Removal of Ionic Liquid (IL) from Herbal Materials After Extraction with IL and Comprehensive Investigation
Abstract
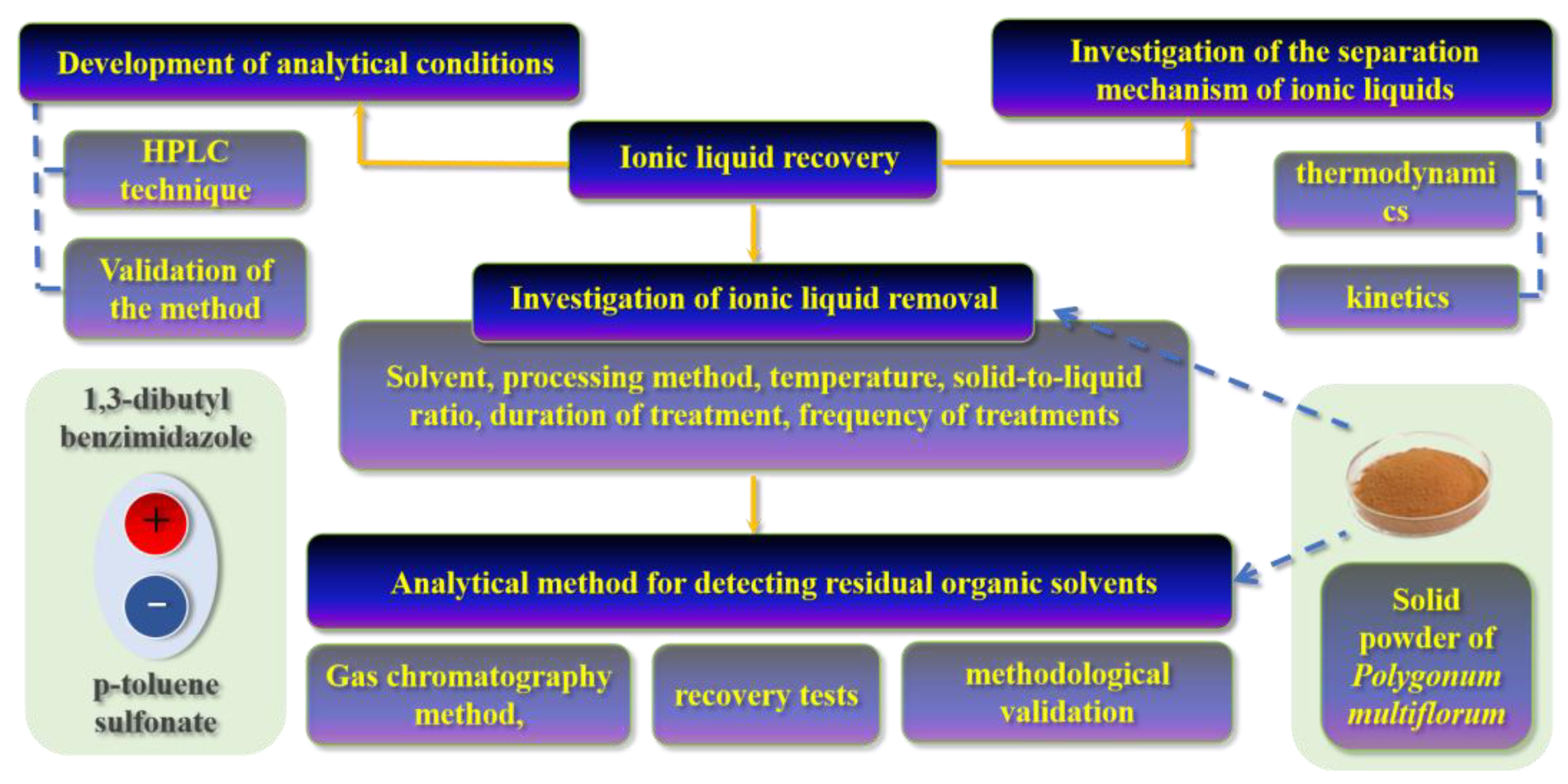
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instruments
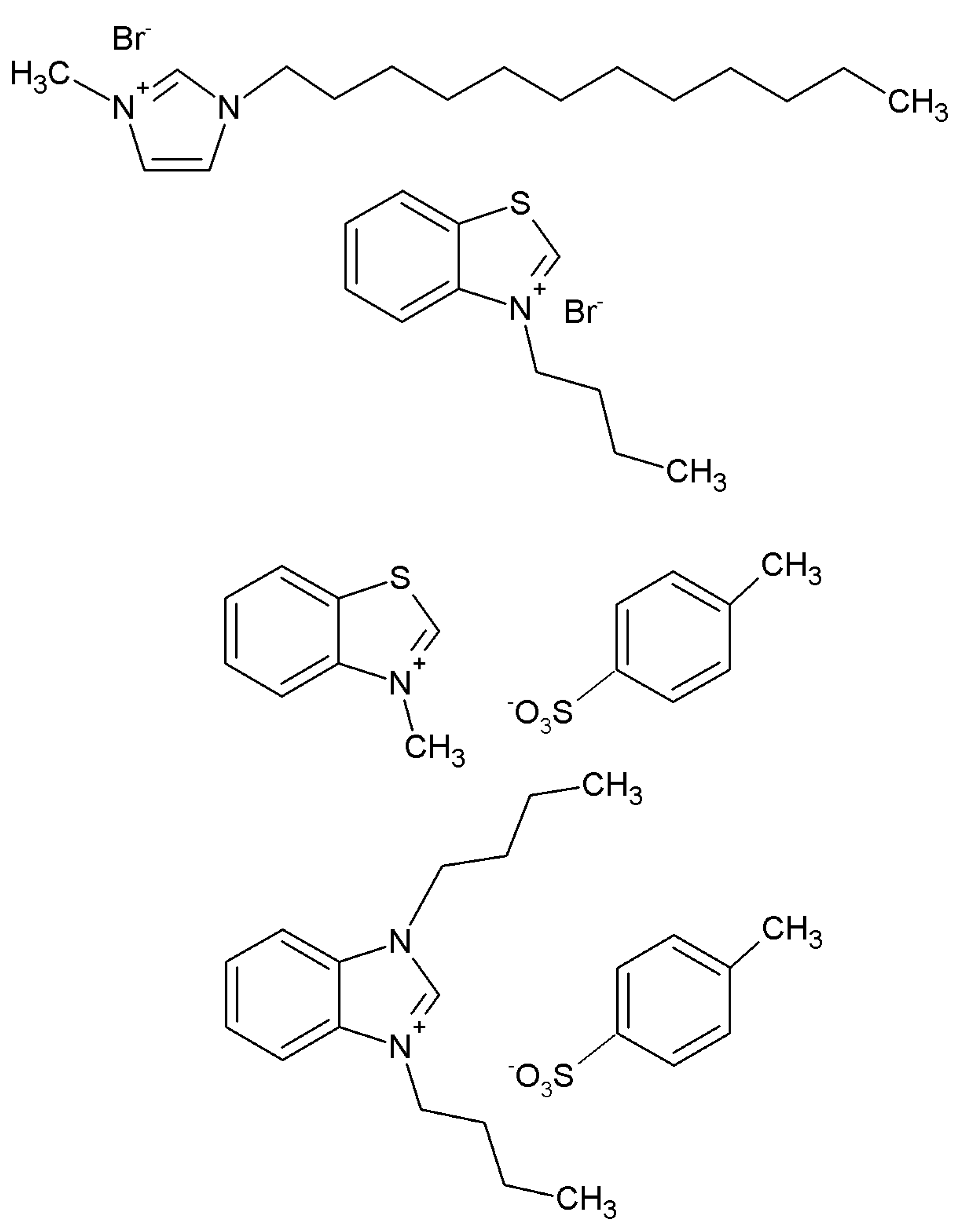
2.3. Extraction of Toxic Anthraquinones in Polygonum Multiflorum by Using [C4Bim][PTSA]
2.4. Development of Analytical Conditions for IL Analysis
2.5. Method for Recovery of IL
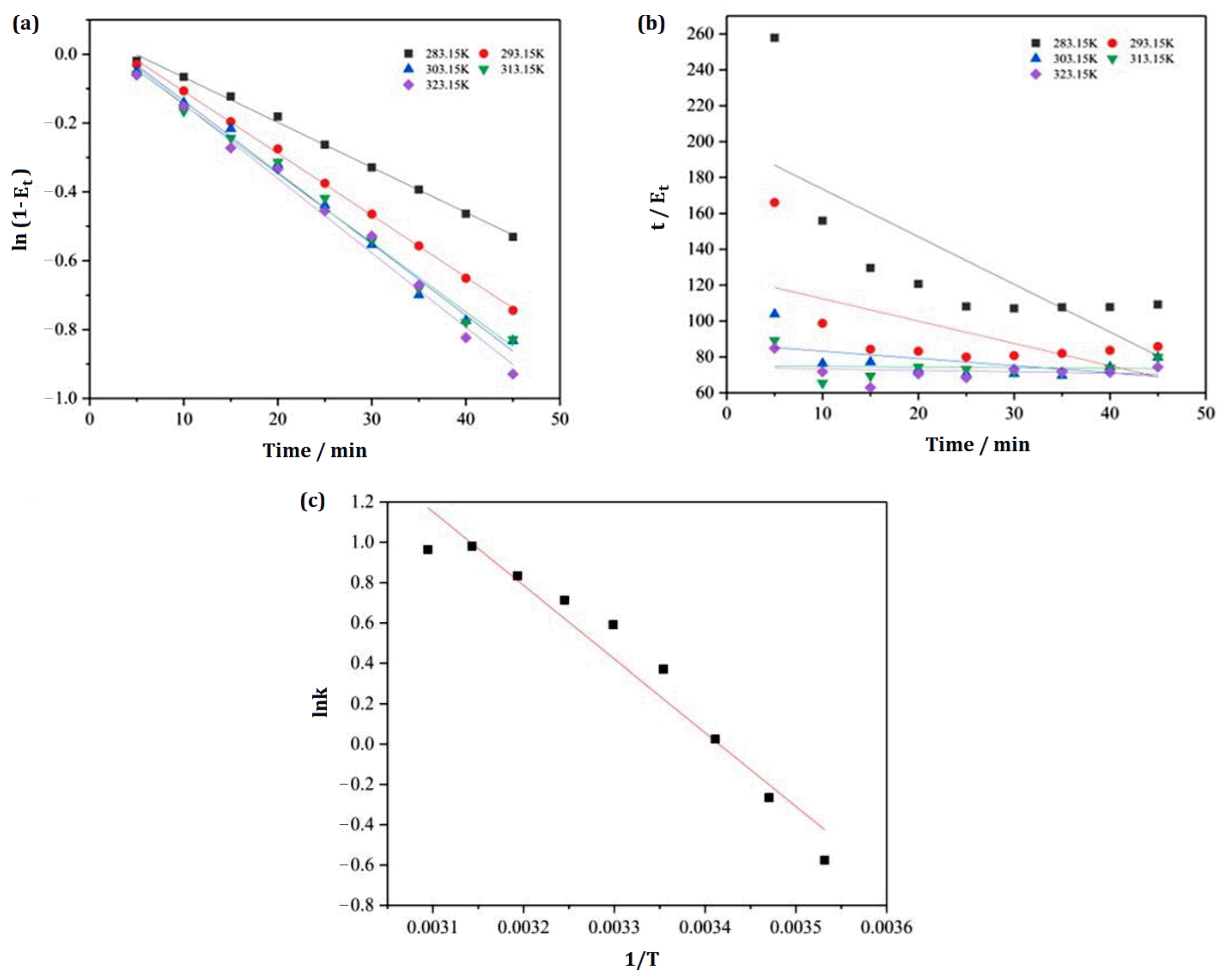
2.6. Kinetic and Thermodynamic Investigation of IL Removal
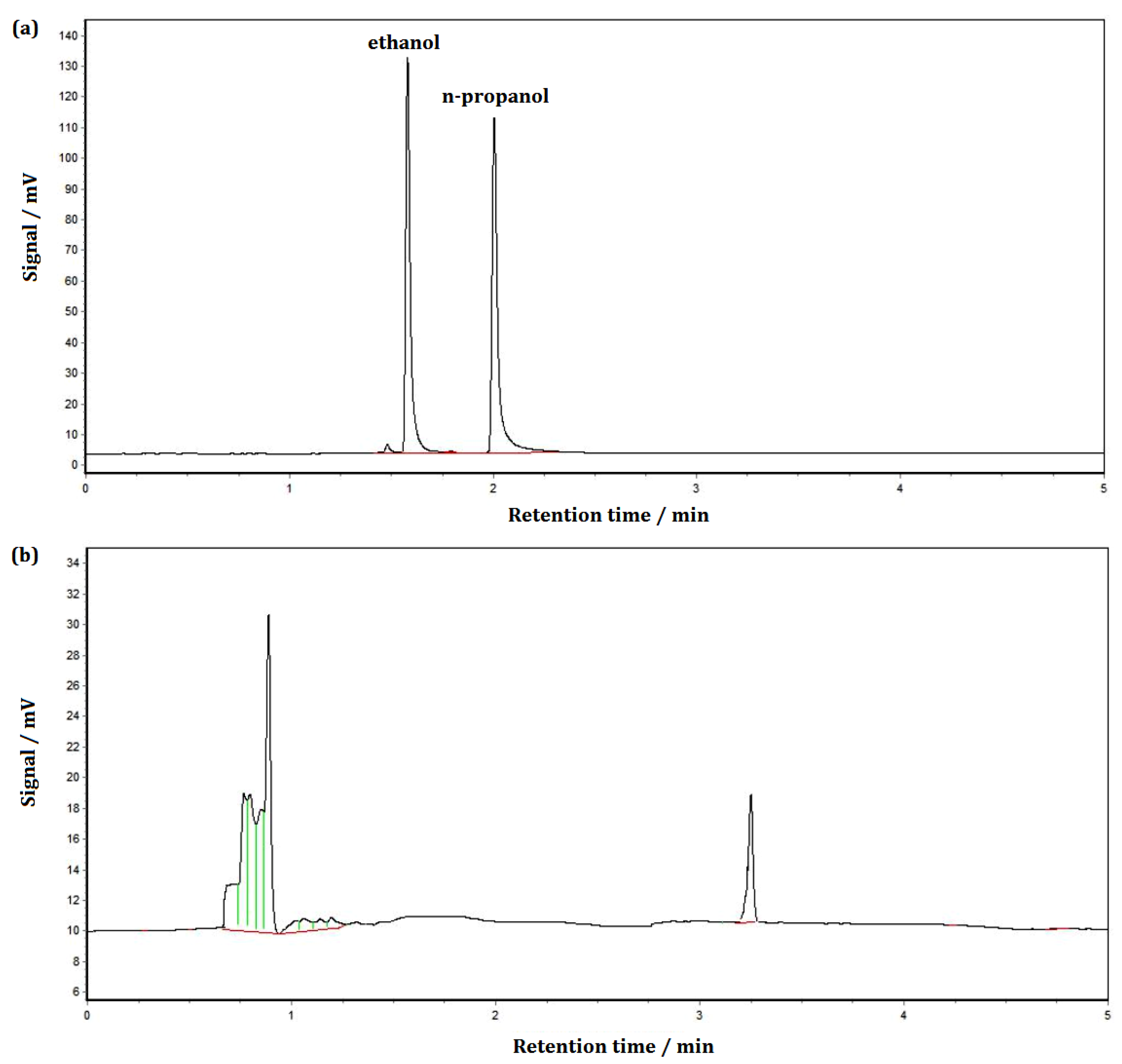
2.7. Development of Analytical Method for Detecting Residual Organic Solvents in Solid Powders
3. Results and Discussion
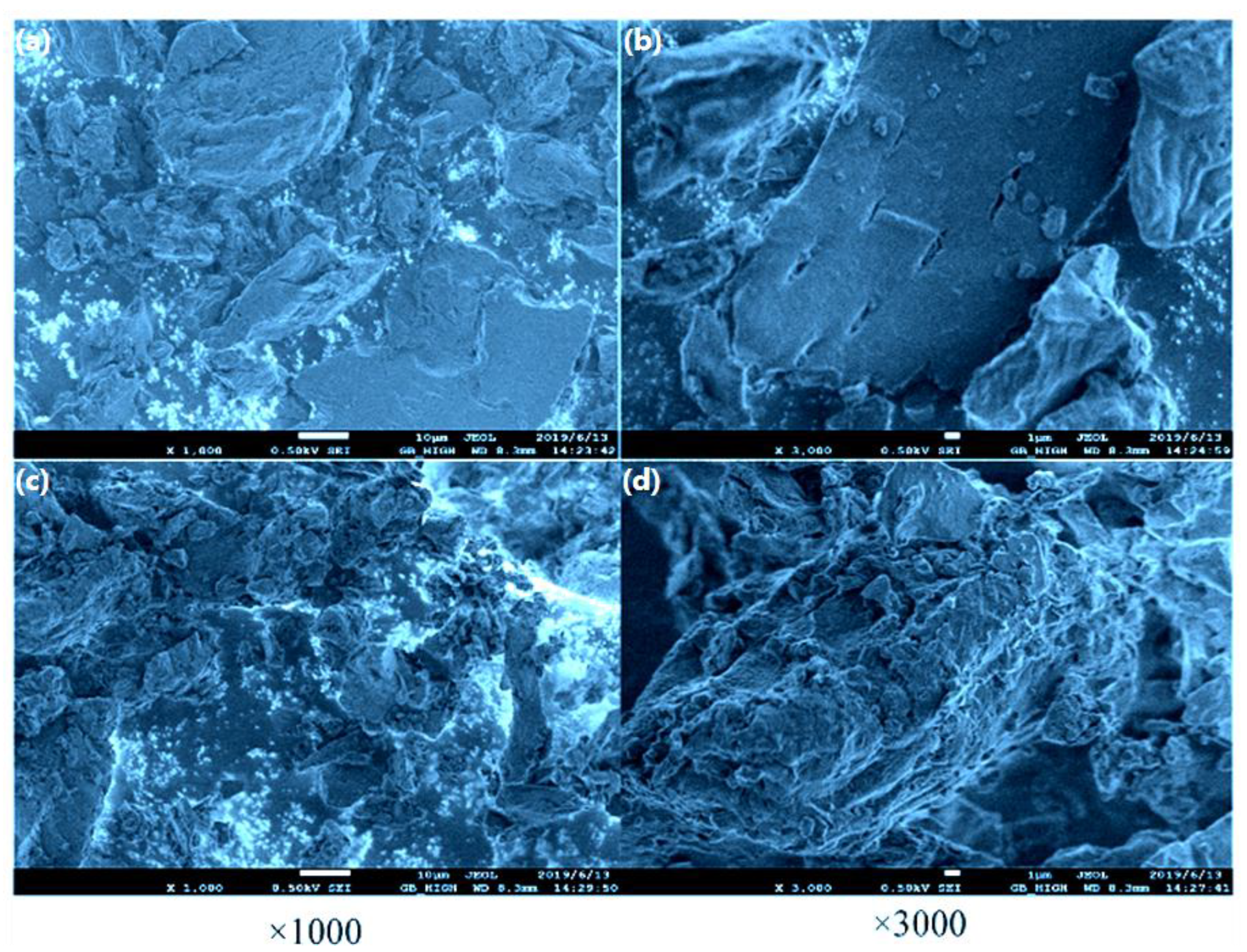
3.1. Analysis of Residual Raw Material and Extract After Extraction
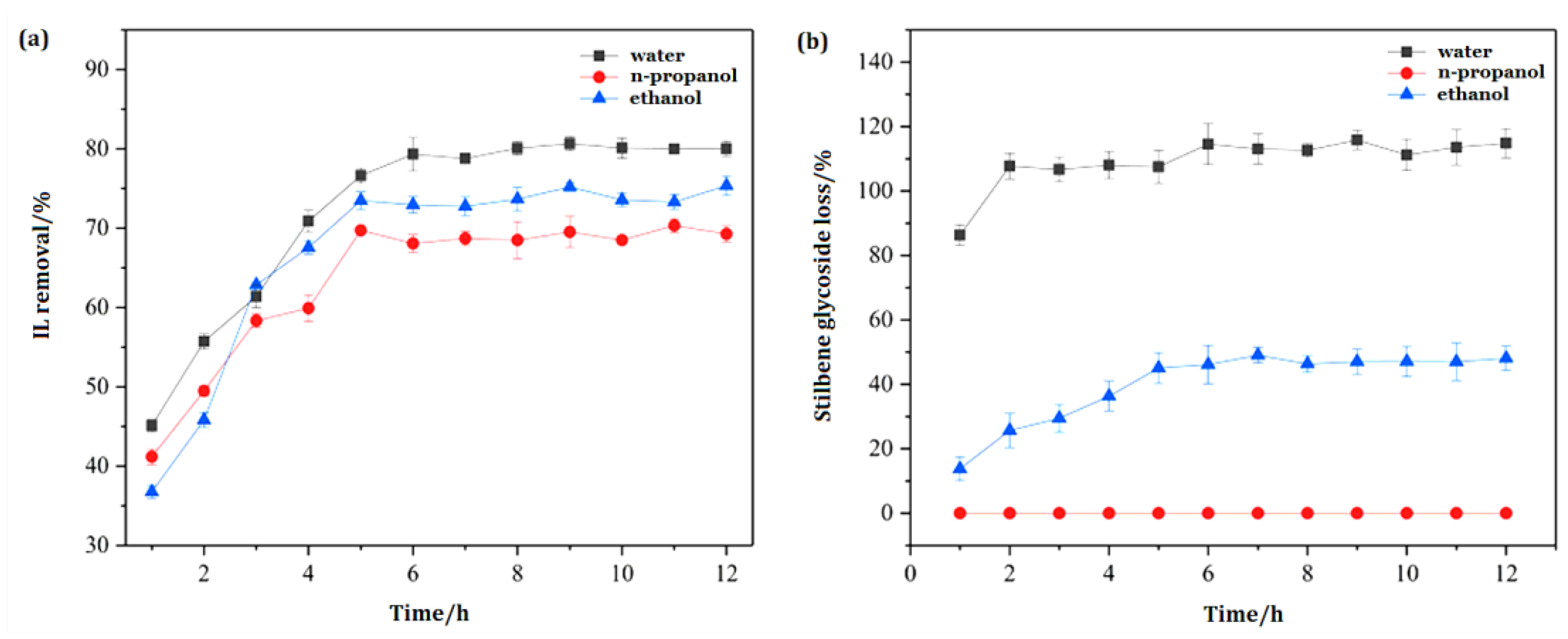
3.2. Screening of Removal Solvent for IL
3.3. Screening of Removal Methods for IL
3.4. Selection of Removal Temperature and Solid–Liquid Ratio
3.5. Selection of Operation Duration
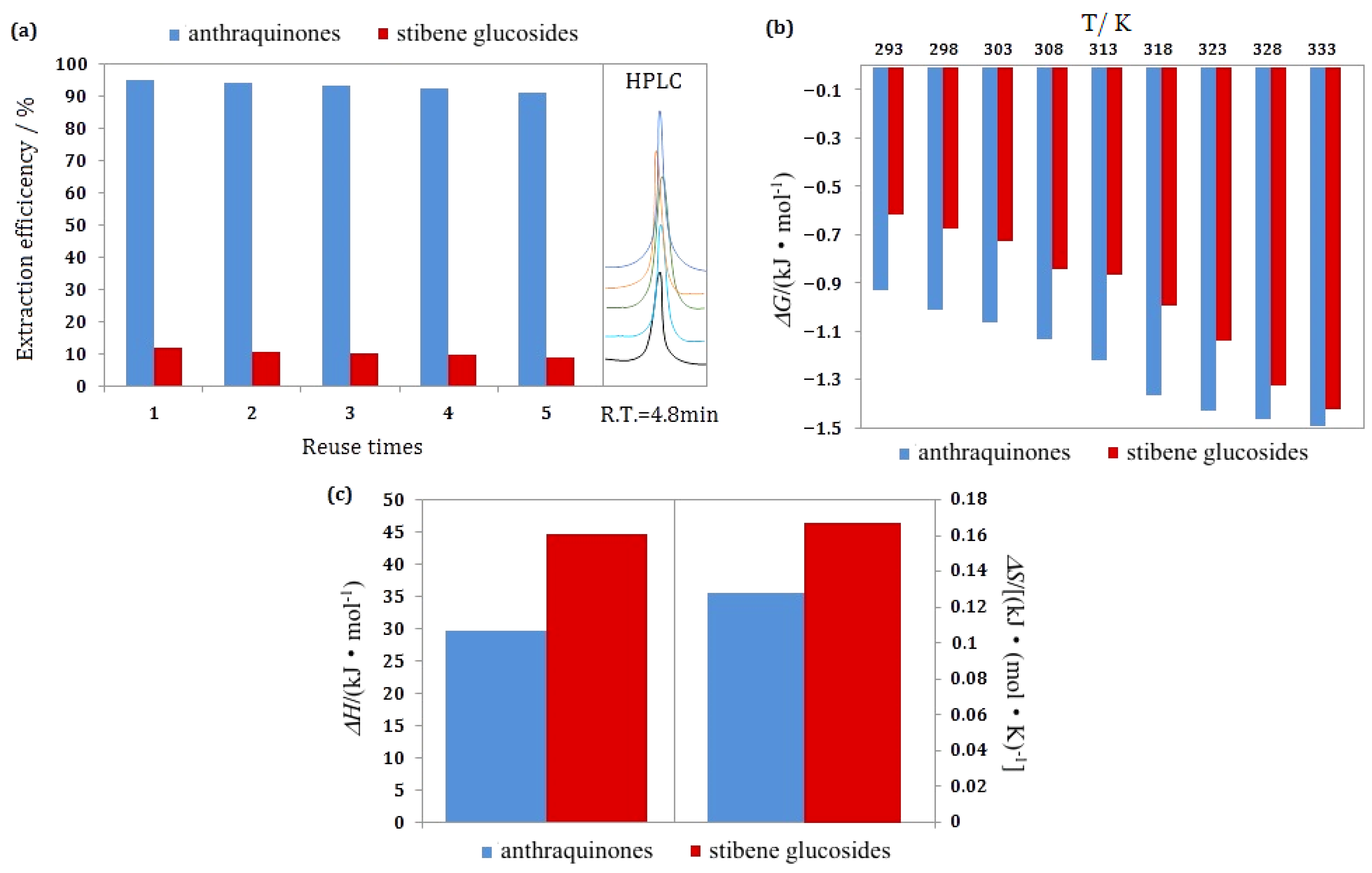
3.6. Selection of Operation Times
3.7. Study on Removal Kinetic Process
3.8. Thermodynamic Study
3.9. Determination of Solvent Residue
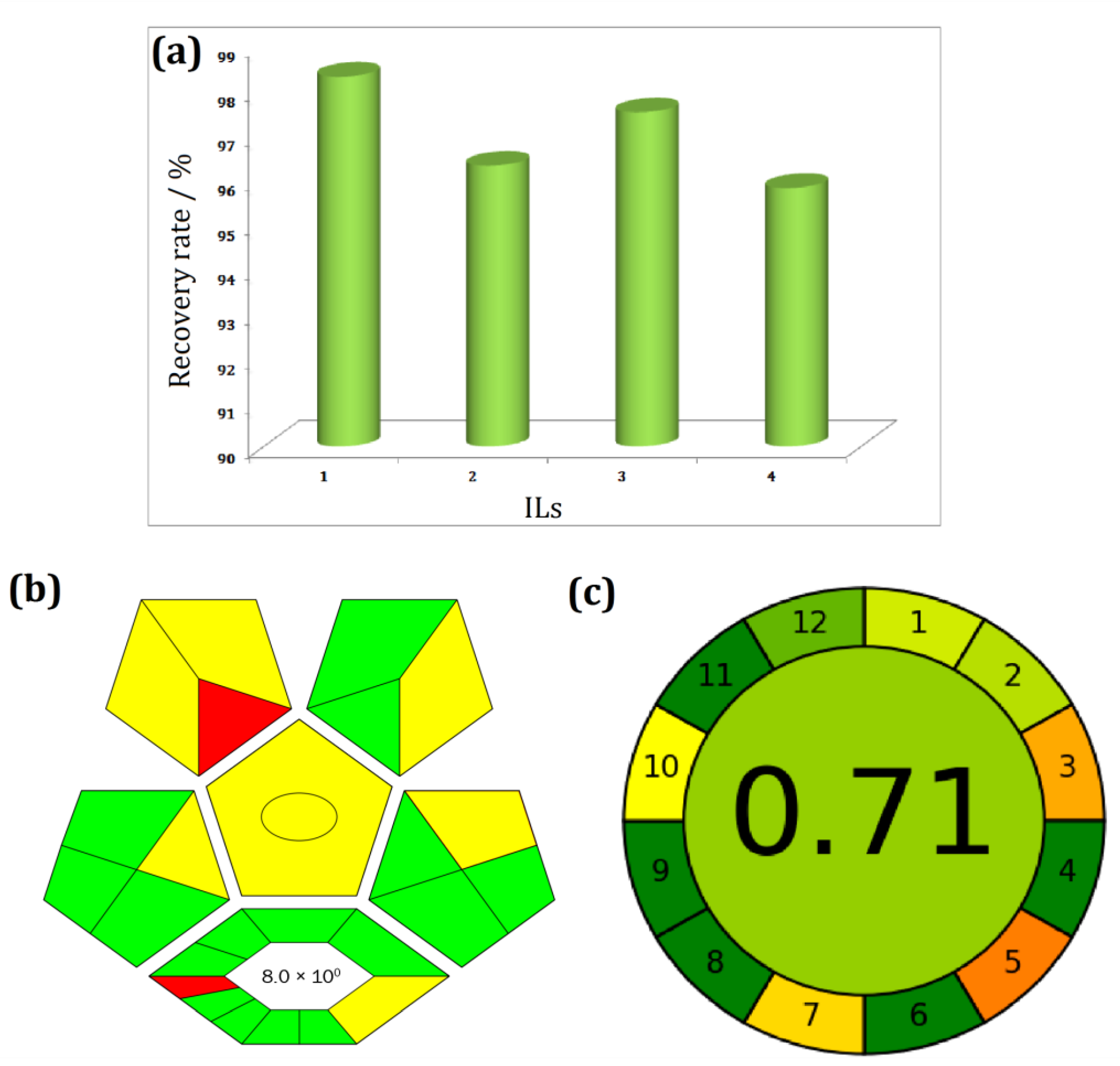
3.10. Analysis of Recovered IL and Its Reuse Performance
3.11. General Applicability and Greenness Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ventura, S.P.M.; eSilva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Belchior, D.C.V.; Duarte, I.F.; Freire, M.G. Ionic Liquids in Bioseparation Processes. Adv. Biochem. Eng. -Biotechnol. 2019, 168, 1–29. [Google Scholar] [CrossRef]
- Chang, K.L.; Chen, X.M.; Wang, X.Q.; Han, Y.J.; Potprommanee, L.; Liu, J.Y.; Liao, Y.L.; Ning, X.A.; Sun, S.Y.; Huang, Q. Impact of Surfactant Type for Ionic Liquid Pretreatment on Enhancing Delignification of Rice Straw. Bioresour. Technol. 2017, 227, 388–392. [Google Scholar] [CrossRef]
- Gu, L.; Ran, C.; Chao, L.; Bao, Y.; Hui, W.; Wang, Y.; Chen, Y.; Gao, X.; Song, L. Designing Ionic Liquids as the Solvent for Efficient and Stable Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 22870–22878. [Google Scholar] [CrossRef]
- Miao, W.; Chan, T.H. Ionic-Liquid-Supported Synthesis: A Novel Liquid-Phase Strategy for Organic Synthesis. Acc. Chem. Res. 2006, 39, 897–908. [Google Scholar] [CrossRef]
- Li, L.; Chang, L.; Zhang, X.; Liu, H.; Jiang, L. Surface Charge-Induced Efficient Recovery of Ionic Liquids from Aqueous Phase. ACS Appl. Mater. Interfaces 2017, 9, 29355–29362. [Google Scholar] [CrossRef]
- Pérez, R.L.; Ayala, C.E.; Opiri, M.M.; Ezzir, A.; Li, G.; Warner, I.M. Recycling Thermoset Epoxy Resin Using Alkyl-Methyl-Imidazolium Ionic Liquids as Green Solvents. ACS Appl. Polym. Mater. 2021, 3, 5588–5595. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Wang, H.; Li, Z.; Wang, J. Recovery of Ionic Liquids with Aqueous Two-Phase Systems Induced by Carbon Dioxide. ChemSusChem 2012, 5, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wilkins, M.R.; Wang, D. A Review on Strategies to Reduce Ionic Liquid Pretreatment Costs for Biofuel Production. Bioresour. Technol. 2022, 364, 128045. [Google Scholar] [CrossRef]
- Zhe, Y.; Gao, S.; Cao, Z.; Chen, X.; Yu, G. Recovery of Ionic Liquids from Methanol by Pervaporation with Polydimethylsiloxane Membrane. Chem. Pap. 2020, 74, 1331–1337. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.K.; Morsy, M.A.; Abdalla, A.M.; Hamouda, A.H.; Alhaider, I.A. Mechanisms of Thymoquinone Hepatorenal Protection in Methotrexate-Induced Toxicity in Rats. Mediat. Inflamm. 2014, 2015, 859383. [Google Scholar] [CrossRef]
- Xiao, M.Y.; Jun, S.L.; Qi, Q.L.; Guo, X.H.; Liu, J.Y. Evaluation of the Potential Toxicity of Anthraquinone Derivatives in Chinese Herbal Medicines by the Resonance Light Scattering Spectrum. Asian J. Chem. 2011, 23, 3631–3634. Available online: https://asianpubs.org/index.php/ajchem/article/view/10700 (accessed on 29 April 2011).
- Ho, T.-T.; Murthy, H.N.; Dalawai, D.; Bhat, M.A.; Paek, K.-Y.; Park, S.-Y. Attributes of Polygonum multiflorum to Transfigure Red Biotechnology. Appl. Microbiol. Biotechnol. 2019, 103, 3317–3326. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Chen, S.; Chen, X.; Yao, S.; Wang, X.; Song, H.; Zhu, M. High Effective Extraction of Selected Anthraquinones from Polygonum multiflorum Using Ionic Liquids with Ultrasonic Assistance. J. Mol. Liq. 2020, 314, 113342. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, J.; Zhang, C.; Zhang, J.; Zhu, L.; Du, Z.; Wang, J. Determination of Residual Concentration of Ionic Liquids with Different Anions and Alkyl-Chain Lengths in Water and Soil Samples. Anal. Chem. 2017, 89, 10520–10528. [Google Scholar] [CrossRef]
- Lu, J.; Li, W.; Zhu, M.; Ma, L. GC Determination of Residual Organic Solvents in Total Glucosides of Paeony Active Pharmaceutical Ingredient. Chin. J. Mod. Appl. Pharm. 2018, 35, 1709–1712. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Casañas Pimentel, R.G.; San Martin Martinez, E. UV-Vis Spectroscopic Quantification of Residual Acetone during the Development of Nanoparticulate Drug Delivery Systems. Pharm. Dev. Technol. 2019, 24, 751–760. [Google Scholar] [CrossRef]
- Sun, R.; Hu, C.; Dou, Q.; Luan, L. Simultaneous Determination of Nine Residual Solvents in Sorafenib Tosylate by Gas Chromatography. J. AOAC Int. 2021, 104, 1005–1009. [Google Scholar] [CrossRef]
- Noorbasha, K.; Shaik, A.R. Determination of Residual Solvents in Paclitaxel by Headspace Gas Chromatography. Future J. Pharm. Sci. 2021, 7, 40. [Google Scholar] [CrossRef]
- Yang, C.; Wang, S.; Guo, X.; Sun, J.; Liu, L.; Wu, L. Simultaneous Determination of Seven Anthraquinones in Rat Plasma by Ultra High Performance Liquid Chromatography-Tandem Mass Spectrometry and Pharmacokinetic Study after Oral Administration of Semen Cassiae Extract. J. Ethnopharmacol. 2015, 169, 305–313. [Google Scholar] [CrossRef]
- Dai, Q.; Ma, J.; Ma, S.; Wang, S.; Li, L.; Zhu, X.; Qiao, X. Cationic Ionic Liquids Organic Ligands Based Metal-Organic Frameworks for Fabrication of Core-Shell Microspheres for Hydrophilic Interaction Liquid Chromatography. ACS Appl. Mater. Interfaces 2016, 8, 21632–21639. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P. Use of Ionic Liquids in Chitin Biorefinery: A Systematic Review. Front. Bioeng. Biotechnol. 2020, 8, 11. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, C.; Wang, J.; Liu, Q.; Li, R.; Yang, P.; Zhang, M. Removal of Uranium(VI) from Aqueous Solutions by Magnetic Schiff Base: Kinetic and Thermodynamic Investigation. Chem. Eng. J. 2012, 198–199, 412–419. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Huyiligeqi; Ni, J. Emodin: A Review of Its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef]
- Porto, T.S.; Porto, C.S.; Cavalcanti, M.T.H.; Filho, J.L.L.; Pessoa, A. Kinetic and Thermodynamic Investigation on Ascorbate Oxidase Activity and Stability of a Cucurbita Maxima Extract. Biotechnol. Prog. 2010, 22, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Sribudda, D.; Sunsandee, N.; Ramakul, P.; Pancharoen, U.; Phatanasri, S. Separation of Cd(II) from Industrial Wastewater via HFSLM: Equilibrium, Kinetic and Thermodynamic Investigation. J. Ind. Eng. Chem. 2015, 25, 22–28. [Google Scholar] [CrossRef]
- Brandt, A.; Ray, M.J.; To, T.Q.; Leak, D.J.; Murphy, R.J.; Welton, T. Ionic Liquid Pretreatment of Lignocellulosic Biomass with Ionic Liquid–Water Mixtures. Green Chem. 2011, 13, 2489–2499. [Google Scholar] [CrossRef]
- Berg-Schlosser, D.; Badie, B.; Morlino, L.; Beck, N. Methodology: Qualitative and Quantitative Approaches; SAGE Publications Ltd.: London, UK, 2020; pp. 423–436. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia (2020 edition); The Medicine Science and Technology Press of China: Beijing, China, 2020; Volume 4, p. 151. Available online: https://ydz.chp.org.cn/#/item?bookId=4&entryId=5476 (accessed on 24 September 2025).
- Saha, K.; Dwibedi, P.; Ghosh, A.; Sikder, J.; Chakraborty, S.; Curcio, S. Extraction of Lignin, Structural Characterization and Bioconversion of Sugarcane Bagasse after Ionic Liquid Assisted Pretreatment. 3 Biotech. 2018, 8, 374. [Google Scholar] [CrossRef]
- Buarque, F.S.; Gautério, G.V.; Coelho, M.A.Z.; Lemes, A.C.; Ribeiro, B.D. Aqueous Two-Phase Systems Based on Ionic Liquids and Deep Eutectic Solvents as a Tool for the Recovery of Non-Protein Bioactive Compounds—A Review. Processes 2023, 11, 31. [Google Scholar] [CrossRef]
- Norfarhana, A.S.; Ilyas, R.A.; Ngadi, N.; Othman, M.H.D.; Misenan, M.S.M.; Norrrahim, M.N.F. Revolutionizing Lignocellulosic Biomass: A Review of Harnessing the Power of Ionic Liquids for Sustainable Utilization and Extraction. Int. J. Biol. Macromol. 2024, 256, 128256. [Google Scholar] [CrossRef]
- Zhou, J.; Sui, H.; Jia, Z.; Yang, Z.; He, L.; Li, X. Recovery and Purification of Ionic Liquids from Solutions: A Review. RSC Adv. 2018, 8, 32832–32864. [Google Scholar] [CrossRef]
- Hingston, F.J. Activity of Polyphenolic Constituents of Leaves of Eucalyptus and Other Species in Complexing and Dissolving Iron Oxide. Soil Res. 1963, 1, 63–73. [Google Scholar] [CrossRef]
- Feng, X.T.; Song, H.; Dong, B.; Yang, Y.; Yao, S. Sequential Extraction And Separation Using Ionic Liquids For Stilbene Glycoside And Anthraquinones in Polygonum multiflorum. J. Mol. Liq. 2017, 241, 27–36. [Google Scholar] [CrossRef]
- Du, K.Z.; Chen., Y.; Li, J.; Tang, G.; Tian, F.; He, J.; Chang, Y.X. Quantification of eight active ingredients in crude and processed radix polygoni multiflori applying miniaturized matrix solid-phase dispersion microextraction followed by UHPLC. J. Sep. Sci. 2018, 41, 3486–3495. [Google Scholar] [CrossRef] [PubMed]

| T/K | First-Order Kinetics | Second-Order Kinetics | ||||
|---|---|---|---|---|---|---|
| k1/min−1 | R2 | RMSE | k2/min−1 | R2 | RMSE | |
| 283.15 | 0.01309 | 0.9971 | 9.0895 × 10−3 | 2.6547 | 0.5444 | 31.3524 |
| 293.15 | 0.01800 | 0.9992 | 6.6355 × 10−3 | 1.2455 | 0.3799 | 20.5434 |
| 303.15 | 0.02077 | 0.9929 | 2.2641 × 10−2 | 0.4007 | 0.2705 | 8.4946 |
| 313.15 | 0.02109 | 0.9919 | 2.3510 × 10−2 | 0.0376 | 0.00565 | 6.4418 |
| 323.15 | 0.02162 | 0.9912 | 2.6247 × 10−2 | 0.07736 | 0.03317 | 5.3913 |
| T/K | PartitionCoefficient (k) | ΔG/(kJ·mol−1) | ΔH/(kJ·mol−1) | ΔS/[(kJ·(mol·K−1)] |
|---|---|---|---|---|
| 283.15 | 0.5619 | 0.9907 | 30.3534 | 0.1037 |
| 288.15 | 0.7669 | 0.4722 | ||
| 293.15 | 1.0249 | −0.0463 | ||
| 298.15 | 1.4493 | −0.5648 | ||
| 303.15 | 1.8055 | −1.0833 | ||
| 308.15 | 2.0388 | −1.6018 | ||
| 313.15 | 2.3011 | −2.1203 | ||
| 318.15 | 2.6652 | −2.6388 | ||
| 323.15 | 2.6212 | −3.1573 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Mahmood, S.; Cao, Y.; Yao, S. Removal of Ionic Liquid (IL) from Herbal Materials After Extraction with IL and Comprehensive Investigation. Separations 2025, 12, 302. https://doi.org/10.3390/separations12110302
Zhang Z, Mahmood S, Cao Y, Yao S. Removal of Ionic Liquid (IL) from Herbal Materials After Extraction with IL and Comprehensive Investigation. Separations. 2025; 12(11):302. https://doi.org/10.3390/separations12110302
Chicago/Turabian StyleZhang, Zhaojin, Subhan Mahmood, Yu Cao, and Shun Yao. 2025. "Removal of Ionic Liquid (IL) from Herbal Materials After Extraction with IL and Comprehensive Investigation" Separations 12, no. 11: 302. https://doi.org/10.3390/separations12110302
APA StyleZhang, Z., Mahmood, S., Cao, Y., & Yao, S. (2025). Removal of Ionic Liquid (IL) from Herbal Materials After Extraction with IL and Comprehensive Investigation. Separations, 12(11), 302. https://doi.org/10.3390/separations12110302







