Abstract
This study has evaluated the removal efficiencies of phosphate ions (PO43−) using pristine (TB) and chemical-activated tangerine peel biochars. The adsorption kinetics and isotherm presented that the enhanced physicochemical properties of TB surface through the chemical activation with CaCl2 (CTB) and FeCl3 (FTB) were helpful in the adsorption capacities of PO43− (equilibrium adsorption capacity: FTB (1.655 mg g−1) > CTB (0.354 mg g−1) > TB (0.104 mg g−1)). The adsorption kinetics results revealed that PO43− removal by TB, CTB, and FTB was well fitted with the pseudo-second-order model (R2 = 0.999) than the pseudo-first-order model (R2 ≥ 0.929). The adsorption isotherm models showed that the Freundlich equation was suitable for PO43− removal by TB (R2 = 0.975) and CTB (R2 = 0.955). In contrast, the Langmuir equation was proper for PO43− removal by FTB (R2 = 0.987). The PO43− removal efficiency of CTB and FTB decreased with the ionic strength increased due to the compression of the electrical double layer on the CTB and FTB surfaces. Besides, the PO43− adsorptions by TB, CTB, and FTB were spontaneous endothermic reactions. These findings demonstrated FTB was the most promising method for removing PO43− in waters.
1. Introduction
With a significant increase in the amount of nutrients introduced to the water system due to the rapid industrialization and recent population growth, increasing water pollution hinders effective water quality management [1,2]. Nutrients are divided into point and non-point sources, depending on the primary source of inflow. Although most effluents from domestic sewage treatment plants and livestock wastewater treatment plants meet the water quality criteria, agricultural drainage water, a representative non-point source, significantly affects the discharge of nutrients because the increased amounts of compost or fertilizer used to improve agricultural production [3,4]. This oversupply of nutrients into the water system through point and non-point sources increases eutrophication causes algal blooms. It reduces the amount of dissolved oxygen in the aquatic ecosystem, thereby causing the deaths of aquatic organisms [5]. Among the main components of nutrients, nitrate ions (NO3−) are required in relatively large quantities for algal growth, but phosphate ions (PO43−) are a limiting factor that can promote algal growth even when present in small amounts. PO43− can lead to blue-green algal blooms, leading to renal failure through toxicity [6,7].
The anaerobic anoxic aerobic (A2O) process is typically applied for the biological treatment of PO43−. Although this process requires no chemical injection and generates little sludge compared to the amount of phosphate removed [8], the results are significantly affected by the operating conditions. Besides, A2O is not suitable for strict water quality criteria because it cannot protect microorganisms against toxic chemicals present in the influent water. Moreover, microorganisms in the A2O process are subject to more significant technical limitations in treatment efficiency compared with the physicochemical treatment process [6]. There are commonly used physicochemical treatment processes (e.g., Electrodialysis, membrane filtration, coagulation, precipitation, and adsorption) to remove PO43− from water and wastewater treatment plants [9]. These techniques are not appropriate to the full-scale water and wastewater treatment plants due to the high operating costs and energy consumption [10], and the generated sludge may cause secondary environmental pollution in subsequent treatment processes [11]. However, the adsorption process has been used in the water and wastewater treatment plants due to low operation and maintenance costs [12]. The various types of PO43− adsorbents, such as clay minerals [13], fly ashes [14], and metal oxides [15], have been investigated. Das et al. have demonstrated the high PO43− adsorption on clay minerals, including layered double hydroxides [13]. Chen et al. reported the efficient PO43− adsorption on fly ashes [14]. Zhang et al. have fabricated activated carbon fiber with metal oxides for PO43− adsorption [15]. Despite the advantage of these adsorbents, including high efficiency and environment-friendly properties, they were difficult to be applied the full-scale wastewater treatment plant for PO43− adsorption due to the high treatment cost (e.g., recycle and regeneration). Therefore, it was necessary to develop an alternative adsorbent to remove PO43− in water [16].
Biochars, alternative adsorbents, are carbon-rich substances obtained through biomass pyrolysis, such as fruit peels and rice bran, under oxygen-limited conditions. The utilization of biochar shows significant environmental advantages in reducing greenhouse gas emissions and resource recycling technology of agriculture and food residues [17]. The agricultural residues represent an important potential source of reusable products [18,19]. Tangerine peels are common agricultural residues in Korea. The amount of tangerines produced in Jeju Island was 125,343 tons as of 2005, representing approximately 18% of the total global tangerine production. Annually, over 55,000 tons have been discarded as tangerine peels [20]. Tangerine peels can be highly applicable as a raw material for biochars because they are mainly composed of pectin, hemicellulose, and cellulose substances [21,22]. The negative surface charge of pristine biochar has limited their adsorption affinity towards anions, including PO43− [23,24]. Therefore, their adsorption capacities might be considerably enhanced after modification, including chemical activation, surface functionality modification, and biochar impregnated with metals (e.g., CaCl2, MgCl2, FeCl3, and AlOOH) [24,25,26,27,28]. Fang et al. have demonstrated the high PO43− adsorption on MgCl2 modified ground corn biochar [27]. Zhang and Gao. have reported the efficient PO43− adsorption on AlOOH modified cottonwood biochar [24]. Despite the effective adsorption capacities of metal-loaded biochar for PO43−, these biochars preparation demanded high energy for pyrolysis, and the adsorption ability of biochars reduced due to coalescence with water [24,27]. CaCl2 and FeCl3, which are a type of chemical coagulants, are commonly used in the adsorption of PO43− in water. Thus, the modification of biochars with CaCl2 and FeCl3 could significantly improve the adsorption capacities of PO43− in water.
The primary purpose of this study is to evaluate the effect of pretreatment with CaCl2 and FeCl3 on the PO43− removal of biochars made from tangerine peels. Thus, the effects of various conditions, such as the biochar dosage, pH, ionic strength, and temperature, on PO43− removal were evaluated using the pristine tangerine peel biochar (TB), and CaCl2 (CTB) and FeCl3 (FTB) activated tangerine peel biochars. In addition, the PO43− adsorption mechanisms of TB, CTB, and FTB were investigated through adsorption kinetics and adsorption isotherm models.
2. Materials and Methods
2.1. Chemicals and Reagents
Potassium dihydrogen phosphate (KH2PO4, 99.0%), CaCl2 (>99.0%), FeCl3 (>99.0%), sodium chloride (NaCl, 99.0%), sodium hydroxide (NaOH, 99.0%), and hydrochloric acid (HCl, 35%) were purchased from Daejung Chemicals (Siheung-si, Gyeonggi-do, Korea). All chemicals were used without further purification. Deionized (DI) water (resistivity > 18.2 MΩ cm−1, Barnstead Nanopure Water System, Lake Balboa, CA, USA) was applied to make the PO43− stock solution (concentration = 10 mg L−1).
2.2. Preparation of Tangerine Peel Biochars
Tangerine peels were purchased from a local food store on Jeju Island (Jeju-do, Korea). After dried tangerine peels were crushed to 0.5–1.0 mm using a blender, they were several rinsed (ten times) with DI water to remove impurities and then dried in an oven at 105 °C for 12 h. The crushed tangerine peels were immersed in 200 mL solutions of 1 M CaCl2 and 1 M FeCl3, respectively. They were then stirred at 80 °C for 1 h and dried in an oven at 105 °C for 24 h. The pristine and chemical activated tangerine peels were pyrolyzed at 800 °C for 1 h using a tubular furnace (PyroTech, Namyangju, Gyeonggi-do, Korea) under N2 gas (the flow rate = 0.25 L min−1) atmospheric conditions (heating rate = 5 °C min−1) [29]. After cooling to room temperature (20 ± 0.5 °C), the fabricated tangerine peel biochars were rinsed using DI water until no impurities were observed and dried in an oven at 80 °C for 24 h. The dried tangerine peel biochars (i.e., TB, CTB, and FTB) were sieved to obtain a homogenized particle size of 150 µm and then stored in a desiccator prior to use.
2.3. Characteristics of Tangerine Peel Biochars
Total carbon (C), nitrogen (N), and hydrogen (H) contents of the pristine and chemical activated tangerine peel biochars were analyzed using a CHN element analyzer (Flash 2000, Thermo Fisher, Waltham, MA, USA). The average pore size (nm) and specific surface area (m2 g−1) were measured using a Brunauer–Emmett–Teller (BET; BELSORP-mini II, Microtrac BEL, Osaka, Japan) analyzer. An X-ray diffractometer (XRD; D/Max-2500, Rigaku, Tokyo, Japan) was used to analyze the surface crystallinity of TB, CTB, and FTB. The surface morphologies of TB, CTB, and FTB were observed using a ultra-high resolution scanning electron microscope (UH-SEM; S-4800, Hitachi, Tokyo, Japan), and the atomic-resolution chemical mapping of calcium and iron ions were identified using energy-dispersive X-ray spectroscopy (EDX; Link ISIS 300, Oxford Instruments, Abingdon, UK).
2.4. Adsorption Experiments
2.4.1. Optimal Dosage
The adsorption of PO43− was examined to determine the optimal adsorbent dosages of TB, CTB, and FTB. Each adsorbent dosage (TB = 0.2–2.0 g L−1; CTB = 0.2–12 g L−1; FTB = 0.2–2.0 g L−1) was added to Erlenmeyer flasks containing 25 mL of the PO43− solution (initial concentration = 1 mg L−1, pH = 7.0) The sample solutions were stirred at 25 °C and 150 rpm for 24 h using a shaking incubator (VS-8480, Vision Scientific, Daejeon-Si, Korea). Upon completing the adsorption experiment, the sample solutions were filtered using a glass fiber filter (GF/F, Whatman, Maidstone, UK) with a nominal pore size of 0.7 μm to remove adsorbents. The PO43− concentration was analyzed at UV absorbances of 880 nm using the ascorbic acid method (UV-Vis Spectrophotometer, UV-1280, Shimadzu, Kyoto, Japan) [30]. The experiment was performed in triplicate to minimize errors.
2.4.2. Adsorption Kinetics
The adsorption kinetics was conducted by adding the optimal dosage of each TB, CTB, and FTB to Erlenmeyer flasks containing 25 mL of the PO43− solution (initial concentration = 1 mg L−1, pH = 7.0). The sample solutions were stirred at 150 rpm during a certain period (0.5–48 h) at 25 °C in a shaking incubator. After the adsorption kinetics experiment, the sample solutions were filtered using GF/F. The concentrations of PO43− at the initial and equilibrium states were measured using a UV-Vis spectrophotometer. The experiment was performed in triplicate to minimize errors. All adsorption experiments are repeated three times to minimize errors. The amount of PO43− adsorbed per unit mass of the TB, CTB, and FTB at equilibrium, Qe (mg g−1), was calculated using the following Equation (1):
where V is the volume of the solution (L). C0 and Ce are the initial and equilibrium concentrations of PO43− solution (mg L−1), and M (g) is the mass of the used adsorbent.
The PO43− removal efficiency was calculated using Equation (2):
The PO43− adsorption characteristics and adsorption capacity of each TB, CTB, and FTB were investigated using the following Equations (3) and (4) [31]:
where Qt (mg g−1) is the amount of the adsorbed PO43− on the TB, CTB, and FTB at the time t, t (min) is the adsorption time. k1 (min−1) is the constant of the pseudo-first-order model and k2 (g mg−1∙min) is the constant of the pseudo-second-order model.
2.4.3. Adsorption Isotherm
To investigate the adsorption isotherm of PO43− by TB, CTB, and FTB, the adsorption isotherm experiment was performed by adjusting the PO43− concentrations (0.5–10 mg L−1) and adding each optimal adsorbent dosage under controlled conditions (agitation speed = 150 rpm, contact time = 24 h, pH = 7.0, and temperature = 25 °C). The adsorption isotherm results were analyzed using the Langmuir isotherm and Freundlich isotherm models [32].
where Qmax (mg g−1) is the maximum adsorption capacity in the Langmuir isotherm model, and KL (L mg−1) is the equilibrium constant of the linearized Langmuir isotherm model. RL = 1/(1 + KLC0), derived from KL, can be used to compare the adsorption affinity of Langmuir isotherms [33]:
where KF (mg−1/nL1/n g−1) is the Freundlich isotherm adsorption constant related to the relative maximum adsorption capacity, and n is the dimensionless adsorption intensity.
2.4.4. Effects of pH and Ionic Strength
The effects of pH and ionic strength on the adsorptions of the PO43− by the TB, CTB, and FTB were evaluated by adjusting solution pH (pH = 3.0–9.0) and ionic strengths (ionic strength = 0–0.5 M) using 0.1 N HCl and 0.1 N NaOH, and NaCl, respectively (initial concentration of PO43− solution = 1 mg L−1, agitation speed = 150 rpm, contact time = 24 h). The removal efficiencies of PO43− using TB, CTB, and FTB were calculated by Equation (2).
2.4.5. Effects of Temperature
The effects of the temperature of the solution on the PO43− removal efficiency of the TB, CTB, and FTB were performed under various temperature (15–35 °C) conditions (initial concentration of PO43− solution = 1 mg L−1, agitation speed = 150 rpm, contact time = 24 h, and pH 7.0). The removal efficiencies of PO43− using TB, CTB, and FTB were followed by Equation (2).
The thermodynamic parameters of the PO43− adsorption are calculated using the following Equations (7)–(9) [34]:
where Kd (L g−1) is the partition coefficient. ∆G° in (kJ mol−1), ∆H° in (kJ mol−1), and ∆S° in (J mol−1·K) are the Gibbs free energy, enthalpy, and entropy, respectively. R is the ideal gas constant (8.314 J mol−1·K), and T is the absolute temperature (K). ∆H° and ∆S° were calculated as the slope and intercept in the linear graph of ln Kd and 1/T, respectively.
3. Results and Discussions
3.1. Characterization of TB, CTB, and FTB
3.1.1. Elemental Composition and Functionality Analyses
The elemental composition (i.e., C, H, O, and N) and surface properties (i.e., specific surface area pore volume and average pore size) of TB, CTB, and FTB associated with the adsorption capacity of PO43− are presented in Table 1. Although the H (TB: 1.50%, CTB: 1.94%, and FTB: 1.63%) and N (TB: 2.09%, CTB: 1.31%, and FTB: 1.05%) contents of TB, CTB and FTB were similar, CTB and FTB had lower C (CTB: 69.42% and FTB: 53.81%) content and higher O (CTB: 7.61% and FTB: 8.39%) content than those of TB (C content = 81.12%, O content = 4.40%). The atomic ratios of H/C, O/C, and (O + N)/C are generally distinguished for carbonization, surface hydrophobicity, and polarity, respectively [35]. The CTB and FTB showed higher values of the H/C (CTB: 0.34 and FTB: 0.36), O/C (CTB: 0.08 and FTB: 0.12), and (O + N)/C (CTB: 0.10 and FTB: 0.12) ratios compared to the TB (H/C = 0.22, O/C = 0.04, and (O + N)/C = 0.06). These results showed that FTB contained relatively less aromatic functional groups than those of TB and CTB [36]. Furthermore CTB and FTB exhibited larger specific surface areas (TB = 9.21 m2 g−1; CTB = 342.11 m2 g−1; 558.71 m2 g−1), larger pore volumes (TB = 0.01 cm3 g−1; CTB = 0.36 cm3 g−1; FTB = 0.18 cm3 g−1), and smaller average pore sizes (TB = 6.07 nm; CTB = 3.67 nm; FTB = 3.64 nm) compared with TB, indicating that activation process with CaCl2 and FeCl3 was effective in improving the physicochemical characteristics of the tangerine peel biochars related to PO43− adsorption [37].

Table 1.
The physicochemical properties of TB, CTB, and FTB.
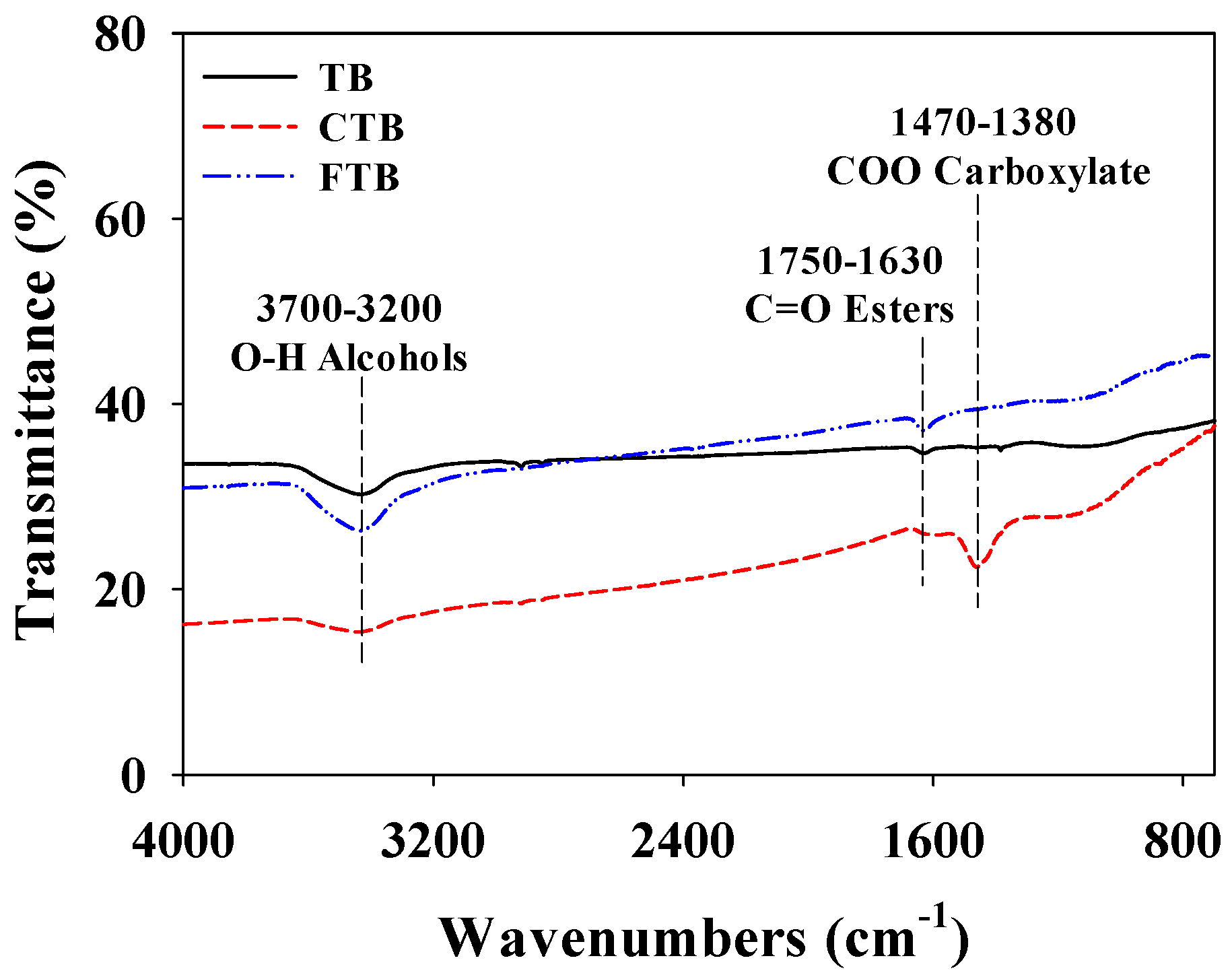
The functional groups of TB, CTB, and FTB are revealed by FT-IR analysis (Figure 1). The main differences between TB and chemical activated TB (i.e., CTB and FTB) are the existence of C=O stretching of esters and -COO carboxylates. These functional groups might enhance the adsorption capacities of the phosphate ions using CTB and FTB by working as an electron acceptor [38,39].
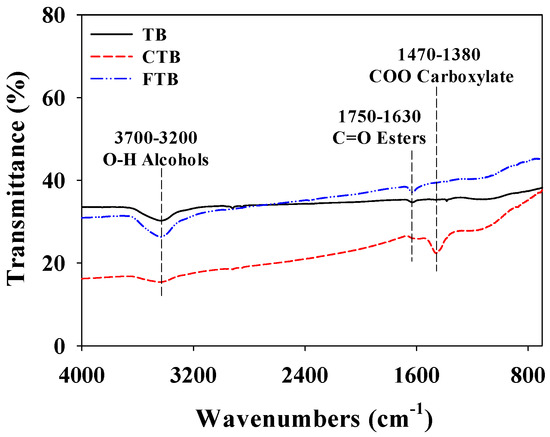
Figure 1.
The FT-IR spectra of TB, CTB, and FTB.
3.1.2. UH-SEM-EDX and XRD Analyses
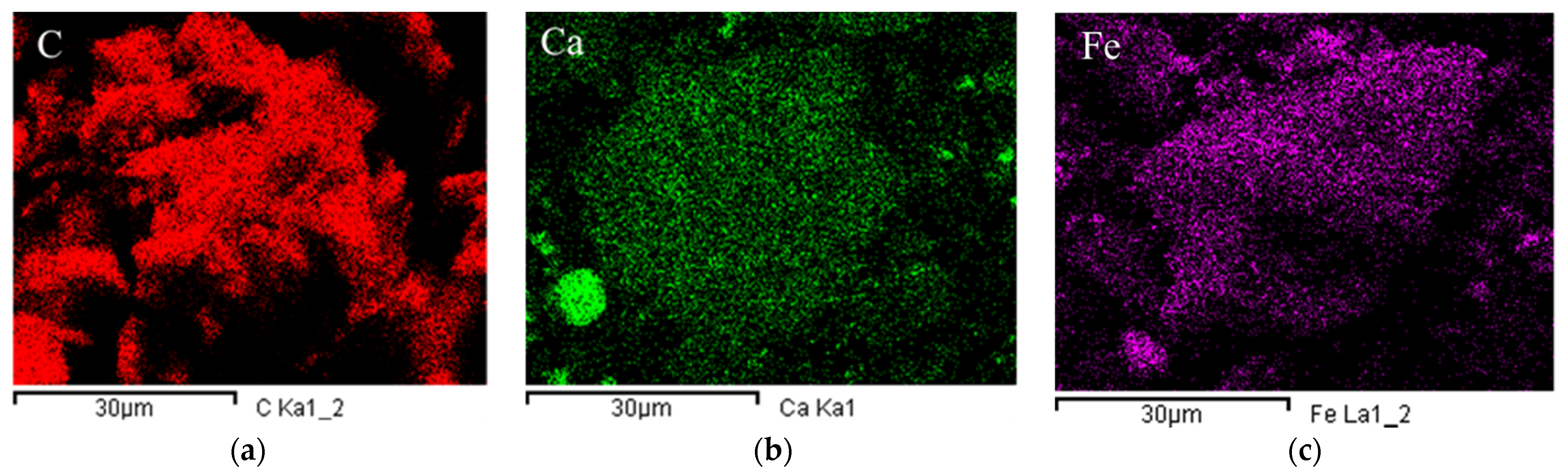
The surfaced morphologies of TB, CTB, and FTB are shown in Figure 2. The surfaces of the CTB (Figure 2b) and FTB (Figure 2c) exhibited much coarser compared to the surface of TB (Figure 2a). These observations are in agreement with the result of measuring the specific surface area using BET. Figure 3 shows the EDX mapping images of the TB, CTB, and FTB surfaces. The surface of TB was mostly composed of carbon (Figure 3a), whereas calcium and iron salts were evenly distributed on the surfaces of CTB and FTB (Figure 3b,c). Moreover, the results of EDX mapping were in good agreement with the atomic percentage of elements in TB, CTB, and FTB (Table 2). These observations indicate that calcium and iron salts were successfully impregnated in the surface of the tangerine peel biochars through pretreatment with CaCl2 and FeCl3.
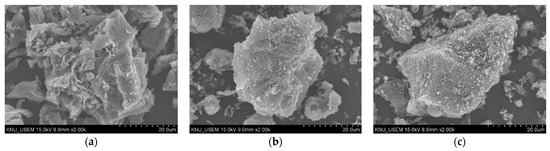
Figure 2.
The UH-SEM images of (a) TB; (b) CTB; and (c) FTB.
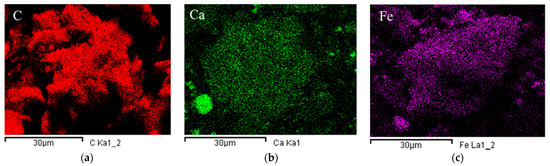
Figure 3.
The UH-SEM-EDX mapping of (a) TB; (b) CTB; and (c) FTB (magnification = 2000).

Table 2.
Atomic percentage of elements in TB, CTB, and FTB.
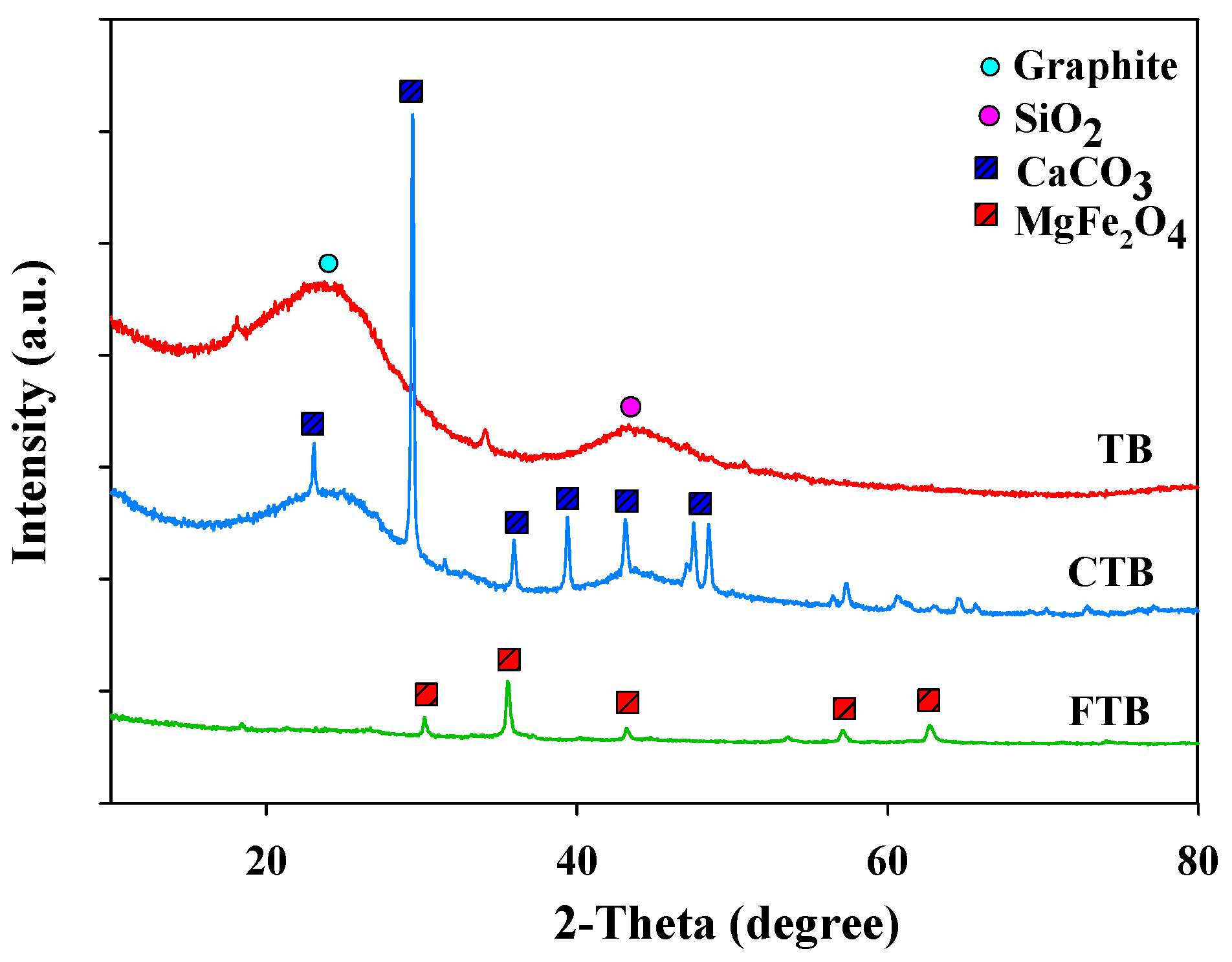
The crystallinities of TB, CTB, and FTB were analyzed using XRD (Figure 4). The XRD peaks of TB related to graphite and quartz (SiO2) were found at 2θ = 23° and 43°, respectively [40]. The XRD peaks of CTB and FTB related to calcium and iron species (e.g., CaCO3, MgFe2O4) were found (CaCO3 at 2θ = 35°, 57°, and 65°; MgFe2O4 at 2θ = 30°, 35°, 43°, 57°, and 63°) [41]. These findings were in good agreement with the SEM-EDX analysis results of TB, CTB, and FTB.
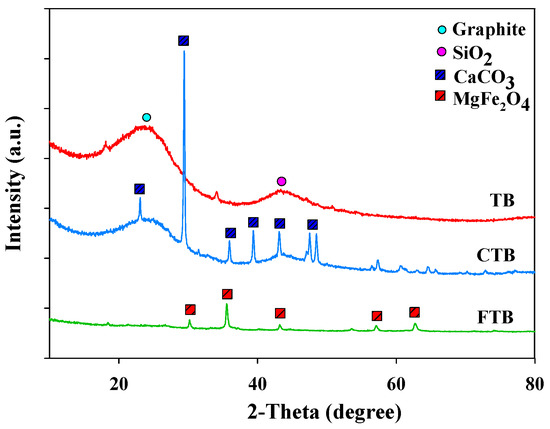
Figure 4.
The XRD of TB, CTB, and FTB.
3.2. Effects of Tangerine Peel Biochar Dosage
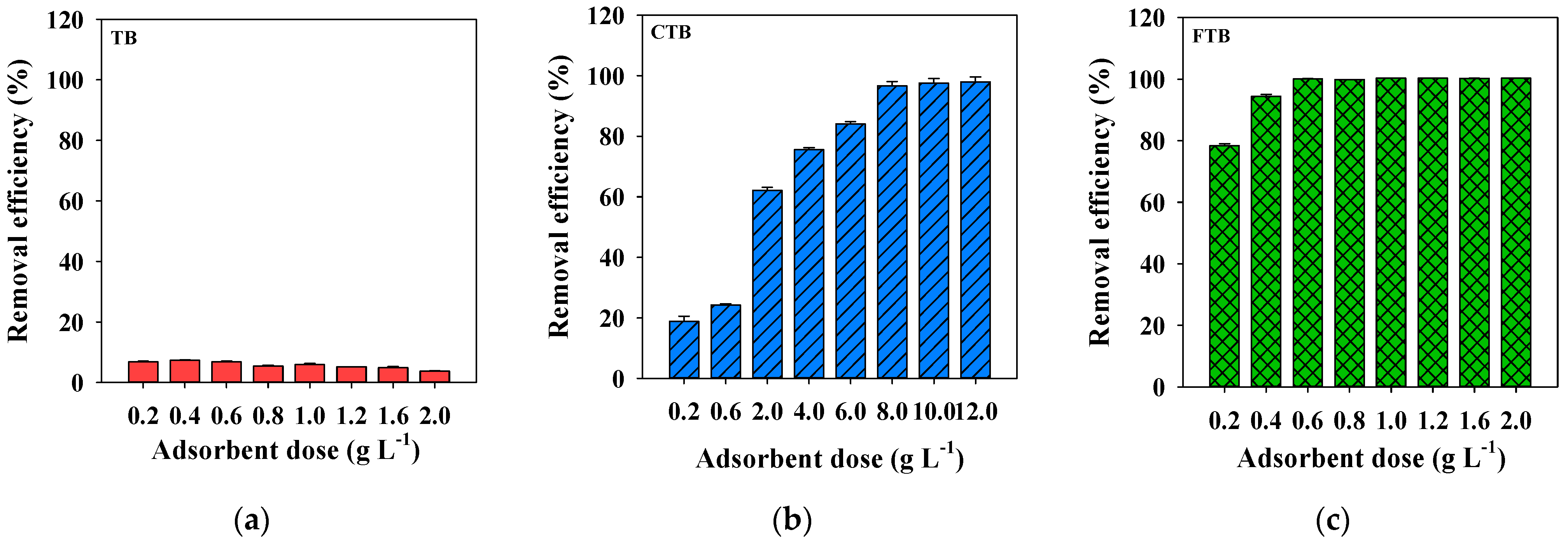
The adsorbent dosage is one of the critical factors which affect the adsorption of PO43−. Figure 5 presents the effects of the dosages of TB, CTB, and FTB on the removal efficiency of PO43−. In the case of TB, the removal efficiency of PO43− decreased as the adsorbent dosage was increased beyond 0.6 g·L−1. These results indicated that the decreased adsorption capacity of TB for PO43− was caused by reducing the total number of binding sites on TB surfaces due to the aggregation of TB particles as increasing adsorbent dosage [42]. However, the removal efficiencies of the PO43− by CTB and FTB increased with the dosage increase. These results indicated that the activated binding sites of the adsorbents capable of PO43− adsorption increased with increasing dosage [43]. Furthermore, FTB was more effective in removing PO43− than that of CTB because the binding capacity of iron salts is higher than that of calcium [44]. Based on these experiments on the PO43− removal efficiency according to the TB, CTB, and FTB dosages, 0.6 g·L−1 was selected as the optimal dosage and applied to subsequent experiments.
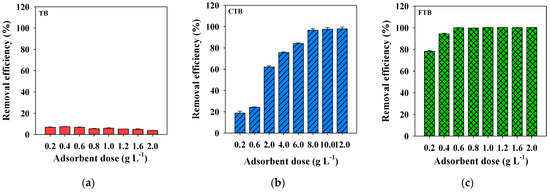
Figure 5.
The effects of adsorbent doses on the removal efficiency of PO43− by (a) TB, (b) CTB and (c) FTB (PO43− = 1 mg L−1; agitation speed = 150 rpm; temperature = 25 °C; contact time = 24 h; pH = 7.0).
3.3. Adsorption Kinetics
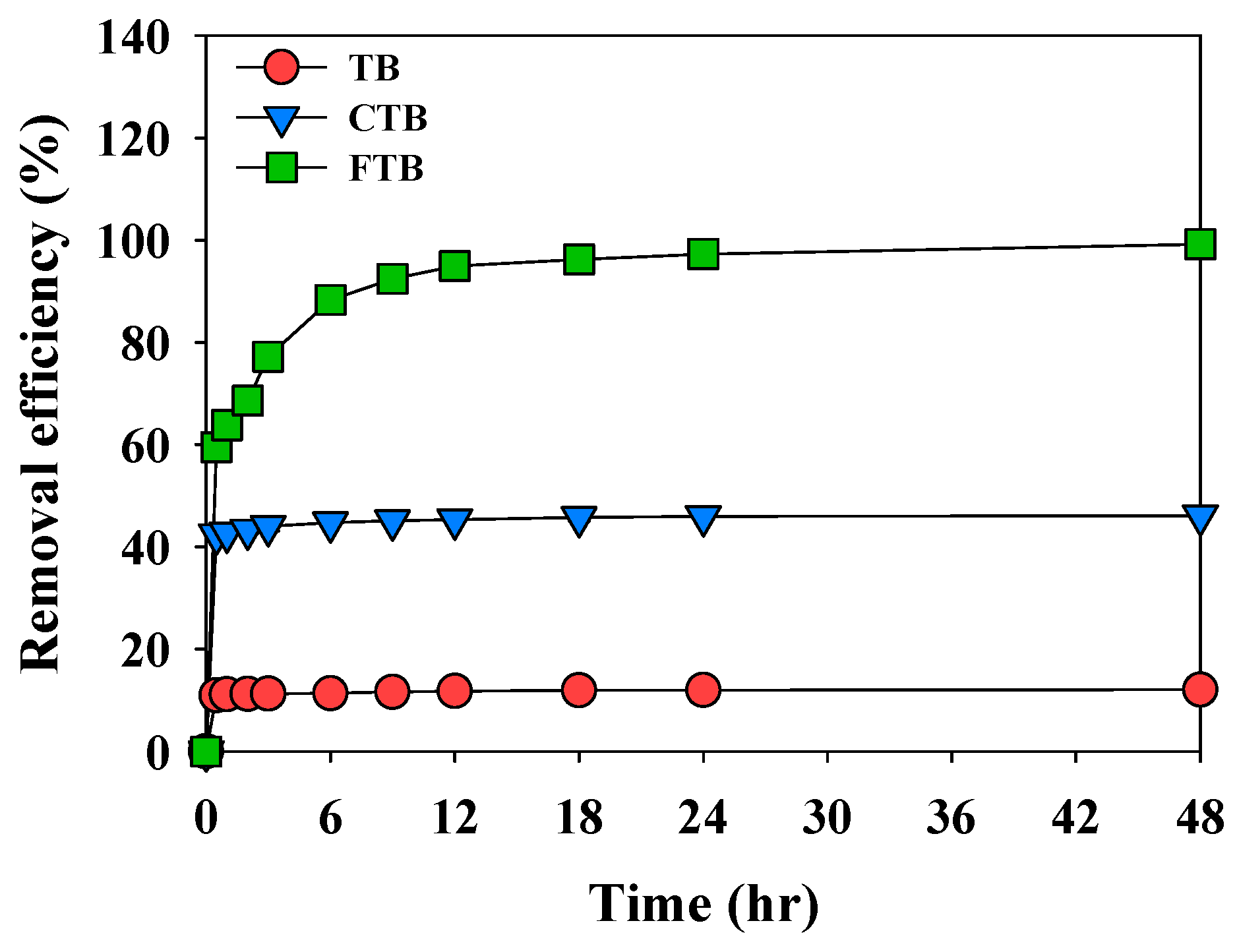
Figure 6 shows the adsorption kinetics of PO43− by TB, CTB, and FTB. The adsorption process of PO43− is comprised of fast and slow reaction stage. The fast adsorption reaction was completed in about 0.5 h for TB, CTB, and FTB as the activated sites on the surface of the biochars were saturated.
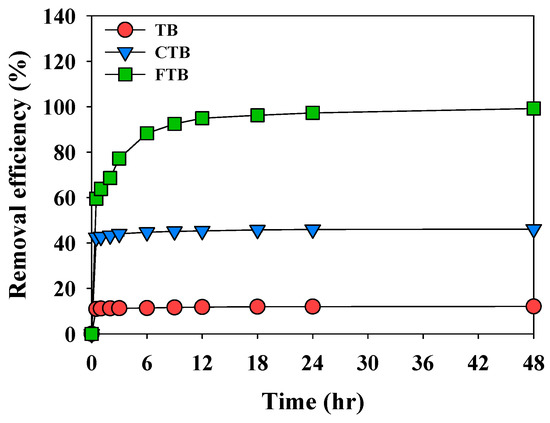
Figure 6.
The adsorption kinetics of PO43− onto the TB, CTB, FTB (adsorbent dose = 0.6 g L−1; PO43− = 1 mg L−1; agitation speed = 150 rpm; temperature = 25 °C; contact time = 24 h; pH = 7).
The fast adsorption reaction was completed in about 0.5 h for TB, CTB, and FTB as the activated sites on the surface of the biochars were saturated. The TB (Qe,exp = 0.104 mg g−1) and CTB (Qe,exp = 0.354 mg g−1) with relatively low removal efficiency compared to FTB, adsorption equilibrium was reached after 2 h. However, the adsorption equilibrium of FTB (Qe,exp = 1.655 mg g−1) was completed in 18 h due to many activated sites on the surface [45]. Table 3 presents the results of calculating the constant and correlation coefficient of adsorption kinetics. The adsorption of PO43− by TB, CTB, and FTB was well fitted for the pseudo-second-order model (R2 = 0.999) than the pseudo-first-order model (R2 ≥ 0.929). These results indicated that the adsorption of TB, CTB, and FTB is caused by chemical adsorption [46].

Table 3.
The kinetic parameters for the removal of the PO43− using TB, CTB, and FTB.
3.4. Adsorption Isotherms
The adsorption behaviors of PO43− by the TB, CTB, and FTB were examined using the Langmuir and Freundlich adsorption isotherm models (Table 4). The adsorption of PO43− by TB was well fitted to the Freundlich isotherm model with the high R2 values (R2 of Langmuir isotherm = 0.887; R2 of Freundlich isotherm = 0.975). This is evidence that the multilayer adsorption played a critical role in removing the PO43− toward the heterogeneous surfaces of the TB [47]. For the CTB and FTB, the adsorption of PO43− followed both Langmuir (R2 of CTB = 0.889; R2 of FTB = 0.987) and Freundlich (R2 of CTB = 0.955; R2 of FTB = 0.912) isotherm models. These observations could explain that the chemical activation with CaCl2 and FeCl3 might change the adsorption mechanism (i.e., multilayer adsorption → monolayer adsorption) of PO43− by the TB. A similar result was previously observed for the removal of the pharmaceuticals with NaOH-activated biochars [48]. The adsorption affinities of PO43− to the TB, CTB, and FTB were evaluated using the n values (dimensionless adsorption intensity) of the Freundlich isotherm model: (i) n > 1.0 (favorable), (ii) n = 1.0 (linear), and (iii) n < 1.0 (unfavorable) [49]. The adsorption of PO43− by TB (n value = 0.766) was unfavorable, whereas the adsorptions of PO43− by CTB (n value = 1.523) and FTB (n value = 7.530) were favorable. The RL value (maximum adsorption capacity; RL = 1/(1 + KLC0)) of the Langmuir isotherm model: (i) RL = 0 (irreversible), (ii) 1 > RL > 0 (favorable), (iii) RL = 1 (linear), and (iv) RL > 1 (unfavorable), was assessed to the adsorption affinities of PO43− toward TB, CTB, and FTB [50]. The adsorption of PO43− by FTB (RL = 0.209) followed the Langmuir isotherm model and seemed to be favorable for the monolayer adsorption [51]. Moreover, these results are comparable to the maximum adsorption capacity (mg g−1) calculated using different adsorbents as shown in Table 5.

Table 4.
The isotherm parameters for the removal of the PO43− using TB, CTB, and FTB.

Table 5.
Summary of available results related to PO43− adsorption by biochars.
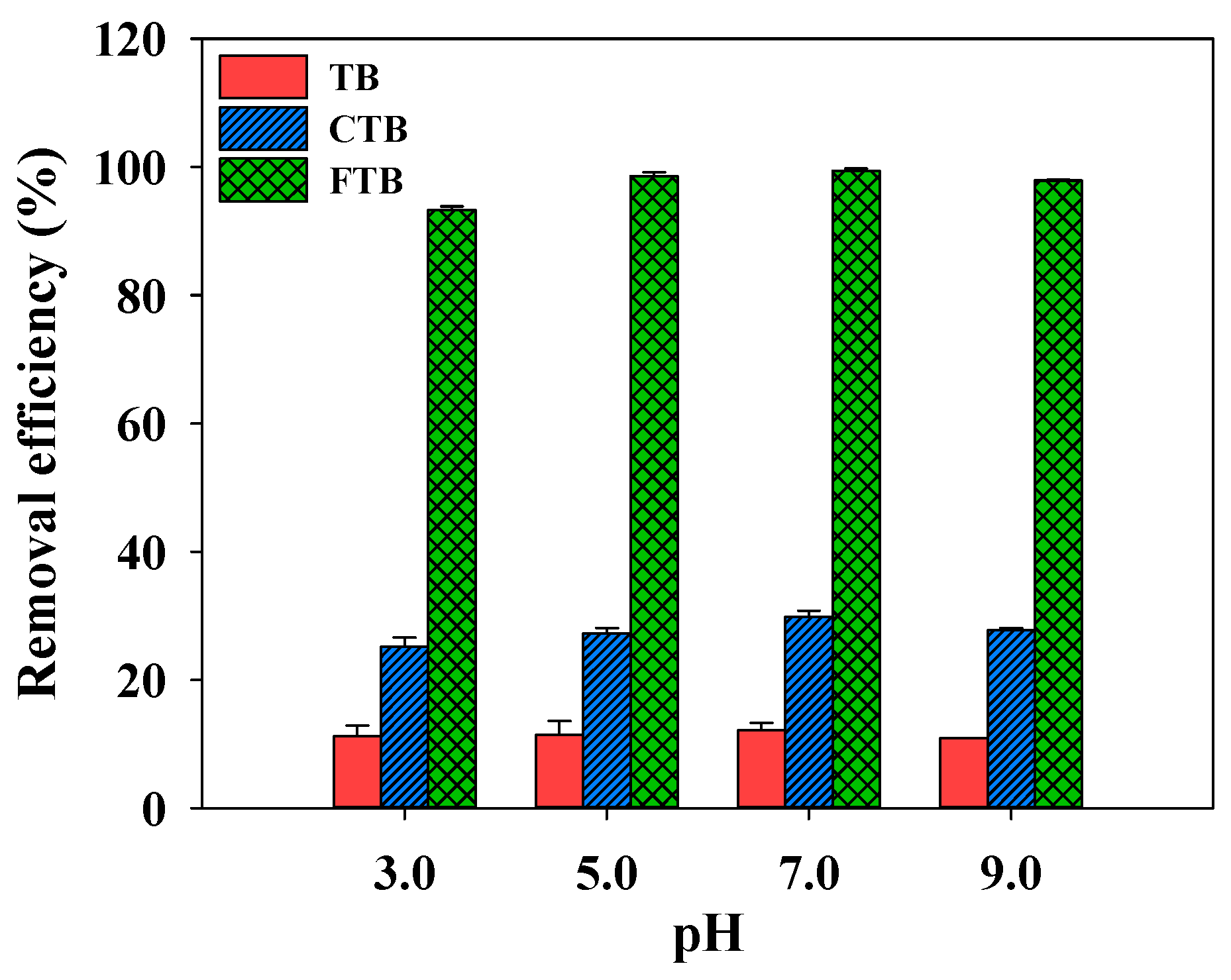
3.5. Effects of pH on Adsorption of PO43−
Figure 7 illustrates the effect of pH (pH = 3–9) on the adsorption of PO43− using TB, CTB, and FTB. It was presented that the removal efficiency of PO43− by TB, CTB, and FTB was not significantly affected by the pH change (removal efficiency of TB = 10.9–12.1%; removal efficiency of CTB = 25.1–29.8%; removal efficiency of FTB = 93.3–99.4%). These results indicated that TB, CTB, and FTB could be used to effectively remove PO43− from wastewater with a wide range of pH [54].
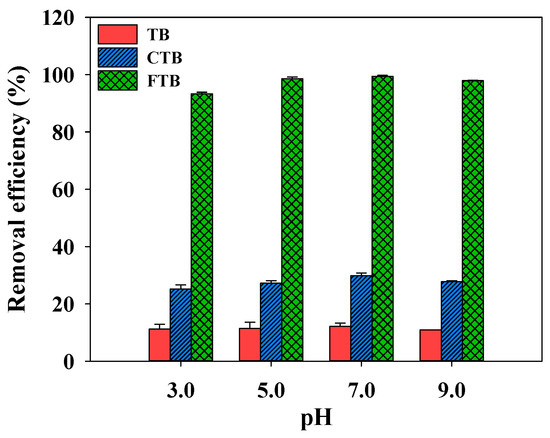
Figure 7.
The effects of pH on the removal efficiency of PO43− by TB, CTB and FTB (adsorbent dose = 0.6 g L−1; PO43− = 1 mg L−1; agitation speed = 150 rpm; temperature = 25 °C; contact time = 24 h).
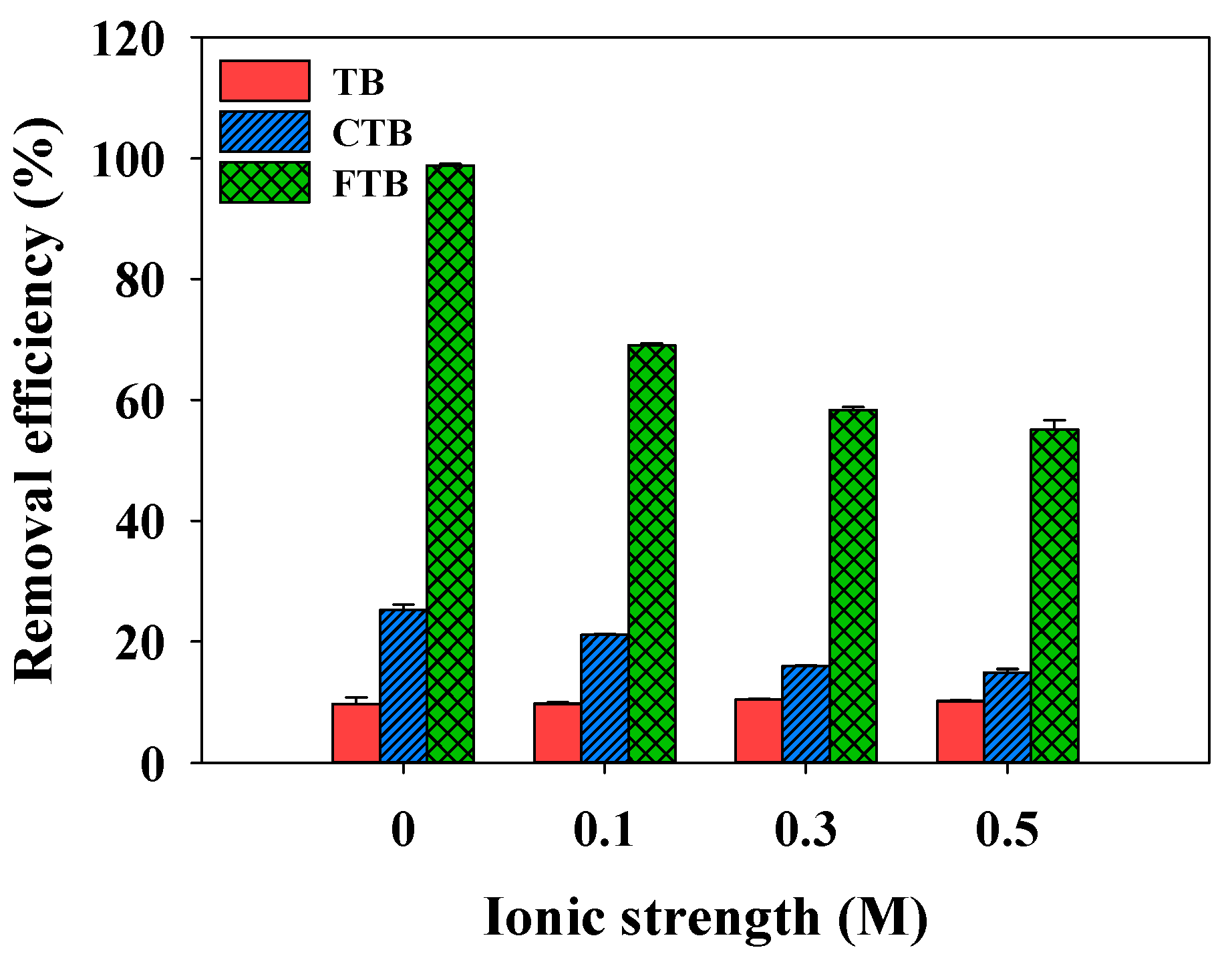
3.6. Effects of Ionic Strength on Adsorption of PO43−
The effects of ionic strength (ionic strength = 0–0.5 M) on the adsorption of PO43− by TB, CTB, and FTB are shown in Figure 8. The removal efficiency of PO43− by TB was not significantly affected by the ionic strength change (the removal efficiency of PO43− = 9.7%→10.5%). However, the removal efficiencies of PO43− using the CTB and FTB were gradually decreased with increasing ionic strengths (CTB: the removal efficiency of PO43− = 25.2%→14.8%; FTB: the removal efficiency of PO43− = 98.8%→55.1%). These observations suggested that increases in ionic strength might reinforce the electrostatic repulsion between PO43− and adsorbent surfaces, and activated adsorption sites on the surface might be reduced due to the compression of the electrical double layer on the adsorbent surfaces [55].
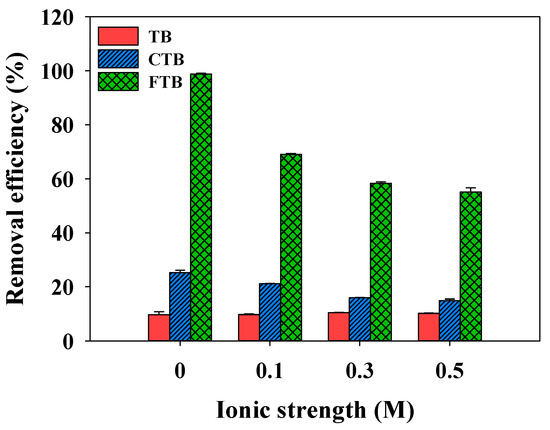
Figure 8.
The effects of ionic strength on the removal efficiency of PO43− by TB, CTB and FTB (adsorbent doses = 0.6 g L−1; PO43− = 1 mg L−1; agitation speed = 150 rpm; contact time = 24 h; pH = 7.0).
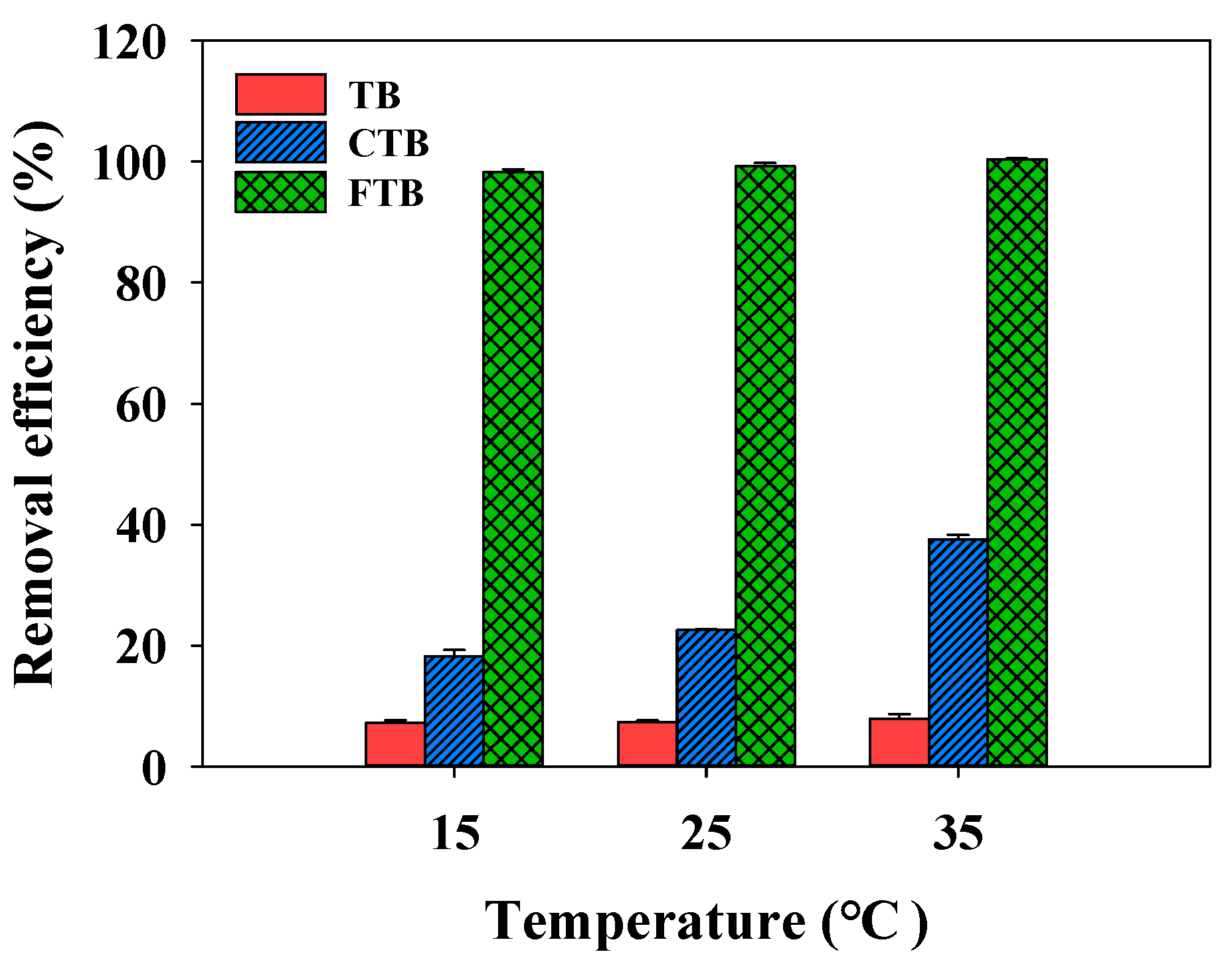
3.7. Effects of Temperature and Thermodynamic Analysis
The effects of the temperature on the removal efficiencies of the PO43− by TB, CTB, and FTB are compared in Figure 9 (temperature = 15–35 °C). The adsorption of PO43− on the CTB gradually increased with increasing temperature (Figure 9, the removal efficiency of CTB = 18.2%→37.5%). A possible explanation for these results is that increasing temperature cause more strong intermolecular motion and PO43− diffusion rate to the surface of CTB, which promoted the adsorption of PO43− on the CTB [56]. However, the removal efficiencies of PO43− by TB and FTB were not significantly affected by the temperature change (removal efficiency of TB = 7.3–7.9%; removal efficiency of FTB = 98.2–100.0%). Table 6 shows the values of the thermodynamic parameters (∆G°, ∆H°, and ∆S°) for PO43− removal by TB, CTB, and FTB according to the temperature (15–35 °C). The ∆G° < 0 and ∆H° > 0 suggested that the adsorption of PO43− on the TB, CTB, and FTB was a spontaneous and endothermic reaction [57,58]. Furthermore, ∆S° > 0 indicated that the adsorption of PO43− on the TB, CTB, and FTB was irreversible, which was conducive to the adsorption stability [56].
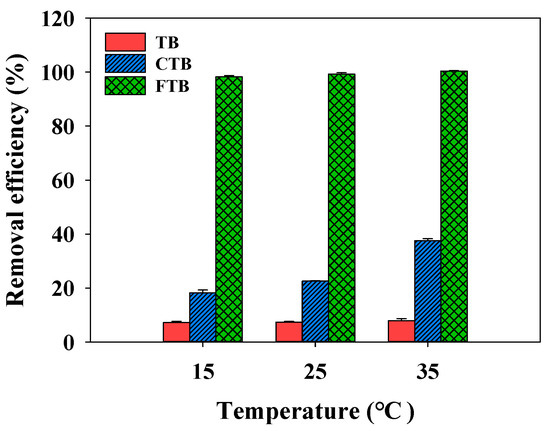
Figure 9.
The effects of temperature on the removal efficiency of PO43− by TB, CTB and FTB (adsorbent doses = 0.6 g L−1; PO43− = 1 mg L−1; agitation speed = 150 rpm; contact time = 24 h; pH = 7.0).

Table 6.
The thermodynamic parameters of PO43− adsorption onto TB, CTB, and FTB.
4. Conclusions
This study verified that pretreatment with CaCl2 and FeCl3 could improve the surface characteristics of tangerine peel biochars related to the adsorption behaviors of PO43−. The FTB might more effectively remove the PO43− (Qe, exp = 1.655 mg g−1) than TB (Qe, exp = 0.104 mg g−1) and CTB (Qe, exp = 0.354 mg g−1) due to the considerable enhancement of the physicochemical characteristics (specific surface area and surface characteristics). The removal efficiencies of PO43− by TB (R2 = 0.975) and CTB (R2 = 0.955) were more suitable for the Freundlich adsorption model (multilayer adsorption) and the FTB was well fitted to the Langmuir adsorption model (R2 = 0.987, monolayer adsorption). Furthermore, the thermodynamic analysis presented that the adsorption of PO43− for the FTB was more spontaneously endothermic than that for the TB and CTB under various pH and ionic strength conditions. These results are evidence that the chemical activation with FeCl3 might be a promising option to make the pristine tangerine peel biochar practically more relevant for the removal of PO43− in the aqueous solutions.
Author Contributions
Conceptualization, C.S.; methodology, W.A.; validation, I.J.; formal analysis, G.L.; data curation, C.S.; writing—original draft preparation, C.S.; writing—review and editing, Y.-G.L. and K.C.; supervision, K.C.; funding acquisition, Y.-G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A1A01073157).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kılıc, M.; Kırbıyık, C.; Çepelioğullar, Ö.; Pütün, A.E. Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl. Surf. Sci. 2013, 283, 856–862. [Google Scholar] [CrossRef]
- Bishop, M.J.; Powers, S.P.; Porter, H.J.; Peterson, C.H. Benthic biological effects of seasonal hypoxia in a eutrophic estuary predate rapid coastal development. Estuar. Coast. Shelf Sci. 2006, 70, 415–422. [Google Scholar] [CrossRef]
- Withers, P.J.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and eutrophication: Where do we go from here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Bennett, E.M.; Carpenter, S.R.; Caraco, N.F. Human impact on erodable phosphorus and eutrophication: A global perspective: Increasing accumulation of phosphorus in soil threatens rivers, lakes, and coastal oceans with eutrophication. BioScience 2001, 51, 227–234. [Google Scholar] [CrossRef]
- Oguz, E. Thermodynamic and kinetic investigations of PO3−4 adsorption on blast furnace slag. J. Colloid Interface Sci. 2005, 281, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Chubar, N.; Kanibolotskyy, V.; Strelko, V.; Gallios, G.; Samanidou, V.; Shaposhnikova, T.; Milgrandt, V.; Zhuravlev, I. Adsorption of phosphate ions on novel inorganic ion exchangers. Colloids Surf. A Physicochem. Eng. Asp. 2005, 255, 55–63. [Google Scholar] [CrossRef]
- Abu-Alhail, S.; Lu, X.W. Experimental investigation and modeling of innovative five-tank anaerobic-anoxic/oxic process. Appl. Math. Model. 2014, 38, 278–290. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Seida, Y.; Nakano, Y. Removal of phosphate by layered double hydroxides containing iron. Water Res. 2002, 36, 1306–1312. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Champagne, P.; Mabee, W. Overview of current biological and thermo-chemical treatment technologies for sustainable sludge management. Waste Manag. Res. 2014, 32, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Ayranci, E.; Hoda, N. Adsorption of bentazon and propanil from aqueous solutions at the high area activated carbon-cloth. Chemosphere 2004, 57, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Patra, B.S.; Baliarsingh, N.; Parida, K.M. Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl. Clay Sci. 2006, 32, 252–260. [Google Scholar] [CrossRef]
- Chen, J.; Kong, H.; Wu, D.; Chen, X.; Zhang, D.; Sun, Z. Phosphate immobilization from aqueous solution by fly ashes in relation to their composition. J. Hazard. Mater. 2007, 139, 293–300. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, L.; Chang, N.; Liu, J.; Duan, C.; Zhou, Q.; Li, X.; Wang, X. Removal of phosphate from water by activated carbon fiber loaded with lanthanum oxide. J. Hazard. Mater. 2011, 190, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Tong, S.; Zhao, S.; Jia, C.Q. Adsorption of polycyclic aromatic hydrocarbons from water using petroleum coke-derived porous carbon. J. Hazard. Mater. 2010, 181, 1115–1120. [Google Scholar] [CrossRef]
- Yin, Q.; Ren, H.; Wang, R.; Zhao, Z. Evaluation of nitrate and phosphate adsorption on Al-modified biochar: Influence of Al content. Sci. Total Environ. 2018, 631–632, 895–903. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, B.; Zimmerman, A.R.; Chen, H.; Zhang, M.; Cao, X. Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Bioresour. Technol. 2014, 152, 538–542. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lee, H.W.; Lee, S.-H.; Kim, S.-S.; Park, S.H.; Jeon, J.-K.; Kim, S.; Park, Y.-K. Pyrolysis properties and kinetics of mandarin peel. Korean J. Chem. Eng. 2011, 28, 2012–2016. [Google Scholar] [CrossRef]
- Biswas, B.K.; Inoue, J.-i.; Inoue, K.; Ghimire, K.N.; Harada, H.; Ohto, K.; Kawakita, H. Adsorptive removal of As(V) and As(III) from water by a Zr(IV)-loaded orange waste gel. J. Hazard. Mater. 2008, 154, 1066–1074. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Cao, X.; Lu, D.; Luo, F.; Shao, W. Preparation and evaluation of orange peel cellulose adsorbents for effective removal of cadmium, zinc, cobalt and nickel. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 512–521. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, B. Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem. Eng. J. 2013, 226, 286–292. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered biochar reclaiming phosphate from aqueous solutions: Mechanisms and potential application as a slow-release fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-K.; Jang, H.M.; Kan, E.; Wallace, A.R.; Sun, W. Adsorption of phosphate in water on a novel calcium hydroxide-coated dairy manure-derived biochar. Environ. Eng. Res. 2019, 24, 434–442. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.-f.; Wang, Y.-c. Application of magnesium modified corn biochar for phosphorus removal and recovery from swine wastewater. Int. J. Environ. Res. Public Health 2014, 11, 9217–9237. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, N.; Li, L.; An, J.-K.; Zhao, L.; Ren, N.-Q. Granulation and ferric oxides loading enable biochar derived from cotton stalk to remove phosphate from water. Bioresour. Technol. 2015, 178, 119–125. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, W.; Yan, J.; Han, L.; Gao, W.; Liu, R.; Chen, M. Effective removal of heavy metal by biochar colloids under different pyrolysis temperatures. Bioresour. Technol. 2016, 206, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Towns, T.G. Determination of aqueous phosphate by ascorbic acid reduction of phosphomolybdic acid. Anal. Chem. 1986, 58, 223–229. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’souza, S. Removal of copper ions by the filamentous fungus, Rhizopus oryzae from aqueous solution. Bioresour. Technol. 2008, 99, 3829–3835. [Google Scholar] [CrossRef]
- Gorgievski, M.; Božić, D.; Stanković, V.; Štrbac, N.; Šerbula, S. Kinetics, equilibrium and mechanism of Cu2+, Ni2+ and Zn2+ ions biosorption using wheat straw. Ecol. Eng. 2013, 58, 113–122. [Google Scholar] [CrossRef]
- Lee, J.-J. Study on equilibrium, kinetic and thermodynamic for adsorption of quinoline yellow by granular activated carbon. Clean Technol. 2014, 20, 35–41. [Google Scholar] [CrossRef][Green Version]
- Chen, S.; Qin, C.; Wang, T.; Chen, F.; Li, X.; Hou, H.; Zhou, M. Study on the adsorption of dyestuffs with different properties by sludge-rice husk biochar: Adsorption capacity, isotherm, kinetic, thermodynamics and mechanism. J. Mol. Liq. 2019, 285, 62–74. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Wu, X.; Zhou, W.; Zhu, L. Impact of mineral components in cow manure biochars on the adsorption and competitive adsorption of oxytetracycline and carbaryl. RSC Adv. 2017, 7, 2127–2136. [Google Scholar] [CrossRef]
- Luo, L.; Wang, G.; Shi, G.; Zhang, M.; Zhang, J.; He, J.; Xiao, Y.; Tian, D.; Zhang, Y.; Deng, S. The characterization of biochars derived from rice straw and swine manure, and their potential and risk in N and P removal from water. J. Environ. Manag. 2019, 245, 1–7. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Q.; Chen, J.; Zhang, L.; Chang, N. Phosphate adsorption on hydroxyl–iron–lanthanum doped activated carbon fiber. Chem. Eng. J. 2013, 215, 859–867. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, X.-J.; Domene, X.; Alcañiz, J.-M.; Liao, X.; Palet, C. Comparison of biochars derived from different types of feedstock and their potential for heavy metal removal in multiple-metal solutions. Sci. Rep. 2019, 9, 9869. [Google Scholar] [CrossRef]
- Binda, G.; Spanu, D.; Bettinetti, R.; Magagnin, L.; Pozzi, A.; Dossi, C. Comprehensive comparison of microalgae-derived biochar from different feedstocks: A prospective study for future environmental applications. Algal Res. 2020, 52, 102103. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Shan, R.; Wang, Y.; Yuan, H. A novel peat biochar supported catalyst for the transesterification reaction. Energy Convers. Manag. 2017, 139, 89–96. [Google Scholar] [CrossRef]
- Shakir, I.; Sarfraz, M.; Ali, Z.; Aboud, M.F.; Agboola, P.O. Magnetically separable and recyclable graphene-MgFe2O4 nanocomposites for enhanced photocatalytic applications. J. Alloy Compd. 2016, 660, 450–455. [Google Scholar] [CrossRef]
- Banerjee, S.; Mukherjee, S.; LaminKa-ot, A.; Joshi, S.R.; Mandal, T.; Halder, G. Biosorptive uptake of Fe2+, Cu2+ and As5+ by activated biochar derived from Colocasia esculenta: Isotherm, kinetics, thermodynamics, and cost estimation. J. Adv. Res. 2016, 7, 597–610. [Google Scholar] [CrossRef]
- Almasi, A.; Omidi, M.; Khodadadian, M.; Khamutian, R.; Gholivand, M.B. Lead (II) and cadmium (II) removal from aqueous solution using processed Walnut shell: Kinetic and equilibrium study. Toxicol. Environ. Chem. 2012, 94, 660–671. [Google Scholar] [CrossRef]
- Yan, H.; Shih, K. Effects of calcium and ferric ions on struvite precipitation: A new assessment based on quantitative X-ray diffraction analysis. Water Res. 2016, 95, 310–318. [Google Scholar] [CrossRef]
- Pellera, F.-M.; Giannis, A.; Kalderis, D.; Anastasiadou, K.; Stegmann, R.; Wang, J.-Y.; Gidarakos, E. Adsorption of Cu (II) ions from aqueous solutions on biochars prepared from agricultural by-products. J. Environ. Manag. 2012, 96, 35–42. [Google Scholar] [CrossRef]
- Mistar, E.; Ahmad, S.; Muslim, A.; Alfatah, T.; Supardan, M. Preparation and characterization of a high surface area of activated carbon from Bambusa vulgaris—Effect of NaOH activation and pyrolysis temperature. In Proceedings of the 3rd International Conference on Chemical Engineering Sciences and Applications 2017, Banda Aceh, Indonesia, 20–21 September 2017. [Google Scholar]
- Lee, K.-H.; Lee, Y.-G.; Shin, J.; Chon, K.; Lee, S.-H. Selective immobilization of antimony using brucite-rich precipitate produced during in situ hypochlorous acid formation through seawater electrolysis in a nuclear power plant. Energies 2020, 13, 4493. [Google Scholar] [CrossRef]
- Shin, J.; Kwak, J.; Lee, Y.-G.; Kim, S.; Choi, M.; Bae, S.; Lee, S.-H.; Park, Y.; Chon, K. Competitive adsorption of pharmaceuticals in lake water and wastewater effluent by pristine and NaOH-activated biochars from spent coffee wastes: Contribution of hydrophobic and π-π interactions. Environ. Pollut. 2021, 270, 116244. [Google Scholar] [CrossRef]
- Kim, H.; Ko, R.-A.; Lee, S.; Chon, K. Removal efficiencies of manganese and iron using pristine and phosphoric acid pre-treated biochars made from banana peels. Water 2020, 12, 1173. [Google Scholar] [CrossRef]
- Hayati, B.; Mahmoodi, N.M. Modification of activated carbon by the alkaline treatment to remove the dyes from wastewater: Mechanism, isotherm and kinetic. Desalination Water Treat. 2012, 47, 322–333. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y.; Lu, X. Biochars with excellent Pb (II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour. Technol. 2017, 232, 204–210. [Google Scholar] [CrossRef]
- Sarkhot, D.V.; Ghezzehei, T.A.; Berhe, A.A. Effectiveness of biochar for sorption of ammonium and phosphate from dairy effluent. J. Environ. Qual. 2013, 42, 1545–1554. [Google Scholar] [CrossRef]
- Liu, F.; Zuo, J.; Chi, T.; Wang, P.; Yang, B. Removing phosphorus from aqueous solutions by using iron-modified corn straw biochar. Front. Environ. Sci. Eng. 2015, 9, 1066–1075. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, W.-J.; Zhang, N.; Li, Y.-S.; Jiang, H.; Sheng, G.-P. Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution. Bioresour. Technol. 2014, 169, 403–408. [Google Scholar] [CrossRef]
- Behera, S.; Oh, S.; Park, H. Sorptive removal of ibuprofen from water using selected soil minerals and activated carbon. Int. J. Environ. Sci. Technol. 2012, 9, 85–94. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Zhang, Y.; Liu, S.; Wang, C.; Chen, W.; Liu, C.; Chen, Z.; Zhang, Y. ZnCl2 modified biochar derived from aerobic granular sludge for developed microporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2020, 297, 122381. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, K.; Meng, X.; Li, J.; Guan, X.; Sun, Q.; Sun, Y.; Wang, W.; Lin, M.; Liu, M.; et al. New use for spent coffee ground as an adsorbent for tetracycline removal in water. Chemosphere 2019, 215, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jiang, Y.-s.; Li, F.-y.; Yang, D.-y. Preparation of biochar by simultaneous carbonization, magnetization and activation for norfloxacin removal in water. Bioresour. Technol. 2017, 233, 159–165. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).