Abstract
The present study evaluates a surface dielectric barrier discharge (SDBD) plasma system utilizing porous metal electrodes to enhance the performance of non-thermal plasma (NTP)-based water treatment. A custom high-voltage, variable-frequency power driver was developed to operate SDBD reactors featuring novel porous electrode configurations aimed at enhancing plasma–liquid interaction. Three types of porous metal electrodes—copper (60 ppi), copper (20 ppi), and nickel (60 ppi)—were investigated as ground electrodes to evaluate their impact on discharge behavior and treatment performance. Electrical characterization via Lissajous plot analysis and optical emission spectroscopy (OES) was used to assess plasma power and reactive species generation. Ozone measurement and hydroxyterephthalic acid (HTA) dosimetry confirmed the formation of O3 and hydroxyl radicals (·OH), while methylene blue (MB) removal experiments quantified pollutant removal percentage and energy yield. Among the tested electrodes, the copper (20 ppi) configuration achieved the highest MB removal percentage of 95.07%, followed by nickel (60 ppi) with 90.53%, and copper (60 ppi) with only 27.55%. Correspondingly, the energy yield () reached 0.349 g/kWh for copper (20 ppi) at 15 min of plasma exposure, 0.19 g/kWh for nickel (60 ppi) at 20 min, and 0.049 g/kWh for copper (60 ppi) at 15 min. These results highlight the potential of porous metal electrodes as effective design choices for optimizing plasma–liquid interaction in SDBD systems. The findings support the development of compact, energy-efficient plasma water purification technologies using air-fed, surface DBD configurations.
1. Introduction
Non-thermal plasma (NTP) has emerged as a promising advanced oxidation process (AOP) for water treatment due to its ability to generate a rich mixture of highly reactive chemical species such as hydroxyl radicals (·OH), ozone (O3), hydrogen peroxide (H2O2), and energetic electrons. These reactive oxygen species are capable of degrading a wide spectrum of organic pollutants and inactivating pathogens without requiring chemical additives or generating harmful byproducts [1,2]. In addition to these, there are other major factors involved, like UV radiation, physical effects from electromagnetic fields, and shock waves [3,4]. Compared to conventional methods such as chlorination, ozonation or UV-based disinfection, NTP offers higher oxidative potential and operational flexibility under ambient conditions, making it particularly attractive for decentralized or mission-critical applications [5,6]. This could pave the way for a reliable alternative or supplement in water In Situ Resource Utilisation (ISRU) system in space exploration missions, including the Moon and Mars [7].
The principles, underlying plasma chemistry and classes of contaminants addressed by non-thermal plasma-based water treatment have been comprehensively reviewed in the literature. Thagard and Locke outlined the major classes of NTP systems, namely direct discharge, plasma jets, and plasma-activated water (PAW), and discussed the corresponding chemical reaction pathways and applications [8]. Foster provided mechanistic insight into the roles of ·OH radicals, showing their impact in initiating oxidation via hydrogen abstraction or electron transfer pathways [5]. Gott et al. demonstrated NTP’s viability for space mission applications by benchmarking different plasma reactor configurations using methylene blue (MB) as a model contaminant. They highlighted the relationship between MB removal percentage, gas flow rates, and energy consumption under operational constraints relevant to spacecraft [7].
Recent studies have increasingly focused on reactor-level optimizations for water treatment applications. Stratton et al. introduced a general design principle based on maximizing the plasma–liquid interfacial area, observing that gas-phase discharge reactors generally perform better than liquid-phase ones due to more efficient plasma species transfer and lower electrical losses [3]. Tang et al. further explored indirect treatment strategies involving printed surface dielectric barrier discharge (DBD) plasma systems coupled with hydrophobic PTFE bubbling reactors, emphasizing simplified reactor design and higher species transfer efficiency [9]. Jakob et al. investigated how electrode geometry and inter-electrode spacing influence plasma uniformity and efficiency [10], while Allabakshi et al. demonstrated scalable planar DBD setups [11].
As shown by Tang et al. and Robinson et al. [9,12], bubbling ionized air or plasma-activated gas into the liquid medium is a promising approach in indirect treatment setups by maximising plasma–liquid interfacial area. This method improves mass transfer of reactive species and allows the discharge to be maintained outside the liquid, thereby enhancing process reliability and extending component lifetimes [13,14]. Shahsavari et al. implemented a microbubble-type plasma reactor in a continuous water treatment loop and examined removal pathways of MB, providing a practical approach for scaling NTP-based systems [13].
Among commonly used model contaminants, methylene blue (MB) has become a standard due to its stability, detectability via UV-VIS absorption, and structural representativeness of organic dye pollutants [15]. Several studies have established ·OH radicals as the dominant species responsible for MB removal, with peroxides and ozone playing supplementary roles [16,17]. Recent studies have highlighted the importance of reactive nitrogen species (RNS), in addition to reactive oxygen species (ROS), in plasma-assisted water treatment. Air-fed non-thermal plasmas generate a variety of nitrogen radicals such as nitric oxide (NO), nitrogen dioxide (NO2), and peroxynitrite (ONOO−), which can participate in the removal of organic dyes like methylene blue (MB) [4,16]. Although hydroxyl radicals (·OH) are recognized as the most potent oxidizing species, the contribution of RNS enhances the oxidative environment and supports dye decomposition through secondary reactions and synergistic effects [17]. These insights support the application of air-fed plasma systems as an energy-efficient approach to water purification due to their capacity to generate both ROS and RNS. Analytical methods such as optical emission spectroscopy (OES) and hydroxyl radical quantification via hydroxyterephthalic acid (HTA) probing are increasingly adopted to understand plasma chemistry and optimize the conditions [4,18,19].
Different NTP reactors have been developed and benchmarked for MB removal. These include atmospheric pressure DBD [9], surface DBD [11], and plasma microbubble configurations [10]. Factors such as applied voltage, initial dye concentration, treatment time, and pH have all been optimized using response surface methodology or energy efficiency indices [20]. Kooshki et al. demonstrated the ability to selectively tune reactive oxygen and nitrogen species (RONS) through electrode configuration and power input to improve energy efficiency in water treatment reactors [18]. A few commonly used DBD plasma source configurations are illustrated in Figure 1 [21,22].
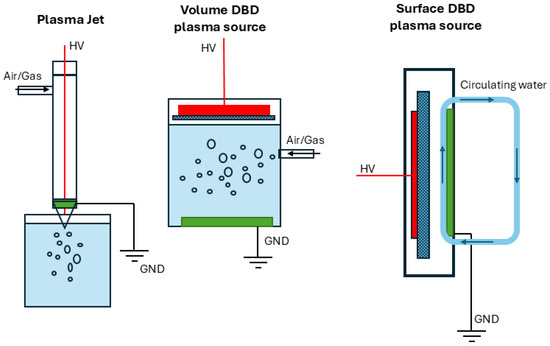
Figure 1.
Schematic diagrams of different DBD plasma sources used for MB removal.
Surface dielectric barrier discharge (SDBD) reactors have gained attention in NTP applications for their planar structure, ease of integration, and relatively lower power consumption [6]. However, lab-scale SDBD systems rely on printed electrode designs with thin dielectric layers, which face thermal stress and limited operational lifetime under continuous discharge. Our laboratory’s recent efforts—utilizing PTFE-based bubbling designs and surface DBD configurations [9,10] have demonstrated feasibility but revealed limitations in thermal endurance and discharge uniformity.
To address these challenges, the present work proposes a modified SDBD design featuring porous metal electrodes and thick alumina dielectric sheets. This configuration allows the input air to flow through the pores of the metal electrodes, thereby increasing the effective plasma–liquid interaction area while maintaining mechanical robustness and thermal durability. Compared to printed plasma sources, the thicker alumina layer offers improved heat dissipation and longer operational life, critical attributes for space and high-duty-cycle applications [7]. In this study, we systematically evaluated three types of porous metal electrodes in a bubbling-mode SDBD reactor for MB removal, with attention to species characterization, removal kinetics, and reactor longevity. Copper and nickel were selected as representative electrode materials owing to their complementary properties: copper provides high electrical conductivity and availability in multiple pore sizes, while nickel offers superior oxidation resistance and thermal durability.
2. Materials and Methods
2.1. Plasma Power Driver System
A custom-designed high-voltage, high-frequency variable power supply was developed to energize the plasma reactors. The schematic of the power driver circuit and associated diagnostic instrumentation is shown in Figure 2. The system employs a two-stage amplification strategy. In the first stage, a low-voltage sinusoidal signal from a function generator (Tektronix MDO34, Tektronix Inc., Beaverton, OR, USA) was amplified using a Class-D power amplifier (Crown CDi DriveCore 2|600, Crown Audio, Elkhart, IN, USA). This amplified signal was then stepped up using a ferrite-core high-voltage transformer (CMI 4967, Corona Magnetics Inc., Corona, CA, USA) with a turns ratio of 1:360. The output voltage retained the frequency of the input signal, adjustable between 1 kHz and 20 kHz. The output amplitude could be varied from 0 to 20 kV by tuning the input signal, allowing full control of plasma excitation parameters from the low-voltage side.
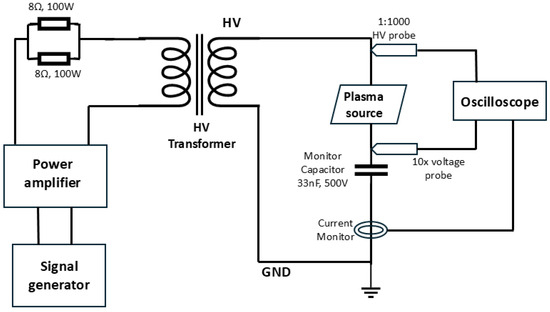
Figure 2.
Circuit schematic of the plasma power driver with electrical diagnostic instrumentation.
The applied voltage was monitored using a 1:1000 high-voltage probe (Tektronix P6015A, Tektronix Inc., Beaverton, OR, USA). Discharge power was estimated using the charge–voltage Lissajous method, where charge transfer was derived from the voltage measured across a 33 nF, 500 V (Mica capacitor; RS Components Ltd., Corby, UK) monitoring capacitor placed in series with the plasma reactor’s ground path [9,20]. A current monitor (Pearson 6585, Pearson Electronics Inc., Palo Alto, CA, USA) was included to capture half-cycle current waveforms, providing insight into discharge behavior [19].
2.2. Surface DBD Plasma Sources
A planar surface dielectric barrier discharge (SDBD) configuration was adopted in this study. The schematic of the plasma source is illustrated in Figure 3.
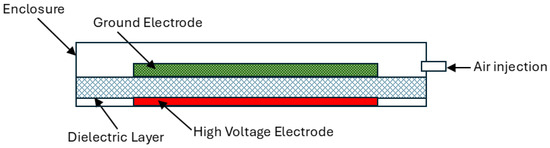
Figure 3.
Schematic of the surface DBD plasma source with porous metal ground electrode.
The SDBD assembly consisted of two circular electrodes (30 mm diameter) that sandwiched a 0.25 mm thick square-shaped alumina dielectric sheet (50 mm × 50 mm). The high-voltage electrode was a 0.1 mm thick copper adhesive sheet, while a porous metal disc was used as the ground electrode. Three porous ground electrodes were investigated to study their influence on discharge and water treatment:
- Copper porous metal (60 ppi)—designated Cu (60 ppi);
- Copper porous metal (20 ppi)—designated Cu (20 ppi);
- Nickel porous metal (60 ppi)—designated Ni (60 ppi).
Copper and nickel were selected as electrode materials for their complementary properties: copper offered high electrical conductivity and was available in tunable pore structures, while nickel provided superior oxidation resistance and thermal stability under prolonged discharge. Silver, despite its excellent conductivity and reactivity, was excluded due to its high cost and susceptibility to oxidation and sulfidation, which limited its feasibility for scalable water treatment applications. Each porous metal electrodes were machine-cut from commercially supplied sheets (Cambridge Energy Solutions Ltd., Cambridge, UK) into 30 mm diameter discs and bonded to the alumina dielectric using epoxy adhesive. The electrode edges were left unmodified to preserve the natural open-cell morphology. Manufacturer specifications for the porous electrodes are as follows:
- Cu (60 ppi): thickness = 2 mm, pore density = 60 ppi (fine), average pore size = 0.25–0.5 mm, porosity = 90–95%, bulk density = 0.15–0.30 g/cm3, purity = 99.9–99.99%.
- Cu (20 ppi): thickness = 5 mm, pore density = 20 ppi (coarse), average pore size = 1–1.5 mm, porosity = 90–98%, bulk density = 0.15–0.45 g/cm3, purity = 99.9–99.99%.
- Ni (60 ppi): thickness = 2 mm, pore density = 60 ppi (fine), average pore size = 0.25–0.5 mm, porosity = 90–98%, bulk density = 0.25–0.45 g/cm3, purity = 99.7–99.9%.
Here, “ppi” denotes pores per inch, a measure of pore fineness that governs gas permeability and plasma distribution. All foams exhibited at least 98% open-cell connectivity, ensuring effective gas transport through the electrode and enhanced plasma–liquid interaction [9]. The alumina dielectric offered high thermal stability, enabling sustained operation without significant degradation, thereby extending the lifetime of the reactor [7,18]. Photographs of the electrodes and plasma generated are provided in Figure 4.
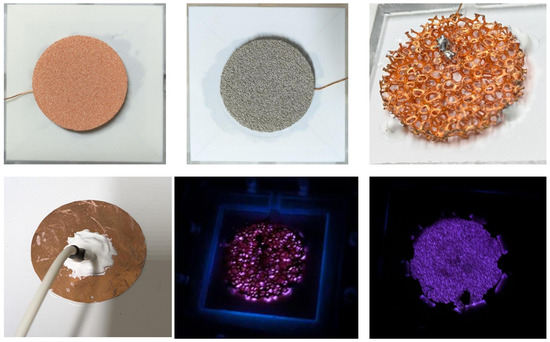
Figure 4.
Photographs of the porous and sheet electrodes in the SDBD plasma sources and the generated plasma discharge.
The electrode surface temperature during plasma operation was monitored using a thermal infrared camera (FLIR Systems Inc., Wilsonville, OR, USA) to evaluate heating behaviour under continuous discharge.
2.3. Experimental Setup
Figure 5 presents a schematic of the plasma-based water treatment system, which consisted of a high-voltage power driver and a liquid test rig integrated with the SDBD plasma source housed in a 3D-printed enclosure. A photograph of the experimental setup is also included. High-voltage and ground connections were routed through sealed ports in the enclosure to ensure electrical safety. Both the enclosure and liquid chamber were 3D-printed using epoxy resin and assembled with M4 bolts, rubber gaskets, and silicone adhesive to achieve an airtight seal. The top section of the enclosure incorporated a threaded coupling for attaching the cylindrical liquid test rig, which had a length of 15 cm, an outer diameter of 4 cm, and a wall thickness of 2 mm.
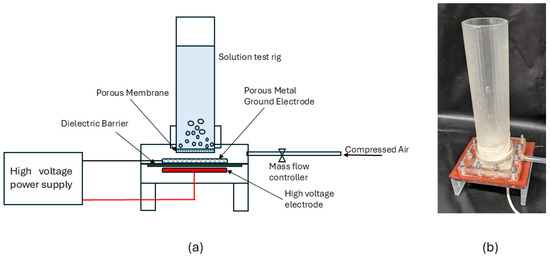
Figure 5.
(a) Experimental setup for plasma treatment of the methylene blue solution. (b) Liquid solution test rig with SDBD plasma source.
A hydrophobic PTFE membrane filter (pore size = 0.22 μm, thickness = 150 μm, diameter = 47 mm; Sigma Aldrich, St. Louis, MO, USA) was fixed at the base of the liquid test rig using silicone adhesive. The membrane had an air flow capacity of 5 L min−1 cm−2, ensuring that the selected operating flow rate (2 slpm) remains well below the critical limit, preventing back pressure or liquid intrusion into the plasma zone. This membrane served both as a gas diffuser and a physical barrier, allowing ionized air to be delivered uniformly into the solution while isolating the plasma region from direct contact with liquid. Air was supplied through a mass flow controller (Alicat MC series, Alicat Scientific Inc., Tucson, AZ, USA) into the enclosure, flowing through the porous metal electrode at a flow rate of 2 slpm. This configuration enabled stable bubbling of ionized gas, enhancing reactive species transfer and improving plasma–liquid contact efficiency [13,15]. The 3D-printed liquid test rig and plasma enclosure were designed as modular, detachable units connected through a threaded coupling. This configuration enabled convenient removal of the cylindrical liquid chamber for post-experimental inspection and replacement of the PTFE membrane and the SDBD assembly. Periodic inspection confirmed that the hydrophobic PTFE membrane, positioned above the discharge region, remained intact without rupture, leakage, or discoloration after repeated 30 minute operating cycles. As a safety precaution, users are advised to avoid direct plasma exposure to polymeric components to prevent possible thermal or chemical degradation.
2.4. Plasma Diagnostics and Water Treatment Tests
The non-thermal plasma generated in the SDBD sources was characterized using both electrical and optical diagnostic techniques. Electrical measurements included monitoring the voltage waveform with a 1:1000 high-voltage probe and oscilloscope (Tektronix MDO34, Tektronix Inc., Beaverton, OR, USA), as well as determining the charge using a 10× voltage probe across the monitoring capacitor. These measurements enabled the construction of charge–voltage Lissajous plots, which were used to estimate energy consumption and plasma discharge power [20]. Optical diagnostics (OES) employed an Andor Shamrock 500i-A (Andor Technology Ltd., Belfast, UK) spectrometer equipped with an ICCD to capture the emission spectra for each electrode configuration. The spectral features were compared with known gas-phase emission lines to identify reactive species in the discharge [16,19]. Ozone concentrations in the treated gas stream were quantified using a ThermoFisher 49iQ (Thermo Fisher Scientific, Waltham, MA, USA) ozone analyzer, which operates on UV photometric detection at 254 nm. The sampling probe was positioned 10 mm vertically above the plasma source ground electrode to capture the effluent gas. This setup enabled real-time monitoring of ozone generation and comparison across the different electrode configurations. To quantify hydroxyl radical (·OH) generation, hydroxyterephthalic acid (HTA) dosimetry was employed. Plasma-treated terephthalic acid solution (2 mM TA in 5 mM NaOH) (CAS: 100-21-0, Thermo Scientific Inc., Geel, Belgium) was excited using 310 nm UV light (LSM-310A with controller LDC-1, Ocean Insight, Orlando, FL, USA), and the resulting fluorescence emission was recorded using an Oceanview HR4000 (Ocean Insight, Orlando, FL, USA) spectrometer. To quantify hydroxyl radical (·OH) concentrations, a calibration curve was established using known concentrations of hydroxyterephthalic acid (HTA), the fluorescent product formed by the reaction between terephthalic acid and ·OH. Standard HTA solutions (CAS: 636-94-2, Sigma Aldrich, St. Louis, MO, USA) were prepared, and their fluorescence intensities were measured at an excitation wavelength of 310 nm and emission at 425 nm using the spectrometer. The resulting calibration curve and the linear regression equation were subsequently used to determine ·OH concentrations in plasma-treated samples. This fluorescence-based detection method for ·OH radicals is widely established in plasma–liquid studies and provides reliable quantification of transient species [4,16,17,23,24].
Methylene blue (MB) was chosen as the model contaminant owing to its strong optical absorbance at approximately 665 nm, which allowed straightforward monitoring of removal using UV–VIS spectroscopy. Its aromatic molecular structure and high reactivity toward plasma-generated reactive oxygen and nitrogen species, particularly hydroxyl radicals (·OH), make it a representative compound for assessing plasma-driven oxidation processes. Furthermore, MB served as a benchmark dye in advanced oxidation studies, enabling direct comparison of removal percentage across different plasma reactor configurations. A 10 mg/L stock solution of MB (C.I. 52015, Scientific Laboratory Supplies Ltd., Nottingham, UK) was prepared by dissolving the dye in deionized water under magnetic stirring. Plasma treatment was performed in batch mode, and the removal process was monitored using the Oceanview HR4000 spectrometer operated through the OceanView software (Version 1.6.7). The same spectrometer was used for both absorbance and fluorescence measurements by switching the illumination source and acquisition mode within the software. A halogen light source (DH-MINI, Ocean Insight, Orlando, FL, USA) was employed for absorbance spectroscopy over a wavelength range of 200–1100 nm, while a UV LED source (310 nm) was used to excite fluorescence emission from hydroxyterephthalic acid (HTA). A cuvette holder (Square One, Ocean Insight, USA) facilitated sequential measurement of MB absorbance and HTA fluorescence without altering the optical setup.
The experimental procedures were carried out sequentially as follows: (1) electrical and optical characterization of SDBD plasma sources, (2) ozone measurement and HTA dosimetry for O3 and ·OH radical quantification, and (3) MB removal studies. In all experiments, the air flow rate was maintained at 2 slpm, while the ambient laboratory conditions were controlled at 20 °C and 50% relative humidity. These parameters ensured stable discharge behavior and reproducible plasma–liquid interaction throughout the study.
2.5. Methylene Blue Removal Percentage and Energy Yield Calculations
The removal percentage of methylene blue (MB) was calculated using the equation:
where and are the initial and final MB concentrations determined from absorbance at 664 nm, a standard method for plasma dye removal analysis [25,26].
Energy yield () in (g/kWh), measuring pollutant removal per unit energy, was calculated as follows:
where V is volume (L), and are in grams per litre (g/L), P is power input (W), and t is treatment time (s) [9,10,25].
2.6. Quantification of Methylene Blue and Calibration Curve
The concentration of methylene blue (MB) in the plasma-treated and control samples was determined spectrophotometrically at 664 nm using the spectrometer (Oceanview HR4000, Ocean Insight, Orlando, FL, USA) with a 1 cm path length quartz cuvette. A calibration curve was established by diluting a 10 mg/L MB stock solution with deionized water to obtain standard concentrations of 0.2, 0.5, 1, 2, 5, and 10 mg/L. The absorbance of each standard was measured, and the resulting data were best described by a second-order polynomial relationship rather than a linear Beer–Lambert dependence at higher concentrations, likely due to matrix and inner-filter effects. The calibration curve is shown in Figure 6, and the corresponding regression equation is as follows:
where A is the absorbance and C is the concentration (mg/L). The polynomial fitting yielded a correlation coefficient of . For unknown samples, the MB concentration was obtained by solving the inverse relation of Equation (3) for C. This approach provided more accurate concentration estimates across the full calibration range and ensured reliable quantification of MB removal percentage in plasma-treated solutions. This calibration procedure enabled accurate determination of MB removal in accordance with established protocols for plasma–liquid treatment studies [25,26].
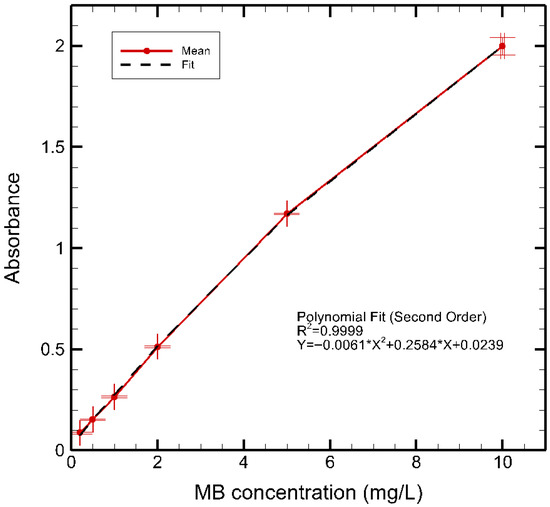
Figure 6.
Calibration plot of methylene blue dye solution at various concentrations.
3. Results
The characterisation of SDBD plasma sources with the porous metal ground electrodes was undertaken in this work. The optical emission spectrum, as well as ozone and hydroxyl radical quantification of these plasma sources under AC high voltage excitation, were obtained. The performance of each plasma source in degrading Methylene Blue dye solution is also presented in this section.
3.1. Plasma Inception and Discharge Power
The applied voltage and discharge current in the plasma sources were the first line of parameters observed for ensuring plasma ignition and sustenance. The plasma source was energized with a sinusoidal voltage and a frequency of 10 kHz. The applied voltage was increased in steps until filamentary discharge currents were observed in the ground path. The discharge currents occurred during each half cycle of the applied high voltage waveform and were viewed in the oscilloscope. The peak values of these current waveforms ranged from tens to hundreds of milliamperes. The applied voltage and discharge current waveforms of three plasma sources are shown in Figure 7 for a voltage amplitude of 6 kVpp. The inception voltages of each plasma source were around 3 kV to 3.5 kV, and visible plasma discharge was observed in violet/blue glow in and around the porous metal electrodes. A higher voltage than that of the inception was selected for conducting water treatment experiments.
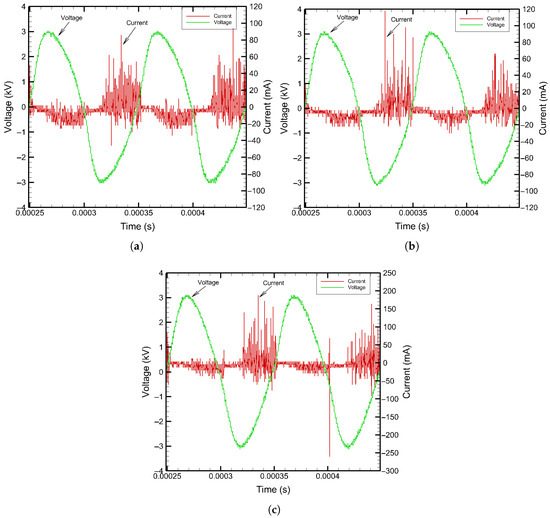
Figure 7.
Voltage and discharge current plots: (a) SDBD with Cu (60 ppi). (b) SDBD with Cu (20 ppi). (c) SDBD with Ni (60 ppi).
The impact of plasma discharge on MB removal can be assessed using discharge power. The plasma discharge power in the SDBD was calculated using charge–voltage (q–V) Lissajous plots obtained with the circuit arrangement in Figure 2. The voltage across the monitoring capacitor was obtained and converted into charge. The transported charge q,
where is the voltage measured across the monitoring capacitor . The area under the q–V Lissajous plot is given by
The average discharge power inside the DBD reactor can be calculated as the energy dissipated per unit time,
where T is the time period of the applied high voltage waveform, and f is the frequency of the applied voltage in Hz. The q–V Lissajous plots of the three SDBDs considered in this work, when operated at 6 kVpp, 10 kHz, are shown in Figure 8 which provides the discharge power in these configurations. Discharge power in the three SDBD plasma sources was calculated from the area of q–V Lissajous plots and Equation (6).
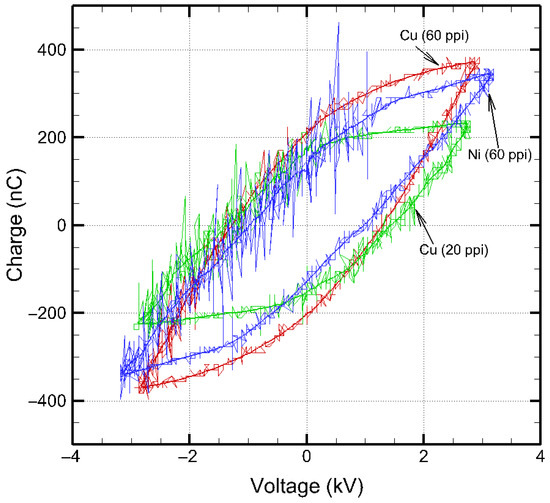
Figure 8.
Charge–voltage Lissajous plot of SDBD plasma sources.
The applied voltage magnitude was increased in steps, and corresponding plasma discharge powers were calculated and plotted (Figure 9). The discharge power in Cu (60 ppi) (15.17 W) and Ni (60 ppi) (11.13 W) was found to be comparatively higher than that of Cu (20 ppi) (10.95 W) at similar applied voltages. This property was owing to the denser electrode structure and resulting capacitance with the porous metal electrodes. In the following MB removal experiments, a common applied voltage of 6 kVpp at 10 kHz was used for all the plasma sources.
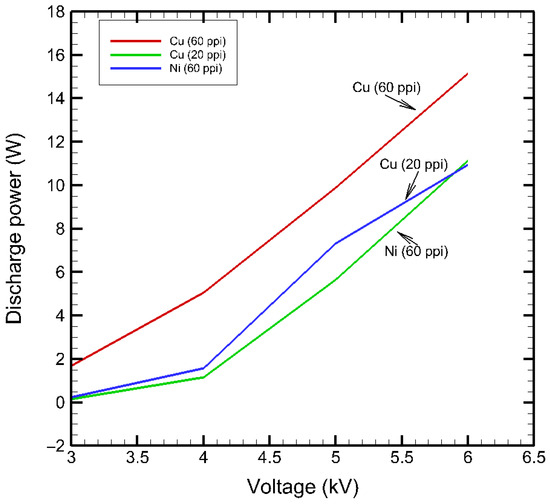
Figure 9.
Plasma discharge power in Cu (60 ppi), Cu (20 ppi), and Ni (60 ppi) SDBDs.
The charge-–voltage (q–V) Lissajous plots were averaged over seven consecutive discharge cycles to improve signal stability. The burning voltage for each SDBD plasma source was extracted as the transition point between the linear dielectric and discharge-active regions, corresponding to the onset of plasma microdischarges. This point was determined from the slope change in the q–V Lissajous plot, identified via derivative-based analysis of using MATLAB R2025a (25.1). The burning voltage represented the minimum applied voltage required to sustain plasma microdischarges during each cycle, serving as an indicator of discharge stability and energy coupling efficiency between the electrode and dielectric. Among the electrodes tested, Cu (20 ppi) exhibited the lowest burning voltage (2.74 kV), indicating easier plasma initiation and superior energy coupling within its coarser porous network. In contrast, Cu (60 ppi) and Ni (60 ppi) showed higher burning voltages (2.87 kV and 3.13 kV, respectively), consistent with their finer pore structures and higher surface impedance, which reduce discharge uniformity and plasma power transfer efficiency.
3.2. Optical Emission Spectrum
In order to identify the active chemical species generated by the SDBD plasma sources, the emission spectra of the three electrode configurations were examined. Figure 10 shows the emission spectrum of three SDBD plasma sources when operated at the specified electrical parameters.
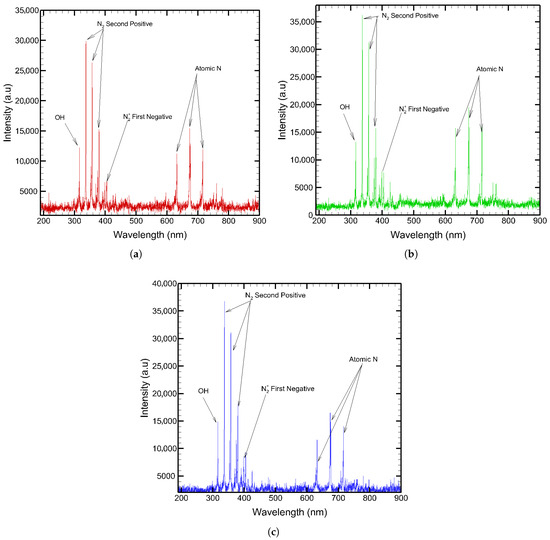
Figure 10.
Optical emission spectra: (a) SDBD with Cu (60 ppi). (b) SDBD with Cu (20 ppi). (c) SDBD with Ni (60 ppi).
The optical emission spectra (OES) revealed that most of the detected species correspond to excited states of nitrogen, as expected since the working gas used was compressed air. A characteristic ·OH (A2Σ+− X2Π) peak was observed at 309 nm in all SDBD plasma sources, confirming the generation of hydroxyl radicals. Ozone could not be directly detected in the spectra, as O3 is a stable secondary product formed downstream of the discharge and does not emit in the UV–VIS range accessible to OES. In addition to the dominant molecular emissions (N2 second positive system in the 300–400 nm range and
first negative system around 391 nm), notable differences were observed in the higher wavelength region (600–800 nm). While the Ni (60 ppi) spectrum was dominated by molecular nitrogen emissions, both Cu-based electrodes showed additional atomic emission lines, particularly around 742–746 nm (neutral atomic nitrogen) and near 777 nm (atomic oxygen). These atomic lines were weak or absent in the Ni (60 ppi) configuration, suggesting reduced dissociation of N2 and O2 with nickel electrodes.
Since ozone formation in air plasmas relies on O atom precursors, the lower O emission in Ni (60 ppi) spectra implies a diminished ozone generation potential relative to Cu electrodes. This behavior can be attributed to the lower work function and higher secondary electron emission yield of Cu compared to Ni (60 ppi), which favors electron-impact dissociation and thus enriches the production of reactive atomic species. Consequently, Cu electrodes enable a richer pool of RONS, complementing the molecular excitations observed, and are expected to be more effective in pollutant removal.
3.3. Ozone Concentrations
The generation of ozone in plasma sources was measured using an ozone analyser (Thermo Fischer 49iQ, Thermo Fisher Scientific, Waltham, MA, USA). The ozone monitor probe was placed 10 mm vertically above the plasma source, and the level of ozone produced was monitored for a time period of 30 min. Figure 11 shows the temporal evolution of ozone concentration during plasma treatment using the three porous metal electrodes.
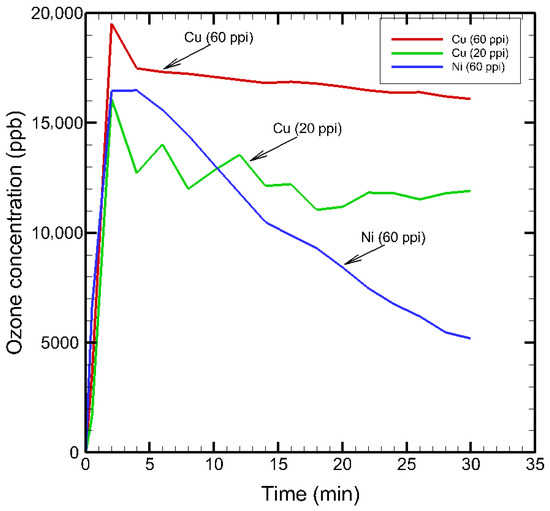
Figure 11.
Ozone concentrations in the SDBD plasma sources.
All configurations exhibited a rapid increase in ozone generation within the first few minutes, reaching peak values between 15,000–20,000 ppb. Among them, Cu (60 ppi) produced the highest initial ozone concentration, followed by Cu (20 ppi) and Ni (60 ppi). After reaching the maximum, the concentrations gradually decreased over time, with Ni (60 ppi) showing the steepest decline, while Cu (60 ppi) and Cu (20 ppi) maintained relatively higher levels throughout the 30 min plasma-on time.
3.4. HTA Dosimetry
100 mL solution of 2 mM TA in 5 mM NaOH was treated in the bubbling solution test rig with porous metal electrodes for 30 min. The ·OH radicals generated chemically react with TA molecules and convert them into HTA. Since the process is highly selective and only involves ·OH radicals and not ozone, this method provides the concentrations of hydroxyl radicals. The fluorescence of treated TA solution was examined at time steps of 5 min over 30 min total NTP treatment period using each of the three SDBD plasma sources. Figure 12 provides the calculated cumulative ·OH concentrations in each of the SDBD plasma sources. The cumulative ·OH concentrations were estimated directly from the measured HTA concentrations, assuming a 1:1 stoichiometric conversion with terephthalic acid in excess. Potential effects such as photobleaching, secondary radical reactions, or incomplete trapping were neglected, consistent with commonly adopted practice in ·OH dosimetry using TA.
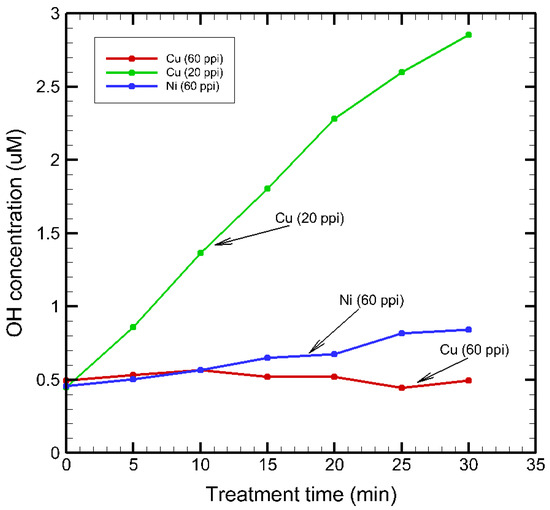
Figure 12.
HTA concentrations in SDBD plasma sources.
The hydroxyl radical concentrations in Cu (20 ppi) were found to be higher than those in Cu (60 ppi) and Ni (60 ppi). This could be due to the superior plasma–air interaction obtained with larger pores in Cu (20 ppi). In Cu (60 ppi), HTA measurements revealed a very low and nearly constant ·OH concentration over 30 min, despite the significantly higher ozone levels detected. This suggests that the discharge favors gas-phase O3 generation while limiting interfacial ·OH formation. HTA fluorescence revealed the effects of material and porosity on ·OH production. Ni (60 ppi) showed similarly low initial ·OH levels but with a gradual increase over time, suggesting slower activation of its porous framework. In contrast, Cu (20 ppi) with larger pores enabled stronger plasma–air exchange and mass transfer, yielding consistently higher ·OH concentrations throughout treatment.
3.5. Methylene Blue Removal
With the knowledge of chemical species included in the water treatment process when porous metal electrodes are used, the time required to remove MB dye was to be experimentally determined. 100 mL MB solution with a concentration of 10 mg/L was treated in the bubbling solution test rig. Methylene blue removal percentage was calculated using Equation (1). Each methylene blue removal experiment was performed in triplicate, and the mean and standard deviation were used to represent the measurement uncertainty. Error bars shown in the MB removal efficiency plots correspond to one standard deviation. The temporal evolution of MB removal percentage under plasma treatment for different porous metal electrodes is illustrated in Figure 13. A distinct variation in removal performance was observed depending on the electrode material and structure.
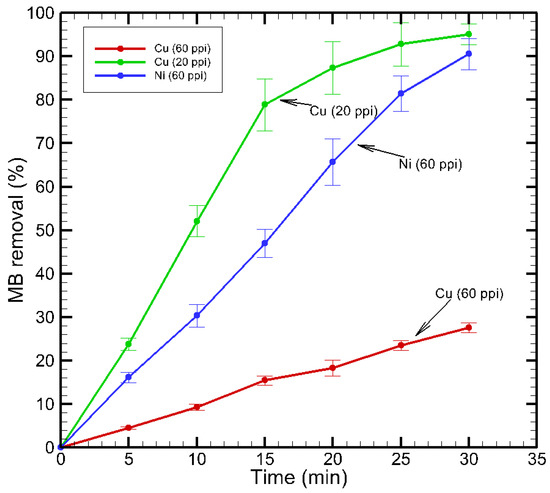
Figure 13.
Effect of MB removal percentage on treatment time.
Among the three tested configurations, Cu (20 ppi) achieved the highest removal percentage, reaching approximately 95.07% within 30 min of treatment. The removal proceeded rapidly in the initial 15 min, with nearly 80% removal percentage, after which the rate plateaued. This behavior suggested that Cu (20 ppi) facilitated efficient plasma–liquid interactions and radical formation early in the treatment. The Ni (60 ppi) also demonstrated significant removal capacity, reaching around 90.53% by the end of the treatment. Its performance was characterized by a steady and linear increase in MB removal, indicating stable plasma activity and consistent generation of reactive species over time. In contrast, the Cu (60 ppi) exhibited the lowest removal percentage, only reaching about 27.55% after 30 min. The limited performance of Cu (60 ppi) could be attributed to reduced discharge activity or less effective diffusion and activation of reactive species within its finer porous structure. These results underlined the critical influence of electrode pore size and material properties on the generation of oxidative species like hydroxyl radicals and ozone, which are key contributors to dye removal in plasma-assisted water treatment processes. The electrode surface temperature, monitored using a FLIR thermal infrared camera (FLIR Systems Inc., Wilsonville, OR, USA), increased during 30 min of plasma operation from 27 °C to 37.8 °C for Cu (20 ppi), 29 °C to 33.8 °C for Cu (60 ppi), and 29 °C to 50 °C for Ni (60 ppi). After repeated plasma operation (5–10 cycles of 30 min each), visible oxidation and discoloration appeared on both copper electrodes, more pronounced in Cu (60 ppi), whereas the Ni (60 ppi) electrode retained its metallic surface appearance. The UV–VIS spectra of the plasma-treated solutions exhibited only the characteristic methylene blue absorption band at 664 nm, with no additional peaks associated with fluorinated organics. This confirmed that the PTFE membrane remained chemically stable and physically undamaged during plasma operation.
Figure 14 shows the energy yield (), defined as the mass of MB removed per unit of energy consumed (g/kWh) (Equation (2)), providing a distinctive assessment of the energy efficiency of the plasma treatment system. As can be seen, Cu (20 ppi) consistently achieved the highest , peaking at approximately 0.349 g/kWh after 15 min of treatment before gradually declining with extended operation. This peak corresponds to the period of most efficient generation and utilization of reactive species, after which recombination or saturation effects reduce marginal MB removal percentage despite continued power input.
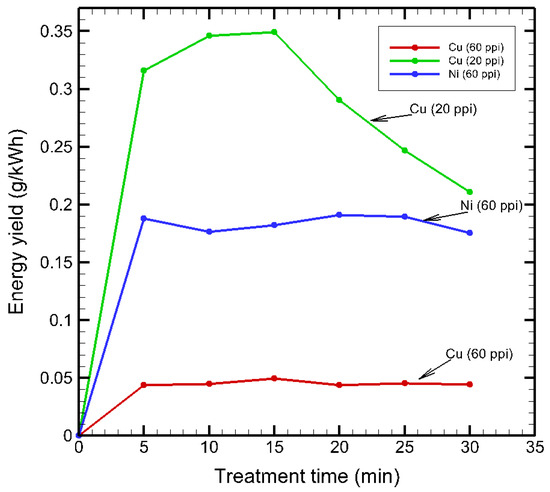
Figure 14.
Effect of energy yield on treatment time.
Ni (60 ppi) showed a steady increase in , reaching a maximum of 0.19 g/kWh at 20 min, indicating sustained energy-efficient performance over time, likely due to the stable micro-discharge behavior of nickel maintaining reactive species generation across prolonged operation. In contrast, Cu (60 ppi) exhibited consistently low energy yields, with a peak of just 0.049 g/kWh during the initial stages, followed by stagnation, suggesting that removal occurs at a high energy cost, likely because of inefficient plasma formation and thermal effects within the more compact porous structure. Ni (60 ppi) exhibited intermediate removal and energy yield compared to copper electrodes. Although it did not reach the maximum value observed with Cu (20 ppi), it maintained a stable energy yield profile throughout the treatment duration. This characteristic suggested that nickel electrodes may be less prone to thermal stress and surface oxidation during plasma operation. Thus, a porous nickel electrode can enhance the operational duration and effectiveness in a practical water treatment scenario.
These results demonstrated that electrode configuration strongly influences both removal effectiveness and operational energy efficiency. The combined analysis of removal performance and highlighted the interdependence between electrode structure, discharge behavior, and plasma–liquid interaction efficiency. Cu (20 ppi), with a coarse 20 ppi structure, was found as the most effective configuration, balancing high removal performance with optimal energy utilization. The larger pore size facilitated enhanced plasma propagation, fluid mixing, and radical diffusion, contributing to higher reaction rates and lower energy consumption per unit of dye degraded. Ni (60 ppi), although slightly less efficient in overall removal, exhibited a steady and reliable increase in , making it a strong candidate for prolonged treatment scenarios due to its consistent discharge characteristics. In contrast, the Cu (60 ppi) showed the lowest removal percentage and energy yield. While pore size and structural compactness likely contributed to limited plasma propagation and mass transport, another contributing factor is thermal-induced surface oxidation during plasma exposure. Copper is known to heat up under dielectric barrier discharge conditions, and the fine pore structure of Cu (60 ppi) may accelerate this heating. Surface oxidation may alter the dielectric properties and increase the surface resistance, which in turn can affect local electric field strength and change the capacitance of the system. Such modifications can inhibit stable plasma generation and reduce the production of reactive species. The q–V Lissajous plots revealed that although Cu (60 ppi) provides high energy into the discharge, its unstable characteristics favor ozone formation over ·OH production. In contrast, Cu (20 ppi) showed more stable discharges with efficient energy transfer to the liquid phase, resulting in higher ·OH concentrations, while Ni (60 ppi) exhibited intermediate behavior due to nickel’s material properties and porosity.
Overall, these results demonstrated that optimal performance in plasma-based water treatment is not solely dependent on the chemical composition of the electrode but also on the thermal, electrical, and structural characteristics that govern discharge dynamics and energy transfer efficiency. This insight is essential for the development of scalable and energy-efficient plasma systems for environmental applications.
4. Discussion
This study systematically evaluated the performance of three porous metal electrodes—Cu (60 ppi), Cu (20 ppi), and Ni (60 ppi) in a surface dielectric barrier discharge (SDBD) plasma setup for the removal of methylene blue in aqueous solutions. The key metrics analyzed were MB removal percentage and energy yield (g/kWh), both as a function of plasma treatment time. These metrics provide insight into the electrode material’s influence on plasma reactivity and overall treatment efficacy.
4.1. MB Removal Percentage
The results showed a steady increase in MB removal percentage with treatment time across all electrode types. Among the three, the Cu (20 ppi) demonstrated the highest performance, achieving nearly complete removal (95.07%) after 30 min of plasma exposure. This superior behavior is likely attributed to the coarser pore structure (20 ppi), which enhances the interaction between the plasma and liquid interface. A larger pore size enabled greater contact area and improved transport of reactive species such as ·OH, O3, and H2O2, which are essential for oxidative removal of MB. The Ni (60 ppi) showed moderate removal percentage, while Cu (60 ppi) displayed the least removal, possibly due to restricted discharge spread and limited gas–liquid interface contact caused by its finer pore structure. In the present study, the optical emission spectra confirmed the presence of dominant nitrogen species, particularly (Second Positive System) and (First Negative System), alongside strong emissions around 309 nm. It is understood that radicals generated through vibrationally excited and electron-impact dissociation of water vapor play a primary role in methylene blue removal in air-fed SDBD reactors. As the nitrogen bands are strong and consistent across the tested electrode configurations, it is inferred that nitrogen species contribute to sustaining the discharge, while radicals remain the dominant oxidative agents for pollutant removal in this setup. The low ·OH production in Cu (60 ppi), combined with its high O3 output, points to altered discharge conditions likely influenced by temperature rise and electrode surface oxidation. These effects reduced the liquid-phase ·OH transfer and explained the lower MB removal percentage compared to the other electrodes. The contrasting ·OH and O3 behaviors emphasized the role of both electrode material and porosity in plasma reactivity. The fine-pored copper (Cu (60 ppi)) favored ozone accumulation but limited ·OH delivery, while the nickel (Ni (60 ppi)) allowed a slower but progressive increase in ·OH production. The large-pored copper (Cu (20 ppi)) provided the most efficient plasma–liquid coupling, resulting in the highest ·OH generation and superior MB removal performance. Cu (60 ppi) generated the highest ozone but showed the lowest MB removal, while Cu (20 ppi), despite lower ozone, achieved the best removal percentage. This confirms that short-lived radicals like ·OH, rather than ozone, dominate the removal process. These observations are in agreement with earlier findings by Stancu et al. [25] and Liang et al. [26], where the morphology of electrodes and reactor configuration were found to influence the availability of reactive species. The steady increase in removal with time also reflected the persistent generation and accumulation of reactive plasma species, especially in systems that favor fluid mixing and radical retention. The discharge behaviors observed in copper and nickel electrodes could be attributed to their intrinsic material properties and the porous structure. Copper, with higher electrical and thermal conductivity, promoted stronger local electric fields along the pore surfaces, resulting in more intense and reactive discharges. However, this also makes copper electrodes, particularly at higher porosity (Cu (60 ppi)), prone to heating and oxidation, which eventually alters capacitance and water treatment performance. In contrast, nickel has lower electrical conductivity and a more oxidation-resistant surface, which tends to maintain stable discharge and MB removal performance. Further, the porous structure also played a role in these: while larger pore sizes in Cu (20 ppi) facilitated enhanced plasma–gas penetration and reactivity, the nickel foam at the same 60 ppi porosity provided steadier plasma–liquid interactions. This explained the superior short-term percentage of copper foams and the more sustainable discharge behavior of nickel foams.
4.2. Energy Yield Analysis
The energy yield, expressed in g/kWh, followed a time-dependent trend consistent with removal percentage but revealed more nuanced dynamics. Cu (20 ppi) exhibited the highest energy yield, peaking at around 0.349 g/kWh before declining slightly toward the end of the treatment period. This peak likely corresponds to an optimal phase where MB concentration remained high enough to sustain active removal while power input remained efficient. As the dye concentration decreased, the incremental removal per unit energy diminished, resulting in a lower yield. The Ni (60 ppi) displayed relatively stable and increasing energy yield, indicating consistent plasma performance and moderate removal activity. In contrast, Cu (60 ppi) had a significantly lower energy yield throughout, which aligns with its weak removal percentage. One notable hypothesis is that Cu (60 ppi), despite being composed of the same base metal as Cu (20 ppi), may undergo surface oxidation due to localized heating during discharge. This oxidation could alter its surface conductivity and capacitance, reducing plasma intensity and stability. The performance of Ni (60 ppi) is of significance in terms of the experimental results. Compared to Cu (60 ppi) with a declining percentage, the Ni electrode maintained a stable removal percentage and energy yield over the entire treatment period. This robustness was reflected in the plasma source characterisation as well, where ·OH concentrations increased gradually, ozone levels remained moderate, and OES spectra were dominated by N2 molecular emissions with comparatively weaker atomic oxygen lines, indicating a more balanced plasma chemistry. These characteristics can be linked to nickel’s resistance to oxidation and superior thermal durability, which promote consistent discharge behaviour. Although the overall efficiency was lower than Cu (20 ppi), the stability of the Ni electrode places it as a reliable option for continuous plasma-based water treatment. The higher temperature rise observed for Ni (60 ppi) can be attributed to its lower electrical and thermal conductivity, which promotes localized resistive heating. However, its oxidation-resistant surface contributed to stable discharge behaviour and consistent treatment performance. In contrast, the copper electrodes—particularly Cu (60 ppi) exhibited pronounced surface oxidation after multiple plasma cycles, indicating dielectric deterioration and reduced plasma uniformity.
4.3. Implications and Future Perspectives
The comparative results between SDBDs with Cu (60 ppi), Cu (20 ppi), and Ni (60 ppi) emphasized the interplay between electrode structure, thermal behavior, and discharge characteristics in plasma systems. The coarser Cu (20 ppi) proved superior in both removal percentage and energy utilization.
The significantly lower performance of Cu (60 ppi) may be attributed to its susceptibility to surface oxidation under continuous plasma exposure. This alters the dielectric environment and could reduce effective plasma power transfer to the discharge zone. Ni (60 ppi), while not achieving the highest removal percentage, maintained stable removal and energy yield throughout treatment, underscoring the role of nickel’s oxidation resistance and thermal durability. These findings suggest that long-term operational reliability may be better supported by nickel electrodes, even if the short-term removal percentage is higher with coarser copper foams. The observed trends suggested that optimal plasma treatment requires balancing removal kinetics, energy efficiency, and material durability—factors that do not always scale proportionally with treatment time.
Subsequent studies should focus on addressing the material and operational limitations identified here. In particular, the short operational lifetime of copper electrodes due to surface oxidation and heating requires systematic aging studies, while the stable performance of nickel electrodes calls for mechanistic investigations into their plasma–material interactions. Further experiments should also explore the influence of pore size in both copper and nickel electrodes, along with the impact of applied voltage, frequency, and discharge duration on electrode integrity. Detailed thermal imaging and in situ surface diagnostics should be employed to correlate electrode temperature rise, oxidation progression, and dielectric changes with long-term plasma stability. Additionally, byproducts potentially formed during plasma treatment with different metal electrodes should be characterized to ensure safe and sustainable application. Future research also needs to investigate the effect of initial contaminant concentrations, air flow rate and humidity on plasma removal efficiency to better understand potential mass-transport limitations and optimize reactor operation under varying pollutant loads. Extending the study to actual wastewater with complex chemical matrices will provide a more realistic assessment of scalability and long-term reactor robustness.
5. Conclusions
In this study, surface dielectric barrier discharge (SDBD) plasma reactors employing porous metal electrodes were developed and integrated into a bubbling-type water treatment system. The aim was to assess the impact of electrode material and pore density on plasma characteristics and the removal performance of methylene blue (MB) dye. Three porous electrodes—two copper discs with porosity levels of 60 ppi (Cu (60 ppi)) and 20 ppi (Cu (20 ppi)), and one nickel disc with 60 ppi (Ni (60 ppi)) were evaluated. The experimental results clearly demonstrated that the bubbling configuration, combined with non-thermal plasma, offers a promising approach for enhancing dye removal in aqueous media. The air flow of 2 slpm facilitated effective dispersion of reactive plasma species into the liquid, enabling greater contact with the dye molecules. Among the tested configurations, Cu (20 ppi) exhibited the highest removal percentage and energy yield, followed by Ni (60 ppi) and Cu (60 ppi). This trend was attributed to the combined effect of higher porosity (in Cu (20 ppi)) and favorable thermal and surface properties of the electrode material, which enhanced plasma generation and reactive oxygen species production. Optical emission spectroscopy (OES) and HTA dosimetry confirmed the generation of key plasma species, including hydroxyl radicals, which play a central role in the oxidation and breakdown of organic dyes. The comparative analysis further revealed that ozone concentration alone does not define treatment performance, underscoring the dominant role of short-lived radicals in MB removal across different electrode configurations. The power analysis revealed that the discharge power was higher in electrodes with higher pore densities, likely due to increased surface area and localized field enhancement. Cu (60 ppi), despite being of the same material as Cu (20 ppi), underperformed due to its lower porosity, and possibly due to heating and surface oxidation effects that altered its capacitance and discharge behavior over time. While Cu (20 ppi) achieved the highest removal percentage, the Ni electrode delivered a more stable and sustainable performance. Although copper electrodes, particularly at lower porosity, excel in short-term performance, porous nickel electrodes offer an oxidation-resistant and thermally robust alternative, a suitable candidate for long-term plasma water treatment applications. Future investigations should focus on long-term electrode stability, byproduct analysis, and the treatment of real wastewater matrices to further validate the practical applicability of such plasma systems. Moreover, scaling up the system while maintaining energy efficiency remains a critical area for future research.
Author Contributions
Conceptualization, E.N.S. and M.K.; methodology, E.N.S. and M.K.; validation, E.N.S. and M.K.; formal analysis, E.N.S. and M.K.; investigation, E.N.S. and M.K.; resources, M.K.; writing—original draft preparation, E.N.S.; writing—review and editing, M.K.; visualization, E.N.S. and M.K.; supervision, M.K.; project administration, E.N.S. and M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the United Kingdom Space Agency (UKSA) Enabling Space Exploration Programme grant number ESE01.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author due to the ongoing research.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| NTP | Non Thermal Plasma |
| SDBD | Surface Dielectric Barrier Discharge |
| OES | Optical Emission Spectroscopy |
| MB | Methylene Blue |
| HTA | Hydroxy Terephthalic Acid |
References
- Xu, Y.; Bassi, A. Non-thermal plasma decontamination of microbes: A state of the art. Biotechnol. Prog. 2025, 41, e3511. [Google Scholar] [CrossRef] [PubMed]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Stratton, G.R.; Bellona, C.L.; Dai, F.; Holsen, T.M.; Thagard, S.M. Plasma-based water treatment: Conception and application of a new general principle for reactor design. Chem. Eng. J. 2015, 273, 543–550. [Google Scholar] [CrossRef]
- Li, S.; Timoshkin, I.V.; Maclean, I.V.; Macgregor, M.; Wilson, S.J.; Given, M.P.; Wang, T.; Anderson, J.G. Fluorescence detection of hydroxyl radicals in water produced by atmospheric pulsed discharges. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1856–1865. [Google Scholar] [CrossRef]
- Foster, J.E. Plasma-based water purification: Challenges and prospects for the future. Phys. Plasmas 2017, 24, 055501. [Google Scholar] [CrossRef]
- Barjasteh, A.; Dehghani, Z.; Lamichhane, P.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Recent progress in applications of non-thermal plasma for water purification, bio-sterilization, and decontamination. Appl. Sci. 2021, 11, 3372. [Google Scholar] [CrossRef]
- Gott, R.P.; Miller, S.D.; Hodges, J.C.; Xu, K.G. Plasma-based water purification for crewed space missions: Laboratory experimental comparisons for on-board applicability. Adv. Space Res. 2021, 68, 1591–1600. [Google Scholar] [CrossRef]
- Thagard, S.M.; Locke, B.R. Electrical discharge plasma for water treatment. In Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications; IWA Publishing: London, UK, 2018; pp. 493–534. [Google Scholar]
- Tang, X.; Júnior, A.D.F.; Karu, K.; Campos, L.C.; Kim, M. Atmospheric pressure dielectric barrier discharge plasma for in-situ water treatment using a bubbling reactor. J. Environ. Manag. 2024, 370, 122574. [Google Scholar] [CrossRef]
- Jakob, H.; Paliwoda, M.; Rovey, J.L.; Kim, M. Surface DBD plasma microbubble reactor for degrading methylene blue. Phys. Scr. 2023, 98, 025603. [Google Scholar] [CrossRef]
- Allabakshi, S.M.; Srikar, P.S.N.S.R.; Gangwar, R.K.; Maliyekkal, S.M. Treatment of azo, direct, and reactive dyes in surface dielectric barrier discharge: Valorization of effluent, the influence of wastewater characteristics, and plasma modeling by Stark broadening technique. J. Water Process Eng. 2023, 56, 104503. [Google Scholar] [CrossRef]
- Robinson, C.D.; Sponsel, N.L.; Stapelmann, K. Bubbling water-treating DBD plasma device optimization using experimental and computational methods. Plasma Processes Polym. 2025, 22, 70000. [Google Scholar] [CrossRef]
- Shahsavari, N.; Zhang, X. Microbubble-enhanced cold plasma activation for water decontamination: Degradation dynamics and energy yield in relation to pollutant concentration, total volume and flow rate of water. J. Water Process Eng. 2023, 55, 104169. [Google Scholar] [CrossRef]
- Kumar, A.; Škoro, N.; Gernjak, W.; Puač, N. Cold atmospheric plasma technology for removal of organic micropollutants from wastewater—A review. Eur. Phys. J. D 2021, 75, 283. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Al-Khazrajy, O.S.; Abdallh, M.; Aadim, K.A.; Al-Mamari, A.; Al-Owaisi, H.; Yousif, E. Removal of dyes from aqueous solutions using non-thermal plasma. Environ. Processes 2023, 10, 63. [Google Scholar] [CrossRef]
- Massima Mouele, E.S.; Tijani, J.O.; Masikini, M.; Fatoba, O.O.; Eze, C.P.; Onwordi, C.T.; Zar Myint, M.T.; Kyaw, H.H.; Al-Sabahi, J.; Al-Abri, M.; et al. Spectroscopic measurements of dissolved O3, H2O2 and OH radicals in double cylindrical dielectric barrier discharge technology: Treatment of methylene blue dye simulated wastewater. Plasma 2020, 3, 59–91. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Nam, W.; Yun, G. Enhanced production of hydroxyl radicals in plasma-treated water via a negative DC bias coupling. J. Phys. D Appl. Phys. 2022, 55, 455201. [Google Scholar] [CrossRef]
- Kooshki, S.; Yousefi, R. Selective tuning of RONS in recirculating NTP reactors for water treatment. Environ. Sci. Pollut. Res. 2024, 31, 1874–1887. [Google Scholar]
- Allabakshi, S.M.; Srikar, P.; Gangwar, R.K.; Maliyekkal, S.M. Feasibility of surface dielectric barrier discharge in wastewater treatment: Spectroscopic modeling, diagnostic, and dye mineralization. Sep. Purif. Technol. 2022, 296, 121344. [Google Scholar] [CrossRef]
- Targhi, R.R.; Qaderi, F. Synergistic effects of process parameters on methylene blue degradation by cold plasma. Int. J. Environ. Sci. Technol. 2025, 22, 4559–4570. [Google Scholar] [CrossRef]
- Rathore, V.; Nagar, A.; Patel, S.; Pandey, A.; Patil, C.N.; Savjani, J.; Butani, S.; Natesan, G.; Dave, H.; Nisoa, M.; et al. Optimizing dielectric barrier discharge pencil plasma jet treatment for efficient degradation of organic contaminants in denim industry wastewater. Plasma Chem. Plasma Process. 2025, 45, 569–595. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Mustafa, F.S.; Omer, K.M.; Shafiq, I. Recent advances in water falling film reactor designs for the removal of organic pollutants by advanced oxidation processes: A review. Water Resour. Ind. 2023, 30, 100227. [Google Scholar] [CrossRef]
- Lee, S.; Kang, H.G.; Kim, M.; Yun, G. Solvated Electrons and Hydroxyl Radicals at Plasma-Liquid Interface. Plasma Processes Polym. 2025, 22, 70005. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Tijani, J.O.; Badmus, K.O.; Pereao, O.; Babajide, O.; Fatoba, O.O.; Zhang, C.; Shao, T.; Sosnin, E.; Tarasenko, V.; et al. A critical review on ozone and co-species, generation and reaction mechanisms in plasma induced by dielectric barrier discharge technologies for wastewater remediation. J. Environ. Chem. Eng. 2021, 9, 105758. [Google Scholar] [CrossRef]
- Stancu, E.C.; Piroi, D.; Magureanu, M.; Dinescu, G. Decomposition of methylene blue by a cold atmospheric pressure plasma jet source. In Proceedings of the 20th International Symposium on Plasma Chemistry, Philadelphia, PA, USA, 24–29 July 2011; p. 375. [Google Scholar]
- Liang, J.P.; Zhao, Z.L.; Zhou, X.F.; Yang, D.Z.; Yuan, H.; Wang, W.C.; Qiao, J.J. Comparison of gas phase discharge and gas-liquid discharge for water activation and methylene blue degradation. Vacuum 2020, 181, 109644. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).