Abstract
Goat milk is an interesting product from a nutritional and health standpoint, although its physico-chemical composition presents some technological challenges, mainly for being less stable than cow’s milk at high temperatures. As pasteurization and ultra-high temperature processing are universally employed to ensure milk quality and safety, non-thermal methods, such as pulsed electric fields (PEFs), reduce the microbial load and eliminate pathogens, representing an interesting alternative for processing this product. This study demonstrates how the combined use of a PEF with short thermal processing and moderate temperature can be effective and energy-efficient in goat milk processing. A combination of thermal treatment at 63 °C after a low-intensity PEF (50 µs pulses, 3 Hz, and 10 kV·cm−1) caused the same reduction effect on the population of Listeria monocytogenes (goat’s raw milk artificially spiked), as compared to a thermal treatment at 72 °C without a PEF. However, z values are significantly higher when PEF is used as a pre-treatment, suggesting that it may induce heat resistance in the survival population of L. monocytogenes. The sensitivity of L. monocytogenes to high temperatures is less pronounced in goat’s milk than cow’s milk, with a more pronounced impact of a PEF on lethality when combined with lower temperatures in goat’s milk. The effect of a PEF on Escherichia coli viability was even more pronounced. It was also observed that thermal treatment energy needs with a PEF as a pre-treatment can be reduced by at least 50% of the total energy requirements.
1. Introduction
According to statistics from 2018 to 2019, goats produce about 15.26 million tons of milk annually [1,2]. In Portugal, in 2021, goat’s milk accounted for only 1.46% of total milk production [3]. The unique composition and physico-chemical characteristics of goat’s milk [1,4,5,6,7] impact its behavior when subjected to processing. In fact, the heat stability of goat’s milk is lower than that of cow’s milk, and its coagulation pattern is highly dependent on initial pH [8], among other factors [9,10], thus being more susceptible to damage when treated with conventional thermal treatments [11]. The current and most common method used to maintain milk’s safety and shelf-life stability is pasteurization using a thermal treatment, which typically ranges from 72 °C to 80 °C or even higher temperatures in the case of UHT milk. These heating temperatures promote the denaturation of whey proteins and the aggregation of casein micelles, which are more severe in goat’s milk due to very small casein micelles and fat globules, leading to a gradual decrease in quality and acceptability of commercial goat milk [12,13,14]. The effect of high-temperature treatments on physical and organoleptic properties, like nutritional losses, color modifications, and flavor changes, has led to an exploration of emerging technologies to produce milk products with better retention of nutrients and fresh-like characteristics of milk components [15,16,17,18]. PEF technology is an emerging technology that has been considered an alternative to conventional pasteurization technology. However, although promising, the industry has not yet adopted this new technology, mostly due to a lack of information concerning investment needs and their scale-up viability [17,19].
PEF equipment generally comprises an electrical pulse-generating device, a chamber where the electric pulses trespass the food product, and circulating pumps. PEF technology consists of an application of high voltage (usually 20–50 kV·cm−1 and even higher) with short pulses (a few to tens of μs) at a pulse-defined frequency (Hz), which will increase temperature (°C) through liquid foods or liquid-immersed foods [19,20]. Thus, the electrical breakdown of the cell membrane will occur, acting as a capacitor filled with a dielectric medium. This is the mechanism by which microbes are inactivated using PEF treatment [21]. These parameters (pulse width and frequency, potential difference between electrodes, and flow rate in the case of continuous treatments) directly affect the viability of microorganisms [20], but the adverse effects of thermal processing are minimized, allowing the preservation of the natural fresh-like characteristics. Moreover, this technology offers other advantages, such as a low processing cost, higher energy efficiency, and environmental sustainability [22,23].
However, some authors state that the industry has shown some resistance to adopting this technology due to the high initial investment costs. Nevertheless, those studies are quite outdated, not taking into consideration the development of the PEF technology and the low maintenance costs [19]. According to Arshad et al. [22], PEF processing is an energy-conservation technique compared to thermal processing. These authors have also outlined that conventional pre-heaters in some food pre-treatments require higher energy than the energy obtained as a consequence of PEF pre-heating and offer an exclusive opportunity to decrease energy expenses through careful targeting of the food matrices [22]. In spite of the investment needs in PEF technology, using thermal treatments in combination with a PEF has been suggested as a strategy to increase its effectiveness and reduce energy costs [24].
The impact of the PEF on microorganisms and milk enzymes can be improved by combining it with other technological methods, such as mild heating, i.e., operating below conventional pasteurization temperatures, acting as complementary to PEF treatment. Although the effects of the PEF on milk and dairy product processing have been recently studied, the specific processing conditions are extensively discussed by several authors, focusing on evaluating the combined impact of these techniques [25].
Concerning milk pasteurization, the PEF is an emerging alternative method studied in bovine milk, including skim and whole milk [26,27,28,29]. However, there are limited published studies with goat milk [30,31,32,33]. Pasteurized goat’s milk is not as commonly found in the market as cow’s milk. However, improvements in the pasteurization of goat milk, especially with processes that allow the maintenance of its nutritional qualities, will certainly have an impact on its availability in the market [32].
Sharma et al. [18] also reported that PEF-treated raw bovine whole milk was microbiologically stable for 21 days at 4 °C and similar to thermally treated milk (63 °C for 30 min or 73 °C for 15 s).
Beyond the inactivation capacity of the PEF treatment, another advantage is the fact that this type of treatment does not have significant repercussions on the physical properties such as pH, color, or particle size distribution, as demonstrated by studies with a PEF treatment of commercial low-fat bovine milk at 10 kV·cm−1 for 30 μs [16].
The objective of the present work was to evaluate the effectiveness of microbial inactivation of Listeria monocytogenes of the PEF-processed goat milk in combination with mild temperature pasteurization and reduce the energy requirements of this step.
2. Materials and Methods
In the following sections, milk samples pasteurized with heat treatment only are referred to as “HT” samples and samples treated with a combination of pulsed electric fields and heat treatment are referred to as “PEF + HT”.
2.1. Milk Samples
UHT cow’s milk (1.5% milk fat) was obtained from a retail store, and goat milk was kindly provided by a local dairy farm of traditional cheese in Melgaço (Prados de Melgaço, Melgaço, Portugal). Samples of raw goat milk were obtained in the morning within 2 h after milking, collected in low-density polyethylene (LDPE) glass bottles, and transported at 4 ± 1 °C, directly to the IPVC Food Processing and Engineering Laboratory, Viana do Castelo, Portugal. All samples were kept at 4 ± 1 °C and used within a maximum of 12 h for their respective treatments and analysis, all of which were carried out in triplicate.
2.2. Regeneration of Bacterial Cultures
Cultures of E. coli (ATCC 11775) and L. monocytogenes ATCC 13932, preserved at −80 °C in a BHI medium with 15% (v/v) glycerol (Merck, Darmstadt, Germany) were used in this work. The regeneration of E.coli and L. monocytogenes was performed in 5 mL of a brain–heart infusion medium, BHI (Biokar Diagnostics, Beauvais, France), at 37 °C overnight.
2.3. Preparation of the Inoculum for Use in Inactivation Studies
Before the use in inactivation studies, microbial cultures were diluted at 1:100 with the respective culture medium and grown at 30 °C until the exponential growth phase was reached. The concentration of the inoculum was adjusted via densitometry until a 0.5 MacFarland suspension was obtained (Biosan DEN-1B, Riga, Latvia) prepared by the direct suspension of four or five morphologically similar colonies, isolated in a TSA medium in a sterile 0.85% (w/v) NaCl solution until turbidity of 0.5 MacFarland was obtained (Biosan DEN-1B, Riga, Latvia).
2.4. Pulsed Electric Field Tests
For non-thermal processing of inoculated samples, PEF equipment was used—EPULSUS®-LPM1A-10 (EnergyPulse Systems, Lda., Lisbon, Portugal) for laboratory scale. This generator produces positive, unipolar rectangular pulses and is equipped with a continuous mode over a collinear treatment chamber with an internal diameter of 1.0 cm and a gap distance of 1.0 cm between the metal (titanium) electrodes. Samples were processed with a PEF in continuous mode, using a fixed electric field of 10 kV·cm−1. The temperature was measured at the entrance and exit of the PEF treatment assembly. Prior to each test, the treatment line was cleaned with distilled water, sanitized by pumping a 70% ethanol solution through it, and then rinsed with sterile distilled water. A peristaltic pump (Watson Marlow 313S, Marlow, United Kingdom) was used to pump the microbial suspension through the system at a flow rate of 2.92 L·h−1. This flow rate was decided after preliminary studies that showed that, under lab conditions, it provided a maximum reduction in the viability of microbial cells. After treatment, the temperature of the treated product was measured, and 5 mL was collected from sterile test tubes, which were immediately placed on ice until the quantification of surviving cells was performed. All tests were performed in triplicate.
2.5. Combined PEF and Milk Thermal Treatments
Inoculated raw goat milk and UHT cow’s milk kept at 4 °C were pre-treated using a low-intensity PEF followed by heat treatments (PEF + HT). PEF conditions were: constant pulse width of 50 µs, frequency of 3 Hz, an electric field strength of 10 kV·cm−1, and a flow rate of 2.92 L·h−1. Thermal treatments were then carried out in a heat exchanger unit FT74XTS HTST/UHT system (Armfield, UK) at a constant flow rate of 10 L·h−1, using temperatures in the range of 63–75 °C (63, 66, 69, 72, and 75 °C), and a holding tube of 2 s.
Samples of goat and cow’s milk subjected only to thermal treatment (HT) were used as controls.
In this combined heat thermal treatment with a PEF, a continuous mode of the PEF was performed using laboratory-scale PEF equipment as described before, with positive rectangular pulses before pasteurization and using only this sequence. Immediately after processing, HT or PEF + HT milk samples were collected in duplicates under sterile conditions and immediately placed in an ice bath until further analysis.
The electrical conductivity of fresh milk at 4 ± 1 °C was measured using a pH-Conductivity Meter Orion 4-Star (Thermo Scientific, Waltham, MA, USA).
2.6. Cleaning-in-Place of Heat Exchanger Unit
The product lines of the heat exchanger were cleaned and sanitized before and after each experiment using a cleaning-in-place procedure involving pre-rinsing, chemical cleaning, disinfection, and final rinsing. Cleaning and sanitation were carried out using distilled water (60 °C in a heat-exchange unit) followed by a 5% (w/v) NaOH solution, sterilized distilled water, a 1% (v/v) HNO3 solution, and finally sterilized distilled water.
2.7. Quantification of the Surviving Cells
After spiking the cow’s and goat’s milk with the test microorganism, a sample was taken immediately before the treatment (HT or PEF + HT) to determine the initial concentration of cells. After treatment, a sample was immediately cooled on ice. Enumeration of the surviving cells was performed after decimal dilutions in a maximum recovery diluent (Liofilchem srl, Roseto degli Abruzzi, Italy) and quantified according to ISO 16649-2:2001 by incorporation into a TBX medium for counting E. coli after incubation at 37 °C for 24 h, and counting L. monocytogenes was performed vis inoculation on the surface of a Listeria chromogenic agar base, ALOA (Biomerieux, France), after incubation at 37 °C for 48 h, as described on ISO 11290-1:2017. The mean values of viable counts and respective standard deviations were estimated and expressed as the logarithm of colony-forming units per mL of product, i.e., log (CFU·mL−1).
Calculations of thermal death kinetic parameters, namely decimal reduction time (D), were estimated using the log-linear equation
where N0 and N are, respectively, the initial population and number of survivors after the treatment. The z value was calculated according to:
Sub-lethally injured cells were quantified using plating cells after milk treatment, in parallel on TSAYE and TSAYE with 5% NaCl [34].
2.8. Measurements of pH, Electrical Conductivity, Titratable Acidity (TA), Total Soluble Solids (TSS), and Viscosity in Goat Milk
The pH, TA, and TSS of HT and PEF + HT samples were measured at room temperature (20 ± 2 °C) according to the AOAC standard method 981.12, 947.05, and 932.12, respectively [35].
Milk viscosity evaluation was carried out using a Thermo Haake rotational viscometer (model VT 550) with concentric cylinders (NV ST 807-0713 CE and NV 807-0702) and collected using the software program Pro RheoWin (version 2.93, Haake). Flow behavior was evaluated according to a previously described method [35]. Flow curves were generated using a shear rate increased from 10.82 s−1 to 221.80 s−1 in the first 2 min, under a controlled temperature of 20.0 ± 0.1 °C, through water circulation in a temperature-controlled bath (Thermo Haake K20) coupled to the equipment. Regarding the physico-chemical characterization, five milk samples collected at the Prados de Melgaço facilities (March 29th and April 4th, 6th, 11th, and 13th) were used. Results are presented as the average of all determinations for each parameter. All the values were obtained in triplicate.
2.9. Heat Treatment and PEF Energy Calculations
Heating energy or sensitive heat, Q, expressed in kJ∙h−1, during thermal treatments was calculated according to the temperature increase (∆T) promoted by the heat exchanger after the PEF treatment. The heat-energy needs were calculated according to other similar studies using Equation (3) [24,27,36].
where m is the mass rate (m3∙h−1) of heat-treated milk and Cp (kJ/(kg∙K)) is the specific heat of milk.
Q = m·Cp·∆T
The mass rate of vapor, mv (kg∙h−1), needed for PEF + HT pasteurization (63 °C) and thermal pasteurization (HT at 72 °C) was calculated using Equation (4).
where Qv (kJ∙h−1) vapor heat energy (kJ∙h−1) and Hv is the energy of 1 kg of steam (kJ), obtained from saturated steam property tables, considering a steam temperature of 70 °C in the case of PEF + HT treatment at 63 °C, and 80 °C in the case of HT treatment at 72 °C.
Qv = mv·Hv
The energy delivered using the PEF equipment, QPEF (kJ∙L−1), was calculated according to Sampedro, 2014 [37] using Equation (5).
where f is the pulse frequency (Hz), Qpulse is the energy per pulse (J), and m is the flow rate of milk (L∙s−1) entering the PEF equipment system.
For the PEF + HT treatment, the total required energy, QT, was then calculated using Equation (6).
QT = QPEF + Q
2.10. Data Analysis
For all treatments, statistical differences were determined using data from three different batches of milk (n = 3) and analyzed using analysis of variance (one-way ANOVA) with a significance level of α = 0.05. Tukey HSD tests were used to evaluate differences following significative ANOVAs. All statistical analyses were performed using the R software (R version 4.3.1, 2023, R Foundation for Statistical Computing). All graphs were produced using the same software.
3. Results and Discussion
Alternative pasteurization methods, which avoid the exposure of milk to high temperatures but achieve the needed reduction of the microbial load, are of great interest. In this sense, the PEF has been used in several food products with success in microbial inactivation.
3.1. Microbial Inactivation
The effectiveness of PEF treatments on inactivating bacteria is highly influenced by the size of the cell. As the cell size decreases, the resistance to PEF treatments increases due to the smaller transmembrane potential created by the external electric field [21,38], which is probably one of the reasons why L. monocytognes is a bacterium with high resistance to PEF treatments [38]. The fact that it is a pathogen and a common contaminant of dairy products, as well as its resistance to heat treatments, determined its choice for the inactivation tests that were conducted on goat’s milk, with the goal of achieving a comparable reduction of 5 log cycles in both types of treatment (with and without PEF). This is the minimum reduction for L. monocytogenes as recommended by the USFDA’s “Control of Listeria monocytogenes in Ready-To-Eat Foods: Guidance for Industry” [39] and its resistance is supported by several previous works on the bacterium’s thermotolerance (e.g., [40]) and thermal resistance in goat’s milk [41].
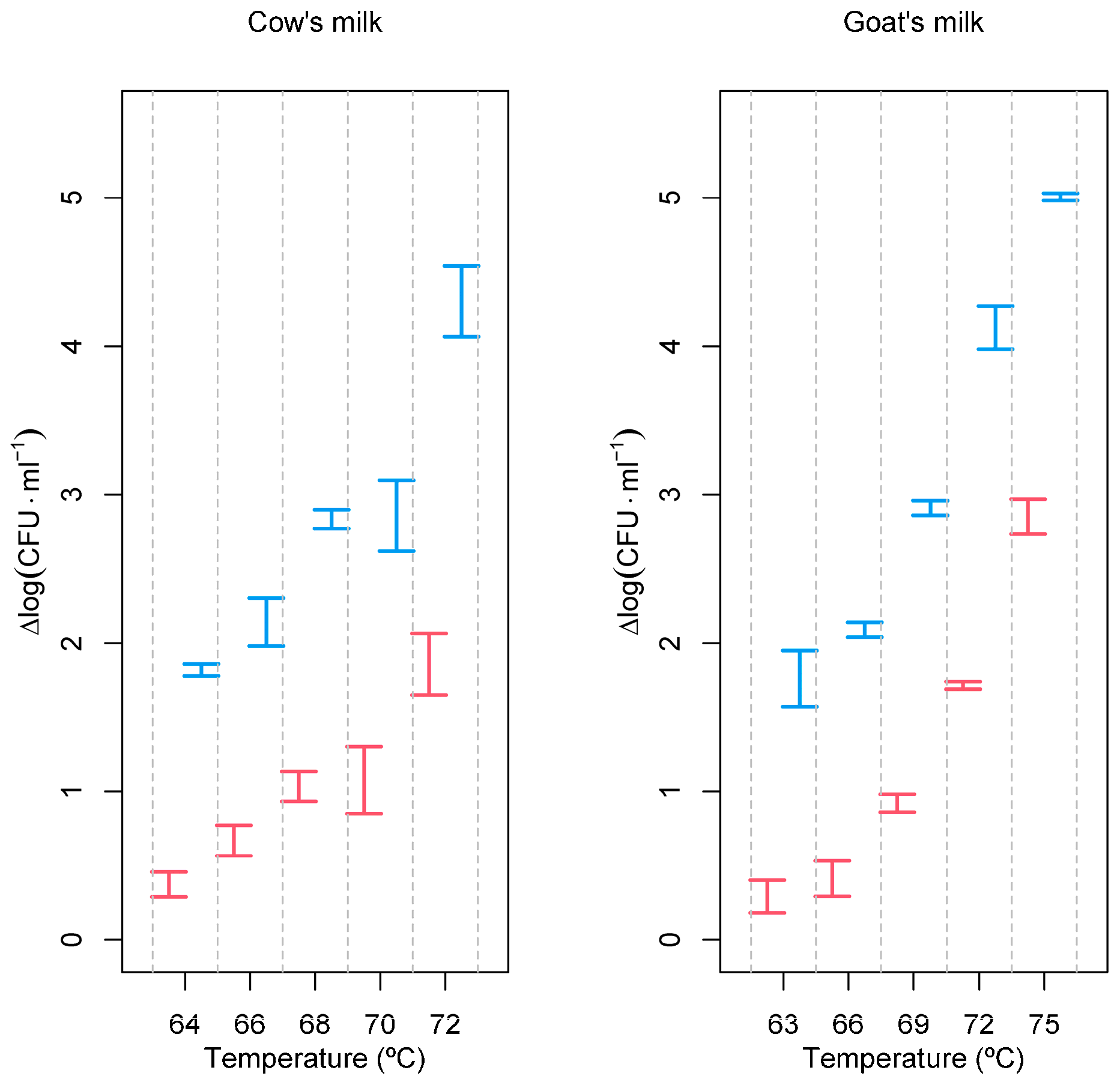
Tests were performed at different temperatures with and without the PEF in cow’s and raw goat’s milk. The milks were inoculated with a L. monocytogenes culture to obtain an initial concentration of at least 8 log (CFU·mL−1), and the reduction in the number of viable cells was determined after a fixed treatment time of 2 s (Figure 1).
Figure 1.
Inactivation of L monocytogenes as a function of processing conditions. Red—heat treatment only; blue—PEF combined with heat treatment. Experimental temperatures ranged between 63 and 75 °C. Inactivation, expressed as Δlog (CFU·mL−1), was calculated according to log (N0/N), where N0 is the initial number of cells and N is the number of cells after the treatment.
As expected, Figure 1 clearly shows that the inactivation of L. monocytogenes increases just by increasing temperature. But, this figure also shows that inactivation is much more evident and more pronounced when the PEF is also used as a pre-treatment, and this is true for both cow’s and goat’s milk. These results are so evident that analysis of variance to compare the effect of temperature, coupled or not with the PEF, would be unnecessary. However, ANOVAs are presented because they facilitate post hoc Tukey HSD tests, comparing all pairs of processing conditions, i.e., all pairs of temperatures with and without PEF. As these involve a total of 45 comparisons, but only a few relevant for discussion are shown in Table 1 and Table 2.

Table 1.
ANOVA table reporting the combined effects of five levels of heat treatment alone and combined with the PEF applied to cow’s milk. The results of three post hoc Tukey HSD tests related to 64 and 72 °C, with and without the PEF, are also shown.

Table 2.
ANOVA table reporting the combined effects of five levels of heat treatment alone and combined with the PEF applied to goat’s milk. The results of four post hoc Tukey HSD tests related to 63, 69, 72, and 75 °C, with and without the PEF, are also shown.
Table 1, referring to cow’s milk, highlights the fact that the maximum temperature used (72 °C, with no PEF) has the same effect over L. monocytogenes as the minimum temperature condition used with the PEF (PEF + 64 °C). After survival counting, calculations of the decimal reduction time (D) allowed the calculation of the z values for both types of treatments (Table 3).

Table 3.
Thermal death behavior of L. monocytogenes in cow’s and goat´s milk.
The application of the PEF alone did not allow for a significant reduction in the L. monocytogenes load (well below the 5 log cycles reduction), and this was why cheeses were not produced with milk only treated with the PEF (as the reduction of viable cells is too low to be considered pasteurization). However, there is a significant number of cells affected by the PEF even though they are not dead: the log reduction after PEF treatment and plating on a medium with and without 5% NaCl showed a significant increase in the number of affected cells (lethal and sub-lethal injured), 3.351 ± 0.008 log (CFU∙mL−1) and 1.765 ± 0.083 log (CFU∙mL−1) reduction, respectively, meaning that after the PEF, 97.4% of survivor L. monocytogenes cells were sub-lethally injured, which is probably why a high efficiency of reducing the population of viable cells using the combined treatment was achieved. Most studies on microbial inactivation induced using the PEF use high electric field strengths (20–50 kV∙cm−1), while low electric field strengths tend to cause sub-lethal injuries to cells. Man-Sheng Wang et al. [42] observed that after a PEF treatment with field strengths of 5–10 kV·cm−1, about 90% of the cells were sub-lethally injured. Several studies have demonstrated the occurrence of a significant number of sub-lethally damaged cells after PEF treatments, and the extent of this damage is influenced by the type of bacteria, the pH of the treatment medium, the duration of treatment, and the strength of the electric field applied [34].
The PEF pre-treatment allowed for a significant increase in the log reduction of the L. monocytogenes population (Figure 1). In goat’s milk, after 2 s of holding time at 66 °C, a reduction of 0.408 ± 0.121 log (CFU∙mL−1) of the L. monocytogenes population was obtained. If pre-treated with the PEF, a reduction of 2.084 ± 0.049 log (CFU∙mL−1) was achieved, corresponding to a very significant increase in the efficacy of the milk processing. The effect of the PEF treatment on cow’s milk was not so pronounced, even though it managed to increase the reduction in viable cells from 0.679 ± 0,10 log (CFU∙mL−1) at 64 °C to 2.125 ± 0.16 log (CFU∙mL−1) at 72 °C.
In goat’s milk, the combined use of the PEF and mild temperature allowed us to obtain a 5.002 ± 0.029 log (CFU∙mL−1) reduction after the PEF treatment combined with a heat treatment for 2 s at 75 °C. In the absence of the PEF pre-treatment, the reduction obtained was just 2.858 ± 0.117 log (CFU∙mL−1).
Both for cow’s and goat’s milk, the increase in the temperature needed to decrease the decimal reduction time is much higher in the PEF pre-treated milk than in milk just subjected to thermal processing (Table 3).
In this study, D values are within the same order of magnitude as values already published, although there is some variation from work to work, depending significantly on how the experimental data were obtained, the type of treatment (continuous or in batch), and the strain used. For example, in cow’s milk, a D65 value of 0.1 min for L. monocytogenes has been reported [43,44]. This is exactly the value obtained for the same microorganism but in goat’s milk. In cow’s milk, its sensitivity to temperature proved to be higher, with a D65 value of 3.89 s. The resistance of L. monocytogenes in cow’s and goat’s milk proved slightly different. The sensitivity of L. monocytogenes to high temperatures is lower in goat’s milk than in cow’s milk, as revealed by the D calculated. This is particularly visible for lower temperatures (e.g., 63 °C), where D63 in goat’s milk is 28% higher than in goat’s milk but only 12.8% higher at 72 °C. The influence of the PEF on lethality is more notorious when combined with lower temperatures and in goat’s milk. At 63 °C, there is a reduction of 83.6% in the decimal reduction time when the PEF is used as a pre-treatment, revealing how efficient this step might be in decreasing the temperature of milk processing.
The PEF pre-treatment significantly increases the z values obtained for the inactivation of L. monocytogenes in cows’ and goat’s milk. In fact, in cows’ milk, the increase in temperature needed to decrease the decimal reduction time by a fraction of 10 is 76.4% higher when the milk is pre-treated with the PEF than when milk is only heat processed. In goats’ milk, this effect is even more pronounced as the estimated increase is 112.8%. This suggests that PEF may induce some heat resistance in the population of cells that are not dead or injured by this treatment. Previous works on the resistance of L. monocytogenes to a PEF have also suggested that the PEF might influence the levels of expression of chaperone proteins, impacting the resistance of the microorganism to mild heat treatments and heat resistance [45].
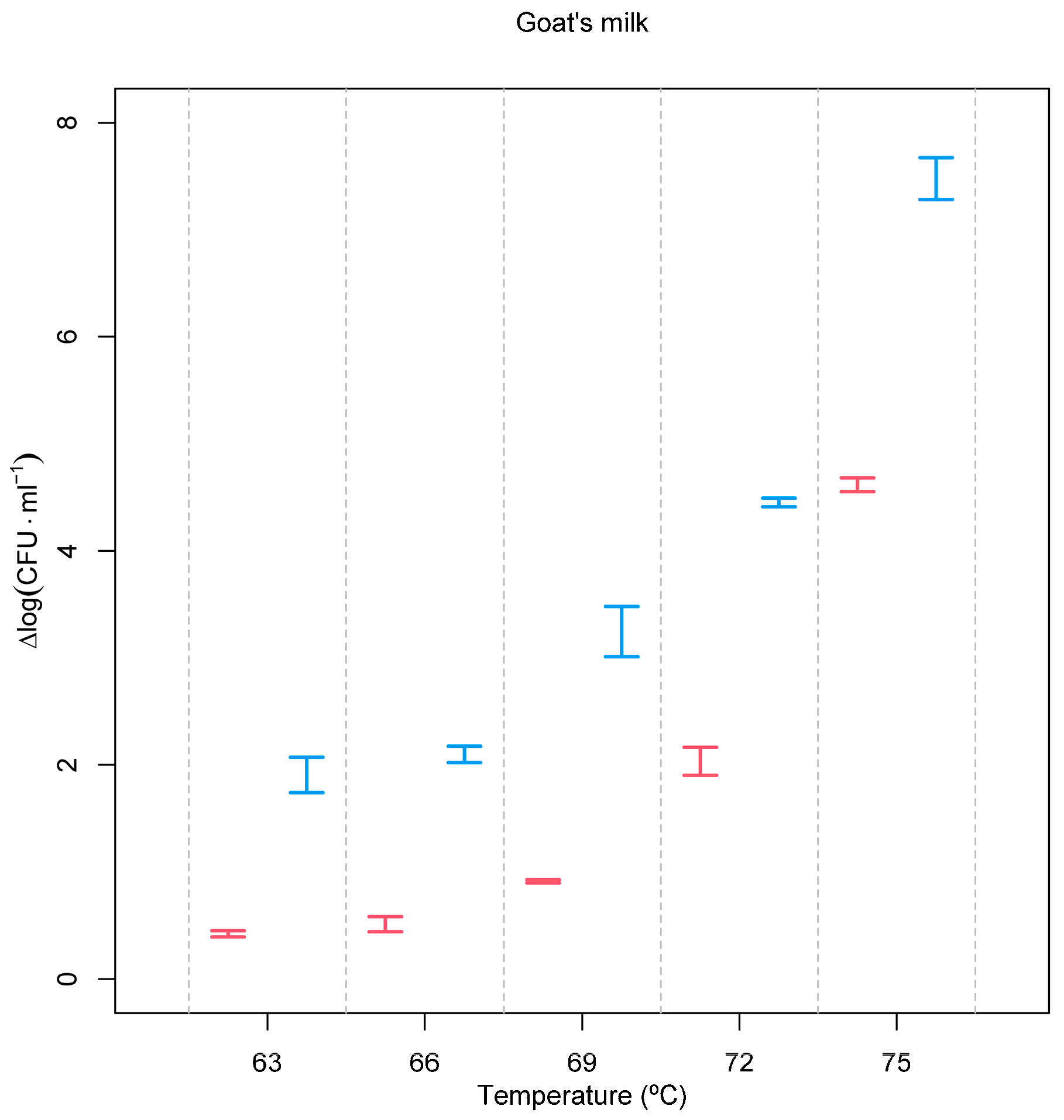
Applying the same treatment conditions to goat’s milk spiked with a common bacterium usually associated with poor hygiene practices, E. coli allowed us to observe its expected lower resistance to both treatments (Figure 2) and an even higher effect of the pre-treatment with the PEF. At 75 °C, a 4.617 ± 0.066 log (CFU·mL−1) reduction was observed, but this value was increased to 7.498 ± 0.198 log (CFU·mL−1) when milk was pre-treated with a PEF. A recent article showed that it is possible to achieve a reduction of 3.87 log (CFU∙mL−1) of E. coli at 40 kV·cm−1 and 13 ms. However, this is a Gram-negative bacteria with little resistance to pulsed electric fields, and a high electric field strength was used [33].
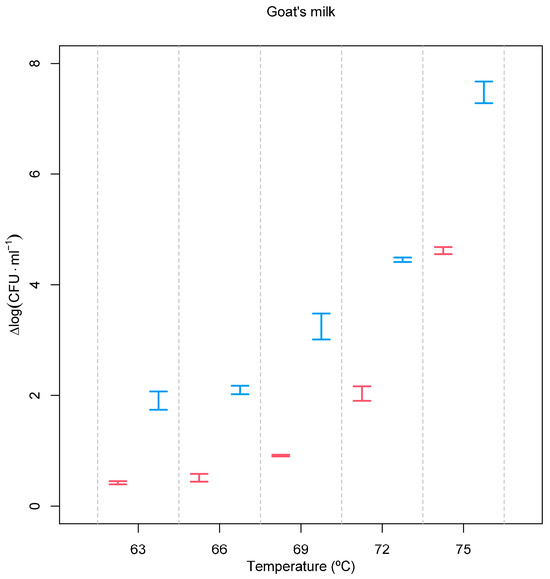
Figure 2.
Inactivation of E. coli as a function of processing conditions. Red = heat treatment only; blue = heat treatment combined with the PEF. Experimental temperatures ranged between 63 °C and 75 °C. Inactivation, expressed as Δlog (CFU·mL−1), was calculated according to log (N0/N), where N0 is the initial number of cells and N is the number of cells after the treatment.
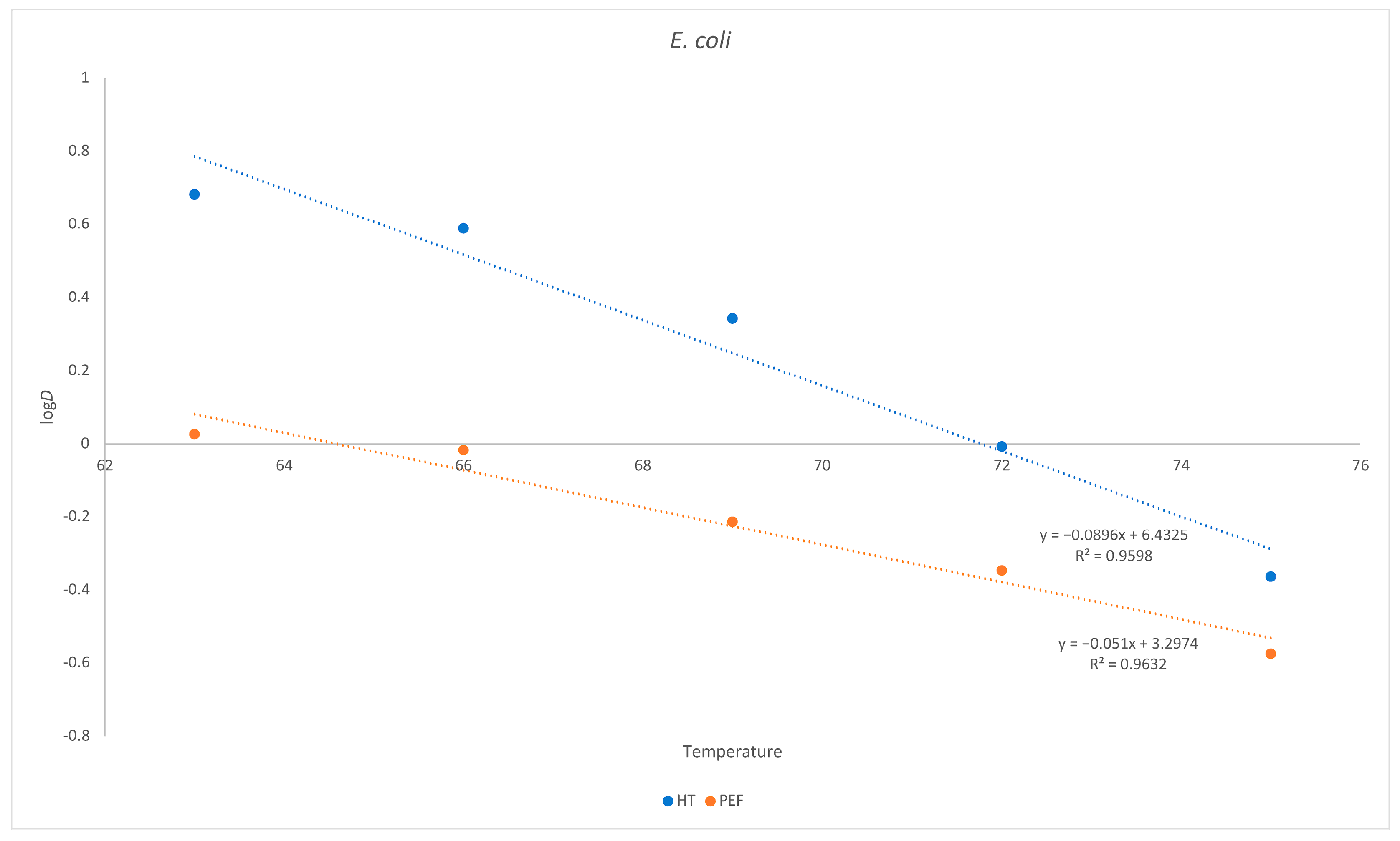
As already demonstrated for L. monocytogenes, the same holds true for E. coli. Despite being more sensitive to thermal death after a PEF treatment, the requirement for a greater temperature increase to reduce the D value is higher when the PEF is applied (Figure 3).
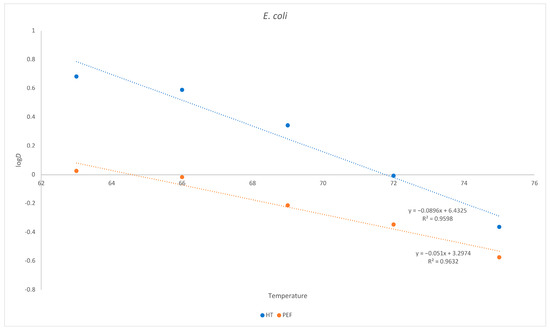
Figure 3.
Inactivation of E. coli as a function of temperature in goat´s milk. Influence of temperature on D. Red = heat treatment only; blue = heat treatment combined with PEF.
3.2. Chemical Analysis
During the experiments with raw goat’s milk, the following chemical parameters were monitored: conductivity, pH, TA, TSS, fat, protein, dry extract (defatted), urea, and lactose, according to the results of Table 4, to ensure the analytical control of raw milk.

Table 4.
Chemical parameters of raw goat’s milk (mean ± standard deviation).
The results in Table 4 demonstrate that the samples of raw goat milk are quite homogeneous over the collected samples, as observed by the low standard deviation values obtained for all the parameters analyzed.
At the same time, some physical parameters of goat´s milk were also analyzed after thermal treatments to evaluate the effect of temperature on the final characteristics.
Table 5 highlights the results of pH, electrical conductivity, titratable acidity, and TSS after thermal treatments (HT and PEF + HT).

Table 5.
Physico-chemical parameters of raw goat’s milk and after thermal treatment (all values expressed as mean ± standard deviation).
The average pH values (6.76 ± 0.03) and total soluble solids (10.70 ± 0.26) in raw milk did not change after the treatment. The titratable acidity decreased from a maximum of 0.150 ± 0.003% (% in lactic acid) in raw milk to values below 0.136% for both pasteurized and combined treatment samples. Conductivity decreased as a function of the increase in temperature for both treatments.
Regarding the viscosity values, they did not differ between treated and untreated samples, with goat’s milk showing a non-Newtonian fluid behavior. The viscosity decreases with increasing shear rate, showing a pseudoplastic behavior in all samples analyzed.
3.3. Energy Considerations
Considering the equipment used in this study, the energy per unit time (kJ∙h−1) needed for the HT stage, with and without the PEF pre-treatment (QPEF+HT and QHT, respectively), was calculated. In this case, the milk flow rate of the heat exchanger was 10 L∙h−1. To determine mass rate, it was assumed that goat and cow milk density was 1007.245 kg∙m−3 (T = 40 °C) and 1030.8 kg∙m−3 (T = 50 °C), respectively. The Cp values considered for goat’s milk and cow’s milk were 3.79 kJ∙(kg∙K)−1 and3.77 kJ∙(kg∙K)−1, respectively [30,46,47,48,49]. Table 6 summarizes the energy needed to raise milk temperatures in the thermal step of the studied processing conditions.

Table 6.
Sensitive heat necessary to raise milk temperature and complete pasteurization. QT = QPEF + QPH.
It can be observed that the energy requirement by the heat treatment stage without the PEF is much higher. In this study, this means almost three times less energy requirements in the heat exchanger at a milk flow rate of 10 L∙h−1. Similar conclusions have been reported by Sharma et al. [27]. The economic advantage in industrial applications can be much more significant as the need for thermal energy for the thermal treatment with the PEF as a pre-treatment can be reduced to a great extent. These needs depend on several factors, with a special reference to the efficiencies of heat exchangers and the steam supply equipment, noting, e.g., that boilers in industrial plants are major energy consumers. The results obtained in this study with laboratory-scale equipment revealed that energy conservation can reach 50%. The steam flow rate (mv) required to complete the pasteurization operation using PEF + HT at 63 °C is 0.33 kg·h−1, which is three times lower than that required using the HT treatment at 75 °C (approx. 0.97 kg∙h−1), assuring a similar decimal reduction in the number of viable microorganisms.
Moreover, considering the total energy requirement for the PEF operation, it can be observed that it has no great expression compared to the steam supply. The value of QPEF was determined, taking into consideration the equipment and the PEF conditions, which represents 159.8 kJ·L−1 of treated milk, amounting to approximately 466.6 kJ∙h−1. In Table 3, this value was added to QPEF + HT to calculate QTotal. As expected, the latter is much lower than in the case of the HT treatment.
Despite equipment investment, many authors report that they can be quickly recovered since the installation cost is negligible and the production of steam needed for the heat treatments will be significantly lower [37]. Thus, PEF + HT treatment has a much lower environmental impact than conventional thermal pasteurization [19,22,23,24,25].
4. Conclusions
The results obtained as the outcome of this research provide useful information to dairy industries, especially those seeking technologies with the potential to increase energy efficiency. In this sense, pulsed electric fields (PEFs) show great potential as a pasteurization method for goat’s milk and cheese processing.
In this work, L. monocytogenes, because of its high resistance and predominance in dairy products, was used to evaluate the impact of PEF treatments on reducing the number of viable bacterial cells in cow’s milk and mainly in goat’s milk. It was observed that there was a similar reduction in the number of viable L. monocytogenes cells when using a typical 72 °C pasteurization or a 64 °C pasteurization with a PEF pre-treatment. The use of the PEF, along with a mild temperature (2 s at 64 °C), enabled us to obtain a 5 log (CFU∙mL−1) reduction of viable cells in comparison to the 2.9 log (CFU∙mL−1) obtained without the PEF pre-treatment.
E. coli was also included in this study because it serves as a general hygiene indicator. It was observed that the effect of the pre-treatment with the PEF on this bacterial species was even more noticeable, with a reduction of 7.5 log (CFU∙mL−1) of viable cells, while a reduction of only 4.6 ± 0.198 log (CFU∙mL−1) was obtained without the PEF pre-treatment.
The combined application of the PEF and mild heating demonstrated its effectiveness in inactivating L. monocytogenes and E. coli in goat’s milk, serving as an interesting alternative to traditional heat treatment within the dairy industry. Concerning energy conservation, it has been confirmed that employing the PEF as a pre-heating process significantly reduces the heating energy requirements in thermal treatment by approximately three times. This, in turn, results in substantial water vapor conservation and subsequently reduces the workload and costs associated with boilers. Furthermore, utilizing PEF technology is not only cost-effective but also enhances the preservation of product quality attributes. Despite the potential of the PEF as an alternative to high temperature, its influence on the nutritional and functional quality of goat’s milk, as well as on the sensory quality and consumer acceptability, still needs to be clarified.
Author Contributions
Conceptualization, C.B., A.A., M.R.A., and P.F.; methodology, C.B., A.A., M.R.A., and P.F.; software, M.R.A.; validation, C.B., A.A., and P.F.; formal analysis, C.B., A.A., M.R.A., and P.F.; investigation, A.R.; resources, M.R.A., A.A., and P.F.; data curation, C.B., A.A., M.R.A., and P.F.; writing—original draft preparation, C.B., A.A., M.R.A., and P.F.; writing—review and editing, C.B., A.A., M.R.A., and P.F.; supervision, C.B., A.A., and P.F.; project administration, M.R.A. and A.A.; funding acquisition, M.R.A., A.A., and P.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by UIDB/05937/2020—CISAS funded by national funds, through FCT—Fundação para a Ciência e a Tecnologia. Alexandre Romão was supported by grant BI_01_2021_CISAS.
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.
Acknowledgments
We are grateful to Victor Monteiro, Carla Ramos, and Susana Rocha for the assistance they provided on the laboratory activities. We are deeply gratefull to Prados de Melgaço and Verónica Solheiro.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nayik, G.A.; Jagdale, Y.D.; Gaikwad, S.A.; Devkatte, A.N.; Dar, A.H.; Ansari, M.J. Nutritional Profile, Processing and Potential Products: A Comparative Review of Goat Milk. Dairy 2022, 3, 622–647. [Google Scholar] [CrossRef]
- Morales, F.D.A.R.; Genís, J.M.C.; Guerrero, Y.M. Current status, challenges and the way forward for dairy goat production in Europe. Asian-Australas. J. Anim. Sci. 2019, 32, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Ine, P.D. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_indicadores&indOcorrCod=0000919&contexto=bd&selTab=tab2&xlang=pt (accessed on 20 January 2023).
- Hammam, A.R.A.; Salman, S.M.; Elfaruk, M.S.; Alsaleem, K.A. Goat Milk: Compositional, Technological, Nutritional, and Therapeutic Aspects. Asian J. Dairy Food Res. 2022, 41, 367–376. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, M.; Zhang, X.; Li, X.; Chen, D.; Qin, Y.; Wang, J.; Wang, C. The effect of heat treatment on the microstructure and functional properties of whey protein from goat milk. J. Dairy Sci. 2020, 103, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, C.; Chessa, S.; Rignanese, D.; Gigliotti, C.; Pagnacco, G.; Terracciano, L.; Fiocchi, A.; Restani, P.; Caroli, A. Goat milk allergenicity as a function of αS1-casein genetic polymorphism. J. Dairy Sci. 2011, 94, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Roncada, P.; Gaviraghi, A.; Liberatori, S.; Canas, B.; Bini, L.; Greppi, G.F. Identification of caseins in goat milk. Proteomics 2002, 2, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Anema, S.G.; Stanley, D.J. Heat-induced, pH-Dependent Behaviour of Protein in Caprine Milk. Int. Dairy J. 1998, 8, 917–923. [Google Scholar] [CrossRef]
- Leconte, N.; Graet, Y.; Garem, A. Heat-induced coagulation of goat milk: Modification of the environment of the casein micelles by membrane processes. Lait 2002, 82, 673–681. [Google Scholar]
- Moatsou, G. Heat treatment of goat milk—A review. Int. Dairy J. 2023, 139, 105569. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Litwińczuk, Z.; Florek, M.; Barłowska, J. The effects of breed and other factors on the composition and freezing point of cow’s milk in Poland. Int. J. Dairy Technol. 2011, 64, 336–342. [Google Scholar] [CrossRef]
- Prasantha, B.D.R.; Wimalasiri, K.M.S. Effect of HTST Thermal Treatments on End-Use Quality Characteristics of Goat Milk. Int. J. Food Sci. 2019, 2019, 1801724. [Google Scholar] [CrossRef] [PubMed]
- Lucey, J.A.; Teo, C.T.; Munro, P.A.; Singh, H. Microstructure, permeability and appearance of acid gels made from heated skim milk. Food Hydrocoll. 1998, 12, 159–165. [Google Scholar] [CrossRef]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Tiwari, S.; Kadyan, S. Applications of emerging processing technologies for quality and safety enhancement of non-bovine milk and milk products. LWT 2021, 149, 111845. [Google Scholar] [CrossRef]
- Lee, G.J.; Han, B.K.; Choi, H.J.; Kang, S.H.; Baick, S.C.; Lee, D.-U. Inactivation of Escherichia coli, Saccharomyces cerevisiae, and Lactobacillus brevis in Low-fat Milk by Pulsed Electric Field Treatment: A Pilot-scale Study. Korean J. Food Sci. Anim. Resour. 2015, 35, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Sharma, P.; Oey, I.; Bremer, P.; Everett, D.W. Microbiological and enzymatic activity of bovine whole milk treated by pulsed electric fields. Int. J. Dairy Technol. 2018, 71, 10–19. [Google Scholar] [CrossRef]
- Sampedro, F.; McAloon, A.; Yee, W.; Fan, X.; Zhang, H.; Geveke, D. Cost analysis of commercial pasteurization of orange juice by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 72–78. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; Bekhit, A.E.-D.; Liu, Z.-W.; Aadil, R.M. Pulsed electric field: A potential alternative towards a sustainable food processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]
- Zimmermann, U.; Pilwat, G.; Riemann, F. Dielectric breakdown of cell membranes. Biophys. J. 1974, 14, 881–899. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M.H.; Jusoh, Y.M.; Bekhit, A.E.-D.; Roobab, U.; Manzoor, M.F.; Aadil, R.M. Electrical systems for pulsed electric field applications in the food industry: An engineering perspective. Trends Food Sci. Technol. 2020, 104, 1–13. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Gullon, P.; Hesari, J.; Gullón, B.; Alirezalu, K.; Lorenzo, J. Quality aspects and safety of pulsed electric field (PEF) processing on dairy products: A comprehensive review. Food Rev. Int. 2022, 38, 96–117. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, J.; Sepulveda, D.R.; Góngora-Nieto, M.M.; Swanson, B.; Barbosa-Cánovas, G.V. Milk thermization by pulsed electric fields (PEF) and electrically induced heat. J. Food Eng. 2010, 100, 56–60. [Google Scholar] [CrossRef]
- Alirezalu, K.; Munekata, P.E.S.; Parniakov, O.; Barba, F.J.; Witt, J.; Toepfl, S.; Wiktor, A.; Lorenzo, J.M. Pulsed electric field and mild heating for milk processing: A review on recent advances. J. Sci. Food Agric. 2020, 100, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Butt, M.Z.; Aadil, R.M.; Inam-Ur-Raheem, M.; Abdullah; Bekhit, A.E.; Guimarães, J.T.; Balthazar, C.F.; Rocha, R.S.; Esmerino, E.A.; et al. Impact of nonthermal processing on different milk enzymes. Int. J. Dairy Technol. 2019, 72, 481–495. [Google Scholar] [CrossRef]
- Sharma, P.; Bremer, P.; Oey, I.; Everett, D. Bacterial inactivation in whole milk using pulsed electric field processing. Int. Dairy J. 2014, 35, 49–56. [Google Scholar] [CrossRef]
- Walkling-Ribeiro, M.; Rodríguez-González, O.; Jayaram, S.; Griffiths, M.W. Microbial inactivation and shelf life comparison of ‘cold’ hurdle processing with pulsed electric fields and microfiltration, and conventional thermal pasteurisation in skim milk. Int. J. Food Microbiol. 2011, 144, 379–386. [Google Scholar] [CrossRef] [PubMed]
- McAuley, C.M.; Singh, T.K.; Haro-Maza, J.F.; Williams, R.; Buckow, R. Microbiological and physicochemical stability of raw, pasteurised or pulsed electric field-treated milk. Innov. Food Sci. Emerg. Technol. 2016, 38, 365–373. [Google Scholar] [CrossRef]
- Hariono, B.; Wijaya, R.; Kurnianto, M.; Sutrisno; Seminar, K.; Brilliantina, A. Quality of Goat’s Milk Exposed Ultraviolet and High Pulsed Electric Field. IOP Conf. Ser. Earth Environ. Sci. 2020, 411, 012052. [Google Scholar] [CrossRef]
- Buffa, M.; Guamis, B.; Saldo, J.; Trujillo, A.J. Changes in organic acids during ripening of cheeses made from raw, pasteurized or high-pressure-treated goats’ milk. LWT Food Sci. Technol. 2004, 37, 247–253. [Google Scholar] [CrossRef]
- Mohamad, A.; Shah, N.N.A.K.; Sulaiman, A.; Adzahan, N.M.; Aadil, R.M. Impact of the pulsed electric field on physicochemical properties, fatty acid profiling, and metal migration of goat milk. J. Food Process. Preserv. 2020, 44, e14940. [Google Scholar] [CrossRef]
- Mohamad, A.; Shah, N.N.A.K.; Sulaiman, A.; Adzahan, N.M.; Aadil, R.M. Pulsed electric field of goat milk: Impact on Escherichia coli ATCC 8739 and vitamin constituents. J. Food Process. Eng. 2021, 44, e13779. [Google Scholar] [CrossRef]
- García, D.; Gómez, N.; Mañas, P.; Condón, S.; Raso, J.; Pagán, R. Occurrence of sublethal injury after pulsed electric fields depending on the micro-organism, the treatment medium ph and the intensity of the treatment investigated. J. Appl. Microbiol. 2005, 99, 94–104. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Debon, J.; Prudêncio, E.S.; Petrus, J.C.C. Rheological and physico-chemical characterization of prebiotic microfiltered fermented milk. J. Food Eng. 2010, 99, 128–135. [Google Scholar] [CrossRef]
- Sampedro, F.; McAloon, A.; Yee, W.; Fan, X.; Geveke, D.J. Cost Analysis and Environmental Impact of Pulsed Electric Fields and High Pressure Processing in Comparison with Thermal Pasteurization. Food Bioprocess Technol. 2014, 7, 1928–1937. [Google Scholar] [CrossRef]
- Lvarez, I.; Condón, S.; Raso, J. Microbial Inativation by Pulsed Eletric Fields. In Pulsed Electric Fields Technology for the Food Industry; Food Engineering Series; Heinz, J.R.A.V., Ed.; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Center for Food Safety and Applied Nutrition. Draft Guidance for Industry: Control of Listeria monocytogenes in Ready-To-Eat Foods; FDA: Rockville, MD, USA, 2017. [Google Scholar]
- Ells, T.C.; Speers, R.A.; Hansen, L.T. Insertional mutagenesis of Listeria monocytogenes 568 reveals genes that contribute to enhanced thermotolerance. Int. J. Food Microbiol. 2009, 136, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Migeemanathan, S.; Bhat, R.; Wan-Abdullah, W.N.; Chye, F.Y. Influence of temperature variations on growth, injury survival and inactivation of Listeria monocytogenes in goat milk samples at laboratory scale. Int. J. Dairy Technol. 2014, 67, 437–447. [Google Scholar] [CrossRef]
- Wang, M.-S.; Zeng, X.-A.; Sun, D.-W.; Han, Z. Quantitative analysis of sublethally injured Saccharomyces cerevisiae cells induced by pulsed electric fields. LWT 2015, 60, 672–677. [Google Scholar] [CrossRef]
- Eckner, K.F. Fluorometric Analysis of Alkaline Phosphatase Inactivation Correlated to Salmonella and Listeria Inactiviation. J. Food Prot. 1992, 55, 960–963. [Google Scholar] [CrossRef]
- Walstra, P. Dairy Technology: Principles of Milk Properties and Processes, 1st ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Lado, B.H.; Bomser, J.A.; Dunne, C.P.; Yousef, A.E. Pulsed electric field alters molecular chaperone expression and sensitizes Listeria monocytogenes to heat. Appl. Environ. Microbiol. 2004, 70, 2289–2295. [Google Scholar] [CrossRef]
- Franco, J.; Saravia, L.; Javi, V.; Caso, R.; Fernandez, C. Pasteurization of goat milk using a low cost solar concentrator. Sol. Energy 2008, 82, 1088–1094. [Google Scholar] [CrossRef]
- Gabas, A.L.; Cabral, R.A.F.; de Oliveira, C.A.F.; Telis-Romero, J. Density and rheological parameters of goat milk. Food Sci. Technol. 2012, 32, 381–385. [Google Scholar] [CrossRef]
- Kljajevic, N.V.; Tomasevic, I.B.; Miloradovic, Z.N.; Nedeljkovic, A.; Miocinovic, J.B.; Jovanovic, S.T. Seasonal variations of Saanen goat milk composition and the impact of climatic conditions. J. Food Sci. Technol. 2018, 55, 299–303. [Google Scholar] [CrossRef]
- Parmar, P.; Lopez-Villalobos, N.; Tobin, J.T.; Murphy, E.; McDonagh, A.; Crowley, S.V.; Kelly, A.L.; Shalloo, L. The Effect of Compositional Changes Due to Seasonal Variation on Milk Density and the Determination of Season-Based Density Conversion Factors for Use in the Dairy Industry. Foods 2020, 9, 1004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).