Abstract
Aronia melanocarpa, a plant with nutrient-rich fruits, with application in the food and pharmaceutical industry, has been extensively investigated but, nevertheless, the exploration of the secondary metabolites profile from its by-products remains quite limited. The main objective of this study was to evaluate the possibility of using some different natural mineral waters from Romania, as green solvents, for the extraction of bioactive compounds from aronia stems and fruits by applying eco-compatible working techniques (maceration for 24 h, and ultrasonication at room temperature and 50 °C for 30 min). The effect of five natural mineral waters (one with medium and four with low mineral content) on the extraction capacity and phytochemical profile of stems and fruits’ extracts was monitored using fast and efficient analysis techniques (electrochemical, spectroscopic, and chromatographic) and compared with that of classical solvents. The results showed that, in the case of stems, extraction by maceration was, for all types of water used, the most efficient, followed by ultrasonication at room temperature. Also, at the same time, in most cases, all mineral waters showed better performance than distilled water, and the highest efficiency of the extraction process was recorded for natural water with a medium mineralization level. The similarity observed in the phytochemical profiles of aqueous extracts from the aronia stems and the fruits highlights both the potential of this by-product as a source of bioactive compounds and the efficiency of natural mineral waters as green extraction solvents.
1. Introduction
Plants are considered rich sources of biologically active compounds with antioxidant, antibacterial, antifungal, anti-inflammatory, and antitumor effects, etc. On the other hand, plant by-products, usually used for low-value applications, can also constitute a relevant source for various bioactive compounds (primary and secondary metabolites), useful in obtaining cosmetics, pharmaceuticals, supplements, or food products valuable in improving human nutrition, health, and well-being [1].
One of the most known and studied classes of bioactive compounds is represented by the polyphenols. These secondary metabolites have proven various nutritional values and beneficial effects on health due to their antioxidant and anti-inflammatory properties [2,3,4,5].
Black chokeberry (Aronia melanocarpa), also called aronia, a species of shrubs belonging to the Rosaceae family, native to eastern parts of North America, is cultivated around the world for its medicinal and nutritional value. Aronia, considered a superfruit, has a positive impact on health due to its rich and varied content of bioactive components, such as vitamins, minerals, and polyphenolic compounds.
Its anti-inflammatory, antibacterial, hypolipidemic, hypoglycemic, anticancer, antidepressant, and antifatigue effects promoted it over the past decade as a raw material for a wide variety of juices, wines, teas, effervescent tablets, and nutritional supplements [6,7,8,9,10,11,12].
While chokeberry fruits are well characterized from a compositional point of view, their by-products (leaves, stems, etc.) have been less studied, with the number of investigations on this subject being limited [13,14,15].
Nowadays, there is growing interest in obtaining bioactive compounds from plants, residues of food industries, and by-products through green methods [16,17,18,19]. In this regard, one of the biggest challenges for the recovery of secondary metabolites from plants is to develop an appropriate working system (equipment, extraction protocol, and solvent), simultaneously compatible with the chemical complexity of plant by-products and ecologically sustainable.
The efficiency of the extraction process, conducted through conventional (decoction, infusion, maceration, Soxhlet extraction, percolation, etc.) or modern methods (ultrasonication, microwave-assisted extraction, extraction with pressurized liquids, extraction with subcritical or supercritical fluids and accelerated solvent extraction, electrotechnologies such as pulsed electric field and high-voltage electrical discharges, enzyme-assisted extraction, etc.), is influenced by numerous factors related to the solvent, the composition of the plant material, and to the extraction process parameters (time, temperature, plant–solvent ratio, etc.) [1,20,21,22].
Taking into account the concept of green chemistry, lately, attention has been directed toward the use of non-toxic solvents that do not harm human health and the environment. Among them, water can be considered the most risk-free and the least expensive solvent.
Only few studies regarding the efficiency of extraction techniques for biologically active compounds using natural mineral waters and the influence of their composition on the biological activity of the obtained extracts are available [23,24,25].
On the other hand, a series of previous studies indicate that factors such as pH value or presence of dissolved salts (especially those of calcium and magnesium) and bicarbonate ions can affect the bioactive compounds’ extraction, particularly that of polyphenols [24,26].
In Romania, there are over 2000 mineral water springs (over 40% of the European reserve of natural mineral waters [27]), whose chemical diversity is given by the particularly complex geological conditions in which they were formed. Important Romanian mineral water deposits are located in mountainous areas and in intermountain depressions, far from the pollution sources characteristic of industrial areas or where intensive agriculture is practiced. Most springs presenting carbonated mineral waters are bottled and commercially available. When consumed with food or shortly before a meal, they stimulate digestive functions and excite gastric secretion and motility, intestinal, pancreatic, and biliary secretions, favoring the acceleration of food absorption.
The beneficial role of natural mineral waters on the healthy constitution and functioning of the human body is amplified by their content in mineral salts, and by their purity, characterized by the total absence of artificial contaminants. These waters are rich in minerals, such as calcium (Ca), magnesium (Mg), sodium (Na), and potassium (K), and ions, such as bicarbonate (HCO3−), chlorine (Cl−), and sulfate (SO42−). At the trace level, metals and metalloids, such as manganese (Mn), iron (Fe), silicon (Si), selenium (Se), and zinc (Zn), are also present.
Our research concerns are mainly focused on the superior valorization of regional resources (NE Romania), namely, the natural mineral waters in the area [28,29,30], as well as the diversity of plant-based foods and their by-products as potential sources of bioactive compounds [31,32,33,34].
From this perspective, the present work was designed as a qualitative and comparative study concerning the potential of commercial natural mineral waters of Romania to efficiently extract polyphenols, especially from aronia by-products (stems) but also from fruits, in order to initiate future research on their functionalities and bioactivities. The effects of the environmentally friendly solvents used and of the extraction techniques (maceration and ultrasound) were evaluated by safe, efficient, and less expensive analytical methods (electrochemical, spectroscopic, and chromatographic methods). The use of instrumental fingerprinting techniques (UV-Vis and FTIR spectroscopy) was considered for a rapid screening of the extracts’ phytochemical profiles in order to highlight differences induced by the solvent type and the extraction method.
2. Materials and Methods
2.1. Plant Material
Aronia fruits and stems used in the experiments were collected in the hilly area of Bacau County, northeast region (latitude: 46.598553 N; longitude: 26.843002 E) of Romania, in August 2024. They were naturally dried in a cool and dark place until reaching a moisture content of 10–12%. Then, the fruits (F) were separated from stems (S), packed in sealed glass containers, protected with aluminum foil, and stored in a dark and cool place. Before use, they were grinded with an electric grinder (Heinner, model HCG-150SS, 150 W, Heinner, Bucharest, Romania). The resulted powders were sieved through a digital electromagnetic sieve shaker (FILTRA Vibratión FTS-0200, Barcelona, Spain). Fractions with size of 0.200 mm for stems and 0.250 mm for fruits were retained for extraction. Figure 1 shows the macroscopic photographs together with images obtained under stereomicroscope (Optika Microscopes, Model ST-30FX, Ponteranica, Italy) of the vegetal material in powder form.

Figure 1.
Macroscopic and stereomicroscopic images of powdered aronia ((A) fruits and (B) stems).
2.2. Extraction Solvents
- Ethanol 96° (Chemical Company S.A., Iași, Romania) was mixed with distilled water (40/60 v/v) and used for hydroalcoholic extraction (HA).
- Distilled water (aqua destillata, AD) and five commercial natural mineral waters (NMWs) were used for aqueous extraction.
- The sources of natural mineral waters, one with medium mineral content (500–1500 mg/L) and four with low mineral content (50–500 mg/L) [35], were as follows:
- Tușnad (T), source FH 35bis, Tușnad, Harghita, carbonated.
- Aquavia (AQV), source A1—A2R, Bologa, Cluj, non-carbonated.
- Aqua Carpatica (AC), source AQUA, Broșteni, Suceava, non-carbonated.
- Aqua Carpatica Kids (ACK), source Izvor BAJENARU, Paltiniș, Suceava, non-carbonated.
- Perla Harghitei (PH), source FH Artezia 2, Sânsimion, Harghita, non-carbonated.
The main characteristics included on the labels of commercial mineral waters used in extraction are presented in Table 1 (according to the producers’ statements).

Table 1.
Characteristics of commercial mineral waters used in extraction.
2.3. Extraction Procedure
Two grams of powdered plant sample and 40 mL of solvent were submitted to 30 min extractions performed by two techniques: maceration (M) at room temperature for 24 h and ultrasonication (US) in a digital ultrasonic cleaner, Biobase model UC-40A (Biobase Biodustry (Shandong) Co., Ltd., Jinan, China), at room temperature (USRT) and at 50 °C (US50). The extracts were separated by filtration through Whatman filter paper No. 42 (Marlborough, MA, USA). The resulted 42 extracts (Table 2) were stored at 4 °C until further analyses.

Table 2.
Coding of the 42 samples.
2.4. Solvent and Extract Characterization
The electrochemical parameters, pH, conductivity (EC), salinity (SAL), and total dissolved solids (TDSs), of solvents and extracts were measured using a Thermo Scientific™ Orion™ Versa Star Pro™ Multiparameter Benchtop Meter (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a ROSS Ultra pH/ATC electrode and DuraProbe conductivity cell 013005MD.
The extracts were further analyzed through ultraviolet-visible (UV-Vis) and Fourier transform infrared (FTIR) spectroscopy. In the first case, a UV-Vis spectrophotometer (Shimadzu UV-1280, Kyoto, Japan) was used for scanning the samples in the wavelength ranging from 190 to 1100 nm. In the second case, the analyses were performed with an IRSpirit FTIR spectrometer with attenuated total reflectance single-reflection (QATR-S) auxiliary (Shimadzu, Bucharest, Romania) employed between 4000 and 400 cm−1 (50 scans/min) with a resolution of 8 cm−1. The cleaning of the QATR-S accessory was realized with ethanol. The high-performance thin-layer chromatography (HPTLC) technique was used for a rapid screening of the extracted compounds [36,37] using a CAMAG HPTLC system (CAMAG, Muttenz, Switzerland) equipped with a Semi Auto Sample Applicator CAMAG®Linomat 5, TLC Visualizer, CAMAG TLC Scanner 3 UV with visionCats CAMAG HPTLC software (version 3.0). The HPTLC plates (20 cm × 10 cm silica gel 60 F254—Merck, Darmstadt, Germany) were used without any pretreatment. The length of the bands was 8 mm, the application rate was 5 µL/s, and the application volume was 2 µL. The plates were developed with an eluent adapted from Asfaw et al. [38] containing toluene:ethyl acetate:ethanol:formic acid (20:12:8:4 v/v/v/v). After development, the plates were removed, dried at room temperature, and visualized under UV light at 254 nm and 366 nm.
2.5. Statistical Analysis
All the experiments were performed in triplicate, and the obtained results were reported as mean values with standard deviation (mean ± SD). The variables’ relationship was tested using linear regression and quantified by calculating the Pearson correlation coefficient. Microsoft Office Excel 2016 (Microsoft, Redmond, WA, USA) was used for this purpose. Principal component analysis (PCA), permutational multivariate analysis of variance (PERMANOVA), and partial least squares–discriminant analysis (PLS-DA) were carried out with MetaboAnalyst 6.0.
3. Results and Discussion
The aqueous and hydroalcoholic extracts were obtained through two extraction techniques, maceration and ultrasonication.
Maceration, although a traditional, simple, and inexpensive method that allows the solvent to gradually diffuse into plant tissues, extracting bioactive compounds through a concentration gradient, requires a long interaction time [21,22].
Ultrasonication is an accessible, modern, rapid, and non-polluting extraction technique that helps to release bioactive compounds from plant materials to a solvent, exposed to high-frequency sound waves. The process relies on ultrasonic waves to create cavitation bubbles in the liquid. As these bubbles collapse, they generate intense localized heat and pressure that can be harnessed to facilitate the extraction process [21,39,40].
Aqueous and hydroalcoholic extracts of aronia fruits and stems were subjected to analyses through physicochemical, UV-Vis, FTIR, and HPTLC evaluations.
3.1. Electrochemical Analyses
Both the solvents and the crude extracts of aronia fruits and stems were analyzed by physicochemical methods. The results obtained in terms of representative physicochemical parameters, such as pH, electrical conductivity (EC), total dissolved solids (TDSs), and salinity (SAL), are summarized in Figure 2, Figure 3, Figure 4 and Figure 5. Data are shown as mean ±SD.
3.1.1. pH
The pH of the natural mineral waters used in the experiment ranged from 5.60 ± 0.002 (T) to 9.41 ± 0.002 (AQV).
Regarding the pH values, extracts from both the stems and the fruits obtained using non-carbonated mineral waters (AQV, AC, ACK, and PH), distilled water, and hydroethanolic mixture were acidic, while the extracts obtained using carbonated mineral water (T) were neutral.
As shown in Figure 2, a decrease in the pH of the extracts compared to the initial solvents was observed (both in the case of non-carbonated natural waters with low mineral content, AQV, AC, ACK, and PH, from the basic to the acidic range, and in the case of AD and HA), with the exception of Tușnad (carbonated natural water with medium mineral content), where an opposite behavior was seen.
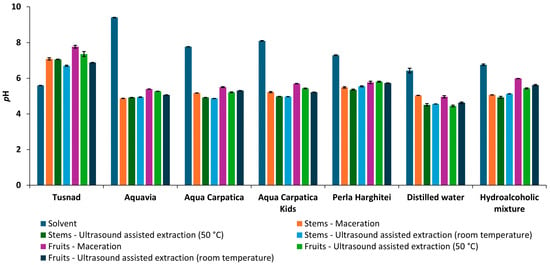
Figure 2.
pH of solvents and extracts obtained from aronia (stems and fruits).
The smallest pH variations were recorded in stem extracts when using Tușnad natural mineral water as a solvent (1.1−1.5 pH units) and in hydroalcoholic fruit extracts (0.8−1.2 pH units). Also, in the case of AQV natural mineral water, the pH variation was the highest, registering a decrease between 4 and 4.5 pH units for both stem and fruit extracts, both macerated and ultrasonicated.
In terms of pH, the values for stem extracts were slightly lower than the ones for fruits.
3.1.2. Conductivity
Among all the extracts obtained (Figure 3), the lowest conductivity values were presented by the hydroalcoholic ones (in the range of 121.10 ± 0.264 to 200.71 ± 0.065 µS/cm), followed by those in distilled water, then relatively close values were presented by the extracts in ACK, AC, and AQV, followed by the PH extracts, while the extracts with T had significantly higher conductivity values, between 1484.33 ± 2.52 and 1919.33 ± 2.08 µS/cm.
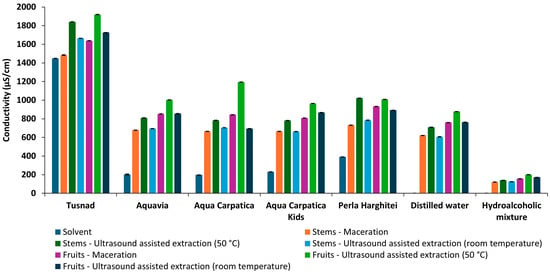
Figure 3.
Conductivity of solvents and extracts obtained from aronia (stems and fruits).
Considering the low ion content of distilled water and hydroethanolic solution, the largest increases in conductivity were observed in the case of extraction with the two solvents (about 550-fold and 240-fold higher, respectively), which denotes a much better extraction power of ions from plant material compared to natural mineral waters.
In the case of mineral waters as solvents, conductivity increases were much smaller, ranging between 1.15 and 4.6 times. The smallest increase was recorded for Tușnad natural mineral water, the only carbonated water, classified as water with a medium mineral content, while for the most alkaline mineral water, AQV, the variation was the largest.
For all samples, the ultrasonic extraction method at 50 °C favored an increase in the rate of ion solubilization, their mobility, and, therefore, their concentration in solution, inducing a greater increase in conductivity compared to maceration and ultrasonication at room temperature.
Due to the higher mineral content of fruits compared to stems [13,41], it was expected that the increases in the conductivity values of the extracts would also be greater for fruits.
3.1.3. Total Dissolved Solids (TDSs) and Salinity (SAL)
In accordance with conductivity, the trends of TDS and salinity measurements were similar to those of conductivity (Figure 4 and Figure 5).
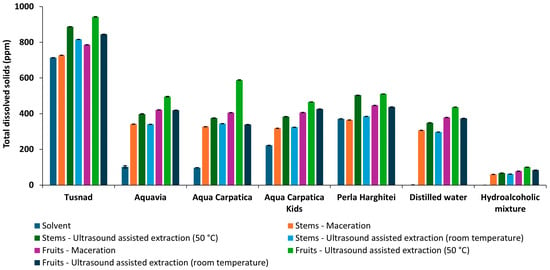
Figure 4.
Total dissolved solids of solvents and extracts obtained from aronia (stems and fruits).
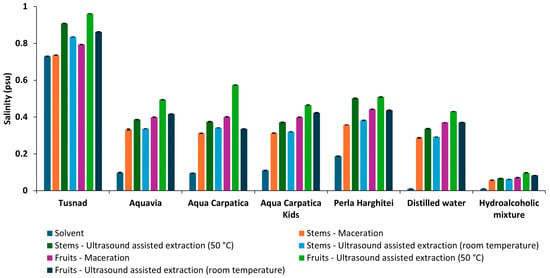
Figure 5.
Salinity of solvents and extracts obtained from aronia (stems and fruits).
The Pearson correlation coefficients (Table 3) were calculated for physicochemical parameters of the extracts obtained using the five natural mineral waters (T, AQV, AC, ACK, and PH), distilled water (AD), and hydroalcoholic mixture (HA).

Table 3.
Pearson correlation coefficient matrix of four measured parameters for aronia (stems and fruits) extracts obtained using the five natural mineral waters, distilled water, and hydroalcoholic mixture.
Very strong correlations between EC and TDS (over 0.99) and EC and SAL (over 0.95) can be observed for all extracts. The highest negative value of coefficient correlation, pH–EC (−0.8869), pH–TDS (−0.9014), and pH–SAL (−0.888), can be observed for the extracts with PH natural mineral water. In the case of the most alkaline mineral water (AQV), weak positive correlations of pH with the other parameters were recorded (0.229–0.347).
3.2. Phytochemical Profiles
3.2.1. FTIR Analysis
The FTIR spectra for 28 extract samples of Aronia melanocarpa, 14 stem extracts (Figure 6a: 7/S-M and 7/S-US50) and 14 fruit extracts (Figure 6b: 7/F-M and 7/F-US50), respectively, recorded in the wavenumber range 4000–400 cm−1, are presented comparatively in Figure 6.
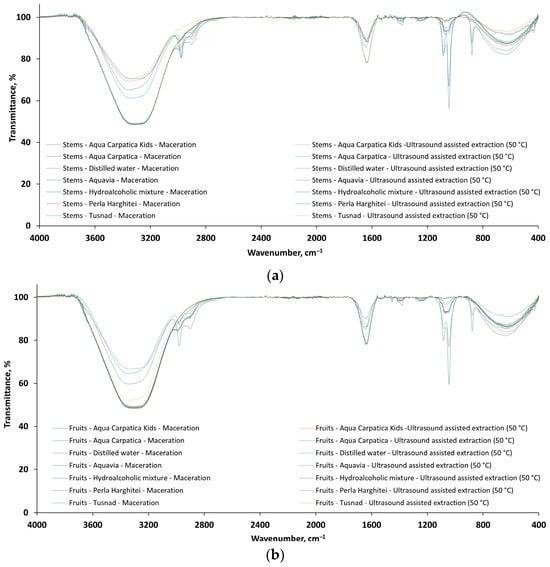
Figure 6.
The FTIR spectra of the 28 extracts from aronia: (a) S—stems and (b) F—fruits.
From Figure 6, it can be seen that the shape of the FTIR spectra for both stem (Figure 6a) and fruit (Figure 6b) extracts is similar and, in accordance with previous studies [42,43,44,45,46,47], shows absorption bands for functional groups at frequencies characteristic of polyphenolic components usually present in aronia. The dominant bands present in the FTIR spectra of the 28 analyzed extracts are given in Table 4.

Table 4.
The main absorption bands from FTIR spectra of aronia extracts (S—stems; F—fruits).
Additionally, the weak and small peaks observed at 2990/2954 (stem extracts) and 2980/2897 (fruit extracts) where possibly originating from –CH, –CH2, and –CH3 groups (stretching vibrations of C–H bonds from carbohydrates). The peaks around 1400 cm−1 and 1250 cm−1 can be assigned to phenolic C–O stretching of the pyran nucleus, typical for flavonoid C-rings [42,45].
3.2.2. UV-Vis Analysis
After UV-Vis scanning of the samples, a comparative spectrophotometric fingerprint of the chokeberry fruit and stem extracts was recorded in the representative range 200–700 nm (Figure 7).
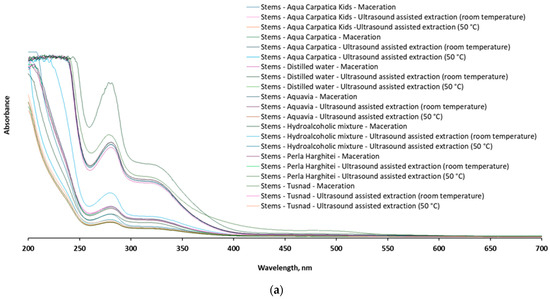
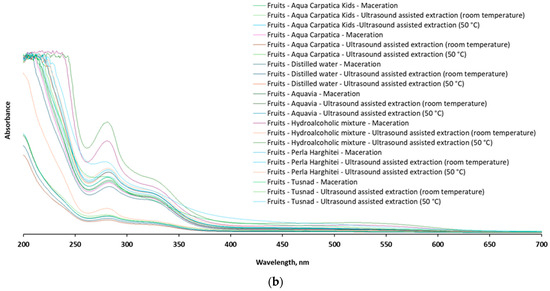
Figure 7.
The UV-Vis profile of the 42 extracts from aronia: (a) stems and (b) fruits.
The qualitative profiles of the UV-Vis spectra recorded for both stem and fruit extracts, respectively, the position (λmax) and intensity of the spectral bands, are similar and in full agreement with other available data reported for the content of polyphenolic compounds in chokeberry [8,14,15,48,49,50,51,52,53], confirming at the same time the fact that stem extracts also constitute a valuable source of such bioactive compounds.
From the examination of the spectral profile, it is observed that the extracts from the stems are also characterized by the presence of intense absorption bands in the ranges of 270–290 nm and 320–340 nm, respectively, considered specific to different types of phenolic acids and flavonoids, while the signals in the range of 480–520 nm, attributed mainly to anthocyanins [49,50], are absent in the case of the stems or much less represented (C-T-M: 470–780 nm) compared to those in the fruit spectrum.
PCA (Figure 8) performed on the full UV-Vis spectra (200–700 nm) differentiates aronia fruit extracts from those obtained from stems.
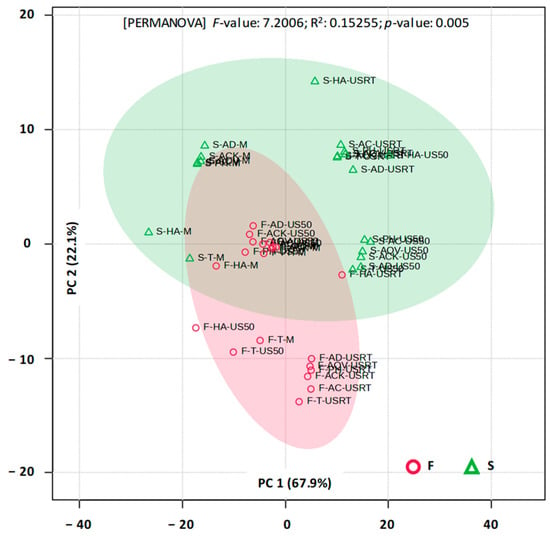
Figure 8.
2D PCA score plot for UV-Vis (200–700 nm) profiles of aronia stems (S) and fruits (F). ACK—Aqua Carpatica Kids; AC—Aqua Carpatica; AD—aqua destillata (distilled water); AQV—Aquavia; HA—hydroalcoholic mixture; PH—Perla Harghitei; T—Tusnad; M—maceration; USRT—ultrasound-assisted extraction (room temperature); US50—ultrasound-assisted extraction (50 °C).
The first two principal components explained 90% of the total variance. The separation was statistically confirmed by PERMANOVA, demonstrating that the plant material significantly influences the overall spectral profile. PLSDA further supported this observation (the first two components jointly explained 89% of the variance), indicating that multiple spectral regions contribute to fruit–stem discrimination rather than a single dominant absorbance feature. Based on these results, it can be inferred that the two plant matrices should not be treated interchangeably when evaluating the extraction of bioactive compounds. In order to confirm or invalidate this hypothesis, a separate multivariate analysis was conducted. PCA and PLSDA realized for UV-Vis spectra of fruit and stem extracts revealed no significant differences for wavelengths between 200 and 700 nm. On the other hand, various differences were detected when the analyses were restricted to the regions specific for phenolic acids (270–290 nm), flavonoids (320–340 nm), and anthocyanins (480–520 nm).
For aronia stems (Figure 9a), PCA and PERMANOVA highlighted differences in multivariate structures among the individual sample types (ACK, AD, AC, AQV, HA, PH, and T) and across analytical configurations. In the first analysis (270–290 nm), PCA revealed strong separation among sample groups, with the first two components explaining 98.1% of the total variance. HA samples formed a distinct cluster, separated from the remaining sample types, while ACK, AD, AC, AQV, PH, and T were differentiated along both PC1 and PC2, with T samples showing greater internal dispersion. This structuring was sustained by PERMANOVA results (F-value = 7.21, R2 = 0.7555, p-value = 0.002). The second PCA (320–340 nm) showed that although PC1 explained 93.7% of the variance, AC, AQV, ACK, AD, PH, and HA samples largely overlapped, and T samples were not consistently separated from the other groups, reflecting weak discrimination among sample types. The lack of structure was consistent with the PERMANOVA data (F-value = 0.8936, R2 = 0.2769, p-value = 0.537). In the third analysis (480–520 nm), multivariate separation was again evident (PC1 84.9% and PC2 4.9%). T samples shifted toward negative PC1 values and exhibited extensive dispersion.
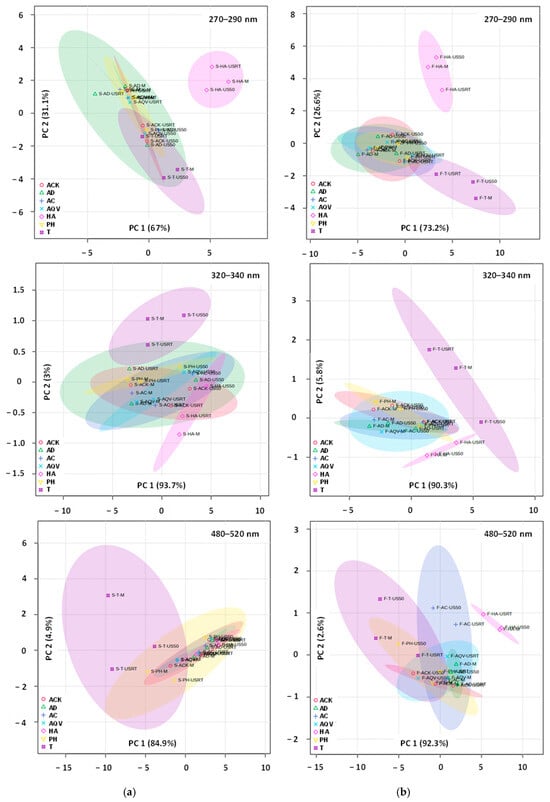
Figure 9.
2D PCA score plot for UV-Vis profiles of aronia stems (a) and fruits (b). ACK—Aqua Carpatica Kids; AC—Aqua Carpatica; AD—aqua destillata (distilled water); AQV—Aquavia; HA—hydroalcoholic mixture; PH—Perla Harghitei; T—Tusnad; M—maceration; USRT—ultrasound-assisted extraction (room temperature); US50—ultrasound-assisted extraction (50 °C).
The three PCA score plots for aronia fruits (Figure 9b) revealed a consistent pattern of multivariate differentiation among groups, with separation mainly driven along PC1 (73.2%, 90.3%, and 92.3%, respectively) and a more reduced contribution recorded for PC2. In the first and third plots, several groups formed separated and compact clusters. The overlap was limited with HA and T ellipses clearly displaced along PC1 and/or PC2. ACK, AD, AC, AQV, and PH showed varying degrees of overlap, suggesting partial similarity and shared variance, though their centroids remained distinguishable, especially when PC2 was considered. The PCA plot specific to the 320–340 nm UV-Vis range showed a greater overlap among most groups and a visibly broader dispersion of ellipses, indicating weaker yet noticeable structuring. These remarks are in agreement with PERMANOVA results, which were statistically significant (F-value = 11.9, 3.76, and 10.16; p-value = 0.001, 0.019, and 0.001) for all three wavelength ranges, confirming that group centroids differ beyond random expectation. The R2 values (0.8324, 0.6171, and 0.8132) indicate that group identity explains a substantial proportion of total multivariate variance, particularly in the cases of 270–290 nm and 480–520 nm. PLSDA, with the first two components jointly explaining 99.8%, 95.6%, and, respectively, 94.6% of the variance, validated these outcomes.
AC, AQV, ACK, AD, PH, and HA samples clustered and followed a structured gradient, with only partial separation. This pattern was statistically supported by PERMANOVA (F-value = 5.65, R2 = 0.7078, p-value = 0.003), confirming that differences among sample types, particularly the distinct behavior of T and HA relative to AC, AQV, and AD, were dominant in the multivariate variation. PLSDA sustained these observations, with the first two components jointly explaining 98.1%, 96.6%, and, respectively, 88.3% of the variance.
The multivariate statistical analyses applied to the UV-Vis spectra of aronia stem and fruit extracts emphasize that the extraction technique, particularly when combined with appropriate solvents, enhances the recovery of bioactive compounds. The discrimination among mineral waters suggests that ionic composition and mineral content influence the extraction performance, particularly for phenolic acids. This effect is more prominent in targeted spectral regions than for the full UV-Vis range, reinforcing the importance of chemically selective analysis.
3.2.3. HPTLC Analysis
In addition to the spectrophotometric analysis, HPTLC testing of the aronia fruit and stem extracts was also performed. HPTLC is a relatively simple, inexpensive, and reproducible testing method that can provide essential information on the comparative compositional quality of plant extracts.
HPTLC silica gel plates were developed with toluene:ethyl acetate:ethanol:formic acid (20:12:8:4 v/v/v/v), and then the chromatograms were scanned at 254 nm and 366 nm.
The captured HPTLC plate images were converted into densitograms.
From the qualitative profiles of the aronia stem extracts (Figure 10), it can be seen that the extraction fingerprint using natural mineral waters as solvents is similar to the case of using classic solvents, such as distilled water or hydroalcoholic mixture.
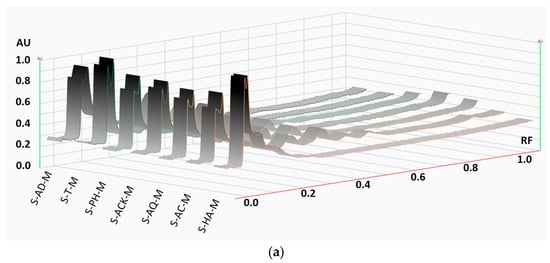
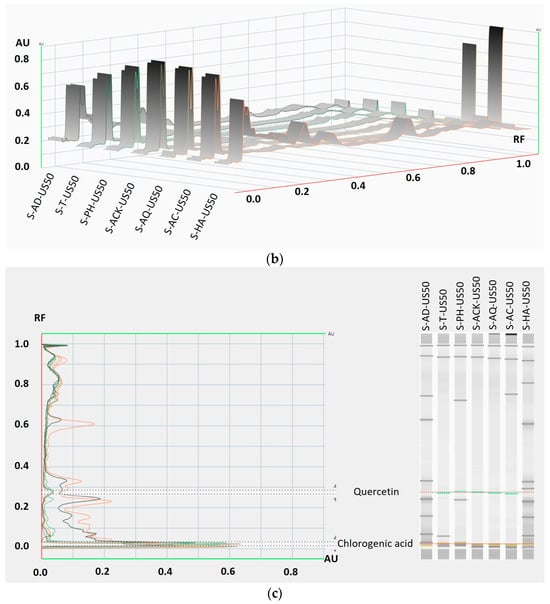
Figure 10.
HPTLC profile of aronia stem extracts at 366 nm: densitograms of the stem extracts obtained by (a) maceration, (b) ultrasonication at 50 °C, and (c) the tracks of 7 extracts’ US50 bands attributed to quercetin and chlorogenic acid, compared to reference compound mixtures.
According to other studies [54], it must be noted that both the extraction conditions and the nature of the solvent influence the profile of aronia extracts, and among the representative polyphenolic compounds, the assignment of bands was performed using the standards for quercetin and chlorogenic acid [13,14,55,56].
3.3. The Influence of Solvents and Experimental Methodology on the Extraction Process of Bioactive Compounds
The results obtained from spectrophotometric and chromatographic analyses highlight the fact that regardless of the working technique used (M or US), the efficiency of the extraction process in the presence of natural mineral waters is superior to distilled water in most cases for both stems and fruits.
FTIR and UV-Vis analyses were employed to qualitatively compare spectral profiles and identify characteristic absorption bands, rather than to perform quantitative spectral analysis. The obtained spectra showed a high degree of overlap, with only negligible variations in band position and relative intensity. This fact visually demonstrates the reproducibility of the extraction and analytical procedures and the qualitative similarity among replicates.
The best performance recorded for all extracts using the hydroalcoholic mixture (water/ethanol: 40/60 v/v) confirms the increased capacity to solubilize polyphenolic compounds in such environments. However, the use of ultrasound brings the extraction capacity of some mineral waters (ACK, AC, and T) closer to that of HA.
Thus, in the case of stems, maceration proved to be more efficient, followed by USRT for all natural mineral waters, while under US50 conditions only two out of the five mineral waters (AC and T) showed an extraction capacity superior to distilled water, with the other three (ACK, AQV, and PH) having behavior closer to that of AD.
In contrast, for fruits, the efficiency of the extraction process under the action of ultrasound and temperature (US50) is noteworthy, followed by M and USTA. In all working conditions, of the five mineral waters, Tușnad water proved to be the most efficient solvent.
Regarding the behavior of different waters used in the extraction of bioactive compounds, previous studies have reported that the presence of alkaline and alkaline earth metal cations in small quantities, respectively, low-mineralized waters, caused an increase in extraction efficiency [24,25]. Physicochemical parameters, such as pH, electrical conductivity (EC), total dissolved solids (TDSs), and salinity, jointly reflect the ionic strength and buffering capacity of the solvent, which can influence solvent–solute interactions, cell wall permeability, and the stability of the extracted compounds. Polyphenols are known to be sensitive to pH variations, with an acidic environment being favorable and known as limiting the oxidative degradation.
The pH also influences, both qualitatively and quantitatively, the extraction process of polyphenolic compounds, with a low pH value stabilizing the concentration of polyphenols [26,57].
Although the initial pH values of all the solvents used ranged from acidic to alkaline (T: 5.60, AD: 6.43, HA: 6.76, PH: 7.29, AC: 7.77, ACK: 8.10, and AQV: 9.41), the pH of the resulting extracts converged toward acidic (AQV, AC, ACK, and PH), and distilled water and hydroethanolic mixture were acidic (4.44–5.99) or neutral values (6.70–7.77 for the carbonated mineral water—T), which may have contributed to the preservation of phenolic structures during extraction.
According to these results, all non-carbonated natural waters with low mineral content presented a positive influence on the extraction process (ACK ≥ AC > AQV ≥ PH) compared with distillated water regardless of the methodology of extraction and plant material used. Even if the initial pH range of these waters was 7.29–9.41, the pH values of the obtained extracts varied between 4.87 and 5.81.
The increase of EC, TDSs, and SAL after extraction is an indicator for the solubilization of ionic species and plant-derived compounds [23,24]. Distilled water and the hydroalcoholic mixture exhibited the largest relative increases in conductivity due to their initially low ionic content, while for natural mineral waters moderate variations were observed, suggesting a buffering effect associated with their intrinsic mineral composition.
The Pearson correlation coefficients calculated for the physicochemical parameters (pH, EC, TDSs, and SAL) helped us to create an image regarding the relationship between these indicators during the extraction process. The efficiency of the electrochemical method, for monitoring such closely related parameters as EC, the amount of TDSs, and salinity, for which the Pearson correlation coefficient value was close to unit for all extracts obtained, respectively, EC–TDS (over 0.99) and EC–SAL (over 0.95), should be noted.
Differences detected among the natural mineral waters can be linked to the mineralization level and ionic composition. Thus, the presence of ions, such as calcium, magnesium, and bicarbonate, may enhance the extraction of phenolic compounds by modifying ionic strength, facilitating mass transfer, or stabilizing phenolic structures through weak complexation mechanisms [24,25,52]. The comparatively better extraction behavior observed for Tușnad water, characterized by medium mineralization and dissolved CO2, may result from a combined effect of mineral content, buffering capacity, and pH modulation.
It should be emphasized that since the present study provides qualitative and comparative evidence, a quantitative assessment of the contribution of individual ions remains to be carried out to confirm the above hypothesis.
4. Conclusions
In the context of sustainable development, the use of plant by-products through the superior valorization of their bioactive compounds and green extraction methods is essential.
The extraction of biologically active compounds from aronia by-products was evaluated in the present study in terms of its dependence on the level of mineralization of the natural mineral waters used as solvents, and the Pearson correlation coefficients were calculated for physicochemical parameters (pH, EC, TDSs, and SAL).
The results obtained from UV-Vis, FTIR, and HPTLC analyses revealed that the similar phytochemical profiles of both the stem and fruit extracts indicates a good extraction capacity in the case of natural mineral waters, most of the time superior to distilled water, regardless of the extraction conditions (maceration and ultrasonication at room temperature). For ultrasonication at 50 °C, only two out of the five mineral waters (AC and T) showed an extraction capacity superior to distilled water.
Moreover, this study highlighted the potential of combining spectroscopic analysis techniques (UV-Vis and FTIR) with chromatographic (HPTLC) and electrochemical ones to obtain the phytochemical fingerprint of the extracts. The existence of a significant content of active principles in the stem extracts was also reconfirmed.
The efficiency of the ultrasonic extraction technique was likewise observed, with the extraction process taking place with very good results, in a short time (30 min), at RT for stems and at 50 °C for fruits, compared to those obtained after 24 h by maceration.
It was also noted that Tușnad water, carbonated and with a medium mineral content, was the most efficient extraction solvent among all the waters analyzed.
The absence of organic solvent residues, combined with the food-grade nature of the solvent, allows the resulting extracts to be potentially used directly in food, nutraceutical, or cosmetic formulations. The approach proposed for extracting bioactive compounds from aronia fruits and stems is in agreement with clean-label and sustainability principles intensively promoted in food and ingredient development.
Given the different behaviors of the analyzed waters depending on the nature of the plant material used (stems or fruits), additional studies are required in order to confirm the extraction properties of the respective natural mineral waters.
Overall, it can be concluded that natural mineral waters represent a promising alternative to conventional solvents, particularly for the valorization of plant by-products intended for food-related applications. The use of these waters contributes simultaneously to a greener extraction process and to the production of greener extracts.
Author Contributions
Conceptualization, A.-L.F., I.-L.I. and O.-I.P.; methodology, A.-L.F., I.-L.I. and O.-I.P.; software, C.-G.G. and I.-L.I.; formal analysis, I.-L.I., O.-I.P. and C.-G.G.; investigation, I.A., I.-L.I., O.-I.P., A.-L.F. and C.-G.G.; writing—original draft preparation, A.-L.F., I.-L.I. and O.-I.P.; writing—review and editing, I.-L.I., O.-I.P., A.-L.F. and C.-G.G.; visualization, I.-L.I., O.-I.P. and C.-G.G.; supervision, A.-L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Dobros, N.; Zielińska, A.; Siudem, P.; Zawada, K.D.; Paradowska, K. Profile of bioactive components and antioxidant activity of Aronia melanocarpa fruits at various stages of their growth, using chemometric methods. Antioxidants 2024, 13, 462. [Google Scholar] [CrossRef]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa products and by-products for health and nutrition: A review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, R.; Dong, J.; Wang, Y.; Huang, X.; Chen, C. Research on the extraction, purification and determination of chemical components, biological activities, and applications in diet of black chokeberry (Aronia melanocarpa). Chin. J. Anal. Chem. 2023, 51, 100301. [Google Scholar] [CrossRef]
- Xu, J.; Li, F.; Zheng, M.; Sheng, L.; Shi, D.; Song, K. A comprehensive review of the functional potential and sustainable applications of Aronia melanocarpa in the food industry. Plants 2024, 13, 3557. [Google Scholar] [CrossRef]
- Jelska, A.; Polecka, A.; Zahorodnii, A.; Olszewska, E. The role of oxidative stress and the potential therapeutic benefits of Aronia melanocarpa supplementation in obstructive sleep apnea syndrome: A comprehensive literature review. Antioxidants 2024, 13, 1300. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, B.-S.; Oprea, O.C.; Negreanu-Pirjol, T.; Roncea, F.N.; Prelipcean, A.-M.; Craciunescu, O.; Iosageanu, A.; Artem, V.; Ranca, A.; Motelica, L.; et al. Health benefits of antioxidant bioactive compounds in the fruits and leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot. Antioxidants 2023, 12, 951. [Google Scholar] [CrossRef]
- Chamberlin, M.L.; Peach, J.T.; Wilson, S.M.G.; Miller, Z.T.; Bothner, B.; Walk, S.T.; Yeoman, C.J.; Miles, M.P. Polyphenol-rich Aronia melanocarpa fruit beneficially impact cholesterol, glucose, and serum and gut metabolites: A randomized clinical trial. Foods 2024, 13, 2768. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Mašković, P.; Đurović, S.; Zengin, G.; Delerue-Matos, C.; Lozano-Sánchez, J.; Jakšić, A. Chemical and biological insights on aronia stems extracts obtained by different extraction techniques: From wastes to functional products. J. Supercrit. Fluids 2017, 128, 173–181. [Google Scholar] [CrossRef]
- Cvetanović, A.; Zengin, G.; Zeković, Z.; Švarc-Gajić, J.; Ražić, S.; Damjanović, A.; Mašković, P.; Mitić, M. Comparative in vitro studies of the biological potential and chemical composition of stems, leaves and berries Aronia melanocarpa’s extracts obtained by subcritical water extraction. Food Chem. Toxicol. 2018, 121, 458–466. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Cerdà, V.; Clavijo, S.; Suárez, R.; Zengin, G.; Cvetanović, A. Chemical and bioactivity screening of subcritical water extracts of chokeberry (Aronia melanocarpa) stems. J. Pharm. Biomed. Anal. 2019, 164, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Carciochi, R.A.; D’Alessandro, L.G.; Vauchel, P.; Rodriguez, M.M.; Nolasco, S.M.; Dimitrov, K. Valorization of agrifood by-products by extracting valuable bioactive compounds using green processes. In Ingredients Extraction by Physicochemical Methods in Food; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Academic Press Inc.: Oxford, UK, 2017; Volume 4, pp. 191–228. [Google Scholar] [CrossRef]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green extraction techniques of bioactive compounds: A state-of-the-art review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Shahbaz, M.; Riaz, M.; Momal, U.; Rasool, I.F.U.; Naeem, H.; Raza, N.; Moreno, A.; Khalid, W.; Esatbeyoglu, T. Green solvent extraction and eco-friendly novel techniques of bioactive compounds from plant waste: Applications, future perspective and circular economy. Appl. Food Res. 2025, 5, 101434. [Google Scholar] [CrossRef]
- Herrero, M. Towards green extraction of bioactive natural compounds. Anal. Bioanal. Chem. 2024, 416, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, D.; Lante, A. Water an eco-friendly crossroad in green extraction: An overview. Open Biotechnol. J. 2019, 13, 155–162. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Puzovic, A.; Mikulic-Petkovsek, M. Comparative evaluation of conventional and emerging maceration techniques for enhancing bioactive compounds in aronia juice. Foods 2024, 13, 3255. [Google Scholar] [CrossRef]
- Korotkova, I.; Romashko, T.; Khakhel’, O.; Zvenihorodska, T.; Yaprynets, T.S.; Liashenko, V. Effect of the water origin on the biological properties of sage (Salvia officinalis L.) aqueous extracts. J. Multidiscip. Appl. Nat. Sci. 2025, 5, 74–86. [Google Scholar] [CrossRef]
- Wyrostek, J.; Kowalski, R. The effect of water mineralization on the extraction of active compounds from selected herbs and on the antioxidant properties of the obtained brews. Foods 2021, 10, 1227. [Google Scholar] [CrossRef]
- Gomes, C.P.; Oliveira, A.S.; Rolo, J.; da Silveira, T.F.F.; Palmeira de Oliveira, R.; Alves, M.J.; Plasencia, P.; Palmeira de Oliveira, A. Natural mineral water–plant extract combinations as potential anti-aging ingredients: An in vitro evaluation. Cosmetics 2025, 12, 113. [Google Scholar] [CrossRef]
- Chethan, S.; Malleshi, N.G. Finger millet polyphenols: Optimization of extraction and the effect of pH on their stability. Food Chem. 2007, 105, 862–870. [Google Scholar] [CrossRef]
- Available online: https://food.ec.europa.eu/document/download/ec4fbcc0-7185-4dce-820a-27f7e2653dad_en?filename=labelling-nutrition_mineral-waters_list_eu-recognised.pdf (accessed on 20 October 2025).
- Misaila, L.; Barsan, N.; Raducanu, D.; Grosu, L.; Patriciu, O.-I.; Alexa, I.-C.; Finaru, A.-L. Eco-friendly and efficient monitoring of physico-chemical parameters of some mineral water from Slanic Moldova (Romania) during storage in different conditions—A case study. Ovidius Univ. Ann. Chem. 2023, 34, 72–82. [Google Scholar] [CrossRef]
- Misaila, L.; Raducanu, D.; Barsan, N.; Alexa, I.-C.; Patriciu, O.-I.; Grosu, L. Assessment of the microbiota of some mineral water form Slanic Moldova (Romania): Seasonal evaluation and after storage in different conditions. Sci. Stud. Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2023, 24, 97–110. [Google Scholar]
- Misaila, L.; Barsan, N.; Nedeff, F.M.; Raducanu, D.; Radu, C.; Grosu, L.; Patriciu, O.-I.; Gavrila, L.; Finaru, A.-L. Perspectives for quality evaluation of some mineral waters from Slanic Moldova. Water 2022, 14, 2942. [Google Scholar] [CrossRef]
- Roșcan, A.G.; Ifrim, I.-L.; Patriciu, O.-I.; Fînaru, A.-L. Exploring the therapeutic value of some vegetative parts of Rubus and Prunus: A literature review on bioactive profiles and their pharmaceutical and cosmetic interest. Molecules 2025, 30, 3144. [Google Scholar] [CrossRef]
- Sandu-Bălan (Tăbăcariu), A.; Ifrim, I.-L.; Patriciu, O.-I.; Ștefănescu, I.-A.; Fînaru, A.-L. Walnut by-products and elderberry extracts—Sustainable alternatives for human and plant health. Molecules 2024, 29, 498. [Google Scholar] [CrossRef]
- Nicută, D.; Grosu, L.; Alexa, I.-C.; Fînaru, A.-L. Sustainable characterization of some extracts of Origanum vulgare L. and biosafety evaluation using Allium cepa assay. Horticulturae 2024, 10, 504. [Google Scholar] [CrossRef]
- Rosales, T.K.O.; Fabi, J.P. Valorization of polyphenolic compounds from food industry by-products for application in polysaccharide-based nanoparticles. Front. Nutr. 2023, 10, 1144677. [Google Scholar] [CrossRef]
- European Union. Directive 2009/54/EC of the European Parliament and of the Council of 18 June 2009 on the exploitation and marketing of natural mineral waters. Off. J. Eur. Union 2009, 164, 45–58. Available online: http://data.europa.eu/eli/dir/2009/54/oj (accessed on 30 May 2025).
- Reich, E.; Schibli, A. High-Performance Thin-Layer Chromatography for the Analysis of Medicinal Plants; Thieme Medical Publisher: New York, NY, USA, 2007; pp. 160–174. [Google Scholar]
- Jug, U.; Glavnik, V.; Kranjc, E.; Vovk, I. HPTLC–densitometric and HPTLC–MS methods for analysis of flavonoids. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 329–341. [Google Scholar] [CrossRef]
- Asfaw, T.B.; Tadesse, M.G.; Tessema, F.B.; Woldemariam, H.W.; Legesse, B.A.; Esho, T.B.; Bachheti, A.; AL-Huqail, A.A.; Taher, M.A.; Zouidi, F.; et al. Optimization, identification, and quantification of selected phenolics in three underutilized exotic edible fruits using HPTLC. Sustainability 2023, 15, 9568. [Google Scholar] [CrossRef]
- Singirala, S.K.; Dubey, P.K.; Roy, S. Extraction of bioactive compounds from Withania somnifera: The biological activities and potential application in the food industry: A review. Int. J. Food Sci. 2025, 2025, 9922626. [Google Scholar] [CrossRef] [PubMed]
- Ifrim, I.L.; Suceveanu, E.-M.; Ștefănescu, I.-A. The impact of ultrasound treatment on the quality of different wine varieties. Sci. Stud. Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2024, 25, 301–311. [Google Scholar] [CrossRef]
- Pavlović, A.N.; Brcanović, J.M.; Veljković, J.N.; Mitić, S.S.; Tošic, S.B.; Kaličanin, B.M.; Kostić, D.A.; Ðorđević, M.S.; Velimirović, D.S. Characterization of commercially available products of aronia according to their metal content. Fruits 2015, 70, 385–393. [Google Scholar] [CrossRef]
- Corciovă, A.; Mircea, C.; Fifere, A.; Turin-Moleavin, I.-A.; Roşca, I.; Macovei, I.; Ivănescu, B.; Vlase, A.-M.; Hăncianu, M.; Burlec, A.F. Biogenic synthesis of silver nanoparticles mediated by Aronia melanocarpa and their biological evaluation. Life 2024, 14, 1211. [Google Scholar] [CrossRef]
- Ćujić, N.; Trifković, K.; Bugarski, B.; Ibrić, S.; Pljevljakušić, D.; Šavikin, K. Chokeberry (Aronia melanocarpa L.) extract loaded in alginate and alginate/inulin system. Ind. Crops Prod. 2016, 86, 120–131. [Google Scholar] [CrossRef]
- Donmez, S. Phyto-mediated synthesis of zinc oxide nanoparticles using black chokeberry fruit (Aronia melanocarpa L.) extracts for promising antioxidant, antibacterial, antidiabetic, and photocatalytic activities. J. Clust. Sci. 2025, 36, 119. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Yu, H.; Li, R.-Y.; Wang, R.-Q.; Wang, R.-J.; Zhang, Z.-R.; Jiang, G.-Q. Green and efficient extraction of polyphenols from Aronia melanocarpa using deep eutectic solvents. Microchem. J. 2024, 207, 112228. [Google Scholar] [CrossRef]
- Tirla, A.; Timar, A.V.; Becze, A.; Memete, A.R.; Vicas, S.I.; Popoviciu, M.S.; Cavalu, S. Designing new sport supplements based on Aronia melanocarpa and bee pollen to enhance antioxidant capacity and nutritional value. Molecules 2023, 28, 6944. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H. Effect of biomordanting with Aronia melanocarpa leaf extract on coloring and functionalizing of wool and cotton fabrics dyed with A. melanocarpa fruit extract. Polym. Bull. 2024, 81, 9235–9251. [Google Scholar] [CrossRef]
- Boggia, R.; Casolino, M.C.; Hysenaj, V.; Oliveri, P.; Zunin, P. A screening method based on UV–Visible spectroscopy and multivariate analysis to assess addition of filler juices and water to pomegranate juices. Food Chem. 2013, 140, 735–741. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Petreska Stanoeva, J.; Damjanovski, V.; Cichna-Markl, M.; Stefova, M. Anthocyanin fingerprinting as an authentication testing tool for blueberry, aronia, and pomegranate juices. Eur. Food Res. Technol. 2024, 250, 751–762. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Rodríguez-Werner, M.; Winterhalter, P. Fractionation and isolation of polyphenols from Aronia melanocarpa by countercurrent and membrane chromatography. Eur. Food Res. Technol. 2017, 243, 1261–1275. [Google Scholar] [CrossRef]
- Borowska, S.; Tomczyk, M.; Strawa, J.W.; Brzóska, M.M. Estimation of the chelating ability of an extract from Aronia melanocarpa L. berries and its main polyphenolic ingredients towards ions of zinc and copper. Molecules 2020, 25, 1507. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Wen, R. Kinetic study of the thermal stability of tea catechins in aqueous systems using a microwave reactor. J. Agric. Food Chem. 2006, 54, 5924–5932. [Google Scholar] [CrossRef]
- Pirvu, L.; Panteli, M.; Rasit, I.; Grigore, A.; Bubueanu, C. The leaves of Aronia melanocarpa L. and Hippophae rhamnoides L. as source of active ingredients for biopharmaceutical engineering. Agric. Agric. Sci. Procedia 2015, 6, 593–600. [Google Scholar] [CrossRef]
- Stanek, N.; Misiak, I.J. HPTLC Phenolic Profiles as useful tools for the authentication of honey. Food Anal. Methods 2018, 11, 2979–2989. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Plaza, M.; Marina, M.L. High-performance thin-layer chromatography and direct analysis in real time-high resolution mass spectrometry of non-extractable polyphenols from tropical fruit peels. Food Res. Int. 2021, 147, 110455. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Ma, M.; Li, C.; Luo, L. Stability of tea polyphenols solution with different pH at different temperatures. Int. J. Food Prop. 2017, 20, 1–18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.