A Chitosan-Binding Protein Mediated the Affinity Immobilization of Enzymes on Various Polysaccharide Microspheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Strains, Plasmids, and Genes
2.2. Gene Cloning and Expression
2.3. Preparation of Chitin, Chitosan, and Cellulose Microspheres
2.4. Simultaneous Purification and Immobilization of Enzymes Using CMs
2.5. Characterization of the Immobilized Enzymes
2.6. Enzyme Activity Assays
2.7. Effect of Temperature and pH on Enzyme Activity
2.8. The Reusability of the Immobilization Enzymes
2.9. Analytical Methods
3. Results and Discussion
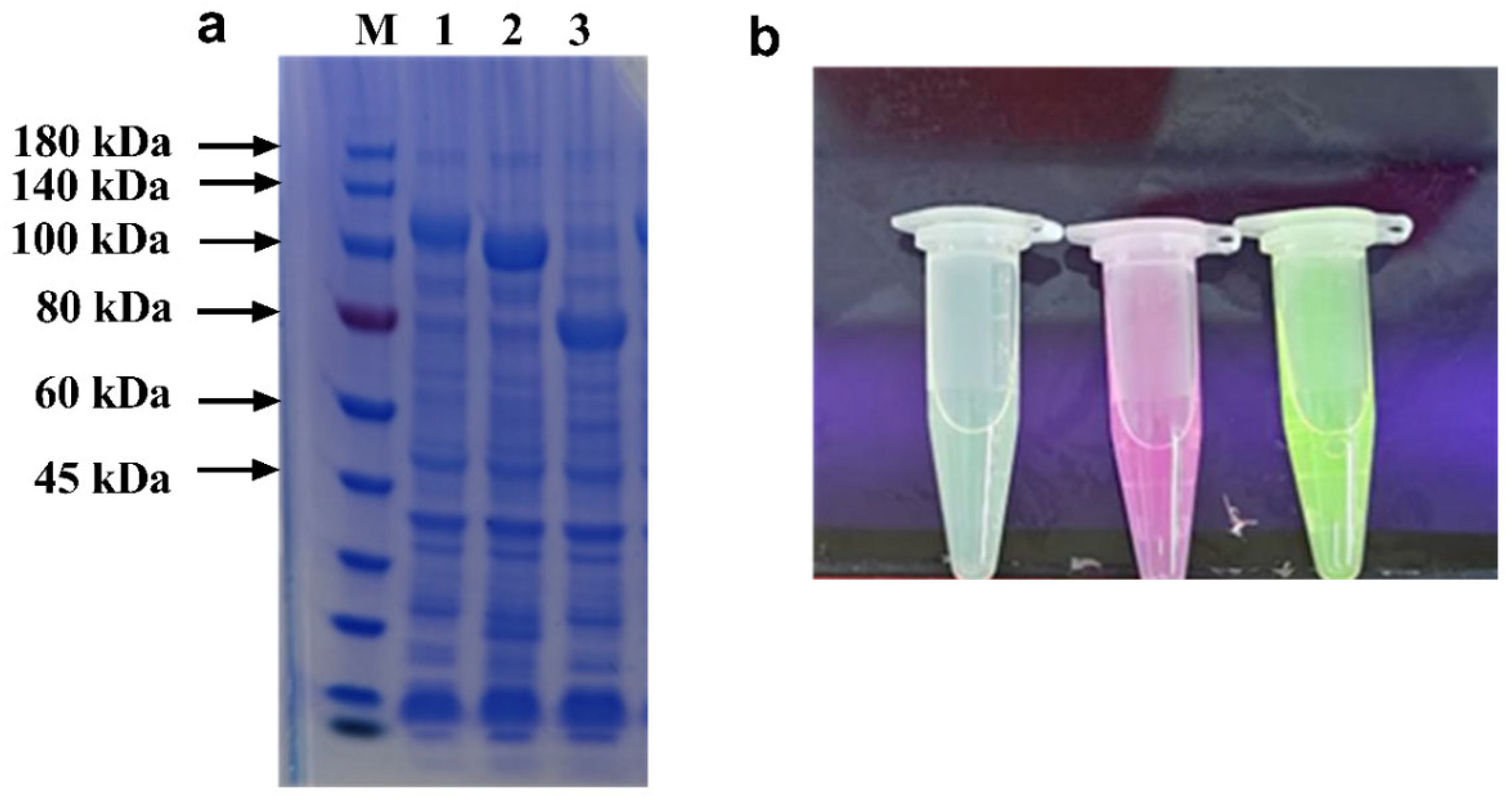
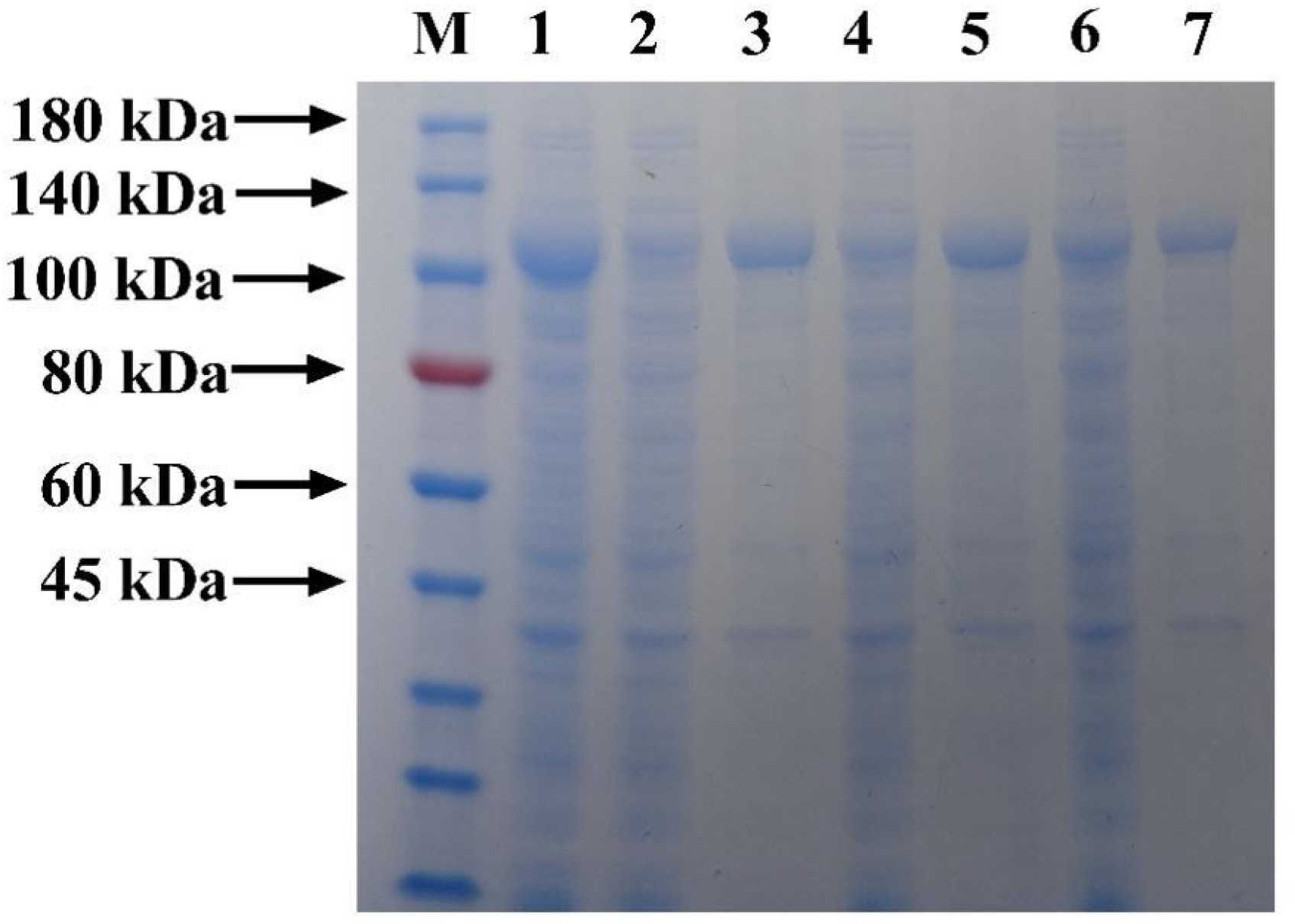
3.1. Fusion of Carbohydrate-Binding Module and Target Proteins
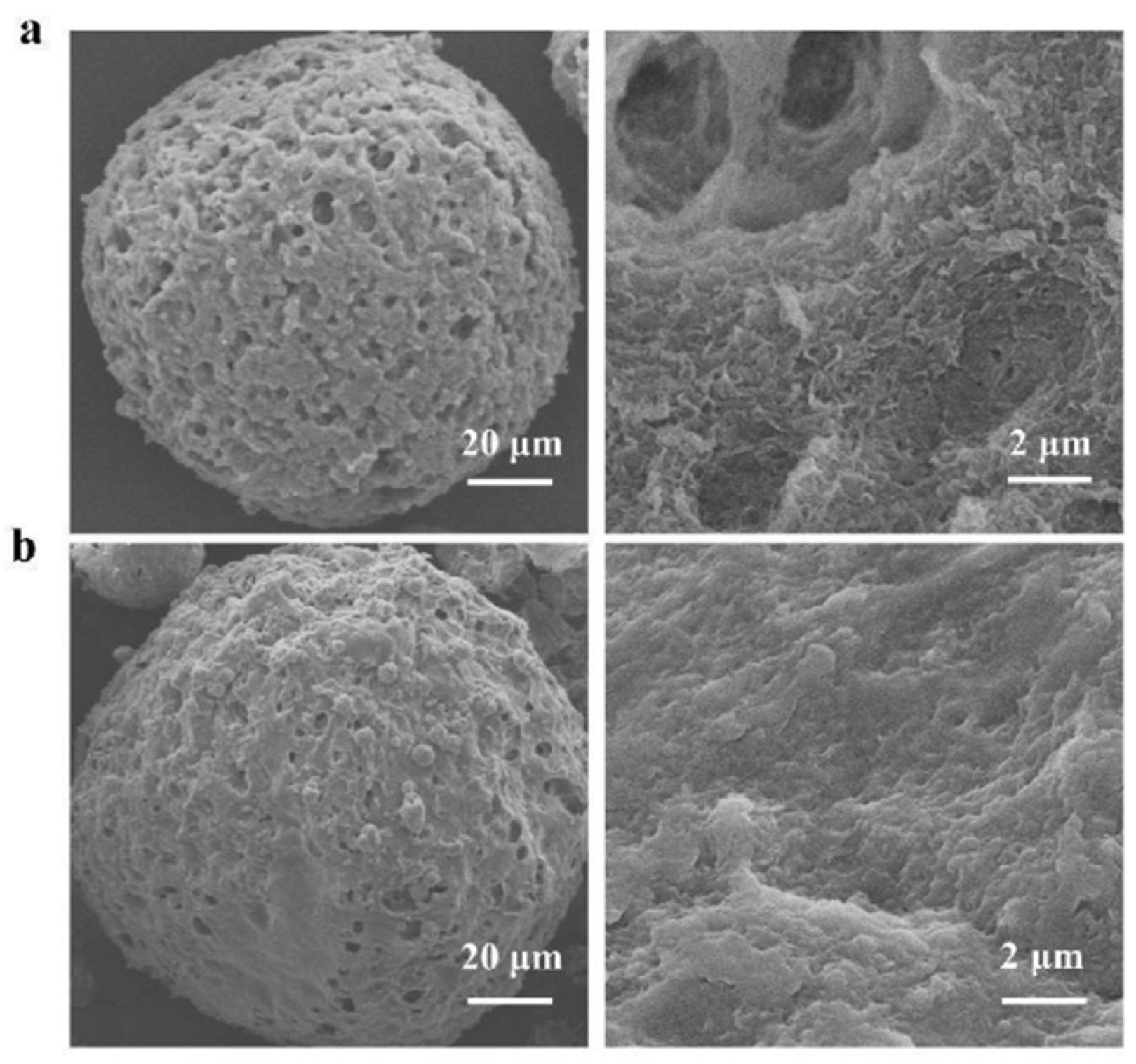
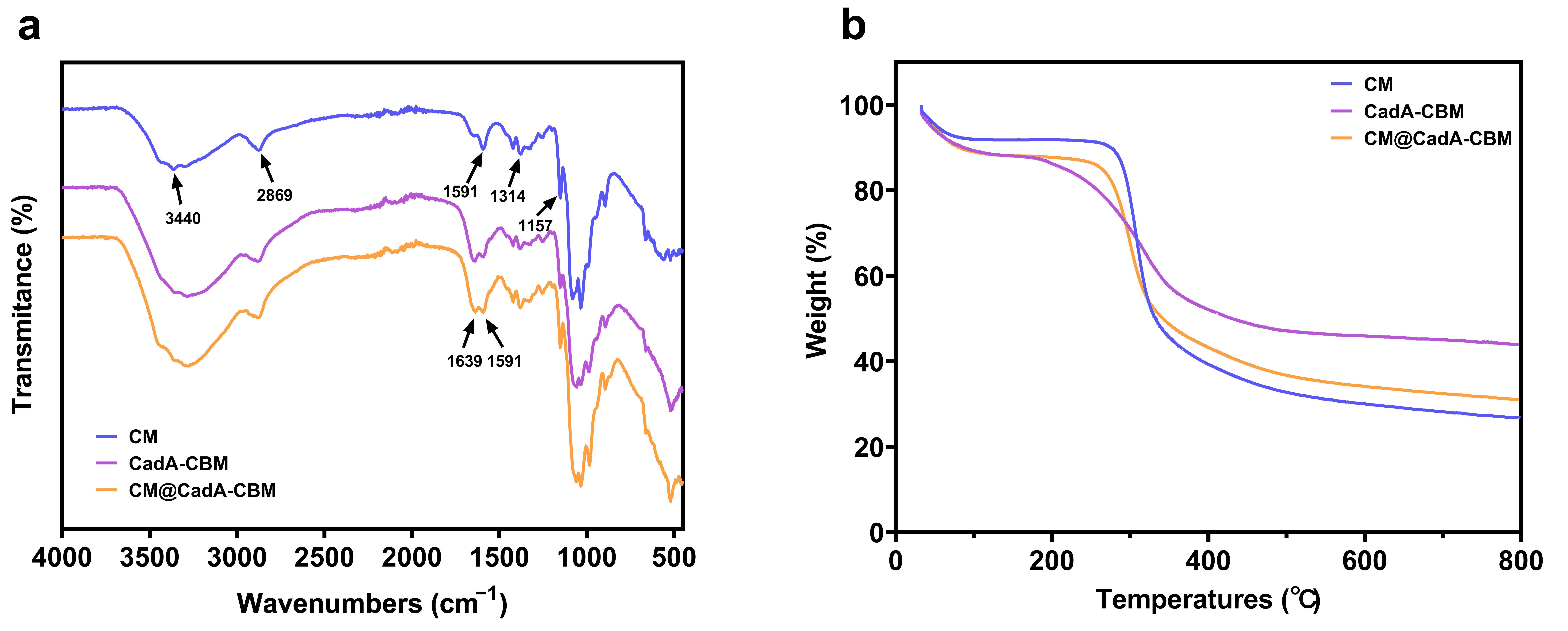
3.2. Preparation and Characterization of Three Polysaccharide Microspheres
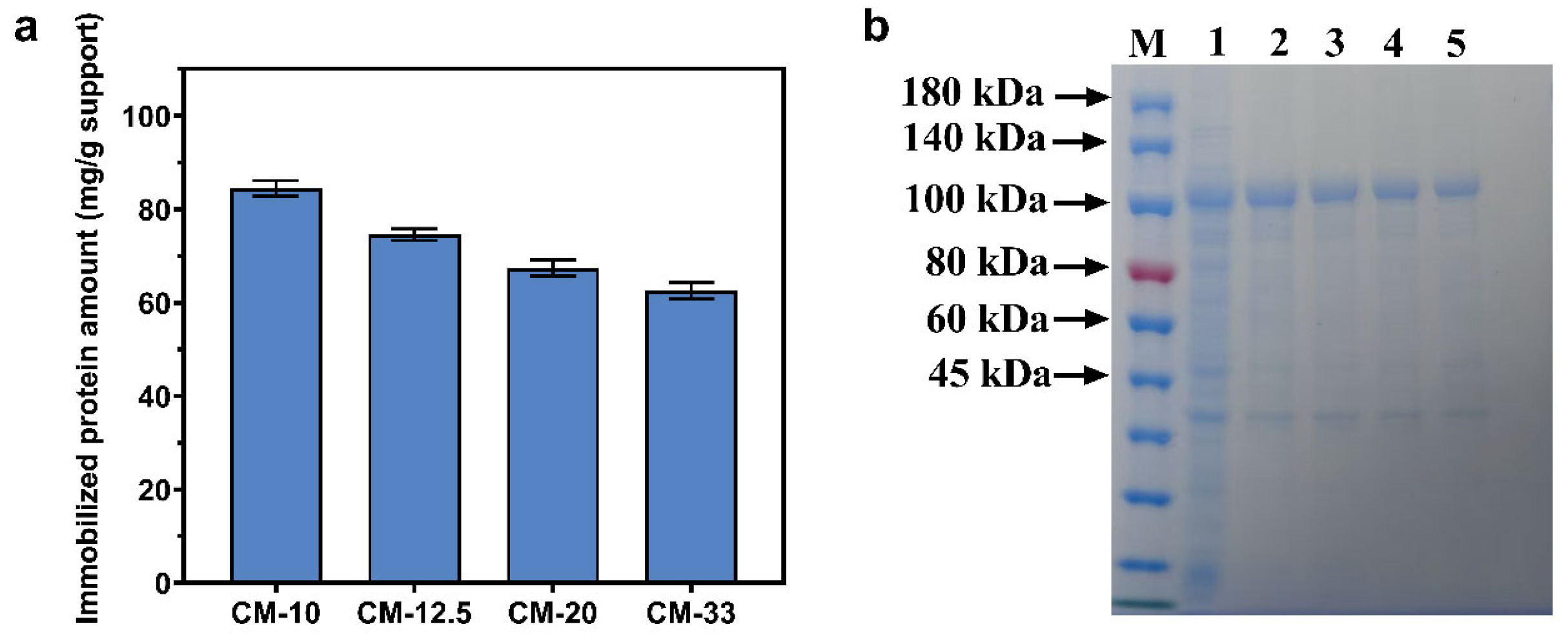
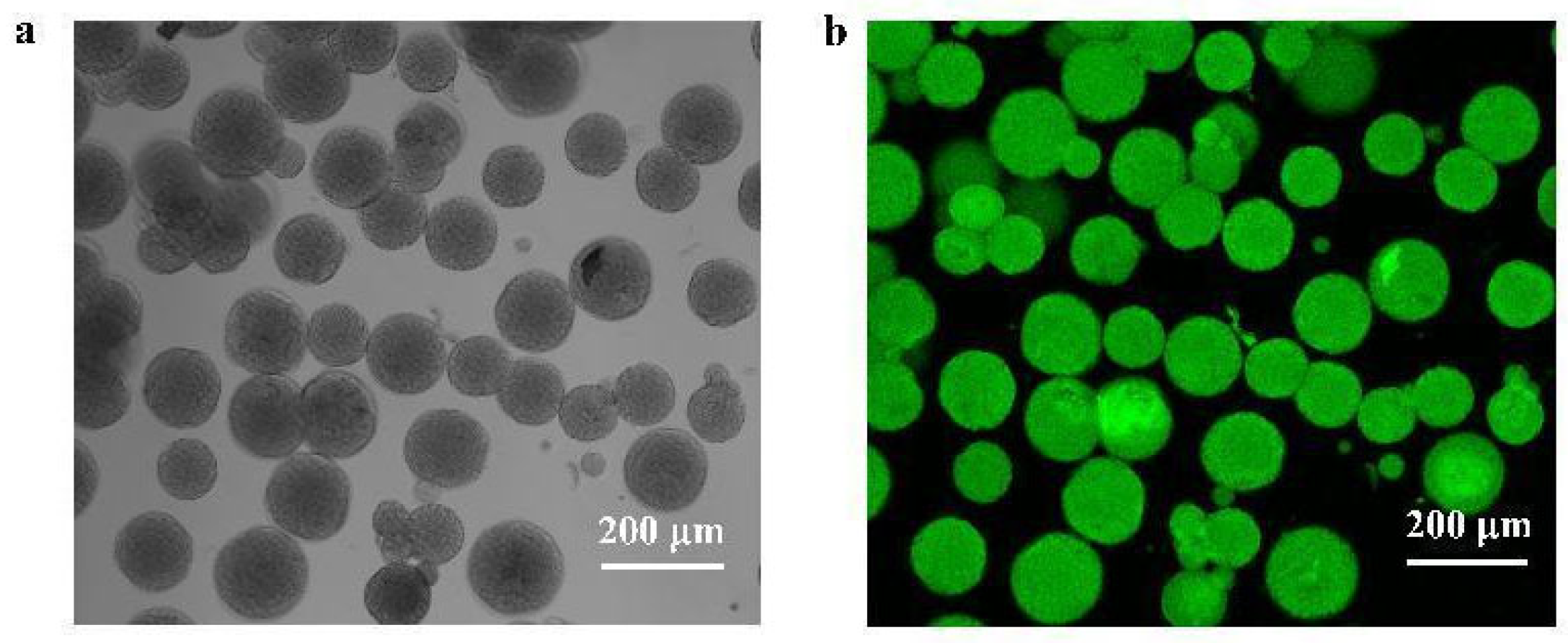
3.3. Simultaneous Purification and Immobilization of Fusion Protein Based on Three Microspheres
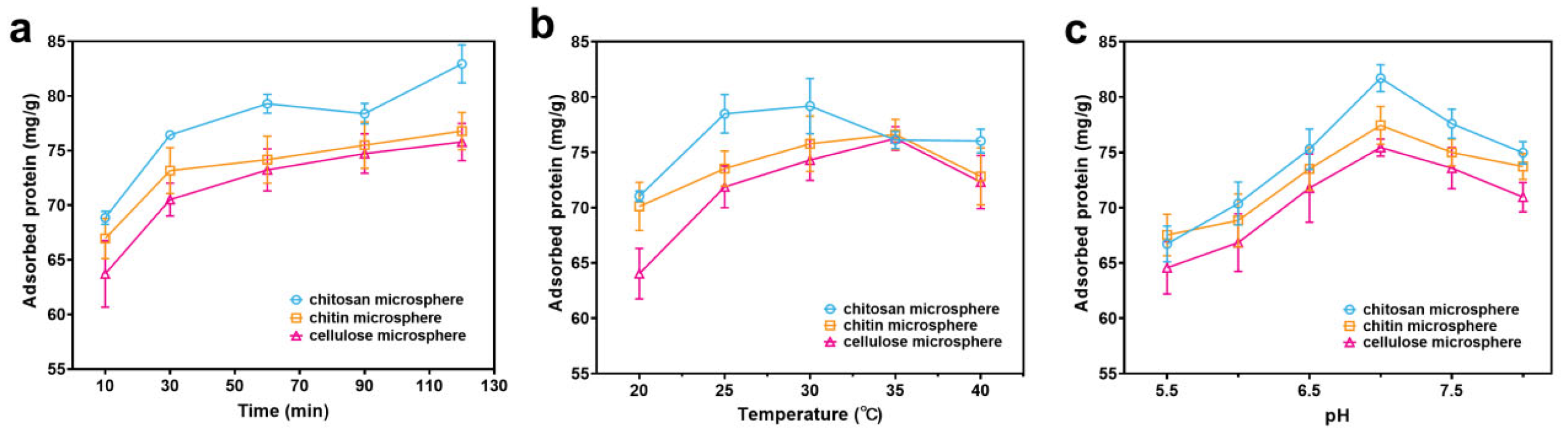
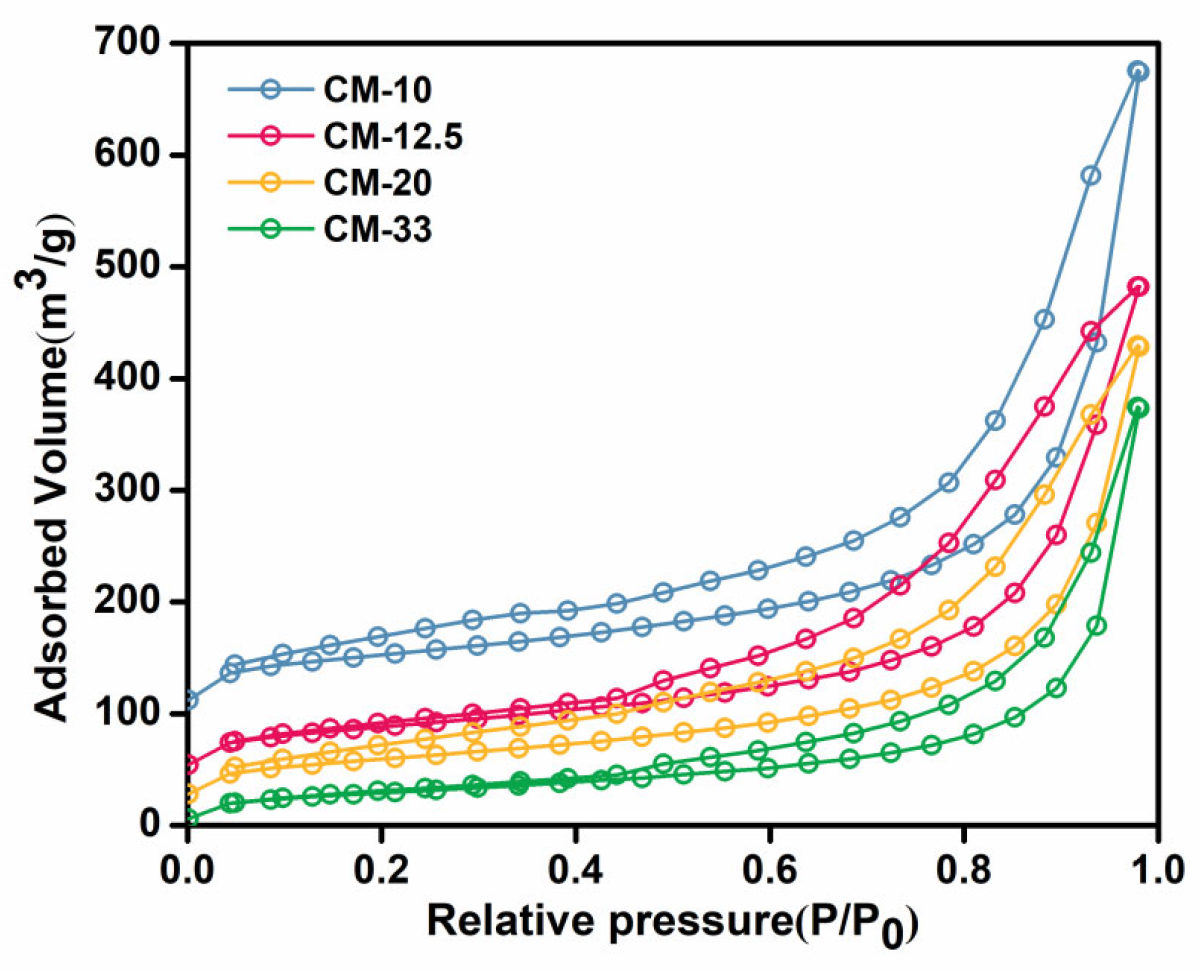
3.4. The Specific Surface Areas of Chitosan Microspheres on Protein Adsorption Capacity
3.5. Characterization of Enzyme Immobilization on Chitosan Microspheres
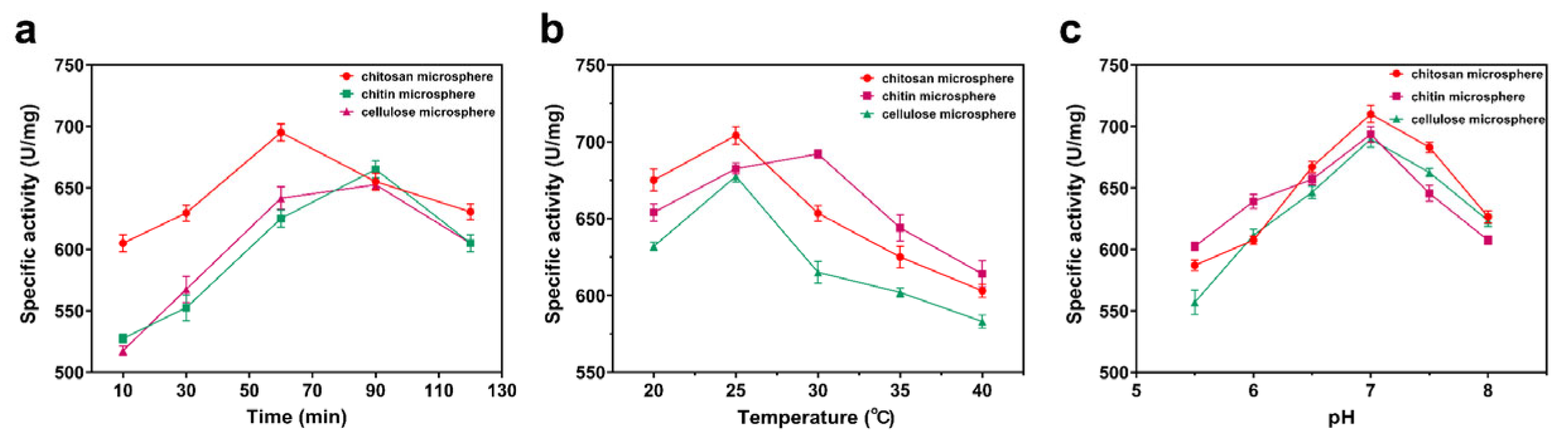
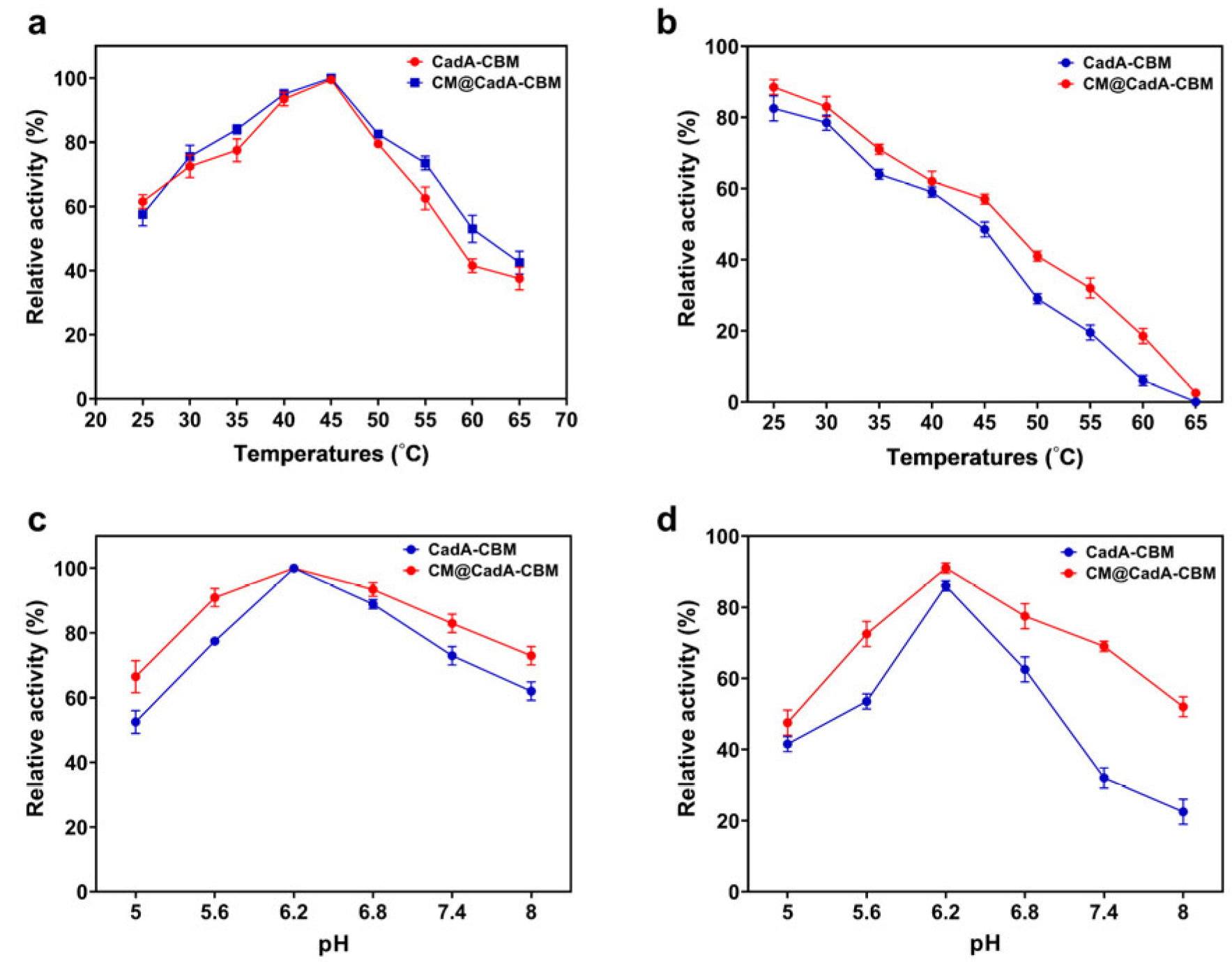
3.6. Enzymatic Properties of Immobilized Enzymes
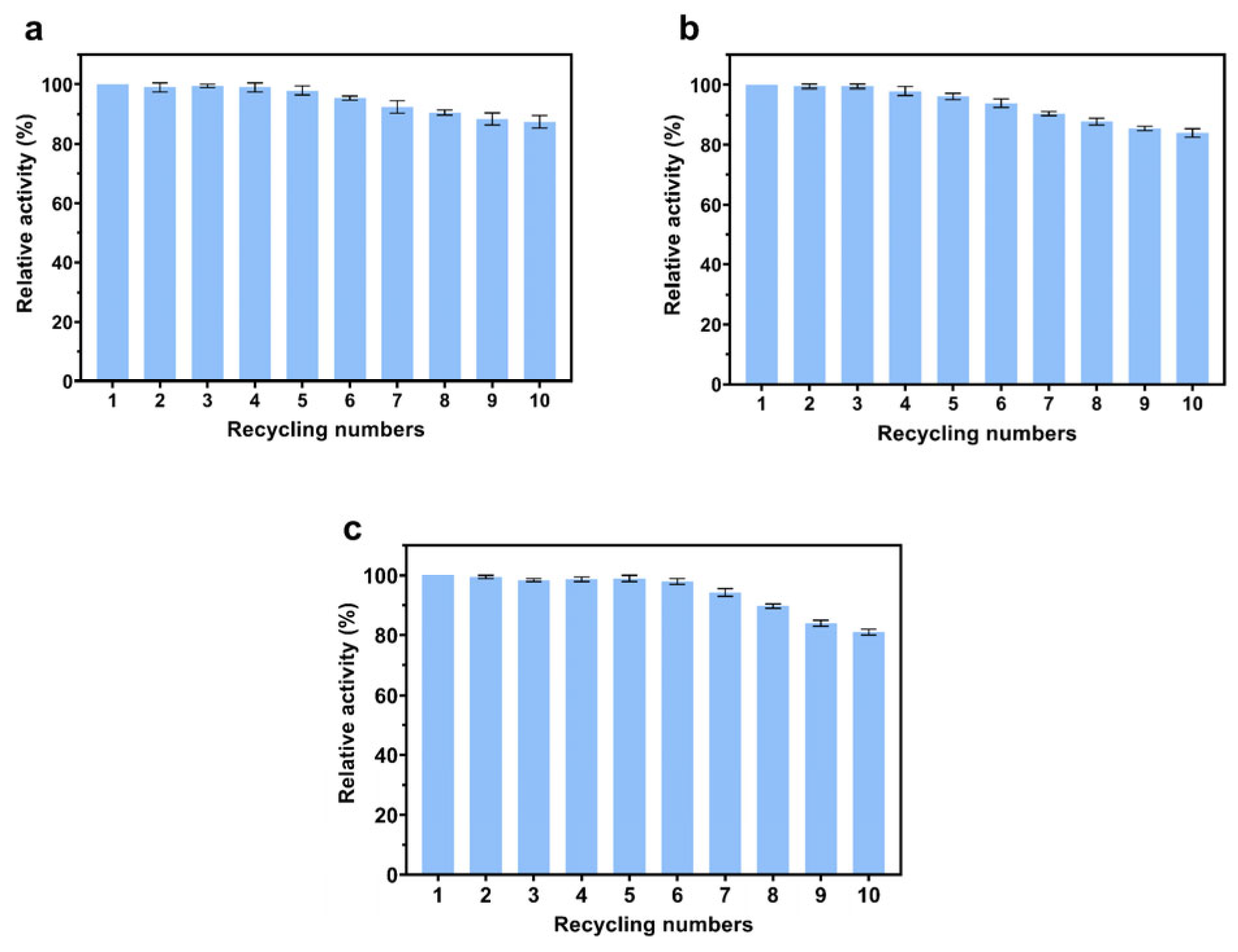
3.7. Reusability of Immobilized Enzymes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buller, R.; Lutz, S.; Kazlauskas, R.J.; Snajdrova, R.; Moore, J.C.; Bornscheuer, U.T. From nature to industry: Harnessing enzymes for biocatalysis. Science 2023, 382, eadh8615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhou, N.; Wu, C.; Wu, B.; Chen, F.; Zhang, A.; Chen, K. The application of chitin materials in enzymatic catalysis: A review. Carbohydr. Polym. 2025, 352, 123172. [Google Scholar] [CrossRef]
- Chettri, D.; Verma, A.K.; Selvaraj, M.; Verma, A.K. Recent advancements for enhanced biocatalyst and biotransformation. Mol. Biotechnol. 2025, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chatzigeorgiou, S.; Jílková, J.; Korecká, L.; Janyšková, R.; Hermannová, M.; Šimek, M.; Čožíková, D.; Slováková, M.; Bílková, Z.; Bobek, J.; et al. Preparation of hyaluronan oligosaccharides by a prokaryotic beta-glucuronidase: Characterization of free and immobilized forms of the enzyme. Carbohydr. Polym. 2023, 317, 121078. [Google Scholar] [CrossRef]
- Ran, L.; Lu, Y.; Chen, L.; He, M.; Deng, Z. Design, Synthesis, and Application of Immobilized Enzymes on Artificial Porous Materials. Adv. Sci. 2025, 2500345. [Google Scholar] [CrossRef]
- Liu, D.-M.; Dong, C. Recent advances in nano-carrier immobilized enzymes and their applications. Process Biochem. 2020, 92, 464–475. [Google Scholar] [CrossRef]
- Liu, D.; Yang, X.; Zhang, L.; Tang, Y.; He, H.; Liang, M.; Tu, Z.; Zhu, H. Immobilization of biomass materials for removal of refractory organic pollutants from wastewater. Int. J. Environ. Res. Public Health 2022, 19, 13830. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme immobilization technologies and industrial applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Zheng, M.M.; Chen, F.F.; Li, H.; Li, C.X.; Xu, J.H. Continuous production of ursodeoxycholic acid by using two cascade reactors with co-immobilized enzymes. Chembiochem 2018, 19, 347–353. [Google Scholar] [CrossRef]
- Freitas, A.I.; Domingues, L.; Aguiar, T.Q. Tag-mediated single-step purification and immobilization of recombinant proteins toward protein-engineered advanced materials. J. Adv. Res. 2022, 36, 249–264. [Google Scholar] [CrossRef]
- Leonhardt, F.; Gennari, A.; Paludo, G.B.; Schmitz, C.; da Silveira, F.X.; Moura, D.C.D.A.; Renard, G.; Volpato, G.; Volken de Souza, C.F. A systematic review about affinity tags for one-step purification and immobilization of recombinant proteins: Integrated bioprocesses aiming both economic and environmental sustainability. 3 Biotech 2023, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-J.; Li, R.-F.; Li, X.-Y.; Zhang, Y.-W. One-step selective affinity purification and immobilization of His-tagged enzyme by recyclable magnetic nanoparticles. Eng. Life Sci. 2021, 21, 364–373. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Meligy, A.M.A.; Yeo, K.B.; Lee, C.-S.; Pack, S.P. Silaffin-3-derived pentalysine cluster as a new fusion tag for one-step immobilization and purification of recombinant Bacillus subtilis catalase on bare silica particles. Int. J. Biol. Macromol. 2020, 159, 1103–1112. [Google Scholar] [CrossRef]
- Yu, Q.; Yang, J.; Liu, L.; Huang, Y.; Wang, E.; Li, D.; Yuan, H. One-step immobilization of chitosanase on microcrystalline cellulose using a carbohydrate binding module family 2. Carbohydr. Polym. 2025, 353, 123291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Harindintwali, J.D.; Yang, W.; Han, M.; Deng, B.; Luan, H.; Zhang, W.; Liu, X.; Yu, X. Engineering of a chitosanase fused to a carbohydrate-binding module for continuous production of desirable chitooligosaccharides. Carbohydr. Polym. 2021, 273, 118609. [Google Scholar] [CrossRef]
- Roberts, A.D.; Payne, K.A.P.; Cosgrove, S.; Tilakaratna, V.; Penafiel, I.; Finnigan, W.; Turner, N.J.; Scrutton, N.S. Enzyme immobilisation on wood-derived cellulose scaffolds via carbohydrate-binding module fusion constructs. Green Chem. 2021, 23, 4716–4732. [Google Scholar] [CrossRef]
- Drula, E.; Garron, M.L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- You, Y.; Kong, H.; Li, C.; Gu, Z.; Ban, X.; Li, Z. Carbohydrate binding modules: Compact yet potent accessories in the specific substrate binding and performance evolution of carbohydrate-active enzymes. Biotechnol. Adv. 2024, 73, 108365. [Google Scholar] [CrossRef]
- Hu, S.; Wang, D.; Hong, J. A simple method for beta-glucosidase immobilization and its application in soybean isoflavone glycosides hydrolysis. Biotechnol. Bioprocess Eng. 2018, 23, 39–48. [Google Scholar] [CrossRef]
- Qin, Z.; Lin, S.; Qiu, Y.; Chen, Q.; Zhang, Y.; Zhou, J.; Zhao, L. One-step immobilization-purification of enzymes by carbohydrate-binding module family 56 tag fusion. Food Chem. 2019, 299, 125037. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zhang, A.; Wei, G.; Yang, S.; Xu, S.; Chen, K.; Ouyang, P. Cadaverine production from L-Lysine with chitin-binding protein-mediated lysine decarboxylase immobilization. Front. Bioeng. Biotechnol. 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Zhao, L.; Du, K. Construction of hierarchically porous chitin microspheres via a novel dual-template strategy for rapid and high-capacity removal of heavy metal ions. Chem. Eng. J. 2020, 393, 124818. [Google Scholar] [CrossRef]
- Gao, L.; Xiong, L.; Xu, D.; Cai, J.; Huang, L.; Zhou, J.; Zhang, L. Distinctive construction of chitin-derived hierarchically porous carbon microspheres/polyaniline for high-rate supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 28918–28927. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kallel, F.; Bettaieb, F.; Khiari, R.; García, A.; Bras, J.; Chaabouni, S.E. Isolation and structural characterization of cellulose nanocrystals extracted from garlic straw residues. Ind. Crops Prod. 2016, 87, 287–296. [Google Scholar] [CrossRef]
- Abbott, D.W.; Eirín-López, J.M.; Boraston, A.B. Insight into ligand diversity and novel biological roles for family 32 carbohydrate-binding modules. Mol. Biol. Evol. 2008, 25, 155–167. [Google Scholar] [CrossRef]
- Hanazono, Y.; Takeda, K.; Niwa, S.; Hibi, M.; Takahashi, N.; Kanai, T.; Atomi, H.; Miki, K. Crystal structures of chitin binding domains of chitinase from Thermococcus kodakarensis KOD1. FEBS Lett. 2016, 590, 298–304. [Google Scholar] [CrossRef]
- Kusaoke, H.; Shinya, S.; Fukamizo, T.; Kimoto, H. Biochemical and biotechnological trends in chitin, chitosan, and related enzymes produced by Paenibacillus IK-5 Strain. Int. J. Biol. Macromol. 2017, 104, 1633–1640. [Google Scholar] [CrossRef]
- Perrakis, A.; Tews, I.; Dauter, Z.; Oppenheim, A.B.; Chet, I.; Wilson, K.S.; Vorgias, C.E. Crystal structure of a bacterial chitinase at 2.3 Å resolution. Structure 1994, 2, 1169–1180. [Google Scholar] [CrossRef]
- McLean, B.W.; Bray, M.R.; Boraston, A.B.; Gilkes, N.R.; Haynes, C.A.; Kilburn, D.G. Analysis of binding of the family 2a carbohydrate-binding module from Cellulomonas fimi xylanase 10 Å to cellulose: Specificity and identification of functionally important amino acid residues. Protein Eng. Des. Sel. 2000, 13, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Shiiba, H.; Hayashi, S.; Yui, T. Molecular dynamics study of carbohydrate binding module mutants of fungal cellobiohydrolases. Carbohydr. Res. 2013, 374, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, Q.; Guan, Y.; Li, W.; Xie, S.; Tong, J.; Li, M.; Ren, L. Synthesis and characterization of porous chitosan/saccharomycetes adsorption microspheres. Polymers 2022, 14, 2292. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Meng, Q.; Wang, Q. Cellulose and chitosan based magnetic nanocomposite microspheres and its application. J. Appl. Polym. Sci. 2021, 138, 51512. [Google Scholar] [CrossRef]
- Liao, J.; Zhou, Y.; Zhao, X.; Hou, B.; Zhang, J.; Huang, H. Chitin microspheres: From fabrication to applications. Carbohyd Polym. 2024, 329, 121773. [Google Scholar] [CrossRef]
- Zhang, A.; Gao, C.; Wang, J.; Chen, K.; Ouyang, P. An efficient enzymatic production of N-acetyl-d-glucosamine from crude chitin powders. Green Chem. 2016, 18, 2147–2154. [Google Scholar] [CrossRef]
- Zhou, N.; Wei, G.; Chen, X.; Wu, B.; Li, H.; Lu, Q.; Cao, X.; Zhang, A.; Chen, K.; Ouyang, P. Self-sufficient biocatalysts constructed using chitin-based microspheres. Chem. Eng. J. 2023, 459, 141660. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, P.; Zeng, H.; Rui, Z. Construction of porous chitosan macrospheres via dual pore-forming strategy as host for alkaline protease immobilization with high activity and stability. Carbohydr. Polym. 2023, 305, 120476. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; Zhang, J.; Tang, Y.; Ge, Y.; Cui, X. A low-cost biomimetic heterostructured multilayer membrane with geopolymer microparticles for broad-spectrum water purification. ACS Appl. Mater. Interfaces 2020, 12, 12133–12142. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Z.; Ma, M.; Sheng, L.; Huang, X. Effect of eggshell membrane as porogen on the physicochemical structure and protease immobilization of chitosan-based macroparticles. Carbohydr. Polym. 2020, 242, 116387. [Google Scholar] [CrossRef]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Sofi, K.; Sargin, I.; Mujtaba, M. Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohydr. Polym. 2016, 145, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Chen, S.; Liu, Y.; Sun, H.; Jia, S.; Shi, J.; Pedersen, C.M.; Wang, Y.; Hou, X. Pyrolysis of chitin biomass: TG–MS analysis and solid char residue characterization. Carbohydr. Polym. 2015, 133, 163–170. [Google Scholar] [CrossRef] [PubMed]

| Strains | CAZyme Source | Ligands | Sequence ID | References | |
|---|---|---|---|---|---|
| 1 | Chitinibacter sp. GC72 | Chitinase | Chitin | WP_157670862.1 | [22] |
| 2 | Chitinolyticbacter meiyuanensis | Chitinase | Chitin | WP_148716959.1 | [30] |
| 3 | Cellulomonas fimi | β-1,4-glucanase | Cellulose | AAB34464.1 | [31] |
| 4 | Trichoderma reesei | Cellobiohydrolase I | Cellulose | 1CBH_A | [32] |
| 5 | Paenibacillus sp | Chitosanase | Chitosan | BAB64835.1 | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Peng, S.; Chen, F.; Zhang, A.; Chen, K. A Chitosan-Binding Protein Mediated the Affinity Immobilization of Enzymes on Various Polysaccharide Microspheres. Foods 2025, 14, 1981. https://doi.org/10.3390/foods14111981
Zhao D, Peng S, Chen F, Zhang A, Chen K. A Chitosan-Binding Protein Mediated the Affinity Immobilization of Enzymes on Various Polysaccharide Microspheres. Foods. 2025; 14(11):1981. https://doi.org/10.3390/foods14111981
Chicago/Turabian StyleZhao, Dexin, Shiguo Peng, Feifei Chen, Alei Zhang, and Kequan Chen. 2025. "A Chitosan-Binding Protein Mediated the Affinity Immobilization of Enzymes on Various Polysaccharide Microspheres" Foods 14, no. 11: 1981. https://doi.org/10.3390/foods14111981
APA StyleZhao, D., Peng, S., Chen, F., Zhang, A., & Chen, K. (2025). A Chitosan-Binding Protein Mediated the Affinity Immobilization of Enzymes on Various Polysaccharide Microspheres. Foods, 14(11), 1981. https://doi.org/10.3390/foods14111981





