Numerical Modeling and Analysis of Harvesting Atmospheric Water Using Porous Materials
Abstract
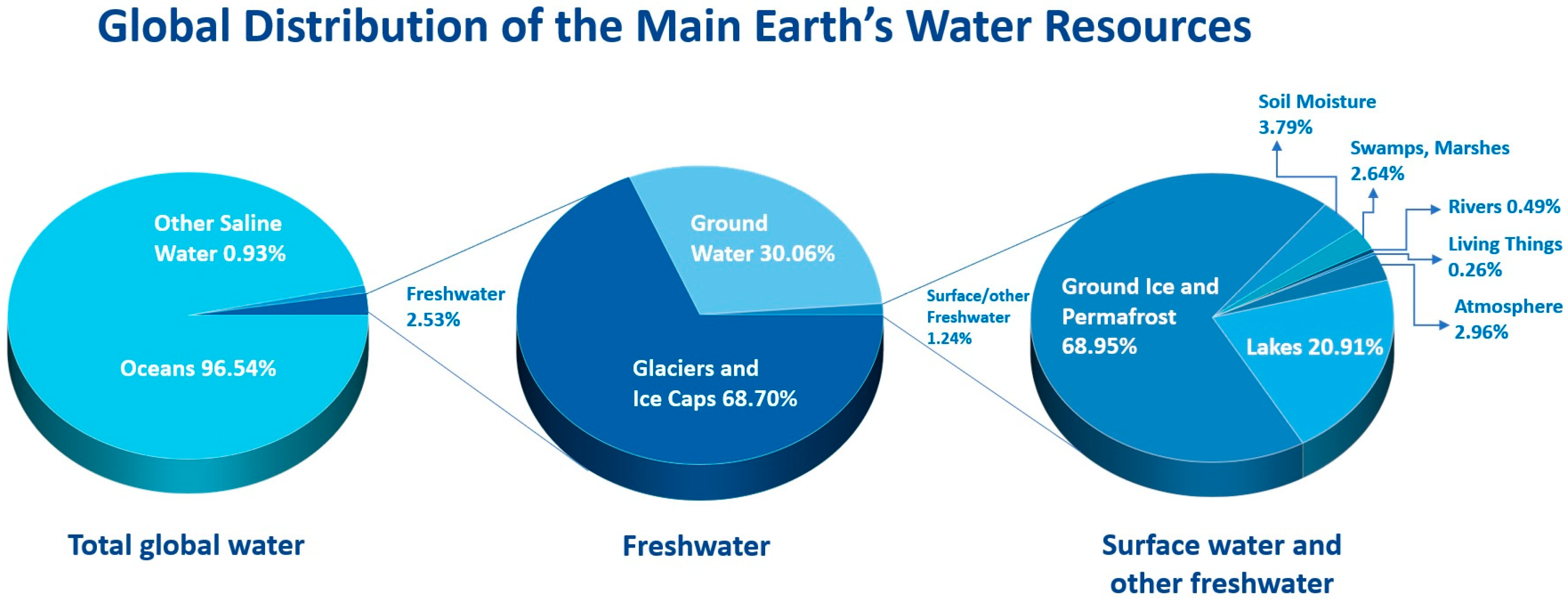
1. Introduction
1.1. Overview
1.2. Research Motivation and Objectives
1.3. Paper Outline
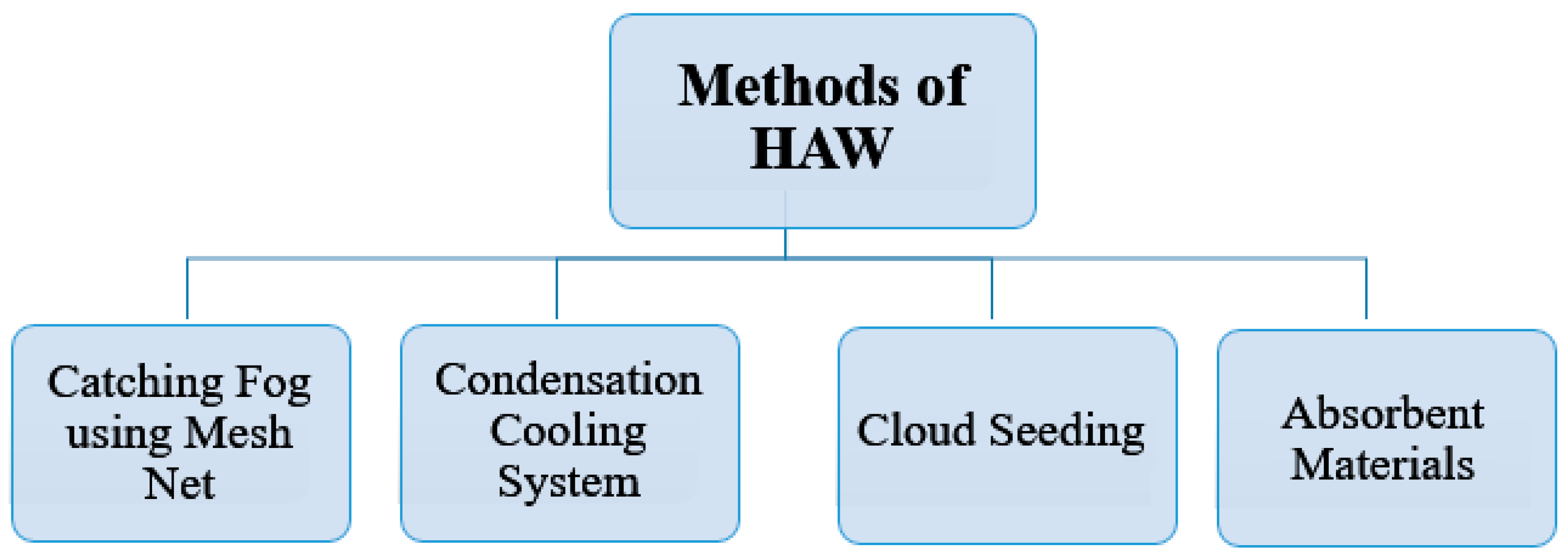
2. Literature Review
3. Mathematical Modeling
4. Porous Materials Used in HAW Model
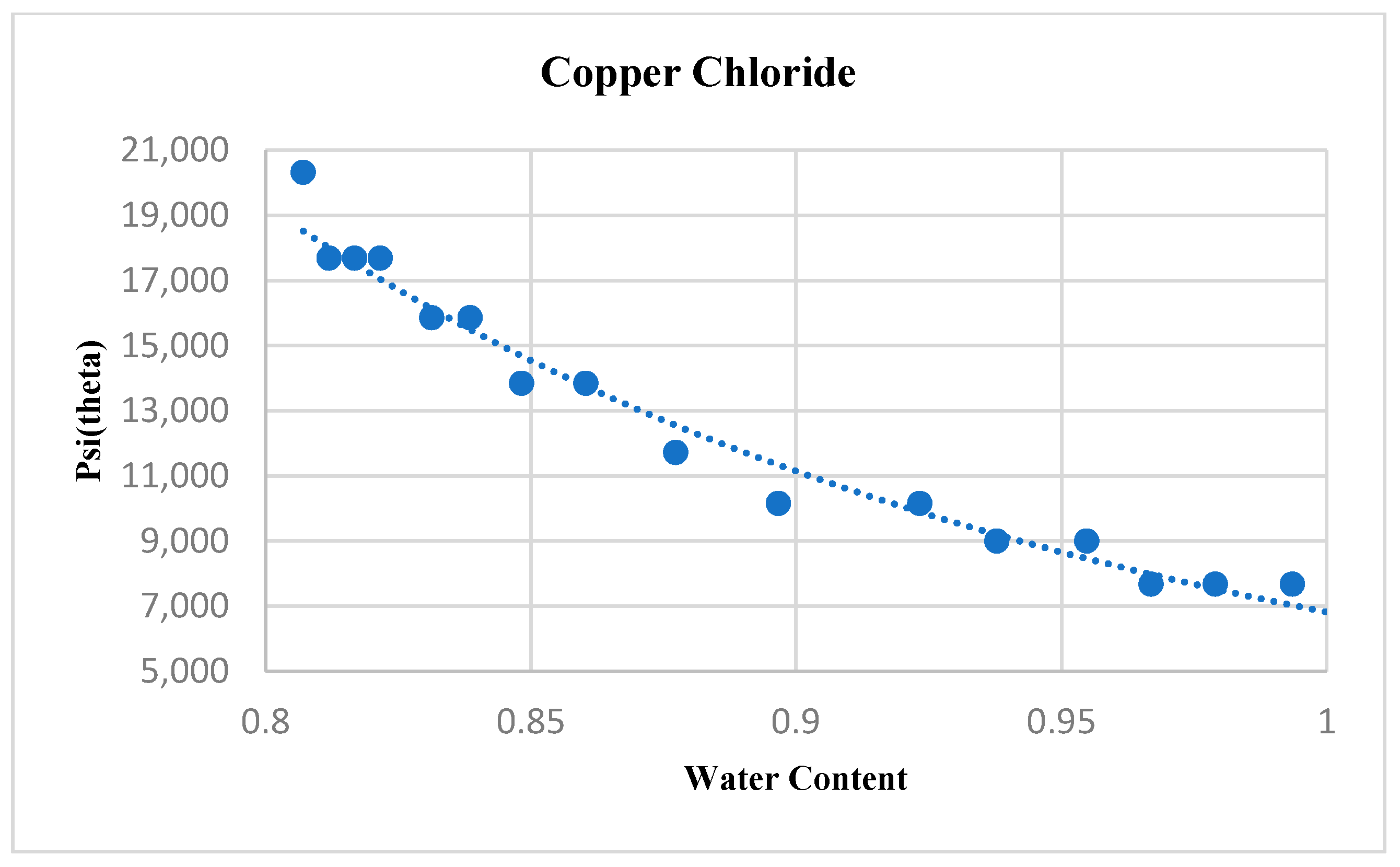
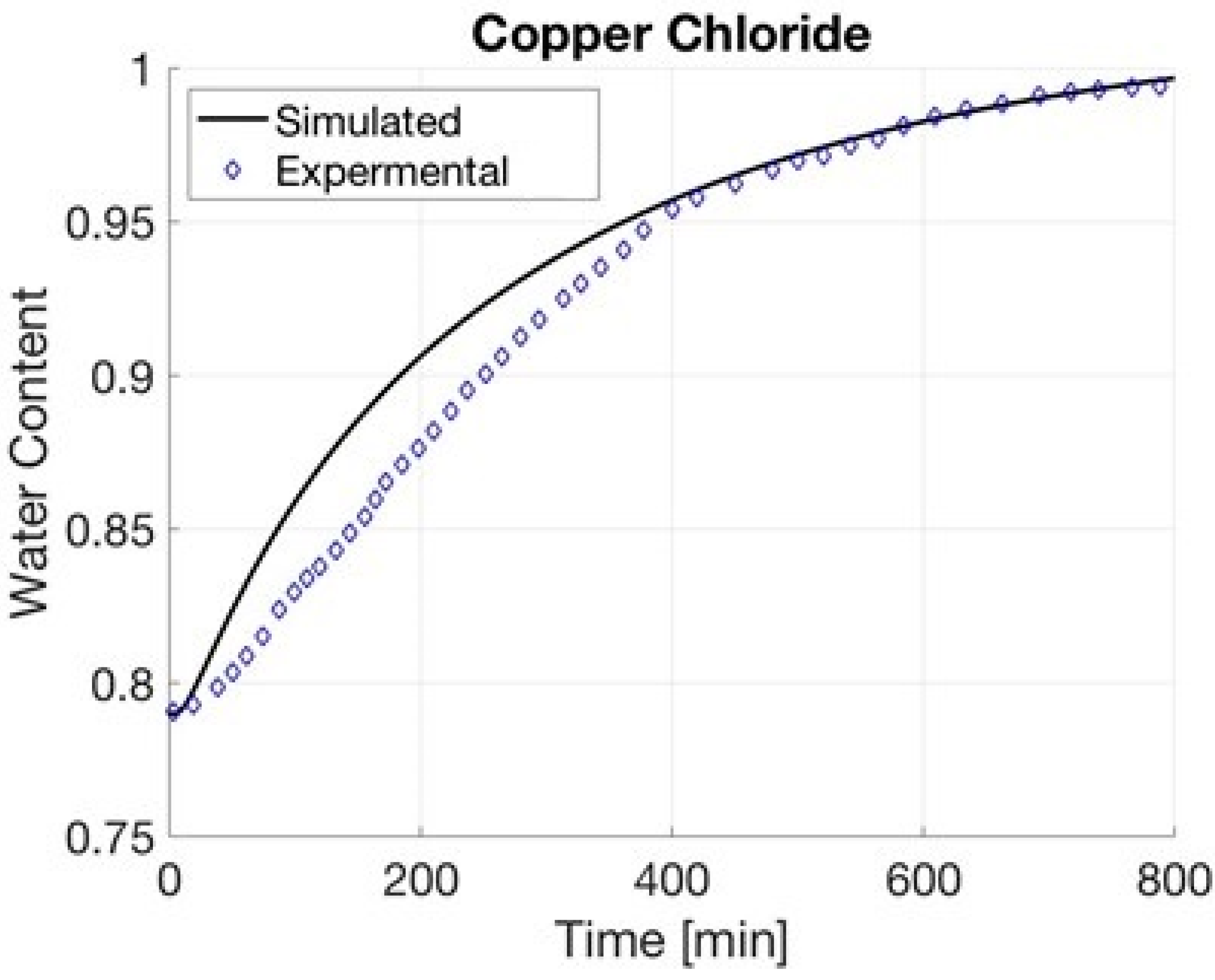
4.1. Copper Chloride
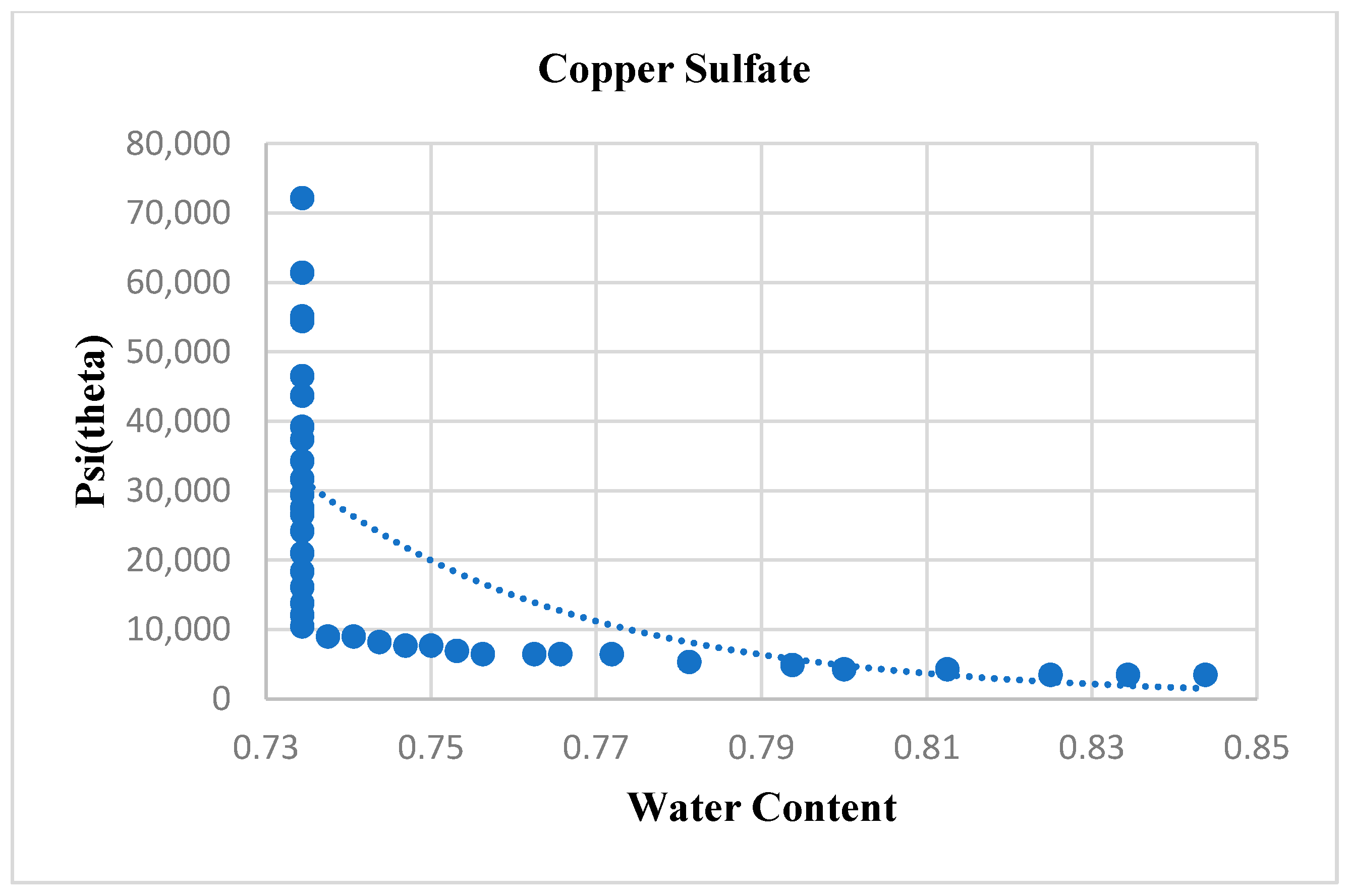
4.2. Copper Sulfate
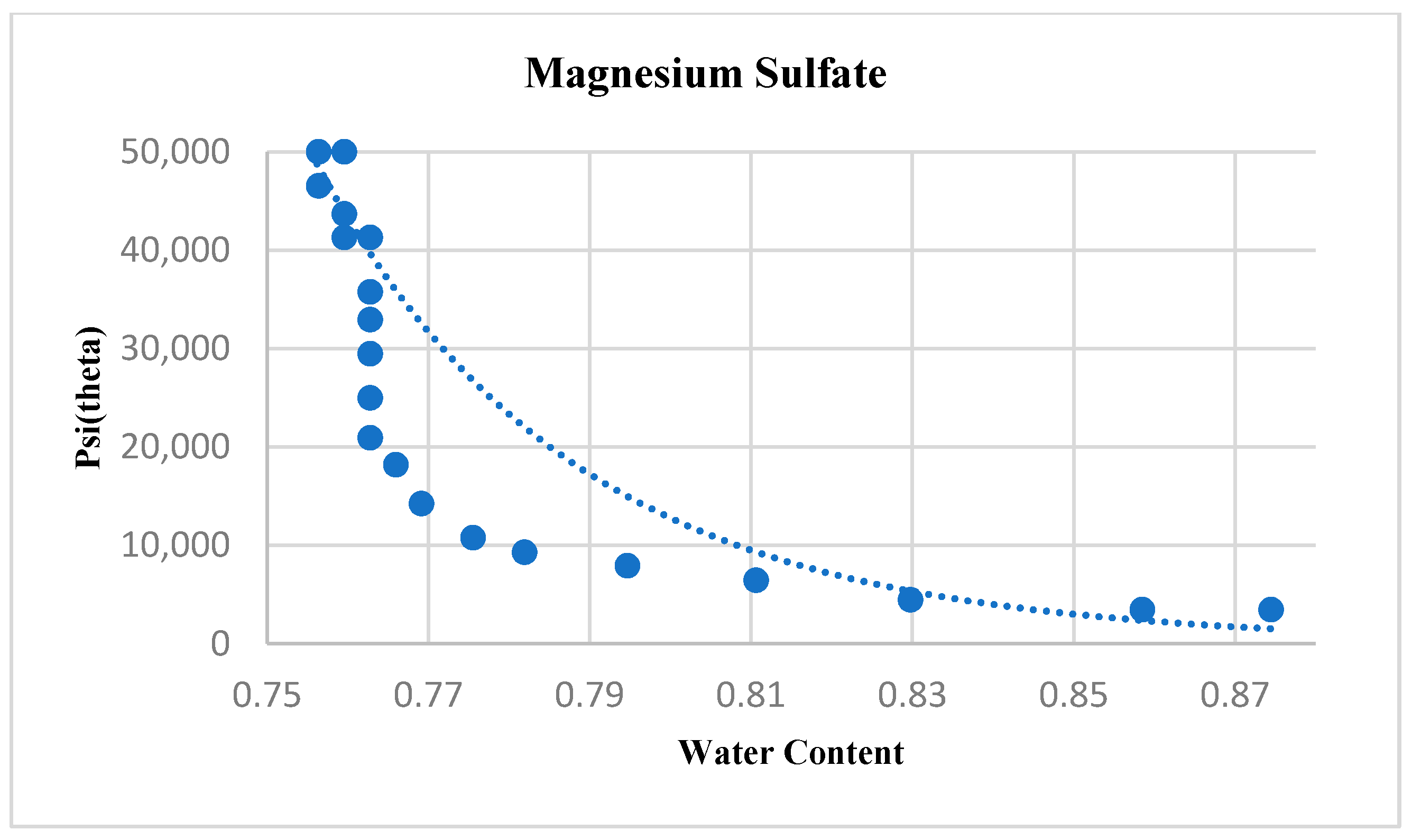
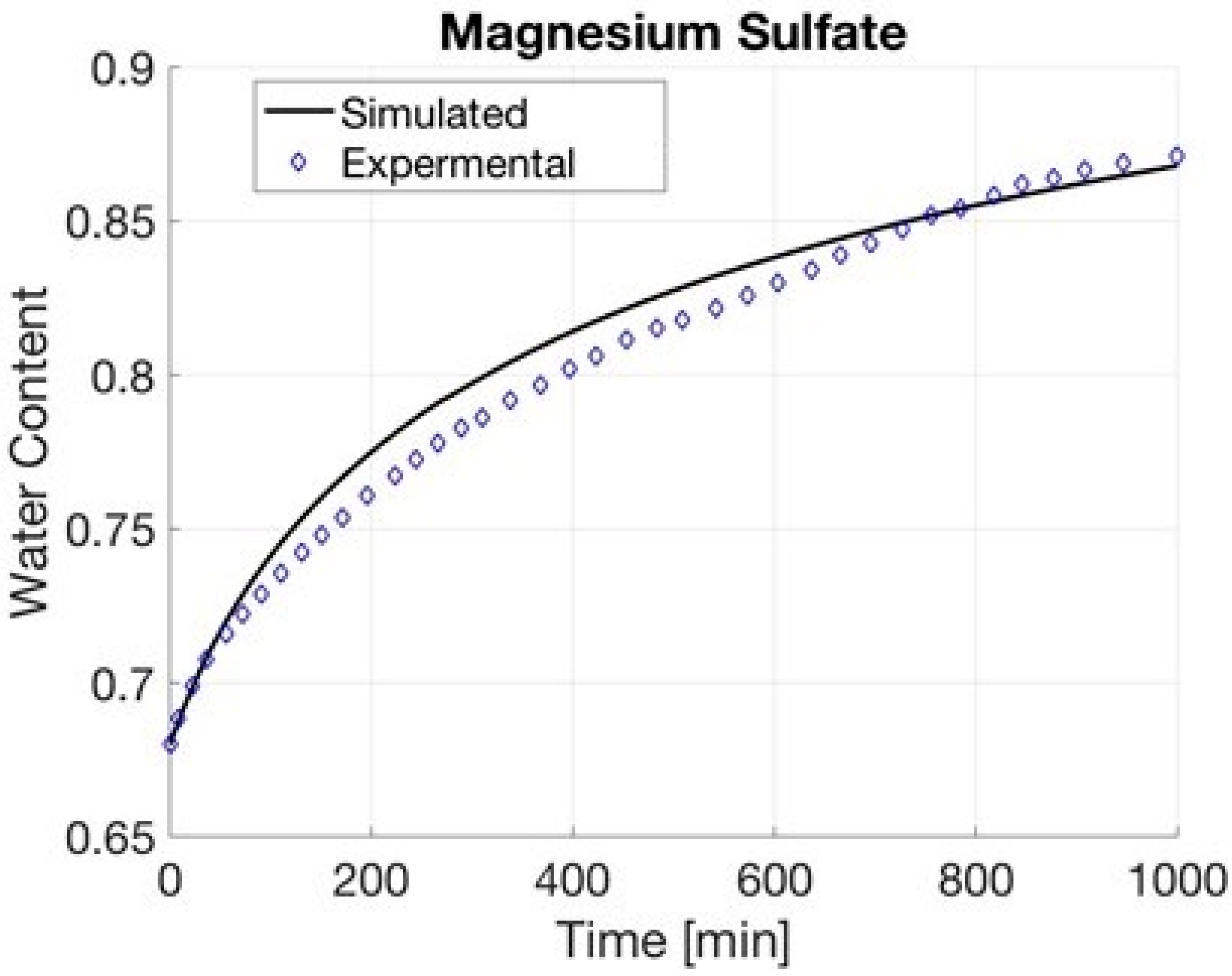
4.3. Magnesium Sulfate
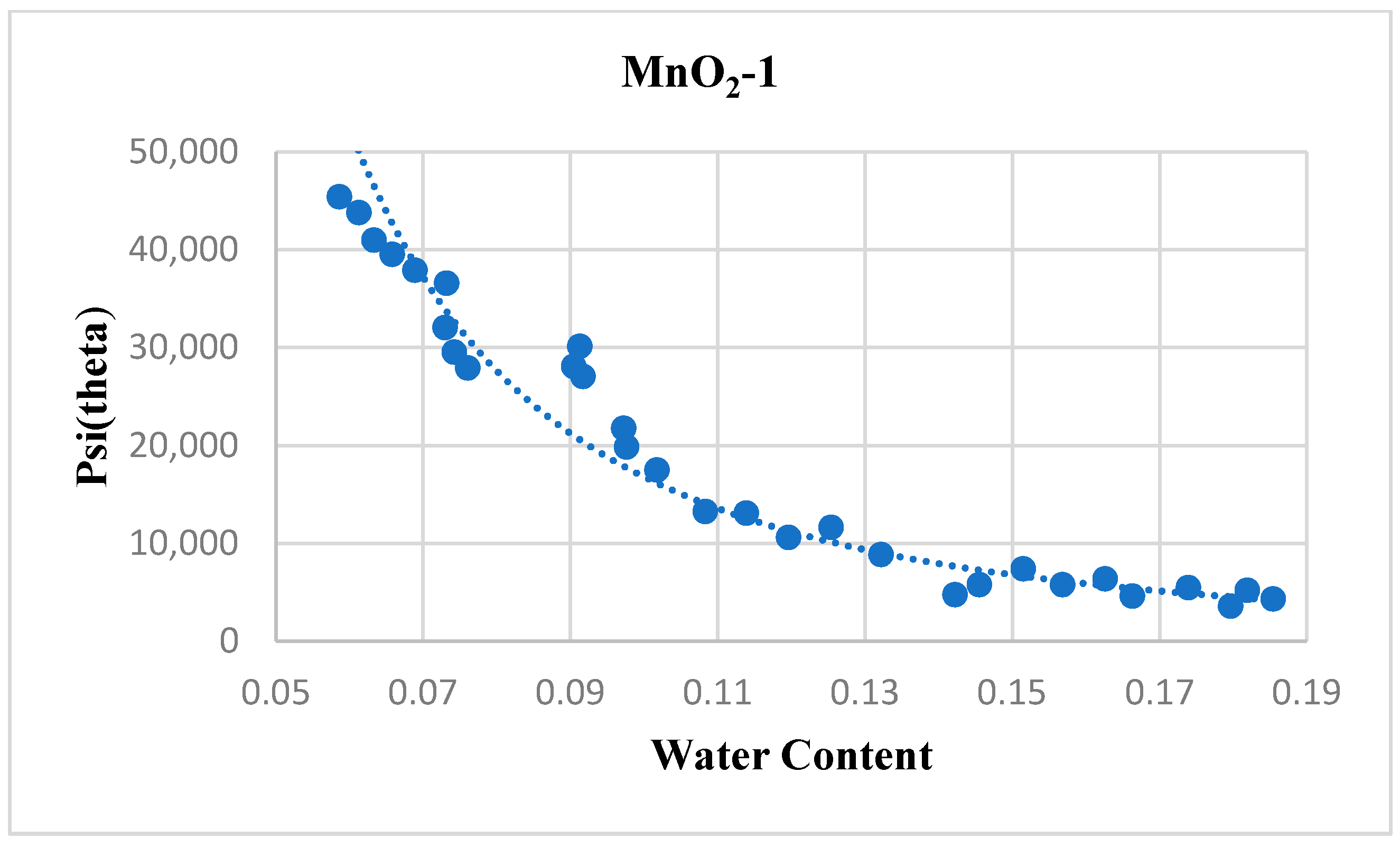
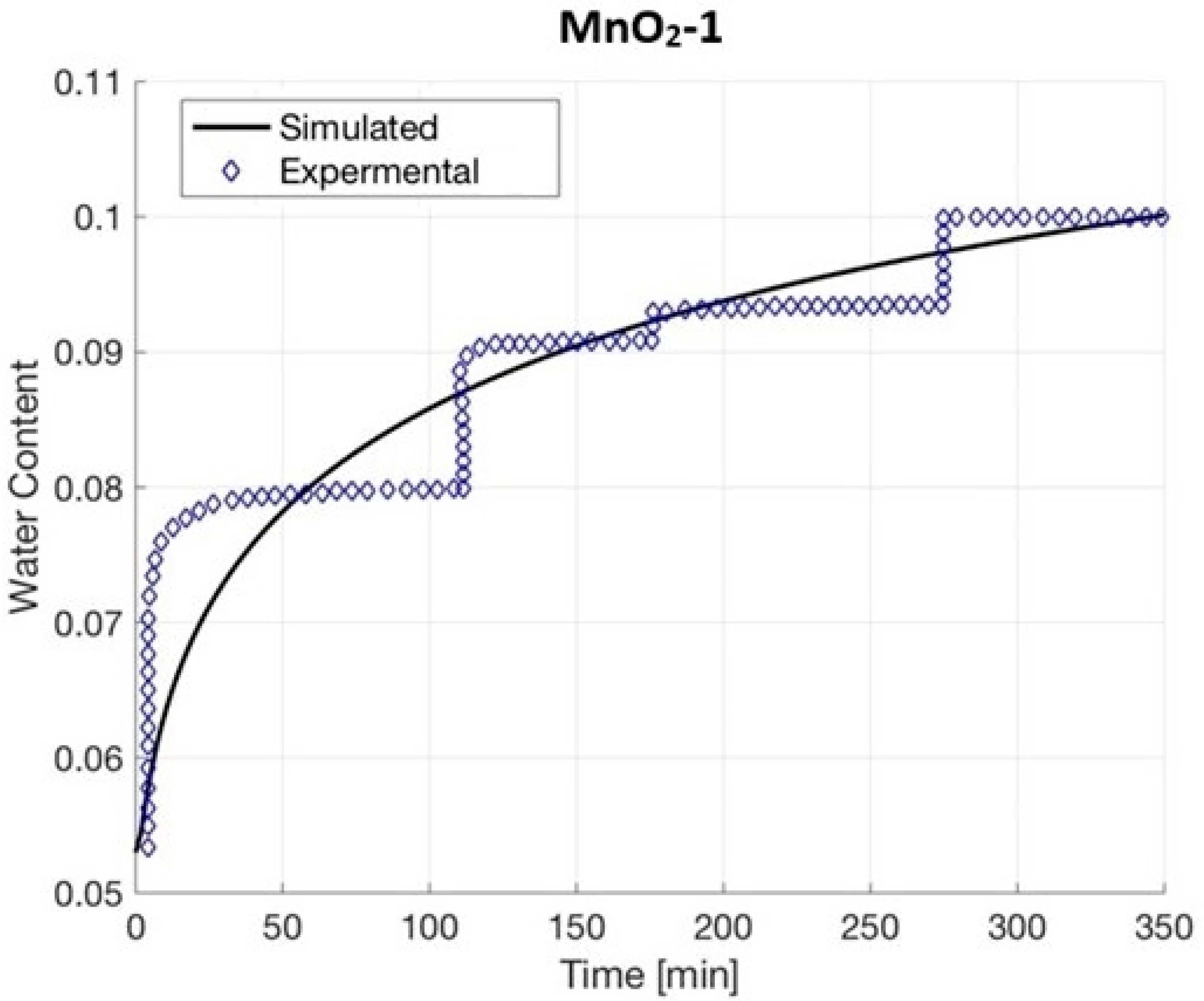
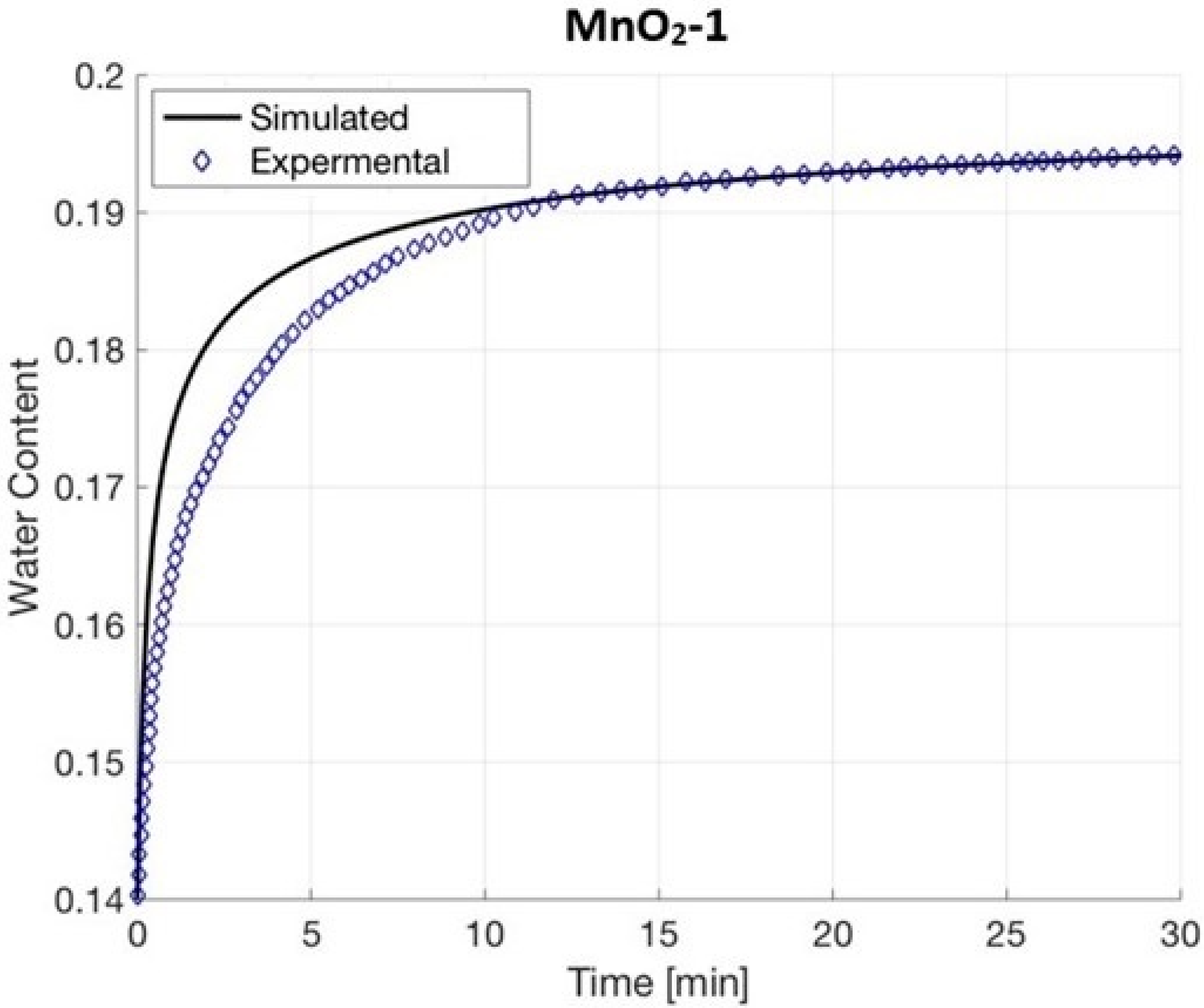
4.4. MnO2-1
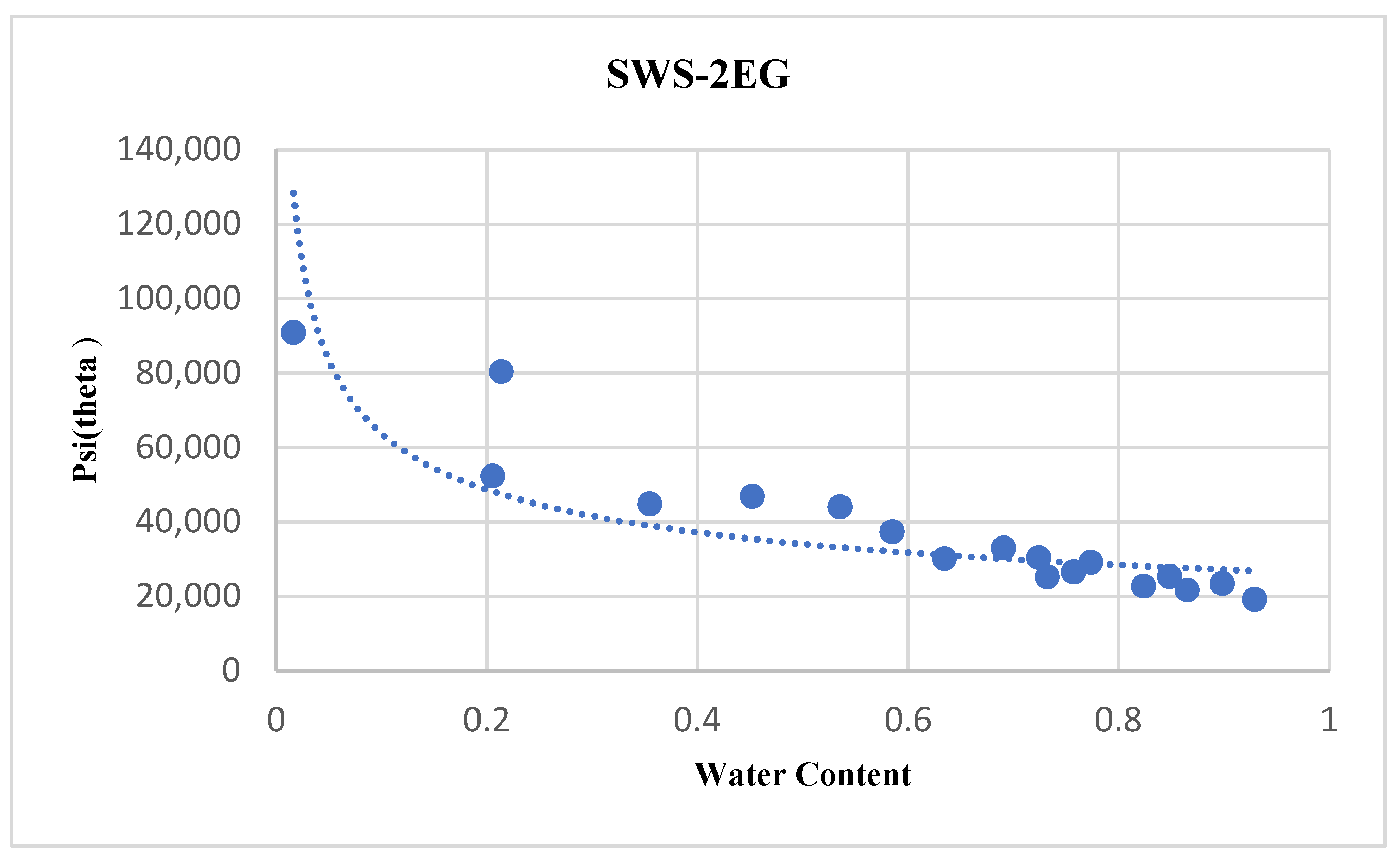
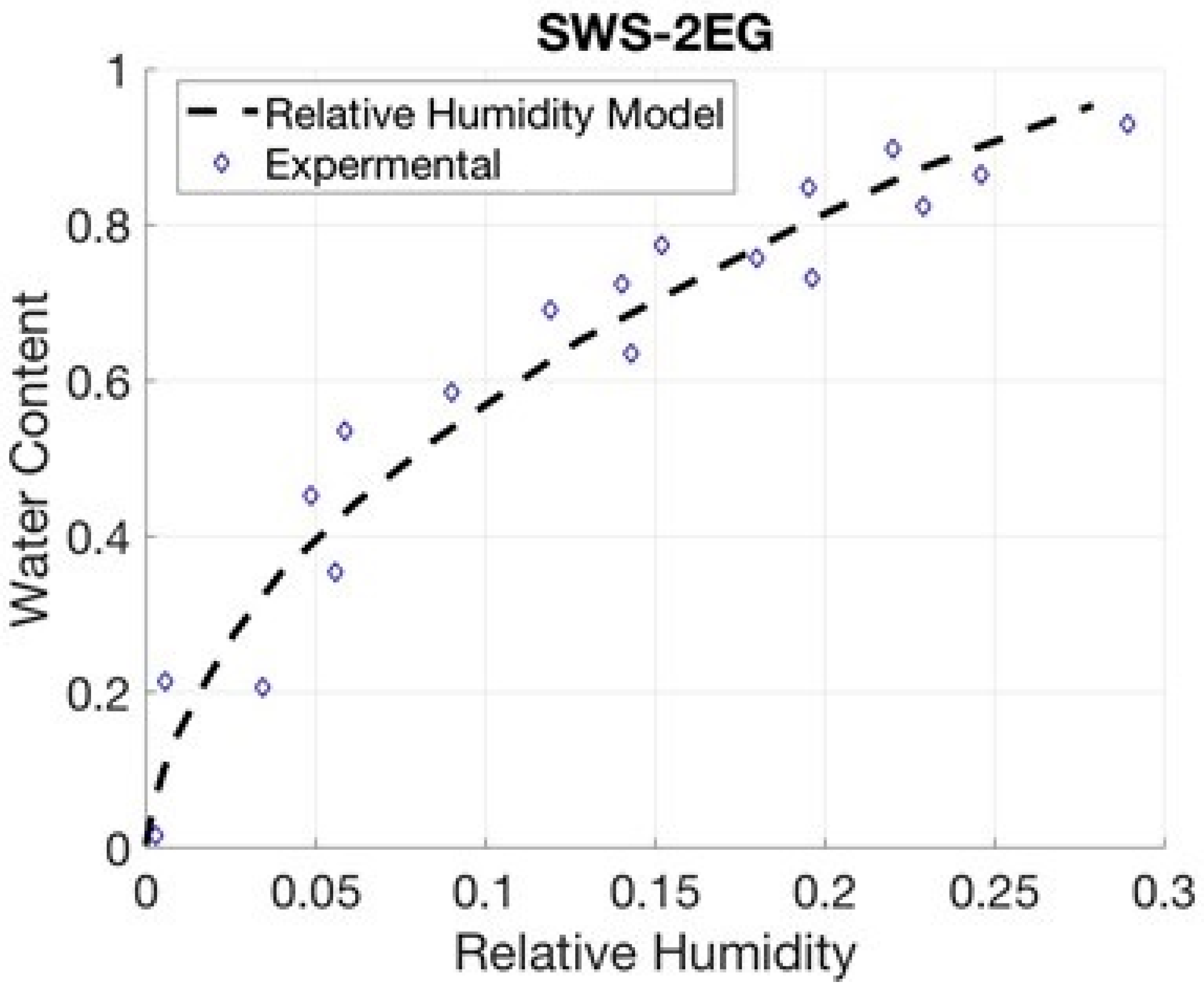
4.5. SWS-2EG
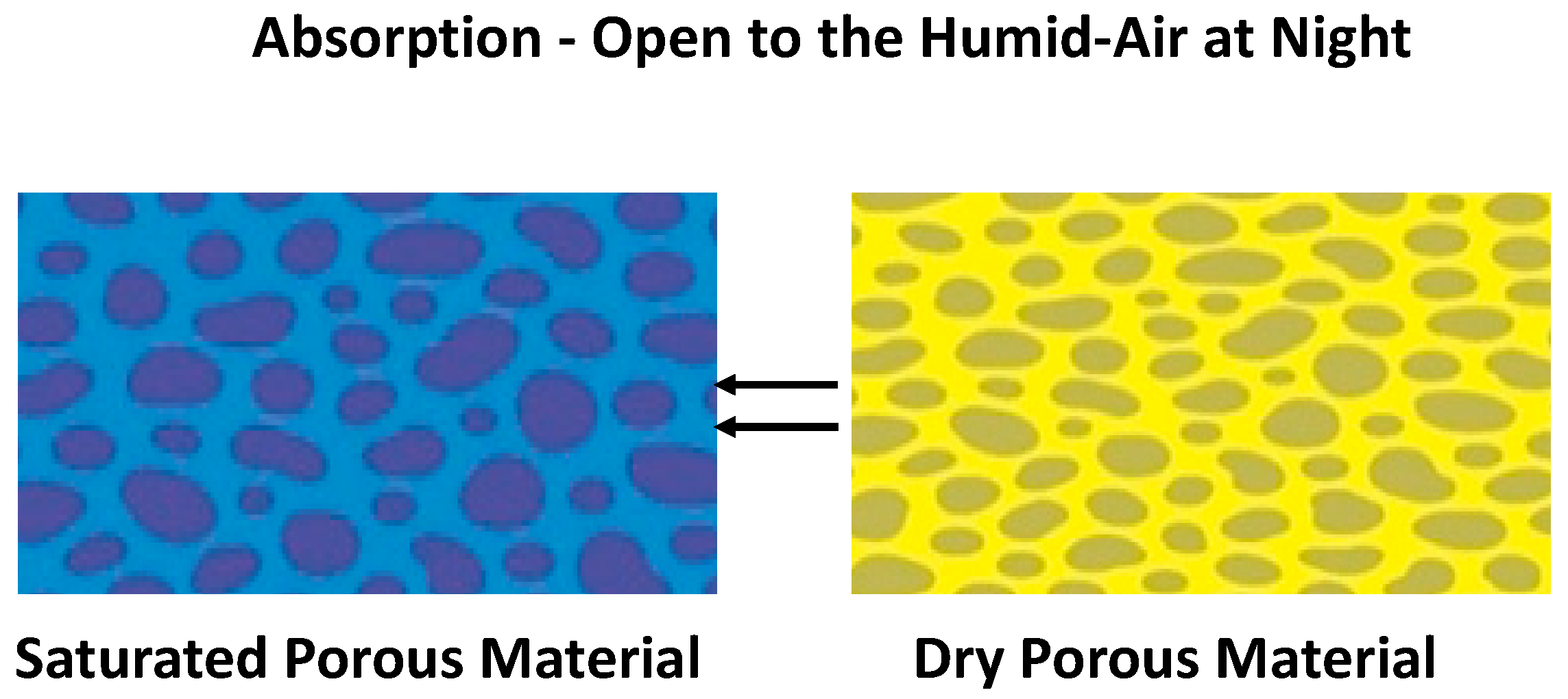
5. Relative Humidity Model
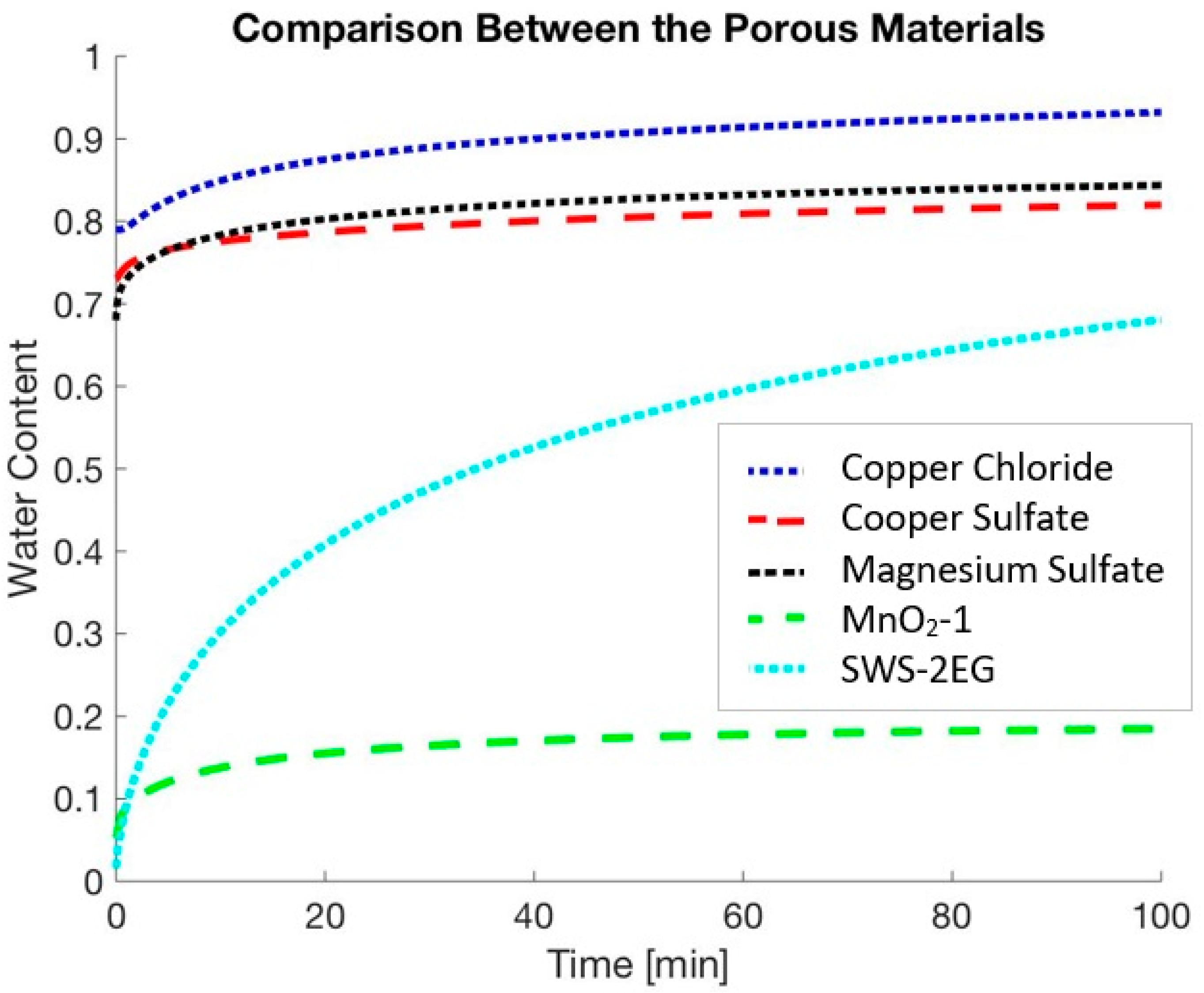
6. Results and Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- UNESCO. The United Nations World Water Development Report 2021: Valuing Water. UNESCO: Paris, France, 2021; Available online: https://unesdoc.unesco.org/ark:/48223/pf0000375724 (accessed on 14 June 2022).
- Gleick, P.H. (Ed.) Water in Crisis: A Guide to the World’s Fresh Water Resources, Illustrated ed.; Oxford University Press: New York, NY, USA, 1993. [Google Scholar]
- UNICEF. Billions of People Will Lack Access to Safe Water, Sanitation and Hygiene in 2030 unless Progress Quadruples–Warn WHO, UNICEF. UNICEF for Every Child. 1 July 2021. Available online: https://www.unicef.org/press-releases/billions-people-will-lack-access-safe-water-sanitation-and-hygiene-2030-unless (accessed on 30 November 2021).
- Jarimi, H.; Powell, R.; Riffat, S. Review of sustainable methods for atmospheric water harvesting. Int. J. Low-Carbon Technol. 2020, 15, 253–276. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, R.; Zhang, Y.; Wang, J. Progress and Expectation of Atmospheric Water Harvesting. Joule 2018, 2, 1452–1475. [Google Scholar] [CrossRef]
- Cherif, H.; Belhadj, J. Chapter 15-Environmental Life Cycle Analysis of Water Desalination Processes. In Sustainable Desalination Handbook; Gude, V.G., Ed.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 527–559. [Google Scholar] [CrossRef]
- Curto, D.; Franzitta, V.; Guercio, A. A Review of the Water Desalination Technologies. Appl. Sci. 2021, 11, 670. [Google Scholar] [CrossRef]
- Dhakal, N.; Salinas-Rodriguez, S.G.; Hamdani, J.; Abushaban, A.; Sawalha, H.; Schippers, J.C.; Kennedy, M.D. Is Desalination a Solution to Freshwater Scarcity in Developing Countries? Membranes 2022, 12, 381. [Google Scholar] [CrossRef]
- Sleiti, A.K.; Al-Khawaja, H.; Al-Khawaja, H.; Al-Ali, M. Harvesting water from air using adsorption material—Prototype and experimental results. Sep. Purif. Technol. 2020, 257, 117921. [Google Scholar] [CrossRef]
- Wang, J.; Dang, Y.; Meguerdichian, A.G.; Dissanayake, S.; Kankanam-Kapuge, T.; Bamonte, S.; Tobin, Z.M.; Achola, L.A.; Suib, S.L. Water Harvesting from the Atmosphere in Arid Areas with Manganese Dioxide. Environ. Sci. Technol. Lett. 2019, 7, 48–53. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Torregrosa, A.; Weiss-Penzias, P.S.; Zhang, B.J.; Sorensen, D.; Cohen, R.E.; McKinley, G.H.; Kleingartner, J.; Oliphant, A.; Bowman, M. Fog Water Collection Effectiveness: Mesh Intercomparisons. Aerosol Air Qual. Res. 2018, 18, 270–283. [Google Scholar] [CrossRef]
- Cruzat, D.; Jerez-Hanckes, C. Electrostatic fog water collection. J. Electrost. 2018, 96, 128–133. [Google Scholar] [CrossRef]
- GENAQ. Atmospheric Water Generator by GENAQ. A New Water Supply Alternative. Available online: http://www.genaq.com/water/ (accessed on 28 November 2021).
- Shourideh, A.H.; Ajram, W.B.; Al Lami, J.; Haggag, S.; Mansouri, A. A comprehensive study of an atmospheric water generator using Peltier effect. Therm. Sci. Eng. Prog. 2018, 6, 14–26. [Google Scholar] [CrossRef]
- Precipitation Formation from Orographic Cloud Seeding. PNAS. Available online: https://www.pnas.org/content/115/6/1168 (accessed on 20 September 2021).
- World Economic Forum. Unusual Methods to Harvest Earth’s Untapped Water Sources. Available online: https://www.weforum.org/agenda/2021/02/water-scarcity-shortage-sustainability-climate-change-innovation-resources/ (accessed on 20 September 2021).
- Gado, M.G.; Nasser, M.; Hassan, A.A.; Hassan, H. Adsorption-based atmospheric water harvesting powered by solar energy: Comprehensive review on desiccant materials and systems. Process Saf. Environ. Prot. 2022, 160, 166–183. [Google Scholar] [CrossRef]
- Qi, S.; Zhuang, H.; Wang, X.; Li, X.; Liu, K.; Liu, J.; Zhang, H. Advances in solar-driven hygroscopic water harvesting. Glob. Chall. 2021, 5, 2000085. [Google Scholar]
- Asim, N.; Badiei, M.; Alghoul, M.A.; Mohammad, M.; Samsudin, N.A.; Amin, N.; Sopian, N.K. Sorbent-based air water-harvesting systems: Progress, limitation, and consideration. Rev. Environ. Sci. Bio/Technol. 2021, 20, 257–279. [Google Scholar] [CrossRef]
- Bilal, M.; Sultan, M.; Morosuk, T.; Den, W.; Sajjad, U.; Aslam, M.M.; Farooq, M.M. Adsorption-based atmospheric water harvesting: A review of adsorbents and systems. Int. Commun. Heat Mass Transf. 2022, 133, 105961. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, X.; Liu, Y.; Shi, Y.; Dai, Y.; Yu, G. Super moisture- absorbent gels for all-weather atmospheric water harvesting. Adv. Mater. 2019, 31, 1806446. [Google Scholar]
- Sultan, M.; Bilal, M.; Miyazaki, T.; Sajjad, U.; Ahmad, F. Adsorption-Based Atmospheric Water Harvesting: Technology Fundamentals and Energy-Efficient Adsorbents. In Technology in Agriculture; IntechOpen: London, UK; Available online: https://www.intechopen.com/chapters/76511 (accessed on 14 June 2022).
- Kode, V.R.; Stuckenberg, D.J.; Went, E.K.; Erickson, O.M.; Plumer, E. Techno-economic analysis of atmospheric water generation by hybrid nanofluids to mitigate global water scarcity. Liquids 2022, 2, 183–195. [Google Scholar] [CrossRef]
- Siegel, N.P.; Conser, B. A Techno-Economic Analysis of Solar-Driven Atmospheric Water Harvesting. J. Energy Resour. Technol. 2021, 143, 090902. [Google Scholar] [CrossRef]
- Adeyanju, A.A. Theoretical and Experimental Analysis of Atmospheric Water Harvesting Device. Prog. Can. Mech. Eng. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Sibie, S.; El-Amin, M.; Sun, S. Modeling of Water Generation from Air Using Anhydrous Salts. Energies 2021, 14, 3822. [Google Scholar] [CrossRef]
- Li, R.; Shi, Y.; Shi, L.; Alsaedi, M.; Wang, P. Harvesting Water from Air: Using Anhydrous Salt with Sunlight. Environ. Sci. Technol. 2018, 52, 5398–5406. [Google Scholar] [CrossRef]
- Gordeeva, L.; Restuccia, G.; Freni, A.; Aristov, Y. Water sorption on composites “LiBr in a porous carbon”. Fuel Process. Technol. 2002, 79, 225–231. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, Y.; Sun, Q.; Cao, X.; Sun, L. Steady-state equation of water vapor sorption for CaCl2-based chemical sorbents and its application. Sci. Rep. 2016, 6, 34115. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, M.; Haldar, S.; Dharmalingam, K.; Muthukumar, P.; Anandalakshmi, R. Evaluation of Thermo-Kinetic and Absorption Characteristics of Pure Desiccants and Desiccant Mixtures. Mater. Today Proc. 2020, 26, 1967–1971. [Google Scholar] [CrossRef]
- Elashmawy, M.; Alshammari, F. Atmospheric water harvesting from low humid regions using tubular solar still powered by a parabolic concentrator system. J. Clean. Prod. 2020, 256, 120329. [Google Scholar] [CrossRef]
- Almasarani, A.; Ahmad, I.K.; El-Amin, M.F.; Brahimi, T. Experimental Investigations and Modeling of Atmospheric Water Generation Using a Desiccant Material. Energies 2022, 15, 6834. [Google Scholar] [CrossRef]
- Milly, P.C.D. Moisture and heat transport in hysteretic, inhomogeneous porous media: A matric head-based formulation and a numerical model. Water Resour. Res. 1982, 18, 489–498. [Google Scholar] [CrossRef]
- Zeng, Y.; Su, Z.; Wan, L.; Wen, J. Numerical analysis of air-water-heat flow in unsaturated soil: Is it necessary to consider airflow in land surface models? J. Geophys. Res. Earth Surf. 2011, 116, 1–18. [Google Scholar] [CrossRef]
- Philip, J.R.; De Vries, D.A. Moisture movement in porous materials under temperature gradients. Trans. Am. Geophys. Union 1957, 38, 222–232. [Google Scholar] [CrossRef]
- Saito, H.; Šimůnek, J.; Mohanty, B.P. Numerical Analysis of Coupled Water, Vapor, and Heat Transport in the Vadose Zone. Vadose Zone J. 2006, 5, 784–800. [Google Scholar] [CrossRef]
- Solve 1-D Parabolic and Elliptic PDEs-MATLAB Pdepe. Available online: https://www.mathworks.com/help/matlab/ref/pdepe.html (accessed on 9 December 2021).

| Porous Materials | ||||
|---|---|---|---|---|
| Copper Chloride | 6821 | 4.661 | 0.98780 | 0.98926 |
| Copper Sulfate | 36.635 | 21.9 | 0.52737 | 0.73621 |
| Magnesium Sulfate | 63.849 | 23.75 | 0.86528 | 0.90572 |
| MnO2-1 | 79.219 | 2.338 | 0.91026 | 0.95684 |
| SWS-2EG | 26051 | 0.388 | 0.75640 | 0.92507 |
| Name | Symbol | Value | Unit |
|---|---|---|---|
| Density of water | 997 | ||
| Temperature | T | 320 | [K] |
| Specific gas constant for water vapor | 461.5 |
| Depth (m) | Time (min) | |||||
|---|---|---|---|---|---|---|
| Copper Chloride | 0.04 | 1000 | 0.37 | 0.99 | 0.79 | |
| Copper Sulfate | 0.15 | 1000 | 0.33 | 0.85 | 0.73 | |
| Magnesium Sulfate | 0.16 | 1000 | 0.41 | 0.87 | 0.68 | |
| MnO2-1 | 0.08 | 300 | 0.48 | 0.193 | 0.053 | |
| SWS-2EG | 0.08 | 1500 | 0.42 | 0.93 | 0.016 |
| Porous Materials | Maximum Error % | Minimum Error % | Average for All the Relative Error Points % |
|---|---|---|---|
| Copper Chloride | 1.28605756% | 0.0251514% | 0.49353302% |
| Copper Sulfate | 0.76765625% | 0.01021925% | 0.31534476% |
| Magnesium Sulfate | 1.33724095% | 0.0013657% | 0.6653724% |
| MnO2-1 | 7.72054771% | 0.00105762% | 1.87852984% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkinani, S.S.; El-Amin, M.F.; Brahimi, T. Numerical Modeling and Analysis of Harvesting Atmospheric Water Using Porous Materials. Separations 2022, 9, 364. https://doi.org/10.3390/separations9110364
Alkinani SS, El-Amin MF, Brahimi T. Numerical Modeling and Analysis of Harvesting Atmospheric Water Using Porous Materials. Separations. 2022; 9(11):364. https://doi.org/10.3390/separations9110364
Chicago/Turabian StyleAlkinani, Sadeem S., Mohamed F. El-Amin, and Tayeb Brahimi. 2022. "Numerical Modeling and Analysis of Harvesting Atmospheric Water Using Porous Materials" Separations 9, no. 11: 364. https://doi.org/10.3390/separations9110364
APA StyleAlkinani, S. S., El-Amin, M. F., & Brahimi, T. (2022). Numerical Modeling and Analysis of Harvesting Atmospheric Water Using Porous Materials. Separations, 9(11), 364. https://doi.org/10.3390/separations9110364








