Abstract
Tungsten is a commercially important metal element that usually coexists with a variety of non-ferrous metals, which makes its extraction difficult. Scheelite is a commonly occurring tungsten-containing ore with the formula CaWO4. Improving the surface properties of scheelite to increase its adsorption of the collector for flotation separation is the focus of our current research. In this paper, the effects of manganese ions on scheelite flotation in benzohydroxamic acid (BHA) system were studied by micro-flotation tests, adsorption tests, fourier transform infrared spectroscopy (FTIR), zeta potential, and X-ray photoelectron spectroscopy (XPS) analysis. The addition of Mn2+ was found to improve the recovery of scheelite. The addition of Mn2+ greatly improved the recovery of scheelite. Infrared spectroscopy, adsorption tests, zeta potential measurements and XPS analysis all confirmed that BHA had a higher adsorption capacity and a stronger bond to the surface of scheelite after the addition of manganese ions, increasing the floatability of scheelite particles. Therefore, Mn2+ shows great potential for the improvement of the flotation index of scheelite in a system with BHA.
1. Introduction
Tungsten is a significant metal which has been incorporated in a variety of high-tech materials, including high temperature resistant alloys, electronic optical materials, new functional materials, and organometallic compounds [1,2]. It is widely used in electronic computers, modern communication technology, aerospace development, photoelectric materials, medical and health applications, photosensitive materials, new energy sources, and catalytic materials [3,4,5]. However, tungsten is a typical rare metal: Although it is widely distributed in almost all kinds of rocks, its content is low. The content of tungsten in the earth’s crust is only 0.001%, and it usually coexists with a variety of non-ferrous metals, which makes its extraction difficult [6,7,8]. Nonetheless, the development of metallurgical and mineral processing technology and equipment and in analytical detection technology, as well as the expansion of the scale of rare metal production, has enabled expansion of the applications of tungsten.
Scheelite, CaWO4, is an important tungsten-containing resource; it is usually recovered by foam flotation. With the increasing depletion of wolframite resources, the use of scheelite has become more and more important [9,10,11]. Fatty acids are the most commonly used collector in scheelite flotation. These reagents for the collection of scheelite are based on the chemical interaction between Ca2+ ions on the surface of the particles and the carboxyl group in the collector, but these collectors are less selective and cannot effectively separate minerals [12,13,14]. Therefore, it is often necessary to use with depressants. As an example, when sodium oleate was used as a collector, scheelite and calcite have similar recovery [15]. Compared with conventional fatty acids and amine collectors, which have the disadvantage of low selectivity, hydroxamic acid as a collector has much better selectivity. However, the collection capacity is limited when only a single hydroxamic acid collector is used. Therefore, an important research direction is to improve the surface properties of scheelite and thereby to enhance its interaction with the collector.
The addition of metal ions in the flotation process is a common technical means to improve the flotation index. Metal ions can interact with the mineral surface and flotation reagents to activate or depress minerals, so that they can float better and therefore be better separated [16]. For example, lead ions can activate sphalerite very well, thereby increasing its flotation, whereas calcium ions can effectively enhance the inhibition of carboxymethyl cellulose on talc in flotation [17,18,19].
Previous studies on the flotation of scheelite showed that benzohydroxamic acid (BHA) was effective as a collector for scheelite flotation. It was worth noting that the chelation reaction between BHA with calcium ions on minerals surface showed excellent selectivity, providing a practical protocol for the selective flotation of scheelite. However, the effective improvement of scheelite recovery was still a great challenge [20]. There had been previous studies, Pb-BHA could be formed in solution by adding metal ions (lead ions) in the flotation process, it could be attracted to the negatively charged surface of the scheelite by electrostatic force. Adsorption of these basic aqueous complexes could then occur by emitting water, which was formed by combining hydrogen from the complex and an adsorbed hydroxyl. The W-O-Pb-collector might be the main adsorption structure, which was significantly different from that of fatty acid. [Replacing Petrov’s process with atmospheric flotation using Pb-BHA complexes for separating scheelite from fluorite] It indicated that Pb-BHA system not only had good selectivity, but also had strong collecting ability of scheelite. However, the effect of other metal ions on the flotation of scheelite remains to be further studied.
In the research reported herein, benzohydroxamic acid (BHA) was used as a collector for the flotation of scheelite. To explore the effect of Mn2+ on the flotation process of scheelite, the mechanism of action between Mn2+ and the surface of scheelite was explored by micro-flotation tests, ultraviolet-visible spectrophotometer measurements, zeta potential measurements, fourier transform infrared (FTIR) spectroscopy, and X-ray photoelectron spectroscopy (XPS) analysis. The results of these studies are expected to provide a reference for the mechanism of metal ion activation and flotation of scheelite.
2. Experimental
2.1. Materials and Reagents
Pure mineral samples of scheelite from Yunnan Province, China were obtained by grinding in a ceramic grinding machine (Xiamen Chenggong Mining Equipment Co., Ltd., Sanming, China). Screening < 74 μm samples for experiments and testing. Mineral samples with a particle size of approximately 38 microns were taken for the determination of chemical composition and X-ray diffraction. The results recorded in Table 1 and Figure 1 show that the detected scheelite has high purity (>97%). BHA as a collector, MnCl2 as a Mn2+ source, and NaOH as a cosolvent (10%) were analytical-grade reagents. Pine alcohol oil was used as a frother. Deionized water (resistivity = 18.25 MΩ·cm) was used for experiments and testing.

Table 1.
Chemical analysis of the pure scheelite sample. (% composition).
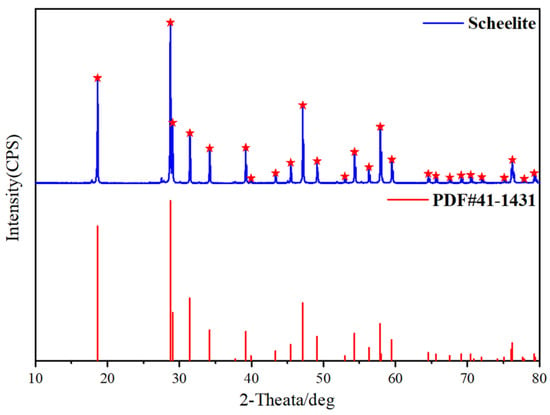
Figure 1.
X-ray diffraction pattern of pure scheelite.
2.2. Micro–Flotation Tests
The micro-flotation tests were carried out using a 40-mL FGC suspension tank flotation machine (Wuhan Rock Grinding Equipment Manufacturing Co., Ltd., Wuhan, China) with a spindle speed of 1800 r/min. The particle size of the pure mineral samples used in the test was in the range of 38 to 74 μm. For the first set of experiments, the concentration of BHA was set at 1 × 10−3 mol/L and the concentration of Mn2+ was increased in increments of 5 × 10−5 mol/L from 0 to 3 × 10−4 mol/L, for a total of 7 samples. For the second set of experiments, two series of samples were prepared in which the concentration BHA was increased in increments of 2.5 × 10−4 mol/L, from 2.5 × 10−4 mol/L to 2.25 × 10−3 mol/L, for a total of 9 concentrations. For one series, no Mn2+ was added. For the other series, the concentration of Mn2+ was set at 2 × 10−4 mol/L. The general experimental process was as follows: BHA was dissolved in deionized water and a small amount of NaOH, and MnCl2 was dissolved with deionized water. The 2.0 g mineral sample was weighed and put into a 40-mL flotation cell, and an appropriate amount of deionized water was added and mixed for 1 min. Then added MnCl2, then added BHA with the mineral for 3 min each, and then the frother was added for 1 min. The flotation time was 3 min. Finally, the concentrate and tailings were filtered, dried, and weighed, and the percent recovery was calculated.
2.3. UV–Vis Spectrophotometer Measurements
The adsorption spectra were obtained on a UV-2700 UV-visible spectrophotometer for samples with varying collector concentrations to test for the adsorption of BHA in the presence or absence of Mn2+. Two series of samples were prepared. The general experimental process was as follows: 1 g of a mineral sample was weighed into a beaker, to which 40 mL of deionized water was added. Added the reagents according to the flotation reagent system The mixture was stirred for 10 min, allowed to stand for 5 min, and then filtered and centrifuged. The residual BHA concentration in the supernatant was then obtained via spectrophotometric determination, using the following formula:
where τ is the adsorption of BHA on the scheelite surface in units of mol/g; C0 is the initial concentration of BHA in the solution in units of mol/L; C is the concentration of BHA in the solution after reaction in units of mol/L; V is the volume of the solution in mL; and m is the weight of the mineral sample in units of g.
2.4. Fourier Infrared Spectroscopy Analysis
The Nicolet FTIR-740 FTIR spectrometer (Nicolet iS50, Thermo Fisher, Waltham, MA, USA) was used for analysis by the diffuse reflectance method with a wavelength measurement range of 400–4000 cm−1 at ambient temperature, 20 ± 5 °C. Four samples–one of pure scheelite; one of pure BHA; one of scheelite and BHA; and one of scheelite, BHA, and Mn2+–were prepared according to the following procedure. Each 2.0-g mineral sample (<38 μm) was placed in a 100-mL beaker, and then deionized water was added to a total volume of 40 mL. Added the reagents according to the flotation reagent system. The reaction mixture was stirred for 30 min after each reagent was added, then allowed to stand, and filtered and dried. The dried ore sample and potassium bromide powder were ground evenly in an agate mortar and pressed.
2.5. Zeta Potential Measurements
The zeta potentials were measured by a Malvern Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK). After grinding the scheelite to a particle size of less than 2 μm, four samples were prepared by adding 50 mg of the sample to 100 mL of a KCl solution (5 × 10−3 mol/L). The pH was adjusted by adding HCl and NaOH. Four samples–one of pure scheelite; one of scheelite and BHA; one of scheelite and Mn2+; and one of scheelite, BHA and Mn2+–were prepared according to the following procedure: The reagent was added, and each reagent was stirred for 5 min. Finally, the solution was allowed to stand for five minutes, and then an appropriate amount of supernatant was transferred to the electrophoresis pool for measurement of the zeta potential. Each sample was measured no less than three times under the same conditions; the average measurement is used in the analysis.
2.6. XPS Analysis
Four samples–one of pure scheelite; one of scheelite and BHA; one of scheelite and Mn2+; and one of scheelite, BHA and Mn2+–were prepared according to the following procedure: A 2.0 g sample of ground scheelite was placed into a beaker with 40 mL of deionized water, and flotation reagents were added. The mixture was stirred for 30 min after each reagent was added. Finally, the mixture was isolated by gravity filtration using qualitative filter paper (The filter paper brand is “New Star” brand, is a medium speed qualitative filter paper) and dried. The sample was then analyzed on a PHI5000 Versaprobe II multifunctional scanning imaging photoelectron spectrometer (PHI 5000, ULVAC-PHI, Osaka, Japan). The experimental data were analyzed by MultiPak software (Version 9.9.2, ULVAC-PHI, Osaka, Japan), using C 1 s (284.8 eV) to standardize the energy correction. The composition of the elements on the mineral surface was obtained by peak fitting, and the atomic concentration ratio of the elements on the mineral surface was calculated semi-quantitatively.
3. Results and Discussions
3.1. Micro–Flotation Tests
Before studying the effect of Mn2+, we first studied the effect of pulp pH on scheelite flotation in BHA system. The effects of pH on flotation recovery of scheelite at the BHA concentration of 1.0 × 10−3 mol/L were exhibited in Figure 2. The results showed that the best flotation index of scheelite was obtained in BHA system under alkaline conditions. The recovery rate between pH 8–10 was the highest, which was consistent with the previous research results [21,22]. Therefore, in subsequent experiments, we have chosen this pH range for the experiment.
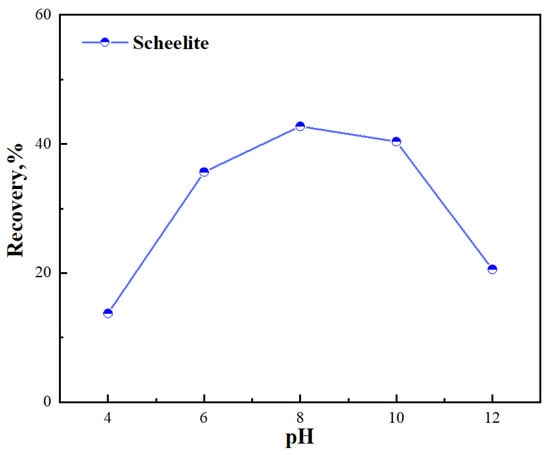
Figure 2.
Effect of pulp pH on flotation recovery of scheelite in BHA system.
To explore the effect of the addition of Mn2+ on scheelite flotation, the concentration of BHA was set to 1 × 10−3 mol/L and the concentration of Mn2+ was varied from 0 to 3 × 10−4 mol/L. The results, plotted in Figure 3, showed that the flotation recovery of scheelite, which occurred at a rate of 41.84% recovery in the absence of Mn2+, increases with the increase in Mn2+ concentration within this range: the recovery could be increased from 41.84% to 84.86% by increasing the concentration of Mn2+ from 0 to 3 × 10−4 mol/L. The value of the increase in the flotation recovery appeared to level off at higher concentrations; the recovery of scheelite was 82.28% with an Mn2+ concentration of 2 × 10−4 mol/L, but the recovery increased only slightly, to 84.86%, when the concentration of Mn2+ reached 3 × 10−4 mol/L. For future tests, setting the concentration of Mn2+ to 2 × 10−4 mol/L would optimize the recovery while minimizing the consumption of Mn2+. These results implied that Mn2+ could enhance the adsorption capacity of BHA onto the surface of scheelite, which then can float better and achieve a better flotation index and thus a higher recovery.
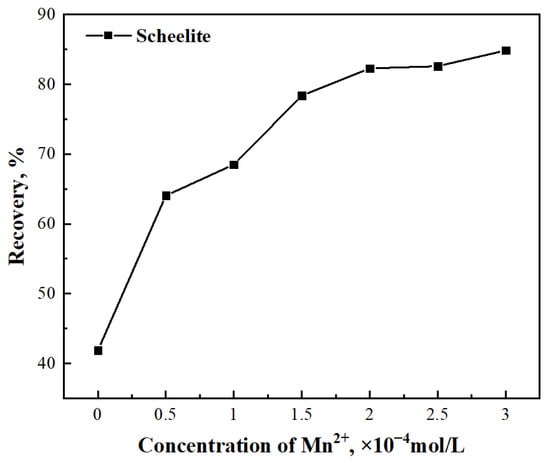
Figure 3.
Effect of Mn2+ concentration on the recovery of scheelite flotation.
In the third set of micro–flotation experiments, the Mn2+ concentration was set to 2 × 10−4 mol/L, and the initial concentration of BHA was varied, as described in Section 2.1. The plot in Figure 4. showed the percent recovery of BHA for each initial concentration of BHA without (represented by the blue squares) or with (represented by the red circles) added Mn2+. The results showed that without the addition of Mn2+, when the concentration of BHA increased within the range of 2.5 × 10−4 mol /L to 2.25 × 10−3 mol/L, the recovery of scheelite increased from 4.25% to 78.43% and tended to level off at higher concentrations. However, in the same BHA concentration range, after adding Mn2+, the recovery of scheelite had improved significantly, from 6.87% to 98.40%. In the absence of Mn2+, the maximum recovery of scheelite was only 78.43%, but after adding Mn2+, a significantly greater quantity of scheelite could be floated, leading to improved percent recovery. When the concentration of BHA was 1.0 × 10−3 mol/L, the recovery was increased by 38.93% in the sample with Mn2+ compared with that in the sample without Mn2+. Thus, the absorption of Mn2+ on the surface of scheelite was shown to increase the activation of scheelite significantly. The initial concentration of BHA required for a recovery of approximately 80% was 2.25 × 10−3 mol/L in the absence of Mn2+, but the required concentration of BHA was reduced to 1.0 × 10−3 mol/L when Mn2+ was added, proving that the addition of Mn2+ could reduce the amount of BHA consumption, thereby reducing costs.
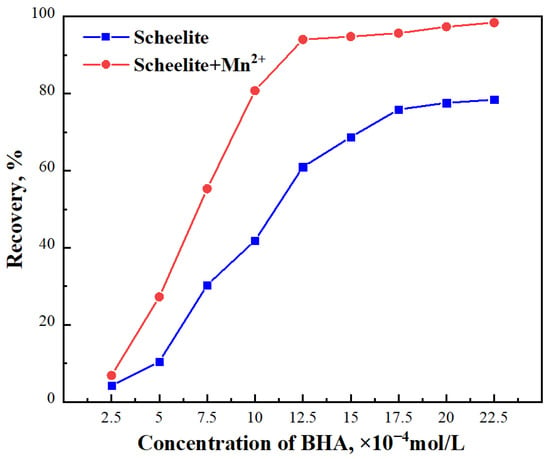
Figure 4.
Effect of BHA concentration on the recovery of scheelite flotation.
3.2. UV–Vis Analysists
To further explored the flotation test results, the adsorption amount of BHA on the surface of scheelite was determined for different reagent systems, as described below. To study the effect of Mn2+ on the adsorption of BHA, the concentration of Mn2+ was set to 2 × 10−4 mol/L, and the concentration of BHA was increased (Figure 5, pink bars), giving a concomitant increased in the adsorption capacity of BHA that was greater than the increase with concentration of BHA observed in samples without Mn2+ (Figure 5, blue bars). In the range of 5.0 × 10−4 mol/L to 1.5 × 10−3 mol/L, the adsorption capacity increased from 1.44 mol/g to 4.16 mol/g. Moreover, the adsorption capacity of Mn2+ treated BHA on the surface of scheelite was significantly higher than that of the untreated one. In the same BHA concentration range, the adsorption capacity increased from 2.88 mol/g to 7.04 mol/g upon the addition of Mn2+. When the BHA concentrations were 1.25 × 10−3 mol/L and 1.5 × 10−3 mol/L, the adsorption capacity increased the most dramatically, up to 2.88 mol/g. The results, graphed in Figure 5, showed that the adsorption amount of BHA on the surface of scheelite increases with the increase in BHA concentration and increases further with the addition of Mn2+. In general, the addition of Mn2+ to a scheelite sample could reduce the dosage of BHA required to achieve a desired recovery relative to a sample with similar BHA adsorption capacity but with no Mn2+, which was consistent with the micro–flotation results.
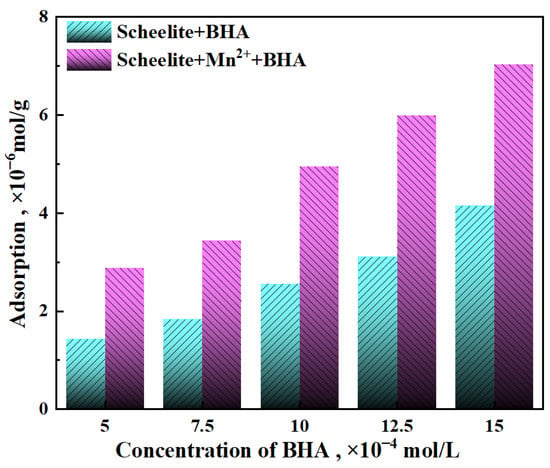
Figure 5.
Effect of Mn2+ (2 × 10−4 mol/L) on the adsorption of BHA on minerals surfaces.
3.3. Infrared Spectral Analysis
Different chemical bonds or functional groups have characteristic infrared absorption frequencies [23,24]. The mechanism of action of Mn2+ and BHA on the surface of scheelite can be studied by the detection of the characteristic frequencies of specific bonds or functional groups through infrared spectroscopy. The infrared spectra of samples of pure scheelite, pure BHA, scheelite with added BHA, and scheelite with added BHA and Mn2+ were shown in Figure 6.
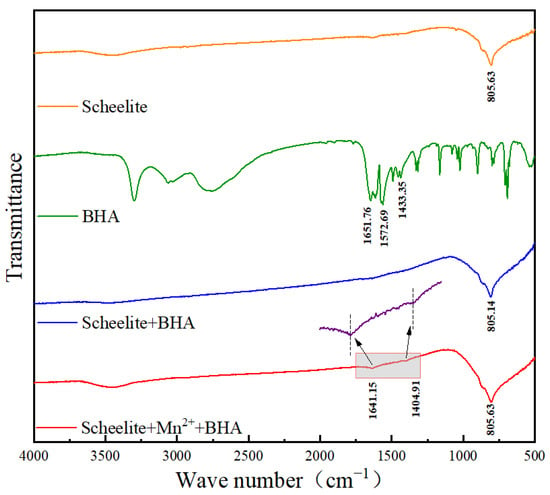
Figure 6.
Infrared spectra of pure scheelite; pure BHA; scheelite with added BHA; and scheelite with added BHA and Mn2+.
The asymmetric stretching vibration peak and out–of–plane bending vibration absorption peak of the tungstate W–O bond appeared at approximately 805 cm−1 [15]. The infrared spectrum of scheelite after its reaction with BHA did not change significantly, indicating that the reaction between scheelite and BHA was weak [25]. However, after pretreatment with Mn2+ followed by treatment with BHA, new peaks appeared at 1641.15 cm−1 and 1404.91 cm−1 that can be attributed to C=O and C–H bonds from BHA [26,27,28]. The presence of these bonds indicates that, after adding Mn2+, BHA can be better adsorbed on the surface of scheelite, as represented by Mn–BHA. The infrared spectra of scheelite with and without Mn2+ showed that the characteristic peak of BHA could be detected at a high intensity on the surface of scheelite after adding Mn2+. This result confirmed that BHA had high adsorption capacity and strength on the surface of scheelite in the presence of Mn2+ [29].
3.4. Zeta Potential Measurements
To further illustrated the effect of Mn2+ addition on the flotation behavior of scheelite, the surface zeta potentials were obtained for the four samples prepared as described in Section 2.5: pure scheelite; scheelite with added BHA; scheelite with added Mn2+; and scheelite with added Mn2+ and BHA. Figure 7 showed the zeta potential for each of these samples over a pH range of 4 through 12. The zeta potential of the scheelite in the absence of BHA and Mn2+ was −21.5 mV at pH = 4; as the pH increased, the potential gradually decreased. When the pH = 12, the potential dropped to −29.37 mV, which was consistent with the observations of previous researchers [30], indicating that the negatively charged species in the solution were adsorbed on the surface of scheelite. After adding only BHA, the zeta potential had decreased to −23 mV at pH = 4. As the pH increased, the surface potential had been shifted negatively relative to that obtained in the absence of BHA. This was because the solution of C7H6NO2− adsorbed on the scheelite surface, causing the scheelite surface zeta potential negative shift, which proved that the scheelite surface could adsorb BHA [31,32]. After adding only Mn2+, the positively charged Mn2+ ions were adsorbed on the scheelite surface, causing a positive shift of the zeta potential; at pH = 10, the potential reached its highest value: −9.4 mV. In the presence of both Mn2+ and BHA, the zeta potential of the scheelite surface decreased significantly compared to that in the sample containing Mn2+ alone; again, the trend of decreasing zeta potential with increasing pH was observed, and the potential at pH = 10 was −20.37 mV. Compared to pure scheelite, after Mn2+ and BHA treatment of scheelite surface potential was on the rise. This indicated that the dominant species adsorbed on the surface of scheelite were positively charged [33]. combined with infrared spectroscopy, the surface of scheelite should be Mn-BHA adsorption, and this adsorption is stronger than the BHA alone. The zeta potential difference between the samples with and without BHA was only −1.23 mV at pH = 10, but in the presence of Mn2+, the corresponding potential difference between the samples with and without BHA increased significantly, reaching −10.97 mV. The results of the zeta potential analysis indicated that more BHA could be adsorbed on the scheelite surface after adding Mn2+, which was consistent with the UV results and the micro−flotation results.
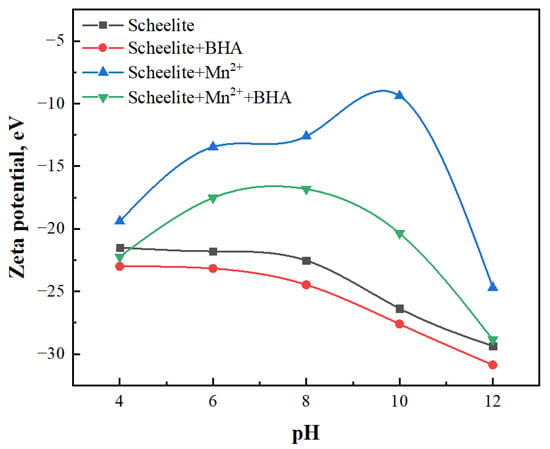
Figure 7.
Zeta potential of scheelite in the presence of different flotation reagents.
3.5. XPS Analysis
The above techniques revealed the effect of substituents on the mineral surface of scheelite on flotation behavior after interaction with reagents. XPS testing was conducted to obtain detailed chemical information about the presence of various elements and the mineral surface composition. Therefore, we carried out XPS detection to illustrate the adsorption mechanism of Mn2+ and BHA on the surface of scheelite. Figure 8 showed the full XPS spectra for the four samples under study, and Figure 9 presented pie charts of the relative atomic composition of the surfaces of each sample. The four samples analyzed were prepared as described in Section 2.6: pure scheelite; scheelite with added BHA; scheelite with added Mn2+; and scheelite with added Mn2+ and BHA.
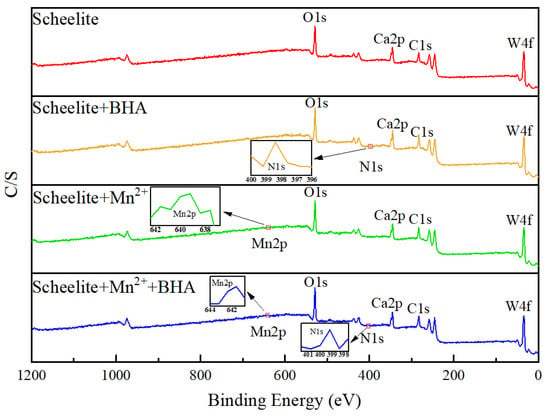
Figure 8.
XPS spectra of scheelite treated with different conditions.
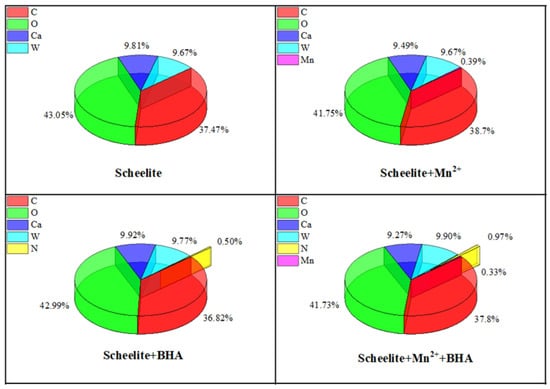
Figure 9.
Relative atomic content on the surface of minerals.
As shown in Figure 8, the Mn 2p peak appeared on the mineral surface after Mn2+ treatment. After adding BHA, a new N 1 s peak appeared on the mineral surface [34]. After adding BHA to the scheelite treated with Mn2+, new N 1 s and Mn 2p peaks appeared on the mineral surface. However, the new peaks of N 1 s appeared when only BHA was added to the scheelite surface, or when Mn2+ was added before BHA, which did not explain the specific mechanism of the addition of Mn2+ to scheelite. Therefore, further analysis of different elements was needed.
As can be seen from Figure 9, the concentration of N atoms on the surface of scheelite increased from 0.50% to 0.97% after the addition of Mn2+, indicating that the addition of Mn2+ can promote the reaction between the scheelite and BHA, thereby improving the surface properties of scheelite and enhancing its flotation [35]. As shown in Figure 10, the C 1 s peak on the surface of the non–BHA–treated scheelite had only two separate peaks after peak fitting, which were caused by hydrocarbon contamination on the surface of the scheelite at 284.78 eV and 284.79 eV, as well as the carbon-oxygen compounds at 287.09 eV and 286.98 eV [14,36]. After the addition of BHA, a new peak appears on the surface of the scheelite, which was attributed to the C-N bond in the BHA structure at 288.69 eV [5]. The appearance of the C-N species indicated that BHA experienced adsorption on the scheelite surface. Based on the information in Table 2, the atomic concentration of C-N species at 288.54 eV increased by 0.58% after the addition of Mn2+. The significant increase in C-N species on the surface indicated that the adsorption was enhanced after the addition of Mn2+.
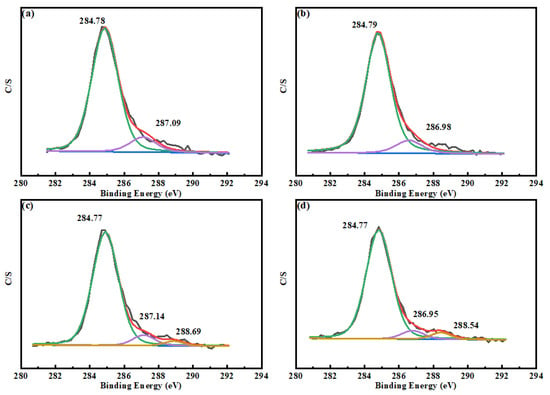
Figure 10.
C 1 s XPS spectra on the surface of (a) pure scheelite, (b) scheelite with added Mn2+, (c) scheelite with added BHA, and (d) scheelite with added Mn2+ and BHA.

Table 2.
Atomic concentration of C species on scheelite surfaces: (c) scheelite with added BHA and (d) scheelite with added Mn2+ and BHA.
Figure 11 gives the O 1 s XPS spectra for the four samples under study. As can be seen from Figure 11a, two peaks appear after the peak fitting of the O 1 s peak of the untreated scheelite, which are attributed to the W–O (530.43 eV) and Ca–O (532.06 eV) species on the surface of the scheelite [13,37]. After Mn2+ treatment (Figure 11b), no new peak was generated on the scheelite surface, but the content of the metal oxide species on the scheelite surface increased by 2.89%. It may be that the newly added Mn2+ adsorbed on the O species on the surface of scheelite and formed the Mn–O species [38]. A new peak appeared at 532.81 eV in scheelite after BHA treatment (Figure 11c), which was attributed to the adsorption of BHA on scheelite surface, thereby form a new hydrophobic component, and the new peak was due to OH in the hydrophobic component [1,39,40]. The binding energy of Ca–O was also shifted by 0.29 eV, indicating that there was a chemical interaction between BHA and Ca components, which also indicated that BHA formed a chemisorption on scheelite surface and formed a new substance. And that also led to the concentration of W–O and Ca–O bonds decreased by 2.57% and 3.85%, respectively. The appearance of the hydrophobic component indicated that BHA was adsorbed on the surface of scheelite. In the presence of both Mn2+ and BHA (Figure 11d), the content of metal oxide species increased by 3.08% compared with the content in the presence of only Mn2+, and the concentration of OH atoms increased by 1.61% compared with the content in the presence of only BHA. The binding energy of Ca–O was shifted by 0.21 eV, which indicated that the newly generated Mn-BHA can also chemically interact with scheelite surface [41]. According to the data in Table 3, the adsorption amount of Mn–BHA on scheelite surface was more than that of BHA alone. The results indicated that the presence of Mn2+ enhanced the adsorption of BHA on the surface of scheelite. The above results were consistent with the FTIR spectra, adsorption tests, and zeta potential results.
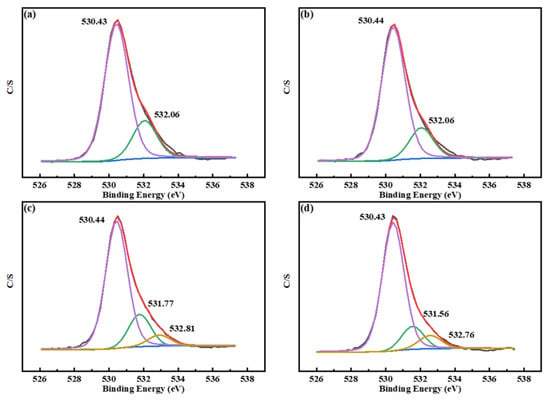
Figure 11.
O 1 s XPS spectra on the surface of (a) pure scheelite, (b) scheelite with added Mn2+, (c) scheelite with added BHA, and (d) scheelite with added Mn2+ and BHA.

Table 3.
Quantification of O 1 s species on scheelite surfaces: (a) pure scheelite, (b) scheelite with added Mn2+, (c) scheelite with added BHA, and (d) scheelite with added Mn2+ and BHA.
4. Conclusions
This paper reported a detailed study on the activation flotation of scheelite by Mn2+ in a BHA system. The activation mechanism of Mn2+ was inferred from the results of micro–flotation tests, adsorption tests, infrared measurements, zeta potential measurements and XPS analysis. The conclusions are as follows:
- (1)
- Mn2+ had a significant activation effect on the flotation of scheelite. The micro–flotation test and the adsorption test showed that the addition of Mn2+ was beneficial to the flotation of scheelite. The addition of Mn2+ increased the adsorption strength of BHA on the scheelite surface and can achieve the best flotation recovery (98.40%).
- (2)
- In order to further study the interaction mechanism between Mn2+ and scheelite surface, adsorption tests and infrared spectral analysis were carried out. These results showed that BHA could form Mn-BHA adsorption on scheelite surface after the addition of Mn2+, and this adsorption effect was stronger than that of BHA alone. And they implied that Mn2+ can increase the number of active sites on the scheelite surface, so that the more BHA was adsorbed, given rise to the higher strength of its characteristic peaks.
- (3)
- XPS analysis showed that when Mn2+ was added, the adsorption of BHA on scheelite surface increased, relative to the study in which only BHA were added, and therefore the amount of observed C–N species was also increased. After the addition of Mn2+, Mn–O chemical bonds were formed on the surface of the scheelite and promote the BHA to combine with scheelite surface to form a new hydrophobic component. The binding energy of Ca–O was shifted by 0.21 eV, which indicated that the newly generated Mn-BHA can also interact with scheelite surface, and the adsorption capacity of Mn-BHA on scheelite surface was more than that of BHA alone. The XPS results, combined with zeta potential analysis, indicated that adding Mn2+ can enhance BHA adsorption on the mineral surface and improve its adsorption stability.
The addition of Mn2+ promoted more adsorption of BHA on the scheelite surface, which contributed to the hydrophobic floating of the scheelite. These results provide research directions and theoretical basis for the flotation of scheelite and directions for further research. Further studies of the activation effect of some metal ions on scheelite and the effect of the interaction between activated scheelite and BHA on the flotation of scheelite are necessary to enable the faster application of this knowledge to the actual production of desirable tungsten–containing minerals.
Author Contributions
X.W.: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing—original draft, Writing—review & editing. S.W.: Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing—review & editing. Q.Z.: Formal analysis, Investigation, Methodology, Visualization, Writing—review & editing. R.L.: Investigation, Methodology, Visualization, Writing—review & editing. S.M.: Investigation, Methodology, Visualization, Writing—review & editing. Y.T.: Formal analysis, Investigation, Methodology. Z.S.: Visualization, Writing—review & editing. Q.F.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
The research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available within this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiao, F.; Dong, L.; Qin, W.; Liu, W.; Hu, C. Flotation separation of scheelite from calcite using pectin as depressant. Miner. Eng. 2019, 136, 120–128. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W.; et al. Application of a new amidoxime surfactant in flotation separation of scheelite and calcite: Adsorption mechanism and DFT calculation. J. Mol. Liq. 2022, 364, 120036. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, H.; Feng, B.; Zhang, L.; Chen, Y.; Gao, Z. Flotation separation of scheelite and apatite by polysaccharide depressant xanthan gum. Miner. Eng. 2021, 170, 107045. [Google Scholar] [CrossRef]
- Wang, X.; Jia, W.; Yang, C.; He, R.; Jiao, F.; Qin, W.; Cui, Y.; Zhang, Z.; Li, W.; Song, H. Innovative application of sodium tripolyphosphate for the flotation separation of scheelite from calcite. Miner. Eng. 2021, 170, 106981. [Google Scholar] [CrossRef]
- Liu, C.; Ni, C.; Yao, J.; Chang, Z.; Wang, Z.; Zeng, G.; Luo, X.; Yang, L.; Ren, Z.; Shao, P.; et al. Hydroxypropyl amine surfactant: A novel flotation collector for efficient separation of scheelite from calcite. Miner. Eng. 2021, 167, 106898. [Google Scholar] [CrossRef]
- Dong, L.; Wei, Q.; Qin, W.; Jiao, F. Effect of iron ions as assistant depressant of citric acid on the flotation separation of scheelite from calcite. Chem. Eng. Sci. 2021, 241, 116720. [Google Scholar] [CrossRef]
- Qi, J.; Zhao, G.; Liu, S.; Chen, W.; Liu, G. Strengthening flotation enrichment of Pb(Ⅱ)-activated scheelite with N-[(3-hydroxyamino)-propoxy]-N-hexyl dithiocarbamate. J. Ind. Eng. Chem. 2022, 114, 338–346. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.; Zhang, M.; Cui, R.; Shi, J.; Ning, J. Effects of Pb2+ ions on the flotation behavior of scheelite, calcite, and fluorite in the presence of water glass. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127826. [Google Scholar] [CrossRef]
- Kuang, J.; Zou, Z.; Huang, Z.; Liu, P.; Yuan, W.; Zhu, L. Surface dissolution of scheelite under different regulators and its effect on flotation behavior. Miner. Eng. 2021, 164, 106811. [Google Scholar] [CrossRef]
- Chen, C.; Sun, W.; Zhu, H.; Liu, R. A novel green depressant for flotation separation of scheelite from calcite. Trans. Nonferrous Met. Soc. China 2021, 31, 2493–2500. [Google Scholar] [CrossRef]
- Foucaud, Y.; Collet, A.; Filippova, I.V.; Badawi, M.; Filippov, L.O. Synergistic effects between fatty acids and non-ionic reagents for the selective flotation of scheelite from a complex tungsten skarn ore. Miner. Eng. 2022, 182, 107566. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, C.; Yin, W.; Luo, B. An investigation of the mechanism of using iron chelate as a collector during scheelite flotation. Miner. Eng. 2019, 131, 146–153. [Google Scholar]
- Wang, J.; Gao, Z.; Han, H.; Sun, W.; Gao, Y.; Ren, S. Impact of NaOL as an accelerator on the selective separation of scheelite from fluorite using a novel self-assembled Pb-BHA-NaOL collector system. Appl. Surf. Sci. 2021, 537, 147778. [Google Scholar] [CrossRef]
- Wang, C.; Ren, S.; Sun, W.; Wu, S.; Tao, L.; Duan, Y.; Wang, J.; Gao, Z. Selective flotation of scheelite from calcite using a novel self-assembled collector. Miner. Eng. 2021, 171, 107120. [Google Scholar] [CrossRef]
- Guan, Z.; Lu, K.; Zhang, Y.; Yang, H.; Li, X. Mechanism of manganese ion interaction with the surface of scheelite and calcite and its effect on flotation separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129397. [Google Scholar] [CrossRef]
- He, J.; Sun, W.; Zeng, H.; Fan, R.; Hu, W.; Gao, Z. Unraveling roles of lead ions in selective flotation of scheelite and fluorite from atomic force microscopy and first-principles calculations. Miner. Eng. 2022, 179, 107424. [Google Scholar] [CrossRef]
- Gao, Z.; Jiang, Z.; Sun, W.; Gao, Y. Typical roles of metal ions in mineral flotation: A review. Trans. Nonferrous Met. Soc. China 2021, 31, 2081–2101. [Google Scholar] [CrossRef]
- Liu, J.; Ejtemaei, M.; Nguyen, A.V.; Wen, S.; Zeng, Y. Surface chemistry of Pb-activated sphalerite. Miner. Eng. 2020, 145, 106058. [Google Scholar] [CrossRef]
- Jin, S.; Shi, Q.; Li, Q.; Ou, L.; Ouyang, K. Effect of calcium ionic concentrations on the adsorption of carboxymethyl cellulose onto talc surface: Flotation, adsorption and AFM imaging study. Powder Technol. 2018, 331, 155–161. [Google Scholar] [CrossRef]
- Zhao, G.; Zhong, H.; Qiu, X.; Wang, S.; Gao, Y.; Dai, Z.; Huang, J.; Liu, G. The DFT study of cyclohexyl hydroxamic acid as a collector in scheelite flotation. Miner. Eng. 2013, 49, 54–60. [Google Scholar] [CrossRef]
- Xiao, W.; Shao, Y.; Yu, J.; Zhang, B.; Shu, H.; Zhang, Y. Activation of ilmenite flotation by Al3+ in the benzohydroxamic acid (BHA) system. Sep. Purif. Technol. 2022, 299, 121770. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Han, H.; Cao, J.; Gui, X.; Xing, Y.; Zhang, C.; Zou, J. Enhanced electronic effect improves the collecting efficiency of benzohydroxamic acid for scheelite flotation. Miner. Eng. 2020, 152, 106308. [Google Scholar] [CrossRef]
- Han, G.; Wen, S.; Wang, H.; Feng, Q. Sulfidization regulation of cuprite by pre-oxidation using sodium hypochlorite as an oxidant. Int. J. Min. Sci. Technol. 2021, 31, 1117–1128. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, M.; Yang, B.; Feng, Q.; Liu, D. Enhanced sulfidization flotation mechanism of smithsonite in the synergistic activation system of copper–ammonium species. Miner. Eng. 2022, 187, 107796. [Google Scholar] [CrossRef]
- Han, H.; Hu, Y.; Sun, W.; Li, X.; Chen, K.; Zhu, Y.; Anh, V.N.; Tian, M.; Wang, L.; Yue, T.; et al. Novel catalysis mechanisms of benzohydroxamic acid adsorption by lead ions and changes in the surface of scheelite particles. Miner. Eng. 2018, 119, 11–22. [Google Scholar] [CrossRef]
- Sun, W.; Lan, L.; Zeng, H.; Zhou, J.; Ahmed, K.S.; Wang, L. Study on the flotation separation mechanism of diaspore from kaolinite using mixed NaOL/BHA collector. Miner. Eng. 2022, 186, 107719. [Google Scholar] [CrossRef]
- Eileen, R.L.; Espiritu, S.N.; Kristian, E.W. Surface chemistry and flotation behavior of dolomite, monazite and bastnäsite in the presence of benzohydroxamate, sodium oleate and phosphoric acid ester collectors. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 254–265. [Google Scholar]
- Al-Saadi, A.A. Conformational analysis and vibrational assignments of benzohydroxamic acid and benzohydrazide. J. Mol. Struct. 2012, 1023, 115–122. [Google Scholar] [CrossRef]
- Fu, J.; Han, H.; Wei, Z.; Liu, R.; Li, W.; Xu, T.; Ji, D. Selective separation of scheelite from calcite using tartaric acid and Pb-BHA complexes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126657. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.; Zhang, M.; Cui, R.; Shi, J.; Ning, J. Decoupling the effects of solid and liquid phases in a Pb-water glass mixture on the selective flotation separation of scheelite from calcite. Miner. Eng. 2020, 154, 106423. [Google Scholar] [CrossRef]
- Tian, M.; Liu, R.; Gao, Z.; Chen, P.; Han, H.; Wang, L.; Zhang, C.; Sun, W.; Hu, Y. Activation mechanism of Fe (III) ions in cassiterite flotation with benzohydroxamic acid collector. Miner. Eng. 2018, 119, 31–37. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Yang, Y.; Sun, W.; Hu, Y. Effect of Pb2+ ions on ilmenite flotation and adsorption of benzohydroxamic acid as a collector. Appl. Surf. Sci. 2017, 425, 796–802. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Wang, J. Selective flotation of scheelite from calcite using Al-Na2SiO3 polymer as depressant and Pb-BHA complexes as collector. Miner. Eng. 2018, 120, 29–34. [Google Scholar] [CrossRef]
- Luo, L.; Wu, H.; Xu, L.; Meng, J.; Lu, J.; Zhou, H.; Huo, X.; Huang, L. An in situ ATR-FTIR study of mixed collectors BHA/DDA adsorption in ilmenite-titanaugite flotation system. Int. J. Min. Sci. Technol. 2021, 31, 689–697. [Google Scholar] [CrossRef]
- Meng, Q.; Xu, Y.; Yuan, Z.; Zhao, X.; Du, Y. Separation mechanism of ilmenite from titanaugite with mixed BHA/NaOL collector. Miner. Eng. 2022, 176, 107363. [Google Scholar] [CrossRef]
- Huang, Z.; Kuang, J.; Yuan, W.; Yu, M.; Wang, X. Regulation mechanism of ultrasonication on surface hydrophobicity of scheelite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127412. [Google Scholar] [CrossRef]
- Wang, R.; Wei, Z.; Han, H.; Sun, W.; Hu, Y.; Wang, J.; Wang, L.; Liu, H.; Yang, Y.; Zhang, C.; et al. Fluorite particles as a novel calcite recovery depressant in scheelite flotation using Pb-BHA complexes as collectors. Miner. Eng. 2019, 132, 84–91. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, Z.; Meng, Q.; Zhao, X.; Du, Y. Study on the flotation behavior and interaction mechanism of ilmenite with mixed BHA/NaOL collector. Miner. Eng. 2021, 170, 107034. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, L.; Gao, Z.; Sun, W.; Cao, X. Activation mechanism of zinc ions in cassiterite flotation with benzohydroxamic acid as a collector. Miner. Eng. 2020, 156, 106523. [Google Scholar] [CrossRef]
- Shu, K.; Xu, L.; Wu, H.; Peng, L.; Xu, Y.; Luo, L.; Yang, J.; Tang, Z. In situ adsorption of mixed collectors BHA/DDA in spodumene-feldspar flotation system. Sep. Purif. Technol. 2020, 251, 117325. [Google Scholar] [CrossRef]
- Meng, Q.; Feng, Q.; Shi, Q.; Ou, L. Studies on interaction mechanism of fine wolframite with octyl hydroxamic acid. Miner. Eng. 2015, 79, 133–138. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).