Abstract
Background: Pomegranate peel (Punica granatum L.) is a rich source of phenols, particularly ellagitannins, highlighting punicalagin, a bioactive compound with recognized antioxidant potential. However, efficient recovery and purification methods are required to enable its application in food and health-related products. This study aimed to obtain a partially purified fraction of punicalagin from pomegranate peel using optimized extraction and purification strategies. Methods: A Taguchi L9 (3)3 experimental design was employed to optimize ultrasound-assisted extraction, evaluating extraction time (10, 20, 30 min), ethanol concentration (20, 40, 80%), and solid-to-solvent ratio (1:12, 1:14, 1:16). Total polyphenol content was quantified using the Folin–Ciocalteu method. Extracts obtained under optimized conditions were concentrated by rotary evaporation and subjected to semipurification using flash chromatography with Amberlite XAD-16 resin. Subsequently, the fractions were lyophilized and analyzed by HPLC/ESI/MS. Results: The Statistica software determined the optimal conditions for polyphenol extraction (20 min, 40% ethanol, 1:12), with the signal-to-noise (S/N) ratio reaching 88.43 ± 0.66, surpassing the predicted value of 77.42. Flash chromatography yielded four fractions, and HPLC/ESI/MS analysis revealed the presence of ellagitannins in all of them, with fraction number 2 showing the highest relative abundance of punicalagin (89.25%). Conclusions: The combination of ultrasound-assisted extraction and flash chromatography proved effective for obtaining punicalagin-rich fractions from pomegranate peel, supporting its potential for nutraceutical applications.
1. Introduction
The fruit Punica granatum L., commonly known as pomegranate, is native to Asia and the Mediterranean region. It is currently cultivated in North and tropical Africa, North and South America, and the Caucasus area []. The fruit is characterized by its nutritional benefits and therapeutic effects due to the high content of bioactive compounds present in the juice, seeds, leaves, flowers, and peel [].
Pomegranate peel accounts for 43–50% of the total fruit weight and is considered an agro-industrial residue during pomegranate juice production, generating approximately 1.9 million tons of waste worldwide [,]. The peel is rich in many polyphenolic compounds, among which ellagitannins stand out, specifically punicalagin, punicalin, and ellagic acid [].
Punicalagin is the most abundant polyphenolic compound in pomegranate peel. It is an ellagitannin, a type of hydrolyzable tannin, with a molecular weight of 1084.7 g/mol []. It is water-soluble and naturally occurs in two anomeric forms, α and β []. The literature reports that punicalagin could exert various positive health effects, primarily antioxidant, anti-inflammatory, and anticancer activities, among others [,,]. Moreover, beneficial effects have also been reported on chronic metabolic conditions such as cardiovascular diseases, diabetes, and obesity [,,,]. These findings highlight the great potential of this molecule for applications in the pharmaceutical and food industries.
Several extraction methods have been reported in the literature to obtain crude extracts rich in polyphenolic compounds from pomegranate peel, using both conventional techniques such as maceration and infusion, as well as advanced technologies including ultrasound-assisted extraction, microwave-assisted extraction, supercritical fluids, pressurized liquids, and enzyme-assisted extraction, among others [,]. On the other hand, various purification or semipurification techniques have also been employed, such as medium-pressure liquid chromatography (MPLC), high-speed countercurrent chromatography (HSCCC), as well as methods based on conventional liquid chromatography (column chromatography, preparative HPLC, and Semi-preparative HPLC) [,,].
In this context, flash chromatography emerges as an efficient and versatile alternative that, through the combination of liquid phases and the simultaneous use of two detectors ultraviolet (UV) and evaporative light scattering detector (ELSD) allows the purification or semipurification of organic compounds in significantly shorter times compared to conventional liquid chromatography methods. Its usefulness has increased notably with the development of automated systems, which have optimized their performance and expanded their applications in the purification of bioactive compounds []. The aim of this work was to extract, purify, and characterize the ellagitannins from pomegranate peel.
2. Materials and Methods
2.1. Raw Material
The Valencia pomegranate variety was collected in the municipality of Nombre de Dios, Durango, Mexico, from a private orchard located in the town of Cardenchos (latitude 23°51′ N, longitude 104°15′ W, altitude 1755 m above sea level) during the May season by M.C. Karina Guadalupe Reyes-Morales. The fruit components were manually separated, and the pomegranate peels were placed in a forced-air tray dryer at 60 °C for 48 h [,]. Subsequently, the dried peels underwent two grinding stages due to the hardness of the material: an initial grinding with an industrial drum mill followed by a secondary grinding with a blade mill (IKA MF-10, Wilmington, NC, USA). The resulting powder was then sieved to obtain a particle size of 500 µm (Humboldt sieve no. 35) and finally stored in vacuum-sealed plastic bags in the dark at room temperature.
2.2. Extraction Bio-Compounds of Pomegranate Peel by Taguchi Methodology
In this study, the Taguchi methodology was used to optimize the polyphenol extraction process from pomegranate peel. An L9 (33) orthogonal array was applied, testing the three main factors: time (min), ethanol concentration (%), and mass/solvent ratio (Table 1). This array allowed for 9 experimental runs in which the 3 factors were combined at their respective levels (Table 2) []. After performing the extractions, the total polyphenol content was evaluated for each treatment, and all treatments were carried out in triplicate.

Table 1.
Factors and levels for the extraction of phenolic compounds from pomegranate peel.

Table 2.
Experimental matrix of the Taguchi l9 (33) design for ultrasound-assisted extraction of phenolic compounds from Pomegranate peel.
The data obtained were analyzed using Statistica 7.0 software (StatSoft, St. Tulsa, OK, USA) by applying the “higher the better” function. The signal-to-noise ratio (S/N) was used as an indicator of treatment reproducibility and quality, and was calculated using the following equation:
In this equation, the factor −10 ensures that the ratio reflects the inverse of “bad quality,” where y represents the experimental value obtained in each trial and N is the total number of samples.
The analysis of variance (ANOVA) was used to determine the percentage contribution of each factor, calculated as follows:
where P represents the percentage of contribution, SSi is the individual sum of squares, SST is the total sum of squares, MSi is the individual mean square, and dfi represents the individual degrees of freedom [].
2.3. Ultrasound-Assisted Extraction
Polyphenol extraction from pomegranate peel was carried out using an ultrasonic bath (model 3800, BRANSON brand, Brookfield, CT, USA) at a frequency of 40 kHz, following the conditions and treatments specified in Table 1 and Table 2, with each treatment performed in triplicate. After extraction, the samples were filtered using Whatman No. 1 filter paper [].
2.4. Total Polyphenol Content (Folin–Ciocalteu)
The extracted samples were diluted at a 1:100 ratio. To initiate the reaction, 125 µL of the sample was placed in a test tube, followed by the addition of 500 µL of distilled water and 125 µL of Folin–Ciocalteu reagent. The reaction was incubated for 6 min, protected from light and at room temperature. Then, 1250 µL of 7% Na2CO3 solution and 1000 µL of distilled water were added. The reaction was incubated again in the dark and at room temperature for 90 min. Absorbance was measured at 760 nm using a spectrophotometer (Genesys 10S UV-Vis Thermo Scientific, Waltham, MA, USA). For the result expression, a calibration curve was prepared from a gallic acid stock solution ranging from 100 to 0 mg/L. The results were expressed as mEq of gallic acid/L [].
2.5. Flash Chromatography and Semipurification of Ellagitannins
For the fractionation of the polyphenolic extract obtained from pomegranate peel, a method for the semipurification of ellagitannins was developed using a Flash Pure C-850 liquid chromatograph equipped with a binary pump, a UV–visible detector, a light scattering detector (ELSD), and an EcoFlex column (40 g) packed with Amberlite XAD-16. An injection volume of 10 mL was used. The eluents were water (solvent A) and 96% ethanol (solvent B), and the elution of polyphenolic compounds was monitored at 254, 280 and 365 nm. The following gradient was applied: 0% B (linear), 0–5 min; 5% B, 5–6 min; 5% B (linear), 6–16 min; 20% B (linear), 16–17 min; 20% B (linear), 17–22 min; 20% B (linear), 22–24 min; 100% B (linear), 24–27 min; 100% B (linear) [].
2.6. Lyophilization of Polyphenols
The fractions recovered in 50 mL Falcon tubes were frozen and subsequently lyophilized using a freeze dryer (Labconco Corporation, Kansas City, MO, USA) at a vacuum pressure of 0.340 mBar and a temperature of −49 °C, in order to preserve sample stability of the recovered compounds [,].
2.7. Characterization of Polyphenolic Compounds by HPLC/ESI/MS and Quantification of Punicalagin
The characterization of the polyphenolic compounds present in the pomegranate peel extract and in the four fractions recovered after fractionation was performed by high-performance liquid chromatography coupled to mass spectrometry (HPLC-MS). The analyzed samples were filtered using 0.45 µm nylon membranes and placed in 2 mL vials. High-performance liquid chromatography (HPLC) analyses in reverse phase were performed on a Varian HPLC system, which included an autosampler (Varian ProStar 410, Palo Alto, CA, USA), a ternary pump (Varian ProStar 230I, Palo Alto, CA, USA), and a PDA detector (Varian ProStar 330, Palo Alto, CA, USA). Additionally, an ion-trap mass spectrometer (Varian 500-MS IT Mass Spectrometer, Palo Alto, CA, USA) equipped with an electrospray ionization (ESI) source was used. A 10 µL sample was injected into a Denali C18 column (150 mm × 2.1 mm, 3 µm, Grace, Palo Alto, CA, USA), with the oven temperature maintained at 30 °C. Formic acid (0.2%, v/v; solvent A) and acetonitrile (solvent B) were used as eluents, applying the following gradient: initial, 3% B; 0–5 min, 9% B linear; 5–15 min, 16% B linear; 15–45 min, 50% B linear. Subsequently, the column was washed and reconditioned. The flow rate was maintained at 0.2 mL/min, and elution was monitored at 245, 280, 320, and 550 nm. The entire effluent (0.2 mL/min) was injected into the mass spectrometer source without splitting. All MS experiments were performed in negative mode. Nitrogen [M-H] was used as the nebulizer gas, and helium served as the damping gas. Ion source parameters included a spray voltage of 5.0 kV, with capillary voltage and temperature set to 90.0 V and 350 °C, respectively. Data were collected and processed using MS Workstation software (V 6.9). Samples were analyzed in full-scan mode, acquired over an m/z range of 50–2000. MS/MS analyses were performed on a series of selected precursor ions [].
3. Results and Discussion
3.1. Total Polyphenol Content from Pomegranate Peel Extracts
In the present study, the Folin–Ciocalteu assay was used to optimize the extraction of phenolic compounds from pomegranate peel through ultrasound-assisted extraction. The Taguchi orthogonal array allowed the exploration of extraction conditions while reducing the number of experiments to nine effective treatments. Figure 1 shows the total polyphenol content, highlighting treatments 2, 5, 6, and 8 for their high content and reproducibility. A statistical analysis of means comparison using Tukey’s test (p < 0.05) showed no significant differences among treatments, indicating that none exhibited statistical superiority. However, since the main objective of the Taguchi design is to identify the combination of factors that maximizes the system response, the signal-to-noise ratio was used to evaluate the effect of each parameter and establish the optimal extraction conditions.

Figure 1.
Average S/N ratio and total polyphenol content in the nine treatments from the Taguchi methodology.  mEq of GA/L and
mEq of GA/L and  S/N.
S/N.
3.2. Relative Influence of Individual Factors
Figure 2 shows the relative influence of individual factors on the extraction of polyphenols from pomegranate peel. Ethanol is observed to be the most decisive factor, contributing 82.97%, followed by the mass-to-volume ratio at 12.20%. Extraction time has a minor impact, accounting for only 1.83%, while the margin of error is 3% (Table 3). These results indicate that solvent selection is the key factor for optimizing the extraction of the target compounds, as it influences the solubility and release of polyphenols from the plant matrix []. Its high influence (82.97%) suggests that the efficiency of the process largely depends on the concentration and solvent properties of ethanol. The mass-to-volume ratio (12.20%) also plays an important role, as a greater amount of material in a fixed volume may affect the availability of compounds for dissolution []. Extraction time (1.83%) has a negligible effect, suggesting that prolonging the process does not significantly improve compound recovery. The margin of error (3%) reflects experimental variations.
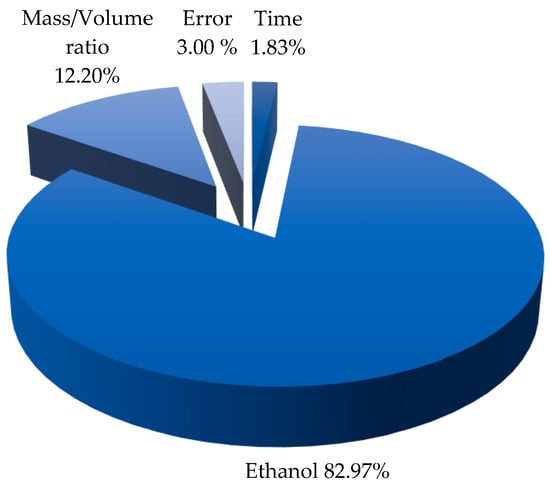
Figure 2.
Relative influence of individual factors.  Time,
Time,  Ethanol,
Ethanol,  Mass/Volume and
Mass/Volume and  Error.
Error.

Table 3.
Analysis of variance (ANOVA).
3.3. Optimal Conditions for the Extraction of Total Polyphenol Content
Figure 3 shows a main effects analysis of the signal-to-noise ratio (S/N), used to evaluate the extraction efficiency of polyphenols. It was observed that extraction time at level 2 (20 min), ethanol concentration at level 2 (40%), and the mass-to-volume ratio at level 1 (1:12) were the combinations that offered the best response in terms of S/N. Under these conditions, the software predicted an S/N value of 77.43 (Table 4). After performing the extraction under the optimal conditions and experimentally validating the process, an S/N value of 88.43 ± 0.66 (8801.89 ± 621.41 mEq of GA/L) was obtained, exceeding the value predicted by the model.
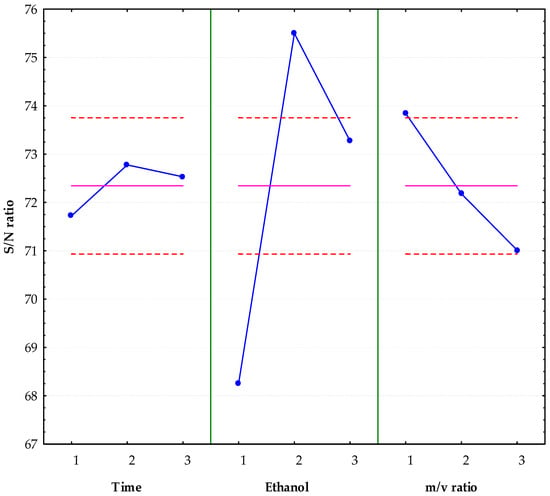
Figure 3.
Graph of main effects for S/N ratio.  Levels,
Levels,  Average S/N value for each factor,
Average S/N value for each factor,  Standard error range (±2 SE). The most relevant effects are those whose changes between levels exceed this margin.
Standard error range (±2 SE). The most relevant effects are those whose changes between levels exceed this margin.

Table 4.
Predicted values and experimental results of total polyphenol content.
This result indicates a high concentration of total polyphenols in the extract and demonstrates the notable reproducibility of the method. Furthermore, the optimal factor levels found are consistent with literature reports, which suggest that ethanol concentration should be between 40 and 60%, the mass-to-solvent ratio between 1:10 and 1:30, and extraction time between 20 and 40 min to maximize punicalagin yield [].
3.4. Flash Chromatography and Semipurification of Ellagitannins
The chromatogram obtained during the fractionation stage using flash chromatography shows the separation of four fractions composed of molecules present in the pomegranate peel extract. Most peaks likely correspond to ellagitannins and other polyphenols, as these are characteristic compounds of this plant matrix (Figure 4) []. There are some reports in the literature where flash chromatography has been used for the separation of polyphenolic compounds from pomegranate peel with EcoFlex C18 and RediSep C-18 columns; however, the use of Amberlite XAD-16 in flash chromatography for the separation of these compounds has not been previously reported in the literature [,]. This resin, employed in adsorption chromatography, favors the retention of polar and hydrophobic compounds such as ellagitannins and other polyphenols, facilitating their separation during the process [].
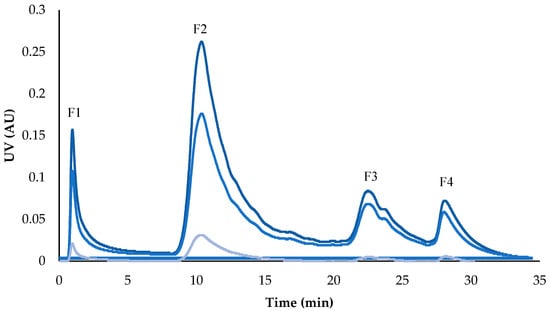
Figure 4.
Flash fractionation of compounds in pomegranate peel.  UV1 λ: 254 nm,
UV1 λ: 254 nm,  UV2 λ: 280 nm,
UV2 λ: 280 nm,  UV3 λ: 365 nm. F1: Fraction 1, F2: Fraction 2, F3: Fraction 3, and F4: Fraction 4.
UV3 λ: 365 nm. F1: Fraction 1, F2: Fraction 2, F3: Fraction 3, and F4: Fraction 4.
3.5. Characterization of the Compounds Present in the Fractions Obtained
As shown in Table 5, the molecules present in the fractions are mainly ellagitannins, which is consistent with reports in the literature stating that ellagitannins are the most abundant phenolic compounds in pomegranate peel []. Furthermore, other authors have reported a similar compound profile after a semipurification process using Amberlite XAD-16, indicating that ellagitannins have a high affinity for this chromatographic resin [].

Table 5.
Compounds present in the fractions obtained by flash chromatography.
Fraction number two (Figure 5) showed the highest punicalagin content with 89.25% and the presence of only ellagitannins (Table 6). From 191 g of dry matter (pomegranate peel), 1.5 g of this fraction was obtained, corresponding to a yield of 0.78%. Previous studies have identified punicalagin α and β as the main compounds after purification processes using UHPLC []. Purifications performed by mass-directed semi-preparative ESI-AP single quadrupole LC-MS have achieved punicalagin recoveries with purities above 95%, while preparative HPLC has reported purities up to 98.05% [,]. Nevertheless, in this study, a considerable concentration of punicalagin was obtained using flash chromatography, a more accessible, faster, and scalable technique that operates at high flow rates and low pressure. This high concentration reflects the strong selectivity of the chromatographic system, enabling the recovery of an enriched fraction without resorting to more expensive purification methods, thus supporting the feasibility of its industrial application [].

Figure 5.
Chromatographic profile of pomegranate peel ellagitannins present in Fraction 2.

Table 6.
Characterization of pomegranate peel ellagitannins present in Fraction 2.
4. Conclusions
The extraction of phenolic compounds from pomegranate peel was successfully carried out, demonstrating the effectiveness of both the experimental design and the extraction method used. The crude extract was efficiently fractionated using flash chromatography, yielding four fractions rich in hydrolyzable tannins. Furthermore, characterization by HPLC/ESI/MS confirmed the presence of ellagitannins in all four fractions, with fraction number two standing out due to its high punicalagin content. These results support the potential of pomegranate peel as a valuable source of ellagitannins, with promising applications in the pharmaceutical and food industries.
Author Contributions
E.M.R.-M.: Conceptualization, Investigation, Writing—Original Draft, Visualization. P.A.-Z.: Conceptualization, Investigation, Review and Editing, Visualization, Resources. L.S.: Writing—Review and Editing. M.R.M.: Writing—Review and Editing, A.I.: Review and Editing, C.N.A.: Review and Editing, Supervision, and J.A.A.-V.: Conceptualization, Investigation, Review and Editing, Visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Tecnológico Nacional de México through the following projects: 20024.24-P Estudio de la degradación de elagitaninos por enzimas glicolíticas and 22037.25-P Estudio de la degradación de elagitaninos con enzimas digestivas asociadas a su degradación.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Secretaria de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI) of Mexico for the scholarship granted as financial support with scholarship number 1319543 and the Department of Food Research of the Autonomous University of Coahuila.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mo, Y.; Ma, J.; Gao, W.; Zhang, L.; Li, J.; Li, J.; Zang, J. Pomegranate Peel as a Source of Bioactive Compounds: A Mini Review on Their Physiological Functions. Front. Nutr. 2022, 9, 887113. [Google Scholar] [CrossRef] [PubMed]
- Maphetu, N.; Unuofin, J.O.; Masuku, N.P.; Olisah, C.; Lebelo, S.L. Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punica granatum L. (pomegranate) plant extracts: A review. Biomed. Pharmacother. 2022, 153, 113256. [Google Scholar] [CrossRef] [PubMed]
- Podetti, C.; Riveros-Gomez, M.; Román, M.C.; Zalazar-García, D.; Fabani, M.P.; Mazza, G.; Rodríguez, R. Polyphenol-Enriched Pectin from Pomegranate Peel: Multi-Objective Optimization of the Eco-Friendly Extraction Process. Molecules 2023, 28, 7656. [Google Scholar] [CrossRef] [PubMed]
- Azmat, F.; Safdar, M.; Ahmad, H.; Khan, M.R.J.; Abid, J.; Naseer, M.S.; Aggarwal, S.; Imran, A.; Khalid, U.; Zahra, S.M.; et al. Phytochemical profile, nutritional composition of pomegranate peel and peel extract as a potential source of nutraceutical: A comprehensive review. Food Sci. Nutr. 2024, 12, 661–674. [Google Scholar] [CrossRef]
- Ruan, J.H.; Li, J.; Adili, G.; Sun, G.Y.; Abuduaini, M.; Abdulla, R.; Maiwulanjiang, M.; Aisa, H.A. Phenolic Compounds and Bioactivities from Pomegranate (Punica granatum L.) Peels. J. Agric. Food Chem. 2022, 70, 3678–3686. [Google Scholar] [CrossRef]
- Wang, W.; Long, P.; He, M.; Luo, T.; Li, Y.; Yang, L.; Zhang, Y.; Wen, X. Pomegranate polyphenol punicalagin as a nutraceutical for mitigating mild cognitive impairment: An overview of beneficial properties. Eur. J. Pharmacol. 2024, 977, 176750. [Google Scholar] [CrossRef]
- Oudane, B.; Boudemagh, D.; Bounekhel, M.; Sobhi, W.; Vidal, M.; Broussy, S. Isolation, characterization, antioxidant activity, and protein-precipitating capacity of the hydrolyzable tannin punicalagin from pomegranate yellow peel (Punica granatum). J. Mol. Struct. 2018, 1156, 390–396. [Google Scholar] [CrossRef]
- Sun, Y.-q.; Tao, X.; Men, X.-m.; Xu, Z.-w.; Wang, T. In vitro and in vivo antioxidant activities of three major polyphenolic compounds in pomegranate peel: Ellagic acid, punicalin, and punicalagin. J. Integr. Agric. 2017, 16, 1808–1818. [Google Scholar] [CrossRef]
- Venusova, E.; Kolesarova, A.; Horky, P.; Slama, P. Physiological and Immune Functions of Punicalagin. Nutrients 2021, 13, 2150. [Google Scholar] [CrossRef]
- Hassan, M.H.U.; Shahbaz, M.; Momal, U.; Naeem, H.; Imran, M.; Abdelgawad, M.A.; Ghoneim, M.M.; Mostafa, E.M.; El-Ghorab, A.H.; Alsagaby, S.A.; et al. Exploring Punicalagin Potential Against Cancers: A Comprehensive Review. Food Sci. Nutr. 2025, 13, e70072. [Google Scholar] [CrossRef]
- Shabir, I.; Dar, A.H.; Dash, K.K.; Manzoor, S.; Srivastava, S.; Pandey, V.K.; Shams, R.; Bashir, I.; Khan, S.A.; SAMukarram Kovács, B. Bioactive potential of punicalagin: A comprehensive review. Appl. Food Res. 2024, 4, 100572. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Cao, Y.; An, X.; Chen, J.; Yang, L. Punicalagin Protects against Diabetic Liver Injury by Upregulating Mitophagy and Antioxidant Enzyme Activities. Nutrients 2022, 14, 2782. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Zhang, Y.; Cao, Y.; Chen, J.; Qin, H.; Yang, L. Punicalagin Protects Diabetic Nephropathy by Inhibiting Pyroptosis Based on TXNIP/NLRP3 Pathway. Nutrients 2020, 12, 1516. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Kim, C.Y.; Hwang, J.; Jo, K.; Kim, S.; Suh, H.J.; Choi, H.S. Punicalagin, a Pomegranate-Derived Ellagitannin, Suppresses Obesity and Obesity-Induced Inflammatory Responses Via the Nrf2/Keap1 Signaling Pathway. Mol. Nutr. Food Res. 2019, 63, 1900574. [Google Scholar] [CrossRef]
- Lampakis, D.; Skenderidis, P.; Leontopoulos, S. Technologies and Extraction Methods of Polyphenolic Compounds Derived from Pomegranate (Punica granatum) Peels. A Mini Review. Processes 2021, 9, 236. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, S.; Liu, M.; Tian, S. Simultaneous process optimization of ultrasound-assisted extraction of polyphenols and ellagic acid from pomegranate (Punica granatum L.) flowers and its biological activities. Ultrason. Sonochem. 2021, 80, 105833. [Google Scholar] [CrossRef]
- Huang, Z.; Foo, S.C.; Choo, W.S. A review on the extraction of polyphenols from pomegranate peel for punicalagin purification: Techniques, applications, and future prospects. Sustain. Food Technol. 2025, 3, 396–413. [Google Scholar] [CrossRef]
- Seeram, N.; Lee, R.; Hardy, M.; Heber, D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep. Purif. Technol. 2005, 41, 49–55. [Google Scholar] [CrossRef]
- Lu, J.; Wei, Y.; Yuan, Q. Preparative separation of punicalagin from pomegranate husk by high-speed counter-current chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 857, 175–179. [Google Scholar] [CrossRef]
- Kasprowiak, A.; Cazier-Dennin, F.; Danjou, P.E. Flash Chromatography System: A Practical Tool for Demonstrating the Influence of Column Characteristics on Chromatographic Resolution. J. Chem. Educ. 2020, 97, 1145–1150. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Amjad, S.; Ashraf, S.; Khawar, L.; Safdar, M.N.; Jabbar, S.; Murtaza, M.A. Extraction of Polyphenols from Apple and Pomegranate Peels Employing Different Extraction Techniques for the Development of Functional Date Bars. Int. J. Fruit Sci. 2020, 20, S1201–S1221. [Google Scholar] [CrossRef]
- Tamborlin, L.; Sumere, B.R.; de Souza, M.C.; Pestana, N.F.; Aguiar, A.C.; Eberlin, M.N.; Simabuco, F.M.; Rostagno, M.A.; Luchessi, A.D. Characterization of pomegranate peel extracts obtained using different solvents and their effects on cell cycle and apoptosis in leukemia cells. Food Sci. Nutr. 2020, 8, 5483–5496. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, A.Y.; Aguilar-Zárate, P.; Muñiz-Márquez, D.B.; Wong-Paz, J.E.; Rojas, R.; Ascacio-Valdés, J.A.; Martínez-Ávila, G.C.G. Effect of ultrasound treatment on the extraction of antioxidants from Ardisia compressa Kunth fruits and identification of phytochemicals by HPLC-ESI-MS. Heliyon 2019, 5, e03058. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.R.; Aguilar-Zárate, M.; Perales-Rosas, D.; Martínez-Ávila, G.C.G.; Gómez-García, R.; Tafolla-Arellano, J.C.; Rojas, R.; Aguilar-Zárate, P. Enhancing the insecticidal efficacy of Allium sativum extracts through microencapsulation via complex coacervation. Plant Sci. Today 2024, 11, 625–633. [Google Scholar] [CrossRef]
- Michel, M.R.; Pacheco-Lara, M.; Rojas, R.; Martínez-Ávila, G.C.G.; Ascacio-Valdés, J.A.; Aguilar-Zárate, M.; Aguilar-Zárate, P. Antioxidant Activity of Pomegranate Husk Ellagitannins in Enhancing Oxidative Stability of Canola Oil During Frying. Foods 2025, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Nossa González, D.L.; Talero Pérez, Y.V.; Rozo Núñez, W.E. Determinación del contenido de polifenoles y actividad antioxidante de los extractos polares de consuelda (Symphytum officinale L.). Rev. Cuba. Plantas Med. 2016, 21, 125–132. [Google Scholar]
- Vázquez-Nuñez, M.d.l.Á.; Rocha-Guzmán, N.E.; Aguilar-Zárate, P.; Rojas, R.; Martínez-Ávila, G.C.G.; Reyes, A.; Michel, M.R. Biopolymer-Based Microencapsulation of Procyanidins from Litchi Peel and Coffee Pulp: Characterization, Bioactivity Preservation, and Stability During Simulated Gastrointestinal Digestion. Polymers 2025, 17, 687. [Google Scholar] [CrossRef]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda, L.; Estrada-Gil, L.E.; Torres-León, C.; Chávez-González, M.L.; Aguilar, C.N.; Ascacio-Valdés, J.A. Enhancing the Release of Ellagic Acid from Mexican Rambutan Peel Using Solid-State Fermentation. Biomass 2024, 4, 1005–1016. [Google Scholar] [CrossRef]
- Guaita, M.; Motta, S.; Messina, S.; Casini, F.; Bosso, A. Polyphenolic Profile and Antioxidant Activity of Green Extracts from Grape Pomace Skins and Seeds of Italian Cultivars. Foods 2023, 12, 3880. [Google Scholar] [CrossRef]
- Ascacio-Valdés, A.; Barrera-Martínez, C.L.; Ascacio-Valdés, J.A.; Sepúlveda, L. Bioproduction of Nordihydroguaiaretic and Ellagic Acid from Creosote Bush Leaves (Larrea tridentata) Using Solid-State Fermentation with Aspergillus niger GH1. Fermentation 2025, 11, 229. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Carpena, M.; Pereira, A.G.; Chamorro, F.; Soria-Lopez, A.; Perez, P.G.; Otero, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; et al. Critical Variables Influencing the Ultrasound-Assisted Extraction of Bioactive Compounds—A Review. Chem. Proc. 2021, 5, 50. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, K.W.; Wu, D.T.; Liu, H.Y.; Li, H.B.; Zhang, J.R.; Gan, R.Y. Pomegranate peel-derived punicalagin: Ultrasonic-assisted extraction, puri-fication, and its α-glucosidase inhibitory mechanism. Food Chem. 2022, 374, 131635. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, F.; Núñez-Gómez, D.; Legua, P.; Del Bubba, M.; Giordani, E.; Melgarejo, P. Qualitative and varietal charac-terization of pomegranate peel: High-value co-product or waste of production? Sci. Hortic. 2022, 291, 110601. [Google Scholar] [CrossRef]
- Glazer, I.; Masaphy, S.; Marciano, P.; Bar-Ilan, I.; Holland, D.; Kerem, Z.; Amir, R. Partial identification of antifungal compounds fro5m Punica granatum peel extracts. J. Agric. Food Chem. 2012, 60, 4841–4848. [Google Scholar] [CrossRef]
- Invernizzi, L.; Moyo, P.; Cassel, J.; Isaacs, F.J.; Salvino, J.M.; Montaner, L.J.; Tietjen, I.; Maharaj, V. Use of hyphenated analytical techniques to identify the bioactive constituents of Gunnera perpensa L., a South African medicinal plant, which potently inhibit SARS-CoV-2 spike glycoprotein–host ACE2 binding. Anal. Bioanal. Chem. 2022, 414, 3971–3985. [Google Scholar] [CrossRef]
- Seif Zadeh, N.; Zeppa, G. Recovery and Concentration of Polyphenols from Roasted Hazelnut Skin Extract Using Macroporous Resins. Foods 2022, 11, 1969. [Google Scholar] [CrossRef]
- Middha, S.K.; Usha, T.; Pande, V. HPLC Evaluation of Phenolic Profile, Nutritive Content, and Antioxidant Capacity of Extracts Obtained from Punica granatum Fruit Peel. Adv. Pharmacol. Sci. 2013, 2013, 296236. [Google Scholar] [CrossRef]
- Setlhodi, R.; Mashile, B.; Izu, G.O.; Gbashi, S.; Mashele, S.S.; Bonnet, S.L.; Makhafola, T.J.; Chukwuma, C.I. Effect of solvent extraction on the antioxidant and phytochemical profiles of ellagitannins from “wonderful” pomegranate peel: An advanced chemometrics analysis. Eur. Food Res. Technol. 2023, 249, 1807–1820. [Google Scholar] [CrossRef]
- Gosset-Erard, C.; Zhao, M.; Lordel-Madeleine, S.; Ennahar, S. Identification of punicalagin as the bioactive compound behind the antimicrobial activity of pomegranate (Punica granatum L.) peels. Food Chem. 2021, 352, 129396. [Google Scholar] [CrossRef]
- Barbieri, M.; Heard, C.M. Isolation of punicalagin from Punica granatum rind extract using mass-directed semi-preparative ESI-AP single quadrupole LC-MS. J. Pharm. Biomed. Anal. 2019, 166, 90–94. [Google Scholar] [CrossRef]
- Lu, J.; Ding, K.; Yuan, Q. One-step purification of punicalagin by preparative HPLC and stability study on punicalagin. Sep. Sci. Technol. 2011, 46, 147–154. [Google Scholar] [CrossRef]
- Li, J.L.; Yu, J.H.; Li, W.Z.; Deng, D.J.; Xin, Y.; Reaney, M.J.T.; Cai, Z.Z.; Wang, Y. Optimized two-step flash chromatography method for large-scale isolation of linusorb and its antioxidant capacity evaluation. Food Res. Int. 2025, 207, 116082. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).