Assessing the Impact of Microplastic Filaments Contaminated with PAHs on Mytilus coruscus Larvae through Surface Contact
Abstract
1. Introduction
2. Method and Materials
2.1. Chemicals and Larval Mussels
2.2. Exposure of PAHs-Contaminated Filaments to Larvae
2.3. Sampling and PAHs Analysis
2.4. Statistical Analysis
3. Results and Discussion
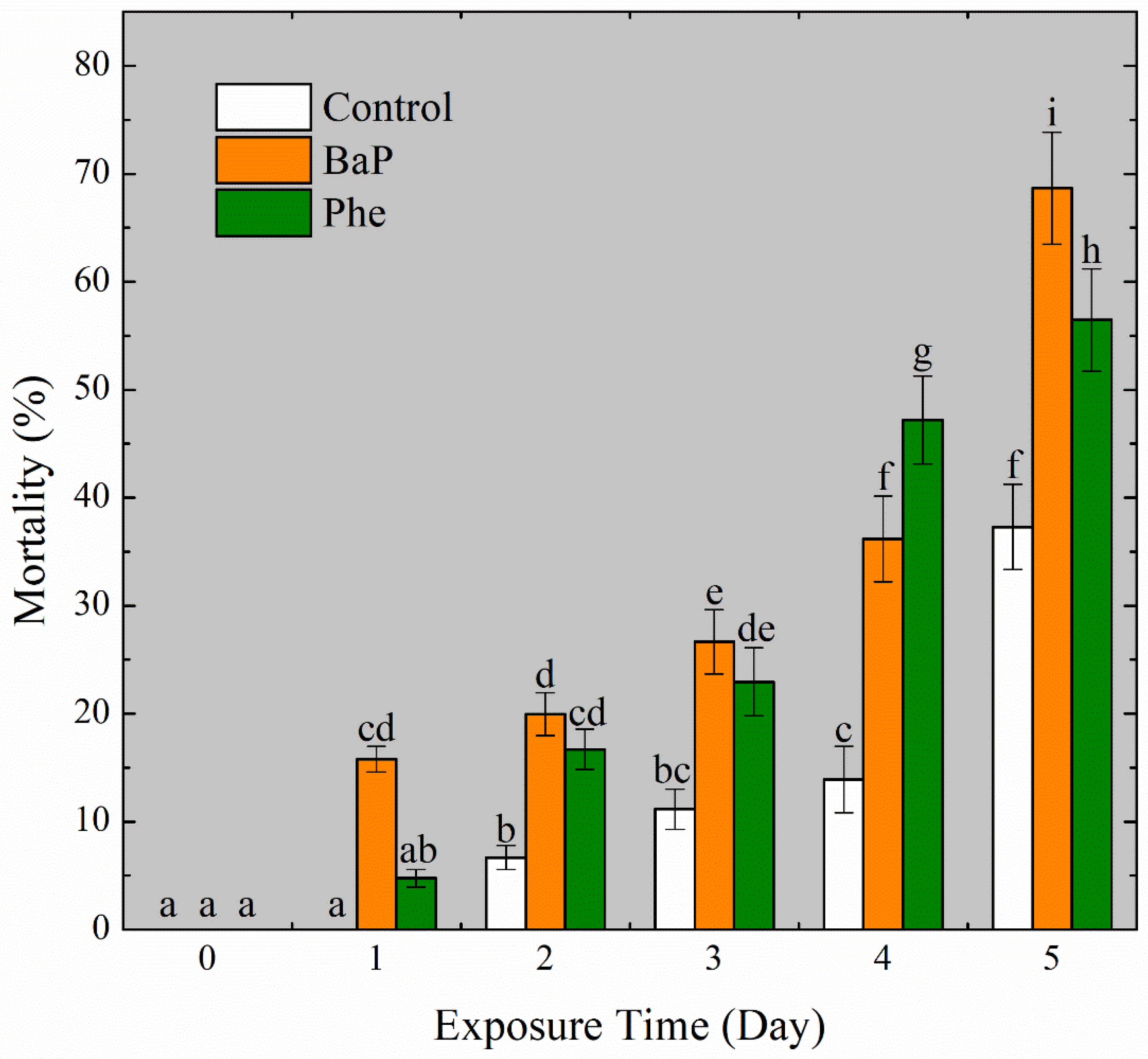
3.1. The Toxic Effects of Contaminated Microplastic Filaments on Larval Mussels
3.2. Ecotoxic Risk of Contaminated Rope on Larvae in Mussel Aquaculture
3.3. Pollutant Transfer Process of Toxic Effects of Contaminated Plastic Filaments on Mussels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Sun, C.; Wang, Y.; Cai, H.; Li, L.; Li, J.; Shi, H. Fusion of microplastics into the mussel byssus. Environ. Pollut. 2019, 252, 420–426. [Google Scholar] [CrossRef]
- Rist, S.; Baun, A.; Hartmann, N.B. Ingestion of micro- and nanoplastics in Daphnia magna—Quantification of body burdens and assessment of feeding rates and reproduction. Environ. Pollut. 2017, 228, 398–407. [Google Scholar] [CrossRef]
- EFSA. Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Qu, C.; Albanese, S.; Lima, A.; Hope, D.; Pond, P.; Fortelli, A.; Romano, N.; Cerino, P.; Pizzolante, A.; De Vivo, B. The occurrence of OCPs, PCBs, and PAHs in the soil, air, and bulk deposition of the Naples metropolitan area, southern Italy: Implications for sources and environmental processes. Environ. Int. 2019, 124, 89–97. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, C.; Zhang, J.; Zhi, L.; Tang, T.; Yao, H.; Li, W.; Shi, C.; Qi, S. Constructing model-averaging species sensitivity distributions of Phenanthrene based on reproductive fitness: Implications for assessing ecological risk in urban watershed. J. Hazard. Mater. 2023, 443, 130296. [Google Scholar] [CrossRef]
- Qu, C.; Albanese, S.; Cicchella, D.; Fortelli, A.; Hope, D.; Esposito, M.; Cerino, P.; Pizzolante, A.; Qi, S.; De Vivo, B. The contribution of persistent organic pollutants to the environmental changes in Campania region, Italy: Results from the Campania Trasparente project. J. Geochem. Explor. 2022, 241, 107071. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Karapanagioti, H.K.; Klontza, I. Testing phenanthrene distribution properties of virgin plastic pellets and plastic eroded pellets found on Lesvos island beaches (Greece). Mar. Environ. Res. 2008, 65, 283–290. [Google Scholar] [CrossRef]
- Devriese, L.I.; De Witte, B.; Vethaak, A.D.; Hostens, K.; Leslie, H.A. Bioaccumulation of PCBs from microplastics in Norway lobster (Nephrops norvegicus): An experimental study. Chemosphere 2017, 186, 10–16. [Google Scholar] [CrossRef]
- Bakir, A.; O’Connor, I.A.; Rowland, S.J.; Hendriks, A.J.; Thompson, R.C. Relative importance of microplastics as a pathway for the transfer of hydrophobic organic chemicals to marine life. Environ. Pollut. 2016, 219, 56–65. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Springer: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Qu, C.; Li, J.; Albanese, S.; Lima, A.; Wang, M.; Sacchi, M.; Molisso, F.; De Vivo, B. Polycyclic aromatic hydrocarbons in the sediments of the Gulfs of Naples and Salerno, Southern Italy: Status, sources and ecological risk. Ecotoxicol. Environ. Saf. 2018, 161, 156–163. [Google Scholar] [CrossRef]
- Chizhova, T.; Hayakawa, K.; Tishchenko, P.; Nakase, H.; Koudryashova, Y. Distribution of PAHs in the northwestern part of the Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2013, 86–87, 19–24. [Google Scholar] [CrossRef]
- Engler, R.E. The complex interaction between marine debris and toxic chemicals in the ocean. Environ. Sci. Technol. 2012, 46, 12302–12315. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, D.; Jia, Y.; Schiwy, S.; Legradi, J.; Yang, S.; Hollert, H. Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total Environ. 2017, 609, 1312–1321. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, A.; Cao, S.; Sun, F.; Wang, L.; Guo, H.; Ji, R. Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ. Pollut. 2016, 219, 166–173. [Google Scholar] [CrossRef]
- Ziccardi, L.M.; Edgington, A.; Hentz, K.; Kulacki, K.J.; Kane Driscoll, S. Microplastics as vectors for bioaccumulation of hydrophobic organic chemicals in the marine environment: A state-of-the-science review. Environ. Toxicol. Chem. 2016, 35, 1667–1676. [Google Scholar] [CrossRef]
- Huang, W.; Song, B.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D.L. Impact of Microplastic Beads and Fibers on Waterflea (Ceriodaphnia dubia) Survival, Growth, and Reproduction: Implications of Single and Mixture Exposures. Environ. Sci. Technol. 2017, 51, 13397–13406. [Google Scholar] [CrossRef]
- Weber, A.; Scherer, C.; Brennholt, N.; Reifferscheid, G.; Wagner, M. PET microplastics do not negatively affect the survival, development, metabolism and feeding activity of the freshwater invertebrate Gammarus pulex. Environ. Pollut. 2018, 234, 181–189. [Google Scholar] [CrossRef]
- Teuten, E.L.; Rowland, S.J.; Galloway, T.S.; Thompson, R.C. Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 2007, 41, 7759–7764. [Google Scholar] [CrossRef]
- Zhang, L. Review of studies on living habits of mussels in foreign countries. Foreign Aquac. 1964, 5, 7–15. [Google Scholar]
- Ma, P.; Wei Wang, M.; Liu, H.; Feng Chen, Y.; Xia, J. Research on ecotoxicology of microplastics on freshwater aquatic organisms. Environ. Pollut. Bioavailab. 2019, 31, 131–137. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Tallec, K.; Gonzalez-Fernandez, C.; Lambert, C.; Vincent, D.; Mazurais, D.; Zambonino-Infante, J.-L.; Brotons, G.; Lagarde, F.; Fabioux, C. Constraints and priorities for conducting experimental exposures of marine organisms to microplastics. Front. Mar. Sci. 2018, 5, 252. [Google Scholar] [CrossRef]
- McCarthy, J.F.; Roberson, L.E.; Burrus, L.W. Association of benzo (a) pyrene with dissolved organic matter: Prediction of Kdom from structural and chemical properties of the organic matter. Chemosphere 1989, 19, 1911–1920. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef]
- Haque, M.N.; Alam, M.M.; Cho, D.; Kwon, S. Sensitivity of veliger larvae of Mytilus edulis and mussel of various sizes to chlorination. Toxicol. Environ. Chem. 2015, 97, 931–945. [Google Scholar] [CrossRef]
- Abbasi, S.; Moore, F.; Keshavarzi, B. PET-microplastics as a vector for polycyclic aromatic hydrocarbons in a simulated plant rhizosphere zone. Environ. Technol. Innov. 2021, 21, 101370. [Google Scholar] [CrossRef]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef]
- Dhavamani, J.; Beck, A.J.; Gledhill, M.; El-Shahawi, M.S.; Kadi, M.W.; Ismail, I.M.; Achterberg, E.P. The effects of salinity, temperature, and UV irradiation on leaching and adsorption of phthalate esters from polyethylene in seawater. Sci. Total Environ. 2022, 838, 155461. [Google Scholar] [CrossRef]
- Yang, J.; Shen, P.; Wang, C.; Li, Y.; Liang, X.; Shen, H.; Xu, C.; Li, J. Effects of biofilms on settlement of plantigrades of the mussel Mytilus coruscus. J. Fish. China 2013, 37, 904–909. [Google Scholar] [CrossRef]
- Bhagwat, G.; O’Connor, W.; Grainge, I.; Palanisami, T. Understanding the fundamental basis for biofilm formation on plastic surfaces: Role of conditioning films. Front. Microbiol. 2021, 12, 687118. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef]
- Von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef]
- Piarulli, S.; Airoldi, L. Mussels facilitate the sinking of microplastics to bottom sediments and their subsequent uptake by detritus-feeders. Environ. Pollut. 2020, 266, 115151. [Google Scholar] [CrossRef]

| Steps | Groups | |||
|---|---|---|---|---|
| Control | DMSO | BaP | Phe | |
| 1: Contaminants solution | one liter seawater | one-liter DMSO solution in seawater (500 μL/L) | BaP stock solution prepared in DMSO (1.0 mg/mL); one-liter BaP seawater solution prepared (200 μg/L, 200 μL stock solution used) | Phe stock solution prepared in DMSO (1.0 mg/mL); one-liter Phe seawater solution prepared (500 μg/L, 500 μL stock solution used) |
| 2: Contaminants adsorption | 5 10-cm-long filaments soaked in above solutions for 24 h, removed from solutions and air-dried | |||
| 3: Exposure | the 5 dried filaments exposed in larvae suspension (10 ind/mL) in 1600 mL seawater for 5 days | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Huang, J.; Ye, Y.; Lü, J.; Mao, S.; Bai, J.; Qi, P.; Guo, B.; Qu, C.; Jiang, H. Assessing the Impact of Microplastic Filaments Contaminated with PAHs on Mytilus coruscus Larvae through Surface Contact. Toxics 2023, 11, 554. https://doi.org/10.3390/toxics11070554
Li J, Huang J, Ye Y, Lü J, Mao S, Bai J, Qi P, Guo B, Qu C, Jiang H. Assessing the Impact of Microplastic Filaments Contaminated with PAHs on Mytilus coruscus Larvae through Surface Contact. Toxics. 2023; 11(7):554. https://doi.org/10.3390/toxics11070554
Chicago/Turabian StyleLi, Jiji, Ji Huang, Yingying Ye, Jiayin Lü, Shuai Mao, Jie Bai, Pengzhi Qi, Baoying Guo, Chengkai Qu, and Hongchen Jiang. 2023. "Assessing the Impact of Microplastic Filaments Contaminated with PAHs on Mytilus coruscus Larvae through Surface Contact" Toxics 11, no. 7: 554. https://doi.org/10.3390/toxics11070554
APA StyleLi, J., Huang, J., Ye, Y., Lü, J., Mao, S., Bai, J., Qi, P., Guo, B., Qu, C., & Jiang, H. (2023). Assessing the Impact of Microplastic Filaments Contaminated with PAHs on Mytilus coruscus Larvae through Surface Contact. Toxics, 11(7), 554. https://doi.org/10.3390/toxics11070554









