Abstract
To address the challenges posed by extensive sample pretreatment and significant matrix interference in conventional metal quantification methods for cyanobacterial culture media, an automated online metal capture and enrichment system was developed and integrated with inductively coupled plasma mass spectrometry (ICP-MS). This system enabled the simultaneous determination of nine metal elements—Cd, Pb, V, Mn, Fe, Co, Ni, Cu, and Zn—within the culture medium. Through systematic optimization and validation, the method demonstrated exceptional analytical performance: calibration curves for all analytes exhibited correlation coefficients (r) exceeding 0.999; repeatability tests yielded relative standard deviations (RSD) below 3% (n = 6); and recoveries at low, medium, and high spike levels ranged from 93.98% to 108.70%. The procedure is characterized by simplicity, high automation, low detection limits, and robust accuracy, making it an effective platform for multi-element contamination monitoring and metal metabolic studies in cyanobacterial cultivation. This approach holds significant potential for applications in algal resource utilization and environmental restoration.
1. Introduction
The rapid global industrialization has exacerbated heavy metal contamination in aquatic environments. These persistent pollutants, with their high toxicity, bioaccumulative nature, and resistance to degradation, pose significant threats to ecological stability and human health, becoming a central concern in international environmental management [1,2]. Consequently, considerable global efforts and resources have been invested in developing advanced technologies for the monitoring, treatment, and restoration of heavy metal-contaminated water systems [3,4]. Among the various remediation strategies, algal-based approaches have emerged as highly promising due to their environmental compatibility, cost-effectiveness, and high heavy metal adsorption capacity. Algae, as primary producers in aquatic ecosystems, are widespread, exhibit rapid reproduction, and possess exceptional metal ion enrichment capabilities, making them invaluable for research and practical applications in water purification [5,6,7]. Current research primarily focuses on three key areas: investigating the toxic effects and mechanisms of trace metals (e.g., iron, copper, manganese, zinc, lead, cadmium, etc.) on algal growth, metabolism, photosynthesis, and antioxidant systems [8,9,10]; exploring algal tolerance, adsorption kinetics, enrichment behavior, and molecular detoxification pathways [11,12]; and developing in situ and ex situ bioremediation frameworks and engineering applications using algal systems [13]. Evidence suggests that metal uptake, transport, and speciation dynamics vary significantly across algal growth stages, while metal content and speciation within the culture medium are continuously transforming [14]. Thus, establishing a precise and efficient analytical method to monitor metal dynamics in algal cultures is crucial for advancing understanding of algae-metal interactions, optimizing bioremediation processes, evaluating treatment efficacy, and ensuring aquatic environmental safety.
Existing analytical methods for detecting metal elements in algal culture systems include spectrophotometry [15,16], anodic stripping voltammetry [17], and inductively coupled plasma mass spectrometry (ICP-MS) [18,19], each with distinct limitations. Spectrophotometry involves labor-intensive procedures and lacks the ability to simultaneously quantify multiple elements. Anodic stripping voltammetry relies on mercury-based electrodes, posing toxic risks and operational challenges. While ICP-MS offers rapid and sensitive multi-element detection, its sample introduction system is limited to low salinity (total dissolved solids, TDS < 0.2%), making it unsuitable for high-salinity algal media. High salinity can lead to nebulizer and orifice clogging, increased matrix interference, and unstable signals, thereby reducing analytical accuracy [20]. Conventional corrective methods are insufficient: internal standardization fails to prevent damage to the sample introduction system [21], dilution diminishes analyte concentrations below detection limits while introducing additional uncertainties [22], and resin-based preconcentration and separation provide a more effective solution [23]. Chelating resins with iminodiacetic acid (IDA) and ethylenediaminetetraacetic acid (EDTA) functional groups exhibit strong selectivity through stable five-membered ring complexation with metal ions and are widely used [24,25]. However, most current implementations still rely on manual operation, limiting analytical efficiency and hindering high-throughput analysis.
To address these analytical challenges, the coupling of online solid-phase extraction (SPE) with ICP-MS has emerged as a robust and versatile solution [26,27,28]. This field is currently advancing along two key paths: the development of advanced sorbent materials and the design of integrated, automated systems. A new generation of functional materials is showing exceptional performance. For example, amine-functionalized polyacrylonitrile fibers provide specialized chelation sites [29], while ion-imprinted polymers utilize molecular recognition through custom-designed cavities for specific metal ions [30]. Expanding on these, Danthron-impregnated carbon nanotubes take advantage of synergistic interactions on high-surface-area platforms [31], and polymer-grafted chitosan microspheres combine the natural biopolymer backbone with synthetic functional groups for enhanced performance [32]. At the same time, innovations in system design include 3D-printed columns with monolithic packings, which enable custom flow-path geometries to enhance extraction efficiency [33], and fully automated online ion exchange systems that efficiently handle complex sample matrices, increasing throughput and reproducibility [34]. Techniques like magnetic solid-phase microextraction further elevate extraction control by utilizing external fields to accelerate the process and improve kinetics [35].
Despite these advancements, challenges remain when applying existing online-SPE-ICP-MS methods to complex, high-salinity algal cultivation media. Although novel sorbents show improved selectivity in controlled conditions, their performance diminishes significantly in biological matrices due to fouling by extracellular polymeric substances and competitive ion effects. System robustness is another concern, as salt deposition in 3D-printed columns and automated systems can lead to clogging, necessitating frequent maintenance and undermining throughput. Additionally, many current methods focus on a limited number of toxic metals (typically 3–4) and fail to account for essential nutrient elements critical to understanding algal-metal interactions. These gaps highlight the need for more comprehensive approaches tailored to complex biological matrices.
This study presents an integrated online process designed to address these limitations for analyzing cyanobacterial culture media. Three key innovations are introduced: (1) a systematically optimized chelating resin and flow protocol that ensures exceptional stability against matrix effects and prevents salt-induced clogging; (2) a comprehensive analytical method for the simultaneous determination of nine trace metals (Cd, Pb, V, Mn, Fe, Co, Ni, Cu, Zn) with high precision at ultra-trace levels; and (3) a fully automated platform that eliminates manual preprocessing, reducing sample preparation time by over 60% and minimizing contamination risks. This approach not only provides a robust solution for monitoring metal dynamics in algal systems but also significantly enhances the applicability of online-SPE-ICP-MS for complex biological matrices, opening new avenues for environmental remediation research.
2. Materials and Methods
2.1. Materials
All reagents used were of analytical grade or higher, unless otherwise specified. The materials were sourced as follows: ultrapure water with a resistivity of 18.2 MΩ·cm was employed throughout the experiments; electronic-grade nitric acid, ammonia solution, and acetic acid were provided by Jingrui Electronic Materials (Suzhou, China); HPLC-grade ammonium acetate solution was obtained from Prin-Cen Scientific (Guangzhou, China); and sodium chloride (NaCl), calcium chloride (CaCl2), and potassium dihydrogen phosphate (KH2PO4) were supplied by Sinopharm Chemical Reagent (Shanghai, China). A mixed standard solution containing Cd, Pb, V, Mn, Fe, Co, Ni, Cu, and Zn (10 mg/L each) was acquired from PerkinElmer (Waltham, MA, USA).
Cyanobacterial strains and the culture medium were obtained from the Freshwater Algae Culture Collection at the Institute of Hydrobiology (FACHB), Chinese Academy of Sciences (Wuhan, China). The species used were Microcystis (FACHB1347), Planktothrix (FACHB1355), Dolichospermum (FACHB1389), and Aphanizomenon (FACHB1398), all cultured in BG-11 medium [36]. The cyanobacterial culture medium was filtered using a polyethersulfone (PES) syringe filter (SCAA-02, 13 mm × 0.22 μm) from Shanghai Anpel Experimental Technology (Shanghai, China).
2.2. Instrumentation
2.2.1. Metal Capture/Enrichment
An MSS-2 online metal capture and enrichment system (Prin-Cen Scientific, Guangzhou, China) was used for automated desalination and enrichment. The setup included a metal capture column (IDA/EDTA, 50 mm × 4 mm, 10 μm) and a metal enrichment column (IDA/EDTA, 50 mm × 3 mm, 10 μm), designed to reduce matrix interference and concentrate target metal ions, respectively. The operational workflow is outlined in Figure 1. Briefly, the 2 mol/L ammonium acetate solution (pH 6.0) and ultrapure water were purified online by passing through the capture column, thereby reducing the reagent blank. The autosampler aspirated the sample, mixed it with the ammonium acetate solution online, and transferred it to the enrichment column, where the target metals were selectively retained through chelation while salts and other contaminants were discarded. Ultrapure water was then passed through the tubing and enrichment column to remove residual ammonium acetate. The retained metals were eluted using a 5% nitric acid solution, and the eluate was directly transferred to the ICP-MS for quantification. The entire process followed a precise timing sequence: 0–1.5 min, ammonium acetate solution at 1.0 mL/min; 1.5–3.0 min, ultrapure water at 1.0 mL/min; 3.0–4.5 min, 5% nitric acid solution at 1.0 mL/min; 4.5–6.0 min, ammonium acetate solution at 1.0 mL/min to recondition the system.
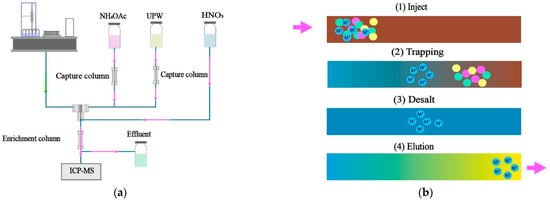
Figure 1.
Schematic illustration of the metal capture/enrichment system: (a) operational workflow and (b) enrichment process. Pink arrows indicate the direction of fluid flow.
2.2.2. ICP-MS
A NexION-300X ICP-MS (PerkinElmer, Waltham, MA, USA) was employed for multi-element analysis of the cyanobacterial culture medium. Prior to analysis, the instrument was optimized using a 1 μg/L tuning solution containing 9Be, 114In, 140Ce, and 238U to meet the following performance criteria: 9Be > 2000, 114In > 40,000, 238U > 30,000, with double charge and oxide ratios maintained below 3%. The final operating conditions of the ICP-MS included a concentric nebulizer, conventional plasma mode, an 8 mm sampling depth, radio frequency (RF) power of 1400 W, spray chamber temperature of 2 °C, plasma gas flow rate of 15.0 L/min, carrier gas flow rate of 1.12 L/min, and auxiliary gas flow rate of 0.20 L/min.
2.2.3. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
The sample solution obtained from the metal capture/enrichment process was diluted tenfold with a 2% nitric acid solution before analysis. An OPTIMA 8000DV ICP-OES (PerkinElmer, Waltham, MA, USA) was used to quantify the signal intensities of sodium (Na), calcium (Ca), and potassium (K) ions in the diluted sample. For accurate and stable measurements, detection wavelengths were set to 589.592 nm for Na, 317.933 nm for Ca, and 766.49 nm for K. A uniform radial viewing mode was maintained throughout the detection process to ensure consistency and reliability of the data.
2.3. Sample Preparation and Pretreatment
2.3.1. Sample Preparation
Cyanobacterial stock cultures were inoculated into BG-11 medium and maintained under strictly controlled conditions: pH 7.1–7.2, temperature 25 °C, light intensity of 25 μmol/(m2·s), and a 12 h light–dark cycle. Cultures were collected during the logarithmic growth phase for subsequent analysis.
Simulated Test Solution: A 100 mL mixture was prepared using ultrapure water with 1.5% NaCl, 0.25% CaCl2, and 0.25% KH2PO4 to simulate the ionic composition of actual saline matrices. Standard solutions of Cd, Pb, V, Mn, Fe, Co, Ni, Cu, and Zn were added to achieve a final concentration of 5 μg/L for each metal.
2.3.2. Sample Pretreatment
A 30 mL aliquot of the cyanobacterial culture medium was transferred to a 50 mL centrifuge tube and centrifuged at 11,000 rpm for 10 min at 4 °C. The resulting supernatant was filtered through a 0.22 μm membrane into a clean 50 mL centrifuge tube for further analysis.
3. Results and Discussion
3.1. Optimization of Detection Conditions
3.1.1. Optimization of Buffer pH
The pH of the buffer system is a critical factor influencing both the recovery efficiency of target elements and the removal of matrix salts during chelating resin-based enrichment. Previous studies have shown that mildly acidic conditions enhance the resin’s affinity for most metal ions; however, the buffer pH must remain within a precisely controlled range to ensure optimal performance [37]. If the pH is too low, protonation of the resin’s functional groups occurs, significantly reducing their complexation capacity and thereby decreasing recovery efficiency [38]. Conversely, excessively high pH levels diminish the resin’s selectivity for abundant salt matrix ions (e.g., Na+, K+, Ca2+), leading to insufficient desalting and increased matrix interference, which compromises the accuracy of subsequent analytical detection.
To systematically assess the synergistic influence of pH on metal enrichment efficiency and salt elimination, the Simulated Test Solution described in Section 2.3.1 was selected as the study matrix. A series of ammonium acetate buffers (2 mol/L) with pH values adjusted across a gradient of 4.5–7.0 using acetic acid or ammonia solution was employed. While the theoretical optimal buffering ranges for ammonium acetate are around pH 4.76 and 9.25, the high concentration used in this study enhances substantial buffer capacity, maintaining a stable chelation environment across the investigated pH range [39]. The signal intensities of the nine target metal elements under varying pH conditions were quantified using the online metal capture/enrichment–ICP-MS system. Simultaneously, the effluent was collected, and the remaining Na+, K+, and Ca2+ concentrations were determined by ICP-OES to evaluate the salt matrix removal efficiency. The experimental results are shown in Figure 2.
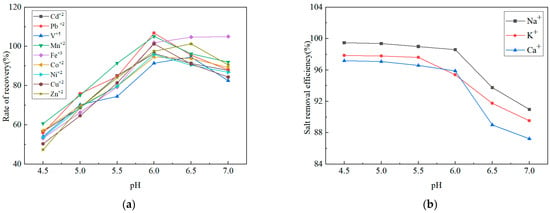
Figure 2.
Effect of buffer pH on (a) recovery rates of nine metal elements and (b) salt removal efficiency.
The results revealed that when the buffer pH was below 6.0, retention of all nine target metals on the enrichment column was significantly inadequate, resulting in low recoveries. As the pH increased, recoveries of Zn and Co improved, but salt removal efficiency decreased, indicating higher residual matrix content. A comprehensive evaluation of both parameters identified pH 6.0 as the optimal condition, offering high enrichment efficiency for all target metals and effective salt removal. This condition balanced detection sensitivity and resistance to matrix interference, providing a reliable pretreatment foundation for precise subsequent analysis.
3.1.2. Optimization of Elution Conditions
Following adsorption of the target metal ions onto the enrichment column and subsequent desalting through rinsing, efficient elution with an appropriately concentrated nitric acid solution is essential to ensure the effective transfer of enriched analytes into the ICP-MS system. Optimization of the elution parameters constitutes a critical step for maintaining analytical accuracy and sensitivity. In this study, nitric acid served as the eluent, with five concentration gradients (1%, 2%, 5%, 6%, and 8% (v/v)) established for evaluation. Elution performance was systematically assessed by measuring the signal responses of nine target metal elements in the simulated experimental solution described in Section 2.3.1, with the results presented in Figure 3.
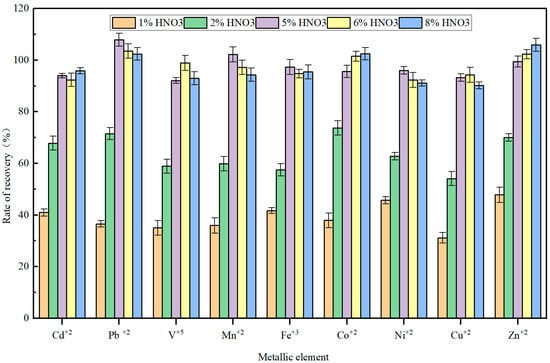
Figure 3.
Effect of nitric acid eluent concentration on the recovery rates of nine metal elements.
The experimental data revealed that nitric acid concentration significantly impacts metal recovery efficiency. At lower concentrations (1–2%), the hydrogen ion activity is insufficient to effectively compete with the chelating sites on the resin, resulting in incomplete desorption of metal ions. This leads to pronounced tailing of elution peaks and weak detection signals for the target elements. As nitric acid concentration increased to 5% or higher, signal intensities of metal elements stabilized, with no further improvement at 6% or 8%, indicating that 5% nitric acid is sufficient for complete elution. However, excessively high concentrations (e.g., 8%) accelerate corrosion of critical components such as nebulizers, spray chambers, central tubes, and sampling or skimmer cones, while also promoting the formation of polyatomic ion interferences (e.g., ArO+, ClO+), thereby compromising long-term analytical stability and data reliability [20].
Balancing elution efficiency with instrument durability, 5% (v/v) nitric acid was identified as the optimal eluent. This concentration ensures complete metal ion elution and high recovery, while minimizing chemical degradation of the injection system, thereby ensuring both analytical accuracy and operational safety.
3.1.3. Optimization of Flow Rates
In online preconcentration systems, the sample loading flow rate is a critical parameter for balancing analytical efficiency with preconcentration recovery. To systematically evaluate the combined effects of flow rate on metal adsorption and salt removal, optimization experiments were performed within a flow rate range of 0.8 to 1.2 mL/min. Extraction efficiency was assessed by measuring the recovery rates of nine target elements, while the residual concentrations of Na+, K+, and Ca2+ in the collected eluate were determined via ICP-OES to evaluate matrix removal performance.
As shown in Figure 4, both metal recovery rates and salt removal efficiency increased as the flow rate was raised from 0.8 to 1.0 mL/min. This trend indicates that at the lower flow rate of 0.8 mL/min, the insufficient volume of eluent passing through the resin per unit time resulted in inadequate salt ion removal, limiting desalting efficiency. A moderate increase in flow rate enhanced fluid mass transfer, significantly improving salt removal while maintaining effective metal adsorption kinetics. However, at 1.2 mL/min, the recovery rates of target elements decreased sharply, despite sustained desalting efficiency. The high flow rate reduced the contact time between metal ions and the resin, leading to incomplete adsorption.
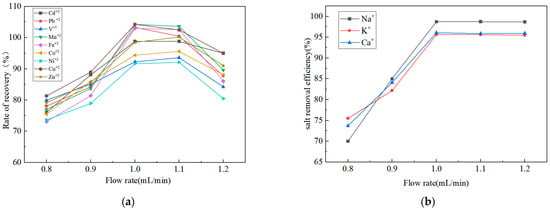
Figure 4.
Effect of flow rates on (a) recovery rates of nine metal elements and (b) salt removal efficiency.
Based on these results, a flow rate of 1.0 mL/min was selected as the optimal condition for subsequent experiments. This parameter strikes a balance between high metal recovery rates and effective salt matrix removal, ensuring efficient preconcentration while minimizing the risk of flow path blockage caused by high-salinity matrices. Under this optimized flow rate, a 1.5 min desalting duration was established and validated, effectively removing residual salts without causing analyte loss.
3.2. Linear Range and Limit of Detection (LOD)
Under optimized conditions, the method’s linearity, sensitivity, and detection selectivity were systematically evaluated. A mixed standard stock solution containing the nine target elements was serially diluted with 1% nitric acid to prepare calibration standards at six concentration levels. Calibration curves were constructed by plotting the chromatographic peak area (y) against the corresponding concentration (x), all of which demonstrated excellent linearity, with correlation coefficients (R) exceeding 0.999 across their respective ranges.
The LODs were determined at a signal-to-noise ratio (S/N) of 3, ranging from 0.1000 to 114.1 ng/L. To demonstrate the method’s detection capability and selectivity at trace levels, chromatograms acquired at the lower limit of quantification (LLOQ) for each element are presented in Figure 5. All peaks at the LLOQ were clearly distinguishable from the baseline, with S/N ratios exceeding 10, confirming the method’s excellent selectivity and robustness for accurate quantification at ultra-trace concentrations. Key analytical performance parameters, including retention time, regression equation, linear range, correlation coefficient, and LOD, are summarized in Table 1.
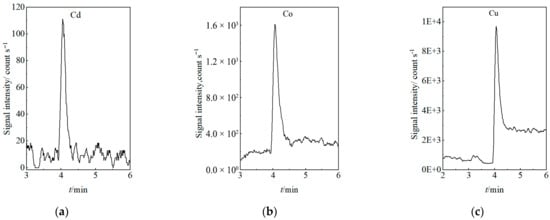
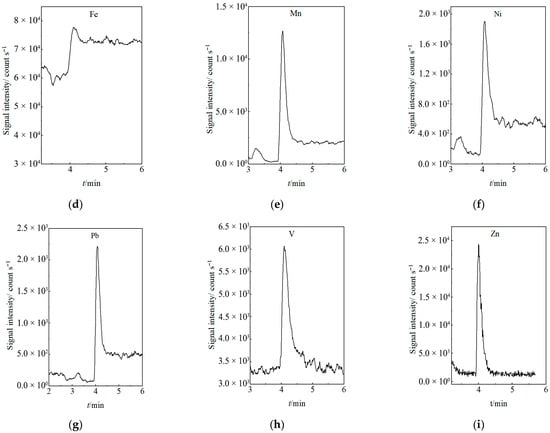
Figure 5.
Chromatograms of the Nine Target Elements at LLOQ: (a) Cd, (b) Co, (c) Cu, (d) Fe, (e) Mn, (f) Ni, (g) Pb, (h) V, (i) Zn.

Table 1.
Linear equations, correlation coefficients, linear ranges, and LOD for the Nine Target Elements.
3.3. Precision Experiment
Method precision was evaluated by spiking Simulated Test Solutions with the nine metal standard solutions at low, medium, and high quality control (QC) levels, according to the procedure outlined in Section 2.3.1. Six parallel samples were analyzed for each level. As shown in Table 2, the relative standard deviation (RSD) for all elements at each QC level (n = 6) was below 2%. These results confirm that the method exhibits excellent repeatability and measurement accuracy within the validated concentration range.

Table 2.
Results of Precision Experiment (n = 6).
3.4. Spike Recovery Experiment
An aliquot of Dolichospermum culture medium was treated according to the procedure outlined in Section 2.3.2 and subsequently analyzed. A spike recovery assessment for nine metal elements was conducted at three concentration levels (n = 3). The corresponding detection data and recovery rates are summarized in Table 3. Average recoveries ranged from 93.98% to 108.7%, meeting the accepted accuracy criteria for trace metal determination and confirming the method’s precision and reliability. This approach effectively minimizes element loss and external contamination during sample pretreatment, ensuring stable and accurate quantification of target metal elements in cyanobacterial culture media.

Table 3.
Recovery Rates of the Nine Target Elements in the Spiked Dolichospermum Culture Medium.
3.5. Evaluation of Analytical Carryover
Potential carryover was evaluated by analyzing ultrapure water blanks immediately following the analysis of a high-concentration standard solution prepared at the upper limit of each analyte’s calibration curve. Carryover was calculated as the percentage of analyte signal detected in the blank relative to the high standard. The carryover for all target metals was found to be below 0.1%, which is considered negligible. Specifically, the carryover values were quantified as follows: V (<0.04%), Mn (<0.06%), Co (<0.01%), Cu (<0.02%), Cd (<0.02%), Fe (<0.04%), Pb (<0.02%), Ni (<0.02%), and Zn (<0.05%). This result confirms that the optimized elution and washing steps in the online procedure effectively prevent cross-contamination between runs, ensuring the reliability of the automated sequential analysis.
3.6. Evaluation of Matrix Effect
The method’s tolerance to complex matrices was systematically evaluated by introducing an online internal standard for real-time monitoring. After desalting and preconcentration with the metal capture system, a 10 μg·L−1 rhodium (Rh) internal standard was added via a T-connector and peristaltic pump, mixing online with the sample eluent before ICP-MS analysis. A simulated test solution and four typical algal culture media (Microcystis, Planktothrix, Dolichospermum, Aphanizomenon) were selected as test subjects and analyzed sequentially. The fluctuation in the Rh signal intensity during the ICP-MS analysis served as the key metric for quantifying interference from the different algal culture media matrices.
As shown in Figure 6, the Rh internal standard signal intensities remained consistent across all tested samples after desalting and preconcentration, with an RSD of ≤3%. No significant signal enhancement or suppression was observed. This result robustly demonstrates that the method effectively mitigates interference from matrix components such as organic biomacromolecules and high-salinity constituents in algal culture media. Therefore, the method exhibits exceptional anti-interference capability and analytical stability across diverse sample matrices.
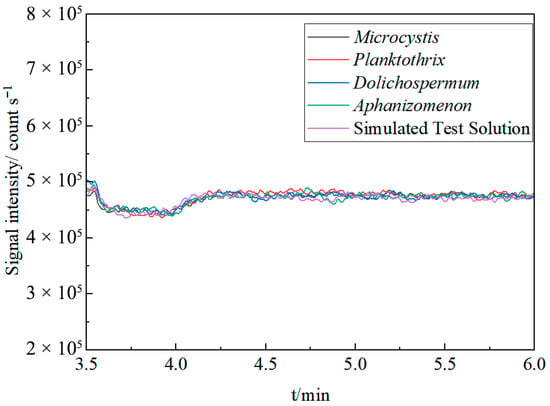
Figure 6.
Stability of Rh internal standard signal in different matrix samples.
3.7. Detection in Various Cyanobacteria Culture Media
Under optimized analytical conditions, the concentrations of nine metal elements were quantified in the culture media of four cyanobacterial species—Microcystis, Planktothrix, Dolichospermum, and Aphanizomenon, as summarized in Table 4. Comparative analysis revealed distinct interspecies variations in metal uptake capacities, likely influenced by species-specific biological characteristics such as cellular architecture, metabolic processes, and the diversity and abundance of membrane-associated metal transport proteins.

Table 4.
Determination of Nine Metal Elements in Cyanobacteria Culture Media (μg/L).
This analytical approach refines the methodology for assessing metal dynamics in cyanobacterial systems, providing a solid theoretical and empirical foundation for selecting strains with specific metal accumulation traits. Furthermore, it enhances the potential of cyanobacteria as biological agents for mitigating metal contamination in aquatic ecosystems.
3.8. Method Comparison
A comparative analysis with recently developed online SPE coupled with ICP-MS (online SPE-ICP-MS) methods highlights the distinct advantages of the present approach in handling complex biological matrices (Table 5). While established techniques, such as 3D-printed monolith SPE and magnetic-assisted SPME, offer favorable analytical speed (5–7 min per sample) and precision in environmental water analysis, their applications have been primarily limited to groundwater, river water, and seawater matrices. To date, no successful application has been reported for complex biological matrices characterized by high salinity and organic content.

Table 5.
Performance comparison of the developed method with similar published approaches.
This study addresses the challenges posed by cyanobacterial culture media—a complex system enriched with inorganic salts and organic biomacromolecules. Through systematic optimization of chelating resin performance and flow path parameters, an efficient automated analytical method was developed without relying on novel functional materials or sophisticated instrumentation. The method achieves a single-run analysis time of just 6.5 min, with RSD below 3% for all target elements, demonstrating excellent analytical precision. Moreover, it exhibits outstanding sensitivity, with detection limits for most metal elements in the 0.1–0.8 ng/L range.
4. Conclusions
This study established an automated online analytical approach for the simultaneous quantification of multiple metal elements (Cd, Pb, V, Mn, Fe, Co, Ni, Cu, Zn) in cyanobacterial culture media. The method combined online metal capture and enrichment with ICP-MS, achieving high analytical sensitivity and precision. Systematic optimization of enrichment parameters identified an ammonium acetate buffer (pH = 6.0) and a 5% nitric acid eluent as the optimal combination, facilitating efficient metal accumulation while removing matrix salts, ensuring accurate quantification.
Method validation confirmed excellent linearity (r > 0.999), high precision (RSD < 3%), and acceptable spike recoveries (93.98–108.7%), demonstrating both analytical reliability and operational robustness. Comparative analysis of four cyanobacterial culture media revealed distinct metal absorption capacities across species. These results provide crucial data for understanding cyanobacteria–metal interactions and establish a solid methodological foundation for exploring cyanobacteria-based strategies for mitigating metal contamination in aquatic ecosystems.
Author Contributions
Conceptualization, J.M. and Y.Z.; methodology, J.M. and S.W.; software, S.W. and F.Z.; validation, F.Z. and Z.Q.; formal analysis, X.C. and Z.Q.; investigation, J.M. and L.Z.; resources, L.Z.; data curation, S.W. and X.C.; writing—original draft preparation, J.M. and Y.Z.; writing—review and editing, Y.Z.; supervision, F.G.; project administration, J.M. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors gratefully acknowledge the technical support provided by Yan Wang, Manyi Wang, and Min Wang at the Analytical Testing Center of the Institute of Hydrobiology, Chinese Academy of Sciences.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Heavy metals in Iberian soils: Removal by current adsorbents/amendments and prospective for aerogels. Adv. Colloid. Interface Sci. 2016, 237, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Cozma, P.; Roșca, M.; Minuț, M.; Gavrilescu, M. Phytoremediation: A sustainable and promising bio-based approach to heavy metal pollution management. Sci. Total Environ. 2025, 1001, 180458. [Google Scholar] [CrossRef]
- Liu, K.; Li, X.; Lei, P.; Wang, H.; Yuan, S.; Li, L.; Dai, X. Heavy metals removal from stormwater runoff by bioretention cells: Recent advances and future prospects. J. Water Process Eng. 2025, 75, 108027. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Salama, E.; Roh, H.; Dev, S.; Khan, M.A.; Abou-Shanab, R.A.I.; Chang, S.W.; Jeon, B. Algae as a green technology for heavy metals removal from various wastewater. World J. Microbiol. Biotechnol. 2019, 35, 75. [Google Scholar] [CrossRef]
- Singh, D.V.; Bhat, R.A.; Upadhyay, A.K.; Singh, R.; Singh, D.P. Microalgae in aquatic environs: A sustainable approach for remediation of heavy metals and emerging contaminants. Environ. Technol. Innov. 2021, 21, 101340. [Google Scholar] [CrossRef]
- Facey, J.A.; Violi, J.P.; King, J.J.; Sarowar, C.; Apte, S.C.; Mitrovic, S.M. The Influence of Micronutrient Trace Metals on Microcystis aeruginosa Growth and Toxin Production. Toxins 2022, 14, 812. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Y.; Wu, J.; Kang, X.; Sheng, X.; Tan, Z. Integration of bioaccumulations, chemical forms and gene expression responses to understand the transformation and detoxification of inorganic arsenic, cadmium and lead in the brown seaweed Sargassum fusiforme. Ecotoxicol. Environ. Saf. 2025, 300, 118453. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B. Heavy metal-induced stress in eukaryotic algae-mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef]
- Xiao, X.; Li, W.; Jin, M.; Zhang, L.; Qin, L.; Geng, W. Responses and tolerance mechanisms of microalgae to heavy metal stress: A review. Mar. Environ. Res. 2023, 183, 105805. [Google Scholar] [CrossRef]
- Khatiwada, B.; Hasan, M.T.; Sun, A.; Kamath, K.S.; Mirzaei, M.; Sunna, A.; Nevalainen, H. Proteomic response of Euglena gracilis to heavy metal exposure—Identification of key proteins involved in heavy metal tolerance and accumulation. Algal Res. 2020, 45, 101764. [Google Scholar] [CrossRef]
- Rempel, A.; Gutkoski, J.P.; Nazari, M.T.; Biolchi, G.N.; Cavanhi, V.A.F.; Treichel, H.; Colla, L.M. Current advances in microalgae-based bioremediation and other technologies for emerging contaminants treatment. Sci. Total Environ. 2021, 772, 144918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, L.; Deng, X.; Gao, K.; He, H.; Mao, B. Passive leakage dominates cadmium-induced release of dissolved extracellular organic matters from Chlorella vulgaris. Bioresour. Technol. 2025, 437, 133079. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Peng, F.; Zhang, L.; Ying, G. Biosorption of zinc and copper from aqueous solutions by two freshwater green microalgae Chlorella pyrenoidosa and Scenedesmus obliquus. Environ. Sci. Pollut. Res. 2011, 19, 2918–2929. [Google Scholar] [CrossRef]
- Makhanya, B.N.; Nyandeni, N.; Ndulini, S.F.; Mthembu, M.S. Application of green microalgae biofilms for heavy metals removal from mine effluent. Phys. Chem. Earth Parts A/B/C 2021, 124, 103079. [Google Scholar] [CrossRef]
- Vasconcelos, M.T.S.D.; Leal, M.F.C. Exudates of different marine algae promote growth and mediate trace metal binding in Phaeodactylum tricornutum. Mar. Environ. Res. 2008, 66, 499–507. [Google Scholar] [CrossRef]
- De Paepe, J.; Garcia Gragera, D.; Arnau Jimenez, C.; Rabaey, K.; Vlaeminck, S.E.; Godia, F. Continuous cultivation of microalgae yields high nutrient recovery from nitrified urine with limited supplementation. J. Environ. Manag. 2023, 345, 118500. [Google Scholar] [CrossRef]
- Clagnan, E.; D’Imporzano, G.; Dell’Orto, M.; Bani, A.; Dumbrell, A.J.; Parati, K.; Acien-Fernandez, F.G.; Portillo-Hahnefeld, A.; Martel-Quintana, A.; Gomez-Pinchetti, J.L.; et al. Centrate as a sustainable growth medium: Impact on microalgal inocula and bacterial communities in tubular photobioreactor cultivation systems. Bioresour. Technol. 2022, 363, 127979. [Google Scholar] [CrossRef]
- Agatemor, C.; Beauchemin, D. Matrix effects in inductively coupled plasma mass spectrometry: A review. Anal. Chim. Acta 2011, 706, 66–83. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, X.; Suo, L.; Ma, L.; Hu, G.; Xia, X.; Li, M.; Huang, W.; Jin, Y. Application of Online Multi-Internal Standard Calibration for Determination of Iodine by ICP-MS. J. Mass Spectrom. 2025, 60, e5109. [Google Scholar] [CrossRef]
- Hein, C.; Sander, J.M.; Kautenburger, R. New approach of a transient ICP-MS measurement method for samples with high salinity. Talanta 2017, 164, 477–482. [Google Scholar] [CrossRef]
- Burevska-Atkovska, K.; Olivieri, F.; Avolio, R.; Castaldo, R.; Cocca, M.; Errico, M.E.; Gentile, G.; Grozdanov, A. Amino-modified microporous hyper-crosslinked resins for heavy metal ions adsorption. Colloid Surf. A-Physicochem. Eng. Asp. 2024, 700, 134720. [Google Scholar] [CrossRef]
- Ndung, U.K.; Franks, R.P.; Bruland, K.W.; Flegal, A.R. Organic complexation and total dissolved trace metal analysis in estuarine waters: Comparison of solvent-extraction graphite furnace atomic absorption spectrometric and chelating resin flow injection inductively coupled plasma-mass spectrometric analysis. Anal. Chim. Acta 2003, 481, 127–138. [Google Scholar] [CrossRef]
- Gao, Y.; Oshita, K.; Lee, K.; Oshima, M.; Motomizu, S. Development of column-pretreatment chelating resins for matrix elimination/multi-element determination by inductively coupled plasma-mass spectrometry. Analyst 2002, 127, 1713–1719. [Google Scholar] [CrossRef]
- Shih, T.; Chen, W.; Sun, Y. Open-channel chip-based solid-phase extraction combined with inductively coupled plasma-mass spectrometry for online determination of trace elements in volume-limited saline samples. J. Chromatogr. A 2011, 1218, 2342–2348. [Google Scholar] [CrossRef]
- Guo, F.; Zeng, P.; Liu, J.; Hu, H.; Zhu, W.; Wang, Y.; Cheng, H. Simultaneous preconcentration and quantification of ultra-trace tin and lead species in seawater by online SPE coupled with HPLC-ICP-MS. Anal. Chim. Acta 2024, 1294, 342294. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Kajiya, T.; Aihara, M.; Honda, K.; Shikino, O. Determination of rare earth elements in seawater by on-line column preconcentration inductively coupled plasma mass spectrometry. Talanta 2002, 58, 1185–1194. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, J.; Wang, J.; Ren, H.; Hong, Z.; Wu, K. Amine functionalized polyacrylonitrile fibers for the selective preconcentration of trace metals prior to their on-line determination by ICP-MS. Anal. Methods 2021, 13, 2504–2511. [Google Scholar] [CrossRef]
- El Ouardi, Y.; Giove, A.; Laatikainen, M.; Branger, C.; Laatikainen, K. Benefit of ion imprinting technique in solid-phase extraction of heavy metals, special focus on the last decade. J. Environ. Chem. Eng. 2021, 9, 106548. [Google Scholar] [CrossRef]
- Shahryari, T.; Singh, P.; Raizada, P.; Davidyants, A.; Thangavelu, L.; Sivamani, S.; Naseri, A.; Vahidipour, F.; Ivanets, A.; Hosseini-Bandegharaei, A. Adsorption properties of Danthron-impregnated carbon nanotubes and their usage for solid phase extraction of heavy metal ions. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 641, 128528. [Google Scholar] [CrossRef]
- Cao, Y.; Qin, J.; Su, Z.; Cai, L.; Fang, G.; Wang, S. Novel poly (N-methacryloyl-L-alanine acid) grafted chitosan microspheres based solid-phase extraction coupled with ICP-MS for simultaneous detection of trace metal elements in food. Food Chem. X 2023, 20, 100926. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Lin, J. 3D-Printed Column with Porous Monolithic Packing for Online Solid-Phase Extraction of Multiple Trace Metals in Environmental Water Samples. Anal. Chem. 2020, 92, 9640–9648. [Google Scholar] [CrossRef]
- Wang, W.; Evans, R.D.; Newman, K.; Toms, A. Automated separation and measurement of 226Ra and trace metals in freshwater, seawater and fracking water by online ion exchange chromatography coupled with ICP-MS. Microchem. J. 2021, 167, 106321. [Google Scholar] [CrossRef]
- Suo, L.; Dong, X.; Gao, X.; Xu, J.; Huang, Z.; Ye, J.; Lu, X.; Zhao, L. Silica-coated magnetic graphene oxide nanocomposite based magnetic solid phase extraction of trace amounts of heavy metals in water samples prior to determination by inductively coupled plasma mass spectrometry. Microchem. J. 2019, 149, 104039. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Yokota, Y.; Gemmei-Ide, M.; Inoue, Y.; Kagaya, S. Automated rapid solid-phase extraction system for separation and preconcentration of trace elements using carboxymethylated polyethyleneimine-type chelating resin. Anal. Sci. 2023, 39, 589–600. [Google Scholar] [CrossRef]
- Prabhakaran, D.; Subramanian, M.S. A new chelating sorbent for metal ion extraction under high saline conditions. Talanta 2003, 59, 1227–1236. [Google Scholar] [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis, 9th ed.; Macmillan: New York, NY, USA, 2016; pp. 201–202. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).