The Health Impact of Cocoa from Cultivation to the Formation of Biogenic Amines: An Updated Review
Abstract
1. Introduction
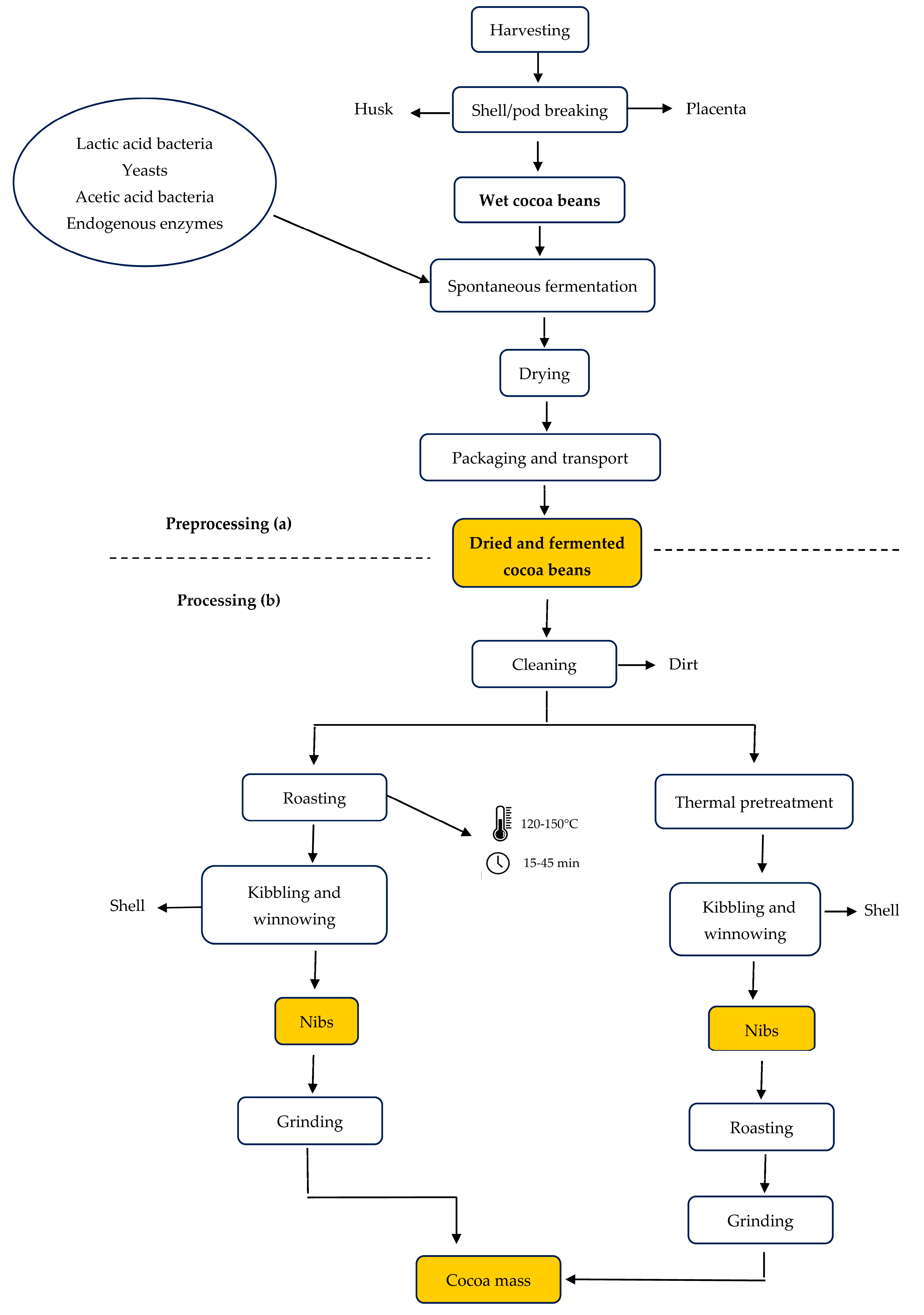
2. Cocoa Bean Processing
- -
- cocoa butter contains not more than 1.75% free fatty acid content (expressed as oleic acid) and not more than 0.5 except in the case of press cocoa butter, where it shall not be more than 0.35% unsaponifiable matter (determined using petroleum ether);
- -
- cocoa powder contains not less than 20% cocoa butter, calculated according to the weight of the dry matter and not more than 9% water;
- -
- chocolate contains not less than 35% total dry cocoa solids, including not less than 18% cocoa butter and not less than 14% of dry non-fat cocoa solids.
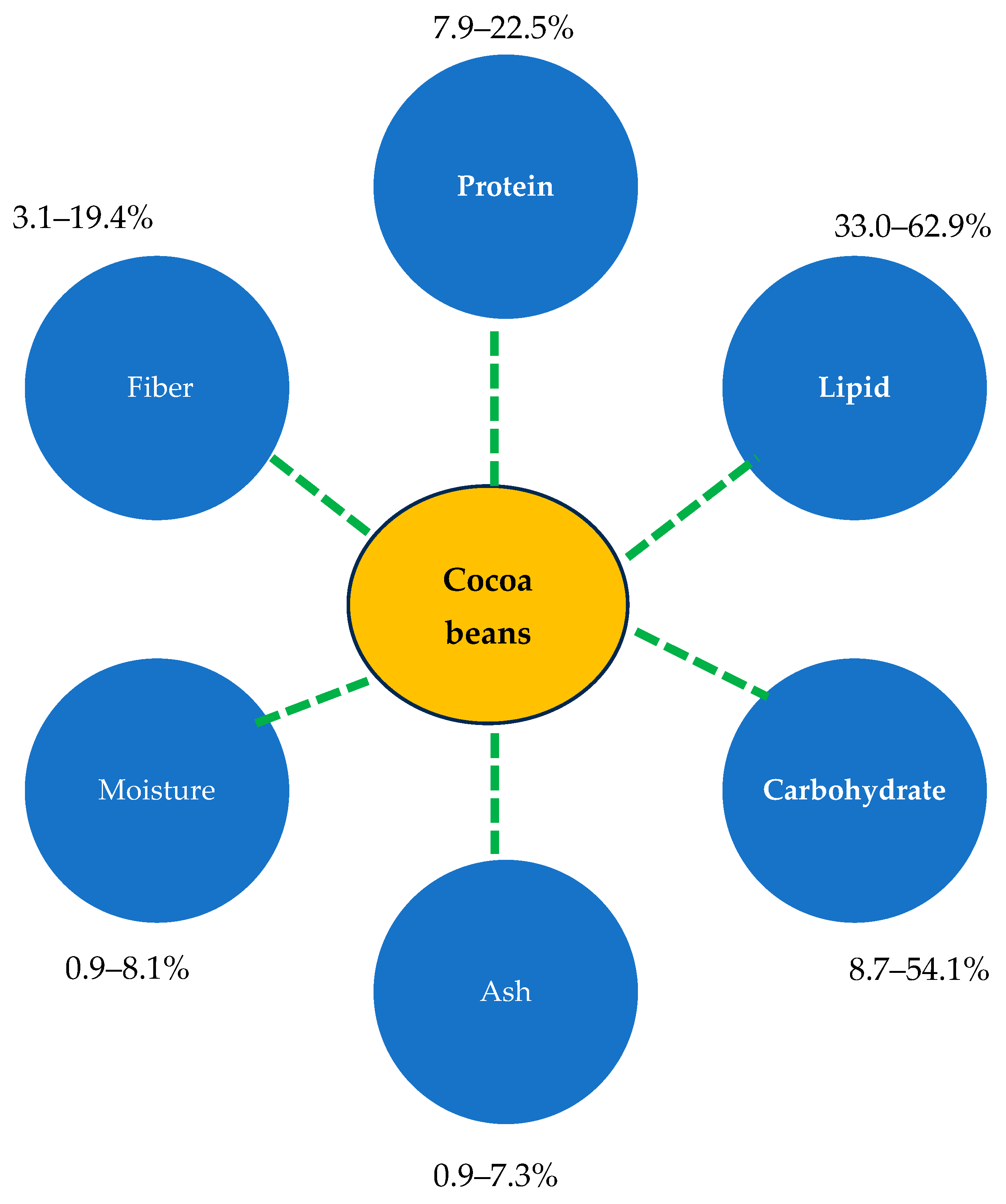
3. Chemical Composition of Cocoa Beans
- Theobromine and Caffeine
- Polyphenols
4. Health Benefits and Potential Risks of Cocoa Products
5. Occurrence and Role of Biogenic Amines in Cocoa Products
6. Formation of Biogenic Amines During Cocoa Fermentation
7. Limits and Standards for Biogenic Amines in Cocoa Products
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- dos Santos, F.C.G.; Fontes, M.J.V. Do cacau ao chocolate: Estudo de caso em pequena indústria familiar da cadeia de valor do cacau no território litoral sul da Bahia—TLS. Rev. Livre Sustentabiliade Empreendedorismo 2020, 5, 196–216. [Google Scholar]
- Castro-Alayo, E.M.; Idrogo-Vásquez, G.; Siche, R.; Cardenas-Toro, F.P. Formation of aromatic precursors during fermentation of Criollo and Forastero cocoa. Heliyon 2019, 5, e01157. [Google Scholar] [CrossRef] [PubMed]
- Żyżelewicz, D.; Budryn, G.; Oracz, J.; Antolak, H.; Kręgiel, D.; Kaczmarska, M. The effect on bioactive components and characteristics of chocolate by functionalization with raw cocoa beans. Food Res. Int. 2018, 113, 234–244. [Google Scholar] [CrossRef]
- Kongor, J.E.; Owusu, M.; Oduro-Yeboah, C. Cocoa production in the 2020s: Challenges and solutions. CABI A&B 2024, 5, 102. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Quao, J.; Budu, A.S.; Takrama, J.; Saalia, F.K. Effect of pulp preconditioning on acidification, proteolysis, sugars and free fatty acids concentration during fermentation of cocoa (Theobroma cacao) beans. Int. J. Food Sci. Nutr. 2011, 62, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Hurko-Romeyko, I.; Kowalska, J.; Pochitskaya, I. Cocoa powder as source of phenolic compounds, determining factors—A review. Postępy Tech. Przetwórstwa Spożywczego 2020, 30, 117–125. [Google Scholar]
- Kongor, J.E.; Hinneh, M.; Van de Walle, D.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Beg, M.S.; Ahmad, S.; Jan, K.; Bashir, K. Status, supply chain and processing of cocoa—A review. Trends Food Sci. Technol. 2017, 66, 108–116. [Google Scholar] [CrossRef]
- Dillinger, T.L.; Barriga, P.; Escárcega, S.; Jimenez, M.; Salazar Lowe, D.; Grivetti, L.E. Food of goods: Cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J. Nutr. 2000, 130, 2057S–2072S. [Google Scholar] [CrossRef]
- Gavrilova, N.G. Contemporary global production and consumption of cocoa: An assessment. IOP Conf. Ser. Earth Environ. Sci. 2021, 839, 022095. [Google Scholar] [CrossRef]
- Ishaq, S.; Jafri, L. Biomedical importance of cocoa (Theobroma cacao): Significance and potential for the maintenance of human health. Matrix Sci. Pharma 2017, 1, 1–5. [Google Scholar] [CrossRef]
- ICCO (International Cocoa Organization). Quarterly Bulletin of Cocoa Statistics 2023, 49, No. 4, Cocoa 2022/2023. Available online: https://www.icco.org/wp-content/uploads/Production_QBCS-XLIX-No.-4.pdf (accessed on 15 December 2024).
- Anning, A.K.; Ofori-Yeboah, A.; Baffour-Ata, F.; Owusu, G. Climate change manifestations and adaptations in cocoa farms: Perspectives of smallholder farmers in the Adansi South District, Ghana. Curr. Res. Environ. Sustain. 2022, 4, 100196. [Google Scholar] [CrossRef]
- Sparacino, A.; Merlino, V.M.; Bru, F.; Borra, D.; Blanc, S.; Massaglia, S. Corporate social responsibility communication from multinational chocolate companies. Sustain. Futures 2024, 7, 100151. [Google Scholar] [CrossRef]
- Cocoa Prices Surge, Making Chocolate Pricier than Ever Before! 2024. Available online: https://straitsresearch.com/statistic/cocoa-prices-surge-globally-driving-chocolate-costs-higher (accessed on 15 December 2024).
- ICCO (International Cocoa Organization). Quarterly Bulletin of Cocoa Statistics 2024, 50, No. 3. Available online: https://www.icco.org/november-2024-quarterly-bulletin-of-cocoa-statistics/ (accessed on 15 December 2024).
- Shahbandeh, M. Cocoa Industry—Statistics & Facts. Statista 2024. Available online: https://www.statista.com/topics/3211/cocoa-industry/#topicOverview (accessed on 15 December 2024).
- Observatory of Economic Complexity (OEC). Cocoa Beans. 2022. Available online: https://oec.world/en/profile/hs/cocoa-beans (accessed on 15 December 2024).
- Sobiech, M.; Lulińki, P.; Synoradzki, K.; Bednarchuk, T.J.; Janczura, M.; Provorova, V.; Giebułtowicz, J. Implementing magnetic molecularly imprinted solid phase extraction to analytical method for determination of 2-phenethylamine in cocoa powder and chocolate by LC-MS/MS system. Microchem. J. 2024, 205, 111155. [Google Scholar] [CrossRef]
- Deus, V.L.; Resende, L.M.; Bispo, E.S.; Franca, A.S.; Gloria, M.B.A. FTIR and PLS-regression in the evaluation of bioactive amines, total phenolic compounds and antioxidant potential of dark chocolates. Food Chem. 2021, 357, 129754. [Google Scholar] [CrossRef] [PubMed]
- Deus, V.L.; Bispo, E.S.; Franca, A.S.; Gloria, M.B.A. Influence of cocoa clones on the quality and functional properties of chocolate—Nitrogen compounds. LWT 2020, 134, 110202. [Google Scholar] [CrossRef]
- Gloria, M.B.A.; Deus, V.L.; Franca, A. Bioactive amines during cocoa fermentation. In Proceedings of the XXI ENAAL & VII Congresso Latino-Americano de Analistas de Alimentos, Florianópolis, SC, Brasil, 26–39 May 2019; pp. 1–6. [Google Scholar]
- Oracz, J.; Nebesny, E. Influence of roasting conditions on the biogenic amine content in cocoa beans of different Theobroma cacao cultivars. Food Res. Int. 2014, 55, 1–10. [Google Scholar] [CrossRef]
- Thompson, S.O.; Rough, S.L. The densification of cocoa beans shells for bioenergy purposes. Biomass Bioenergy 2021, 148, 106057. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Puerta-Polanco, L.F.; Grande-Tovar, C.D.; Cuervo, R.A.; Navia-Porras, D.P.; Poveda-Perdomo, L.G.; Fernández-Daza, F.F.; Chaves-López, C. Exploring the core microbiota of four different traditional fermented beverages from the Colombian Andes. Fermentation 2022, 8, 733. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Functional role of yeasts, lactic acid bacteria and acetic acid bacteria in cocoa fermentation processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef] [PubMed]
- Suazo, Y.; Davidov-Pardo, G.; Arozarena, I. Effect of fermentation and roasting on the phenolic concentration and antioxidant activity of cocoa from Nicaragua. J. Food Qual. 2014, 37, 50–56. [Google Scholar] [CrossRef]
- Petyaev, I.M.; Bashmakov, Y.K. Cocobiota: Implications for human health. J. Nutr. Metab. 2016, 2016, 7906927. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ospina, J.; Acquaticci, L.; Molina-Hernandez, J.B.; Rantsiou, K.; Martuscelli, M.; Kamgang-Nzekoue, A.F.; Vittori, S.; Paparella, A.; Chaves-López, C. Exploring the capability of yeasts isolated from Colombian fermented cocoa beans to form and degrade biogenic amines in a lab-scale model system for cocoa fermentation. Microorganisms 2021, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Farrera, L.; Colas de la Noue, A.; Strub, C.; Guibert, B.; Kouame, C.; Grabulos, J.; Montet, D.; Teyssier, C. Towards a starter culture for cocoa fermentation by the selection of acetic acid bacteria. Fermentation 2021, 7, 42. [Google Scholar] [CrossRef]
- Rahayu, E.S.; Triyadi, R.; Khusna, R.N.B.; Djaafar, T.F.; Utami, T.; Marwati, T.; Hatmi, R.U. Indigenous yeast, lactic acid bacteria, and acetic acid bacteria from cocoa bean fermentation in Indonesia can inhibit fungal-growth-producing mycotoxins. Fermentation 2021, 7, 192. [Google Scholar] [CrossRef]
- Domínguez-Pérez, L.A.; Beltrán-Barrientos, L.M.; González-Córdova, A.F.; Hernández-Mendoza, A.; Vallejo-Cordoba, B. Artisanal cocoa bean fermentation: From cocoa bean proteins to bioactive peptides with potential health benefits. J. Funct. Foods 2020, 73, 104134. [Google Scholar] [CrossRef]
- Illeghems, K.; Weckx, S.; De Vuyst, L. Applying meta-pathway analyses through metagenomics to identify the functional properties of the major bacterial communities of a single spontaneous cocoa bean fermentation process sample. Food Microbiol. 2015, 50, 54–63. [Google Scholar] [CrossRef]
- Christian, K.A.; Houphouet, K.R.R.; Koné, M.K.M.; Fontana, A.; Noël, D.; Montet, D.; Guéhi, T.S.S. Effect of the reduction of ochratoxin A and free fatty acids production in dry fermented cocoa beans using Bacillus sp. on the sensory quality of chocolate produced thereof. In Current Perspectives in Agriculture and Food Science; B P International: Hong Kong, China, 2024; Volume 7, pp. 121–141. [Google Scholar] [CrossRef]
- Díaz-Muñoz, C.; Van der Voorde, D.; Tuenter, E.; Lemarcq, V.; Van de Walle, D.; Soares Maio, J.P.; Mencía, A.; Hernandez, C.E.; Comasio, A.; Sioriki, E.; et al. An in-depth multiphasic analysis of the chocolate production chain, from bean to bar, demonstrates the superiority of Saccharomyces cerevisiae over Hanseniaspora opuntiae as functional starter culture during cocoa fermentation. Food Microbiol. 2023, 109, 104115. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, G.V.; Soccol, V.T.; Soccol, C.R. Current state of research on cocoa and coffee fermentations. Curr. Opin. Food Sci. 2016, 7, 50–57. [Google Scholar] [CrossRef]
- Díaz-Muñoz, C.; Van De Voorde, D.; Comasio, A.; Verce, M.; Hernandez, C.E.; Weckx, S.; De Vuyst, L. Curing of cocoa beans: Fine-scale monitoring of the starter cultures applied and metabolomics of the fermentation and drying steps. Front. Microbiol. 2021, 11, 616875. [Google Scholar] [CrossRef] [PubMed]
- da Veiga Moreira, I.M.; de Figueiredo Vilela, L.; da Cruz Pedroso Miguel, M.; Santos, C.; Lima, N.; Freitas Schwan, R. Impact of a microbial cocktail used as a starter culture on cocoa fermentation and chocolate flavor. Molecules 2017, 22, 766. [Google Scholar] [CrossRef] [PubMed]
- Koné, M.K.; Guéhi, S.T.; Durand, N.; Ban-Koffi, L.; Berthiot, L.; Tachon, A.F.; Brou, K.; Boulanger, R.; Montet, D. Contribution of predominant yeasts to the occurrence of aroma compounds during cocoa bean fermentation. Food Res. Int. 2016, 89, 910–917. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Zhao, J.; Fleet, G. Yeasts are essential for cocoa bean fermentation. Int. J. Food Microbiol. 2014, 174, 72–87. [Google Scholar] [CrossRef]
- Crafack, M.; Mikkelsen, M.B.; Saerens, S.; Knudsen, M.; Blennow, A.; Lowor, S.; Takrama, J.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Influencing cocoa flavour using Pichia Kluyvery and Kluyveromyces marxianus in a defined mixed starter culture for cocoa fermentation. Int. J. Food Microbiol. 2013, 167, 103–116. [Google Scholar] [CrossRef]
- Leal, G.A., Jr.; Gomes, L.H.; Efraim, P.; de Almeida Tavares, F.C.; Figueira, A. Fermentation of cacao (Theobroma cacao L.) seeds with a hybrid Kluyveromyces marxianus strain improved product quality attributes. FEMS Yeast Res. 2008, 8, 788–798. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, A.B.; da Cruz, M.L.; de Souza Oliveira, F.A.; Soares, S.E.; Druzian, J.I.; de Santana, L.R.R.; de Souza, C.O.; da Silva Bispo, E. Influence of under-fermented cocoa mass in chocolate production: Sensory acceptance and volatile profile characterization during the processing. LWT 2021, 149, 112048. [Google Scholar] [CrossRef]
- Gertner, J. Eat chocolate, live longer? New York Times, 10 October 2004. Available online: https://www.nytimes.com/2004/10/10/magazine/eat-chocolate-live-longer.html (accessed on 15 December 2024).
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- do Carmo Brito, B.D.N.; Campos Chisté, R.; da Silva Pena, R.; Abrue Gloria, M.B.; Santos Lopes, A. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chem. 2017, 228, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Batista, N.N.; de Andrade, D.P.; Ramos, C.L.; Dias, D.R.; Schwan, F.R. Antioxidant capacity of cocoa beans and chocolate assessed by FTIR. Food Res. Int. 2016, 90, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, C.; Martuscelli, M.; Sacchetti, G.; Beheydt, B.; Mastrocola, D.; Pittia, P. Effect of different conching processes on procyanidins content and antioxidant properties of chocolate. Food Res. Int. 2014, 63, 367–372. [Google Scholar] [CrossRef]
- Di Mattia, C.D.; Sacchetti, G.; Mastrocola, D.; Serafini, M. From cocoa to chocolate: The impact of processing on in vitro antioxidant activity and the effects of chocolate on antioxidant markers in vivo. Front. Immunol. 2017, 8, 1207. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Ieri, F.; Campo, M.; Paolino, D.; Restuccia, D.; Romani, A. Biogenic amines, phenolic, and aroma-related compounds of unroasted and roasted cocoa beans with different origin. Foods 2019, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Farah, D.M.H.; Zaibunnisa, A.H.; Misnawi, J. Optimization of cocoa beans roasting process using response surface methodology based on concentration of pyrazine and acrylamide. Int. Food Res. J. 2012, 19, 1355–1359. [Google Scholar]
- Mota-Gutierrez, J.; Botta, C.; Ferrocino, I.; Giordano, M.; Bertolino, M.; Dolci, P.; Canno, M.; Cocolin, L. Dynamica and biodiversity of bacterial and yeasts communities during fermentation of cocoa beans. Appl. Environ. Microbiol. 2018, 84, e01164-18. [Google Scholar] [CrossRef]
- Zahouli, G.I.B.; Guehi, S.T.; Fae, A.M.; Ban-Koffi, L.; Nemlin, J.G. Effect of drying methods on the chemical quality traits of cocoa raw material. Adv. J. Food Sci. Technol. 2010, 2, 184–190. [Google Scholar]
- Beckett, S.T. The Science of Chocolate, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2008; ISBN 978-0-85404-970-7. [Google Scholar]
- Ioannone, F.; Di Mattia, C.D.; De Gregorio, M.; Sergi, M.; Serafini, M.; Sacchetti, G. Flavanols, proanthocyanidins and antioxidant activity changes during cocoa (Theobroma cacao L.) roasting as affected by temperature and time of processing. Food Chem. 2015, 174, 256–262. [Google Scholar] [CrossRef]
- Krysiak, W.; Adamski, R.; Żyżelewicz, D. Factors affecting the color of roasted cocoa bean. J. Food Qual. 2013, 36, 21–31. [Google Scholar] [CrossRef]
- 2000/36/EC; Directive 2000/36/EC of the European Parliament and of the Council of 23 June 2000 Relating to Cocoa and Chocolate Products Intended for Human Consumption. European Parliament and of the Council: Luxembourg, 2000.
- Soares, T.F.; Oliveira, M.B.P.P. Cocoa by-products: Characterization of bioactive compounds and beneficial health effects. Molecules 2022, 27, 1625. [Google Scholar] [CrossRef] [PubMed]
- Salazar, E.; Valenzuela, R.; Aguilar, M.; Aranda, N.; Sotelo, A.; Chire-Fajardo, G.C.; Ureña, M. Physicochemical properties and microbial group behavior of postharvest Peruvian cocoa bean (Theobroma cacao L.). Enfoque Ute 2020, 11, 48–56. [Google Scholar] [CrossRef]
- Djikeng, F.T.; Teyomnou, W.T.; Tenyang, N.; Tiencheu, B.; Morfor, A.T.; Touko, B.A.H.; Houketchang, S.N.; Boungo, G.T.; Karuna, M.S.L.; Ngoufack, F.Z.; et al. Effect of traditional and oven roasting on the physicochemical properties of fermented cocoa beans. Heliyon 2018, 4, e00533. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Fisk, I.D. Total lipid prediction in single intact cocoa beans by hyperspectral chemical imaging. Food Chem. 2021, 344, 128663. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, D.; Corno, M.; Ulrich, M.S.; Kuhnert, N. Characterization of triacylglycerol profiles in unfermented and dried fermented cocoa beans of different origins. Food Res. Int. 2018, 111, 361–370. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Wang, S.; Zhu, F. Chemical and biological properties of cocoa beans affected by processing: A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 8403–8434. [Google Scholar] [CrossRef] [PubMed]
- Barišić, V.; Kopjar, M.; Jozinović, A.; Flanjak, I.; Aćkar, D.; Miličević, B.; Šubarić, D.; Jokić, S.; Babić, J. The chemistry behind chocolate production. Molecules 2019, 24, 3163. [Google Scholar] [CrossRef]
- Santader Muñoz, M.; Rodríguez Cortina, R.; Vaillant, F.E.; Escobar, P.S. An overview of the physical and biochemical transformation of cocoa seeds to beans and to chocolate: Flavor formation. Crit. Rev. Food Sci. Nutr. 2020, 60, 1593–1613. [Google Scholar] [CrossRef] [PubMed]
- Caligiani, A. Cocoa: Production, chemistry, and use. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 185–190. ISBN 9780123849533. [Google Scholar]
- Bandeira, C.M.; Evangelista, W.P.; Gloria, M.B.A. Bioactive amines in fresh, canned and dried sweet corn, embryo and endosperm and germinated corn. Food Chem. 2012, 131, 1355–1359. [Google Scholar] [CrossRef]
- Silva, G.S.; Dala-Paula, B.; Bispo, E.S.; Gloria, M.B.A. Bioaccessibility of bioactive amines in dark chocolates made with different proportions on under-fermented and fermented cocoa beans. Food Chem. 2023, 404, 134725. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, D.; Spizzirri, U.G.; Puoci, F.; Picci, N. Determination of biogenic amines profiles in conventional and organic cocoa-based products. Food Addit. Contam. Part A 2015, 32, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O. Chocolate Science and Technology; Wiley-Blackwell Publishers: Oxford, UK, 2010; pp. 3–22. [Google Scholar]
- Biehl, B.; Ziegleder, G. Cocoa: Chemistry of processing. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1436–1448. [Google Scholar]
- Ascrizzi, R.; Flamini, G.; Tessieri, C.; Pistelli, L. From the raw seed to chocolate: Volatile profile of Blanco de Criollo in different phases of the processing chain. Microchem. J. 2017, 133, 474–479. [Google Scholar] [CrossRef]
- Owusu, M.; Petersen, M.A.; Heimdal, H. Effect of fermentation method, roasting and conching conditions on the aroma volatiles of dark chocolate. J. Food Process. Preserv. 2012, 36, 446–456. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Quao, J.; Takrama, J.; Budu, A.S.; Saalia, F.K. Chemical composition and physical quality characteristics of Ghanaian cocoa beans as affected by pulp pre-conditioning and fermentation. J. Food Sci. Technol. 2013, 50, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.C.; Dominguez-Perles, R.; Moreno, D.A.; Muries, B.; Alcaraz-López, C.; Bastías, E.; García-Viguera, C.; Carvajal, M. Minerals in plant food: Effect of agricultural practices and role in human health. A review. Agron. Sustain. Dev. 2010, 30, 295–309. [Google Scholar] [CrossRef]
- Champagne, C.M. Magnesium in hypertension, cardiovascular disease, metabolic syndrome, and other conditions: A review. Nutr. Clin. Prac. 2008, 23, 142–151. [Google Scholar] [CrossRef]
- Borchers, A.T.; Keen, C.L.; Hannun, S.M.; Gershwin, M.E. Cocoa and chocolate: Composition, bioavailability and health implication. J. Med. Food 2000, 3, 77–105. [Google Scholar] [CrossRef]
- Oracz, J.E.; Nebesny, E.; Żyżelewicz, D. Effect of roasting conditions on the fat, tocopherol, and phytosterol content and antioxidant capacity of the lipid fraction from cocoa bans of different Theobroma cacao L. cultivars. Eur. J. Lipid Sci. Technol. 2014, 116, 1002–1014. [Google Scholar] [CrossRef]
- Kühn, J.; Schröter, A.; Hartmann, B.M.; Stangi, G.I. Cocoa and chocolate are sources of vitamin D2. Food Chem. 2018, 15, 318–320. [Google Scholar] [CrossRef]
- Perez, M.; Lopez-Yerena, A.; Vallverdú-Queralt, A. Traceability, authenticity and sustainability of cocoa and chocolate products: A challenge for the chocolate industry. Crit. Rev. Food Sci. Nutr. 2022, 62, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Penido, F.C.L.; Lourenço, I.C.R.; Silva, L.M.d.; Garcia, C.F.; Rezende, F.A.G.G. Chemical composition, antioxidant activity, and fatty acid profile of cocoa nibs. Int. J. Res. Granthaalayah 2021, 9, 168–176. [Google Scholar] [CrossRef]
- Panak Balentić, J.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef]
- Rios, L.Y.; Gonthier, M.P.; Remesy, C.; Mila, I.; Lapierre, C.; Lazarus, S.A.; Williamson, G.; Scalbert, A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am. J. Clin. Nutr. 2003, 77, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Jean-Marie, E.; Bereau, D.; Robinson, J.-C. Benefits of polyphenols and methylxanthines from cocoa beans on dietary metabolic disorders. Foods 2021, 10, 2049. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, K.; Jegadeeswari, V. Evaluating the processed beans of different cocoa (Theobroma cacao L.) accessions for quality parameters. J. Phytol. 2019, 11, 1–4. [Google Scholar]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor chemistry of cocoa and cocoa products—An overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.; Bareuther, S.; Hofmann, T. Molecular definition of the taste of roasted cocoa nibs (Theobroma cacao) by means of quantitative studies and sensory experiments. J. Agric. Food Chem. 2006, 54, 5530–5539. [Google Scholar] [CrossRef] [PubMed]
- Wiyono, T.; Nurhayati, R.; Herawati, E.R.N.; Laila, U. The effect of time, pH and solvent composition on cocoa shell polyphenol extraction and its antioxidant activity: Response surface method approach. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, England, 2020; Volume 462, p. 012029. [Google Scholar]
- Jacobs, D.M.; Smolders, L.; Lin, Y.; de Roo, N.; Trautwein, E.A.; van Duynhove, J.; Mensink, R.P.; Plat, J.; Mihaleva, V.V. Effetc of theobromine consumption on serum lipoprotein profiles in apparently healthy humans with low HDL-cholesterol concentrations. Front. Mol. Biosci. 2017, 4, 59. [Google Scholar] [CrossRef]
- Fanning, E.; Eyres, G.; Frew, R.; Kebede, B. Linking cocoa quality attributes to its origin using geographical indications. Food Control 2023, 151, 109825. [Google Scholar] [CrossRef]
- Jairami, H.; Al-Mutarid, M.; Penson, P.E.; Faris, M.A.-I.; Saif, Z.; Hammad, L. Intake of caffeine and its association with physical and mental health status among university students in Bahrain. Foods 2020, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kim, Y.J.; Lee, H.; Lee, C.Y. Cocoa has more phenolic phytochemical and a higher antioxidant capacity than teas and red wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, F.M.; Bearden, M.M.; Kee, C.L. Cocoa and chocolate flavonoids: Implications for cardiovascular health. J. Am. Diet. Assoc. 2003, 103, 215–223. [Google Scholar] [CrossRef]
- Rusconi, M.; Conti, A. Theobroma cacao L., the Food of the Gods: A scientific approach beyond myths and claims. Pharmacol. Res. 2010, 61, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Hannum, S.M.; Erdman, J.W. Emerging health benefits from cocoa and chocolate. J. Med. Food 2000, 3, 73–75. [Google Scholar] [CrossRef]
- Urbańska, B.; Kowalska, J. Comparison of the total polyphenol content and antioxidant activity of chocolateobtained form roasted and unroasted cocoa beans from different regions of the world. Antioxidants 2019, 8, 283. [Google Scholar] [CrossRef]
- Tamimi, K.A.; Hidayat, C.; Utami, T.; Witasari, L.D. Flavor precursor of non-fermented forastero cocoa beans after flavourzyme® and gluose treatment. LWT 2023, 184, 114910. [Google Scholar] [CrossRef]
- Khan, N.; Khymenets, O.; Urpí-Sardá, M.; Tulipani, S.; Garcia-Aloy, M.; Monagas, M.; Mora-Cunillos, X.; Llorach, R.; Andres-Lacueva, C. Cocoa polyphenols and infiammatory markers of cardiovascular disease. Nutrients 2014, 6, 844–880. [Google Scholar] [CrossRef]
- Hii, C.L.; Law, C.L.; Suzannah, S.; Misnawi, J.; Cloke, M. Polyphenols in cocoa (Theobroma cacao L.). Asian J. Food Agro-Ind. 2009, 2, 702–722. [Google Scholar]
- Heiss, C.; Dejam, A.; Kleinbongard, P.; Schewe, T.; Sies, H.; Kelm, M. Vascular effects of cocoa rich in flavan-3-ols. JAMA 2003, 290, 1030–1031. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Osakabe, N.; Kato, Y.; Natsume, M.; Yasuda, A.; Kido, T.; Fukuda, K.; Muto, Y.; Kondo, K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am. J. Clin. Nutr. 2007, 85, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Natsume, M.; Yasuda, A.; Nakamura, Y.; Tamura, T.; Osakabe, N.; Kanegae, M.; Kondo, K. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J. Nutr. 2007, 137, 1436–1441. [Google Scholar] [CrossRef]
- Rein, D.; Lotito, S.; Holt, R.R.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G. Epicatechin in human plasma: In vivo determination and effect of chocolate consumption on plasma oxidation status. J. Nutr. 2000, 130, 2109S–2114S. [Google Scholar] [CrossRef] [PubMed]
- García-Blanco, T.; Dávalos, A.; Visioli, F. Tea, cocoa, coffee, and affective disorders: Vicious or virtuous cycle? J. Affect. Disord. 2017, 224, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Mai, F.; De Feo, M.; Barnabei, R.; Carducci, A.; Desideri, G.; Necozione, S.; Allegaert, L.; Bernaert, H.; Ferri, C. Cocoa consumption decreases oxidative stress, proinflammatory mediators and lipid peroxidation in healthy subjects: A randomized placebo-controlled dose-response clinical trial. High Blood Press. Cardiovasc. Prev. 2023, 30, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lamuela-Raventós, R.M.; Romero-Pérez, A.I.; Andrés-Lacueva, C.; Tornero, A. Health effects of cocoa flavonoids. Food Sci. Technol. Int. 2005, 11, 159–176. [Google Scholar] [CrossRef]
- Ludovici, V.; Barthelmes, J.; Nägele, M.; Enseleit, F.; Ferri, C.; Flammer, A.J.; Ruschitzka, F.; Sudano, I. Cocoa, blood pressure, and vascular function. Front. Nutr. 2017, 4, 36. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Modification of the Authorization of a Health Claim Related to Cocoa Flavanols and Maintenance of Normal Endothelium-Dependent Vasodilation Pursuant to Article 13 of Regulation (EC) No 1924/2006 Following a Request in Accordance with Article 19 of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 3654. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2014.3654 (accessed on 15 December 2024).
- Miller, K.B.; Stuart, D.A.; Smith, N.L.; Lee, C.Y.; McHale, N.L.; Flanagan, J.A.; Boxin, O.U.; Hurst, W.J. Antioxidant activity and polyphenol and procyanidin contents of select commercially available cocoa-containing and chocolate products in United States. J. Agric. Food Chem. 2006, 54, 4062–4068. [Google Scholar] [CrossRef]
- Scapagnini, G.; Davinelli, S.; Di Renzo, L.; De Lorenzo, A.; Olarte, H.H.; Micali, G.; Cicero, A.F.; Gonzalez, S. Cocoa bioactive compounds: Significance and potential for the maintenance of skin health. Nutrients 2014, 6, 3202–3213. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavanoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Ried, K.; Fakler, P.; Stocks, N.P. Effect of cocoa on blood pressure. Cochrane Database Syst. Rev. 2017, 4, CD008893. [Google Scholar] [CrossRef]
- Berry, N.M.; Davison, K.; Coates, A.M.; Buckley, J.D.; Howe, P.R. Impact of cocoa flavanol consumption on blood pressure responsiveness to exercise. Br. J. Nutr. 2010, 103, 1480–1484. [Google Scholar] [CrossRef]
- Arranz, S.; Valderas-Martinez, P.; Chiva-Blanch, G.; Casas, R.; Urpi-Sarda, M.; Lamuela-Raventós, R.M.; Estruch, R. Cardioprotective effects of cocoa: Clinical evidence from randomized clinical intervention trials in humans. Mol. Nutr. Food Res. 2013, 57, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Sarriá, B.; Gomez-Juaristi, M.; López, S.M.; Cordero, J.G.; Bravo, L.; Briz, M.R.M. Cocoa colonic phenolic metabolites are related to HDL-cholesterol raising effects and methylxanthine metabolites and insoluble dietary fibre to anti-inflammatory and hypoglycemic effects in humans. PeerJ 2020, 8, e9953. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.D.; Sathyapalan, T.; Kilpatrick, E.S.; Beckett, S.; Atkin, S.L. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in Type 2 diabetes patients. Diabet. Med. 2010, 27, 1318–1321. [Google Scholar] [CrossRef]
- Pearson, D.A.; Paglieroni, T.G.; Rein, D.; Wun, T.; Schramm, D.D.; Wang, J.F.; Holt, R.R.; Gosselin, R.; Schmitz, H.H.; Keen, C.L. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb. Res. 2002, 106, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Balcázar-Zumaeta, C.R.; Castro-Alayo, E.M.; Muñoz-Astecker, L.D.; Cayo-Colca, I.S.; Velayarce-Vallejos, F. Food technology forecasting: A based bibliometric update in functional chocolates. Heliyon 2023, 9, e19578. [Google Scholar] [CrossRef]
- Fact.MR. Cocoa Market. Available online: https://www.factmr.com/report/44/cocoa-market (accessed on 15 December 2024).
- Singh, P.K.; Khedkar, R.D.; Chandra, S. Chocolate: An overview of functional potential and recent trends in fortification. Braz. J. Food Technol. 2024, 27, e2023118. [Google Scholar] [CrossRef]
- Becerra, L.D.; Quintanilla-Carvajal, M.X.; Escobar, S.; Ruiz Pardo, R.Y. From controlled transformed cocoa beans to chocolate: Bioactive properties, metabolomic profile, and in vitro bioaccessibility. Food Chem. 2024, 433, 137321. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Duman, H.; Pekdemir, B.; Rocha, J.M.; Oz, F.; Karav, S. Functional chocolate: Exploring advances in production and health benefits. Int. J. Food Sci. Technol. 2024, 59, 5303–5325. [Google Scholar] [CrossRef]
- InsightAce Analytic 2024. Bioactive Formulation for Functional F&B Market, Share & Trends Analysis Report, by Ingredient Type (Prebiotics, Probiotics, Adaptogens, Polyphenols, Omega-3 Fatty Acids, Antioxidants), by Formulation Technology (Encapsulation, Microemulsions, Liposomal Delivery), by Application, by Region, and Segment Forecasts, 2024–2031, Report ID 2736, p. 180. Available online: https://www.insightaceanalytic.com/report/bioactive-formulation-for-functional-fb-market/2736 (accessed on 15 December 2024).
- Barišić, V.; Jozinović, A.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Doko, K.; Ačkar, Đ. Difficulties with use of cocoa bean shell in food production and high voltage electrical discharge as a possible solution. Sustainability 2020, 12, 3981. [Google Scholar] [CrossRef]
- Rossin, D.; Barbosa-Pereira, L.; Iaia, N.; Sottero, B.; Danzero, A.C.; Poli, G.; Zeppa, G.; Biasi, F. Protective effect of cocoa bean shell against intestinal damage: An example of byproduct valorization. Antioxidants 2021, 10, 2080. [Google Scholar] [CrossRef] [PubMed]
- Shashikiran, N.; Subba Reddy, V.; Srikanth, R. Chocolate mouth rinse: Effect on plaque accumulation and mutans streptococci counts when used by children. J. Indian Soc. Pedod. Prev. Dent. 2008, 26, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Anoraga, S.B.; Shamsudin, R.; Hamzah, M.H.; Sharif, S.; Saputro, A.D. Cocoa by-products: A comprehensive review on potential uses, waste management, and emerging green technologies for cocoa pod husk utilization. Heliyon 2024, 10, e35537. [Google Scholar] [CrossRef]
- Farag, M.A.; Hariri, M.L.M.; Ehab, A.; Homsi, M.N.; Zhao, C.; von Bergen, M. Cocoa seeds and chocolate products interaction with gut microbiota; mining microbial and functional biomarkers from mechanistic studies, clinical trials and 16S rRNA amplicon sequencing. Crit. Rev. Food Sci. Nutr. 2024, 64, 3122–3138. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa polyphenols and gut microbiota interplay: Bioavailability, prebiotic effect, and impact on human health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.; Dias, J.; Lourenço, V.; Partidário, A.; Lageiro, M.; Lampreia, C.; Fernandes, J.; Lidon, F.; Reboredo, F.; Alvarenga, N. Development of a functional dark chocolate with baobab pulp. Foods 2023, 12, 1711. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.J.; Williams, C.M. Polyphenols and cognition in humans: An overview of current evidence from recent systematic reviews and meta-analyses. Brain Plast. 2021, 6, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Lua, P.L. Chocolate: Food for moods. Malays. J. Nutr. 2011, 17, 259–269. [Google Scholar]
- Benton, D. Carbohydrate consumption, mood and anti-social behaviour. In Lifetime Nutritional Influences on Cognition, Behaviour and Psychiatric Illness; Benton, D., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2011; Volume 7, pp. 160–179. [Google Scholar]
- Silveira, P.T.D.S.; Glória, M.B.A.; Tonin, I.P.; Martins, M.O.P.; Efraim, P. Varietal influence on the formation of bioactive amines during the processing of fermented cocoa with different pulp contents. Foods 2023, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- Esposito, L.; Perillo, M.; Di Mattia, C.D.; Scroccarello, A.; Della Pelle, F.; Compagnone, D.; Sacchetti, G.; Mastrocola, D.; Martuscelli, M. A survey on potentially beneficial and hazardous bioactive compounds in cocoa powder samples sourced from the European market. Foods 2024, 13, 2457. [Google Scholar] [CrossRef]
- Turna, N.S.; Chung, R.; McIntyre, L. A review of biogenic amines in fermented foods: Occurrence and health effects. Heliyon 2024, 10, e24501. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- del Rio, B.; Fernandez, M.; Redruello, B.; Ladero, V.; Alvarez, M.A. New insights into the toxicological effects of dietary biogenic amines. Food Chem. 2024, 435, 137558. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Risk-Based Control of Biogenic Amine Formation in Fermented Foods. EFSA J. 2011, 9, 2393. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2011.2393 (accessed on 15 December 2024). [CrossRef]
- Deus, V.L.; Bispo, E.S.; Franca, A.S.; Gloria, M.B.A. Understanding amino acid and bioactive amines changes during on-farm cocoa fermentation. J. Food Compos. Anal. 2021, 97, 103776. [Google Scholar] [CrossRef]
- Doeun, D.; Davaatseren, M.; Chung, M.S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Alvarez, M.A. The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Sci. Rep. 2019, 9, 120. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Zimmermann, A.; Schroeder, S.; Pendl, T.; Harger, A.; Stekovic, S.; Schipke, J.; Magnes, C.; Schmidt, A.; et al. Dietary spermidine for lowering high blood pressure. Autophagy 2017, 13, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ospina, J.; Molina-Hernández, J.B.; Chaves-López, C.; Romanazzi, G.; Paparella, A. The role of fungi in the cocoa production chain and the challenge of climate change. J. Fungi 2021, 7, 202. [Google Scholar] [CrossRef]
- Ekici, K.; Omer, A.K. Biogenic amines formation and their importance in fermented foods. In BIO Web of Conferences; EDP Sciences: Paris, France, 2020; Volume 17, p. 00232. [Google Scholar]
- Granvogl, M.; Bugan, S.; Schieberle, P. Formation of amines and aldehydes from parent amino acids during thermal processing of cocoa and model systems: New insights into pathways of the strecker reaction. J. Agric. Food Chem. 2006, 54, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Kosman, V.M.; Stankevich, N.M.; Makarov, V.G.; Tikhonov, V.P. Biologically active substances in grated cocoa and cocoa butter. Vopr. Pitan. 2007, 76, 62–67. [Google Scholar] [PubMed]
- Baranowska, I.; Płonka, J. Simultaneous determination of biogenic amines and methylxanthines in foodstuff—Sample preparation with HPLC-DAD-FL analysis. Food Anal. Methods 2015, 8, 963–972. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; De Luca, M.; Parisi, O.I.; Picci, N. Biogenic amine as quality marker in organic and fair-trade cocoa-based products. Sustainability 2016, 8, 856. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Di Mattia, C.D.; Paparella, A.; Mastrocola, D.; Martuscelli, M.; Chaves-Lòpez, C. Effect of fermentation, drying and roasting on biogenic amines and other biocompounds in Colombian Criollo cocoa beans and shells. Foods 2020, 9, 520. [Google Scholar] [CrossRef]
- Tuenter, E.; Sakavitsi, M.E.; Rivera-Mondragón, A.; Hermans, N.; Foubert, K.; Halabalaki, M.; Pieters, L. Ruby chocolate: A study of its phytochemical composition and quantitative comparison with dark, milk and white chocolate. Food Chem. 2021, 343, 128446. [Google Scholar] [CrossRef]
- Korcari, D.; Fanton, A.; Ricci, G.; Rabitti, N.S.; Laureati, M.; Hogenboom, J.; Pellegrino, L.; Emide, D.; Barbiroli, A.; Fortina, M.G. Fine cocoa fermentation with selected lactic acid bacteria: Fermentation performance and impact on chocolate composition and sensory properties. Foods 2023, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ospina, J.; Molina-Hernandez, J.B.; Viteritti, E.; Maggio, F.; Fernández-Daza, F.F.; Sciarra, P.; Serio, A.; Rossi, C.; Paparella, A.; Chaves-López, C. Advances in understanding the enzymatic potential and production of ochratoxin A of filamentous fungi isolated from cocoa fermented beans. Food Microbiol. 2022, 104, 103990. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Menichini, F.; Picci, N.; Puoci, F.; Spizzirri, U.G.; Restuccia, D. Technological aspects and analytical determination of biogenic amines in cheese. Trends Food Sci. Technol. 2013, 30, 38–55. [Google Scholar] [CrossRef]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Santos Lopes, A. The microbiota diversity identified during the cocoa fermentation and benefits of the starter cultures use: An overview. Int. J. Food Sci. Technol. 2021, 56, 544–552. [Google Scholar] [CrossRef]
- Ganeswari, I.; Khairul Bariah, S.; Amizi, M.A.; Sim, K.Y. Effects of different fermentation approaches on the microbiological and physicochemical changes during cocoa bean fermentation. Int. Food Res. J. 2015, 22, 7076. [Google Scholar]
- Miguel, M.G.; de Castro Reis, L.V.; Efraim, P.; Santos, C.; Lima, N.; Freitas Schwan, R. Cocoa fermentation: Microbial identification by MALDI-TOF MS, and sensory evaluation of produced chocolate. LWT 2017, 77, 362–369. [Google Scholar] [CrossRef]
- Lappa, I.K.; Terpou, A.; Bosnea, L.A.; Papadaki, A. Chapter 10—Lactic acid bacteria and biogenic amines in food: Biological importance and human health. In Lactic Acid Bacteria in Food Biotechnology; Ray, R.C., Paramithiotis, S., de Carvalho Azevedo, V.A., Montet, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 181–194. ISBN 9780323898751. [Google Scholar]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Spanjer, M.C.; van Roode, B.A.S.W. Towards a regulatory limit for biogenic amines in fish, cheese and sauerkraut. Ware (N)-Chem. 1991, 21, 139–167. [Google Scholar]
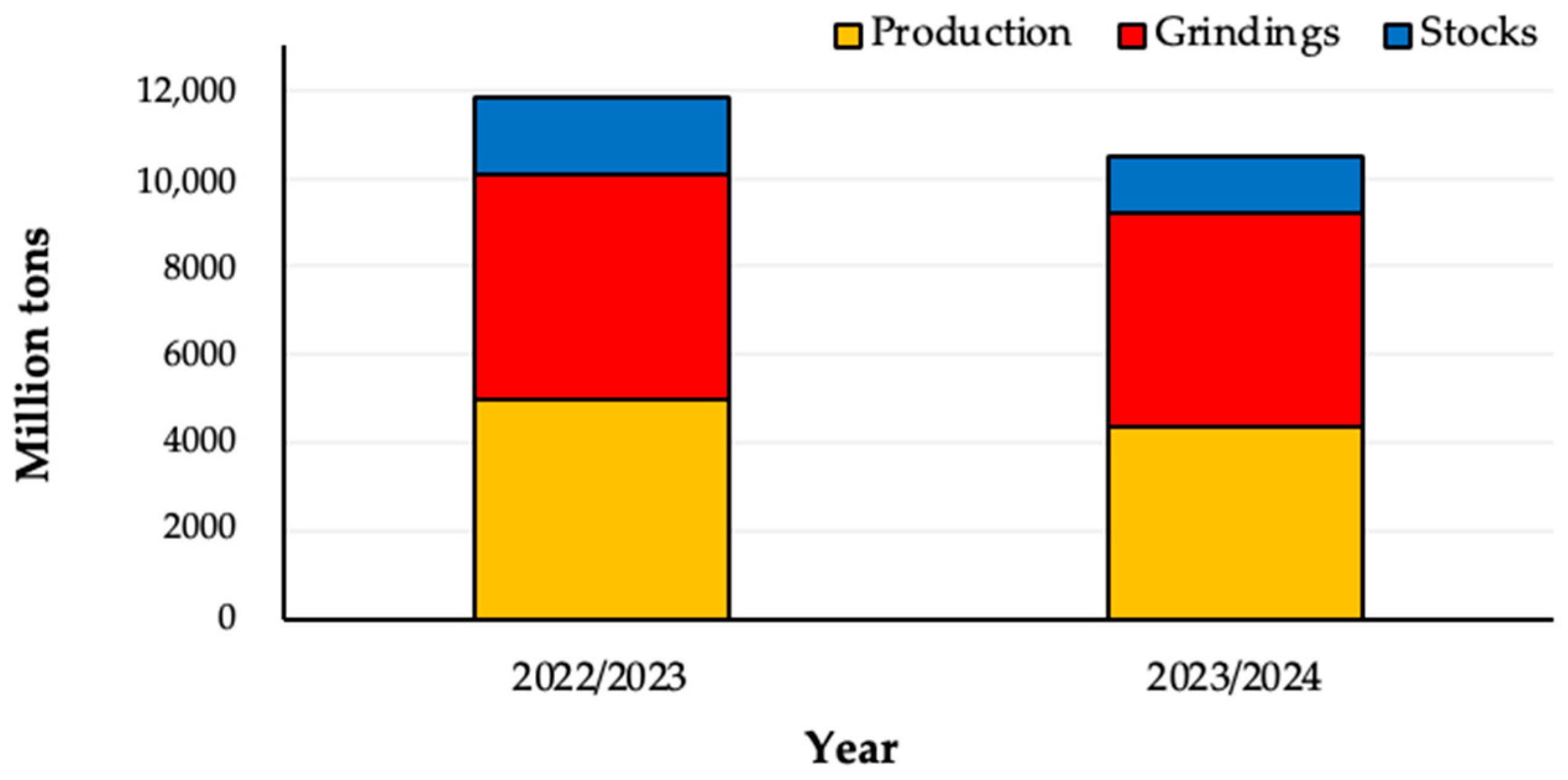
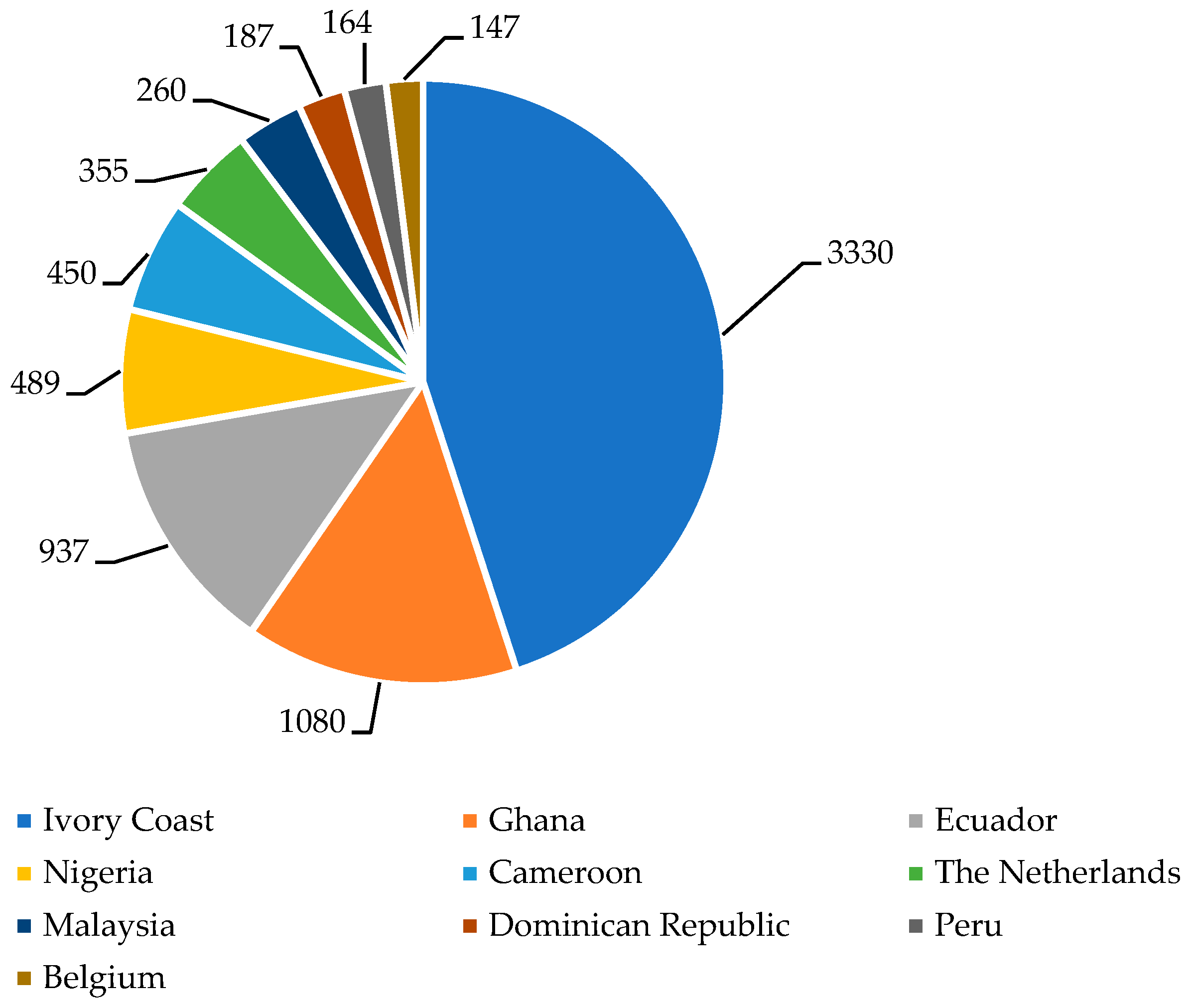
| Countries | Year | |||
|---|---|---|---|---|
| 2020/2021 | 2021/2022 | 2022/2023 | 2023/2024 | |
| Ivory Coast | 2248 | 2121 | 2241 | 1800 |
| Ghana | 1047 | 683 | 654 | 580 |
| Other Africa | 761 | 785 | 774 | 788 |
| Americas | 935 | 973 | 1061 | 1035 |
| Asia and Oceania | 254 | 265 | 266 | 247 |
| World total | 5245 | 4827 | 4996 | 4450 |
| Average Price * | 2.43 | 2.39 | 3.20 | 4.40 |
| Reference | Samples | Biogenic Amines (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|
| CAD | HIM | PEA | PUT | SPD | TYM | Total Content | ||
| Granvogl et al. 2006 [146] | Raw cocoa beans | 0.2–1.2 | ||||||
| Roasted cocoa beans | 0.6–10.2 | |||||||
| Dark chocolate | ||||||||
| Kosman et al. 2007 [147] | Grated cocoa | 2.8–14.9 | 9.6–71.7 | |||||
| Oracz and Nebesny 2014 [23] | Raw cocoa beans | 2.7–11.4 | ||||||
| Fermented and dried cocoa beans | 1.7–18.4 | 1.0–7.4 | 4.4–33.5 | |||||
| Baranowska and Płonka 2015 [148] | Chocolate 90% | 47.6 | ||||||
| Cocoa | 46.8 | |||||||
| Broad bean | 22.6 | |||||||
| Bean | 31.0 | |||||||
| Restuccia et al. 2016 [149] | Cocoa-based products | nd–5.3 | 2.4–38.1 | nd–2.0 | nd–32.7 | nd–9.7 | 1.3–31.7 | 5.7–79.0 |
| Cocoa powder | 0–6.1 | 2.2–23.8 | 0–3.2 | 0–10.1 | 1.0–10.2 | 1.3–13.1 | 5.7–72.3 | |
| Dark chocolate | 0 | 2.9–35.0 | 0–1.1 | 0.9–6.1 | 1.0–9.8 | 1.6–12.4 | 7.7–65.0 | |
| Milk chocolate | 0 | 7.3–45.4 | 0 | 0.8–3.9 | 1.0–6.1 | 1.8–19.6 | 12.4–68.7 | |
| White chocolate | 0 | 9.5–38.1 | 0 | 0 | 2.0–6.2 | 3.0–20.6 | 16.4–75.3 | |
| Powder to prepare cocoa drink | 0 | 9.8 | 0 | 0.9 | 0 | 7.9 | 20.3 | |
| Powder to prepare cocoa mousse | 0 | 25.1 | 0 | 13.3 | 7.4 | 19.7 | 71.5 | |
| Powder to prepare cocoa pudding | 0 | 15.0–23.4 | 0 | 3.7–10.1 | 3.6–4.9 | 12.9–31.7 | 38.0–79 | |
| do Carmo Brito et al. 2017 [46] | Fermented cocoa beans | - | - | - | - | 3.3–19.4 | 2.2–11.8 | 12.8–39.6 |
| Spizzirri et al. 2019 [50] | Fermented and roasted cocoa beans | 1.1–2.6 | 3.1–12.8 | nd–2.1 | 1.5–10.9 | nd–9.1 | nd–11.1 | 12.9–58.3 |
| Delgado-Ospina et al. 2020 [150] | Fermented and dried cocoa beans | nd–66.6 | nd–41.9 | nd | nd | 0.3–48.7 | nd–0.2 | |
| Roasted cocoa beans (120 °C for 22 min) | nd–5.5 | nd–59.8 | 10.4–18.8 | nd–6.5 | nd–2.6 | 9.6–26.5 | ||
| Roasted cocoa beans (135 °C for 15 min) | nd–5.5 | nd–17.1 | 6.0–26.2 | nd–62.6 | nd–2.6 | 0.8–16.9 | ||
| Deus et al. 2021 [140] | Dark monoclonal chocolates | 0–8.2 | 2.7–8.9 | 0.6–10.4 | ||||
| Tuenter et al. 2021 [151] | White chocolate | <0.3 | ||||||
| Ruby chocolate | <0.3 | |||||||
| Milk chocolate | 1.0 | |||||||
| Dark chocolate | 2.8 | |||||||
| Silva et al. 2023 [68] | Chocolate | 2.3–7.2 | 0.6–1.2 | 0.8–2.2 | 2.3–5.7 | 3.2–5.6 | 1.4–2.8 | 14.9–34.7 |
| Silveira et al. 2023 [134] | Roasted cocoa beans | nd–18.0 | nd–2.5 | nd–10.5 | nd–5.7 | nd–4.3 | ||
| Liquor | nd–5.2 | nd–0.6 | nd–2.6 | nd–4.4 | nd–1.5 | nd–3.9 | ||
| Chocolate | nd–6.5 | nd | nd–1.5 | nd–4.0 | nd–2.0 | nd–3.6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paparella, A.; Schirone, M.; López, C.C. The Health Impact of Cocoa from Cultivation to the Formation of Biogenic Amines: An Updated Review. Foods 2025, 14, 255. https://doi.org/10.3390/foods14020255
Paparella A, Schirone M, López CC. The Health Impact of Cocoa from Cultivation to the Formation of Biogenic Amines: An Updated Review. Foods. 2025; 14(2):255. https://doi.org/10.3390/foods14020255
Chicago/Turabian StylePaparella, Antonello, Maria Schirone, and Clemencia Chaves López. 2025. "The Health Impact of Cocoa from Cultivation to the Formation of Biogenic Amines: An Updated Review" Foods 14, no. 2: 255. https://doi.org/10.3390/foods14020255
APA StylePaparella, A., Schirone, M., & López, C. C. (2025). The Health Impact of Cocoa from Cultivation to the Formation of Biogenic Amines: An Updated Review. Foods, 14(2), 255. https://doi.org/10.3390/foods14020255