Abstract
Previous research has shown that freshwater edible fish imported into Australia are not compliant with Australian importation guidelines and as a result may be high risk for bacterial contamination. In the present study, the outer surface of imported freshwater fish were swabbed, cultured, confirmatory tests performed and antimicrobial patterns investigated. Channidae fish (Sp. A/n = 66) were contaminated with zoonotic Salmonella sp./Staphylococcus aureus (n = 1/66) and other bacteria implicated in cases of opportunistic human infection, these being Pseudomonas sp. (including P. mendocina and P. pseudoalcaligenes (n = 34/66)); Micrococcus sp. (n = 32/66); Comamonas testosteroni (n = 27/66) and Rhizobium radiobacter (n = 3/66). Pangasiidae fish (Species B/n = 47) were contaminated with zoonotic Vibrio fluvialis (n = 10/47); Salmonella sp. (n = 6/47) and environmental bacteria Micrococcus sp. (n = 3/47). One sample was resistant to all antimicrobials tested and is considered to be Methicillin Resistant S. aureus. Mud, natural diet, or vegetation identified in Sp. A fish/or packaging were significantly associated with the presence of Pseudomonas spp. The study also showed that visibly clean fish (Sp. B) may harbour zoonotic bacteria and that certain types of bacteria are common to fish groups, preparations, and contaminants. Further investigations are required to support the development of appropriate food safety recommendations in Australia.
1. Introduction
The growth of the fish farming (FF) sector in many countries, the ‘blue revolution’ [1], occurred without regulatory guidance or enforcement of environmental or food safety standards [2]. High stocking density/fish stress [3,4,5], lack of appropriate biosecurity barrier controls [6] and inadequate isolation of seed stock [7,8,9] have all contributed to the spread of bacteria in FF. As a consequence, the use of prophylactic and therapeutic antibiotics has increased to compensate for inadequate sanitary practices [10,11,12,13,14,15]. Approximately 90% of global FF takes place in countries that have no or few effective regulations for antibiotic use [16,17,18,19,20]. Despite the advances in bacterial control in FF, phytobiotics to improve disease tolerance [21,22], phytocompounds with antimicrobial activity [23], vaccines and improved husbandry practices [24,25,26], phage therapy and research into antimicrobial peptides [27] and biosynthesized nanoparticles [28,29], antibiotics are still injudiciously applied to control bacterial disease in some countries [30,31]. Antibiotics used to inactivate or inhibit bacteria may accumulate in the ponds, sediments and fish raised in the FF system [2]. A 2016 study showed results which supported the hypothesis that selection pressures present in FF favour rapid alterations in pathogenic bacterial populations. These alterations are likely to have enduring evolutionary effects on pathogen virulence [32]. According to Shi, et al. [33], bacterial pathogens are now the ultimate disease affecting aquatic species. This conclusion is supported by Cai, et al. [34], Hoai, et al. [35] and Reverter, et al. [36].
Many of the world’s largest producers of farmed fish use the VAC system (Vuon: garden, Ao: pond and Chuong: livestock pen), fish systems for commercially intensive production [37], pond–dyke [38,39] and paddy-fish farming [40], lake/reservoir [41] and polyculture systems [42]. Fish may be fed food waste and animal manure [37], pelleted feed or raw feed, soybean and grass often grown in pond sludge [42], homemade diets consisting of food waste, such as chicken bones/viscera, and kitchen remains [43]. According to Chávez-Crooker and Obreque-Contreras [44], sediments organically enriched through intensive FF will produce an ecosystem which is dominated predominantly by bacteria. In addition, the extractive species, such as bivalves, aquatic plants and algae, which utilise water nutrients also accumulate antibiotics administered to other species in these intensive production systems [45]. Aquatic pollution discharged from households, farmed livestock, hospitals and untreated sewerage are also potential sources of contamination of resistant genes into water and sediment in FF facilities [46]. Preena et al. [47] notes that antibiotic resistance patterns can be transferred between clinically important strains through horizontal gene transfer, and that the majority of farmed fishes are contaminated with a broad spectrum of multiply resistant bacteria. In Bangladesh, a pattern of resistance was observed for Klebsiella pneumoniae and to a lesser extent E. coli and P. aeruginosa isolated from urinary tracts of infected women in Sylhet [48] and other recently isolated clinical samples in Jessore [49]. High patterns of resistance were correspondingly shown in K. pneumoniae and E. coli isolates from farmed Hilsa shad (Tenualosa ilisha) in the Dhaka and Chittagong regions [46] against ten antimicrobials [46,49]. According to Slettemeås, et al. [50], the global trade in animal products and food may represent significant dissemination routes which enable important resistant bacterial types to be introduced into geographic areas outside usual areas of endemicity.
In recent years many environmental bacteria previously considered of no consequence to human health have emerged as serious human pathogens. Certainly, ‘high risk environments’ for bacterial genetic resistance exchange are sewage, wastewater, discharge from hospitals, aquaculture and waste from livestock, agriculture and abattoirs [51,52,53,54], all of which are present in many of the main producers of freshwater fishery products (FP) exported to Australia.
A variety of zoonotic bacterial species have been identified in imported vacuum packed and frozen catfish (Pangasius) fillets [55,56,57]. Vibrio fluvialis and Salmonella spp., which cause intense diarrhoea, have been identified on fresh and frozen fish offered for retail sale [58,59,60] or in water or fish at FF facilities [61,62,63].
Zoonotic bacteria contaminating FP are able to infect humans in three ways: contact with the aquatic animal, FP or water that they inhabit [64], via consumption of under cooked FP [65], or from the toxin produced by the bacteria contaminating the FP [66]. Opportunistic infection of humans with non-zoonotic bacteria has also been reported following contact with contaminated environmental sources [67,68,69,70,71,72,73]. Zoonotic bacteria may not only cause illness if consumed, but according to Economou and Gousia [74], due to the over/misuse of antimicrobials in food production a reservoir of antimicrobial resistant bacteria can be transmitted to humans via contact, both direct or indirect. Bortolaia, et al. [75] hypothesises that humans may acquire antimicrobial resistant bacteria after consumption or handling of contaminated food. Therefore, in order to protect consumers and vulnerable members of the community, it is expected that testing procedures for detection of zoonotic bacteria in imported farmed fish should be robust.
As per the ‘Imported Food Control Regulations 2019’ [76] and the ‘Imported Food Control Act 1992’ [77], the relevant Australian minister may classify a certain imported edible product as ‘risk’ food and this is based on the product in question being a high or medium risk to public health. In Australia at the present time (2022), the only additional test applied to ‘risk’ imported fish, cooked, smoked, smoke flavoured or vacuum packed [78], is for the zoonotic bacterium Listeria monocytogenes (Figure 1) as per Schedule 27 ‘Microbiological limits in food’ in the Australia and New Zealand Food Standards Code [79].
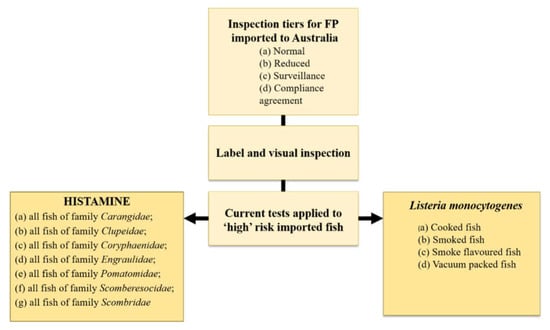
Figure 1.
The current tests applied to high risk imported fish on entry to Australia. Listeria monocytogenes at present is the only additional test for bacteria applied to imported fish on entry to Australia. Original figure based on information in the Imported Food Control Order, 2020.
Any additional tests applied to imported seafood must be justified according to the global trade agreements signed by Australia [80]. Williams et al. [81] developed a risk scoring system to identify countries as high risk for supply chain breaches which may impact the safety of edible finfish imported into Australia and to justify and prioritise examination of these products for supply chain breaches, such as zoonotic parasites/bacteria and physical and other biological hazards. Six predictor variables were scored to achieve an outcome variable of ‘Freshwater fish high risk’. Each country was allocated a unique numerical and anonymous identifier. Imported freshwater fish were examined from two of the highest scoring countries for supply chain breaches identified as Country 20 and 22 in Williams et al. [81] and in the present study. The Williams et al. [81] study identified a number of supply chain breaches, such as mud, vegetation, natural diet, snails and other physical hazards from poorly processed ‘consumer ready’ fish and in the same study the outer surface of each fish was swabbed for bacterial culture in the present study.
The aims of the current study were to identify and compare selected bacterial flora of imported edible freshwater finfish, Sp. A/Country 22 and Sp. B/Country 20, and describe the antimicrobial resistance pattern for identified bacteria. In addition, associations between the presence of bacteria and contaminants, such as mud, vegetation, and natural diet, were also explored.
The purpose of this study was to identify bacteria on edible fish imported into Australia, not to disadvantage an exporting country. Therefore, information that may lead to the identification of an included country, such as fish species names, has been omitted from the manuscript and auxiliary tables. Any published literature cited in text omitted to maintain country confidentiality is indicated with (*).
2. Materials and Methods
2.1. Sampling
A total of 60 entire imported Sp. A fish, 41 fillets of imported Sp. B fish and thawed ice samples ‘bag water’ from each of the 12 separate bags of fish were swabbed for bacterial culture using a sterile culture swab as per Saito et al. [82]. ‘Bag water’ and Sp. A and Sp. B results were combined to reflect 66 and 47 samples, respectively (Sp. A: 6 individual bags of fish (n = 60 fish) + 6 ‘bag water’ samples = 66 samples in total, and Sp. B (6 individual bags of fish (n = 41 fish) + 6 ‘bag water’ samples = 47 samples in total)). Both Sp. A and B were pre-packaged, frozen, obtained from a retail outlet and maintained frozen until thawed for sampling. Sample size was based on availability at the time fish were purchased. Species A had been frozen in a single layer (original packaging), in two separate rows, and were embedded in a solid block of ice in each of the 6 bags. Species B fish were frozen individually in each bag and each fillet was glazed with a thin covering of ice. A detailed description of the type and condition of fish packaging is included in Table 1. All fish were thawed, according to packet instructions, in a refrigerated unit (~24 hours (hrs)) in original unopened bags. The bag was split using sterile scissors without touching the fish or bag water. The skins of each fish, which were entire, were swabbed by gently scraping twice along the external surface of the lateral flank using the same sterile swab. For each fish fillet the swab was gently scraped twice along the outer lateral surface. The ‘bag water’ was sampled by dipping one sterile culture swab into the fluid once without touching the bag. Following sampling, the contents of each bag were examined and irregularities, such as the presence of mud, vegetation, food items and other debris, recorded for future statistical analysis.

Table 1.
Processing observations of fish and packaging in this study and corresponding sample numbers.
2.2. Bacterial Species Selection
Due to serious time constraints, it was not possible to culture broadly for all bacteria contaminating the outer surface of Sp. A and Sp. B. Specific bacteria targeted in this investigation were chosen based on the country of origin of the FP, the supply chain breaches, such as mud identified in Williams, et al. [81], water and sanitation information in Wendling, et al. [83], and published literature which describes each bacterium having been identified in FF, sediment/mud, water or FP in Countries 20 and 22 (*). Due to the identification of mud and other debris contaminating FP from Country 22 [81] and the low scores in Wendling, et al. [83] for water and sanitation in both countries, E. coli and Pseudomonas sp. were chosen as total faecal and water indicators as described in Odonkor and Ampofo [84], Rodrigues, et al. [85] and Odjadjare and Ebowemen [86]. Salmonella sp./Staphylococcus aureus were selected as an indicator of faecal/bacterial contamination during processing [87]. Clostridium sp. were selected as an indicator of excreta contamination from human sewerage/non-herbivorous wildlife as demonstrated in Vierheilig et al. [88] or as an indigenous bacterium of fish, from cross contamination during processing [87]. All targeted bacterial species in this study have been identified in either FF or FP in Countries 20 and 22 (*).
2.3. Diluent Preparation, Isolation, Enumeration and Identification
ThermoFisher selective media plates were used for the isolation of target bacteria. Brilliance E. coli/coliform (EC) agar (product code (PC): PP2609) was used to isolate E. coli, Baird Parker agar ((BP) PC: PP2069) was used to isolate S. aureus, TSC No Egg Yolk ((TSC) PC: PP2595) for Clostridium spp., Brilliance Salmonella Chromogenic media ((SAL) PC: PP2351) for Salmonella spp. and for Pseudomonas spp. CFC Pseudomonas selective agar was used ((CFC) PC: PP2161). Peptone water (PC: TM0057) (1.5 mL) was used as a diluent according to manufacturer instructions to inoculate TSC, BP, CFC plates. For SAL plates, the fish swab was added to 1.5 mL of buffered peptone (TM1558) in sterile Eppendorf® tubes and incubated at 40 °C for 24–30 hrs according to product guidelines before inoculating each plate. All selective media plates were inoculated using a sterile 10 µL COPAN inoculating loop (PC: COP-H10) and then incubated at 35 °C for 48 hrs.
Bacterial colonies on plates were counted according to the quadrant method [89]. Bacterial colonies were marked with a highlighter on the culture plate as each colony was counted. As the antibiotics in selective agar can interfere with antimicrobial sensitivity and other confirmatory tests, bacterial isolates obtained from selective media plates (BP; TSC; SAL; CFC) and identified based on Gram stain were diluted in one mL of sterile saline and regrown on two separate Mueller Hinton agar plates ((MH) PCPP2096). One plate was used for antimicrobial disk diffusion susceptibility testing and the other for specific confirmatory tests. All sampling was conducted using a sterile 10 µL COPAN inoculating loop.
2.3.1. Antimicrobial Resistance
Bacterial isolates were prepared according to the Kirby–Bauer Disk Diffusion Susceptibility Test Protocols [90]. Sterile saline was used for the bacterial suspension (variable dilutions) to achieve a 0.5 McFarland turbidity standard against a Wickerham card. The specific antimicrobial diffusion disks used were selected from the WHO [91] as critically important antimicrobials for human medicine. Four of the antimicrobials selected for evaluation were from categories C1 (‘sole, or one of limited available therapies, to treat serious bacterial infections in people’) and C2 (‘used to treat infections caused by bacteria possibly transmitted from non-human sources, or with resistance genes from non-human sources’) and one was in the C2 category only. All antimicrobials selected are used in FF or have been identified contaminating farmed freshwater fish in published literature [91,92,93,94,95,96,97,98,99,100]. Antimicrobial disks used in this study were obtained from ThermoFisher. Availability of some antimicrobial disks during COVID restricted a broader range of resistance testing. The following antimicrobial disks were used: (critically*/highly important#), Cefovecin* (3rd generation Cephalosporin) (30 µg PC: CT1929B), Cefoxitin# (2nd generation Cephalosporin) (30 µg PC: CT0119B), Ciprofloxacin* (Quinolone) (5 µg PC: CT0425B), Fosfomycin* (Phosphonic acid derivative) (50 µg PC: CT01838) and Vancomycin* (Glycopeptide) (30 µg PC: CT0058B) as per Okoh and Igbinosa [101], Tall, et al. [102], Kaysner, et al. [103] and Lesmana, et al. [104]. Only Vibrio fluvialis was tested for sensitivity to Colistin* (Polymyxin) (10 µg PC: CT0017B) and Cephalothin (1st generation Cephalosporin) (30 µg PC: CT0010B) which is not included in WHO [91] lists. Antimicrobial disks were manually placed on MH agar using sterile tissue forceps. Antimicrobial sensitivity evaluation was conducted 24 hrs following MH plate inoculation. Isolates from BP plates were cultured for a further 24 hrs as advised in the Clinical and Laboratory Standards Institute [105] (CLSI). Breakpoint zone measurements where possible were obtained from CLSI [105] and where a suitable category did not exist from Hudzicki [90], Reynolds [106] and Behera, et al. [107]. Breakpoint zones were described as either sensitive, intermediate, or resistant.
2.3.2. Isolate Identification
Bacterial isolates from the second MH culture plate were Gram stained according to Beveridge [108] and were examined at 100X magnification with emersion oil using an Upright Motorized Microscope ECLIPSE Ni-E, Nikon, Japan, fitted with a computer screen and camera to observe bacteria morphology. All reagents were obtained from ThermoFisher. Micrococcus sp. and Staphylococcus aureus were differentiated using Coagulase (slide and tube) (PC: R21051), Oxidase (PC: MB0266B) and Catalase testing. Salmonella sp. was confirmed using the Salmonella Latex Test (DR1108A). Isolates from CFC Pseudomonas plates (15 tested) and Gram-negative oxidase positive isolates from TSC plates with the same growth morphology (12 tests), all pale-yellow small round/globular colonies (3 tests) and purple colonies from SAL plates were tested according to Rapid IDTM NF Plus System (PC: R8311005). For Rapid ID™ testing 1 mL of Inoculation Fluid (PC: R8325102) was added to each bacterial isolate to achieve a visual turbidity of ≥1.0 and <3.0 according to McFarland standard. The bacterial solution was added to each Rapid ID™ rack and incubated for 4 hrs before scoring the ten wells as positive or negative according to the Rapid IDTM NF Plus colour guide. Reagents Rapid NF Plus reagent (PC: R8311005), RapID™ Spot Indole Reagent (PC: R8309002) and RapID Nitrate A. Reagent (PC: R8309003) were then added to appropriate test wells according to product information. Wells 4–10 were rescored against the same colour guide. Results were entered into the Rapid NF Plus Code Compendium (ERICTM) for identification and reports for each test downloaded. Following the 15 biochemical tests to confirm species identity of representative colonies grown on CFC Pseudomonas plates, other colonies were presumptively confirmed as Pseudomonas sp. based on growth on selective CFC media, negative Gram stain, characteristic morphology, positive Catalase and Oxidase, negative Coagulase and resistant to antimicrobials Cefovecin, Cefoxitin and Vancomycin. Comamonas testosteroni not tested using Rapid IDTM NF Plus System (RNF Plus) were identified based on Gram stain, characteristic morphology, negative lactose-fermentation (MacConkey without Salt Agar Plates (PC: PP2016)), positive Catalase and Oxidase.
2.4. Data Analysis
Data were expressed as the actual number of fish contaminated and the range of bacterial colonies for each bacterial species or genera identified in Sp. A and Sp. B. The recovery rate (RR) of bacterial contamination in all fish was calculated according to Bush, et al. [109] and calculation of bacterial colonies based on Smith & Brown [110].
Associations between presence or absence of bacteria and a set of explanatory variables or potential factors were investigated using univariable logistic regression analyses, when sufficient data were available. Explanatory variables in the present study were based on the supply chain breaches identified in Williams, et al. [81] and included ‘mud’, ‘natural diet’, and ‘components of natural diet’ which were absent or present in each bag of fish. Analyses were conducted using statistical software ‘R’ (R Core Team 2020). The explanatory variables used were fish family, mud, vegetation, and natural diet. Best model fit was assessed using the Chi-Square Test and odds ratios and confidence intervals were calculated to quantify the strength of an association between the occurrence of bacteria and predictor. Only relationships that resolved to significance (p < 0.05) are reported in the results section. In some instances, insufficient data were available to develop a robust model. Either count data were one-sided or substantially unbalanced. These data were omitted from statistical analyses.
3. Results
The bacteria (cfu/g = colony forming units per gram) found in imported Sp. A and Sp. B are included in Table 2. Antimicrobial disks, Colistin (CT) and Cephalothin (KF), were tested as diagnostically significant for V. fluvialis only (Table 3). No growth of E. coli or Clostridium sp. was observed on EC or TSC indicator agars, respectively, and are omitted from results.

Table 2.
List and number of bacteria found between the two species of imported fish colonies per gram (cfu/g). Bag water from Sp. A (n = 6) and Sp. B. (n = 6) has been included in the fish overall number. Mean not calculated when only one fish contaminated *.

Table 3.
Growth media and tests for identification of bacteria.
3.1. Species A from Country 22
Mud was present in 46.9% of samples from Sp. A and represented by all fish in three of the six bags of fish examined. Natural diet (snail shells, cricket legs, other small crustaceans, and insect larvae) was identified in 65.1% of the samples, represented by four bags of Sp. A fish, and vegetation (green matter, sticks/twigs, and other decomposing vegetable matter) was identified in 18.1% of fish, all coming from one bag only of the Sp. A examined (Table 1).
Pseudomonas sp. was the dominant bacteria isolated from the skin of imported Sp. A fish with an overall RR of 51.5%, Micrococcus sp. had an RR of 48.5% and S. aureus (S700) an RR of 1.5% (Table 2). Comamonas testosteroni (syn. Delftia testosteroni) had an RR of 40.9%, and Rhizobium radiobacter was isolated from three samples (4.5%) (S468; S655; S690), with Salmonella sp. being isolated from one sample (1.5%) (S469).
3.1.1. Pseudomonas sp.
Only Pseudomonas sp. was grown on CFC selective agar. Of the 66 swabs cultured, 34 were positive for Pseudomonas sp. with a mean cfu/g of 104.0. Of the 15 isolates tested using the RNF Plus, 60% (N = 9) were identified as P. pseudoalcaligenes and 40% (N = 6) as P. mendocina (Table 2). No difference in colony morphology was observed between Pseudomonas pseudoalcaligenes and P. mendocina on either CFC or MH agar at 24 hrs. Remaining samples were presumptively identified as Pseudomonas sp. based on tests in Table 3. Of the six P. mendocina identified using the RNF Plus there were no test contraindications and 100% of the samples tested returned a probability score of 92.11% and bio-score of 1/7 according to the ERICTM Compendium. Species level identification of P. mendocina was confirmed by glucose oxidation. Of the nine P. pseudoalcaligenes identified using the RNF Plus there were no test contraindications for eight of the samples tested and each returned a probability score of 97.21% and bio-score of 1/3. Sample S689 identified as P. pseudoalcaligenes had a probability score of 79.08%, a bio-score of 1/49 and slight overlap with P. stutzeri. Species level identification in all samples of P. pseudoalcaligenes was confirmed by glucose alkalisation.
A complete pattern of resistance was demonstrated by Pseudomonas sp. for Cefovecin, Cefoxitin and as expected to Vancomycin (Table 4).

Table 4.
Percentage of antimicrobials susceptible (S), intermediate (I) or resistant (R). From *Sp. A and from #Sp. B. CVN (Cefovecin), FOX (Cefoxitin), CIP (Ciprofloxacin), FOS (Fosfomycin), VAN (Vancomycin), Colistin (CT) and Cephalothin (KF).
3.1.2. Micrococcus sp. and Staphylococcus aureus
3.1.3. Comamonas testosteroni (syn. Delftia testosteroni)
Colonies on TSC were exclusively C. testosteroni cultured from 27/66 swabs and with a mean of 61.7 cfu/g (Table 2). Of the twelve C. testosteroni identified using the RNF Plus there were no test contraindications for 100% of the samples tested. Ten of the samples returned a probability score of 96.13% and bio-score of 1/9. Samples S439 and S649 returned a probability score of 95.45% and bio-score of 1/24 and showed a slight overlap with Burkholderia cepacia. Species level identification of C. testosteroni in all samples was confirmed by glucose alkalisation. Comamonas testosteroni was then presumptively identified based on negative Gram stain, characteristic morphology, negative lactose-fermentation, positive to Catalase and Oxidase (Table 3).
3.1.4. Rhizobium radiobacter
Rhizobium radiobacter was cultured on MH agar from 3/66 swabs and had a mean of 7.6 cfu/g (Table 2). All A. radiobacter were identified using the RNF Plus and all samples tested returned a probability score of 99.9% and a bio-score of 1/8 (Table 3). All three isolates grew as red colonies on MacConkey agar as described in Chanza, et al. [111] from an isolate in a human pulmonary abscess case, Spain, identified using mass spectrometry MALDI-TOF®, Beckman Coulter® biochemical profile and molecular method.
3.1.5. Salmonella sp.
3.2. Species B Fish from Country 20
Vibrio fluvialis was the dominant bacteria isolated from the outer surface of imported Sp. B fillets with an RR of 21.3%, followed by Micrococcus sp. with an RR of 6.0% and Salmonella sp. with an RR of 1.5%. One sample (S1013) was positive for S. aureus. Vibrio fluvialis was identified from four of the six bags tested, with bags one and four each having four positive samples. Within the ten positive samples of V. fluvialis, three were identified in the ‘bag water’ (S878; S908; S1007). Micrococcus sp. and Salmonella sp. were all recovered from the outer surface of the fillets from two and three bags, respectively (Table 2). Bag three had the most diverse bacterial flora with V. fluvialis (S908; S912), Micrococcus sp. (S911) and Salmonella sp. (S910; S912) identified. Two samples (S912; S1036) were positive for both Salmonella sp. and V. fluvialis. All of the 47-fish examined from six separate bags were clean with no visible mud, food items or vegetation (Table 1).
3.2.1. Vibrio fluvialis
Vibrio fluvialis grew on SAL from 10/47 swabs cultured and a mean of 13.9 cfu/g (Table 2). All samples were identified using RNF Plus. Samples S878 and S879 had a small percentage overlap and S916 one contraindication all for Plesiomonas shigelloides in the RNF Plus system. DNAse activity and negative Lysine decarboxylase (PC: CM0308S) confirmed samples as V. fluvialis (Table 3).
3.2.2. Salmonella sp.
3.2.3. Micrococcus sp. and S. aureus
Micrococcus sp. was cultured from 3/47 swabs with a mean of 4.6 cfu/g and S. aureus was cultured from one swab and 1.0 cfu/g only was observed (Table 2). Micrococcus sp. and S. aureus identification followed steps described in Table 3. The detailed antimicrobial patterns of resistance are included in Table 4. It is noted that Staphylococcus aureus was resistant to all antimicrobials tested.
3.3. Associations between Identified Biosecurity Breeches (Mud, Food Items or Vegetation) and Bacteria
Insufficient data were available for several predictor variables to confidently report associations with presence or absence of bacteria species, due to zero counts for at least one of the predictor levels. For example, Staphylococcus aureus, V. fluvialis and Salmonella species were only present in specimens where mud was absent. Similarly, these bacteria species were only present at low levels in specimens where components of a natural diet or vegetation were absent. Rhizobium (syn. Agrobacterium) radiobacter and Pseudomonas spp. were observed in Sp. A, but not in Sp. B. In contrast, V. fluvialis was reported in Sp. B but not Sp. A (Table 5).

Table 5.
Occurrence of bacteria species by predictor variables. Bracketed numbers in ‘Occurrence’ column show the total count of fish from Sp. A (n = 60) and Sp. B. (n = 41). Bag water not included in statistical evaluation. Predictor variables based on supply chain breaches identified in Williams et al. (2021).
Where adequate data were available, univariable logistic regression identified significant associations between predictor variables and bacteria occurrence (Table 6). The identification of mud in specimens was significantly associated with the presence or absence of Pseudomonas spp. Where mud was recovered from specimens, the recovery of Pseudomonas spp. was 7.58 (CI 2.95, 20.72) more likely (p < 0.001). The presence of environmental contaminants was also associated with an increased likelihood of Pseudomonas spp. contamination. Both the presence of natural diet components (OR 8.46; CI 3.37, 22.93), and the presence of vegetation (OR 13.10; CI 3.10, 90.26) were significantly associated with recovery of Pseudomonas spp. (p < 0.001).

Table 6.
Associations between predictor variables and bacteria. Odds ratios calculated using the first level as a reference. Bracketed numbers in ‘Occurrence’ column show the total count. Sp. A (n = 60) and Sp. B. (n = 41). Bag water not included in statistical evaluation. Predictor variables based on supply chain breaches identified in Williams et al. (2021).
Fish species was significantly associated with some bacteria species. Pseudomonas spp., R. radiobacter and C. testosteroni were only identified in Sp. A and V. fluvialis in Sp. B. One sample of S. aureus was identified in each of the Sp. A and Sp. B samples. The occurrence of Micrococcus sp. was more likely (OR 11.84; CI 3.75, 52.80; p < 0.05) in Sp. A and Salmonella sp. less likely (OR, 0.09; CI 0.005, 0.61; p < 0.001) in Sp. A than in Sp. B.
4. Discussion
The present study aimed to identify if selected bacteria are present on the skin or outer surface of two edible fish products from Countries 20 and 22, and to explore associations between bacteria isolated and a set of explanatory variables which were based on the supply chain breaches identified in Williams, et al. [81]. Zoonotic bacteria, S. aureus and Salmonella sp., C. testosteroni with zoonotic potential and R. radiobacter, Micrococcus sp., P. mendocina and P. pseudoalcaligenes implicated in opportunistic cases of human infection were cultured from the skin/outer surface and the ‘bag water’ of edible imported Sp. A (Country 22). Zoonotic species Vibrio fluvialis, Salmonella sp. and S. aureus and the opportunistic Micrococcus sp. were identified from Sp. B fish (Country 20). Univariable logistic regression analysis demonstrated that Pseudomonas spp. were significantly associated with the presence of mud, natural diet components and presence of vegetation. This infers that these types of breaches to importation regulations have potential to introduce zoonotic/opportunistic bacteria into the Australian food chain. Species A (Country 22) had the greatest diversity of bacteria. Antimicrobial sensitivity testing demonstrated that for some samples of Salmonella sp. and S. aureus, resistance was demonstrated to all antimicrobials tested. This study showed that the outermost surface of imported frozen edible freshwater fish has potential to contaminate hands, work surfaces, kitchen sponges and other kitchen utensils with bacteria which are not only a human health concern but are capable of forming a persistent biofilm. All species or genera of bacteria identified from Sp. A and Sp. B have been implicated in cases of community and/or nosocomial infection, including a greater vulnerability to infection demonstrated in the immune compromised. In this study only a small area of fish skin was swabbed and therefore it is considered that there is significant contamination of the skin of Sp. A with Pseudomonas species (P. mendocina and P. pseudoalcaligenes), C. testosteroni and Micrococcus sp. and with V. fluvialis and Salmonella in Sp. B.
According to Australian importation policy, for Sp. A and Sp. B, all consignments must be clean and be free of ‘live insects, seeds, soil, mud, clay, animal material (such as faeces), plant material (such as straw, twigs, leaves, roots, bark) and other debris prior to arrival into Australian territory’ [112]. There was a significant association between mud, natural diet and vegetation and the presence of Pseudomonas spp. in specimens of Sp. A. As an environmental bacterium, the presence of Pseudomonas spp. in mud seems likely, however the ability to form a biofilm on environmental debris [113,114] may explain the association with natural diet, vegetation and other debris observed in bags of contaminated fish. Whilst this association was identified for Pseudomonas spp., other environmental bacterium which are associated with mud and can adopt a biofilm existence, such as C. testosteroni and R. radiobacter, as identified in this study are also a contamination concern on the aforementioned debris.
The natural diet identified in Sp. A in the present study consisted of Lymnaeid and Planorbid snails both of which prefer lentic environments with stagnant water [115]. Species A were imported into Australia from a country (Country 22) which, according to Wendling, et al. [83], has a country score of 0/100 for ‘Wastewater Treatment’ and less than ‘30/100′ for ‘Water and Sanitation’ (lower scores representing least favourable). Pseudomonas spp. are associated with polluted and eutrophic freshwater [116,117]. It seems mud and this species of bacteria are synonymous in fish from countries with poor environmental controls in FF and the Pseudomonas: mud relationship closely associated with fish habitat and feeding presences. Consequently, failure to comply with Australian importation guidelines has potential to introduce Pseudomonas spp. into the Australian food chain.
As Sp. A and Sp. B are not ‘risk’ foods they are subject to a reduced rate of inspection on entry into Australia. Five percent of samples may be randomly selected for label/visual inspection or sent for analysis to determine if the product is compliant with Australian food safety standards. However, if the exporting country has a compliance agreement with Australia, Sp. A and Sp. B may not be inspected on entry providing the product is accompanied by a declaration of safety compliance ‘under the supervision of the competent authority and/or systems approved by the competent authority’ [76,77,112,118,119]. Whilst microbiological limits are set for some ‘risk’ seafood (shellfish, prawns, crabs) imported into Australia in the FSANZ [120], other than L. monocytogenes [78], the zoonotic bacteria identified in the present study may not be isolated from Sp. A or Sp. B if tested on entry into Australia. According to the international health standards established by Codex Alimentarius [121] the ‘Code of Practice for Fish and Fishery Products’ (CXC 52-2003) states ‘to remove foreign debris and reduce bacterial load prior to gutting’ (whole fish) and glazing dips should be replaced periodically ‘to minimize the bacterial load.’ In section 22.1.2 of the ‘code of practice’, ‘Reception of frozen products at retail’ ‘evidence of filth or contamination’ at the retail level is an ‘unlikely’ hazard [122], although high levels of filth and contamination were identified in Sp. A in the current study.
Of the zoonotic bacteria identified in the present study, Salmonella species and S. aureus are the only bacteria included as a potential hazard for the fish examined in CXC 52-2003 [123]. Vibrio spp. is mentioned, which ‘can be controlled by thorough cooking and preventing cross-contamination of cooked products’ and ‘indigenous pathogenic bacteria, when present on fresh fish, are usually found in fairly low numbers, and food safety hazards are insignificant where products are adequately cooked prior to consumption’. The Codex code of practice for fishery products fails to recognise pathogenic bacteria on the surface of uncooked thawed imported fish as a potential human health hazard. Contamination of hands, cutting surfaces and kitchen utensils prior to cooking is a significant threat and one which has been described in published literature [124,125,126,127,128]. Far from indigenous bacteria occurring in low numbers in the present study, bacteria C. testosteroni, Pseudomonas spp., V. fluvialis and Micrococcus sp. had a high RR. Salmonella sp. had a medium RR which may indicate that water used for glazing fish fillets during processing is not compliant with CXC 52-2003 standards [123].
Species of fish was also significantly associated with certain species of bacteria. Whilst Sp. A had many breaches according to Australian edible fish importation requirements, Sp. B appeared clean and well glazed, indicating separate bacterial hazards exist for both groups of fish from different regions and some of these hazards are not observable. Therefore, targeting high risk fish for bacterial contamination on entry into Australia seems achievable. In addition, in compliance with ‘The General Agreement on Sanitary and Phytosanitary Measures’ [121], developed nations are encouraged to help developing nations to reach food safety compliance. Inexperienced or untrained labour may be responsible for the variable processing standard of Sp. A, and this may be an area where Australia could offer assistance.
Micrococcus sp. was isolated from both Sp. A and Sp. B. Micrococcus spp. are common across aquatic and terrestrial ecosystems, the skin of humans, other endothermic animals [129] and frequently contaminate food. Micrococcus spp. have been identified at farm level in cultured Nile tilapia (Oreochromis niloticus) [130,131], striped catfish (Pangasius hypophthalmus) [56], isolated during filleting and trimming of cultured striped catfish [56] and cassava fish (Pseudotolithus sp.) [132]. Micrococcus spp. contamination has been described in preserved [133] and fermented fish at the retail level in developing countries [134,135]. Pathogenicity of Micrococcus spp. has been multiply described in cases of opportunistically acquired community human infection [69,136,137,138,139,140,141,142], demonstrating the potential for contaminated imported FP to be a health risk to consumers.
In the present study, Micrococcus spp. was resistant to Cefovecin and Vancomycin. Traditionally, Micrococcus spp. are sensitive to a broad range of antimicrobials [138,143,144]; however, as in this study, resistance to Vancomycin has been described in some clinical cases of human infection [140,145]. The occurrence of Micrococcus in edible fish imported to Australia deserves greater attention.
Comamonas testosteroni was identified in Sp. A only. This bacterium is common in the environment [146,147] but has a demonstrated prevalence in anthropogenically impacted environments [148], including polluted lakes/regions, agricultural land, urban sewerage [149] and farm polluted aquifers [116]. Comamonas testosteroni has been identified in cultured common carp (Cyprinus carpio & Carassius auratus) [150], Nile tilapia and African sharp-tooth catfish (Clarias gariepinus) [151]. Community transmission has occurred via minor abrasions from fish and contaminated food [147,152,153]. Smith and Gradon [152] reported a case of human infection from C. testosteroni speculated to have been transferred from diseased tropical fish and the zoonotic potential of this species requires clarification. Due to an ability to utilise nitrate as a substrate, this aerobic species can exist in anaerobic conditions and adopt a biofilm existence [154]. This species has been multiply reported in opportunistic community acquired invasive infections [68,147,153] and imported FP contaminated with this bacterium may be a consumer risk.
In the present study C. testosteroni was sensitive to all antimicrobials, which is supported by sensitivity patterns in clinical cases of human infection [155,156,157]. However, resistance to some commonly used antimicrobials has recently been observed [146,147,156,158] in human clinical cases and the high RR of contamination on the outer surface of imported edible Sp. A may require further attention.
Both P. mendocina and P. pseudoalcaligenes were identified from Sp. A. Pseudomonas spp. are environmental bacteria which demonstrate an extremely high degree of genetic and physiological adaptability [159]. Member species increase yearly [160]. Pseudomonas sp. have been isolated from cultured tilapia (Oreonchromis spp.) and pond water [161] and P. pseudoalcaligenes from water at a freshwater prawn hatchery [162]. Pseudomonas mendocina is able to form a biofilm on environmental microplastics [113,114], is associated with polluted/eutrophic freshwater [116,117], and demonstrates increased antimicrobial resistance in manure treated soils [163]. Cultured freshwater fish are frequently described contaminated with Pseudomonas spp. at the farm level [130,131,161,164,165], during processing [56] and in retail fresh/frozen cultured freshwater fish [55,166,167]. Pseudomonas mendocina has been isolated from processed fish products [168,169]. Pseudomonas pseudoalcaligenes has been isolated from cultured common carp for retail sale [170] and was the causative agent of mass death in cultured Nile tilapia [171].
No published literature has identified Pseudomonas genus as zoonotic. However, the many cases of community acquired opportunistic infection of humans following contact with environmental sources [70,172,173,174] demonstrates the potential of contaminated imported FP to be a health risk to consumers. The case of human infection from a pet bird in which P. mendocina was cultured from the bird’s throat and drinking water also supports the further investigation of this species as a zoonosis [175].
In the present study Pseudomonas sp. demonstrated an expected pattern of antimicrobial sensitivity according to the CLSI [105]. Resistance to a broad range of antimicrobials has also been reported [176] in hospital and other settings [177]. Pseudomonas spp. are able to exchange genes (conjugation/horizontal gene transfer) which may favour increased virulence and spread between species [178].
Rhizobium radiobacter, identified in three samples of Sp. A, is a pathogen that can cause disease in both humans and plants. This species is widely distributed in soil as well as hospital environments [111], although reports of R. radiobacter contaminating fish are absent from literature. In the present study one sample came from a bag where vegetation, mud and natural diet were observed and another where mud and natural diet were both present. The preferred habitat of Sp. A is stagnant waters in muddy streams [179] which may explain the presence of this bacterium. Rhizobium radiobacter in the present study grew during incubation and produced small, bright orange, non-haemolytic colonies on MacConkey agar and it is possible this may have been the clone described in cases of human infection. Chanza, et al. [111], in a report of a pulmonary abscess in a Spanish 64-year-old male, notes growth on MacConkey agar as ‘bright orange, non-haemolytic colonies.’ The ability to grow at high temperature is an emergent trait in this bacterium. According to Hélène, et al. [180], a primary condition for colonization and pathogenicity is living in close proximity to the bacteria. It was suggested by Coenye, et al. [181] that R. radiobacter has potential for person to person transmission, therefore investigation into the spread between consumers via contaminated fish seems warranted. As a biofilm inhabitant, this bacterium contaminating FP may lead to colonisation of kitchen items used during preparation/clean up.
Human infection with R. radiobacter is described as rare in published literature, however Table 7 shows 23 cases. In many cases of human infection, contact with vegetation [72,182] and soil [72,73,183] is described. Rhizobium radiobacter is associated with invasive infections, particularly in the immunodeficient and children [111,184,185,186,187,188] and this bacterium present on FP may be an unacceptable risk.

Table 7.
Cases of human infection associated with zoonotic bacteria identified in this manuscript. N/S indicates not stated in cited publication.
In the present study R. radiobacter was sensitive only to Ciprofloxacin. This bacterium has been identified as sensitive to Ciprofloxacin in a number of studies [184,239,240], as expected, resistant to Vancomycin, and a large range of other antimicrobials [241,242]. According to Edmond, et al. [241], many bacterial species found in soil are innately resistant to antimicrobials and R. radiobacter has been demonstrated to ‘produce multiple antibiotic-inactivating enzymes’.
Vibrio fluvialis was only isolated from imported Sp. B fillets in the present study. The halophilic Vibrio fluvialis has traditionally been recovered from marine/estuarine environments, however V. fluvialis is now reported from cultured freshwater fish [61,62,243,244] and is considered an emerging foodborne pathogen [245]. Vibrio fluvialis has been isolated from river mud samples in Bangladesh [246], human stools, and faecally contaminated [247] as well as treated effluents systems [248] and is able to form a biofilm on environmental microplastics [249]. Vibrio fluvialis has been isolated at the farm level from cultured Nile tilapia and North African catfish [243,250,251], cultured African snakehead (Parachanna africana) and bagrid catfish (Chrysichthys nigrodigitatus) [244], common carp [252], rohu (Labeo rohita) [253,254], fish farm water [61] and in retail frozen/fresh fish [58,255].
Large outbreaks of cholera-like diarrhoea from V. fluvialis have been reported after consumption of contaminated fish/freshwater [225,227,230], although many outbreaks have an unknown origin [233]. Vibrio fluvialis was the causative agent for haemorrhagic cellulitis leading to amputation [231] and a urinary tract infection [238] from heavily contaminated water.
In the present study, all samples were resistant to Colistin, Cephalothin and Vancomycin, which has been previously described in published literature [103,232,256,257] (ref. [257] is a preprint). Vibrio fluvialis has been demonstrated resistant to a wide variety of common antimicrobials and it is considered that a rapid expansion in resistant strains [258] has occurred.
Two samples, one each from Sp. A and Sp. B, were identified as S. aureus, with one sample being resistant to all antimicrobials tested and considered to be MRSA. Staphylococcus aureus is found in the environment and the nose and skin of healthy humans [259]. This bacterium can be spread by direct contact between people and/or contact with contaminated objects [260], and S. aureus on the outer surface of edible fish has potential to contaminate hands/work surfaces or be transmitted between family members/pets. Staphylococcus spp. and S. aureus have been isolated at the farm level from cultured tilapia [130,161,261], striped catfish [56,166], during filleting/trimming of cultured striped catfish [56], and in retail frozen/fresh/fermented fish [134,166,167,262].
Coagulase positive staphylococcal food poisoning is a dominant cause of foodborne illness globally [263] and one of the most common bacterial infections described in other clinical conditions [264,265,266,267,268,269,270,271,272,273,274,275,276].
In the present study Salmonella sp. was identified from Sp. A and Sp. B, although not all species belonging to this genus are pathogenic to humans [277]; the testing of edible food, including fish, which may be carried out on entry to Australia only lists the genus Salmonella, as does Codex ‘Code of Practice for Fish and Fishery Products’ (CXC 52-2003) [123]. Salmonella spp. are found in the digestive tract of a broad range of animals. Humans, cows, pigs, and chickens are recognised carriers [278] and may contaminate food and water sources through the excretion of this bacterium in faeces [279]. Salmonella is spread from consumption of contaminated food, but may also be passed from person-to-person [280,281] and between animals and people [282]. In regions where the intensive freshwater FF system is used particularly in conjunction with poor sanitation standards, Salmonella will be prevalent in both the water and the fish raised in these facilities.
Salmonella sp. has been isolated from the waters where cultured catfish [283], Mozambique tilapia (Oreochromis mossambicus) and catfish [63] are raised. In Vietnam, it is considered that the important contamination sources in FF are ‘livestock and non-livestock-reservoirs, such as faecal-contaminated pond water, fish feed, birds, amphibians and reptiles’ [284]. Salmonella sp. has been isolated at the farm level from tilapias [130,261,285] and African sharp-tooth catfish [63], in retail catfish/other cultured freshwater fish [59,262,286] and in an array of imported seafood [60,287,288,289,290,291]. In the USA, the incidence of Salmonella contamination in imported raw fish was 12.2%, with Vietnam having the highest by country basis [60].
Salmonellosis, which has increased markedly in the last 50 years, is associated with gastroenteritis and many other types of systemic disease [60,292]. Seafood borne salmonellosis outbreaks have occurred in the United Kingdom [223,293], Germany [224], the European Union and the USA, although according to Heinitz, et al. [60] salmonellosis is significantly under reported.
In the present study, Salmonella sp. sample S469 showed a total pattern of resistance for all antimicrobials tested which is supported in recent reports of multi antimicrobial resistance in Salmonella sp. samples from edible fish and other food products [294,295,296].
Seafood related salmonellosis and staphylococcal infection has been recognised globally for some time; however, the emergence of new human pathogens from environmental bacteria, previously not considered a human health threat, is of concern. The international trade in cultured edible fish cannot be disregarded as a significant circulator and propagator of these environmental zoonotic/non-zoonotic bacteria and antimicrobial resistant strains into regions where they have not previously been recognised.
A limitation of the present study was the small sample size and lack of more sophisticated methods of identification for bacteria, due to limitations in time and resources available. It is considered that future studies could use MALDI-TOF®, mass spectrometry for identification of bacteria, and molecular and phenotypic methodology to characterize antimicrobial patterns of resistance.
5. Conclusions
In conclusion, zoonotic bacteria, many with a high RR, were identified contaminating frozen edible Sp. A and Sp. B imported into Australia. It is suggested that further study, using a greater sample size, may support the development of new food safety guidelines for Australian consumers for certain types of imported freshwater fish. It is also suggested that Australia could provide some countries, which produce farmed edible fish, with support to reach greater food safety compliance.
Author Contributions
Conceptualization, M.W., M.H.-J., T.W. and S.S.; methodology, M.W., M.H.-J. and T.W.; software, M.W. and T.W.; validation, M.W., T.W. and M.H.-J.; formal analysis, M.W. and T.W.; investigation, M.W.; resources, M.W. and T.W.; data curation, M.W. and T.W.; writing—original draft preparation, M.W. and T.W.; review, M.H.-J., T.W., S.S. and editing, M.W. and T.W.; visualization, M.W., M.H.-J. and T.W.; supervision, M.H.-J. and S.S.; project administration, M.W., M.H.-J. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are unavailable due to privacy restrictions.
Acknowledgments
Michelle Williams is the grateful recipient of the PhD scholarship from the Australian Research Training Program Scholarship through Charles Sturt University. All authors would like to acknowledge Stephen Dixon, the Thermo Fisher Scientific Microbiology Product Specialist, for his boundless knowledge, enthusiasm, and professionalism. All authors would also like to thank Angus McKinnon for organising this MS to be reviewed and critical comment made on the technical sections of this article by John Prescott.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hishamunda, N.; Ridler, N.B.; Bueno, P.; Yap, W.G. Commercial aquaculture in Southeast Asia: Some policy lessons. Food Policy 2009, 34, 102–107. [Google Scholar] [CrossRef]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Feijoó, C.G.; Navarrete, P. Antibiotics in aquaculture–use, abuse and alternatives. Health Environ. Aquac. 2012, 159, 159–198. [Google Scholar]
- Alfiansah, Y.R.; Hassenrück, C.; Kunzmann, A.; Taslihan, A.; Harder, J.; Gärdes, A. Bacterial abundance and community composition in pond water from shrimp aquaculture systems with different stocking densities. Front. Microbiol. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Mishra, S.S.; Rakesh, D.; Dhiman, M.; Choudhary, P.; Debbarma, J.; Sahoo, S.; Mishra, C. Present status of fish disease management in freshwater aquaculture in India: State-of-the-art-review. J. Aquac. Fish. 2017, 1, 14–23. [Google Scholar]
- Ina-Salwany, M.; Al-saari, N.; Mohamad, A.; Mursidi, F.A.; Mohd-Aris, A.; Amal, M.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in fish: A review on disease development and prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef]
- Mzula, A.; Wambura, P.N.; Mdegela, R.H.; Shirima, G.M. Present status of aquaculture and the challenge of bacterial diseases in freshwater farmed fish in Tanzania; A call for sustainable strategies. Aquac. Fish. 2021, 6, 247–253. [Google Scholar] [CrossRef]
- Rukanda, J.J.; Sigurgeirsson, O. Evaluation of aquaculture development in Tanzania. Nations Univ. Fish. Train. Programme Icel. 2016, 19, 1–39. [Google Scholar]
- Chenyambuga, S.; Mwandya, A.; Lamtane, H.; Madalla, N. Productivity and marketing of Nile tilapia (Oreochromis niloticus) cultured in ponds of small-scale farmers in Mvomero and Mbarali districts, Tanzania. Livest. Res. Rural. Dev. 2014, 26, 3–12. [Google Scholar]
- Zheng, Q.; Zhang, R.; Wang, Y.; Pan, X.; Tang, J.; Zhang, G. Occurrence and distribution of antibiotics in the Beibu Gulf, China: Impacts of river discharge and aquaculture activities. Mar. Environ. Res. 2012, 78, 26–33. [Google Scholar] [CrossRef]
- Granada, L.; Sousa, N.; Lopes, S.; Lemos, M.F. Is integrated multitrophic aquaculture the solution to the sectors’ major challenges?–a review. Rev. Aquac. 2016, 8, 283–300. [Google Scholar] [CrossRef]
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents 2018, 52, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Manage, P.M. Heavy use of antibiotics in aquaculture: Emerging human and animal health problems–a review. Sri Lanka J. Aquat. Sci. 2018, 23, 13–27. [Google Scholar] [CrossRef]
- Grema, H.A.; Kwaga, J.K.P.; Bello, M.; Umaru, O.H. Understanding fish production and marketing systems in North-western Nigeria and identification of potential food safety risks using value chain framework. Prev. Vet. Med. 2020, 181, 105038. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Silva, A.; e Barros, L.S.S. Food Safety and Fish Farming: Serious Issues for Brazil. Food Nutr. Sci. 2020, 11, 123–152. [Google Scholar]
- Ye, L.; Zhang, L.; Li, X.; Shi, L.; Huang, Y.; Wang, H.H. Antibiotic-resistant bacteria associated with retail aquaculture products from Guangzhou, China. J. Food Prot. 2013, 76, 295–301. [Google Scholar] [CrossRef]
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef]
- Mog, M.; Ngasotter, S.; Tesia, S.; Waikhom, D.; Panda, P.; Sharma, S.; Varshney, S. Problems of antibiotic resistance associated with oxytetracycline use in aquaculture: A review. J. Entomol. Zool. Stud. 2020, 8, 1075–1082. [Google Scholar]
- Scarano, C.; Piras, F.; Virdis, S.; Ziino, G.; Nuvoloni, R.; Dalmasso, A.; De Santis, E.; Spanu, C. Antibiotic resistance of Aeromonas sp. strains isolated from Sparus aurata reared in Italian mariculture farms. Int. J. Food Microbiol. 2018, 284, 91–97. [Google Scholar] [CrossRef]
- Le, T.S.; Nguyen, T.H.; Vo, H.P.; Doan, V.C.; Nguyen, H.L.; Tran, M.T.; Tran, T.T.; Southgate, P.C.; Kurtböke, D.İ. Protective effects of bacteriophages against Aeromonas hydrophila causing motile Aeromonas septicemia (MAS) in striped Catfish. Antibiotics 2018, 7, 16. [Google Scholar] [CrossRef]
- Kari, Z.A.; Wee, W.; Hamid, N.K.A.; Mat, K.; Rusli, N.D.; Khalid, H.N.M.; Sukri, S.A.M.; Harun, H.C.; Dawood, M.A.; Hakim, A.H. Recent advances of phytobiotic utilization in carp farming: A review. Aquac. Nutr. 2022, 2022, 7626675. [Google Scholar] [CrossRef]
- García Beltrán, J.M.; Esteban, M.Á. Nature-identical compounds as feed additives in aquaculture. Fish Shellfish Immunol. 2022, 123, 409–416. [Google Scholar] [CrossRef]
- Nik Mohamad Nek Rahimi, N.; Natrah, I.; Loh, J.-Y.; Ervin Ranzil, F.K.; Gina, M.; Lim, S.-H.E.; Lai, K.-S.; Chong, C.-M. Phytocompounds as an alternative antimicrobial approach in aquaculture. Antibiotics 2022, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Shen, Y. An overview of disruptive technologies for aquaculture. Aquac. Fish. 2022, 7, 111–120. [Google Scholar] [CrossRef]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and food safety in aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef] [PubMed]
- Burridge, L.; Weis, J.S.; Cabello, F.; Pizarro, J.; Bostick, K. Chemical use in salmon aquaculture: A review of current practices and possible environmental effects. Aquaculture 2010, 306, 7–23. [Google Scholar] [CrossRef]
- Bhat, R.A.H.; Thakuria, D.; Tandel, R.S.; Khangembam, V.C.; Dash, P.; Tripathi, G.; Sarma, D. Tools and techniques for rational designing of antimicrobial peptides for aquaculture. Fish Shellfish Immunol. 2022, 127, 1033–1050. [Google Scholar] [CrossRef]
- Nabilah Mohd Noor, N.; Hazirah Kamaruzaman, N.; Al-Gheethi, A.; Maya Saphira Radin Mohamed, R.; Hossain, M.S. Degradation of antibiotics in aquaculture wastewater by bio-nanoparticles: A critical review. Ain Shams Eng. J. 2022, 14, 101981. [Google Scholar] [CrossRef]
- Chari, N.; Felix, L.; Davoodbasha, M.; Ali, A.S.; Nooruddin, T. In vitro and in vivo antibiofilm effect of copper nanoparticles against aquaculture pathogens. Biocatal. Agric. Biotechnol. 2017, 10, 336–341. [Google Scholar] [CrossRef]
- Culot, A.; Grosset, N.; Gautier, M. Overcoming the challenges of phage therapy for industrial aquaculture: A review. Aquaculture 2019, 513, 734423. [Google Scholar] [CrossRef]
- Song, C.; Zhang, C.; Fan, L.; Qiu, L.; Wu, W.; Meng, S.; Hu, G.; Kamira, B.; Chen, J. Occurrence of antibiotics and their impacts to primary productivity in fishponds around Tai Lake, China. Chemosphere 2016, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, L.-R.; Ketola, T.; Laanto, E.; Kinnula, H.; Bamford, J.K.; Penttinen, R.; Mappes, J. Intensive aquaculture selects for increased virulence and interference competition in bacteria. Proc. Royal Soc. B 2016, 283, 20153069. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-H.; Chen, J.; Li, C.-H.; Lu, X.-J.; Zhang, D.-M.; Li, H.-Y.; Zhao, Z.-X.; Li, M.-Y. Detection of bacterial pathogens in aquaculture samples by DNA microarray analysis. Aquaculture 2012, 338, 29–35. [Google Scholar] [CrossRef]
- Cai, W.; Willmon, E.; Burgos, F.A.; Ray, C.L.; Hanson, T.; Arias, C.R. Biofilm and Sediment are Major Reservoirs of Virulent Aeromonas hydrophila (vAh) in Catfish Production Ponds. J. Aquat. Anim. Health 2019, 31, 112–120. [Google Scholar] [CrossRef]
- Hoai, T.D.; Trang, T.T.; Van Tuyen, N.; Giang, N.T.H.; Van Van, K. Aeromonas veronii caused disease and mortality in channel catfish in Vietnam. Aquaculture 2019, 513, 734425. [Google Scholar] [CrossRef]
- Reverter, M.; Sarter, S.; Caruso, D.; Avarre, J.-C.; Combe, M.; Pepey, E.; Pouyaud, L.; Vega-Heredía, S.; De Verdal, H.; Gozlan, R.E. Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat. Commun. 2020, 11, 1870. [Google Scholar] [CrossRef]
- Van Huong, N.; Huu Cuong, T.; Thi Nang Thu, T.; Lebailly, P. Efficiency of different integrated agriculture aquaculture systems in the Red River Delta of Vietnam. Sustainability 2018, 10, 493. [Google Scholar] [CrossRef]
- Sasmal, D.; Borah, B.; Kumar, S.; Borah, D.; Bora, M.; Kalita, H. Improvement of farmer’s livelihood through rice-fish-duck integration at Namsai District of Arunachal Pradesh. J. Entomol. Zool. Stud. 2020, 8, 1582–1585. [Google Scholar]
- Debnath, B.; Kandpal, B.; Singh, R. Integrated fish farming modules for income enhancement in South Tripura: A comparative assessment of profitability. Indian J. Ext. Educ. 2020, 56, 228–232. [Google Scholar]
- Mallick, S.; Deka, R.S.; Hoque, J.; Deka, P.M. Krishi Vigyan Kendra′s Integrated Paddy-Cum-Fish Culture Practice (KVK Morigaon, Assam). Agric. Food Newsl. 2022, 4, 260–263. [Google Scholar]
- Newton, R.; Zhang, W.; Xian, Z.; McAdam, B.; Little, D.C. Intensification, regulation and diversification: The changing face of inland aquaculture in China. Ambio 2021, 50, 1739–1756. [Google Scholar] [CrossRef] [PubMed]
- Weiman, M.; Mengqing, L. Analysis of Feeds and Fertilizers for Sustainable Aquaculture Development in China. In Study and Analysis of Feeds and Fertilizers for Sustainable Aquaculture Development; FAO Fisheries Technical Paper: Rome, Italy, 2007; pp. 141–190. [Google Scholar]
- FAO. Report of the FAO Workshop on the on-Farm Feeding and Feed Management in Aquaculture, Manila, Philippines, 13–15 September 2010; Food and Agriculture Organisation of the United Nations: Geneva, Switzerland, 2010; pp. 1–46. [Google Scholar]
- Chávez-Crooker, P.; Obreque-Contreras, J. Bioremediation of aquaculture wastes. Curr. Opin. Biotechnol. 2010, 21, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.; Lemos, M.F.L.; Crespo, D.; Nunes, M.; Freitas, A.; Ramos, F.; Pardal, M.Â.; Leston, S. Integrated multitrophic aquaculture systems–Potential risks for food safety. Trends Food Sci. Technol. 2020, 96, 79–90. [Google Scholar] [CrossRef]
- Foysal, M.; Momtaz, F.; Kawser, A.; Chaklader, M.; Siddik, M.; Lamichhane, B.; Tay, A.; Rahman, M.; Fotedar, R. Microbiome patterns reveal the transmission of pathogenic bacteria in hilsa fish (Tenualosa ilisha) marketed for human consumption in Bangladesh. J. Appl. Microbiol. 2019, 126, 1879–1890. [Google Scholar] [CrossRef]
- Preena, P.G.; Swaminathan, T.R.; Kumar, V.J.R.; Singh, I.S.B. Antimicrobial resistance in aquaculture: A crisis for concern. Biologia 2020, 75, 1497–1517. [Google Scholar] [CrossRef]
- Mahmudunnabi, G.; Majlish, A.N.K.; Momtaz, F.; Foysal, M.J.; Rahman, M.M.; Islam, K. Molecular detection and PCR-RFLP analysis using Pst1 and Alu1 of multidrug resistant Klebsiella pneumoniae causing urinary tract infection in women in the eastern part of Bangladesh. J. Genet. Eng. Biotechnol. 2018, 16, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Rahman, M.S.; Hassan, F.N.; Sarkar, S.; Islam, M.K.; Saha, P.; Alam, M.B.; Sultana, N.; Rahman, K.T.; Sumi, S.S. Antimicrobial resistance in uropathogen isolates from patients with urinary tract infections. Biomed. Res. Ther. 2015, 2, 263–269. [Google Scholar] [CrossRef]
- Slettemeås, J.S.; Urdahl, A.M.; Mo, S.S.; Johannessen, G.S.; Grave, K.; Norström, M.; Steinbakk, M.; Sunde, M. Imported food and feed as contributors to the introduction of plasmid-mediated colistin-resistant Enterobacteriaceae to a ‘low prevalence’ country. J. Antimicrob. Chemother. 2017, 72, 2675–2677. [Google Scholar] [CrossRef]
- Fang, J.; Shen, Y.; Qu, D.; Han, J. Antimicrobial resistance profiles and characteristics of integrons in Escherichia coli strains isolated from a large-scale centralized swine slaughterhouse and its downstream markets in Zhejiang, China. Food Control 2019, 95, 215–222. [Google Scholar] [CrossRef]
- Kenzaka, T.; Tani, K.; Nasu, M. High-frequency phage-mediated gene transfer in freshwater environments determined at single-cell level. ISME J. 2010, 4, 648–659. [Google Scholar] [CrossRef]
- von Wintersdorff, C.J.H.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Waseem, H.; Ali, J.; Jamal, A.; Ali, M. Potential dissemination of antimicrobial resistance from small scale poultry slaughterhouses in Pakistan. Appl. Ecol. Environ. Res. 2019, 17, 3049–3063. [Google Scholar] [CrossRef]
- Noseda, B.; Islam, M.T.; Eriksson, M.; Heyndrickx, M.; De Reu, K.; Van Langenhove, H.; Devlieghere, F. Microbiological spoilage of vacuum and modified atmosphere packaged Vietnamese Pangasius hypophthalmus fillets. Food Microbiol. 2012, 30, 408–419. [Google Scholar] [CrossRef]
- Thi, A.N.T.; Noseda, B.; Samapundo, S.; Nguyen, B.L.; Broekaert, K.; Rasschaert, G.; Heyndrickx, M.; Devlieghere, F. Microbial ecology of Vietnamese Tra fish (Pangasius hypophthalmus) fillets during processing. Int. J. Food Microbiol. 2013, 167, 144–152. [Google Scholar]
- Thi, A.N.T.; Samapundo, S.; Devlieghere, F.; Heyndrickx, M. Microbiota of frozen Vietnamese catfish (Pangasius hypophthalmus) marketed in Belgium. Int. J. Food Contam. 2016, 3, 17. [Google Scholar]
- Yücel, N.; Balci, Ş. Prevalence of Listeria, Aeromonas, and Vibrio species in fish used for human consumption in Turkey. J. Food Prot. 2010, 73, 380–384. [Google Scholar] [CrossRef]
- Lyer, T.S.G.; Shrivastava, K.P. Incidence and low temperature survival of Salmonella in fishery products. Fish Technol. 1989, 26, 39–42. [Google Scholar]
- Heinitz, M.L.; Ruble, R.D.; Wagner, D.E.; Tatini, S.R. Incidence of Salmonella in fish and seafood. J. Food Prot. 2000, 63, 579–592. [Google Scholar] [CrossRef]
- Alkhunni, S.B.A.; Gaballah, M.S.M.; Gultepe, N. Pathogenic bacteria for human and fish isolated from fish farm in Kastamonu, Turkey. J. Aquac. Mar. Biol. 2017, 6, 157. [Google Scholar]
- Bohai, X.; Zhan, Y.; Yushen, W.; Taozhen, C. Studies on the Taxonomy of Pathogenic Bacteria of the Bacterial Hemorrhagic Septicemia in Cultured Fishes in Freshwater (Abstract only). Acta Hydrobiol. Sin. 1993, 3, 259–266. [Google Scholar]
- Budiati, T.; Rusul, G.; Wan-Abdullah, W.N.; Arip, Y.M.; Ahmad, R.; Thong, K.L. Prevalence, antibiotic resistance and plasmid profiling of Salmonella in catfish (Clarias gariepinus) and tilapia (Tilapia mossambica) obtained from wet markets and ponds in Malaysia. Aquaculture 2013, 372, 127–132. [Google Scholar] [CrossRef]
- Haenen, O.L.M.; Evans, J.J.; Berthe, F. Bacterial infections from aquatic species: Potential for and prevention of contact zoonoses. OIE Rev. Sci. Tech. 2013, 32, 497–507. [Google Scholar] [CrossRef]
- Lowry, T.; Smith, S.A. Aquatic zoonoses associated with food, bait, ornamental, and tropical fish. J. Am. Vet. Med. Assoc. 2007, 231, 876–880. [Google Scholar] [CrossRef]
- Bielecki, J. Emerging food pathogens and bacterial toxins. Acta Microbiol. Pol. 2003, 52, 17–22. [Google Scholar]
- De Keukeleire, S.; De Bel, A.; Jansen, Y.; Janssens, M.; Wauters, G.; Piérard, D. Yersinia ruckeri, an unusual microorganism isolated from a human wound infection. New Microbes New Infect. 2014, 2, 134–135. [Google Scholar] [CrossRef]
- Cooper, G.R.; Staples, E.D.; Iczkowski, K.A.; Clancy, C.J. Comamonas (Pseudomonas) testosteroni endocarditis. Cardiovasc. Pathol. 2005, 14, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, M.J.; King, M.H.; Weinberg, R.S.; Guerry, R.K. Micrococcus endophthalmitis. Arch. Ophthalmol. 1990, 108, 1523–1524. [Google Scholar] [CrossRef] [PubMed]
- Aragone, M.; Maurizi, D.; Clara, L.; Estrada, J.N.; Ascione, A. Pseudomonas mendocina, an environmental bacterium isolated from a patient with human infective endocarditis. J. Clin. Microbiol. 1992, 30, 1583–1584. [Google Scholar] [CrossRef]
- Rapsinski, G.J.; Makadia, J.; Bhanot, N.; Min, Z. Pseudomonas mendocina native valve infective endocarditis: A case report. J. Med. Case Rep. 2016, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Levitski-Heikkila, T.V.; Ullian, M.E. Peritonitis with multiple rare environmental bacteria in a patient receiving long-term peritoneal dialysis. Am. J. Kidney Dis. 2005, 46, e119–e124. [Google Scholar] [CrossRef]
- Tsai, S. Rhizobium radiobacter peritonitis revisited: Catheter removal is not mandatory. Perit. Dial. Int. 2013, 33, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Espinosa-Gongora, C.; Guardabassi, L. Human health risks associated with antimicrobial-resistant enterococci and Staphylococcus aureus on poultry meat. Clin. Microbiol. Infect. 2016, 22, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Australian Government. Imported Food Control Regulations 2019; Federal Register of Legislation, Ed.; Australian Government: Canberra, Australia, 2019; pp. 1–26. [Google Scholar]
- Australian Government. Imported Food Control Act 1992. In Number 227. Compilation 27; Federal Register of Legislation, Ed.; Australian Government: Canberra, Australia, 2021; pp. 1–83. [Google Scholar]
- Australian Government. Imported Food Control Order 2020. In In Force 25 November 2020; Federal Register of Legislation, Ed.; Australian Government: Canberra, Australia, 2020; pp. 1–11. [Google Scholar]
- ANZFSC. Standard 1.6.1–Microbiological limits in food. In Food Standards as Amended, Taking into Account Amendments up to Food Standards (Proposal P1048–Code Revision (2018)) Variation; Federal Register of Legislation: Canberra, Australia, 2018. [Google Scholar]
- WTO. Marrakesh Agreement Establishing the World Trade Organisation; Opened for Signature 15 April 1994, 1867 UNTS 3 (entered into force 1 January 1995) Annex 1A (‘Agreement on the Application of Sanitary and Phytosanitary Measures’); WTO: Geneva, Switzerland, 1995; In force 1 January 1995. [Google Scholar]
- Williams, M.; Hernandez-Jover, M.; Williams, T.; Shamsi, S. A risk scoring system for seafood supply chain breaches and examination of freshwater fish imported to Australia. Food Qual. Saf. 2021, 5, fyab004. [Google Scholar] [CrossRef]
- Saito, E.; Yoshida, N.; Kawano, J.; Shimizu, A.; Igimi, S. Isolation of Staphylococcus aureus from raw fish in relation to culture methods. J. Vet. Med. Sci. 2011, 73, 287–292. [Google Scholar] [CrossRef]
- Wendling, Z.A.; Emerson, J.W.; Esty, D.C.; Levy, M.A.; de Sherbinin, A. The Environmental Performance Index; Yale Center for Environmental Law & Policy: New Haven, CT, USA, 2018; pp. 1–200. [Google Scholar]
- Odonkor, S.T.; Ampofo, J.K. Escherichia coli as an indicator of bacteriological quality of water: An overview. Microbiol. Res. 2013, 4, e2. [Google Scholar] [CrossRef]
- Rodrigues, J.G.; Nair, H.P.; O′Kane, C.; Walker, C.A. Prevalence of multidrug resistance in Pseudomonas spp. isolated from wild bird feces in an urban aquatic environment. Ecol. Evol. 2021, 11, 14303. [Google Scholar] [CrossRef]
- Odjadjare, E.; Ebowemen, M. Antibiogram of Pseudomonas isolates and potential public health impact of an abattoir effluent in Benin City, Nigeria. Afr. J. Clin. Exp. Microbiol. 2020, 21, 240–249. [Google Scholar]
- Sarkar, S.; Dey, S.K.; Nipu, M.A.I.; Brishti, P.S.; Billah, M.B. Microbiological assessment of Nile tilapia Oreochromis niloticus collected from different super shops and local market in Dhaka, Bangladesh. J. Fish. 2020, 8, 784–791. [Google Scholar] [CrossRef]
- Vierheilig, J.; Frick, C.; Mayer, R.; Kirschner, A.; Reischer, G.; Derx, J.; Mach, R.; Sommer, R.; Farnleitner, A. Clostridium perfringens is not suitable for the indication of fecal pollution from ruminant wildlife but is associated with excreta from nonherbivorous animals and human sewage. Appl. Environ. Microbiol. 2013, 79, 5089–5092. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol; American Society for Microbiology: Washington, DA, USA, 2009; pp. 1–23. [Google Scholar]
- WHO. Critically Important Antimicrobials for Human Medicine; World Health Organisation Department of Food Safety and Zoonoses: Geneva, Switzerland, 2018; pp. 1–52. [Google Scholar]
- Hussein, M.; Hassan, W. Efficacy of fosfomycin in controlling streptococcosis in Nile tilapia (Oreochromis niloticus). J. Vet. Med. Res. 2011, 21, 59–66. [Google Scholar] [CrossRef]
- Lee, S.W.; Najiah, M.; Wendy, W. Bacteria associated with golden pompano (Trachinotus blochii) broodstock from commercial hatchery in Malaysia with emphasis on their antibiotic and heavy metal resistances. Front. Agric. China 2010, 4, 251–256. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.-Q.; Li, J.; Ma, Y.-F.; Lin, J.; Xiao, Z.-R.; Yu, D.-J. Phenotypic and genotypic analysis of vancomycin-resistant Enterococci strains isolated from different water sources. Afr. J. Bacteriol. Res. 2016, 8, 8–13. [Google Scholar]
- Osman, K.M.; Ali, M.N.; Radwan, I.; ElHofy, F.; Abed, A.H.; Orabi, A.; Fawzy, N.M. Dispersion of the vancomycin resistance genes vanA and vanC of Enterococcus isolated from nile tilapia on retail sale: A public health hazard. Front. Microbiol. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Gibson, J.S.; Wai, H.; Oo, S.S.M.L.; Hmwe, E.M.M.; Wai, S.S.; Htun, L.L.; Lim, H.P.; Latt, Z.M.; Henning, J. Antimicrobials use and resistance on integrated poultry-fish farming systems in the Ayeyarwady Delta of Myanmar. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Gazal, L.E.d.S.; Brito, K.C.T.d.; Kobayashi, R.K.T.; Nakazato, G.; Cavalli, L.S.; Otutumi, L.K.; Brito, B.G.d. Antimicrobials and resistant bacteria in global fish farming and the possible risk for public health. Arq. Inst. Biológico 2020, 87, 1–11. [Google Scholar] [CrossRef]
- Yanong, R.P. Use of antibiotics in ornamental fish aquaculture. Inst. Food Agric. Sci. 2003, 84, 1–8. [Google Scholar] [CrossRef]
- Liu, M.; Sang, Y.; Zhang, J.; Li, J.; Yu, W.; Zhang, F.; Wang, X. Development of a broad-specific competitive ELISA for first-generation cephalosporin antibiotics in animal-derived foods samples. Bull. Environ. Contam. Toxicol. 2021, 107, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Kalasseril, S.G.; Krishnan, R.; Vattiringal, R.K.; Paul, R.; Mathew, P.; Pillai, D. Detection of New Delhi metallo-β-lactamase 1 and cephalosporin resistance genes among carbapenem-resistant Enterobacteriaceae in water bodies adjacent to hospitals in India. Curr. Microbiol. 2020, 77, 2886–2895. [Google Scholar] [CrossRef] [PubMed]
- Okoh, A.I.; Igbinosa, E.O. Antibiotic susceptibility profiles of some Vibrio strains isolated from wastewater final effluents in a rural community of the Eastern Cape Province of South Africa. BMC Microbiol. 2010, 10, 143. [Google Scholar] [CrossRef]
- Tall, B.; Fall, S.; Pereira, M.; Ramos-Valle, M.; Curtis, S.; Kothary, M.; Chu, D.; Monday, S.; Kornegay, L.; Donkar, T. Characterization of Vibrio fluvialis-like strains implicated in limp lobster disease. Appl. Environ. Microbiol. 2003, 69, 7435–7446. [Google Scholar] [CrossRef]
- Kaysner, C.A.; De Paola, A.J. BAM Chapter 9: Vibrio. In Bacteriological Analytical Manual; USA, Food and Drug Administration: Silver Spring, MD, USA, 2004; Volume 8. [Google Scholar]
- Lesmana, M.; Subekti, D.S.; Tjaniadi, P.; Simanjuntak, C.H.; Punjabi, N.H.; Campbell, J.R.; Oyofo, B.A. Spectrum of Vibrio species associated with acute diarrhea in North Jakarta, Indonesia. Diagn. Microbiol. Infect. Dis. 2002, 43, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals. In CLSI supplement VET01S, 5th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; pp. 1–250. [Google Scholar]
- Reynolds, J. Kirby-Bauer (Antibiotic Sensitivity). In LibreTexts-Biology; Richland College: Dallas, TX, USA, 2020. [Google Scholar]
- Behera, B.; Mohanty, S.; Sahu, S.; Praharaj, A.K. In vitro Activity of fosfomycin against Multidrug-resistant urinary and nonurinary Gram-negative isolates. Indian J. Crit. Care Med. 2018, 22, 533. [Google Scholar] [PubMed]
- Beveridge, T.J. Use of the Gram stain in microbiology. Biotech. Histochem. 2001, 76, 111–118. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Smith, H.; Brown, A. Benson’s Microbiological Applications Laboratory Manual, 12th ed.; McGraw Hill: New York, NY, USA, 2011; pp. 1–576. [Google Scholar]
- Chanza, M.; Vidal, S.; Gimeno, C. Rhizobium radiobacter in pulmonary abscess associated with postgripal necrotizing pneumonia. Rev. Esp. Quim. 2017, 30, 50. [Google Scholar]
- BICON. Case: Finfish (Excluding Salmonid) for Human Consumption Effective: 07 April 2020. Available online: https://bicon.agriculture.gov.au/BiconWeb4.0/ImportConditions/Questions/EvaluateCase?elementID=0000067916&elementVersionID=360 (accessed on 15 April 2020).
- Saikia, D.J.; Chattopadhyay, P.; Banerjee, G.; Talukdar, B.; Sarma, D. Identification and pathogenicity of Pseudomonas aeruginosa DJ1990 on tail and fin rot disease in spotted snakehead. J. World Aquac. Soc. 2018, 49, 703–714. [Google Scholar] [CrossRef]
- Wu, X.; Pan, J.; Li, M.; Li, Y.; Bartlam, M.; Wang, Y. Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res. 2019, 165, 114979. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, A.; da Silva, C.L.P.A.C.; Leal, G.B.E.; Baptista, D.F. Habitat preference of freshwater snails in relation to environmental factors and the presence of the competitor snail Melanoides tuberculatus (Müller, 1774). Mem. Inst. Oswaldo Cruz 2005, 100, 169–176. [Google Scholar] [CrossRef]
- Sultana, M.; Härtig, C.; Planer-Friedrich, B.; Seifert, J.; Schlömann, M. Bacterial communities in Bangladesh aquifers differing in aqueous arsenic concentration. Geomicrobiol. J. 2011, 28, 198–211. [Google Scholar] [CrossRef]
- Austin, B.; Stobie, M. Recovery of Serratia plymuthica and presumptive Pseudomonas pseudoalcaligenes from skin lesions in rainbow trout, Oncorhynchus mykiss (Walbaum), otherwise infected with enteric redmouth. J. Fish Dis. 1992, 15, 541–543. [Google Scholar] [CrossRef]
- FSANZ. Advice on imported food. In Australian and New Zealand Food Standards Code; Food Standards Australia and New Zealand: Canberra, Australia, 2019; p. 1. [Google Scholar]
- Australian Government. Integrated Cargo System (ICS); Australian Border Force, Ed.; Australian Government: Canberra, Australia, 2020. [Google Scholar]
- FSANZ. The Australian and New Zealand Food Standards Code. In The Compendium of Microbiological Criteria for Food; Food Standards Australia and New Zealand: Canberra, Australia, 2018. [Google Scholar]
- WTO. Sanitary and Phytosanitary Measures signed Marrakesh on 15 April 1994. Entered into affect by World Trade Organization on 1 January 1995. In The WTO Agreement Series; World Trade Organisation, Ed.; World Trade Organisation: Geneva, Switzerland, 1994; pp. 1–50. [Google Scholar]
- Codex. Code of Practice for Fish and Fishery Products. In Codex International Food Safety Standards; Codex Alimentarius and the Food and Agriculture Organisation of the United Nations and World Health Organisation: Geneva, Switzerland, 2020; Volume CXC 52-2003 (Updated 2020), pp. 1–372. [Google Scholar]
- Codex. Codex food safety standards. In Code of Practice for Fish and Fishery Products; Food and Agriculture Organisation of the United Nations and World Health Organisation: Geneva, Switzerland, 2020; Volume CXC 52-2003 (previously CAC/RCP 52-2003), pp. 1–268. [Google Scholar]
- Miettinen, H.; Aarnisalo, K.; Salo, S.; Sjöberg, A.-M. Evaluation of surface contamination and the presence of Listeria monocytogenes in fish processing factories. J. Food Prot. 2001, 64, 635–639. [Google Scholar] [CrossRef]
- Rao, S.; Ngan, W.Y.; Chan, L.C.; Sekoai, P.T.; Fung, A.H.Y.; Pu, Y.; Yao, Y.; Habimana, O. Questioning the source of identified non-foodborne pathogens from food-contact wooden surfaces used in Hong Kong′s urban wet markets. One Health 2021, 13, 100300. [Google Scholar] [CrossRef]
- Albuquerque, W.F.; Macrae, A.; Sousa, O.V.; Vieira, G.H.F.; Vieira, R.H.S.F. Multiple drug resistant Staphylococcus aureus strains isolated from a fish market and from fish handlers. Braz. J. Microbiol. 2007, 38, 131–134. [Google Scholar] [CrossRef]
- Onjong, H.A.; Wangoh, J.; Njage, P.M.K. Semiquantitative analysis of gaps in microbiological performance of fish processing sector implementing current food safety management systems: A case study. J. Food Prot. 2014, 77, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.S.; Sanjeev, S. Prevalence of enterotoxigenic Staphylococcus aureus in fishery products and fish processing factory workers. Food Control 2007, 18, 1565–1568. [Google Scholar] [CrossRef]
- Nuñez, M. Micrococcus. In Encyclopedia of Food Microbiology; Batt, C.A., Tortorello, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 627–633. [Google Scholar]
- Huicab-Pech, Z.; MR, C.-C.; Lango-Reynoso, F. Pathogenic Bacteria in Oreochromis Niloticus Var. Stirling Tilapia Culture. Fish. Aquac. J. 2017, 8, 1000197. [Google Scholar]
- Wanja, D.W.; Mbuthia, P.G.; Waruiru, R.M.; Bebora, L.C.; Ngowi, H.A.; Nyaga, P.N. Antibiotic and Disinfectant Susceptibility Patterns of Bacteria Isolated from Farmed Fish in Kirinyaga County, Kenya. Int. J. Microbiol. 2020, 2020, 8897338. [Google Scholar] [CrossRef] [PubMed]
- Anihouvi, V.B.; Sakyi-Dawson, E.; Ayernor, G.S.; Hounhouigan, J.D. Microbiological changes in naturally fermented cassava fish (Pseudotolithus sp.) for lanhouin production. Int. J. Food Microbiol. 2007, 116, 287–291. [Google Scholar] [CrossRef]
- Horner, W. Preservation of fish by curing (drying, salting and smoking). In Fish Processing Technology; Chapman and Hall: New York, NY, USA, 1997; pp. 21–39. [Google Scholar]
- Anihouvi, V.; Ayernor, G.; Hounhouigan, J.; Sakyi-Dawson, E. Quality characteristics of Lanhouin: A traditional processed fermented fish product in the Republic of Benin. African J. Food, Agric. Nutr. Dev. 2006, 6, 1–15. [Google Scholar] [CrossRef]
- Nerquaye-Tetteh, G.; Tete-Marmon, J.; Eyeson, K. Studies on Bomone, a Ghanaian fermented fish product. GJAS 1978, 11, 21–26. [Google Scholar]
- Adang, R.P.; Schouten, H.C.; van Tiel, F.H.; Blijham, G.H. Pneumonia due to Micrococcus spp. in a patient with acute myeloid leukaemia. Leukemia 1992, 6, 224–226. [Google Scholar]
- Ambler, M.; Homans, A.; O′Shea, P. An unusual central nervous system infection in a young immunocompromised host. Arch. Pathol. Lab. Med. 1986, 110, 497–501. [Google Scholar] [PubMed]
- Gupta, V.; Kumar, A.C.R.S.; Dhyani, A.; Chakravarty, S. Meningitis caused by Micrococcus luteus: Case report and review of literature. Int. J. Med Microbiol. Trop. Dis. 2019, 5, 63–64. [Google Scholar]
- Magee, J.T.; Burnett, I.A.; Hindmarch, J.M.; Spencer, R.C. Micrococcus and Stomatococcus spp. from human infections. J. Hosp. Infect. 1990, 16, 67–73. [Google Scholar] [CrossRef]
- Miltiadous, G.; Elisaf, M. Native valve endocarditis due to Micrococcus luteus: A case report and review of the literature. J. Med. Case Rep. 2011, 5, 251. [Google Scholar] [CrossRef]
- Salar, A.; Carratalà, J.; Fernández-Sevilla, A.; Marín, D.; Grañena, A. Pneumonia caused by Micrococcus species in a neutropenic patient with acute leukemia. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 546–548. [Google Scholar] [CrossRef]
- Selladurai, B.M.; Sivakumaran, S.; Aiyar, S.; Mohamad, A.R. Intracranial suppuration caused by Micrococcus luteus. Br. J. Neurosurg. 1993, 7, 205–207. [Google Scholar] [CrossRef]
- Khan, A.; Aung, T.T.; Chaudhuri, D. The First Case of Native Mitral Valve Endocarditis due to Micrococcus luteus and Review of the Literature. Case Rep. Cardiol. 2019, 2019, 5907319. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, Q.; Yang, Z.; Liang, Z. Clinical Characteristics of Patients with Micrococcus luteus Bloodstream Infection in a Chinese Tertiary-Care Hospital. Polish J. Microbiol. 2021, 70, 321–326. [Google Scholar] [CrossRef]
- Song, S.H.; Choi, H.S.; Ma, S.K.; Kim, S.W.; Shin, J.-H.; Bae, E.H. Micrococcus aloeverae-a rare cause of peritoneal dialysis-related peritonitis confirmed by 16S rRNA gene sequencing. J. Nippon Med. Sch. 2019, 86, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Farooq, R.; Nahvi, N. Comamonas testosteroni: Is it still a rare human pathogen. Case Rep. Gastroenterol. 2017, 11, 42–47. [Google Scholar] [CrossRef]
- Tiwari, S.; Nanda, M. Bacteremia caused by Comamonas testosteroni an unusual pathogen. J. Lab. Physicians 2019, 11, 87–90. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, W.; Cao, Z.; Xu, B.; Wang, G.; Luo, M. High correlation between genotypes and phenotypes of environmental bacteria Comamonas testosteroni strains. BMC Genom. 2015, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Yani, A.; Amin, M.; Rohman, F.; Suarsini, E.; Putra, W.E. Profiling indigenous lead-reducing bacteria from Tempe Lake, South Sulawesi, Indonesia as bioremediation agents. Biodiversitas 2020, 21, 4778–4786. [Google Scholar] [CrossRef]
- Preena, P.; Arathi, D.; Raj, N.S.; Arun Kumar, T.; Arun Raja, S.; Reshma, R.; Raja Swaminathan, T. Diversity of antimicrobial-resistant pathogens from a freshwater ornamental fish farm. Lett. Appl. Microbiol. 2020, 71, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Wamala, S.P.; Mugimba, K.K.; Mutoloki, S.; Evensen, Ø.; Mdegela, R.; Byarugaba, D.K.; Sørum, H. Occurrence and antibiotic susceptibility of fish bacteria isolated from Oreochromis niloticus (Nile tilapia) and Clarias gariepinus (African catfish) in Uganda. Fish Aquat. Sci. 2018, 21, 6. [Google Scholar] [CrossRef]
- Smith, M.D.; Gradon, J.D. Bacteremia due to Comamonas species possibly associated with exposure to tropical fish. South. Med. J. 2003, 96, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Tsui, T.-L.; Tsao, S.-M.; Liu, K.-S.; Chen, T.-Y.; Wang, Y.-L.; Teng, Y.-H.; Lee, Y.-T. Comamonas testosteroni infection in Taiwan: Reported two cases and literature review. J. Microbiol. Immunol. Infect. 2011, 44, 67–71. [Google Scholar] [CrossRef]
- Wu, Y.; Zaiden, N.; Cao, B. The Core- and Pan-Genomic Analyses of the Genus Comamonas: From Environmental Adaptation to Potential Virulence. Front. Microbiol. 2018, 9, 3096. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.M.; Simon, G.L. Comamonas testosteroni bacteremia: A case report and review of the literature. Infect Dis. Clin. Pract. 2007, 15, 272–273. [Google Scholar] [CrossRef]
- Farshad, S.; Norouzi, F.; Aminshahidi, M.; Heidari, B.; Alborzi, A. Two cases of bacteremia due to an unusual pathogen, Comamonas testosteroni in Iran and a review literature. J. Infect. Dev. Ctries. 2012, 6, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Ciragil, P.; Bulbuloglu, E.; Aral, M.; Alkis, S.; Ezberci, F. Comamonas testosteroni bacteremia in a patient with perforated acute appendicitis. Acta Microbiol. Immunol. Hung. 2007, 54, 317–321. [Google Scholar] [CrossRef]
- Cetin, S.; Baslarli, S.; Celik, B.; Celik, I. A Case of Pneumonia Caused by Comamonas testosteroni in the Pediatric Intensive Care Unit. Eurasian J. Med. Oncol. 2018, 2, 251–253. [Google Scholar]
- Spiers, A.J.; Buckling, A.; Rainey, P.B. The causes of Pseudomonas diversity. Microbiology 2000, 146, 2345–2350. [Google Scholar] [CrossRef]
- Gomila, M.; Peña, A.; Mulet, M.; Lalucat, J.; García-Valdés, E. Phylogenomics and systematics in Pseudomonas. Front. Microbiol. 2015, 6, 214. [Google Scholar] [CrossRef]
- Newaj-Fyzul, A.; Mutani, A.; Ramsubhag, A.; Adesiyun, A. Prevalence of bacterial pathogens and their anti-microbial resistance in tilapia and their pond water in Trinidad. Zoonoses Public Health 2008, 55, 206–213. [Google Scholar] [CrossRef]
- Monghit-Camarin, M.-A.A.; Cruz-Lacierda, E.R.; Pakingking, R., Jr.; Cuvin-Aralar, M.L.; Traifalgar, R.F.; Añasco, N.C.; Austin, F.; Lawrence, M. Bacterial microbiota of hatchery-reared freshwater prawn Macrobrachium rosenbergii (de Man, 1879). Asian Fish. Sci. 2020, 33, 241–248. [Google Scholar] [CrossRef]
- Udikovic-Kolic, N.; Wichmann, F.; Broderick, N.A.; Handelsman, J. Bloom of resident antibiotic-resistant bacteria in soil following manure fertilization. Proc. Natl. Acad. Sci. USA 2014, 111, 15202–15207. [Google Scholar] [CrossRef]
- Hossain, M.; Rahman, M.; Mondal, S.; Shadat Mondal, A.; Chowdhury, M. Isolation of some emergent bacterial pathogens recovered from captured and culture fisheries in Bangladesh. Bangladesh Res. Publ. J. 2011, 6, 77–90. [Google Scholar]
- Sarter, S.; Kha Nguyen, H.N.; Hung, L.T.; Lazard, J.; Montet, D. Antibiotic resistance in Gram-negative bacteria isolated from farmed catfish. Food Control 2007, 18, 1391–1396. [Google Scholar] [CrossRef]
- Boss, R.; Overesch, G.; Baumgartner, A. Antimicrobial resistance of Escherichia coli, Enterococci, Pseudomonas aeruginosa, and Staphylococcus aureus from raw fish and seafood Imported into Switzerland. J. Food Prot. 2016, 79, 1240–1246. [Google Scholar] [CrossRef]
- Gufe, C.; Canaan Hodobo, T.; Mbonjani, B.; Majonga, O.; Marumure, J.; Musari, S.; Jongi, G.; Makaya, P.V.; Machakwa, J. Antimicrobial Profiling of Bacteria Isolated from Fish Sold at Informal Market in Mufakose, Zimbabwe. Int. J. Microbiol. 2019, 2019, 8759636. [Google Scholar] [CrossRef] [PubMed]
- Festus, F.I.; Damilola, M. Occurrence, antibiotic susceptibility pattern and physiological studies of Pseudomonas species isolated from ready to eat foods in Ibadan, Oyo State, Nigeria. J. Appl. Life Sci. Int. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Yi, S.; Li, J.; Zhu, J.; Lin, Y.; Fu, L.; Chen, W.; Li, X. Effect of tea polyphenols on microbiological and biochemical quality of Collichthys fish ball. J. Sci. Food Agric. 2011, 91, 1591–1597. [Google Scholar] [CrossRef]
- Geraldine, A.R.; Rosidah, R.; Herawati, H.; Bioshina, I.B. Isolation and identification of potential pathogenic bacteria in living carp (Cyprinus carpio Linnaeus, 1758) sold in supermarkets in Cimahi City, Java. World News Nat. Sci. 2020, 32, 21–35. [Google Scholar]
- Abadi, A.S.; Hismayasari, I.B.; Supriatna, I.; Yani, A.; Sayuti, M. The mass death of Nile tilapia (Oreochromis niloticus) in Sorong District, West Papua, Indonesia. Aquac Aquar Conserv Legis 2020, 13, 1906–1916. [Google Scholar]
- Chi, C.-Y.; Lai, C.-H.; Fung, C.-P.; Wang, J.-H. Pseudomonas mendocina spondylodiscitis: A case report and literature review. Scand. J. Infect. Dis. 2005, 37, 950–953. [Google Scholar] [CrossRef]
- Chiu, L.Q.; Wang, W. A case of unusual Gram-negative bacilli septic arthritis in an immunocompetent patient. Singap. Med. J. 2013, 54, e164–e168. [Google Scholar] [CrossRef]
- Gani, M.; Rao, S.; Miller, M.; Scoular, S. Pseudomonas mendocina bacteremia: A case study and review of literature. Am. J. Case Rep. 2019, 20, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Nseir, W.; Taha, H.; Abid, A.; Khateeb, J. Pseudomonas mendocina sepsis in a healthy man. Isr. Med. Assoc. J. 2011, 13, 375–376. [Google Scholar]
- Ioannou, P.; Vougiouklakis, G. A Systematic Review of Human Infections by Pseudomonas mendocina. Trop. Med. Infect. Dis. 2020, 5, 71. [Google Scholar] [CrossRef]
- Flores Ribeiro, A.; Bodilis, J.; Alonso, L.; Buquet, S.; Feuilloley, M.; Dupont, J.-P.; Pawlak, B. Occurrence of multi-antibiotic resistant Pseudomonas spp. in drinking water produced from karstic hydrosystems. Sci. Total Environ. 2014, 490, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z. Bacteria: Pseudomonas. In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 490–500. [Google Scholar]
- Froese, R.; Pauly, D. FishBase, Version February 2018. Available online: https://www.fishbase.se/search.php (accessed on 1 February 2019).
- Hélène, M.; Anne-Laure, M.; Estelle, J.-B. Atypical bacteria in the CF airways: Diversity, clinical consequences, emergence and adaptation. In Cystic Fibrosis–Renewed Hopes through Research; InTech: Rijeka, Croatia, 2012; pp. 225–252. [Google Scholar]
- Coenye, T.; Goris, J.; Spilker, T.; Vandamme, P.; LiPuma, J.J. Characterization of unusual bacteria isolated from respiratory secretions of cystic fibrosis patients and description of Inquilinus limosus gen. nov., sp. nov. J. Clin. Microbiol. 2002, 40, 2062–2069. [Google Scholar] [CrossRef]
- Balasoiu, A.T.; Zlatian, O.M.; Ghenea, A.E.; Davidescu, L.; Lungu, A.; Golli, A.L.; Udriștoiu, A.-L.; Balasoiu, M. A Rare Case of Endophthalmitis with Rhizobium radiobacter, Soon after a Resolved Keratitis: Case Report. Antibiotics 2022, 11, 905. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-L.; Zhao, L.-D.; Li, L.-J.; Zhou, M.-J. Septic shock caused by Rhizobium radiobacter in an elderly woman: A case report. Medicine 2019, 98, 49. [Google Scholar] [CrossRef]
- Christakis, G.B.; Alexaki, P.; Alivizatos, A.S.; Chalkiopoulou, I.; Athanasiou, A.E.; Zarkadis, I.K. Primary bacteraemia caused by Rhizobium radiobacter in a patient with solid tumours. J. Med. Microbiol. 2006, 55, 1453–1456. [Google Scholar] [CrossRef]
- Dunne, W.M.; Tillman, J.; Murray, J.C. Recovery of a strain of Agrobacterium radiobacter with a mucoid phenotype from an immunocompromised child with bacteremia. J. Clin. Microbiol. 1993, 31, 2541–2543. [Google Scholar] [CrossRef]
- Lai, C.-C.; Teng, L.-J.; Hsueh, P.-R.; Yuan, A.; Tsai, K.-C.; Tang, J.-L.; Tien, H.-F. Clinical and Microbiological Characteristics of Rhizobium radiobacter Infections. Clin. Infect. Dis. 2004, 38, 149–153. [Google Scholar] [CrossRef]
- Minguela, J.; De-Pablos, M.; Castellanos, T.; Ruiz-de-Gauna, R. Peritonitis by Rhizobium radiobacter. Perit. Dial. Int. 2006, 26, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Otag, F.; Tezcan, S.; Ozturhan, H.; Aslan, G.; Kuyucu, N.; Emekdas, G. Emerging non-fermenter gram negative pathogens in paediatric patients: Rhizobium radiobacter bacteremia. J. Pediatr. Infect. 2007, 1, 143–146. [Google Scholar]
- Altun, E.; Kaya, B.; Taktakoğlu, O.; Karaer, R.; Paydas, S.; Balal, M.; Seyrek, N. Comamonas testosteroni peritonitis secondary to dislocated intrauterine device and laparoscopic intervention in a continuous ambulatory peritoneal dialysis patient. Perit. Dial. Int. 2013, 33, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Arda, B.; Aydemir, S.; Yamazhan, T.; Hassan, A.; Tünger, A.; Serter, D. Comamonas testosteroni meningitis in a patient with recurrent cholesteatoma: Case report. Apmis 2003, 111, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Le Moal, G.; Paccalin, M.; Breux, J.-P.; Roblot, F.; Roblot, P.; Becq-Giraudon, B. Central venous catheter-related infection due to Comamonas testosteroni in a woman with breast cancer. Scand. J. Infect. Dis. 2001, 33, 627–628. [Google Scholar]
- Nseir, W.; Khateeb, J.; Awawdeh, M.; Ghali, M. Catheter-related bacteremia caused by Comamonas testosteroni in a hemodialysis patient. Hemodial. Int. 2011, 15, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Orsini, J.; Tam, E.; Hauser, N.; Rajayer, S. Polymicrobial Bacteremia Involving Comamonas testosteroni. Case Rep. Med. 2014, 2014, 578127. [Google Scholar] [CrossRef]
- Parolin, M.; Baraldi, M.; Valentini, E.; Murer, L.; Vidal, E. Comamonas testosteroni-associated peritonitis in a pediatric peritoneal dialysis patient. World J. Nephrol. 2016, 5, 220–223. [Google Scholar] [CrossRef]
- Ruziaki, W.A.; Hashami, H.A. Unusual pathogen Comamonas testosteroni sepsis following gastroenteritis in a 12 months old child: Case report and literature review. Am. J. Med. Case Rep. 2017, 5, 148–150. [Google Scholar] [CrossRef]
- Tartar, A.S.; Tartar, T. A Rare Pathogen in Acute Appendicitis: Two Cases with Comamonas testeroni Infection and Literature Review. J. Pediatr. Infect. Dis. 2020, 15, 110–112. [Google Scholar] [CrossRef]
- Yasayancan, N.; Inonu Koseoglu, H. The 20th Comamonas testosteroni Bacteremia Case in the Literature from Turkey: Mortal and Polymicrobial A Case Report and Literature Review. Eurasian J. Med. Oncol 2017, 1, 168–171. [Google Scholar] [CrossRef]
- Newman, J. Review of septic arthritis throughout the antibiotic era. Ann. Rheum. Dis. 1976, 35, 198–205. [Google Scholar] [CrossRef]
- Nordstrom, K.M.; McGinley, K.J.; Cappiello, L.; Zechman, J.M.; Leyden, J.J. Pitted Keratolysis: The Role of Micrococcus sedentarius. Arch. Dermatol. 1987, 123, 1320–1325. [Google Scholar] [CrossRef]
- Souhami, L.; Feld, R.; Tuffnell, P.G.; Feller, T. Micrococcus luteus pneumonia: A case report and review of the literature. Med. Pediatr. Oncol. 1979, 7, 309–314. [Google Scholar] [CrossRef]
- von Eiff, C.; Kuhn, N.; Herrmann, M.; Weber, S.; Peters, G. Micrococcus luteus as a cause of recurrent bacteremia. Pediatr. Infect. Dis. J. 1996, 15, 711–713. [Google Scholar] [CrossRef]
- Goldberg, M.E.; Blyth, M. Pseudomonas mendocina bacteremia in a hemodialysis patient with a central venous catheter. Cureus 2020, 12, e10853. [Google Scholar] [CrossRef]
- Greenough, N.; Gerry, D. First UK Case Report of a Patient with Pseudomonas mendocina Bacteraemia. Clin. Infect. Pract. 2021, 10, 100065. [Google Scholar] [CrossRef]
- Howe, T.S.; Erlich, G.; Koh, J.S.B.; Ng, A.C.M.; Costerton, W. A case of an atypical femoral fracture associated with bacterial biofilm—Pathogen or bystander? Osteoporos. Int. 2013, 24, 1765–1766. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-R.; Lien, C.-Y.; Tsai, W.-C.; Lai, W.-A.; Hsu, C.-W.; Tsai, N.-W.; Chang, C.-C.; Lu, C.-H.; Chien, C.-C.; Chang, W.-N. The clinical characteristics of adult bacterial meningitis caused by non-Pseudomonas (Ps.) aeruginosa Pseudomonas species: A clinical comparison with Ps. aeruginosa meningitis. Kaohsiung J. Med. Sci. 2018, 34, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Johansen, H.; Kjeldsen, K.; Høiby, N. Pseudomonas mendocina as a cause of chronic infective endocarditis in a patient with situs inversus. Clin. Microbiol. Infect. 2001, 7, 650–652. [Google Scholar] [CrossRef]
- Jerónimo, T.M.; Guedes, A.M.; Stieglmair, S.; Guerreiro, R.; Laranjo, C.; Bernardo, I.; Neves, P.L. Pseudomonas mendocina: The first case of peritonitis on peritoneal dialysis. Nefrologia 2017, 37, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Mert, A.; Yilmaz, M.; Ozaras, R.; Kocak, F.; Dagsali, S. Native valve endocarditis due to Pseudomonas mendocina in a patient with mental retardation and a review of literature. Scand. J. Infect. Dis. 2007, 39, 615–616. [Google Scholar] [CrossRef]
- Suel, P.; Martin, P.; Berthelot, G.; Robaday, S.; Etienne, M.; Chibani, A. A case of Pseudomonas mendocina endocarditis. Med. Mal. Infect. 2010, 41, 109–110. [Google Scholar] [CrossRef]
- Hage, J.; Schoch, P.; Cunha, B. Pseudomonas pseudoalcaligenes Peritoneal Dialysis–Associated Peritonitis. Perit. Dial. Int. 2013, 33, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Ledger, W.J.; Headington, J.T. Isolation of Pseudomonas pseudoalcaligenes from an infection of a pregnant uterus. Int. J. Gynaecol. Obstet. 1972, 10, 87–89. [Google Scholar] [CrossRef]
- Amaya, R.A.; Edwards, M.S. Agrobacterium radiobacter bacteremia in pediatric patients: Case report and review. Pediatr. Infect. Dis. J. 2003, 22, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Bhansali, V.D.; Kalane, S.U.; Mandke, H.; Joe, G. Rhizobium radiobacter–A rare organism causing central line-associated bloodstream infection in a preterm neonate. Indian J. Child Health 2020, 7, 418–420. [Google Scholar] [CrossRef]
- Boceska, B.K.; Osmani, D.; Basovska, B.P.; Petreska, V.K.; Trajkova, Z.A.; Jovanovska, A.; Kocheva, S. Rhizobium radiobacter bacteremia in a two-year-old patient with an acute lymphoblastic leukemia: A case report. Arch. Public Health 2020, 12, 91–94. [Google Scholar]
- Detrait, M.; D’Hondt, L.; André, M.; Lonchay, C.; Holemans, X.; Maton, J.P.; Canon, J.L. Agrobacterium radiobacter bacteremia in oncologic and geriatric patients: Presentation of two cases and review of the literature. Int. J. Infect. Dis. 2008, 12, e7–e10. [Google Scholar] [CrossRef] [PubMed]
- Fenner, B.J.; Kumar, A.; Tan, N.Y.; Ang, M. Case of isolated Rhizobium radiobacter contact lens-related infectious keratitis: A plant microbe now emerging as a human pathogen. Am. J. Ophthalmol. Case Rep. 2019, 15, 100476. [Google Scholar] [CrossRef] [PubMed]
- Landron, C.; Moal, G.l.; Roblot, F.; Grignon, B.; Bonnin, A.; Becq-Giraudon, B. Central venous catheter-related infection due to Agrobacterium radiobacter: A report of 2 cases. Scand. J. Infect. Dis. 2002, 34, 693–694. [Google Scholar] [CrossRef]
- Lui, S.; Lo, W. Agrobacterium radiobacter peritonitis in a Chinese patient on CAPD. Perit. Dial. Int. 2005, 25, 95. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Prasad, K.N.; Singh, K.; Bhadauria, D.; Sharma, R. Rhizobium radiobacter peritonitis: The first case report from India and review. JMM Case Rep. 2014, 1, e004051. [Google Scholar] [CrossRef]
- Paphitou, N.; Rolston, K. Catheter-related bacteremia caused by Agrobacterium radiobacter in a cancer patient: Case report and literature review. Infection 2003, 31, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Patel, W.; Aboud, M.; Alnafisah, H.; Razak, A. Rhizobium Radiobacter Sepsis in a Neonate: A Case Report and Literature Review. J. Neonatol. 2022, 36, 240–243. [Google Scholar] [CrossRef]
- Roy, S.; Basuli, D.; Rahman, E.U.; Adapa, S.; Reddy, S.N. Rhizobium radiobacter-Induced Peritonitis: A Case Report and Literature Analysis. J. Med. Cases 2022, 13, 471–474. [Google Scholar] [CrossRef]
- Cartwright, K.; Evans, B. Salmon as a food-poisoning vehicle-two successive Salmonella outbreaks. Epidemiol. Infect. 1988, 101, 249–257. [Google Scholar] [CrossRef]
- Kuhn, H.; Gericke, B.; Klepp, M.; Fellmann, G.; Rabsch, W. Ausbruch von Salmonella paratyphi B-infektionen im zusammenhang mit dem genuss von räucherfisch. Das Gesundheitswesen 1994, 56, 211–214. [Google Scholar]
- Allton, D.R.; Forgione, M.A., Jr.; Gros, S.P. Cholera-like presentation in Vibrio fluvialis enteritis. South. Med. J. 2006, 99, 765–772. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Bhattacharjee, S.; Bal, B.; Pal, R.; Niyogi, S.K.; Sarkar, K. Is Vibrio fluvialis emerging as a pathogen with epidemic potential in coastal region of eastern India following cyclone Aila? J. Health Popul. Nutr. 2010, 28, 311–317. [Google Scholar]
- Boĭko, A.V. The etiological structure of acute intestinal infections caused by noncholera Vibrios in the Volga delta. Zh. Mikrobiol. Epidemiol. Immunobiol. 2000, 1, 15–17. [Google Scholar]
- Cabrera Rodríguez, L.E.; Palma Monroy, S.; Morier, L.; Ramírez Álvarez, M.M.; Fernández Abreu, A.; Castro Escarpulli, G.; Longa Briceño, A.; Bravo Fariñas, L. Severe otitis due to Vibrio fluvialis in a patient with AIDS: First report in the world. Rev. Cuba. Med. Trop. 2005, 57, 154–155. [Google Scholar]
- Chen, P.-J.; Tseng, C.-C.; Chan, H.-T.; Chao, C.-M. Acute Otitis due to Vibrio fluvialis after Swimming. Case Rep. Emerg. Med. 2012, 2012, 838904. [Google Scholar] [PubMed]
- Chowdhury, G.; Pazhani, G.P.; Dutta, D.; Guin, S.; Dutta, S.; Ghosh, S.; Izumiya, H.; Asakura, M.; Yamasaki, S.; Takeda, Y. Vibrio fluvialis in patients with diarrhea, Kolkata, India. Emerging Infect. Dis. 2012, 18, 1868. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-C.; Wen-Wei Hsu, R. Vibrio fluvialis Hemorrhagic Cellulitis and Cerebritis. Clin. Infect. Dis. 2005, 40, 75–77. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Cheng, C.C.; Huang, T.; Hsu, R. Necrotizing fasciitis and primary sepsis caused by Vibrio fluvialis: A case report. Inj. Extra 2005, 36, 546–549. [Google Scholar] [CrossRef]
- Huq, M.; Alam, A.; Brenner, D.J.; Morris, G.K. Isolation of Vibrio-like group, EF-6, from patients with diarrhea. J. Clin. Microbiol. 1980, 11, 621–624. [Google Scholar] [CrossRef]
- Klontz, K.C.; Desenclos, J.C.A. Clinical and epidemiological features of sporadic infections with Vibrio fluvialis in Florida, USA. J. Diarrhoeal Dis. Res. 1990, 8, 24–26. [Google Scholar]
- Lai, C.-H.; Hwang, C.-K.; Chin, C.; Lin, H.-H.; Wong, W.-W.; Liu, C.-Y. Severe watery diarrhoea and bacteraemia caused by Vibrio fluvialis. J. Infect. 2006, 52, e95–e98. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, J.S.; Oh, S.H.; Kim, H.R.; Lee, J.N.; Shin, J.H. Acute infectious peritonitis caused by Vibrio fluvialis. Diagn. Microbiol. Infect. Dis. 2008, 62, 216–218. [Google Scholar] [CrossRef]
- Liu, W.L.; Chiu, Y.H.; Chao, C.M.; Hou, C.C.; Lai, C.C. Biliary tract infection caused by Vibrio fluvialis in an immunocompromised patient. Infection 2011, 39, 495–496. [Google Scholar] [CrossRef]
- Usta, J.; Araj, G.; Taleb, R. An unusual urinary tract infection caused by Vibrio fluvialis. J. Infect. Dev. Ctries. 2018, 12, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-M.; Tsai, T.-C.; Lai, C.-C. Secondary peritonitis due to Rhizobium radiobacter. Surg. Infect. 2014, 15, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Mantadakis, E.; Kondi, A.; Christidou, A.; Kalmanti, M. Agrobacterium radiobacter bacteremia in a child with acute lymphoblastic leukemia. World J. Clin. Pediatr. 2010, 6, 181–184. [Google Scholar] [CrossRef]
- Edmond, M.B.; Riddler, S.A.; Baxter, C.M.; Wicklund, B.M.; Pasculle, A.W. Agrobacterium radiobacter: A recently recognized opportunistic pathogen. Clin. Infect. Dis. 1993, 16, 388–391. [Google Scholar] [CrossRef]
- Rothe, H.; Rothenpieler, U. Peritonitis due to multiresistant Rhizobium radiobacter. Perit. Dial. Int. 2007, 27, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Al-Sunaiher, A.E.; Ibrahim, A.S.; Al-Salamah, A.A. Association of Vibrio species with disease incidence in some cultured fishes in the Kingdom of Saudi Arabia. World Appl. Sci. J. 2010, 8, 653–660. [Google Scholar]
- Adebayo-Tayo, B.; Okonko, I.; Esen, C.; Odu, N.; Onoh, C.; Igwiloh, N. Incidence of potentially pathogenic Vibrio spp. in fresh seafood from Itu Creek in Uyo, Akwa Ibom State, Nigeria. World Appl. Sci. J. 2011, 15, 985–991. [Google Scholar]
- Ramamurthy, T.; Chowdhury, G.; Pazhani, G.; Shinoda, S. Vibrio fluvialis: An emerging human pathogen. Front. Microbiol. 2014, 5, 91. [Google Scholar] [CrossRef]
- Biswas, J.; Jainab, T.; Hossain, M.; Yasmin, M.; Nessa, J.; Ahsan, C.R. Characterization of ctx gene Negative Vibrio fluvialis Organisms Isolated from the Environment. Bangladesh J. Microbiol. 2019, 36, 91–97. [Google Scholar] [CrossRef]
- Brenner, D.; Hickman-Brenner, F.; Lee, J.; Steigerwalt, A.; Fanning, G.; Hollis, D.; Farmer, J.; Weaver, R.; Joseph, S.; Seidler, R. Vibrio furnissii a new species isolated from human feces and the environment. J. Clin. Microbiol. 1983, 18, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, E.O.; Obi, L.C.; Tom, M.; Okoh, A.I. Detection of potential risk of wastewater effluents for transmission of antibiotic resistance from Vibrio species as a reservoir in a peri-urban community in South Africa. Int. J. Environ. Health Res. 2011, 21, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Kesy, K.; Oberbeckmann, S.; Kreikemeyer, B.; Labrenz, M. Spatial environmental heterogeneity determines young biofilm assemblages on microplastics in Baltic Sea mesocosms. Front. Microbiol. 2019, 10, 1665. [Google Scholar] [CrossRef]
- Abdellrazeq, G.S.; Khaliel, S.A. Molecular characterization and antimicrobial susceptibility of vibrios isolated from healthy and diseased aquacultured freshwater fishes. Glob. Vet. 2014, 13, 397–407. [Google Scholar]
- Matter, A.F.; El Asely, A.M.; Shaheen, A.A.; El-Gawad, E.; El-Abd, H.; Abbass, A.A. Phenotypic and molecular characterization of bacterial pathogens isolated from diseased freshwater fishes. Int. J. Fish. Aquat. Stud 2018, 6, 34–41. [Google Scholar]
- Li, A.; Yang, W.; Hu, J.; Wang, W.; Cai, T.; Wang, J. Optimization by orthogonal array design and humoral immunity of the bivalent vaccine against Aeromonas hydrophila and Vibrio fluvialis infection in crucian carp (Carassius auratus L.). Aquac. Res. 2006, 37, 813–820. [Google Scholar] [CrossRef]
- Mishra, P.; Samanta, M.; Mohanty, S.; Maiti, N. Characterization of Vibrio species isolated from freshwater fishes by ribotyping. Indian J. Microbiol. 2010, 50, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Pushpita, M.; Samanta, M.; Maiti, N.K.; Sarangi, N. Characterization of extracellular cytotoxic protein of Vibrio spp. isolated from freshwater carps and prawns. Indian J. Fish. 2009, 56, 307–311. [Google Scholar]
- Al-Hussainy, K.S.; Al-Tememy, M.S.; Al-Gboory, A.O. Effect of some environmental condition Vibrio cholerae and Vibrio fluvialis bacteria isolating from fish proffered in local markets of Basrah and Nasiriyah city. Diyala Ag. Sci. J 2017, 9, 1–15. [Google Scholar]
- Hickman-Brenner, F.; Brenner, D.; Steigerwalt, A.; Schreiber, M.; Holmberg, S.; Baldy, L.; Lewis, C.; Pickens, N.; Farmer, J. Vibrio fluvialis and Vibrio furnissii isolated from a stool sample of one patient. J. Clin. Microbiol. 1984, 20, 125–127. [Google Scholar] [CrossRef]
- Oluyinka, O.; Airede, K.; Olateju, K.; Stephen, O.; Izevbigie, N.; Iloh, K.; Igbokwe, O.; Osuorah, C.D.I. Incidence, bacteriological profile and antibiotic sensitivity pattern of neonatal sepsis in a tertiary health facility in Abuja, North-central Nigeria. Prepint, Research Square 2020. [Google Scholar] [CrossRef]
- Abdollahi, M.; Beyzaei, H.; Hashemi, S.; Ghasemi, B. Comparative antibacterial activity of synthetic N,S-Heterocyclic derivatives, MgO nanoparticles, and glycine on zoonotic Vibrio fluvialis. J. Rep. Pharm. Sci. 2019, 8, 155–160. [Google Scholar]
- Lowy, F. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Rasigade, J.-P.; Vandenesch, F. Staphylococcus aureus: A pathogen with still unresolved issues. Infect. Genet. Evol. 2014, 21, 510–514. [Google Scholar] [CrossRef]
- Sichewo, P.R.; Gono, R.K.; Sizanobuhle, J. Isolation and identification of pathogenic bacteria in edible fish: A case study of Fletcher Dam in Gweru, Zimbabwe. Int. J. Sci. Res. 2013, 2, 269–273. [Google Scholar]
- Nguyen, D.T.A.; Kanki, M.; Nguyen, P.D.; Le, H.T.; Ngo, P.T.; Tran, D.N.M.; Le, N.H.; Dang, C.V.; Kawai, T.; Kawahara, R.; et al. Prevalence, antibiotic resistance, and extended-spectrum and AmpC β-lactamase productivity of Salmonella isolates from raw meat and seafood samples in Ho Chi Minh City, Vietnam. Int. J. Food Microbiol. 2016, 236, 115–122. [Google Scholar] [CrossRef]
- Hennekinne, J.-A. Chapter 7-Staphylococcus aureus as a Leading Cause of Foodborne Outbreaks Worldwide. In Staphylococcus aureus; Fetsch, A., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 129–146. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Fowler Jr, V.G.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Belthur, M.V.; Birchansky, S.B.; Verdugo, A.A.; Mason Jr, E.O.; Hulten, K.G.; Kaplan, S.L.; Smith, E.B.; Phillips, W.A.; Weinberg, J. Pathologic fractures in children with acute Staphylococcus aureus osteomyelitis. J. Bone Jt. Surg. 2012, 94, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-R.; Kim, T.; Kim, M.-C.; Sup Sung, H.; Kim, M.-N.; Kim, M.J.; Kim, S.H.; Lee, S.-O.; Choi, S.-H.; Woo, J.H. Sternoclavicular septic arthritis caused by Staphylococcus aureus: Excellent results from medical treatment and limited surgery. Infect. Dis. 2019, 51, 694–700. [Google Scholar] [CrossRef]
- Ferry, T.; Leboucher, G.; Fevre, C.; Herry, Y.; Conrad, A.; Josse, J.; Batailler, C.; Chidiac, C.; Medina, M.; Lustig, S. Salvage debridement, antibiotics and implant retention (“DAIR”) with local injection of a selected cocktail of bacteriophages: Is it an option for an elderly patient with relapsing Staphylococcus aureus prosthetic-joint infection? Open Forum Infect. Dis. 2018, 5, ofy269. [Google Scholar] [CrossRef] [PubMed]
- Sumon, Z.E.; Berenson, C.S.; Sellick, J.A.; Bulman, Z.P.; Tsuji, B.T.; Mergenhagen, K.A. Successful cure of daptomycin-non-susceptible, vancomycin-intermediate Staphylococcus aureus prosthetic aortic valve endocarditis directed by synergistic in vitro time-kill study. Infect. Dis. 2019, 51, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Murray, R. Staphylococcus aureus infective endocarditis: Diagnosis and management guidelines. Intern. Med. J. 2005, 35, S25–S44. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Gschiel, E.; Mann, M.; Huhulescu, S.; Ruppitsch, W.; Bohm, G.; Pichler, J.; Lederer, I.; Höger, G.; Heuberger, S. Outbreak of acute gastroenteritis in an Austrian boarding school, September 2006. Euro. Surveil. 2007, 12, 7–8. [Google Scholar] [CrossRef]
- Sung, K.; Kim, J.Y.; Lee, Y.J.; Hwang, E.H.; Park, J.H. High incidence of Staphylococcus aureus and norovirus gastroenteritis in infancy: A single-center, 1-year experience. Pediatr. Gastroenterol. Hepatol. Nutr. 2014, 17, 140. [Google Scholar] [CrossRef]
- Gales, A.C.; Sader, H.S.; Andrade, S.S.; Lutz, L.; Machado, A.; Barth, A.L. Emergence of linezolid-resistant Staphylococcus aureus during treatment of pulmonary infection in a patient with cystic fibrosis. Int. J. Antimicrob. Agents 2006, 27, 300–302. [Google Scholar] [CrossRef]
- Yari, Z.; Mahdavi, S.; Khayati, S.; Ghorbani, R.; Isazadeh, A. Evaluation of antibiotic resistance patterns in Staphylococcus aureus isolates collected from urinary tract infections in women referred to Shahid Beheshti educational and therapeutic center in Maragheh city, year 2016. Med. J. Tabriz Univ. Med. Sci. Health Serv. 2019, 41, 106–112. [Google Scholar] [CrossRef]
- Diamantopoulos, P.T.; Psichogiou, M.; Pantazatou, A.; Zervakis, K.; Rougala, N.; Giannakopoulou, N.; Daikos, G.; Viniou, N.-A. Staphylococcus aureus meningitis in a patient with mantle cell lymphoma under treatment with ibrutinib. Ann. Hematol. 2017, 96, 1049. [Google Scholar] [CrossRef] [PubMed]
- Shahini Shams-Abadi, M.; Halaji, M.; Hoseini-Alfatemi, S.; Gholipour, A.; Mojtahedi, A.; Sedigh Ebrahim-Saraie, H. Epidemiology of toxic shock syndrome toxin-1 harboring Staphylococcus aureus obtained from clinical samples in Iran: A systematic review and meta-analysis. Ann Ig 2018, 30, 391–400. [Google Scholar]
- Pigłowski, M. Pathogenic and Non-Pathogenic Microorganisms in the Rapid Alert System for Food and Feed. Int. J. Environ. Res. Public Health 2019, 16, 477. [Google Scholar] [CrossRef]
- Wray, D.; Davies, R.H. Salmonella infections in cattle. In Salmonella in Domestic Animals; Barrow, P., Methner, U., Eds.; CABI: Oxfordshire, UK, 2000; pp. 169–190. [Google Scholar]
- Gopinath, S.; Carden, S.; Monack, D. Shedding light on Salmonella carriers. Trends Microbiol. 2012, 20, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, M.S. An outbreak of salmonellosis propagated by person-to-person transmission on an Indian reservation. Am. J. Epidemiol. 1975, 102, 257–262. [Google Scholar] [CrossRef]
- Trung, N.; Carrique-Mas, J.; Nghia, N.; Tu, L.; Mai, H.; Tuyen, H.; Campbell, J.; Nhung, N.; Nhung, H.; Minh, P. Non-Typhoidal Salmonella Colonization in Chickens and Humans in the Mekong Delta of Vietnam. Zoonoses Public Health 2017, 64, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.M.; Henderson, H.J.; Smith-Palmer, A. An outbreak of Salmonella Ssaintpaul in a Scottish childcare facility: The influence of parental under-reporting. BMC Infect. Dis. 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, L.E.; Nickelson, R.; Vanderzant, C. Occurrence and control of Salmonella in freshwater catfish. J. Food Sci. 1979, 44, 1067–1073. [Google Scholar] [CrossRef]
- Noor Uddin, G.M.; Larsen, M.H.; Barco, L.; Minh Phu, T.; Dalsgaard, A. Clonal occurrence of Salmonella Weltevreden in cultured shrimp in the Mekong Delta, Vietnam. PLoS ONE 2015, 10, e0134252. [Google Scholar] [CrossRef] [PubMed]
- Hiko, A.; Tasisa, K.; Agga, G.E. Helminthiasis and gram negative enteric bacteria in freshwater fish from selected lakes of Haramaya District, Ethiopia. Fish. Aquac. J. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Andrews, W.; Wilson, C.; Poelma, P.; Romero, A. Bacteriological survey of the channel catfish (Ictalurus punctatus) at the retail level. J. Food Sci. 1977, 42, 359–363. [Google Scholar] [CrossRef]
- Andrews, W.H.; Bruce, V.R.; June, G.; Satchell, F.; Sherrod, P. Salmonella, Chapter 5. In U.S. Food and Drug Administration, Bacteriological Analytical Manual; USA, FDA, AOAC International: Gaithersburg, MD, USA, 1992; p. 1. [Google Scholar]
- D′Aoust, J.-Y.; Sewell, A.; Daley, E.; Greco, P. Antibiotic resistance of agricultural and foodborne Salmonella isolates in Canada: 1986–1989. J. Food Prot. 1992, 55, 428–434. [Google Scholar] [CrossRef]
- Khan, A.A.; Cheng, C.-M.; Van, K.T.; West, C.S.; Nawaz, M.; Khan, S. Characterization of class 1 integron resistance gene cassettes in Salmonella enterica serovars Oslo and Bareily from imported seafood. J. Antimicrob. Chemother. 2006, 58, 1308–1310. [Google Scholar] [CrossRef]
- Pal, A.; Marshall, D.L. Comparison of culture media for enrichment and isolation of Salmonella spp. from frozen Channel catfish and Vietnamese basa fillets. Food Microbiol. 2009, 26, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Datta, A.R.; Ayers, S.; Friedman, S.; Walker, R.D.; White, D.G. Antimicrobial-resistant Salmonella serovars isolated from imported foods. Int. J. Food Microbiol. 2003, 84, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Amagliani, G.; Brandi, G.; Schiavano, G.F. Incidence and role of Salmonella in seafood safety. Food Res. Int. 2012, 45, 780–788. [Google Scholar] [CrossRef]
- Francis, S.; Rowland, J.; Rattenbury, K.; Powell, D.; Rogers, W.; Ward, L.; Palmer, S. An outbreak of paratyphoid fever in the UK associated with a fish-and-chip shop. Epidemiol. Infect. 1989, 103, 445–448. [Google Scholar] [CrossRef]
- Saharan, V.V.; Verma, P.; Singh, A.P. High prevalence of antimicrobial resistance in Escherichia coli, Salmonella spp. and Staphylococcus aureus isolated from fish samples in India. Aquac. Res. 2020, 51, 1200–1210. [Google Scholar] [CrossRef]
- VT Nair, D.; Venkitanarayanan, K.; Kollanoor Johny, A. Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, Q.; Zhang, J.; Huang, J.; Chen, L.; Liu, S.; Yu, S.; Cai, S. Prevalence, enumeration, and characterization of Salmonella isolated from aquatic food products from retail markets in China. Food Control 2015, 57, 308–313. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).