Abstract
Boerhavia diffusa, also known as Punarnava, is a plant of the Nyctaginaceae family that has been utilized in traditional medicine to cure a variety of ailments. The goal of this study was to use response surface methodology (RSM) to optimize the maximum percentage yield of boeravinone B and caffeic acid from Boerhavia diffusa roots, and simultaneous determination of boeravinone B and caffeic acid in newly developed single solvent system and demonstrate the hepatoprotective benefits of boeravinone B and caffeic acid. The extraction process examined extraction time, extraction temperature and solvent concentration, which were optimized via Box–Behnken experimental design. The proposed HPTLC method for the quantification of boeravinone B and caffeic acid were successfully validated and developed. The method was validated in term of linearity and detection limit, quantification limit, range, precision, specificity and accuracy. The separation of boeravinone B and caffeic acid bands was achieved on HPTLC plate using formic acid: ethyl acetate: toluene (1:3:5 v/v) as developing system. Densitometric analyses of boeravinone B and caffeic acid was carried out in the absorbance mode at 254 nm. The maximum percentage yield of caffeic acid and boeravinone B from Boerhavia diffusa require appropriate extraction parameters such as temperature, time, organic solvents and water content, which can be achieved using the Box-Behnken statistical design provide time: temperature: solvent ratio (30:45:40 v/v) for extraction of caffeic acid and 60:60:40 v/v for extraction of boeravinone B. The boeravinone B (200 µg/mL) and caffeic acid (200 µg/mL) showed the most significant hepatoprotective activity compared with standard sylimarin in HepG2 cell induced with galactosamine 40 mM toxicity. The findings supported B. diffusa’s traditional use as a functional food forhuman health benefits.
1. Introduction
Boerhavia diffusa has been traditionally used by Indian and Brazilian indigenous and tribal people. Ayurvedic medicine traditionally uses the plant’s root and leaves to treat a wide range of ailments, such as viral jaundice and liver dysfunction. This plant isreported to have behavioral and neuroendocrine effect and anti-depressant activity [1], anti-angiogenic activity [2], anti-convulsant and anti-epileptic activity [3], anti-inflammatory activity [4], inhibitory effect of prostatic hyperplasia [5], anti-genotoxic effect [6], thrombolytic, cytotoxic and anti-microbial activities [7], arsenic trioxide-induced cardiotoxicity [8], anti-urolithic activity [9], anti-hyperglycaemic and reno-protective effect [10], acetaminophen-induced liver toxicity [11], anti-proliferative and anti-estrogenic effects [12], immunomodulatory and anti-metastatic activity [13,14], radio-protective activity [15], potent anti-breast cancer activity [16], intestinal activity [17], immunosuppressive activity [18], anti-diabetic activity [19], Ca2+ channel antagonistic activity [20], chemo-preventive action [21], glycol-induced urolithiasis [22], and inhibition of human cervical cancer cells [23]. Main Rotenoids area type isoflavone compound (known as boeravinones) that are isolated from the roots of this plant; they showed potent anti-cancer [24,25], anti-inflammtory [26], cardioprotective [27] powerful anti-oxidant and geno-protective properties [28], intestinal motility and spasmolytic activity [29]. Caffeic acid (3,4-dihydroxycinnamic acid) is one of the major polyphenolic compounds found in many plant species, showing anti-viral activity [30], immunostimulatory activity [31], cardioprotective activity [32], hepatoprotective activity [33], anti-cancer activity [34] and anti-hepatocellular carcinoma activity [35]. For detecting phenolic compounds in medicinal plants, several analytical techniques such as liquid chromatography–mass spectrometry (LCMS), high-performance liquid chromatography (HPLC), high-performance thin layer chromatography (HPTLC)and ultra-performance liquid chromatography (UPLC). Boeravinone B and caffeic acid are rarely applied by use of their similar analytical techniques. Ilyas et al. in 2013 reported only identification and quantification of caffeic acid and boeravinone in single-solvent system toluene: ethyl acetate: formic acid: methanol (3:3:0.8:0.2 v/v) but tailing peak and separation and resolution of band of caffeic acid and boeravinone was very poor [36]. Here, a validated high-performance thin layer chromatography (HPTLC) method has been newly developed based on single solvent system (toluene: ethyl acetate: formic acid (5:4:1 v/v) for simultaneous validation, quantification and optimization of boeravinone B and caffeic acid in the plants. The selected HPTLC method is accurate, precise, simple, specific, less time-consuming, cost-effective and can separate boeravinone B and caffeic acid compounds from their other constituents. Because of advantages such as ease of sample preparation, optimization of specific chemicals and comparison of several samples on a single plate, comparable chromatographic techniques are frequently used to evaluate retinoid and phenolic acids. The comparison of the entire chromatograms allows for the detection of minor similarities and differences between the plants under investigation. The objective of the study was to simultaneously quantify, validate, optimize and to evaluate hepatoprotective activity of boeravinone B and caffeic acid in hydroalcoholic extracts of B. diffusa.
2. Materials and Methods
2.1. Chemicals and Instruments
We purchased standard boeravinone B and caffeic acid from NRPL, Bangalore, India. Ethyl acetate, toluene, methanol and formic acid (CDH Labs, Mumbai, India) were used as developing systems for HPTLC analysis. All samples and standards used for the analysis were filtered through a membrane filter, 0.22 μm pore size (HIMEDIA, Mumbai, India). Extraction method using a Soxhlet apparatus (Omega, Mumbai, India) and Rotary evaporator (Buchi R-114, Switzerland). CAMAG HPTLC system (Muttenz, Switzerland) equipped with a Linomat IV sample applicator was used for quantification and optimization. HepG2 cell line (National Centre for Cell Science (NCCS), Pune, India), Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS) and MTT assay kit, trypsin EDTA, penicillin and streptomycin and DMSO (Sigma-Aldrich Co. LLC, St. Louis, MO, USA), Trypan blue solution and, galactosamine and absolute ethanol (HimediaLab Pvt. Ltd., Mumbai, India).Tissue culture flasks, 96- and 24-well microculture plates, eppendorf tube, inverted microscope, serological pipette, heamocytometer (HimediaLab Pvt. Ltd., Mumbai, India), laminar flow hoods (Khera instrument, New Delhi, India), CO2 incubator (NuAire, Plymouth, MN, USA), water bath, Deepfreezer (−20 °C).
2.2. Collection and Extraction of Plant Materials
In February 2011, we collected the roots of B. diffusa in Maruthmallai, Kanyakumari district, Tamilnadu, India. It was identified and authenticated by Dr. V. Chelladurai, Research Officer, Central Council for Research in Ayurveda and Siddha (Govt. of India), Tirunelveli, Tamil Nadu. The dried roots were grinded into a coarse powder and subjected to extraction by using 95% methanol and 50% (v/v) hydro-alcohol for 6 h at 37 °C, respectively. The methanol and hydro-alcoholic extracts were evaporated and dried at 45 °C with help of rotary evaporator under reduced pressure. The yield of the extract prepared with 95% methanol was 5.29% weight-for-weight, while the extract prepared with hydro-alcoholic alcohol yielded 9.9% weight-for-weight.
2.3. HPTLC Instrumentation
2.3.1. Preparation of Sample Solution
A total of 100 mg of extracts were dissolved in 10 mL of methanol and sonicated the solution for 15 min and then makeup with 10 mL HPLC grade methanol, filtered through a membrane filter, 0.22 μm pore size before injecting into the HPTLC system.
2.3.2. Preparation of Plate
Prior to usage, pre-coated silica gel 60 F254 aluminum TLC plates (Merck, Germany) were washed in methanol and dried. The samples were applied in the form of bands of 4 mm width using Linomat V applicator (Muttenz, Switzerland) with a 100 µL syringe. The application rate was kept constant at 200 nL s−1, and the space between the two bands was 9 mm.
2.3.3. Slit and Scanning Speed
The slit size was fixed at 5 0.45 mm, and the scanning speed was set to 5 mm s–1.
2.3.4. Chromatographic Conditions
The mobile phase was 1:3:5 v/v/v formic acid, ethyl acetate, and toluene and the development volume was20 mL. In a 10 × 10 cm twin-trough glass chamber saturated with the mobile phase, linear ascending development was performed. At room temperature, the optimal chamber saturation time for the mobile phase was 20 min. The chromatogram run was 8.5 cm long, and the TLC plates were dried in an oven at 50 °C for 5 min after development. CAMAG TLC Scanner 3 in absorbance-reflectance mode at 254 nm, operated by Win-CATS software, was used for densitometric scanning. The deuterium lamp was used as a source of radiation. The peak area response was compared to the amount of medication in a linear regression.
2.3.5. Preparation of Standards
The standard solution of bioactive compounds was prepared by dissolving 1.0 mg of boeravinone B and caffeic acid in 10 mL of methanol as stock solution and stored at 2 to 8 °C. These boeravinone B and caffeic acid were further diluted as per the requirement to plot a final concentration for quantification.
2.3.6. Analytical Method Evaluation
The lowest diluted solutions of the two reference compounds in the calibration curves were further diluted to a series of concentrations with HPLC grade methanol for the determinations of limits of detection (LOD) and quantification (LOQ). The LOD and LOQ under the present chromatographic conditions were determined at a signal-to-noise (S/N) ratio of 3 and 10, respectively. Calibration curves, regression equation along with LOD and LOQ for each compound have been validated as per the ICH guidelines Q2 (R1) (2005). Linearity was assessed with the aid of serially diluted calibration solutions of all standards. Calibration graphs were plotted on the basis of triplicate analysis of each calibration solutions by using peak area against concentration. To evaluate the accuracy, the pre- analyzed samples were spiked with standard at three different known concentration levels i.e., 50, 100 and 150% and the mixtures were re-analyzed by the proposed method. Precision of the method was determined by carrying out the intra-day and inter-day variation tests. The inter-day and intra-day variations for the determination of boeravinone B and caffeic acid were carried out at different levels of concentrations 100, 200, 400 ng/band. The identification of bands was carried out in triplicate. The R.S.D. was taken as a measure of precision. The resolution between the peaks of caffeic acid and Boeravinone B found satisfactory (2.1 ± 0.2). The developed method resolution found to be good enough to avoid any possible merging between the peaks. Satisfactory recovery values suggest that there was no interference happening between the standard peaks.
2.4. Optimization of Boeravinone B and Caffeic Acidin Hydroalcoholic Extracts
Using the Box-Behnken statistical design, three factors and levels were considered. There were seventeen runs of it. To optimize the design, the Stat-Ease V6 software (Minneapolis, MN, USA) was used (Minneapolis, Stat-Ease Inc., MN, and USA). This style is suitable for investigating constructing second-order polynomial models and quadratic response surfaces. The designed style contains the replicated center point of the four-dimensional cube and a group of purposes lying at the center of every edge that confirmed the region of interest. The dependent and independent variables are recorded in Table 1.

Table 1.
Independent and dependent variables selected in Box–Behnken design.
The quadratic equation created by the Box–Behnken Design-Expert equation
where A0 is the intercept, A1 to A9 are the regression coefficients, R is the dependent variable and B1, B2 and B3 are the independent variables.
R = A0 + A1 B1 + A2 B2 + A3 B3 + A4 B1 B2 + A5 B1 B3 + A6 B2 B3 + A7B12 + A8B22 + A9B32
The data of the maximum percentage yield of boeravinone B and caffeic acid in the hydro-alcoholic extract of plant materials were recorded through arithmetical improvement by using Design-Expert software system. The Box–Behnken Design style spreadsheet is shown in Table 2.

Table 2.
Mentioned responses in Box–ehnken design experiment for 17 analytical trails.
2.5. The Hepatoprotective Activity of Caffeic Acid and Boeravinone B
Cells were seeded at a density of 1 × 105 cells/ in each well of the 24-well plates and incubated overnight. After 24 h, the media was flicked off, the cells were treated with boeravinone B and caffeic acid (100 and 200 µg/mL) in separate wells of a 24-well plate and incubated for 2 h. Silymarin (250, 500 µg/mL) was used as a reference standard. After incubation, the cell was treated with D-galactosamine at a concentration of 40 mM and was allowed to incubate for 2 h. After incubation, the cells were washed and incubated for 1 h with MTT (20 μL of 5 mg/mL of MTT in PBS) was added to each well. Formation of formazan crystal was observed under a microscope after 2 h. In case crystal, formation was not proper, incubation was continued for an hour more. Media was removed and the remaining formazan crystals were dissolved in 200 μLof DMSO in each well. The cell culture plate was kept on a shaker for 15 min; the absorbance was recorded by ELISA reader at 540 nm.
The % hepatoprotection of boeravinone B, caffeic acid and silymarin was obtained by the following formula:
% Hepatoprotection = (Optical Density of Test sample / Optical Density of Control) × 100
3. Results
3.1. Development of Mobile Phase
Separation of standard markers studies were carried out on the working standard solution of boeravinone B and caffeic acid in HPLC-grade methanol. Originally, many trials were established with different developing systems such as ethyl acetate: chloroform (5:5), ethyl acetate: chloroform (7:3), ethyl acetate: toluene (7:3), ethyl acetate: toluene (5:5). Finally, toluene: ethyl acetate: formic acid (5:4:1 v/v) for simultaneous quantification and optimization of boeravinone B and caffeic acid gave a well-defined and sharp peak. We obtained sharp bands upon saturating the twin trough plate development chamber with the developing system at room temperature for 30 min. The band of boeravinone B and caffeic acid was recorded on HPTLC plate scanned at 254 nm (Figure 1).
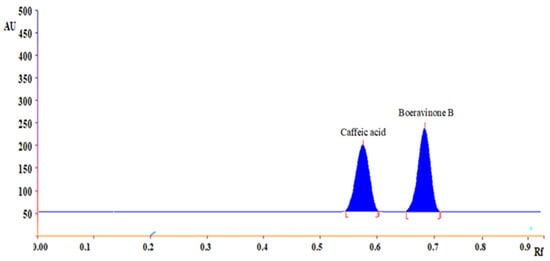
Figure 1.
HPTLC densitogram of caffeic acid (Rf = 0.58), boeravinone B (Rf = 0.71) in formic acid: ethyl acetate: toluene (1:3:5 v/v) presented on HPTLC plate scaned at 254 nm.
3.2. Method of Validation
Figure 1 shows a representative chromatogram of boeravinone b and caffeic acid in the established HPTLC technique. The HPTLC chromatogram in Figure 1 shows a retention factor of caffeic acid (Rf = 0.58), boeravinone B (Rf = 0.71), respectively. The calibration curve’s linear regression data revealed a good linear relationship throughout a concentration range of 60 to 240 μg mL−1 with a correlation coefficient (R2) value of 0.992 indicated acceptable linearity for caffeic acid and 50 to 250 μg mL−1 with a correlation coefficient (R2) value of 0.98.91 indicated acceptable linearity for boeravinone B (Table 2). Correlation coefficient is a statistical tool used to measure the strength or degree of this relationship, and, here, a high correlation coefficient value (a value extremely close to 1.0) indicates a high level of linear relationship between the peak area and concentration of caffeic acid and boeravinone B. The LOD and LOQ were calculated using the ICH Guidelines Q2 (R1) (2005) and were 50 ng, 200 ng for boeravinone B and 40 ng, 200 ng for caffeic acid, respectively (Table 3). The intraday and interday assay was used to determine the repeatability of the proposed HPTLC method, and the results was expressed in the term of %RSD (Table 3) showed that the repeatability of method. The developed HPTLC method’s outstanding precision was suggested by the low-percent RSD for repeatability and intermediate precision. The specificity of the developed HPTLC method for the analysis of boeravinone B and caffeic acid in the hydroalcoholic extract was recorded by comparing the spectra obtained in the standards and sample analyses. These spectra’s peak start, peak apex, and peak end positions were identical. The recovery trials were done to see how sensitive the method for estimating caffeic acid and boeravinone B. The standard addition approach was used to increase the concentration of caffeic acid and boeravinone B in the sample extract by 50%, 100%, and 150%. The percentage recoveries of the three concentrations ranged from 98.91% to 101.11% for boeravinone B and 99.91% to 100.71% for caffeic acid, indicating a high level of precision. The system suitability parameters of HPLC to establish that the total system operated successfully, system suitability criteria such as peak symmetry, resolution (Rs), capacity factor (K), and selectivity was tested. As demonstrated in Table 3, the obtained values were within acceptable ranges.

Table 3.
Method validation parameters for boeravinon b and caffeic acid in B. diffusa.
3.3. Box–Behnken Design-Experiment
A three-level factorial, Box–Behnken Design-Expert applied statistical modeling experimental style was performed by applying 17 investigational trial runs, as recorded in Table 2. Standard boeravinone B and caffeic acid determined that the preferred model was the squared model and also the equivalent values of standard deviation and percentage coefficient of variation for the various planned models is listed in Table 4 together with the regression equations typically for ultimately elite responses. Only statically significant (p < 0.0001) for boeravinone B and caffeic acid respectively coefficient is enclosed in the equations. A positive value indicated the optimization of boeravinone B and caffeic acid in hydroalcoholic extracts, while a negative value represents an inverse relationship between the response and the factor. It is clear from the equations that the factor time (B1), as well as temperature (B2) has an uncooperative effect and 40:60:80 v/v solvent ratios (B3) have a productive on the responses (C1 and C2,). It also indicates that the correlation between factors and response is not always linear. When more than one factor is replaced at the same time, a factor can represent a various degree of response. Relationships of B1 and B2, as well as B1 and B3, induce uncooperative impacts on the response. However, the result in the case of the square root of various factors does not repeat history, as shown by its performance. In the case of the square root of various factors, B22 (extraction temperature) and B23 (solvent concentration) produced positive results, and B21 (time of extraction) showed the negative impact on the responses in Table 4. The last mixture ratios of the extractions were established based on percentage yield of boeravinone B and caffeic acid.

Table 4.
Summary of results of regression analysis for models and responses (Y1-Y2) regression equations for Quadratic models.
3.4. Response of Independent Factors on Boeravinone B (C1)
Regression equation of the fitted quadratic models:
Boeravinone B = 0.00733 + 0.000075 B1−0.0023 B2 0.00812 B3 − 0.0029 B1 B2 + 0.011 B1 B3 + 0.0078 B2 B3 + 0.017 B12 + 0.021 B22 + 0.0054 B32….
Factor B1, which is the time (min) for extraction of plant materials used indicated the percentage yield of boeravinone B in productive direction to that produced with factor B2. The productive coefficient composition of factor A produced the increase in percentage yield of boeravinone B, which improves in factor B1. It was confirmed that, when an improved time of extraction was applied, a higher concentration of the percentage of boeravinone B was obtained (Figure 2A) shows the results of time in min of extraction on boeravinone B percentage yield.
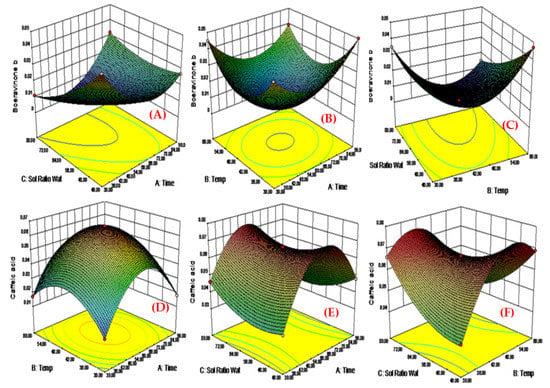
Figure 2.
Response surface plots of factor B2 vs. B1 against boeravinone B (C1) and caffeic acid (C2) respectively. (A) when an improved time of extraction was applied, higher concentrations of percentage of boeravinone B were obtained; (B) as the factor temperature level improved, the boeravinone B percentage yield also showed an uncooperative automatic impact; (C) when an elevated level of solvent ratio was applied, boeravinone B concentration also produced uncooperative impact; (D) when an increase time of extraction was applied, an elevated level of percentage yield of caffeic acid was investigated due to elevated level of penetration of solvent into plant extracts; (E) when the level of temperature increases, the sharp percentage yield of caffeic acid conjointly inflated slowly as a result of thermostable compound; (F) once an elevated level of solvent magnitude relation was applied, the percentage yield of caffeic acid was minimized as a result of the medium polar compound.
Factor B2 (extraction temperature) was found to have an uncooperative result on the boeravinone B percentage yield compared to factor B3 (solvent concentration). As the factor B2 (temperature of extraction) level improved, demonstrated a positive impact on the percentage yield of boeravinone B (Figure 2B), showing that the extraction temperature should be increased to 60 degrees Celsius to get the highest percentage yield of boeravinone B.
In contrast to Factor B3 (extraction temperature), which indicated the maximum percentage yield of boeravinone B, factor B3 (solvent concentration (water: methanol)) produced a favourable impact on the percentage yield of boeravinone B. Boeravinone B concentration increased when a higher solvent concentration ratio was used (Figure 2C), demonstrating the influence of solvent ratio levels on boeravinone B percentage yield.
3.5. Responseof Independent Factors on Caffeic Acid (C2)
As the equation shows that B1 has a productive impact on the caffeic acid percentage yield, When factor B (time of extraction) was increased, a productive level of caffeic acid percentage yield was obtained due to solvent concentration penetrating into the plant materials, as shown in figure. This is because the equation shows that B1 has a productive impact on the caffeic acid percentage yield (Figure 2D).
Caffeic acid = 0.066 + 0.0014 B1 + 0.00093 B2 + 0.000013 B3−0.0016 B1 B2−0.005 B1 B3−0.013 B2 B3−0.031 B12−0.019 B22 + 0.0074 B32
Factor B2 is perceived to have an increased profound impact on the proportion of caffeic acid percentage yield compared to issue B1 and B2. Because the factor B (temperature level) inflated, the sharp percentage yield of caffeic acid conjointly inflated slowly as a result of thermostable compound. Figure 2E shows the impact of temperature levels on caffeic acid proportion yield.
Factor B3 (40:60:80 v/v solvent ratio) indicated a distinguished lower caffeic acid percentage yield as compared to that discovered on the issue B2, which showed a broader negative impact. It had been discovered that, once an elevated level of solvent magnitude relation was applied, the percentage yield of caffeic acid was minimized as a result of the medium polar compound. Figure 2F indicates the impact of solvent magnitude affinity levels on percentage yield of caffeic acid.
3.6. Hepatoprotective Activity of Bioactive Compounds on HpeG2 Cell Induced with Galactosamine
In the study, the above-mentioned fractions were screened for hepatoprotective activity against GalN-induced cytotoxicity, by pre-incubating the cells with or without the extracts or sylimarin. A significant decrease in cell viability was observed upon treatment of HepG2 cells with GalN (40 Mm). Results are expressed as mean ± standard error mean (S.E.M). The percentage protection is represented in Figure 3 and Table 5.
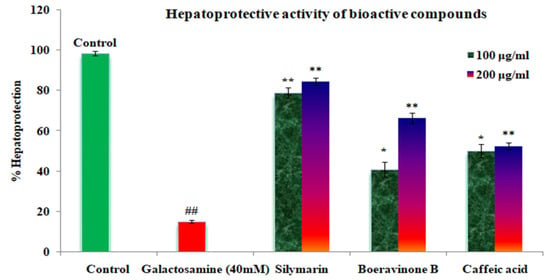
Figure 3.
Effect of the caffeic acid and boeravinone B on GlaN-induced toxicity in HepG2 cells. HepG2 cells were incubated in the presence/ absence of various compounds for 2h, prior to treatment with GlaN (40mm) for 2h. Thereafter, the cells were processed for the MTT assay. The results are expressed as mean ± SEM. ## Galactosamine toxic group significant, ns p > 0.05, ** p < 0.01, * p < 0.05 compared to the GalN (40 mM) control.

Table 5.
In-vitro hepatoprotective activity of potent compounds present in B. diffusa.
4. Discussion
A significant number of medicinal plants are being employed for healing and for the development of new pharmaceuticals all around the world. Understanding the therapeutic significance of natural extracts or their constituents, as well as the standardization of crude pharmaceuticals, necessitate phytochemical study [37]. The literature review supports the fact that many natural products exhibiting hepatoprotective activity need to be standardized and optimized due to degradation of polyphenolic compounds during extraction time.
Several plants that are traditionally used are characterized for the presence of active ingredients using diverse methods [38,39]. Recently, B. diffusa has been increasingly propagated as a medicinal plant. The current study aims to develop and validate high performance thin layer chromatography (HPTLC) method for the simultaneous validation, quantification of boeravinone B and caffeic acid in the hydro alcoholic extract of B. diffusa in single solvent system. The HPTLC method is accurate, precise, simple, specific, less time consuming, cost-effective, and can separate boeravinone B and caffeic acid compounds from their other constituents such as boeravinone A, C, D, E, F, G and flavonoid glycosides, etc.
In this method, the hydroalcoholic extract was found to contain a comparatively elevated amount of boeravinone B and caffeic acid compared to alcoholic and aqueous extracts. This may be because medium polar retinoid and flavonoid glycosides present in hydroalcoholic extract. The percentage yield of extracts depends on the temperature, time, and solvents of the extraction as well as the chemical moiety of secondary metabolites. Under the same time and temperature conditions, the solvent used and the chemical property of the sample are the most important factors. In the present investigation, the application of the Box–Behnken Design for the optimization of boeravinone B and caffeic acid based on temperature of extraction (°C), time of extraction (min) and solvent concentration ratio (%v/v).
Shimma Ibrahim et al., in 2017, reported that natural plants have garnered great interest in the last few decades as therapeutic agents, since they produce inexpensive and less toxic products than synthetic drugs. To date, there is only one protective natural drug (Silymarin) that too is not curative and also has limitations in protecting the liver from viral attacks. Karimi, G. et al., in 2011, established that silymarin is useful forthe treatment of liver cirrhosis and hepatitis, and displaysimmumodulatory activity [40]. However, silymarin’s structure containsa flavonoid moiety. Phenolic compounds (coumarins, flavonoids, xanthones, retinoid and tannins) are widely distributed in plants with potent anti-oxidant and anti-inflammatoryeffects, regulating immunity by interfering with immune cell regulation, proflammatory cytokines, and synthesis and gene expression and inhibitingphsophatidylinostide -3- kinase/ protein kinase [41].In the present study, isolated constituents caffeic acid (100 microgram/mL) and boeravinone B (200 µg/mL) showed significant hepatoprotective activity y compared with standard sylimarin in HepG2 cell induced with galactosamine 40 mM toxicity.
Caffeic acid and boeravinone B have been of interest for theirhealth benefits, and the present HPTLC analytical study could be a future application to purify, identify, quantify, and optimize boeravinone B and caffeic acid in Boerhaviadiffusa.
5. Conclusions
The proposed method is innovative, since it is the first analytical method to report on the validation, quantification, and optimization of boeravinone B and caffeic acid in Boerhavia diffusa (Linn).The proposed HPTLC method was performed according to the guidelines of the International Conference on Harmonization (ICH) for the quantification of boeravinone B and caffeic acid, which were successfully validated and developed. The method was validated in term of linearity and detection limit, quantification limit, range, precision, specificity, and accuracy. The maximum percentage yield of caffeic acid and boeravinone B from Boerhavia diffusa require appropriate extraction parameters such as temperature, time, organic solvents, and water content, which can be achieved using the Box–Behnken statistical design, providing a time: temperature: solvent ratio (30:45:40 v/v) for the extraction of caffeic acid and 60:60:40 v/v for the extraction of boeravinone B. The hepatoprotection of standard silymarin showed ranging from 78.7 percent at 100 µg/mL to 84.34 percent 200 µg/mL, caffeic acid varied from 46.17 percent at 100 µg/mL to 52.34 percent at 200 µg/mL and boeravinone B ranged from 40.89 percent at 100 µg/mL to 62.21 percent at 200 µg/mL. The boeravinone B and caffeic acid showed the most significant hepatoprotective activity compared with standard silymarin in HepG2 cell induced with galactosamine 40 mM toxicity. The findings supported Boerhavia diffusa’s traditional use as a functional food for human health benefits.
Author Contributions
The contribution of the authors is as follows: conceptualization, I.U. and K.Y.T.; methodology, I.U.; investigation, I.U.; data curation, I.U., S.S., M.E.-M., S.N.M.N.U. and A.A.; writing—original draft preparation, I.U., K.Y.T. and M.E.-M.; writing—review and editing, I.U. and M.E.-M.; visualization, I.U.; supervision, K.Y.T.; funding acquisition, K.Y.T., S.N.M.N.U., A.A. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All participants in the study provided their informed permission.
Data Availability Statement
The data that supports the finding of this study are also available from the corresponding author upon request.
Acknowledgments
The whole authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through General Research Project under Grant No: GRP/ 105/43.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dhingra, D.; Valecha, R. Behavioural and neuroendocrine effects of aqueous extract of Boerhaavia diffusa Linn. in mice using tail suspension and forced swim tests—A preliminary study. Indian J. Exp. Biol. 2014, 52, 53–59. [Google Scholar] [PubMed]
- Saraswati, S.; Alhaider, A.A.; Agrawal, S.S. Punarnavine, an alkaloid from Boerhaavia diffusa exhibits anti-angiogenic activity via downregulation of VEGF in vitro and in vivo. Chem. Biol. Interact. 2013, 206, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Gairola, S.; Gaur, R.D.; Painuli, R.M.; Siddiqi, T.O. Ethnomedicinal plants used for treating epilepsy by indigenous communities of sub-Himalayan region of Uttarakhand, India. J. Ethnopharmacol. 2013, 150, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, K.; Singh, I.N.; Roy, S.K.; Grover, J.; Srivastava, A.; Jachak, S.M. Rotenoids from Boerhaavia diffusa as potential anti-inflammatory agents. J. Nat. Prod. 2013, 76, 1393–1398. [Google Scholar] [CrossRef]
- Vyas, B.A.; Desai, N.Y.; Patel, P.K.; Joshi, S.V.; Shah, D.R. Effect of Boerhaavia diffusa in experimental prostatic hyperplasia in rats. Indian J. Pharmacol. 2013, 45, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Aher, V.; Chattopadhyay, P.; Goyary, D.; Veer, V. Evaluation of the genotoxic and antigenotoxic potential of the alkaloid punarnavine from Boerhaavia diffusa. Planta Med. 2013, 79, 939–945. [Google Scholar] [CrossRef]
- Apu, A.S.; Liza, M.S.; Jamaluddin, A.T.; Howlader, M.A.; Saha, R.K.; Rizwan, F.; Nasrin, N. Phytochemical screening and in vitro bioactivities of the extracts of aerial part of Boerhavia diffusa Linn. Asian Pacific J. Trop. Biomed. 2012, 2, 673–678. [Google Scholar] [CrossRef] [Green Version]
- Vineetha, V.P.; Prathapan, A.; Soumya, R.S.; Raghu, K.G. Arsenic trioxide toxicity in H9c2 myoblasts--damage to cell organelles and possible amelioration with Boerhavia diffusa. Cardiovasc. Toxicol. 2013, 13, 123–137. [Google Scholar] [CrossRef]
- Yasir, F.; Waqar, M.A. Effect of indigenous plant extracts on calcium oxalate crystallization having a role in urolithiasis. Urological. Res. 2011, 39, 345–350. [Google Scholar] [CrossRef]
- Singh, P.K.; Baxi, D.; Doshi, A.; Ramachandran, A.V. Antihyperglycaemic and renoprotective effect of Boerhaavia diffusa L. in experimental diabetic rats. J. Comp. Int. Med. 2011, 8, 1–20. [Google Scholar] [CrossRef]
- Olaleye, M.T.; Akinmoladun, A.C.; Ogunboye, A.A.; Akindahunsi, A.A. Antioxidant activity and hepatoprotective property of leaf extracts of Boerhaavia diffusa Linn against acetaminophen-induced liver damage in rats. Food Chem. Toxicol. 2010, 48, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Sreeja, S.; Sreeja, S. An in vitro study on antiproliferative and antiestrogenic effects of Boerhaavia diffusa L. extracts. J. Ethnopharmacol. 2009, 126, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Kuttan, G. Boerhaavia diffusa stimulates cell-mediated immune response by upregulating IL-2 and downregulating the pro-inflammatory cytokines and GM-CSF in B16F-10 metastatic melanoma bearing mice. J. Exp. Ther. Oncol. 2008, 7, 17–29. [Google Scholar] [PubMed]
- Manu, K.A.; Kuttan, G. Anti-metastatic potential of Punarnavine, an alkaloid from Boerhaavia diffusa Linn. Immunobiology 2009, 214, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Leyon, P.V.; Kuttan, G. Studies on the protective effects of Boerhaavia diffusa L. against gamma radiation induced damage in mice. Integr. Cancer Ther. 2007, 6, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Belkacem, A.; Macalou, S.; Borrelli, F.; Capasso, R.; Fattorusso, E.; Taglialatela-Scafati, O.; Di Pietro, A. Nonprenylated rotenoids, a new class of potent breast cancer resistance protein inhibitors. J. Med. Chem. 2007, 50, 1933–1938. [Google Scholar] [CrossRef]
- Borrelli, F.; Milic, N.; Ascione, V.; Capasso, R.; Izzo, A.A.; Capasso, F.; Petrucci, F.; Valente, R.; Fattorusso, E.; Taglialatela-Scafati, O. Isolation of new rotenoids from Boerhaavia diffusa and evaluation of their effect on intestinal motility. Planta Med. 2005, 71, 928–932. [Google Scholar] [CrossRef]
- Pandey, R.; Maurya, R.; Singh, G.; Sathiamoorthy, B.; Naik, S. Immunosuppressive properties of flavonoids isolated from Boerhaavia diffusa Linn. Int. Immunopharmacol. 2005, 5, 541–553. [Google Scholar] [CrossRef]
- Satheesh, M.A.; Pari, L. Antioxidant effect of Boerhavia diffusa L. in tissues of alloxan induced diabetic rats. Indian J. Exp. Biol. 2004, 42, 989–992. [Google Scholar]
- Lami, N.; Kadota, S.; Kikuchi, T.; Momose, Y. Constituents of the roots of Boerhaavia diffusa L. III. Identification of Ca2+ channel antagonistic compound from the methanol extract. Chem. Pharmaceut. Bulletin. 1991, 39, 1551–1555. [Google Scholar] [CrossRef] [Green Version]
- Bharali, R.; Azad, M.R.; Tabassum, J. Chemopreventive action of Boerhaavia diffusa on DMBA-induced skin carcinogenesis in mice. Indain J. Physiol.Pharmacol. 2003, 47, 459–464. [Google Scholar]
- Jarald, E.E.; Kushwah, P.; Edwin, S.; Asghar, S.; Patni, S.A. Effect of Unex on ethylene glycol-induced urolithiasis in rats. Indian J. Pharmacol. 2011, 43, 466–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, R.; Saluja, D.; Dwarakanath, B.S.; Chopra, M. Inhibition of Human Cervical Cancer Cell Growth by Ethanolic Extract of Boerhaavia diffusa Linn. (Punarnava) Root. Evid. -Based Complementary Altern. Med. Ecam 2011, 2011, 427031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Xiao, S.; Huang, J.; Liu, S.; Xue, M.; Lu, F. Chemoprotective Effect of Boeravinone B against DMBA/Croton Oil Induced Skin Cancer via Reduction of Inflammation. J. Oleo. Sci. 2021, 70, 955–964. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Y.; Wang, W.W.; Zhang, L. Boeravinone B a natural rotenoid exerts anticancer activity via inducing internalization and degradation of inactivated EGFR and ErbB2 in human colon cancer cells. Am. J. Transl. Res. 2018, 10, 4183–4192. [Google Scholar]
- Bairwa, K.; Jachak, S.M. Anti-inflammatory potential of a lipid-based formulation of a rotenoid-rich fraction prepared from Boerhavia diffusa. Pharm. Biol. 2015, 53, 1231–1238. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, L.; Shi, S.; Guo, G.; Wen, H. Boeravinone B alleviates gut dysbiosis during myocardial infarction-induced cardiotoxicity in rats. J. Cell Mol. Med. 2021, 25, 6403–6416. [Google Scholar] [CrossRef]
- Aviello, G.; Canadanovic-Brunet, J.M.; Milic, N.; Capasso, R.; Fattorusso, E.; Taglialatela-Scafati, O.; Fasolino, I.; Izzo, A.A.; Borrelli, F. Potent antioxidant and genoprotective effects of boeravinone G, a rotenoid isolated from Boerhaavia diffusa. PLoS ONE 2011, 6, 9628. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Aeri, V.; Gaur, P.K.; Jachak, S.M. Phytochemical, Therapeutic, and Ethnopharmacological Overview for a Traditionally Important Herb: Boerhavia diffusa Linn. Biomed. Res. Int. 2014, 2014, 808302. [Google Scholar] [CrossRef] [Green Version]
- Erdemli, H.K.; Akyol, S.; Armutcu, F.; Akyol, O. Antiviral properties of caffeic acid phenethyl ester and its potential application. J. Intercult Ethnopharmacol. 2015, 4, 344–347. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Hatmal, M.M.; Sattar, K.; Ahmad, S.; Mustafa, M.Z.; Bittencourt, M.C.; Mohamud, R. Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions. Molecules 2020, 25, 5017. [Google Scholar] [CrossRef] [PubMed]
- Bıçakç, N.; Karaboğa, I.; Dökmeci, A.H.; Güzel, S.; Fidanol Erboğa, Z. Cardioprotective effect of caffeic acid phenethyl ester on cardiac contusion following blunt chest trauma in rats. Biotech. Histochem. 2019, 94, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Farahani, M.V.; Hamzehlou, S.; Far, F.B.; Sharifzadeh, S.O.; Samarghandian, S.; Khan, H.; et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.F.; Azab, S.S.; Khalifa, A.E.; Abdel-Rahman, S.Z.; Abdel-Naim, A.B. Caffeic acid phenethyl ester, a promising component of propolis with a plethora of biological activities: A review on its anti-inflammatory, neuroprotective, hepatoprotective, and cardioprotective effects. IUBMB Life 2013, 65, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Brautigan, D.L.; Gielata, M.; Heo, J.; Kubicka, E.; Wilkins, L.R. Selective toxicity of caffeic acid in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2018, 505, 612–617. [Google Scholar] [CrossRef]
- Ilyas, U.K.; Katare, D.P.; Ambardar, N.; Aeri, V. HPTLC densitometric quantification of caffeic acid and boeravinone B in Boerhavia diffusa Linn. Int. J. Phytopharm. 2013, 4, 184–189. [Google Scholar]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Karimi, G.; Vahabzadeh, M.; Lari, P.; Rashedinia, M.; Moshiri, M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran. J. Basic Med. Sci. 2011, 14, 308–317. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



