Abstract
Listeria monocytogenes is an important foodborne pathogen causing listeriosis. L. monocytogenes, existing in the natural environment, can also contaminate food products, which poses a serious threat to human health and life, especially for high-risk groups: pregnant women, newborn babies, and the elderly. Environmental adaptation of L. monocytogenes refers to the various strategies and mechanisms used by this bacterium to survive and thrive in diverse and often hostile environments that include, among others, toxic heavy metals and disinfectants. The aim of this study was to analyze WGS (whole-genome sequencing) data of 45 L. monocytogenes strains isolated from food to compare the prevalence and types of genetic determinants encoding resistance to toxic metals, such as arsenic and cadmium, as well as quaternary ammonium compounds, like benzalkonium chloride. In L. monocytogenes strains, resistance genes were detected for disinfectants, such as benzalkonium chloride (4.4%), as well as for toxic heavy metals, like cadmium (28.9%) and arsenic (24.4%). The bcrABC cassette was found together with the cadA2C2 genes in two strains: 3855-D (IIc, ST9, CC9) and 4315 (IVb, ST6, CC6). The arsenic cassette, encoded by the genes arsR1D2R2A2B1B2, was co-selected with the cadA4C4 genes. The arsenic cassette was prevalent in nine strains of clonal complex CC2 (82%), one strain of CC3 (9%), and one strain of CC11 (9%). In contrast, the benzalkonium chloride cassette was detected in one strain of CC6 and one strain of CC9. The results of the present study demonstrate the need for further research into the characteristics of L. monocytogenes isolated from other sources in order to understand their spread throughout the food chain.
1. Introduction
Listeria monocytogenes is the causative agent of listeriosis in humans and animals, representing one of the major foodborne pathogens, with high rates of mortality of 20–30% [1]. The groups at highest risk of listeriosis infection include pregnant women, newborn babies, the elderly, and people with weakened immune systems resulting from illness or medication. Listeriosis outcomes include septicemia, meningitis, stillbirth, and miscarriages [2,3,4]. In 2022, a total of 2738 confirmed cases of listeriosis were reported, with a case fatality rate of 18.1%. Infections were most frequently reported in the age group >64 years, accounting for 70.9% of all reported cases. The proportion of listeriosis cases has been steadily increasing over the past few years [5]. Strains of certain serotypes (1/2a, 1/2b, and 4b) are responsible for the majority (over 95%) of clinical cases in humans. Serotype 4b strains are responsible for about 50% of human listeriosis cases.
L. monocytogenes is a bacterium widely distributed in the natural environment; it can be found in water, soil, rotting plants, and sewage. Due to its resistance to environmental conditions, it can also contaminate food-processing areas and foodstuffs.
The most commonly affected food products are ready-to-eat (RTE) items, including smoked fish, soft cheeses, deli meats, and salads. It is estimated that nearly 90% of human cases of listeriosis worldwide occur after consuming contaminated food [6,7].
The environmental adaptation of L. monocytogenes is indeed a complex and multifaceted process. This bacterium has evolved multiple mechanisms to adapt to a wide range of stressors, including temperature extremes, pH fluctuations, the presence of antimicrobial agents, disinfectants, and heavy metals.
Disinfectants as quaternary ammonium compounds (QACs), such as benzalkonium chloride (BC), benzethonium chloride (BZC), and cetyltrimethylammonium bromide (CTAB), are a class of antimicrobial agents widely used in the food industry [8]. Their primary function is to control microbial contamination, ensuring food safety and extending shelf life [9]. BC exhibits good antibacterial activity against many important foodborne pathogens [10]. However, some studies have indicated that the use of certain disinfectants has imposed a selective pressure and contributed to the emergence of disinfectant-resistant microorganisms in food environments. When resistance genes are located on mobile genetic elements (MGEs), such as plasmids or transposons, they can potentially be transferred horizontally between bacteria, leading to the dissemination of resistance phenotypes. Recent studies show that L. monocytogenes is resistant to various disinfectants, including organic acids and quaternary ammonium compounds, which poses a serious challenge to the food industry [8]. Several different QAC resistance determinants associated with MGEs leading to increased BC tolerance in L. monocytogenes strains have been discovered: (i) the bcrABC cassette located within a putative composite transposon, which is present in many plasmids, e.g., pLM80 [11]; (ii) qacH encoded within transposon Tn6188 [12]; (iii) emrE in a genomic island (LGI-1) [13]; and (iv) the emrC gene present in plasmid pLMST6 [14].
As previously mentioned, one key aspect of L. monocytogenes’ environmental adaptation is its complex ability to resist heavy metals. Metals, such as arsenic and cadmium, are naturally occurring chemical compounds. They can be present at various levels in the environment, e.g., the soil, water, and the atmosphere. Metals can also occur as residues in food because of their presence in the environment as a result of human activities, such as farming, industry, or car exhausts, or from contamination during food processing and storage [15]. As documented by Nies [16], L. monocytogenes has the ability to survive in environments with high heavy metal content, which may contribute to its survival in polluted industrial areas.
The presence of heavy metal ions in the environment leads bacteria to develop protective mechanisms that enable them to survive in the presence of toxic concentrations. Resistance to heavy metals, especially arsenic and cadmium, has long been recognized as a major adaptation of L. monocytogenes [17,18].
Cadmium resistance determinants are widely distributed and commonly associated with L. monocytogenes strains isolated from different sources [19]. To date, seven different determinants of cadmium resistance (cadAC efflux systems) have been identified in Listeria spp. The CadA protein, a P-type ATPase efflux pump, actively transports cadmium ions out of bacterial cells, thereby lowering the intracellular cadmium concentration. Meanwhile, CadC serves as a regulatory protein that governs the expression of the cadA gene. By sensing toxic compounds in the environment, CadC activates the transcription of cadA, increasing the production of the CadA efflux pump as necessary [20]. The cadA1 gene is located within transposon Tn5422 (Tn3 family) and commonly inserted within plasmids [21]. A second variant of the cadA gene, cadA2, is often found on plasmids and typically associated with the bcrABC cassette [11,22,23]. The third cadmium resistance gene, cadA3, was found in chromosome L. monocytogenes EGD-e located within the integrative and conjugative element ICELm1 (Tn916-like) [24]. Regarding the cadA4 and cadA5 genes, so far, only one has been identified on the large chromosomally located Listeria Genomic Island 2 (LGI2) and LGI2-1 island, respectively [25,26]. The first three variants confer a high level of resistance to cadmium (MIC > 140 μg/mL), whereas cadA4 is responsible for relatively lower resistance levels (MIC < 70 μg/mL) [6]. The new variant cassette cadA6 was detected in Listeria species, including strains of L. monocytogenes, isolated from various countries and sources. Four new spliced or unspliced transposons in plasmids and chromosomes maintain this cassette [27]. Recently, a novel cadmium resistance gene, cadA7, was found in a transposon Tn916 variant inserted in the chromosome L. monocytogenes strain [28]. The CadA7 gene has been also detected on a novel LGI2 variant, LGI2-3, in two L. welshimeri strains [29].
Arsenic resistance in Listeria spp. in most cases is chromosomally encoded, either within aTn554-like transposon (arsCBADR) or genomic islands LGI2 and LGI2-1 (arsR1D2R2A2B1B2 and upstream arsA1D1 cassette) [24,25]. However, the occurrence of arsenic resistance genes within Listeria spp. plasmids has also been observed [24,30,31].
The aim of this study was to analyze WGS data of 45 L. monocytogenes strains isolated from food to identify the genome characteristics and compare the prevalence and types of genetic determinants encoding resistance to toxic metals, such as arsenic and cadmium, as well as quaternary ammonium compounds, like benzalkonium chloride.
2. Materials and Methods
2.1. L. monocytogenes Strains
Listeria monocytogenes strains were isolated from retail food samples in the frame of the Official Control and Monitoring Program by Sanitary and Epidemiological Stations, in accordance with the PN-EN ISO 11290-1 [32] or PN-EN ISO 11290-2 [33] method accredited by the Polish Centre of Accreditation. L. monocytogenes isolates were sent to the National Reference Laboratory for L. monocytogenes for confirmation tests. The L. monocytogenes isolates were stored at −70 °C until further analysis in Brain Heart Infusion medium with 20% sterile glycerol. One strain from the same food matrix, isolated the same year, was selected for whole genome sequencing (WGS) and genomic analysis. In total, 45 isolates from different food products were included.
2.2. DNA Isolation
DNA was isolated using of the E.Z.N.A.® Bacterial DNA Kit (Omega Bio-Tek, Norcross, GA, USA) according to the recommendations of the manufacturer. The DNA concentration was determined by NanoDrop OneC (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Whole Genome Sequencing (WGS)
Sequencing was conducted by Genomed (Warsaw, Poland) using MiSeq paired-end (PE) technology, 2 × 300 nt, with the MiSeq Reagent Kit v3, 600-cycle (Illumina), as per the manufacturer’s protocol. The genomic DNA concentration was measured prior to the library preparation process using Pico-Green reagent (Life Technologies, Thermo Fisher Scientific) with a Tecan Infinite device. Genomic DNA was fragmented using sonication with the Covaris E210, following the recommended parameters for preparing libraries for Illumina sequencing technology. Libraries were then prepared with the NEBNext Ultra II DNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, USA) following the manufacturer’s instructions.
2.4. Genome Assembly and WGS Quality Control
Readings were filtered with Cutadapt version 3.0. Quality control of the sequencing results was performed using FastQC software Galaxy Version 0.72+galaxy1. Denovo assembly was performed with Spades version 3.14.5. For sequence data, an average depth coverage of over 50× was required. The genome size, ranging between 2.8 and 3.1 Mb and consistent with L. monocytogenes parameters, was used as a criterion for assembly quality. Sequences of isolates were submitted to the NCBI GenBank (accession Bio-project numbers: PRJNA1187899).
2.5. MLST, cgMLST
The MLST (Multi-Locus Sequence Typing) and cgMLST (core genome Multi-Locus Sequence Typing) method for L. monocytogenes was performed using an assembly-based sequence in Ridom SeqSphere+. The identity percentage was set at 90%. The analysis was performed following the Ruppitsch scheme [34] with the seed genome EGD-e (NC_003210.1, 17-DEC-2014).
2.6. SNP Analysis
Single Nucleotide Polymorphism (SNP) analysis was conducted using the CSI Phylogeny 1.4 tool from the Center for Genomic Epidemiology (CGE), accessed at www.genomicepidemiology.org accessed on 15 April 2024. Phylogenetic trees were generated based on our datasets using our first genome as the reference. The resulting Newick files were visualized with iTol https://itol.embl.de/ accessed on 15 April 2024.
2.7. In Silico Detection of Heavy Metals and Disinfectant Resistance Genes
The presence of arsenic, cadmium, and benzalkonium chloride resistance genes (casette arsD1A1R1D2R2A2B1B2, cassette arsCBADR, cassette bcrABC, qacH, emrE, cadA1cadC1, cadAcadA2, cad3cadA3, and cadA4cadC4) was detected using Ridom SeqSphere+ softwareVersion 10.0.4. The analysis was conducted with default settings, requiring a reference sequence identity of at least 90% and a base sequence identity of 99%. The presence of plasmids was searched for with the PlasmidFinder v2.0 tool at CGE DTU servers [35]. The sequences of the L. monocytogenes strain were annotated using Prokka Galaxy Version 1.14.6+galaxy1 software on the usegalaxy server. BLAST (https://blast.ncbi.nlm.nih.gov/, accessed on 30 July 2024) was used to verify the presence of MGEs using GenBank sequences HG329628, AADR01000010, LT732640, L28104, FR33648, and CM001159.
3. Results and Discussion
In our study, L. monocytogenes isolates were differentiated into 19 sequence type STs grouped into 15 clonal complexes (CCs) based on the MLST analysis: ST2 (CC2; 15.6%), ST145 (CC2; 15.6%), ST6 (CC6; 11.1%), ST9 (CC9; 6.7%), ST121 (CC121; 6.7%), ST37 (CC37; 6.7%), ST155 (CC155; 6.7%), 2.2% ST8 (CC8; 4,4%), ST10 (CC101; 2,2%), ST16 (CC8; 2,2%), ST20 (CC20; 2.2%), ST91 (CC14; 2.2%), ST101 (CC101; 2.2%), ST193 (CC193; 2.2%), ST394 (CC415; 2.2%), ST412 (CC412; 2.2%), ST3 (CC3; 2.2%), ST451 (CC11; 2.2%), and ST124 (4.4%). Some of these CCs are the most common clones in Europe, such as CC2, CC121, CC9, CC8, CC6, and CC155 [1]. The majority of isolates (n = 25) belonged to lineage II, and 20 isolates belonged to lineage I. This is in agreement with the results of Brown et al. [4].) However, in that study, the percentage of isolates belonging to lineage II was significantly higher (93%). In our study, the dominant serogroups were IIa (n = 22; 48.9%) and IVb (n = 18; 40%), with the remaining serogroups being IIc (n = 3; 6.67%) and IIb (n = 2; 4.4%). Among lineage II isolates, strains belonging to CC2 (n = 14; 31.1%) were clearly predominant (Table 1).

Table 1.
L. monocytogenes isolate characterization and genetic determinants encoding resistance to toxic metals (cadmium and arsenic) and quaternary ammonium compounds (benzalkonium chloride).
The cgMLST analysis performed revealed the presence of five clusters. MST cluster 1 (seven isolates—3115, 3269, 4353, 4247, 3262, 4260, 3400; belonging to serogroup IVb, CC2 and ST145), cluster 2 (two isolates—4314, 4563; belonging to serogroup IVb, CC6 and ST6), cluster 3 (two isolates—4198, 3460; belonging to serogroup IIa, CC37 and ST37), cluster 4 (two isolates—3542, 3543; belonging to serogroup IVb, CC2 and ST2), and cluster 5 (two isolates—3862, 3863; belonging to serogroup IIa, ST124) (Figure 1).
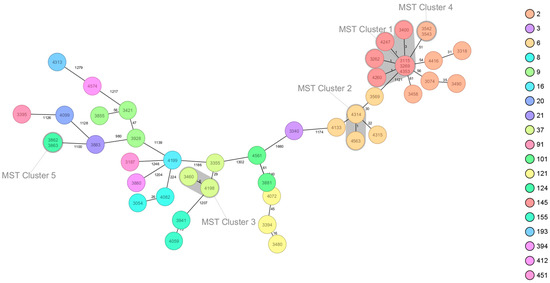
Figure 1.
The 45 strains of L. monocytogenes isolated from food in Poland. Minimum spanning tree of cgMLST allelic profiles. Each circle represents a particular cgMLST type. The distance between the circles represents the genetic divergence. The divergence is given in the number of allelic differences and is indicated on the branch. Colors represent Multi-Locus Sequence Typing (MLST) clonal complexes. The clusters are marked with a loop. The circle label gives the strain identifier and the strain MLST sequence type. Trees of L. monocytogenes were generated using Ridom Seqsphere+.
The visualized SNP phylogeny of the L. monocytogenes results is shown in Figure 2. Interestingly, the pairwise SNP distances between some isolates were small. The genetic differences within some genomes ranged from two to nine pairwise SNP differences, and they are as follows: two SNPs between isolates: 4247 and 4260, 4247 and 4353, and 4353 and 4260; four SNPs: 3542 and 3543; six SNPs: 3863–3862; eight SNPs: 3460–4198; nine SNPs: 3115, 4247, 4260, 4353; 4247–3115; 4260–3115; 4353–3115. This range indicates that the samples are very closely related, suggesting a common origin or recent separation from a common ancestor. In the context of cluster analysis, such small differences in SNPs can indicate that samples belong to the same epidemiological cluster, which is particularly important for tracking disease outbreaks and establishing links between cases. As products have been sampled over several years and strains have been consistently isolated, this suggests that Listeria strains have been continuously present in the food production chain. This may indicate that there are strains that persist in production facilities.

Figure 2.
SNP phylogeny of L. monocytogenes using the CSI Phylogeny 1.4 tool provided by the Center for Genomic Epidemiology, accessed at www.genomicepidemiology.org accessed on 15 April 2024. We generated phylogenetic trees based on our datasets and used our first genome as a reference. To visualize the Newick files that were generated, we utilized iTol (https://itol.embl.de/) accessed on 15 April 2024.
In our study, specific resistance genes were detected in phylogenetically distant lineages I and II. In addition, the presence of resistance genes to arsenic, cadmium, and benzalkonium chloride was associated with specific serotypes and CCs. The reference list used in building the scheme in Ridom SeqSphere+ is presented in Table 1. In the present study, the occurrence of cadmium resistance genes was most prevalent in strains of serogroups IVb and CC2 (n = 9); furthermore, resistance genes were found in the strain of serogroup IVb and CC6 (n = 1), the strain of serogroup IIb and CC3 (n = 1), the strain of serogroup IIa and CC11 (n = 1), and the strain of serogroup IIc and CC9 (n = 1). Similarly, as regards the presence of genes encoding arsenic resistance, strains belonging to serogroup IVb and CC2 (n = 9) are most frequently affected. These genes were also detected in strains belonging to serotypes IIb and CC3 (n = 1) and serotype IIc and CC9 (n = 1). In contrast, benzalkonium chloride resistance genes have only been detected in one strain of serogroup IVb (CC6) and one strain of serogroup IIc (CC9). The results of our study are consistent with the results of previous studies [6,36]. In the study by Gelbicova et al., cadmium and arsenic resistance genes were most frequently identified in L. monocytogenes lineage I strains (27.7% and 16.8%, respectively). Moreover, in these studies, cadmium and arsenic resistance genes were also detected in lineage II strains (28.5% and 14.6%, respectively).
Resistance genes for disinfectants, such as benzalkonium chloride (4.4%), and heavy metals, such as cadmium (28.9%) and arsenic (24.4%), were detected in L. monocytogenes isolates. These results are similar to those obtained in a study of L. monocytogenes strains isolated from food by Gelbicowa et al. [36], where resistance genes were detected for cadmium (36.8%), arsenic (23.6%), and benzalkonium chloride (9.4%). The benzalkonium chloride resistance cassette, bcrABC, was identified in two strains, 3855 (IIc, ST9, CC9) and 4315 (IVb, ST6, CC6), and it was found together with the cadA2C2 genes. As described by Elhanafi et al. [11], bcrABC was first isolated from strains involved in the 1998–1999 foodborne outbreaks. Dutta et al. [22] showed that bcrABC sequences are highly conserved among L. monocytogenes strains of different serotypes, with variability mainly in the flanking regions. Most BCr isolates showed a pLM80-like organization of the bcrABC region, while others contained it chromosomally. The role of the bcrABC cassette in BC resistance exhibited by L. monocytogenes isolated from food processing environments has been reported in numerous studies [11,19,37,38,39]. Our study confirms that L. monocytogenes isolates resistant to BC and carrying the bcrABC cassette are also resistant to cadmium. This cross-resistance, likely mediated by the co-occurrence of cadA2C2 genes, suggests a widespread distribution of bcrABC in L. monocytogenes, independent of serotype or source, and highlights potential mechanisms for its horizontal spread. Our results support the hypothesis of horizontal gene transfer of bcrABC, as proposed by Dutta et al. [22], while emphasizing its association with other resistance genes, such as cadA2. The strains carried the cadA2 gene either alone or together with the cadA1 gene [22]. The arsenic cassette arsR1D2R2A2B1B2, found together with cadA4C4 genes, was detected in nine isolates of the serotype IVb (3262, 3269, 4247, 4260, 3115, 4353, 3074, 3458, 4416), one of IIa (3187), and one of IIb (3340). This simultaneous resistance to arsenic and cadmium is mediated by a 35-kb chromosomal region (LGI-2). LGI-2 also contains genes associated with DNA integration, conjugation, and pathogenicity, making it a critical region for both resistance and virulence [26]. LGI-2 was originally identified in serogroup 4b CC2 strain Scott A [25,26,39]. As shown in recent studies, arsenic and cadmium resistance encoded by genes carried on LGI-2 is strongly associated with its presence in the L. monocytogenes serogroup 4b strains, particularly in the hypervirulent clones CC1, CC2, and CC4. This finding is corroborated by the work of Lee et al. [25], who also identified LGI-2 in these strains as a significant factor contributing to both resistance and virulence. In our study, out of nine serogroup 4b strains, seven belong to clones CC2 and one to CC4.
The emergence of L. monocytogenes strains carrying both disinfectant and heavy metal resistance genes, particularly in food processing environments, is likely to be driven by the selective pressure of frequent exposure to disinfectants and metals. Studies suggest that these environments act as reservoirs for resistant strains, promoting their persistence and potential to cause outbreaks [22].
Author Contributions
Conceptualization, E.M.; methodology, E.M., D.K. and J.K.; software, J.K. validation, E.M., D.K. and J.K.; formal analysis, J.K.; investigation, E.M.; resources, E.M., J.K. and D.K.; data curation, E.M.; writing—original draft preparation, E.M., D.K. and J.K.; writing—review and editing, E.M., D.K. and J.K.; visualization, J.K. and E.M.; supervision, E.M.; project administration, J.P.; funding acquisition, J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported financially by NIPH NIH-NRI (FB-2/2023; FB-2/2024).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Painset, A.; Björkman, J.T.; Kiil, K.; Guillier, L.; Mariet, J.-F.; Félix, B.; Amar, C.; Rotariu, O.; Roussel, S.; Perez-Reche, F.; et al. LiSEQ-Whole-Genome Sequencing of a Cross-Sectional Survey of Listeria monocytogenes in Ready-to-Eat Foods and Human Clinical Cases in Europe. Microb. Genom. 2019, 5, e000257. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.; Bierne, H.; Cossart, P. Listeria monocytogenes: A Multifaceted Model. Nat. Rev. Microbiol. 2006, 4, 423–434. [Google Scholar] [CrossRef]
- Jurkiewicz, A.; Oleszczak-Momot, W. Listeria monocytogenes jako problem zdrowia publicznego. Med. Ogólna Nauk. Zdrowiu 2015, 21, 29–33. [Google Scholar] [CrossRef]
- Brown, S.R.; Bland, R.; McIntyre, L.; Shyng, S.; Weisberg, A.J.; Riutta, E.R.; Chang, J.H.; Kovacevic, J. Genomic characterization of Listeria monocytogenes recovered from dairy facilities in British Columbia, Canada from 2007 to 2017. Front. microbiol. 2024, 15, 1304734. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Amore, G.; Beloeil, P.A.; Boelaert, F.; Fierro, R.G.; Papanikolaou, A.; Rizzi, V.; Stoicescu, A.V. Zoonoses, Foodborne Outbreaks and Antimicrobial Resistance Guidance for Reporting 2023 Data. Eur. Food Saf. Auth. J. 2024, 21, 8586E. [Google Scholar] [CrossRef]
- Lee, S.; Rakic-Martinez, M.; Graves, L.M.; Ward, T.J.; Siletzky, R.M.; Kathariou, S. Genetic Determinants for Cadmium and Arsenic Resistance among Listeria monocytogenes Serotype 4B Isolates from Sporadic Human Listeriosis Patients. Appl. Environ. Microbiol. 2013, 79, 2471–2476. [Google Scholar] [CrossRef]
- Nunes, B.; Barata, A.R.; Oliveira, R.; Guedes, H.; Almeida, C.; da Silva, G.J.; Nogueira, T.; Saavedra, M.J.; Almeida, G. Occurrence and Diversity of Listeria monocytogenes in Portuguese Dairy Farms. Microbe 2024, 3, 100063. [Google Scholar] [CrossRef]
- Morente, E.O.; Fernández-Fuentes, M.A.; Burgos, M.J.G.; Abriouel, H.; Pulido, R.P.; Gálvez, A. Biocide Tolerance in Bacteria. Int. J. Food Microbiol. 2013, 162, 13–25. [Google Scholar] [CrossRef]
- Martínez-Suárez, J.V.; Ortiz, S.; López-Alonso, V. Potential Impact of the Resistance to Quaternary Ammonium Disinfectants on the Persistence of Listeria monocytogenes in Food Processing Environments. Front. Microbiol. 2016, 7, 638. [Google Scholar] [CrossRef]
- Langsrud, S.; Sidhu, M.S.; Heir, E.; Holck, A.L. Bacterial Disinfectant Resistance—A Challenge for the Food Industry. Int. Biodeterior. Biodegrad. 2003, 51, 283–290. [Google Scholar] [CrossRef]
- Elhanafi, D.; Dutta, V.; Kathariou, S. Genetic Characterization of Plasmid-Associated Benzalkonium Chloride Resistance Determinants in a Listeria monocytogenes Strain from the 1998–1999 Outbreak. Appl. Environ. Microbiol. 2010, 76, 8231–8238. [Google Scholar] [CrossRef]
- Müller, A.; Rychli, K.; Muhterem-Uyar, M.; Zaiser, A.; Stessl, B.; Guinane, C.M.; Cotter, P.D.; Wagner, M.; Schmitz-Esser, S. Tn6188—A Novel Transposon in Listeria monocytogenes Responsible for Tolerance to Benzalkonium Chloride. PLoS ONE 2013, 8, e76835. [Google Scholar] [CrossRef]
- Kovacevic, J.; Ziegler, J.; Wałecka-Zacharska, E.; Reimer, A.; Kitts, D.D.; Gilmour, M.W. Tolerance of Listeria monocytogenes to Quaternary Ammonium Sanitizers Is Mediated by a Novel Efflux Pump Encoded by emrE. Appl. Environ. Microbiol. 2015, 82, 939–953. [Google Scholar] [CrossRef]
- Kremer, P.H.; Lees, J.A.; Koopmans, M.M.; Ferwerda, B.; Arends, A.W.; Feller, M.M.; Schipper, K.; Seron, M.V.; van der Ende, A.; Brouwer, M.; et al. Benzalkonium Tolerance Genes and Outcome in Listeria monocytogenes Meningitis. Clin. Microbiol. Infect. 2017, 23, 265.e1–265.e7. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Metals and Contaminants in Food; EFSA: Parma, Italy, 2024; Available online: https://www.efsa.europa.eu/en/topics/topic/metals-contaminants-food (accessed on 28 April 2024).
- Nies, D.H. Efflux-Mediated Heavy Metal Resistance in Prokaryotes. FEMS Microbiol. Rev. 2003, 27, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.L.; Klawitter, L.A.; Bhaduri, S.; Stahl, H.G. Arsenite Resistance in Listeria monocytogenes. Food Microbiol. 1991, 8, 161–166. [Google Scholar] [CrossRef]
- McLauchlin, J.; Hampton, M.; Shah, S.; Threlfall, E.; Wieneke, A.; Curtis, G. Subtyping of Listeria monocytogenes on the Basis of Plasmid Profiles and Arsenic and Cadmium Susceptibility. J. Appl. Microbiol. 1997, 83, 381–388. [Google Scholar] [CrossRef]
- Parsons, C.; Lee, S.; Jayeola, V.; Kathariou, S. Novel Cadmium Resistance Determinant in Listeria monocytogenes. Appl. Environ. Microbiol. 2017, 83, e02580-16. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.; Phung, L.T. Bacterial Heavy Metal Resistance: New Surprises. Annu. Rev. Microbiol. 1996, 50, 753–789. [Google Scholar] [CrossRef]
- Lebrun, M.; Audurier, A.; Cossart, P. Plasmid-Borne Cadmium Resistance Genes in Listeria monocytogenes Are Present on Tn5422, a Novel Transposon Closely Related to Tn917. J. Bacteriol. 1994, 176, 3049–3061. [Google Scholar] [CrossRef] [PubMed]
- Dutta, V.; Elhanafi, D.; Kathariou, S. Conservation and Distribution of the Benzalkonium Chloride Resistance Cassette bcrABC in Listeria monocytogenes. Appl. Environ. Microbiol. 2013, 79, 6067–6074. [Google Scholar] [CrossRef]
- Korsak, D.; Chmielowska, C.; Szuplewska, M.; Bartosik, D. Prevalence of Plasmid-Borne Benzalkonium Chloride Resistance Cassette bcrABC and Cadmium Resistance cadA Genes in Nonpathogenic Listeria spp. Isolated from Food and Food-Processing Environments. Int. J. Food Microbiol. 2019, 290, 247–253. [Google Scholar] [CrossRef]
- Kuenne, C.; Billion, A.; Mraheil, M.A.; Strittmatter, A.; Daniel, R.; Goesmann, A.; Barbuddhe, S.; Hain, T.; Chakraborty, T. Reassessment of the Listeria monocytogenes Pan-Genome Reveals Dynamic Integration Hotspots and Mobile Genetic Elements as Major Components of the Accessory Genome. BMC Genom. 2013, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ward, T.J.; Jima, D.D.; Parsons, C.; Kathariou, S. The Arsenic Resistance-Associated Listeria Genomic Island LGI2 Exhibits Sequence and Integration Site Diversity and a Propensity for Three Listeria monocytogenes Clones with Enhanced Virulence. Appl. Environ. Microbiol. 2017, 83, e01189-17. [Google Scholar] [CrossRef]
- Parsons, C.; Lee, S.; Kathariou, S. Heavy Metal Resistance Determinants of the Foodborne Pathogen Listeria monocytogenes. Genes 2018, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Chmielowska, C.; Korsak, D.; Szmulkowska, B.; Krop, A.; Lipka, K.; Krupińska, M.; Bartosik, D. Genetic Carriers and Genomic Distribution of cadA6—A Novel Variant of a Cadmium Resistance Determinant Identified in Listeria spp. Int. J. Mol. Sci. 2020, 21, 8713. [Google Scholar] [CrossRef]
- Gray, J.A.; Chandry, P.S.; Kaur, M.; Kocharunchitt, C.; Bowman, J.P.; Fox, E.M. Characterisation of Listeria monocytogenes Food-Associated Isolates to Assess Environmental Fitness and Virulence Potential. Int. J. Food Microbiol. 2021, 350, 109247. [Google Scholar] [CrossRef]
- Lee, S.; Parsons, C.; Chen, Y.; Hanafy, Z.; Brown, E.; Kathariou, S. Identification and Characterization of a Novel Genomic Island Harboring Cadmium and Arsenic Resistance Genes in Listeria welshimeri. Biomolecules 2021, 11, 560. [Google Scholar] [CrossRef]
- Hingston, P.; Brenner, T.; Truelstrup Hansen, L.; Wang, S. Comparative analysis of Listeria monocytogenes plasmids and expression levels of plasmid-encoded genes during growth under salt and acid stress conditions. Toxins 2019, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Kuenne, C.; Voget, S.; Pischimarov, J.; Oehm, S.; Goesmann, A.; Daniel, R.; Hain, T.; Chakraborty, T. Comparative Analysis of Plasmids in the Genus Listeria. PLoS ONE 2010, 5, e12511. [Google Scholar] [CrossRef]
- PN EN ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- PN EN ISO 11290-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Part 2: Enumeration Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- Ruppitsch, W.; Pietzka, A.; Prior, K.; Bletz, S.; Fernandez, H.L.; Allerberger, F.; Harmsen, D.; Mellmann, A. Defining and Evaluating a Core Genome Multilocus Sequence Typing Scheme for Whole-Genome Sequence-Based Typing of Listeria monocytogenes. J. Clin. Microbiol. 2015, 53, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In Silico Detection and Typing of Plasmids Using PlasmidFinder and Plasmid Multilocus Sequence Typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Gelbicova, T.; Florianova, M.; Hluchanova, L.; Kalova, A.; Korena, K.; Strakova, N.; Karpiskova, R. Comparative Analysis of Genetic Determinants Encoding Cadmium, Arsenic, and Benzalkonium Chloride Resistance in Listeria monocytogenes of Human, Food, and Environmental Origin. Front. Microbiol. 2021, 11, 599882. [Google Scholar] [CrossRef] [PubMed]
- Minarovicova, J.; Veghova, A.; Mikulasova, M.; Chovanova, R.; Soltys, K.; Drahovska, H.; Kaclíkova, E. Benzalkonium Chloride Tolerance of Listeria monocytogenes Strains Isolated from a Meat Processing Facility Is Related to Presence of Plasmid-Borne bcrABC Cassette. Int. J. Gen. Mol. Microbiol. 2018, 111, 1913–1923. [Google Scholar] [CrossRef]
- Moretro, T.; Schirmer, B.C.T.; Heir, E.; Fagerlund, A.; Hjemli, P.; Langsrud, S. Tolerance to Quaternary Ammonium Compound Disinfectants May Enhance Growth of Listeria monocytogenes in the Food Industry. Int. J. Food Microbiol. 2017, 241, 215–224. [Google Scholar] [CrossRef]
- Briers, Y.; Klumpp, J.; Schuppler, M.; Loessner, M.J. Genome Sequence of Listeria monocytogenes Scott A, a Clinical Isolate from a Food-Borne Listeriosis Outbreak. J. Bacteriol. 2011, 193, 4284–4285. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).