Abstract
Chalcogenide alloys of As2Se3-Ag2Te-GeTe were synthesized using the melt-quenching technique. By the visual and XRD analyses, the state of obtaining alloys was proven (glass, crystalline, glass + crystalline), and the glass formation region in the system was established. The thermal characteristics of some samples were determined—temperatures of glass transition (Tg); crystallization (Tcr); and melting (Tm). The basic physicochemical parameters, such as density (d) and Vickers microhardness (HV), were measured. Compactness (C), as well as some thermomechanical characteristics, such as module of elasticity (E), volume (Vh), and formation energy (Eh) of micro-voids, were calculated, and the influence of the composition on these characteristics was investigated. The addition of silver telluride resulted in a decrease in Tg and HV values and an increase in d and Vh values. No thermochemical effects of crystallization or melting were detected in some of the alloys. The obtained results were in agreement with the available literature data for similar systems.
1. Introduction
Chalcogenide glasses (ChGs), with their characteristic properties—low glass transition temperature; low phonon energy; transparency in the middle infrared region of the electromagnetic spectrum; high refractive index; semiconductor properties; and photosensitivity; combined with their relatively easy production in bulk and thin-film form; have long been widely used in sensors and electronics as active membranes for various types of sensors [1,2,3]; information storage and transmission media [4]; threshold switches [5]; low-cost solar cells [6]; and many other electronic elements.
The interest in glassy chalcogenides with a high content of tellurium is determined by the ability of this class of compounds to transmit light in the middle and far infrared regions of the electromagnetic spectrum—up to 28 μm [7,8,9]. Based on them, optical sensors with good selectivity and sensitivity were obtained for the registration of molecules, biomolecules, bacteria, viruses, and proteins whose absorption bands are located in the region above 12 μm.
The pronounced tendency to crystallize tellurium makes it difficult to obtain stable glasses with its participation. It was established that the introduction of certain components in the composition, such as arsenic, selenium, and gallium—Ge-Te-Se [10,11]; Te-Ge-I and Te-Ge-Ga [7] Ge-As-Te, Ge-As-Te-Se [12]; (GeTe4)100−x(AgI)x [13]; as well as additional purification of the obtained alloys [14]; improve the preparation of compositions in glassy form and reduce their tendency to crystalliz.
Jovari studied the structure of GeTe4-Ag glasses and reported that the concentration of charge carriers increased with increasing Ag content, while the topology of the GeTe4 host framework was largely preserved. Other authors report the structure-terahertz (THz) property relationship for various non-oxide chalcogenide glasses. This comprehensive study combined Raman spectroscopy to examine structural units, connectivity, and glass networks and terahertz time-domain spectroscopy (THz-TDS) to record the THz refractive index, n(THz), across a broad THz bandwidth. Research shows that Ge-Se binary glasses measure increased THz refractive index as <r> increases, with the maximum at <r> = 2.8. Ternary Ge-As-Se glasses record the maximum THz refractive index value at <r> = 2.5 for Ge10As30Se60. Such a correlation is valuable for predicting and designing chalcogenide glasses for integrated optical applications across the THz and IR regions.
Studies for glass forming ability with high thermal stability of glass with composition GexTeySe(100-x-y) and the studied properties prove strongly depending on the Ge/Se ratio and the concentration of Te. The glasses with 15 ≤ x ≤ 40 are the most suitable compositions for applications for making IR lenses and optical fibers [11,15,16].
Multicomponent systems built on the basis of a good glass former, such as As2Se3 and chalcogenide compounds with different properties, imply the creation of a more disordered structure, which increases the probability of obtaining glassy materials in wider areas and with changing properties depending on the composition.
In this regard, the object of the present study is ChGs from the multicomponent system As2Se3-Ag2Te-GeTe.
The compound As2Se3 is a high-resistance semiconductor. It exists in a crystalline and glassy state. At T = 300 K, the crystalline As2Se3 has electrical conductivity σ ≈ 10–13 S cm−1, and the glassy—σ ≈ 10–12 S cm−1. The thermal band gap of the crystalline one is 1.84, and that of the glassy one is 1.90 eV. Under normal synthesis conditions and slow cooling of the melt to room temperature, As2Se3 is obtained in a glassy state [17]. Ismailov et al. study the electrical conductivity of glassy As2Se3. The electrical conductivity of As2Se3 at 300 K is 6 × 10–10 ohm–1 cm–1. The thermal bandgap calculated from the slope of the conductivity curves is ΔE = 1.96 eV [18].
GeTe is a promising material in both crystalline and amorphous forms. Crystalline GeTe is a semiconductor with ΔE = 0.1–0.2 eV [19], has a high specific electrical conductivity at room temperature (3.103 S cm−1 [20]), as well as a high reflection coefficient (65% at 633 nm [21]). On the other hand, amorphous GeTe is a semiconductor with a band gap of 0.8 eV, a low electrical conductivity (almost 106 lower than that of crystalline GeTe [19]), and a lower reflection coefficient (~40% at 633 nm [21]). Ge-Te-based glassy materials are characterized by permeability in the range of 2–20 μm [22]. Germanium is known to increase the network connectivity of glass because it forms relatively strong chemical bonds and improves its thermal and mechanical properties.
Silver (I) telluride is a narrow-bandgap semiconductor with good thermoelectric properties. It exhibits effects of magnetoresistance and high electrical conductivity, which is why it is used in magnetic, electronic, and thermoelectric devices [23]. The inclusion of silver chalcogenides leads to an increase in the plasticity of the material [24], a valuable property in infrared technology. The inclusion of silver chalcogenides leads to an increase in the plasticity of the material, a valuable property in infrared technology for the production of optical fibers and as a material for the optical recording of information. The preparation of As2Se3-based ChGs by the addition of two compounds with different properties—Ag2Te and GeTe—is expected to lead to more stable ChGs in a wider range with improved or new properties.
In this regard, the aim of the present communication is related to the synthesis and investigation of the composition-properties dependence of basic physicochemical and thermomechanical characteristics of ChGs from the As2Se3-Ag2Te-GeTe system.
2. Experimental Procedure
2.1. Materials
Arsenic powder (As, 99,999% Sigma-Aldrich Chemie GmbH, Steinheim, Germany); Silver shot (Ag, 99.999%, Thermo Scientific Chemicals, Alfa Aesar Avocado Research Chemicals, Ltd., Ward Hill, MA, USA); Germanium powder (Ge, 99.999%, Thermo Scientific Chemicals, Fisher Scientific UK); Tellurium powder (Te, 99.999%, Alfa Aesar); Selenium powder (Se, 99.999%, Thermo Scientific Chemicals, Alfa Aesar Avocado Research Chemicals, Ltd., Ward Hill, MA, USA); all these reagents were used without purification.
2.2. Preparation of Glass Samples
The alloys of the As2Se3-Ag2Te-GeTe system, as well as their constituent components, were obtained by direct, three-step, one-temperature synthesis. Se, Te, As, Ag, and Ge of 5N purity were used for the synthesis of the starting materials As2Se3, GeTe, and Ag2Te. The investigated samples with chemical composition (As2Se3)x(Ag2Te)y(GeTe)z, where x, y, and z—mol%; were synthesized in amounts of 4 g. For this purpose, the starting substances were weighed on an analytical balance with an accuracy of ±0.0002 g in quantities corresponding to the chemical composition of the samples, after which they were transferred into quartz ampoules 100 mm long and 10 mm in diameter. The ampoules, evacuated to a residual pressure of 0.133 Pa and soldered on the flame of an oxygen propane-butane torch, were placed in an electric resistance furnace equipped with a vibrating device. The temperature-time characteristics, as well as the method of cooling the melts, are presented in Table 1. The rate of heating from step to step—3–4 °C min−1, 2–3 °C min−1, and 2–3 °C min−1, respectively. Vibration stirring was applied at each isothermal stage as well as at the maximum heating temperature, for better homogenization of the melt. At the end of the last stage, the ampoules are cooled in air to 900–950 °C and sharply quenched in a mixture of water + ice, which provides a cooling rate of 10–15 °C s−1 (depending on the size of the ampoules).

Table 1.
Regimes of synthesis of the initial compounds and samples of the As2Se3-Ag2Te-GeTe system.
2.3. Characterization of Materials
The surface of the freshly synthesized alloys, revealed by mechanical breaking of the ingot, was visually analyzed using a binocular magnifier with a magnification of 4×.
The X-ray phase analysis of the starting substances and samples from the system was carried out on a TUR-M 62 apparatus with a Ka-Cu and Ni filter.
The diffractograms of the studied compositions were obtained under the following conditions: 750 mg of the studied alloy was previously ground in an agate mortar and sieved to obtain a powder sample with particle sizes no larger than 63 μm. The samples were prepared on aluminum cuvettes with a layer thickness of 2 mm.
The thermograms of the vitreous alloys were recorded on a derivatograph with a three-channel Y—t recorder from the company Kutesz; Type 1040. The accuracy of measuring the temperatures of phase transformations is ±5 °C. Pre-calcined Al2O3 served as reference material for this study. The characteristic temperatures of the obtained chalcogenide glasses—softening temperature—Tg; crystallization temperature—Tcr; and melting temperature—Tm of the system were determined during non-isothermal heating carried out in the temperature range from room temperature to 1000 °C, at a rate of 15 °C·min−1.
The density of the chalcogenide glasses in the investigated systems was determined by the hydrostatic method [25]. Working fluid—toluene. Five samples of each composition, with approximately the same mass, were measured. Density values were calculated using the formula:
where m—weight of the air sample; —sample weight in toluene; —relative density of toluene at the measurement temperature; —relative density of water; at the measurement temperature; —relative density of the sample at temperature t; —water density at t = 4 °C and V = const.; λ—relative air density—0.0012. The error of the method used is ±5%.
The compactness—C—of the glasses is a structurally sensitive characteristic that reacts to the occurring changes in the structure. It is calculated by the formula [26]:
where: d—density of glass; ; ; —density; molar mass; and mole fraction of the i-th component.
The microhardness of the voluminous vitreous samples was determined by the Vickers method using a MIM-7 microscope with a PMT-3 microhardness meter built into it. The load on the pyramid was selected experimentally.
The microhardness values were calculated using the formula:
where: P—load force, g; d—diagonal of the footprint, μm2; N—scale division/1 scale division = 0.315 μm; F—surrounding surface from the impression; α—angle at the top of the pyramid; −136°.
d = d1 + d2/2N.0.315; 1 kg f = 9.81 N,
The microhardness of the samples was calculated based on 20 impressions per sample with a measurement accuracy of ±5%.
Sanditov [27] offers the following formula for the microhardness of glasses:
where: и are, respectively, the volume of microvoids in chalcogenide glasses and the energy for their formation.
The moduli of elasticity—E; Vh и Eh are calculated using the formulas:
where: Tg, K.
3. Results and Discussion
3.1. Synthesis and State of the Obtained Alloys—Delineation of the Glass Formation Region in the As2Se3-Ag2Te-GeTe System
Forty compositions of the As2Se3-Ag2Te-GeTe system were synthesized. The resulting samples are dark gray in color. Their condition was determined by visual and X-ray phase analysis according to generally accepted criteria. Vitreous alloys have a smooth, shiny surface; their X-ray patterns are distinguished by a characteristic amorphous “halo” and the absence of diffraction lines. On the surface of the glass crystal samples, two phases are observed—crystalline and vitreous. The X-ray phase analysis registered weak diffraction lines of low intensity. The crystalline samples have a matte and rough surface, and X-rays show the absence of an amorphous “halo”. Well-defined diffraction lines characteristic of the crystalline state are observed in —Figure 1.
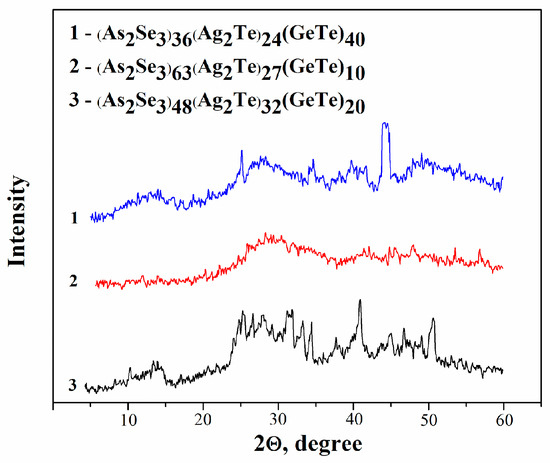
Figure 1.
XRD patterns of the alloys from the As2Se3-Ag2Te-GeTe system.
As a result of the conducted analyses, the condition of the obtained alloys was established—Table 2 and the area of glass formation in the As2Se3-Ag2Te-GeTe system was outlined Figure 2.

Table 2.
Composition (mol %) and condition of the synthesized alloys.
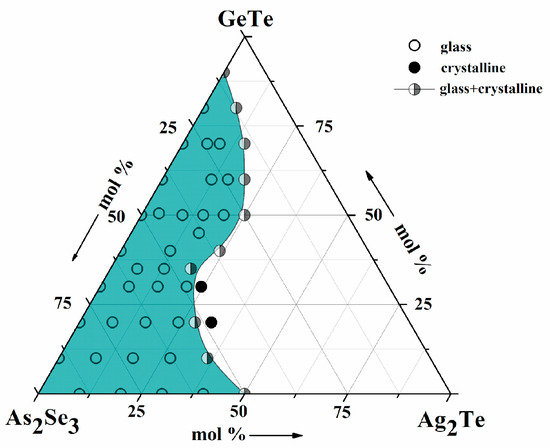
Figure 2.
Glass-forming region in the As2Se3-Ag2Te-GeTe system.
Condition of the samples in the system:
- -
- Specimens in a glassy state (vitrification region)—29 compositions: SG1–SG4; SG6–SG9; SG12–SG15; SG17–SG18; SG20–SG21; SG24–SG28; SG30–SG32; SG34–SG36; SG38;
- -
- Specimens in glass crystal state—SG5; SG10; SG19; SG22−SG23; SG29; SG33; SG39−SG40;
- -
- Samples in the crystalline state, located outside the glass formation region—2 compositions: SG11, SG16.
The glass formation region—Figure 2 is drawn to As2Se3 (peak in the Gibbs concentration triangle). It is located in the range from 0 to 50 mol % Ag2Te [28] in the As2Se3-Ag2Te system and from 0 to 90 mol % GeTe [29] in the As2Se3-GeTe system. Glasses in the Ag2Te-GeTe system, under the given conditions, were not obtained. The As2Se3-Ag2Te couple dissolves up to 70 mol % GeTe. Its solubility in the system has a maximum value in sample SG36 with composition (As2Se3)21.0(Ag2Te)9.0(GeTe)70.0.
Studies by Chen et al. [30] show a solubility of 88 mol % GeTe in the As2Se3-GeTe binary system, which is probably due to the difference in the melt cooling mode—air quenching.
3.2. Study of the Dependence Composition—A Property of Vitreous Samples with a Common Composition (As2Se3)x(Ag2Te)y(GeTe)z
To facilitate the analysis of the composition-property relationship, the magnitude has been introduced: , where: —the molar fraction of Ag2Te; —the molar fraction of As2Se3.
3.2.1. Thermal Characteristics
The thermal analysis was carried out under the conditions described in point 2.2.
It is noticed that in some of the studied compositions, the effects related to crystallization and melting processes are absent—Table 3.

Table 3.
Characteristic temperature of the same of the studied glass samples.
Glass transition temperature (Tg) [29], depending on the composition of ChGs, varies in the range from 160 to 222 °C—Table 3. The introduction of Ag2Te into the system leads to an increase in the metal component of the chemical bond in ChGs, and when its amount increases (at z = const.), the softening temperature decreases. With increasing GeTe content, at m = const., Tg increases. The softening temperature reflects the structure of the glass [31], and a decrease in its value indicates that the integrity of the structure is deteriorating. The pure components As2Se3 и GeTe have Tg = 453–473 K и 413 K, respectively. It can be expected that, at m = const, the addition of GeTe to As2Se3 will lower the Tg. However, in reality, the exact opposite effect is observed (Figure 3).
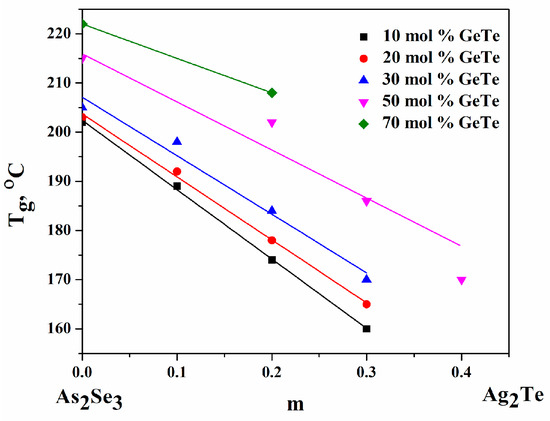
Figure 3.
Dependence of Tg on the contents of Ag2Te, z = const., and GeTe, m = const.
The crystallization temperature (Tcr) varies between 213 and 251 (246) °C, and the melting temperature (Tm) varies between 287 and 387 °C. With increasing concentrations of Ag2Te (at z = const.), Tcr decreases. Crystallization and melting effects were not observed in some of the studied glass compositions—(As2Se3)80.0(Ag2Te)0(GeTe)20.0; (As2Se3)70.0(Ag2Te)0(GeTe)30.0; (As2Se3)63.0(Ag2Te)7.0(GeTe)30.0; (As2Se3)24.0(Ag2Te)6.0(GeTe)70.0. The absence of exothermic effects associated with the crystallization process is an indicator of the good thermal resistance of glassy alloys. For compositions where no crystallization is observed, the endoeffects associated with the melting process are unclear and fuzzy.
3.2.2. Density, Microhardness, Compactness, and Thermo-Mechanical Characteristics
The density (d) depends on the composition of ChGs and varies between 4.31 and 5.58 g cm−1. When increasing the content of Ag2Te (at z = const.) and GeTe (m = const.), respectively, the density of the glasses increases, which is expected since the density of the starting components decreases in the order: d(Ag2Te) > d(GeTe) > d(As2Se3), respectively 8.5 > 6.2 > 4.75 g cm−3—Figure 4.
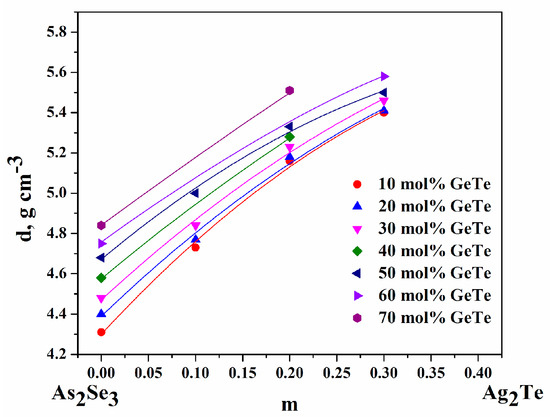
Figure 4.
Dependence of density (d) on glasses’ content.
The microhardness HV of the glasses varies from 73 to 133 kgf mm−2—Table 4. As m increases (at z = const.), HV decreases. As z increases (at m = const.), it increases. This course of the dependences HV(m) and HV(z) is expected because: = 150 kgf mm−2 > HVGeTe = 143 kgf mm−2 [32] > = 38 kgf mm−2—Figure 5.

Table 4.
Measured density (d), microhardness (HV), and compactness (C) of some glass samples.
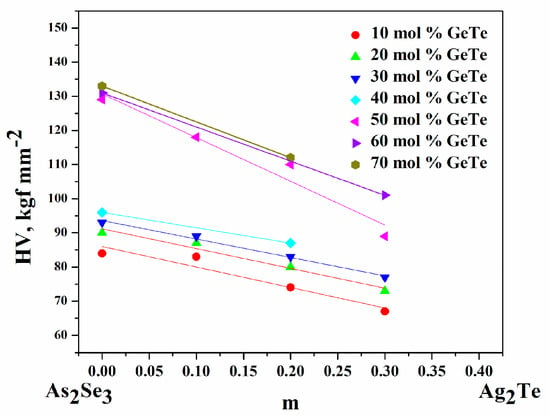
Figure 5.
Dependence of microhardness (HV) on contents of m (z = const.) and z (m = const.).
The modulus of elasticity E (Table 5) varies from 1005 to 1995 kgf mm−2 and reproduces the microhardness of the glasses, following Equation (6).

Table 5.
Thermo-mechanical characteristics—minimum volume of micro-voids (Vh); energy of their formation (Eh); and module of elasticity (E) of some glass samples.
The minimum volume of the microvoids (Vh) varies between 18.76 and 32.57 × 10−3 nm3. Vh increases with increasing amounts of Ag2Te (at z = const.) and decreases with increasing GeTe content (m = const.)—Figure 6. The microvoid formation energy Eh follows the course of Tg and ranges from 13.3 to 15.2 kJ mol−1.
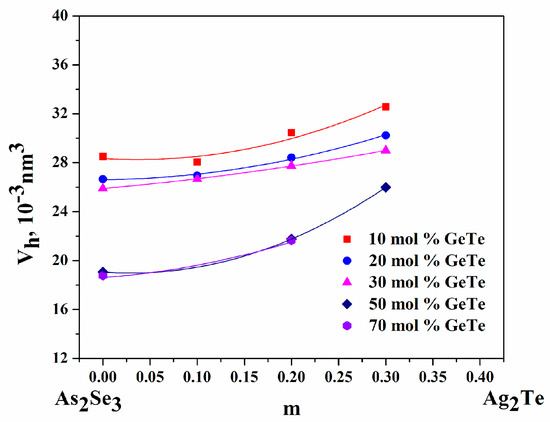
Figure 6.
Minimum volume of micro-voids (Vh) dependence at m (z = const.) and z (m = const.).
According to Tichý [33], compactness (C) expresses the change in volume that is due to the interactions between the components that make up the glass and is therefore related to the free volume and flexibility of the vitreous network. It can also take negative values, which is an indication of greater free volume and flexibility of the vitreous structure. The compactness (C) of the studied samples increases with increasing Ag2Te content in the interval 0.0 < m < 0.2—(Table 5). At m = 0.2, the dependence C (m) at z = const. passes through a maximum. At m > 0.2, the characteristic is weakly dependent on the Ag2Te content. A similar dependence of compactness on composition is also observed in CHGs from other systems [28]. The concentration of GeTe does not have a significant effect on the compactness of the studied compositions.
This course of dependence of Tg, d, HV, Vh, and C (m) and Tg, HV, Vh, and C (z) is closely related to the characteristics of the output components. By introducing Ag2Te into the structure of the chalcogenide glass (at z = const.), chemical bonds are broken, and silver atoms and fragments (-Ag; -Te-Ag) close the broken chains, as a result of which the cross-linking of the structure decreases and the disorder in the system increases, which explains the decrease in Tg and microhardness and the increase in microvoid volume. On the other hand, the introduction of heavier atoms (Ag and Te) to As2Se3 leads to an increase in the average molecular weight of the glass and an increase in density and compactness. GeTe (at m = const.) leads to a compaction of the structure and an increase in the connectivity in the glass network, which is expressed in an increase in the value of Tg, d, and HV and a decrease in the volume of microvoids (Vh) [29] in the studied compositions. A similar effect of GeTe on thermal and mechanical properties such as Tg and HV has been observed in other studies [34,35].
4. Conclusions
Forty samples from the As2Se3-Ag2Te-GeTe system were synthesized by the method of direct mono-temperature synthesis.
With the help of the visual and XRD analyses, the region of the glass formation domain within this system was outlined. Chalcogenide glasses were obtained in the As2Se3-GeTe system in the range of 0 to 90 mol% GeTe, while in the binary As2Se3-Ag2Te system, from 0 to 50 mol % Ag2Te. In the Ag2Te-GeTe system, no glass samples were obtained. It is been found that the maximum solubility is 70 mol % GeTe in the As2Se3-Ag2Te system.
The characteristics and temperatures of some alloys in the studied system were determined. The thermograms showed clearly expressed effects connected to the glass-transition process. It is an indicator of the good thermal resistance of glassy alloys. Thermos-effects of crystallization and melting were not observed in some of the samples.
The composition affects the properties of glassy alloys. The introduction of GeTe leads to a densification of the structure and an increase in the values of microhardness (73 to 133 kgf mm−2) and density (4.31 to 5.58 g cm−1). The values of compactness and thermomechanical characteristics of some of the compositions were calculated, and the influence of the composition on them was investigated. This allows us to suggest that these compounds could be used in the production of thin films for special window glasses or for optical fiber lines for data transmissions.
Author Contributions
Conceptualization, V.K.; Methodology, I.K. and V.I.; Validation, V.K. and V.J.; Investigation, I.K. and V.I.; Resources, V.K. and V.J. Data curation, V.K., V.J., I.K. and V.I.; Writing—original draft preparation, V.K. and I.K.; Writing—review and editing, V.K., V.J., I.K. and V.I.; Visualization, I.K.; Supervision, V.K. and V.J.; Funding acquisition, V.J. All authors have read and agreed to the published version of this manuscript.
Funding
This study is funded by the European Union-Next Generation EU through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No. BG-RRP-2.004-0002, “BiOrgaMCT”.
Data Availability Statement
All the data underlying the results are available as part of this article, and no additional source data are required.
Acknowledgments
The contribution of V. Vassilev to this study of some compositions of the system is sincerely appreciated.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mishra, S.; Jaiswal, P.; Lohia, P.; Dwivedi, D.K. Chalcogenide glasses for sensor application: A Review. In Proceedings of the 2018 5th IEEE Uttar Pradesh Section International Conference on Electrical, Electronics and Computer Engineering (UPCON), Gorakhpur, India, 2–4 November 2018; pp. 1–5. [Google Scholar]
- Bokova, M.; Dumortier, S.; Poupin, C.; Cousin, R.; Kassem, M.; Bychkov, E. Potentiometric Chemical Sensors Based on Metal Halide Doped Chalcogenide Glasses for Sodium Detection. Sensors 2022, 22, 9986. [Google Scholar] [CrossRef] [PubMed]
- Moreno, T.V.; Malacarne, L.C.; Baesso, M.L.; Qu, W.; Dy, E.; Xie, Z.; Fahlman, J.; Shen, J.; Nelson, G. Potentiometric sensors with chalcogenide glasses as sensitive membranes: A short review. J. Non-Cryst. Solids 2018, 49, 8–18. [Google Scholar] [CrossRef]
- Kolobov, A.V.; Tominaga, J. Chalcogenide Glasses in Optical Recording: Recent Progress. J. Optoelectron. Adv. Mater. 2002, 4, 679–686. [Google Scholar]
- Asokan, S.; Lakshmi, K.P. Electrical Switching and Other Properties of Chalcogenide Glasses. J. Indian Inst. Sci. 2011, 91, 319–330. [Google Scholar]
- Boukhris, I. Compositional dependence of physicochemical properties of quaternary (0.9GeS2-0.1CdS)100 x(Sb2S3)x chalcogenide glasses for solar cells and near infrared devices. Mater. Today Commun. 2021, 27, 102414. [Google Scholar] [CrossRef]
- Danto, S.; Houizot, P.; Boussard-Plèdel, C.; Zhang, X.H.; Smektala, F.; Lucas, J. A family of far-infrared transmitting Glasses in the Ga–Ge–Te system for space applications. Adv. Funct. Mater. 2006, 16, 1847–1852. [Google Scholar] [CrossRef]
- Wilhelm, A.A.; Boussard-Plédel, C.; Coulombier, Q.; Lucas, J.; Bureau, B.; Lucas, P. Development of far infrared-transmitting Te based glasses suitable for carbon dioxide detection and space optics. Adv. Mater. 2007, 19, 3796–3800. [Google Scholar] [CrossRef]
- Maurugeon, S.; Bureau, B.; Boussard-Plédel, C.; Faber, A.J.; Lucas, P.; Zhang, X.H.; Lucas, J. Selenium modified GeTe4 based glasses optical fibers for far-infrared sensing. Opt. Mater. 2011, 33, 660–663. [Google Scholar] [CrossRef]
- Svoboda, R.; Brandová, D.; Málek, J. Thermal behavior of Ge20SeyTe80−y infrared glasses (for y up to 8 at.%). J. Alloys Compd. 2018, 680, 427–435. [Google Scholar] [CrossRef]
- Chahal, S.; Ramesh, K. Glass formation, thermal stability and fragility minimum in Ge-Te-Se glasses. Mater. Res. Bull. 2022, 152, 111833. [Google Scholar] [CrossRef]
- Yang, Z.; Lucas, P. Tellurium based far infrared transmitting glasses. J. Am. Ceram. Soc. 2009, 92, 2920–2923. [Google Scholar] [CrossRef]
- Cui, S.; Le Coq, D.; Boussard-Plédel, C.; Bureau, B. Electrical and optical investigations in Te–Ge–Ag and Te–Ge–AgI chalcogenide glasses. J. Alloys Compd. 2015, 639, 173–179. [Google Scholar] [CrossRef]
- Cui, S.; Boussard-Plédel, C.; Lucas, J.; Bureau, B. Te-based glass fiber for far-infrared biochemical sensing up to 16 μm. Opt. Express 2014, 22, 21253–21262. [Google Scholar] [CrossRef] [PubMed]
- Jovari, P.; Nazabal, V.; Boussard, C.; Cui, S.; Kaban, I.; Michalik, S.; Webb, M.A.; Le Coq, D.; Chernikov, R.; Chen, N.; et al. Short- and medium range order in GeTe4-Ag glasses. J. Non-Cryst. Solids 2023, 599, 121–970. [Google Scholar] [CrossRef]
- Tostanoski, N.J.; Heilweil, E.J.; Wachtel, P.F.; Musgraves, J.D.; Sundaram, S.K. Structure-terahertz property relationship and femtosecond laser irradiation effects in chalcogenide glasses. J. Non-Cryst. Solids 2023, 600, 122020. [Google Scholar] [CrossRef]
- Chijikov, D.M.; Schastlivii, V.P. Selenium and Selenides; Nauka: Moscow, Russia, 1964. (In Russian) [Google Scholar]
- Ilyasly, T.; Gahramanova, G.; Abbasova, R.; Veysova, S.; Ismailov, Z. Investigation of the electrical properties glasses of Tm-As-S AND Tm-As-Se SYSTEMS. New Mater. Compd. Appl. 2021, 5, 227–234. [Google Scholar]
- Bahl, S.K.; Chopra, K.L. Amorphous versus crystalline GeTe films. II. Optical properties. J. Appl. Phys. 1969, 40, 4940. [Google Scholar]
- Bahl, S.K.; Chopra, K.L. Amorphous versus crystalline GeTe films. III. Electrical properties and band structure. J. Appl. Phys. 1970, 41, 2196. [Google Scholar] [CrossRef]
- Huber, E.; Marinero, E.E. Laser-induced crystallization of amorphous GeTe: A time-resolved study. Phys. Rev. 1987, B36, 1595. [Google Scholar] [CrossRef]
- Bureau, B.; Danto, S.; Ma, H.L.; Boussard-Pl´edel, C.; Zhang, X.H.; Lucas, J. Tellurium based glasses: A ruthless glass to crystal competition. Solid State Sci. 2008, 10, 427–433. [Google Scholar] [CrossRef]
- Zhu, J.; Pandey, R. Silver tellurides: Structural, elastic, and optical properties of AgTe and Ag2Te. J. Phys. Chem. Solids 2019, 129, 41–45. [Google Scholar] [CrossRef]
- Tveryanovich, Y.S.; Fazletdinov, T.R.; Tverjanovich, A.S.; Pankin, D.V.; Smirnov, E.V.; Tolochko, O.V.; Panov, M.S.; Churbanov, M.F.; Skripachev, I.V.; Shevelko, M.M. Increasing the plasticity of chalcogenide glasses in the system Ag2Se–Sb2Se3–GeSe2. Chem. Mater. 2022, 34, 2743–2751. [Google Scholar] [CrossRef]
- Bonshted-Kupletskaya, E. Opredelenieudelnovowesamineralov; Nauka: Moscow, Russia, 1951. [Google Scholar]
- Aljihmani, L.; Vassilev, V.; Petkov, P. Compositional trends of the physico-chemical properties in pseudoternary chalcogenide glasses. J. Optoelectr. Adv. Mater. 2003, 5, 1187. [Google Scholar]
- Sanditv, D.S. Microhardness and glass transition temperature of inorganic glasses. Fizika Khimiya Stekla. 1977, 3, 14–19. [Google Scholar]
- Vassilev, V.; Karadashka, I.; Parvanov, S. New chalcogenide glasses in the Ag2Te–As2Se3–CdTe system. J. Phys. Chem. Solids 2008, 69, 1835–1840. [Google Scholar] [CrossRef]
- Vassilev, V.; Karadashka, I.; Parvanov, S. Physicochemical properties of glasses in the As2Se3-Ag2Te-GeTe. Int. Sci. Conf. Gabrovo 2007, 2, 285. (In Bulgarian) [Google Scholar]
- Cheng, J.; Chen, W.; Ye, D. Novel chalcohalide glasses in the As-Ge-Ag-Se-Te-I system. J. Non-Cryst. Solids 1995, 184, 124–127. [Google Scholar] [CrossRef]
- Shi, X.Z.; Gu, Y.; Liu, T.Y.; Jiang, Z.H.; Li, R.; Zeng, F.H. Effect of different P2O5/SnF2 ratios on the structure and properties of phosphate glass. J. Non-Cryst. Solids 2022, 578, 121350. [Google Scholar] [CrossRef]
- Perumal, S.; Samanta, M.; Ghosh, T.; Shenoy, U.S.; Bohra, A.K.; Bhattacharya, S.; Singh, A.; Waghmare, U.V.; Biswas, K. Realization of High Thermoelectric Figure of Merit in GeTe by Complementary Co-doping of Bi and In. Joule 2019, 3, 2565–2580. [Google Scholar] [CrossRef]
- Tichý, L.; Tichá, H. On the chemical threshold in chalcogenide glasses. Mater. Lett. 1994, 21, 313. [Google Scholar] [CrossRef]
- Chen, W.; Chen, G.N.; Cheng, J.J. Properties of chalcogenide glasses in the Ge-As-Se-Te-I system. Phys. Chem. Glas. 1997, 38, 156–160. [Google Scholar]
- Chen, W.; Cheng, J.; Chen, G. Formation and properties of chalcohalide glasses in the AsSe-GeTe-CuI system. J. Non-Cryst. Solids 1997, 221, 274–280. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).