1. Introduction
Much of our current knowledge of biological processes at the cellular and subcellular levels has been derived from direct visualization of organelle dynamics and subcellular processes in vivo [
1,
2,
3]. In this regard, fluorescence microscopy is among the most extensively utilized techniques, due to its capability to monitor internal structures within living samples in real time using molecule-specific labeling [
4,
5]. Nevertheless, traditional fluorescence microscopy is limited by its spatial resolution, which is approximately 200–300 nm laterally and 500–700 nm axially due to the diffraction limit of light [
6]. To surpass this limitation, several super-resolution fluorescence techniques have been developed in recent years [
6,
7,
8].
Total Internal Reflection Fluorescence (TIRF) microscopy is recognized as a high-contrast and high-resolution imaging method ideal for visualizing the biological processes occurring at or close to the plasma membrane [
3,
9,
10]. It employs oblique illumination to visualize molecules within a thin region (~200 nm) above the glass coverslip along the
z-axis, offering reduced background and enhanced resolution in live-cell imaging. However, TIRF is less effective when target molecules lie beyond the ~200 nm range from the coverslip, such as cilia, which frequently emerge from the apical membrane of cultured cells.
Primary cilia are solitary, non-motile, microtubule-based organelles extending from the surface of most mammalian cells. They function as critical sensory and signaling structures, coordinating diverse cellular responses to environmental and developmental cues [
11,
12,
13]. Importantly, cells only assemble a primary cilium during the G
0 phase of the cell cycle, when they are quiescent; thus, serum starvation is commonly used to promote ciliogenesis in cultured cell lines [
14]. A fundamental process underpinning the assembly, maintenance, and function of primary cilia is intraflagellar transport (IFT) [
15,
16]. IFT is a bidirectional trafficking system that moves molecular cargo across the transition zone into, within, and out of the cilium. Large multi-subunit assemblies, termed IFT trains, are assembled at the ciliary base from two subcomplexes: IFT-A (six proteins, IFT144–IFT43) and IFT-B (sixteen proteins, including IFT88 and IFT54) [
17,
18]. IFT proteins accumulate at the base of the cilium (IFT protein base pool), where trains are assembled and loaded with cargo, before entering the ciliary shaft. Within the cilium, IFT proteins are organized into trains (IFT trains) that travel along the axoneme and can also be found in a diffusive pool at the tip, where remodeling and turnaround occur [
17,
18]. The structural integrity of cilia is vital for normal physiology, and defects are linked to ciliopathies as well as broader conditions, including trauma, aging, and cancer [
19,
20,
21].
Imaging IFT is difficult because cilia are small compared to the cytoplasm, and many fluorescently tagged ciliary proteins are also present at appreciable levels in the cytoplasm, making it essential to isolate the ciliary signal from the cytoplasmic background. TIRF microscopy has emerged as the preferred method for accomplishing the aforementioned signal separation in both invertebrate and mammalian systems [
22,
23].
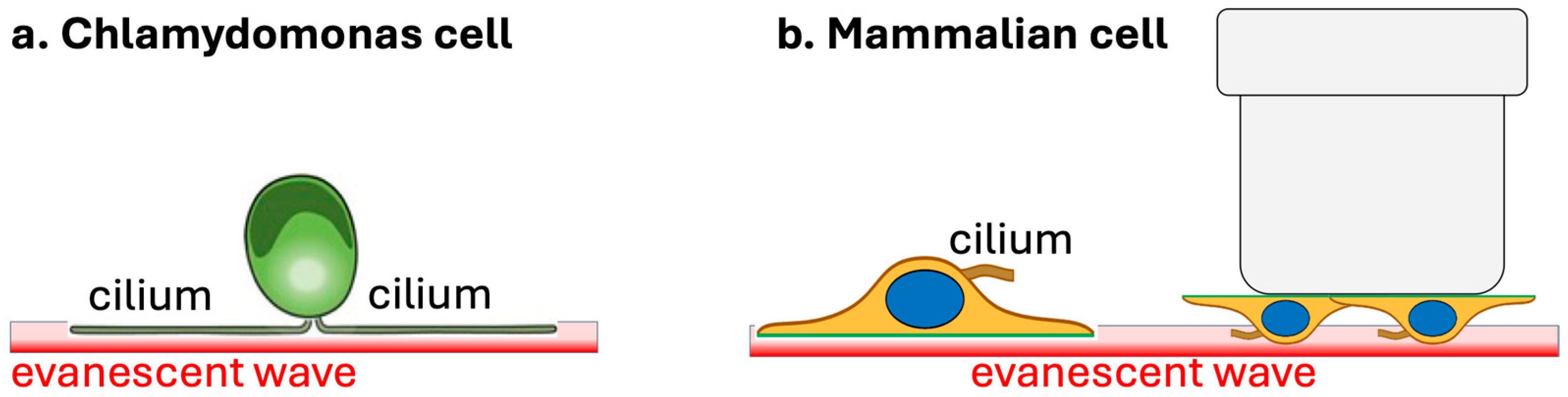
Chlamydomonas cells naturally adhere to smooth surfaces where the TIRF evanescent field forms, as schematically shown in
Figure 1a, making this organism ideally suited for imaging IFT dynamics using TIRF microscopy [
24].
However, this method is not very suitable for imaging IFT in most mammalian cells, as many cells, including patient fibroblasts, grow the primary cilium on the apical cell side. To overcome this, a protocol has been suggested in which cells are seeded on an inverted Transwell insert placed onto a glass-bottom dish [
23], allowing the evanescent field to illuminate cilia on the apical side in contact with the glass surface, as schematically shown in
Figure 1b. However, this technique is limited by trapped air bubbles and compression of the cell and cilium against the glass, which degrades image quality and has prevented its widespread applicability, prompting the need for alternative methods.
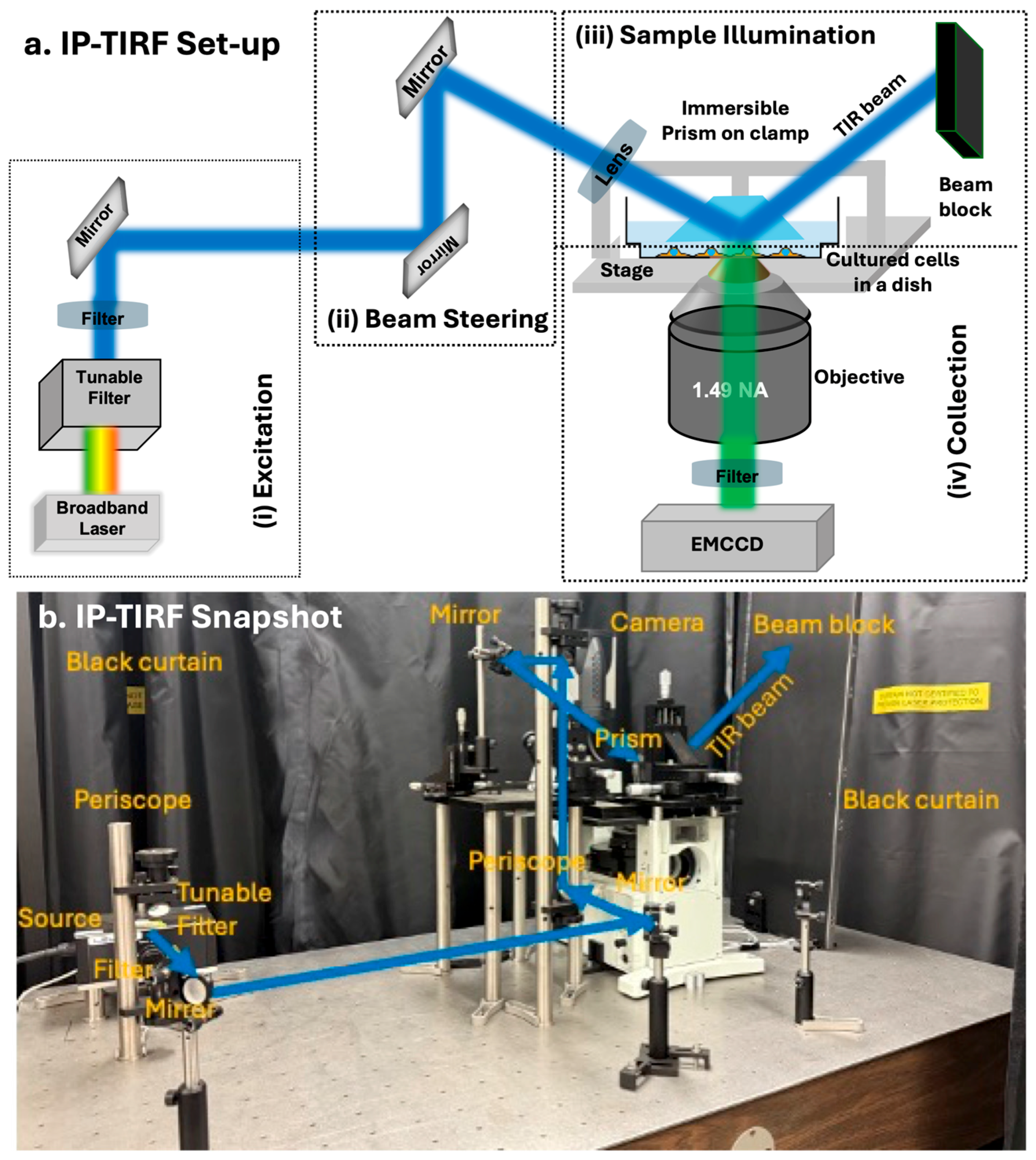
Here, we built a prototype of an immersed-prism TIRF (IP-TIRF) microscope to image the apical membrane of live cells with high contrast and resolution. For IP-TIRF, a prism is placed on top of cells using a micromanipulator and immersed in the cell medium. The total internal reflection (TIR) occurs at the medium–prism interface (as opposed to the medium–substrate interface in conventional TIRF), and the evanescent field associated with TIR selectively illuminates the apical surfaces of cells grown on a glass-bottom dish. The fluorescence signal elicited in the cells expressing, in this case, 3xmNG-IFT88 is captured and imaged by a high numerical aperture (NA) objective and projected onto a highly sensitive EMCCD camera (
Figure 2).
We first imaged fluorescent polystyrene beads using IP-TIRF as a reference sample, followed by imaging fluorescently tagged IFT88 within cilia. Our proof-of-principle experiments with cells expressing 3xmNG-IFT88 demonstrate that the IP-TIRF system can visualize the basal and tip pools of IFT proteins, as well as IFT trains along the axoneme. The performance of IP-TIRF was then compared with scanning confocal microscopy, in cellular regions where both methods can be applied (such as the IFT base pool), revealing that IP-TIRF achieved approximately 1.8-fold improvement in contrast-to-noise ratio (CNR~31) relative to scanning confocal imaging of cilia. We also observed IFT-particle motion in individual cilia with sufficient spatial resolution to detect discrete IFT trains in IP-TIRF image sequences and Kymographs. Kymograph analysis revealed an anterograde transport velocity of 0.156 ± 0.071 µm/s and a retrograde velocity of 0.020 ± 0.007 µm/s. These correspond to roughly one-quarter and less than one-twentieth, respectively, of the reported values for mammalian primary cilia. We attribute the reduced IFT speeds to imaging at room temperature rather than physiological temperature (37 °C). Nevertheless, our results demonstrate that IP-TIRF can be applied to image IFT and, with appropriate modifications, can be extended to other apical surface processes in live cells, providing a valuable tool for advanced cell biology research.
2. Materials and Methods
2.1. General Principle of IP-TIRF
It is well known that, in TIRF microscopy, when the incident light is totally internally reflected, an evanescent field that travels perpendicular to the glass–water interface is created [
25,
26]. This evanescent field, which decays exponentially with the distance from the interface, allows imaging at higher resolution. However, only fluorescent molecules very close (<200 nm) to the interface are excited and observed with reduced background. TIRF microscopy provides high spatiotemporal resolution with single-molecule sensitivity, making it well-suited for live-cell imaging while minimizing background fluorescence from the surrounding fluid environment.
To image fluorescently tagged molecules in live cells or other biological samples, the most widely used method is “objective-type TIRF” [
26,
27]. In this configuration, a laser beam is directed through a high NA microscope objective onto the bottom surface of a glass coverslip or dish (substrate), generating an evanescent field at the medium–substrate interface. Samples are mounted above the interface in aqueous solution, enabling selective excitation of fluorophores within ~100–200 nm of the basal cell membrane. While highly effective for imaging the basal surface of adherent cells, this method cannot resolve the apical surface, which is typically several micrometers above the interface. An alternative, “prism-type TIRF” directs the excitation beam through a prism attached to a glass slide (substrate), producing TIR at the medium–substrate interface [
28,
29]. However, this approach is not compatible with imaging of the apical surface in live cells, as the prism configuration is likely to physically compress the cells. Consequently, existing TIRF approaches are inherently limited for high-resolution imaging of the apical surface of live cells.
The IP-TIRF we built here is based on the prism-type TIRF setup. However, in our approach, the prism is not mounted on the top slide. Instead, the prism is placed on a clamp with translation capability so that it can be immersed in the cell culture medium from the top. In this approach, the cells are grown in glass-bottom dishes, and then the prism is positioned within close proximity (<200 nm) to the apical surface of the cell from the top. This allows the evanescent field associated with the TIR at the interface between the prism (glass) and the medium to image the apical surface of the cell.
2.2. Description of the IP-TIRF
The IP-TIRF microscope system is based on an inverted fluorescence microscope (Olympus, Tokyo, Japan, IX71) platform with desired modifications of a traditional prism-type setup [
28], in terms of optical components for (i) excitation, (ii) beam steering, (iii) sample illumination, and (iv) collection, as schematically shown in
Figure 2a with the snapshot of the setup shown in
Figure 2b.
(i) Excitation: A variable-power supercontinuum laser source (SMHP 4.0, LEUKOS, Limoges, France), emitting white light from the visible to near-infrared range (400–2000 nm), was used for excitation. Wavelength selection was achieved using a mechanical tunable filter in combination with a bandpass filter. In our experiments, a 488 nm wavelength with optimum power is used as an excitation source to illuminate the sample.
(ii) Beam Steering: A periscope assembly made of dielectric mirrors (BB07-E02P, Thorlabs, Newton, NJ, USA) was used to raise and direct the optical beam. The TIRF illumination path was adjusted by controlling the incident angle at the planar side face of the prism, using a combination of an additional mirror and a bi-convex focusing lens (f = 200 mm, Thorlabs), each mounted with xyz-translation capability.
(iii) Sample Illumination: The prepared sample, either fluorescent beads or a dish of live cells expressing 3xmNG–IFT88 that localizes to cilia, was placed on an xy-micrometer stage. A Pellin–Broca prism (11 × 20 × 6.4 mm, EKSMA Optics, Vilnius, Lithuania) was mounted on an xyz-translation stage using a custom-made aluminum clamp. When the incident beam exceeded the critical angle, TIR occurred at the prism–sample interface, and the reflected beam was intercepted by a beam stop to eliminate reflected light. The vertical distance between the prism and the sample was carefully adjusted so that the evanescent field produced the highest image contrast.
(iv) Collection: A high-NA TIRF objective lens (UAPO N TIRF, 100×, NA 1.49, oil immersion, Olympus) was used to collect fluorescence emission from the sample. The emitted light was separated from the excitation beam by a bandpass dichroic mirror (ZET488, Chroma Technology Corp., Bellows Falls, VT, USA) and an emission filter (594rpc-UF2, Chroma Technology Corp.), and then projected onto a high-sensitivity EMCCD camera (Andor iXon-L-888, Oxford Instruments, Abingdon, England) or a qCMOS camera (ORCA-Quest2, Hamamatsu Photonics, Shizuoka, Japan) for image acquisition, enabling low-noise detection of fluorophores. All experiments and setup operations were performed at room temperature.
2.3. Sample Preparation
(i) Fluorescent Beads: Commercially available carboxylate-modified polystyrene microsphere beads with excitation and emission wavelengths (505/515 nm) (Catalog number F8803, Thermofisher, Waltham, MA, USA,) were deposited on glass coverslips and used as a reference sample for alignment and optimization of the IP-TIRF setup.
(ii) Cell Culture: Firstly, immortalized mouse kidney medulla collecting duct cells (mIMCD3s) stably expressing 3xmNG-IFT88 were cultured in D-MEM/RPMI (GIBCO) with 10% Fetal Clone III (HyClone) and GlutaMAX (GIBCO) at 37 °C and 5% CO2. For experiments, cells were seeded onto glass-bottom dishes (P35G-1.5-14-C, MatTek Corporation, Ashland, MA, USA) and 12 h later, the medium was switched to the culture medium containing 1% serum to induce cilium outgrowth (ciliogenesis). Approximately two days later, the medium was changed to Leibovitz’s L-15 Medium (GIBCO) just a few minutes before experiments.
2.4. Scanning Confocal Microscopy
For comparing the performance of the IP-TIRF, we used a scanning confocal (SP8, Leica Microsystems, Wetzlar, Germany) microscope with a 40× water immersion objective (HC PL APO CS2 40x, NA = 1.10), in cellular regions where both methods can be applied (such as the IFT base pool). Excitation lines are selected from a pulsed white-light laser having continuous spectral output in the range of 470–670 nm. mNeonGreen (mNG) emission from cilia, excited at 488 nm, was collected between 500 to 550 nm using a photomultiplier tube (PMT) (DFC7000 T, Leica Microsystems, Wetzlar, Germany).
2.5. Calculation of CNR
All image processing was performed in ImageJ version 1.54p (Fiji distribution). For CNR analysis, circular regions of interest (ROIs) were manually drawn around the accumulation of IFT particles at the base of the cilium (IFT base pool) in both the confocal and IP-TIRF raw images (
Figures S3 and S4 images are included in
Supplementary Materials). An identically sized ROI was then drawn in the background region, consistently chosen approximately 5 pixels away from the ROI within the same image. This procedure was applied uniformly across all datasets. For each ROI, the area, mean intensity, and standard deviation of intensity were obtained using the
Measure function in ImageJ. The histogram data presented in the manuscript were extracted from a single representative image for each method. The Contrast-to-Noise Ratio (CNR), was then calculated as [
30,
31]:
where
is the mean fluorescence intensity at the region of interest (ROI),
is the mean background intensity, and
is the standard deviation of the background.
2.6. Determination of IFT Velocity
IFT particle velocities were determined by kymograph analysis using ImageJ. To generate the kymograph, a time-lapse image stack was opened and the brightness and contrast of each image were adaptively adjusted to 35% saturation using the
Adjust-Brightness/Contrast function, thereby minimizing photobleaching effects during kymograph generation. Next, a segmented line was drawn along the cilium—ensuring consistent overlap throughout the movie—using the
Segmented Line tool. The Multi Kymograph plug-in (ImageJ version 1.54p) was then applied to generate kymographs, with the
x-axis representing distance along the cilium and the
y-axis representing elapsed time. Linear trajectories on the kymograph correspond to individual IFT train movements; their slopes were determined using the
Straight Line tool and quantified with the
Measure function. Velocities (v) were calculated as:
where
is the displacement along the cilium and
is the corresponding elapsed time. Both anterograde and retrograde events were quantified, velocities were averaged over multiple particles, and the standard deviation of individual velocities was assessed.
3. Results and Discussion
Before each experiment, the optical alignment was optimized by adjusting mirrors, lenses, the objective, and the translation stages to minimize background signal and maximize image contrast at the medium–prism interface. Optimal alignment of the prism was achieved through iterative adjustment of the translation stages to maximize the intensity of the TIR beam. This procedure ensured a stable evanescent field with control over penetration depth, enabling selective excitation of fluorophores within ~100–200 nm of the medium–prism interface.
3.1. Imaging Fluorescent Beads Using IP-TIRF
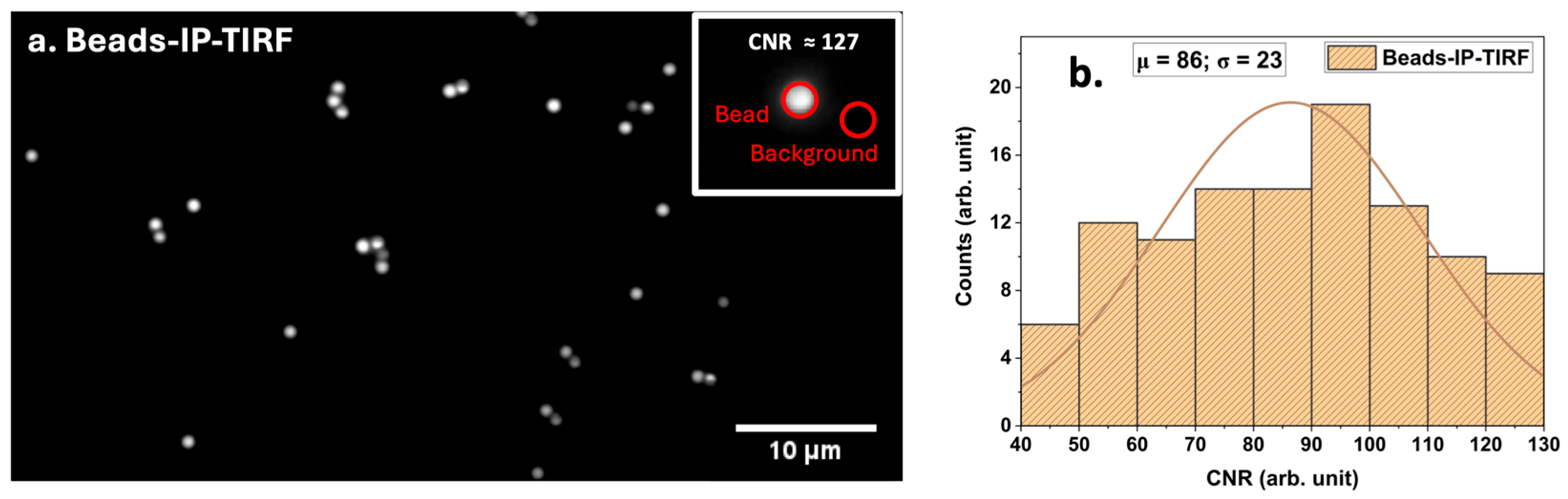
To validate that the evanescent field associated with TIR could indeed be exploited for high-contrast imaging, we used fluorescent polystyrene microspheres (d~1 μm) adhered to the coverslip as a reference sample. The IP-TIRF performance was assessed by calculating the CNR of the recorded image.
Figure 3a shows a representative IP-TIRF image of beads, with the inset highlighting a single bead exhibiting a CNR of ~127.
Figure 3b shows the CNR distribution for N~100 beads, with an average CNR of μ~86 and a standard deviation of σ~23.
The average CNR of μ~86 reflects very high image contrast, where the bead signal is nearly two orders of magnitude stronger than background noise fluctuations. Such values are consistent with the expected performance of TIRF microscopy in well-defined test samples [
10,
32,
33]. High-CNR conditions of this magnitude are often achievable in bead assays due to the strong fluorophore labeling density and stable immobilization, and they provide an important benchmark for the upper limits of imaging performance. In this context, a CNR of ~86 highlights the sensitivity of our optical system and validates its ability to detect much subtler contrasts in live-cell experiments, where intrinsic biological variability and autofluorescence of endogenous, unlabeled molecules reduce achievable CNR values [
23].
3.2. Imaging of IFT Protein Base Pool Using IP-TIRF
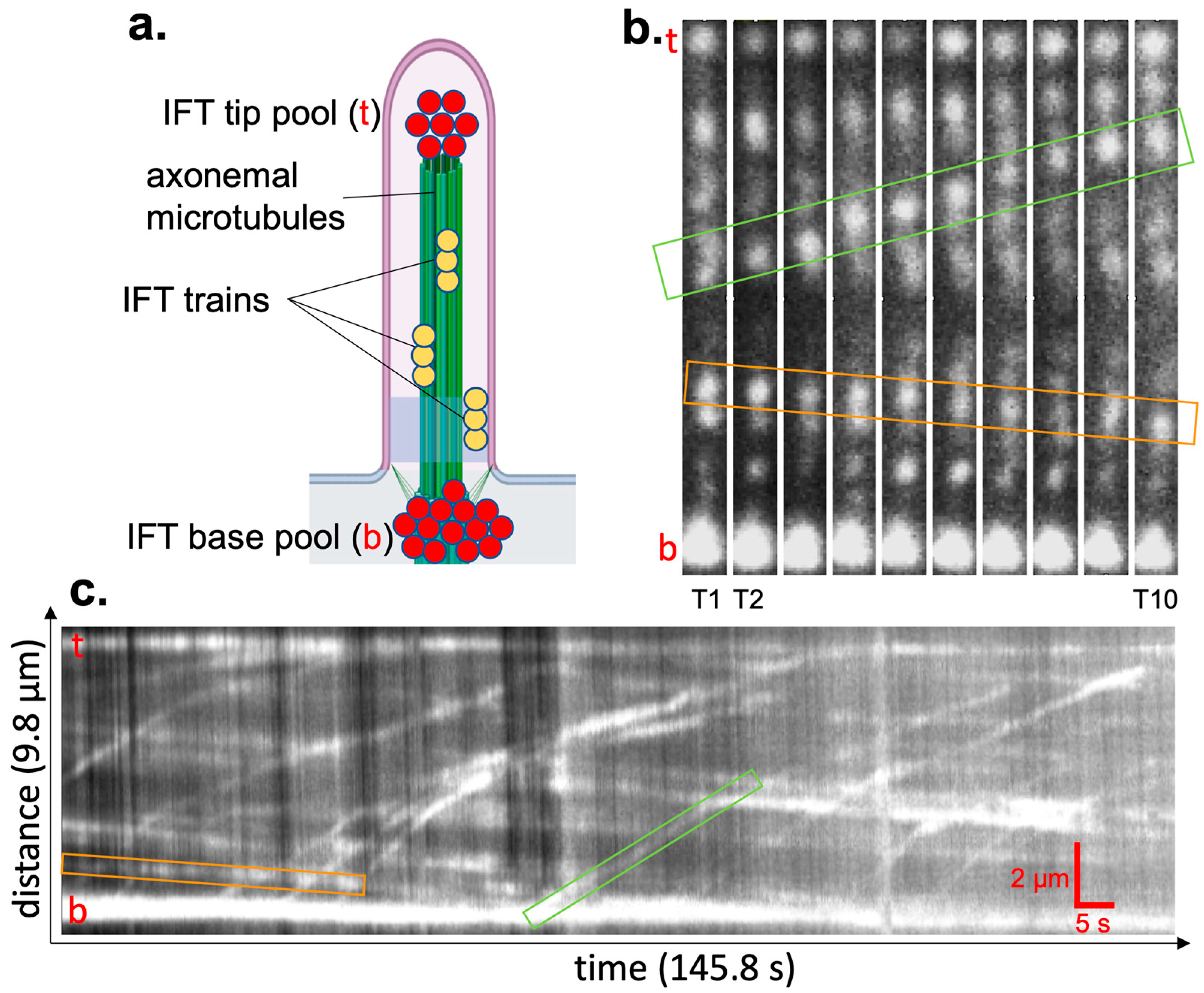
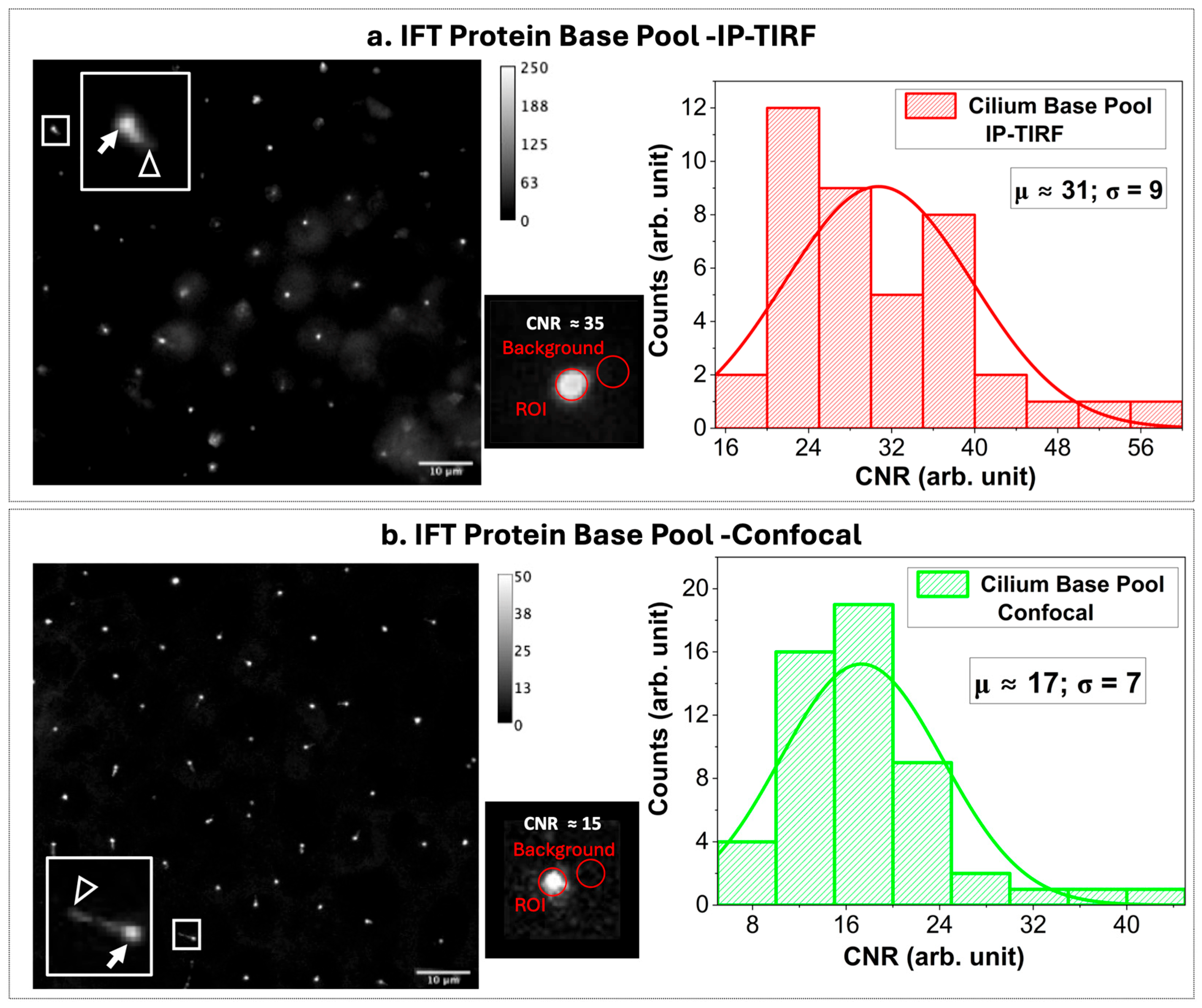
In general, IFT proteins accumulate around the centrioles, and a cilium can extend from one centriole. In the images, this often appears as a bright dot (IFT proteins at the centriole, which we refer to as the IFT protein base pool), and in some cases as a dot with an extending line (the base pool with a cilium), as indicated by solid arrows and empty arrowheads, respectively, in the left-panel images of
Figure 4a,b. As a next step, the time-lapse images of IFT protein base pools of 3xmNG-IFT88 were acquired with 200 ms exposure at 5 frames per second, as shown in the left panel of
Figure 4a for an approximately 100 × 100 μm field of view corresponding to a pixel size of 130 nm/pixel using IP-TIRF. For benchmarking the results, the performance of the IP-TIRF was compared with scanning confocal microscopy for imaging the IFT protein base pool, where both techniques can be applied, as shown in the left panel of
Figure 4b. The middle panels in
Figure 4a,b highlight the IFT protein base pool of single cilium, with CNRs of ~35 and 15 obtained using IP-TIRF and scanning confocal microscopy, respectively. The CNR distributions for N~40 (IFT protein base pools are shown in the left panels) yield a mean CNR of μ~31 and a standard deviation of σ~9 for IP-TIRF, and a mean CNR of μ~17 and a standard deviation of σ~7 for scanning confocal microscopy. Therefore, on average, the mean CNR obtained with IP-TIRF (31 ± 9, N~40) was approximately 1.8-fold higher than that of scanning confocal microscopy (17 ± 7, N~50).
Note that the NA of the objective used for confocal imaging was 1.10, while the NA of the objective used for IP-TIRF imaging was 1.49. The observed improvement in CNR with IP-TIRF relative to confocal was ~1.82×. Based on objective NA alone, the expected gain would be ~1.35× under shot-noise-limited conditions (CNR ∝ NA) and up to ~1.83× under detector-noise-limited conditions (CNR ∝ NA
2) [
34]. The fact that the measured increase approaches this theoretical upper bound indicates that, in addition to photon collection efficiency, selective suppression of cytosolic background by the IP-TIRF excitation geometry makes a critical contribution to the enhanced CNR, underscoring the improved contrast achieved by restricting excitation to the evanescent field.
Also, we would like to reiterate that, although it might seem objective-type TIRF would, in principle, provide a closer comparison to IP-TIRF, it cannot access apical cilia because the evanescent field penetrates only a few hundred nanometers, whereas cilia extend several microns above the basal substrate, as shown in
Figure 1b and discussed in
Section 2.1. Reported studies using objective-TIRF generally involve “dirty” TIRF (a mix of TIRF and epi-fluorescence) or rare cases of basal cilium growth, where the cilium grows beneath the cell body and thus parallel to the coverslip [
35]. Therefore, in the context of imaging cellular regions (such as the IFT base pool) where both techniques can practically be applied, confocal microscopy provides the most meaningful reference point for evaluating IP-TIRF performance. In this regard, in IP-TIRF, bright dots appear with surrounding dim halos due to the exponential decay of the evanescent field along the
z-axis and lateral spread of the point spread function, while regions farther away remain dark where no fluorophores are excited. In confocal microscopy, bright dots are seen against an immediate dark background because the pinhole blocks most local out-of-focus light, but dimmer regions appear laterally due to residual background fluorescence and scattered light that are only partially rejected.
Nevertheless, the mean CNR of ~31 indicates that the IFT protein base pool signal exceeds background fluctuations by more than 31-fold, an excellent level of contrast for dynamic IFT visualization. In the context of cilia biology, achieving this level of CNR is consistent with prior demonstrations of TIRF as a powerful approach for quantitative analysis of IFT dynamics in mammalian cells [
23], and therefore should be adequate to resolve IFT particle transport in real time.
3.3. Imaging Individual Cilia and IFT Using IP-TIRF
We have used IP-TIRF microscopy to directly image individual primary cilia and to visualize both anterograde and retrograde IFT, as illustrated in
Figure 5. Individual IFT trains, along with the base and tip of individual cilia, were clearly resolved with adequate contrast to distinguish transport events, as shown in the representative individual cilium (
Figure 5b). Movies showing the dynamic transport of IFT trains are provided as
Supplementary Materials (time-lapse image stacks with time stamps and scalebar, 2 µm in
Figure S1 (
Figure 5) and
Figure S2).
IFT dynamics are typically quantified by generating kymographs along the ciliary axis, which provide a spatiotemporal representation of particle trajectories and allow direct measurement of transport direction and velocity [
23,
24]. Representative individual transport events are highlighted with a light green (anterograde) and light orange (retrograde) box in
Figure 5b,c. In our analysis, Kymograph traces of 22 anterograde and 14 retrograde trajectories revealed anterograde transport (movement from the cell body to the ciliary tip) with a mean velocity of 0.156 (±0.071) µm/s and a mean retrograde velocity (movement from the tip back to the cell body) of 0.020 (±0.007) µm/s, determined using Equation (2). The standard deviation is shown in parentheses. All 22 anterograde and 14 retrograde transport events are shown in
Figure S6 file in the Supplementary Materials. For anterograde IFT velocity, this is approximately one-quarter, and for retrograde IFT velocity, it is less than twenty times the reported speed for mammalian primary cilia [
36,
37]. We hypothesize that the slowdown of IFT is caused by imaging cells at room temperature rather than at physiological temperature (37 °C). We further observed that the CNR fluctuated significantly between the frames of the movie (
Figure 5c). This is caused by the cilium transiently interacting with the prism (high CNR) and then bending away from the prism (low CNR), with additional details provided in
Section 3.4 below. Nevertheless, our data clearly show IFT movement with very high CNR values in some frames, demonstrating the utility of our microscopy setup. In summary, our results demonstrate that IP-TIRF provides both the spatial resolution and contrast necessary to directly visualize dynamic IFT in intact primary cilia.
3.4. Practical Challenges and Future Directions
We are currently working to improve imaging stability by modifying the prism surface to increase wettability and maximize contact with the cilium, as well as integrating temperature control (37 °C) to maintain physiological conditions, which will facilitate more quantitative investigations of IFT in mammalian cells.
Figure 5.
IFT dynamics revealed via live-cell imaging with the IP-TIRF microscope. (a) Schematic of a primary cilium containing IFT particles in stationary pools at the base and tip (red), and IFT particles assembled to trains moving along the ciliary axoneme (gold). In each panel, the tip is indicated with a red “t” and the base with a red “b”. (b,c). 3xmNeonGreen-IFT88-expressing cells have been seeded on glass-bottom dishes and serum-starved for 2 days before imaging cilia with the IP-TIRF microscope at room temperature with a 200 ms exposure and 5 frames per second. Individual transport events are highlighted with a light green (anterograde) and light orange (retrograde) box. (b) Representative images of the time-lapse images of a primary cilium. T1 = 3 s, T2 = 6 s, …, T10 = 30 s. (c) Kymograph of IFT particle movement using adaptive pixel intensity histogram normalization. Scalebar: horizonal = 5 s, vertical = 2 µm.
Figure 5.
IFT dynamics revealed via live-cell imaging with the IP-TIRF microscope. (a) Schematic of a primary cilium containing IFT particles in stationary pools at the base and tip (red), and IFT particles assembled to trains moving along the ciliary axoneme (gold). In each panel, the tip is indicated with a red “t” and the base with a red “b”. (b,c). 3xmNeonGreen-IFT88-expressing cells have been seeded on glass-bottom dishes and serum-starved for 2 days before imaging cilia with the IP-TIRF microscope at room temperature with a 200 ms exposure and 5 frames per second. Individual transport events are highlighted with a light green (anterograde) and light orange (retrograde) box. (b) Representative images of the time-lapse images of a primary cilium. T1 = 3 s, T2 = 6 s, …, T10 = 30 s. (c) Kymograph of IFT particle movement using adaptive pixel intensity histogram normalization. Scalebar: horizonal = 5 s, vertical = 2 µm.
It should be noted that, due to the unique geometry of the IP-TIRF setup, integrating a Petri dish heating element to maintain 37 °C proved technically challenging. Commercially available 35 mm dishes are too tall, causing the incident beam to strike the dish wall before reaching the critical angle required for TIR, while 50 mm dishes are incompatible with standard heaters. Alternative heating solutions that were tested were also unable to deliver uniform and stable temperature control near the center of the dish, particularly in the presence of the immersed prism, which absorbs and redistributes heat. Although physiological temperature measurements will be pursued in future work with custom-designed hardware, the current proof-of-principle experiments at room temperature clearly demonstrate the feasibility of IP-TIRF for imaging fluorescent beads and IFT particles in live cells.
Furthermore, in the current IP-TIRF setup, alignment of the prism is performed manually, which inherently introduces variability in the incident angle and prism–cell spacing. Such variability can contribute to drift over time and complicate efforts to maintain consistent optical alignment.
Figure S5 in the Supplementary Materials shows the frame-by-frame CNR of a ciliary base pool. These results illustrate an overall trend of exponential decay in intensity, most likely due to contributions from drift and photobleaching, while short-term fluctuations (10–20 s) caused by IFT trains entering and leaving the base pool are superimposed on this variation.
In addition, cilia are not rigidly anchored to the cell body but undergo dynamic fluctuations in position and orientation, leading to frame-to-frame variations in the evanescent field signal. To quantify this effect, we measured CNR on a frame-by-frame basis (
Figure S5 in Supplementary Materials) by tracking bright IFT trains within the cilium. The measured CNR includes contributions from photobleaching, movement of IFT trains (including occasional overlap), and bending of the cilium in and out of the evanescent field.
Therefore, the prism placement stability and reproducibility remain unquantified, and bending of the cilium relative to the prism introduces variability in signal intensity, which should be considered when interpreting the current data. To mitigate such variabilities, we are developing methods to functionalize the prism surface with anti-PDGF receptor α antibodies, which will promote adhesion between the prism and cilia while allowing controlled retraction, as well as integrate a z-axis piezoelectric nano-positioner. These improvements are expected to reduce fluctuations and enable more quantitative studies of alignment tolerances, reproducibility, and excitation depth, while also facilitating broader biological investigations.
4. Conclusions
We developed and optimized a prism-based TIRF microscopy system (IP-TIRF) tailored for high-contrast, apical membrane imaging in live cells. By immersing a prism in the culture medium, TIR is generated at the medium–prism interface, effectively illuminating the apical membrane while reducing cytosolic background signals. In proof-of-principle experiments, we imaged fluorescent beads and mNeonGreen-tagged intraflagellar transport (mNG–IFT88) particles in cilia and compared IP-TIRF with scanning confocal microscopy. Benchmarking revealed that IP-TIRF achieved an approximately 1.8-fold improvement in contrast-to-noise ratio (CNR~31) relative to scanning confocal imaging of cilia. This capability enables direct visualization of individual primary cilia with sufficient resolution to distinguish both anterograde and retrograde IFT. The adaptability, cost-effectiveness, and live-cell compatibility of IP-TIRF establish it as a valuable platform for investigating dynamic processes in the apical surface of live cells. Ongoing improvements include prism surface modification to maximize interfacial interactions, and stably adsorbing the cilium on the prism by coating the prism surface with an antibody against an extracellularly accessible membrane protein. Furthermore, integration of temperature control to maintain physiological conditions will enhance its robustness and establish IP-TIRF as a powerful tool for quantitative studies of IFT, receptor dynamics, and, with appropriate modifications, for imaging other processes occurring at the apical cell membrane. Future work integrating temperature control and improved prism stabilization will be essential for quantitative biological interpretation, while the present study primarily establishes the feasibility of IP-TIRF for apical membrane imaging.