injury, and the risk of calcium urolithiasis: A case-control study. Ecotoxicol. Environ. Saf. 2024, 287, 117322. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Gu, L.; Zhang, T.; Liu, S.; Wang, S.; Wang, Z. Association of urinary phthalate metabolites with renal function among 9989 US adults. Ecotoxicol. Environ. Saf. 2022, 242, 113930. [Google Scholar] [CrossRef]
- Hong, Q.F.; Pu, T.; Li, M.J.; Chen, Z.B.; Liu, X.Y.; Zeng, R.; Zhang, M.Z.; Dai, L.L.; An, S.L.; Shen, X.B.; et al. Association between urinary phthalate metabolites and renal function in late pregnant women. Environ. Sci. Eur. 2024, 36, 98. [Google Scholar] [CrossRef]
- Kang, H.; Lee, J.P.; Choi, K. Exposure to phthalates and environmental phenols in association with chronic kidney disease (CKD) among the general US population participating in multi-cycle NHANES (2005–2016). Sci. Total Environ. 2021, 791, 148343. [Google Scholar] [CrossRef]
- Malits, J.; Attina, T.M.; Karthikraj, R.; Kannan, K.; Naidu, M.; Furth, S.; Warady, B.A.; Vento, S.; Trachtman, H.; Trasande, L. Renal Function and exposure to Bisphenol A and phthalates in children with Chronic Kidney Disease. Environ. Res. 2018, 167, 575–582. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zhu, X.; Shrubsole, M.J.; Fan, H.; Chen, J.; Dong, J.; Hao, C.; Dai, Q. Renal Function, Bisphenol A, and Alkylphenols: Results from the National Health and Nutrition Examination Survey (NHANES 2003–2006). Environ. Health Perspect. 2011, 119, 527–533. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Upson, K.; Cook, N.R.; Weinberg, C.R. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ. Health Perspect. 2016, 124, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Gutleb, A.C.; Bergman, A.; Eriksen, G.S.; Murk, A.J.; Ropstad, E.; Saunders, M.; Skaare, J.U. Reproductive and developmental toxicity of phthalates. J. Toxicol. Environ. Health B 2009, 12, 225–249. [Google Scholar] [CrossRef]
- Day, D.B.; Collett, B.R.; Barrett, E.S.; Bush, N.R.; Swan, S.H.; Nguyen, R.; Szpiro, A.A.; Sathyanarayana, S. Phthalate mixtures in pregnancy, autistic traits, and adverse childhood behavioral outcomes. Environ. Int. 2021, 147, 106330. [Google Scholar] [CrossRef]
- Ejaredar, M.; Nyanza, E.C.; Ten, E.K.; Dewey, D. Phthalate exposure and childrens neurodevelopment: A systematic review. Environ. Res. 2015, 142, 51–60. [Google Scholar] [CrossRef]
- Engel, S.M.; Patisaul, H.B.; Brody, C.; Hauser, R.; Zota, A.R.; Bennet, D.H.; Swanson, M.; Whyatt, R.M. Neurotoxicity of Ortho-Phthalates: Recommendations for Critical Policy Reforms to Protect Brain Development in Children. Am. J. Public. Health 2021, 111, 687–695. [Google Scholar] [CrossRef]
- Wu, M.T.; Wu, C.F.; Wu, J.R.; Chen, B.H.; Chen, E.K.; Chao, M.C.; Liu, C.K.; Ho, C.K. The public health threat of phthalate-tainted foodstuffs in Taiwan: The policies the government implemented and the lessons we learned. Environ. Int. 2012, 44, 75–79. [Google Scholar] [CrossRef]
- Wu, C.F.; Chen, B.H.; Shiea, J.; Chen, E.K.; Liu, C.K.; Chao, M.C.; Ho, C.K.; Wu, J.R.; Wu, M.T. Temporal changes of urinary oxidative metabolites of di(2-ethylhexyl)phthalate after the 2011 phthalate incident in Taiwanese children: Findings of a six month follow-up. Environ. Sci. Technol. 2013, 47, 13754–13762. [Google Scholar] [CrossRef]
- Crocker, J.F.S.; Safe, S.H.; Acott, P. Effects of chronic phthalate exposure on the kidney. J. Toxicol. Environ. Health 1988, 23, 433–444. [Google Scholar] [CrossRef]
- Ito, Y.; Yamanoshita, O.; Asaeda, N.; Tagawa, Y.; Lee, C.; Aoyama, T.; Ichihara, G.; Furuhashi, K.; Kamijima, M.; Gonzalez, F.J.; et al. Di(2-ethylhexyl)phthalate Induces Hepatic Tumorigenesis through a Peroxisome Proliferator-activated Receptor α-independent Pathway. J. Occup. Health 2007, 49, 172–182. [Google Scholar] [CrossRef]
- Toyama, T.; Furuichi, K.; Ninomiya, T.; Shimizu, M.; Hara, A.; Iwata, Y.; Kaneko, S.; Wada, T. The impacts of albuminuria and low eGFR on the risk of cardiovascular death, all-cause mortality, and renal events in diabetic patients: Meta-analysis. PLoS ONE 2013, 8, e71810. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, A.; Yu, C. Correlation between microalbuminuria and cardiovascular events. Int. J. Clin. Exp. Med. 2013, 6, 973–978. [Google Scholar]
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Wu, Y.; Liu, M.; Attina, T.M.; Naidu, M.; Karthikraj, R.; Kannan, K.; Warady, B.A.; Furth, S.; Vento, S.; et al. Serially assessed bisphenol A and phthalate exposure and association with kidney function in children with chronic kidney disease in the US and Canada: A longitudinal cohort study. PLoS Med. 2020, 17, e1003384. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zhu, X.; Shrubsole, M.J.; Yu, C.; Xia, Z.; Dai, Q. Associations of renal function with urinary excretion of metals: Evidence from NHANES 2003-2012. Environ. Int. 2018, 121, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Huang, F.; Wang, H.; Yang, R.; Yang, Q.; He, G.; Chen, B.; Dong, R. Associations of exposure to melamine, cyanuric acid, phthalates with markers of early kidney impairment, and their interactions in US adults: Analyses of NHANES 2003-2004 data. Environ. Sci. Pollut. Res. 2022, 29, 79516–79528. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shi, X.; Zhou, X.; Dong, R.; Yuan, Y.; Wu, M.; Chen, W.; Liu, X.; Jia, F.; Li, S.; et al. Renal function and the exposure to melamine and phthalates in Shanghai adults. Chemosphere 2020, 246, 125820. [Google Scholar] [CrossRef]
- Kang, H.; Kim, S.; Lee, G.; Lee, I.; Lee, J.P.; Lee, J.; Park, H.; Moon, H.; Park, J.; Kim, S.; et al. Urinary metabolites of dibutyl phthalate and benzophenone-3 are potential chemical risk factors of chronic kidney function markers among healthy women. Environ. Int. 2019, 124, 354–360. [Google Scholar] [CrossRef]
- Tsai, H.J.; Chen, B.H.; Wu, C.F.; Wang, S.L.; Huang, P.C.; Tsai, Y.C.; Chen, M.L.; Ho, C.K.; Hsiung, C.A.; Wu, M.T. Intake of phthalate-tainted foods and microalbuminuria in children: The 2011 Taiwan food scandal. Environ. Int. 2016, 89–90, 129–137. [Google Scholar] [CrossRef]
- Trasande, L.; Sathyanarayana, S.; Trachtman, H. Dietary phthalates and low-grade albuminuria in US children and adolescents. Clin. J. Am. Soc. Nephrol. 2014, 9, 100–109. [Google Scholar] [CrossRef]
- Shinshi, H.; Kogo, O. Effect of phthalic acid esters on mouse testes. Toxicol. Lett. 1980, 5, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Ema, M.; Itami, T.; Kawasaki, H. Teratogenic evaluation of butyl benzyl phthalate in rats by gastric intubation. Toxicol. Lett. 1992, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- David, R.M.; Moore, M.R.; Finney, D.C.; Guest, D. Chronic Toxicity of Di(2-ethylhexyl)phthalate in Mice. Toxicol. Sci. 2000, 58, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Song, L.; Wei, J.; Chen, T.; Chen, J.; Lin, Y.; Xia, W.; Xu, B.; Li, X.; Chen, X.; et al. Maternal exposure to di-(2-ethylhexyl)phthalate alters kidney development through the renin–angiotensin system in offspring. Toxicol. Lett. 2012, 212, 212–221. [Google Scholar] [CrossRef]
- Aydemir, D.; Karabulut, G.; Simsek, G.; Gok, M.; Barlas, N.; Ulusu, N.N. Impact of the Di(2-Ethylhexyl) Phthalate Administration on Trace Element and Mineral Levels in Relation of Kidney and Liver Damage in Rats. Biol. Trace Elem. Res. 2018, 186, 474–488. [Google Scholar] [CrossRef]
- Erkekoglu, P.; Rachidi, W.; De Rosa, V.; Giray, B.; Favier, A.; Hincal, F. Protective effect of selenium supplementation on the genotoxicity of di(2-ethylhexyl)phthalate and mono(2-ethylhexyl)phthalate treatment in LNCaP cells. Free Radic. Biol. Med. 2010, 49, 559–566. [Google Scholar] [CrossRef]
- Erkekoglu, P.; Giray, B.; Rachidi, W.; Hininger-Favier, I.; Roussel, A.; Favier, A.; Hincal, F. Effects of di(2-ethylhexyl)phthalate on testicular oxidant/antioxidant status in selenium-deficient and selenium-supplemented rats. Environ. Toxicol. 2014, 29, 98–107. [Google Scholar] [CrossRef]
- Ashari, S.; Karami, M.; Shokrzadeh, M.; Ghandadi, M.; Ghassemi-Barghi, N.; Dashti, A.; Ranaee, M.; Mohammadi, H. The implication of mitochondrial dysfunction and mitochondrial oxidative damage in di (2-ethylhexyl) phthalate induced nephrotoxicity in both in vivo and in vitro models. Toxicol. Mech. Methods 2020, 30, 427–437. [Google Scholar] [CrossRef]
- Nakagomi, M.; Suzuki, E.; Saito, Y.; Nagao, T. Endocrine disrupting chemicals, 4-nonylphenol, bisphenol A and butyl benzyl phthalate, impair metabolism of estradiol in male and female rats as assessed by levels of 15alpha-hydroxyestrogens and catechol estrogens in urine. J. Appl. Toxicol. 2018, 38, 688–695. [Google Scholar] [CrossRef]
- Quasmi, M.N.; Singh, J.; Kumar, D.; Dhingra, D.; Jangra, A. Insights into the molecular mechanisms underlying phthalates-induced nephrotoxicity. Toxicology 2025, 516, 154187. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Shi, H.; Liu, Q.; Chang, Y.; Feng, W.; Zhu, S.; Sun, S. Effects of Lycium barbarum glycopeptide on renal and testicular injury induced by di(2-ethylhexyl) phthalate. Cell Stress Chaperones 2022, 27, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Diwan, B.A.; Ohshima, M.; Hu, H.; Schuller, H.M.; Rice, J.M. Tumor-initiating and promoting activities of di(2-ethylhexyl) phthalate in vivo and in vitro. Environ. Health Perspect. 1986, 65, 279–291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, F.W.; Yang, Z.Y.; Bian, Y.F.; Cui, J.G.; Zhang, H.; Zhao, Y.; Li, J.L. The novel role of the aquaporin water channel in lycopene preventing DEHP-induced renal ionic homeostasis disturbance in mice. Ecotoxicol. Environ. Saf. 2021, 226, 112836. [Google Scholar] [CrossRef] [PubMed]
- Amara, I.; Timoumi, R.; Graiet, I.; Ben, S.I.; Adelou, K.; Abid-Essefi, S. Di (2-ethylhexyl) phthalate induces cytotoxicity in HEK-293 cell line, implication of the Nrf-2/HO-1 antioxidant pathway. Environ. Toxicol. 2019, 34, 1034–1042. [Google Scholar] [CrossRef]
- Amara, I.; Salah, A.; Timoumi, R.; Annabi, E.; Scuto, M.; Trovato, A.; Neffati, F.; Calabrese, V.; Abid-Essefi, S. Effect of di(2-ethylhexyl) phthalate on Nrf2-regulated glutathione homeostasis in mouse kidney. Cell Stress Chaperones 2020, 25, 919–928. [Google Scholar] [CrossRef]
- Erkekoglu, P.; Giray, B.K.; Kizilgun, M.; Rachidi, W.; Hininger-Favier, I.; Roussel, A.M.; Favier, A.; Hincal, F. Di(2-ethylhexyl)phthalate-induced renal oxidative stress in rats and protective effect of selenium. Toxicol. Mech. Methods 2012, 22, 415–423. [Google Scholar] [CrossRef]
- Kamijo, Y.; Hora, K.; Nakajima, T.; Kono, K.; Takahashi, K.; Ito, Y.; Higuchi, M.; Kiyosawa, K.; Shigematsu, H.; Gonzalez, F.J.; et al. Peroxisome proliferator-activated receptor α protects against glomerulonephritis induced by long-term exposure to the plasticizer di-(2-ethylhexyl)phthalate. J. Am. Soc. Nephrol. 2007, 18, 176–188. [Google Scholar] [CrossRef]
- Tang, Y.S.; Zhao, Y.H.; Zhong, Y.; Li, X.Z.; Pu, J.X.; Luo, Y.C.; Zhou, Q.L. Neferine inhibits LPS-ATP-induced endothelial cell pyroptosis via regulation of ROS/NLRP3/Caspase-1 signaling pathway. Inflamm. Res. 2019, 68, 727–738. [Google Scholar] [CrossRef]
- Beltifa, A.; Feriani, A.; Machreki, M.; Ghorbel, A.; Ghazouani, L.; Di Bella, G.; Van Loco, J.; Reyns, T.; Mansour, H.B. Plasticizers and bisphenol A, in packaged foods sold in the Tunisian markets: Study of their acute in vivo toxicity and their environmental fate. Environ. Sci. Pollut. Res. 2017, 24, 22382–22392. [Google Scholar] [CrossRef]
- Sun, C.; Li, F.; Tu, H.; Jia, F.; Li, F. Review on the Impact of Phthalate Esters on Aquatic Food Chain. Asian J. Ecotoxicol. 2016, 11, 12–24. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, C.; Chen, Q.; Wei, W. Progress in the Study of Environmental Pollution and Ecological Behavior and Toxicological Effects of Phthalate Ester. Asian J. Ecotoxicol. 2018, 13, 34–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.; Li, Z.; Tao, Y.; Yang, Y. Hazards of phthalates (PAEs) exposure: A review of aquatic animal toxicology studies. Sci. Total Environ. 2021, 771, 145418. [Google Scholar] [CrossRef]
- Hu, X.; Gu, Y.; Huang, W.; Yin, D. Phthalate monoesters as markers of phthalate contamination in wild marine organisms. Environ. Pollut. 2016, 218, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Tien, C.; Sun, Y.; Hsieh, C.; Lee, C. Occurrence of phthalates in sediment and biota: Relationship to aquatic factors and the biota-sediment accumulation factor. Chemosphere 2008, 73, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Watanuki, H.; Gushiken, Y.; Sakai, M. In vitro modulation of common carp (Cyprinus carpio L.) phagocytic cells by Di-n-butyl phthalate and Di-2-ethylhexyl phthalate. Aquat. Toxicol. 2003, 63, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Li, D.Z.; Guo, R.; Zheng, L.; An, X.L.; Zeng, Z.C. In vivo and in vitro toxicities of diethyl phthalate to flounder fish Paralichthys olivaceus and its gill cell line (FG cells). J. Environ. Biol. 2018, 39, 73–81. [Google Scholar] [CrossRef]
- Oya-Silva, L.F.; Guiloski, I.C.; Vicari, T.; Deda, B.; Marcondes, F.R.; Simeoni, R.D.; Perussolo, M.C.; Martino-Andrade, A.J.; Leme, D.M.; de Assis, H.; et al. Evidence of genotoxicity, neurotoxicity, and antioxidant imbalance in silver catfish Rhamdia quelen after subchronic exposure to diisopentyl phthalate. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2023, 892, 503702. [Google Scholar] [CrossRef]
- Ikele, C.B.; Mgbenka, B.O.; Oluah, N.S. Histopathological effects of diethyl phthalate on Clarias gariepinus juveniles. Anim. Res. Int. 2011, 8, 1431–1438. [Google Scholar]
- Li, P.C.; Li, X.N.; Du, Z.H.; Wang, H.; Yu, Z.R.; Li, J.L. Di (2-ethyl hexyl) phthalate (DEHP)-induced kidney injury in quail (Coturnix japonica) via inhibiting HSF1/HSF3-dependent heat shock response. Chemosphere 2018, 209, 981–988. [Google Scholar] [CrossRef]
- Wang, H.; Guan, T.; Sun, J.; Talukder, M.; Huang, Y.; Li, Y.; Li, J. Di-(2-ethylhexyl) phthalate induced nephrotoxicity in quail (Coturnix japonica) by triggering nuclear xenobiotic receptors and modulating the cytochrome P450 system. Environ. Pollut. 2020, 261, 114162. [Google Scholar] [CrossRef]
- White, S.L.; Perkovic, V.; Cass, A.; Chang, C.L.; Poulter, N.R.; Spector, T.; Haysom, L.; Craig, J.C.; Salmi, I.A.; Chadban, S.J.; et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am. J. Kidney Dis. 2009, 54, 248–261. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, H.; Zhu, Y.; Wood, K.; Li, E.; Sun, W.; Yuan, Q.; Xu, D.; Liu, Z.; Zhao, W.; et al. Reduced Fgf10 / Fgfr2 and androgen receptor (AR) in anorectal malformations male rats induced by di- n -butyl phthalate (DBP): A study on the local and systemic toxicology of DBP. Toxicology 2015, 338, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.L.; Zhu, Y.P.; Ni, X.S.; Jing, D.D.; Yao, Y.T.; Ding, W.; Liu, Z.H.; Ding, G.X.; Jiang, J.T. Potential involvement of Fgf10/Fgfr2 and androgen receptor (AR) in renal fibrosis in adult male rat offspring subjected to prenatal exposure to di-n-butyl phthalate (DBP). Toxicol. Lett. 2018, 282, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Xi, J.; Chen, X.; Huang, J.; Jin, D.; Zhu, X. Curcumin decreases dibutyl phthalate-induced renal dysfunction in Kunming mice via inhibiting oxidative stress and apoptosis. Hum. Exp. Toxicol. 2021, 40, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.M.; Yang, L.; Li, A.Q.; Sun, Y.; Shi, H.Q.; Jin, S.Y.; Xia, L.Z.; Gao, H.T. Glycerin monostearate exacerbates phthalates’ toxicity to liver function and renal function in male rats. J. Toxicol. 2020, 34, 481–485. [Google Scholar]
- Rothenbacher, K.P.; Kimmel, R.; Hildenbrand, S.; Schmahl, F.W.; Dartsch, P.C. Nephrotoxic effects of di-(2-ethylhexyl)-phthalate (DEHP) hydrolysis products on cultured kidney epithelial cells. Hum. Exp. Toxicol. 1998, 17, 336–342. [Google Scholar] [CrossRef]
- Ashari, S.; Karami, M.; Shokrzadeh, M.; Bagheri, A.; Ghandadi, M.; Ranaee, M.; Dashti, A.; Mohammadi, H. Quercetin ameliorates Di (2-ethylhexyl) phthalate-induced nephrotoxicity by inhibiting NF-κB signaling pathway. Toxicol. Res. 2022, 11, 272–285. [Google Scholar] [CrossRef]
- Ye, Q.; Zhao, S.; Zhang, Y.; Su, Y.M.; Chen, M.; Zhao, J.; Jia, G.Z.; Han, B.M.; Jiang, J.T. Activation of the RhoA/ROCK pathway contributes to renal fibrosis in offspring rats induced by maternal exposure to di-n-butyl phthalate. Toxicology 2020, 443, 152573. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, J.T.; Li, D.; Zhu, Y.P.; Xia, S.J.; Han, B.M. Maternal exposure to di-n-butyl phthalate promotes Snail1-mediated epithelial-mesenchymal transition of renal tubular epithelial cells via upregulation of TGF-beta1 during renal fibrosis in rat offspring. Ecotoxicol. Environ. Saf. 2019, 169, 266–272. [Google Scholar] [CrossRef]
- Zhao, S.; Pan, L.; Chen, M.; Zhu, Y.P.; Han, B.M.; Xia, S.J.; Jiang, J.T. In utero di-n-butyl phthalate exposure induced abnormal autophagy in renal tubular cells via hedgehog signaling in newborn rats. Chem.-Biol. Interact. 2020, 328, 109189. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.W.; Zhang, Y.Q.; Sun, W.L.; Hua, S.; Han, B.M.; Jiang, J.T.; Zhu, Y.J.; Jing, Y.F. Dibutyl phthalate induces epithelial-mesenchymal transition of renal tubular epithelial cells via the Ang II/AMPKalpha2/Cx43 signaling pathway. Toxicology 2023, 494, 153584. [Google Scholar] [CrossRef]
- Wood, C.E.; Jokinen, M.P.; Johnson, C.L.; Olson, G.R.; Hester, S.; George, M.; Chorley, B.N.; Carswell, G.; Carter, J.H.; Wood, C.R.; et al. Comparative time course profiles of phthalate stereoisomers in mice. Toxicol. Sci. 2014, 139, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Wang, C.C.; Huang, L.C.; Liu, S.H.; Chiang, C.K. Plasticizer Di-(2-Ethylhexyl)Phthalate Induces Epithelial-to-Mesenchymal Transition and Renal Fibrosis In Vitro and In Vivo. Toxicol. Sci. 2018, 164, 363–374. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Chen, L.; Wang, X.J.; Jiang, Q.H.; Bei, X.Y.; Sun, W.L.; Xia, S.J.; Jiang, J.T. Maternal exposure to di-n-butyl phthalate (DBP) induces renal fibrosis in adult rat offspring. Oncotarget 2017, 8, 31101–31111. [Google Scholar] [CrossRef]
- Ghosh, J.; Das, J.; Manna, P.; Sil, P.C. Hepatotoxicity of di-(2-ethylhexyl)phthalate is attributed to calcium aggravation, ROS-mediated mitochondrial depolarization, and ERK/NF-kappaB pathway activation. Free Radic. Biol. Med. 2010, 49, 1779–1791. [Google Scholar] [CrossRef]
- Wei, Y.; Tang, X.-L.; Zhou, Y.; Liu, B.; Shen, L.-J.; Long, C.-l.; Lin, T.; He, D.-w.; Wu, S.-d.; Wei, G.-h. DEHP exposure destroys blood-testis barrier (BTB) integrity of immature testes through excessive ROS-mediated autophagy. Genes. Dis. 2018, 5, 263–274. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.N.; Xiang, L.R.; Qin, L.; Lin, J.; Li, J.L. Atrazine triggers hepatic oxidative stress and apoptosis in quails (Coturnix C. coturnix) via blocking Nrf2-mediated defense response. Ecotoxicol. Environ. Saf. 2017, 137, 49–56. [Google Scholar] [CrossRef]
- Hsu, C.; Hou, C.; Chen, Y.; Chang-Chien, G.; Lin, S.; Tain, Y. Environmental Nephrotoxicity Across the Life Course: Oxidative Stress Mechanisms and Opportunities for Early Intervention. Antioxidants 2025, 14, 1205. [Google Scholar] [CrossRef]
- Pinkus, R.; Weiner, L.M.; Daniel, V. Role of quinone-mediated generation of hydroxyl radicals in the induction of glutathione S-transferase gene expression. Biochemistry 1995, 34, 81–88. [Google Scholar] [CrossRef]
- Han, S.G.; Han, S.; Toborek, M.; Hennig, B. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicol. Appl. Pharm. 2012, 261, 181–188. [Google Scholar] [CrossRef]
- Dodson, M.; Redmann, M.; Rajasekaran, N.S.; Darley-Usmar, V.; Zhang, J. KEAP1-NRF2 signalling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem. J. 2015, 469, 347–355. [Google Scholar] [CrossRef]
- Ruiz, S.; Pergola, P.E.; Zager, R.A.; Vaziri, N.D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013, 83, 1029–1041. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003, 278, 21592–21600. [Google Scholar] [CrossRef]
- Mohammadi, H.; Ashari, S. Mechanistic insight into toxicity of phthalates, the involved receptors, and the role of Nrf2, NF-kappaB, and PI3K/AKT signaling pathways. Environ. Sci. Pollut. Res. 2021, 28, 35488–35527. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Stachurska, A.; Podkalicka, P.; Sobczak, M.; Mucha, O.; Witalisz-Siepracka, A.; Jozkowicz, A.; Dulak, J. Effect of heme oxygenase-1 on ochratoxin A-induced nephrotoxicity in mice. Int. J. Biochem. Cell Biol. 2017, 84, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, W.; Zhu, H.; Guo, J.; Liu, M.; Xu, S. Eucalyptol relieves the toxicity of diisobutyl phthalate in Ctenopharyngodon idellus kidney cells through Keap1/Nrf2/HO-1 pathway: Apoptosis-autophagy crosstalk and immunoregulation. Fish Shellfish Immunol. 2022, 130, 490–500. [Google Scholar] [CrossRef]
- Ashari, S.; Naghsh, N.; Salari, Y.; Barghi, N.G.; Bagheri, A. Dimethyl Fumarate Attenuates Di-(2-Ethylhexyl) Phthalate-Induced Nephrotoxicity Through the Nrf2/HO-1 and NF-kappaB Signaling Pathways. Inflammation 2023, 46, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Z.; Zhao, Y.; Dai, X.Y.; Talukder, M.; Li, J.L. Lycopene ameliorates DEHP exposure-induced renal pyroptosis through the Nrf2/Keap-1/NLRP3/Caspase-1 axis. J. Nutr. Biochem. 2023, 113, 109266. [Google Scholar] [CrossRef] [PubMed]
- Martinou, J.C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Franco, S.; Padilla-Tejeda, D.E.; Carrillo-Ibarra, S.; Miranda-Diaz, A.G. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int. J. Endocrinol. 2018, 2018, 1875870. [Google Scholar] [CrossRef]
- Hong, G.L.; Liu, J.M.; Zhao, G.J.; Wang, L.; Liang, G.; Wu, B.; Li, M.F.; Qiu, Q.M.; Lu, Z.Q. The reversal of paraquat-induced mitochondria-mediated apoptosis by cycloartenyl ferulate, the important role of Nrf2 pathway. Exp. Cell Res. 2013, 319, 2845–2855. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Liu, F.; Dong, Z. Mitochondrial dysregulation and protection in cisplatin nephrotoxicity. Arch. Toxicol. 2014, 88, 1249–1256. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Li, S.; Gu, X.; Zhang, M.; Jiang, Q.; Xu, T. Di (2-ethylhexyl) phthalate and polystyrene microplastics co-exposure caused oxidative stress to activate NF-kappaB/NLRP3 pathway aggravated pyroptosis and inflammation in mouse kidney. Sci. Total Environ. 2024, 926, 171817. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Gad El-Karim, D.R.S.; Lebda, M.A.; Alotaibi, B.S.; El-kott, A.F.; Ghamry, H.I.; Shukry, M. Lutein Modulates Oxidative Stress, Inflammatory and Apoptotic Biomarkers Related to Di-(2-Ethylhexyl) Phthalate (DEHP) Hepato-Nephrotoxicity in Male Rats: Role of Nuclear Factor Kappa B. Toxics 2023, 11, 742. [Google Scholar] [CrossRef]
- Tripathi, A.; Pandey, V.; Sahu, A.N.; Singh, A.; Dubey, P.K. Di-(2-ethylhexyl) phthalate (DEHP) inhibits steroidogenesis and induces mitochondria-ROS mediated apoptosis in rat ovarian granulosa cells. Toxicol. Res. 2019, 8, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W.; Liu, Y.; Han, B.; Yu, Y.; Lin, H. MEHP induced mitochondrial damage by promoting ROS production in CIK cells, leading to apoptosis, autophagy, cell cycle arrest. Comp. Biochem. Phys. C Toxicol. Pharmacol. 2025, 288, 110064. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, L.; Zhou, L.; Han, W.; Li, Z.; Liu, C. DBP induced autophagy and necrotic apoptosis in HepG2 cells via the mitochondrial damage pathway. Food Chem. Toxicol. 2023, 176, 113782. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, L.; Lei, R.; Zhang, P.; Yang, Y.; Liu, H.; Zhang, Y. DEHP and DBP, common phthalates, induce glucose metabolism disorders in rats via oxidative damage of PI3K/Akt/GLUT4 signaling. Environ. Pollut. 2024, 341, 122948. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, C.; Yang, H.; Deng, J.; Fan, D. Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Pharmacol. Res. 2020, 155, 104746. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, Y.; Shen, L.; Wei, Y.; Long, C.; Fu, Y.; Wu, H.; Wang, J.; Wu, Y.; Wu, S.; et al. Exposure to DEHP induces testis toxicity and injury through the ROS/mTOR/NLRP3 signaling pathway in immature rats. Ecotoxicol. Environ. Saf. 2021, 227, 112889. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Elias, E.E.; Lyons, B.; Muruve, D.A. Gasdermins and pyroptosis in the kidney. Nat. Rev. Nephrol. 2023, 19, 337–350. [Google Scholar] [CrossRef]
- Liu, X.; Xia, S.; Zhang, Z.; Wu, H.; Lieberman, J. Channelling inflammation: Gasdermins in physiology and disease. Nat. Rev. Drug Discov. 2021, 20, 384–405. [Google Scholar] [CrossRef]
- Ding, W.; Huang, S.; Huang, S.; Xu, W.; Wei, W. Di(2-ethylhexyl) phthalate mediates oxidative stress and activates p38MAPK/NF-kB to exacerbate diabetes-induced kidney injury in vitro and in vivo models. Toxicol. Res. 2023, 12, 332–343. [Google Scholar] [CrossRef]
- Cai, M.; Zhuang, W.; Lv, E.; Liu, Z.; Wang, Y.; Zhang, W.; Fu, W. Kaemperfol alleviates pyroptosis and microglia-mediated neuroinflammation in Parkinson’s disease via inhibiting p38MAPK/NF-kappaB signaling pathway. Neurochem. Int. 2022, 152, 105221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, M.; Pan, X.; Zhang, B.; Dou, Q. Binding and detoxification ability of lactobacillus acidophilus towards di-n-butyl phthalate: Change of MAPK pathway in Caco-2 cell model. J. Proteom. 2021, 247, 104333. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Li, B.; Du, J.; Zhang, X.; Zhang, J.; Wang, Q.; Song, M.; Li, Y. Dibutyl phthalate induces liver fibrosis via p38MAPK/NF-kappaB/NLRP3-mediated pyroptosis. Sci. Total Environ. 2023, 897, 165500. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, Y.; Liu, Y.; Chen, W.; Cen, Y.; You, M.; Yang, G. DBP and BaP co-exposure induces kidney injury via promoting pyroptosis of renal tubular epithelial cells in rats. Chemosphere 2023, 314, 137714. [Google Scholar] [CrossRef]
- Williams, G.H. Aldosterone biosynthesis, regulation, and classical mechanism of action. Heart Fail. Rev. 2005, 10, 7–13. [Google Scholar] [CrossRef]
- Nogueira, E.F.; Bollag, W.B.; Rainey, W.E. Angiotensin II regulation of adrenocortical gene transcription. Mol. Cell Endocrinol. 2009, 302, 230–236. [Google Scholar] [CrossRef][Green Version]
- Sasaki, K.; Yamano, Y.; Bardhan, S.; Iwai, N.; Murray, J.J.; Hasegawa, M.; Matsuda, Y.; Inagami, T. Cloning and expression of a complementary DNA encoding a bovine adrenal angiotensin II type-1 receptor. Nature 1991, 351, 230–233. [Google Scholar] [CrossRef]
- Bassett, M.H.; White, P.C.; Rainey, W.E. The regulation of aldosterone synthase expression. Mol. Cell Endocrinol. 2004, 217, 67–74. [Google Scholar] [CrossRef]
- Ge, R.S.; Dong, Q.; Sottas, C.M.; Latif, S.A.; Morris, D.J.; Hardy, M.P. Stimulation of testosterone production in rat Leydig cells by aldosterone is mineralocorticoid receptor mediated. Mol. Cell Endocrinol. 2005, 243, 35–42. [Google Scholar] [CrossRef]
- Martinez-Arguelles, D.B.; Guichard, T.; Culty, M.; Zirkin, B.R.; Papadopoulos, V. In Utero Exposure to the Antiandrogen Di-(2-Ethylhexyl) Phthalate Decreases Adrenal Aldosterone Production in the Adult Rat1. Biol. Reprod. 2011, 85, 51–61. [Google Scholar] [CrossRef]
- Martinez-Arguelles, D.B.; Campioli, E.; Lienhart, C.; Fan, J.; Culty, M.; Zirkin, B.R.; Papadopoulos, V. In Utero Exposure to the Endocrine Disruptor Di-(2-Ethylhexyl) Phthalate Induces Long-Term Changes in Gene Expression in the Adult Male Adrenal Gland. Endocrinology 2014, 155, 1667–1678. [Google Scholar] [CrossRef]
- Uruno, A.; Matsuda, K.; Noguchi, N.; Yoshikawa, T.; Kudo, M.; Satoh, F.; Rainey, W.E.; Hui, X.G.; Akahira, J.; Nakamura, Y.; et al. Peroxisome proliferator-activated receptor-gamma suppresses CYP11B2 expression and aldosterone production. J. Mol. Endocrinol. 2011, 46, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.Q.; Xie, D.; Choudhary, V.; Seremwe, M.; Tsai, Y.Y.; Olala, L.; Chen, X.; Bollag, W.B. The effect of pioglitazone on aldosterone and cortisol production in HAC15 human adrenocortical carcinoma cells. Mol. Cell Endocrinol. 2014, 394, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.R. Interactions between the sympathetic nervous system and the RAAS in heart failure. Curr. Heart Fail. Rep. 2004, 1, 45–50. [Google Scholar] [CrossRef]
- Bernstein, K.E.; Gonzalez-Villalobos, R.A.; Giani, J.F.; Shah, K.; Bernstein, E.; Janjulia, T.; Koronyo, Y.; Shi, P.D.; Koronyo-Hamaoui, M.; Fuchs, S.; et al. Angiotensin-converting enzyme overexpression in myelocytes enhances the immune response. Biol. Chem. 2014, 395, 1173–1178. [Google Scholar] [CrossRef]
- Iosipiv, I.V.; Schroeder, M. A role for angiotensin II AT1 receptors in ureteric bud cell branching. Am. J. Physiol.-Ren. Physiol. 2003, 285, F199–F207. [Google Scholar] [CrossRef]
- Song, R.; Spera, M.; Garrett, C.; El-Dahr, S.S.; Yosypiv, I.V. Angiotensin II AT2 receptor regulates ureteric bud morphogenesis. Am. J. Physiol-Ren. Physiol. 2010, 298, F807–F817. [Google Scholar] [CrossRef]
- Diep, Q.N.; El Mabrouk, M.; Cohn, J.S.; Endemann, D.; Amiri, F.; Virdis, A.; Neves, M.F.; Schiffrin, E.L. Structure, Endothelial Function, Cell Growth, and Inflammation in Blood Vessels of Angiotensin II–Infused Rats. Circulation 2002, 105, 2296–2302. [Google Scholar] [CrossRef]
- Sugawara, A.; Takeuchi, K.; Uruno, A.; Ikeda, Y.; Arima, S.; Kudo, M.; Sato, K.; Taniyama, Y.; Ito, S. Transcriptional suppression of type 1 angiotensin II receptor gene expression by peroxisome proliferator-activated receptor-gamma in vascular smooth muscle cells. Endocrinology 2001, 142, 3125–3134. [Google Scholar] [CrossRef]
- Benkirane, K.; Viel, E.C.; Amiri, F.; Schiffrin, E.L. Peroxisome proliferator-activated receptor gamma regulates angiotensin II-stimulated phosphatidylinositol 3-kinase and mitogen-activated protein kinase in blood vessels in vivo. Hypertension 2006, 47, 102–108. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, J.; Wang, Z.; Liu, N.; Gou, W. PPARgamma agonist, rosiglitazone, regulates angiotensin II-induced vascular inflammation through the TLR4-dependent signaling pathway. Lab. Invest. 2009, 89, 887–902. [Google Scholar] [CrossRef]
- Sugawara, A.; Uruno, A.; Kudo, M.; Matsuda, K.; Yang, C.W.; Ito, S. Effects of PPARgamma on hypertension, atherosclerosis, and chronic kidney disease. Endocr. J. 2010, 57, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Chiang, C.W.; Lin, H.C.; Zhao, J.F.; Li, C.T.; Shyue, S.K.; Lee, T.S. Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Arch. Toxicol. 2016, 90, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Chiolero, A.; Maillard, M.; Nussberger, J.; Brunner, H.R.; Burnier, M. Proximal sodium reabsorption: An independent determinant of blood pressure response to salt. Hypertension 2000, 36, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Arguelles, D.B.; Campioli, E.; Culty, M.; Zirkin, B.R.; Papadopoulos, V. Fetal origin of endocrine dysfunction in the adult: The phthalate model. J. Steroid Biochem. 2013, 137, 5–17. [Google Scholar] [CrossRef]
- Navar, L.G. Physiology: Hemodynamics, endothelial function, renin-angiotensin-aldosterone system, sympathetic nervous system. J. Am. Soc. Hypertens. 2014, 8, 519–524. [Google Scholar] [CrossRef]
- Woods, L.L.; Ingelfinger, J.R.; Nyengaard, J.R.; Rasch, R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr. Res. 2001, 49, 460–467. [Google Scholar] [CrossRef]
- Jangra, A.; Sriram, C.S.; Lahkar, M. Lipopolysaccharide-Induced Behavioral Alterations Are Alleviated by Sodium Phenylbutyrate via Attenuation of Oxidative Stress and Neuroinflammatory Cascade. Inflammation 2016, 39, 1441–1452. [Google Scholar] [CrossRef]
- Jangra, A.; Sriram, C.S.; Dwivedi, S.; Gurjar, S.S.; Hussain, M.I.; Borah, P.; Lahkar, M. Sodium Phenylbutyrate and Edaravone Abrogate Chronic Restraint Stress-Induced Behavioral Deficits: Implication of Oxido-Nitrosative, Endoplasmic Reticulum Stress Cascade, and Neuroinflammation. Cell Mol. Neurobiol. 2017, 37, 65–81. [Google Scholar] [CrossRef]
- Taniguchi, M.; Yoshida, H. Endoplasmic reticulum stress in kidney function and disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 345–350. [Google Scholar] [CrossRef]
- Inagi, R. Endoplasmic reticulum stress as a progression factor for kidney injury. Curr. Opin. Pharmacol. 2010, 10, 156–165. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, X.; Li, Q.; Chen, J.; Wei, Y.; Long, C.; Shen, L.; Zheng, X.; Li, D.; Wang, X.; et al. X-box binding protein 1 caused an imbalance in pyroptosis and mitophagy in immature rats with di-(2-ethylhexyl) phthalate-induced testis toxicity. Genes Dis. 2024, 11, 935–951. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Wang, X.Z.; Wang, T.; Chen, J.J.; Xie, X.Y.; Hu, H.; Yu, F.; Liu, H.L.; Jiang, X.Y.; Fan, H.D. Molecular signal networks and regulating mechanisms of the unfolded protein response. J. Zhejiang Univ.-Sci. B 2017, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Jangra, A.; Kumar, D. Recent advances in toxicological research of di-(2-ethylhexyl)-phthalate: Focus on endoplasmic reticulum stress pathway. Chemosphere 2024, 356, 141922. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lin, Y.; Huang, Q.; Shi, J.; Qiu, L.; Kang, M.; Chen, Y.; Fang, C.; Ye, T.; Dong, S. Di(2-ethylhexyl) phthalate-induced apoptosis in rat INS-1 cells is dependent on activation of endoplasmic reticulum stress and suppression of antioxidant protection. J. Cell Mol. Med. 2015, 19, 581–594. [Google Scholar] [CrossRef]
- Lew, Q.J.; Chu, K.L.; Lee, J.; Koh, P.L.; Rajasegaran, V.; Teo, J.Y.; Chao, S.H. PCAF interacts with XBP-1S and mediates XBP-1S-dependent transcription. Nucleic Acids Res. 2011, 39, 429–439. [Google Scholar] [CrossRef]
- Guo, F.; Xiong, Z.; Lu, X.; Ye, M.; Han, X.; Jiang, R. ATF6 upregulates XBP1S and inhibits ER stress-mediated apoptosis in osteoarthritis cartilage. Cell Signal 2014, 26, 332–342. [Google Scholar] [CrossRef]
- Yin, X.; Ma, F.; Fan, X.; Zhao, Q.; Liu, X.; Yang, Y. Knockdown of AMPKalpha2 impairs epithelial-mesenchymal transition in rat renal tubular epithelial cells by downregulating ETS1 and RPS6KA1. Mol. Med. Rep. 2020, 22, 4619–4628. [Google Scholar] [CrossRef]
- Xu, H.; Wang, M.; Li, Y.; Shi, M.; Wang, Z.; Cao, C.; Hong, Y.; Hu, B.; Zhu, H.; Zhao, Z.; et al. Blocking connexin 43 and its promotion of ATP release from renal tubular epithelial cells ameliorates renal fibrosis. Cell Death Dis. 2022, 13, 511. [Google Scholar] [CrossRef]
- Zhang, Y.; Hui, J.; Xu, Y.; Ma, Y.; Sun, Z.; Zhang, M.; Nie, L.; Ye, L. MEHP promotes liver fibrosis by down-regulating STAT5A in BRL-3A hepatocytes. Chemosphere 2022, 295, 133925. [Google Scholar] [CrossRef]
- Schrier, R.W. Pathogenesis of sodium and water retention in high-output and low-output cardiac failure, nephrotic syndrome, cirrhosis, and pregnancy (2). N. Engl. J. Med. 1988, 319, 1127–1134. [Google Scholar] [CrossRef]
- Bekheirnia, M.R.; Schrier, R.W. Pathophysiology of water and sodium retention: Edematous states with normal kidney function. Curr. Opin. Pharmacol. 2006, 6, 202–207. [Google Scholar] [CrossRef]
- Gamba, G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol. Rev. 2005, 85, 423–493. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Arguelles, D.B.; McIntosh, M.; Rohlicek, C.V.; Culty, M.; Zirkin, B.R.; Papadopoulos, V. Maternal in utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate affects the blood pressure of adult male offspring. Toxicol. Appl. Pharm. 2013, 266, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Tackitt, S.; Gievers, L.; Iragorri, S.; Sage, K.; Cornwall, T.; O’Riordan, D.; Merchant, J.; Rozansky, D. Phthalate-associated hypertension in premature infants: A prospective mechanistic cohort study. Pediatr. Nephrol. 2019, 34, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.F.; Liu, J.X.; Yan, Y.X.; Wang, L.; Wang, Z.Y. Selenium relieves oxidative stress, inflammation, and apoptosis within spleen of chicken exposed to mercuric chloride. Poult. Sci. 2020, 99, 5430–5439. [Google Scholar] [CrossRef]
- Van Eden, W.; Wick, G.; Albani, S.; Cohen, I. Stress, heat shock proteins, and autoimmunity: How immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann. N. Y. Acad. Sci. 2007, 1113, 217–237. [Google Scholar] [CrossRef]

| Phthalate Exposure | ACR | eGFR | References |
|---|---|---|---|
| MECPP, MEHHP, MBP, MBzP | → | ↑ (women) ↓ (men) | Hong et al., 2024 [30] |
| Melamine + MBP, MEP, MBzP, MECPP, MEHHP | ↑ (men) → (women) | – | Li et al., 2023 [27] |
| MBzP, MCPP, MEOHP, MEP, MEHP, MNP, MiBP | – | ↑ (women) ↓ (men) | Wang et al., 2022 [29] |
| MBzP, MCPP(HMW) | – | ↓ | Liu et al., 2022 [23] |
| MMP, MBP, MBzP, MOP | – | ↓ | Liu et al., 2022 [48] |
| Melamine + MMP, MEHHP, MEOHP, MECPP/BBzP, MBzP | ↑ | ↑ | Tsai et al., 2021 [26] |
| MBP, MiBP, MBzP, MECPP, MEHHP | ↑ | ↓ | Kang et al.,2021 [31] |
| DEHP, DBP, BBP | ↑ | ↓ (men) | Lee et al., 2020 [25] |
| MECPP, MEHHP, MBzP | ↑ | – | Chen et al., 2020 [49] |
| ∑LMW phthalates | → | ↑ | Jacobson et al., 2020 [46] |
| MBP, MiBP, MBzP, DEHP | ↑ | – | Kang et al., 2019 [50] |
| MMP, MBP, MiBP, MECPP, MEHHP | ↓ | ↑ | Malits et al., 2018 [32] |
| Modelled phthalate exposure | → | → | Jin et al., 2018 [47] |
| DEHP | ↑ | – | Tsai et al., 2016 [51] |
| MEHHP, MEHP, MECPP, DIDP(HMW) | ↑ | – | Trasande et al., 2014 [52] |
| Phthalate(s) | Species | Observed Renal Effects | Mechanism or Markers Involved | References |
|---|---|---|---|---|
| DEHP | Rat | Kidney injury, fibrosis, loss of podocyte processes | Oxidative stress, ↑ BUN/CRE, ↓ GSH, SOD, GPx1 | David et al., 2000; Wei et al., 2012; Erkekoglu et al., 2010, 2014; Ashari et al., 2020 [55,56,58,59,60] |
| Mouse | Tubular damage, CKD, inflammation | ↓ Nrf2, HO-1, GCLC, GSH | Ward et al., 1986; Jiang et al., 2021; Amara et al., 2019, 2020 [64,65,66,67] | |
| Mouse | Immune complex glomerulonephritis | PPARα pathway, NLRP3 activation | Kamijo et al., 2007; Tang et al., 2019 [69,70] | |
| Aquatic organism | Kidney hemorrhage, epithelial damage | Lipid peroxidation, ROS, ↓ antioxidants | Xiao et al., 2018 [78] | |
| Quail | Glomerular contraction, tubular expansion | PXR activation, cytochrome P450 | Ikele et al., 2011; P. Li et al., 2018; Wang et al., 2020 [80,81,82] | |
| DIBP, DBP | Rat | Renal fibrosis; reduced Fgf10 and Fgfr2 | ↓ Fgf10/Fgfr2 signaling pathway | White et al., 2009; Jiang et al., 2015; Sun et al., 2018 [83,84,85] |
| Mouse | Elevated creatinine and urea; kidney tissue damage | ↑ Bax, ↓ Bcl-2, ↓ Bax/Bcl-2 | Liang et al., 2021 [86] | |
| BBP | Rat | Fetal renal pelvis dilatation | – | Ema et al., 1992 [54] |
| Distal tubule and collecting duct damage | ↑ urinary excretion of 2OHE1 ↓ urinary excretion of E4 | Nakagomi et al., 2018 [61] | ||
| Mouse | Kidney tissue changes | ↑ AST, LDH | Beltifa et al., 2017 [71] | |
| Aquatic organism | BBP accumulation in tissues | short-branched MPEs, ↑ AST, LDH | Hu et al., 2016; Beltifa et al., 2017 [71,75] | |
| DIDP | Aquatic organism | DNA damage in head kidney cells | ↓ antioxidant enzymes | Oya-Silva et al., 2023 [79] |
| DEP | Aquatic organism | Tubular epithelial cell interpretation; hemorrhage | – | Xiao et al., 2018 [78] |
| Mixed | Rat | Apoptosis and necrosis in renal tubular epithelial cells | – | Dai et al., 2020 [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Zhang, M.; Song, J.; Wang, T.; Ma, Y.; Qin, L.; Li, J.; Qian, X.; Chen, J. Nephrotoxicity of Phthalates: A Review Based on Epidemiological and Toxicological Evidence. Toxics 2025, 13, 947. https://doi.org/10.3390/toxics13110947
Wei Y, Zhang M, Song J, Wang T, Ma Y, Qin L, Li J, Qian X, Chen J. Nephrotoxicity of Phthalates: A Review Based on Epidemiological and Toxicological Evidence. Toxics. 2025; 13(11):947. https://doi.org/10.3390/toxics13110947
Chicago/Turabian StyleWei, Yuehang, Minghui Zhang, Jiayuan Song, Tianyue Wang, Yuqin Ma, Liqiang Qin, Jiafu Li, Xiaoyan Qian, and Jingsi Chen. 2025. "Nephrotoxicity of Phthalates: A Review Based on Epidemiological and Toxicological Evidence" Toxics 13, no. 11: 947. https://doi.org/10.3390/toxics13110947
APA StyleWei, Y., Zhang, M., Song, J., Wang, T., Ma, Y., Qin, L., Li, J., Qian, X., & Chen, J. (2025). Nephrotoxicity of Phthalates: A Review Based on Epidemiological and Toxicological Evidence. Toxics, 13(11), 947. https://doi.org/10.3390/toxics13110947