Abstract
Triterpenoid saponins from the Astragalus species possess valuable effects (cytotoxic, adjuvant, hepatoprotective, neuroprotective, antiviral, etc.). Some also have immunomodulatory activities. Astragalus glycyphyllos is distributed in Bulgaria and mainly accumulates cycloartane saponins. From the overground parts of the species, a triterpenoid cyloartane-type saponin (AGOS3) was isolated by different chromatographic techniques. A quantitative LC-MS method for the determination of the saponin was developed and validated. Further, the saponin was loaded in copolymeric micelles based on triblock copolymers of polyethylene oxide and polypropylene oxide (Pluronics). The LC-MS method was applied on the developed micelles to determine their loading degrees. Afterwards, the possible pharmacological effects of free and encapsulated in polymeric nanoparticles of triterpenoid saponin (1, 5, 10, 25, 50, and 100 µg/mL) were evaluated in isolated murine macrophages and lymphocytes in vitro. Free AGOS3 stimulated proliferation only at the highest tested concentrations (50–100 µg/mL), and the effect was more evident in isolated macrophages. Interestingly, AGOS3-loaded polymeric micelles caused concentration dependency and statistically significant increases in the proliferation of both isolated lymphocytes and macrophages, even at a lower concentration (10 µg/mL). These results could serve as the basis for further research on the immunomodulatory effect of this saponin.
1. Introduction
The Astragalus genus is the largest in the Fabaceae family, with more than 3500 species distributed worldwide [1]. Some of the species are reported to possess various pharmacological activities, such as immunostimulant, antiviral, hepatoprotective and neuroprotective activities [1,2]. The chemical composition of the plants is complex, with flavonoids, saponins, and polysaccharides being the main metabolites that accumulate [3]. Recent progress in phytochemical analysis on the representatives of the genus led to the isolation and structural identification of novel triterpenoid saponins [3,4,5,6]. Other representatives of the same group of biologically active compounds have been shown to exhibit significant immunoadjuvant and immune-stimulating properties [7,8]. Interestingly, a purified saponin mixture (PSM) from the aerial parts of A. corniculatus was tested in vivo in hamsters with myeloid Graffi tumour. The results showed that intraperitoneal treatment with PSM significantly increased survival rates and mean survival time, while tumour growth was reduced [9].
Astragalus glycyphyllos is a perennial herb found in Bulgaria that has been used in the folk medicine of the country for centuries as an anti-inflammatory, antihypertensive, and diuretic agent [9]. In the last decade, this species has been the subject of extensive phytochemical and pharmacological research. An extract from the overground parts of the plant was tested in vitro (100 mg/kg) on a model of CCl4-induced liver damage in rats. The levels of malonedialdehyde (MDA), reduced glutathione (GSH), glutathione reductase (GR), glutathione peroxidase (GPx), and glutathione-S-transferase (GST) were measured, and the extract showed similar hepatoprotective effect compared to the control group (same dose of silymarin) [1]. In another study, polysaccharides, obtained from a butanol extract of the aerial parts of the species, were tested (at concentrations of 0.6, 6, and 60 μg/mL) on isolated rat microsomes in the conditions of non-enzyme-induced and enzyme-induced lipid peroxidation. The antioxidant effect was concentration-dependent and, once again, comparable to that of silymarin [1].
A number of studies indicate the promising cytotoxic activity of saponins from A. glycyphyllos. Fractions, rich in saponins, obtained from shoot cultures of the species, were tested on numerous malignant cell lines (T-24, CAL-29, MJ, and HUT-78), and the results were evaluated using a MTT test. The fractions exhibited high efficacy against urinary bladder cancer cells with a constitutive high expression level of the xenobiotic pump gp170 (MDR1) [1]. In a more recent study, the total saponin mixture, a purified saponin fraction, and a cycloartane saponin (17(R),20(R)-3β,6α,16β-trihydroxycycloartanyl-23-carboxylic acid 16-lactone 3-O-β-D-glucopyranoside) from the aerial parts of the plant were tested in different concentrations (12.5–200 µg/mL) against the same cell lines. The purified saponin fraction displayed the strongest cytotoxicity. In the same study, the saponin showed statistically significant immunomodulatory activity in vitro on a murine model of isolated mice lymphocytes and peritoneal macrophages (at concentrations of 1, 10, 20, 60, 100, and 200 µmol/L) [9]. The saponin 17(R),20(R)-3β,6α,16β-trihydroxycycloartanyl-23-carboxylic acid 16-lactone 3-O-β-D-glucopyranoside exhibited considerable neuroprotective activity in different models of cyanotoxin (anatoxin-α)-induced neurotoxicity on subcellular fractions (rat brain microsomes, mitochondria, and synaptosomes) at concentrations of 1, 10, and 100 μM [1]. The compound, together with the rare flavonoid camelliaside A, isolated from this species, displayed significant neuroprotective activity in vitro on a 6-hydroxydopamine-induced neurotoxicity model on isolated rat brain synaptosomes. Also, on human recombinant monoamine oxidase type B enzyme (hMAO-B), the saponin displayed strong inhibiting activity compared to selegiline [9]. Another novel cycloartane saponin (3-O-[α-L-rhamnopyranosyl-(1→2)-β-D-xylopyranosyl]-24-O-α-L-arabinopyranosyl-3β,6α,16β,24(R),25-pentahydroxy-20R-cycloartane), together with the rare saponin astrachrysoside A, both from A. glycyphyllos, were examined for their possible antioxidant and neuroprotective effects on different in vitro sub-cellular models. Both compounds exhibited statistically significant protective activity, while, on the activity of hMAO-B enzyme, they both showed a weak inhibitory effect [5]. In addition to these effects, a double fluorochrome staining study showed that a purified saponin mixture (PSM) from A. glycyphyllos significantly influenced the growth and proliferation of myeloid Graffi tumour cells in vitro. The PSM administered orally on Graffi-tumour-bearing hamsters significantly decreased transplantability and tumour growth, increased survival, and decreased mortality rates amongst animals. The observed pathomorphological changes in tumours were indicative of positive antitumor effect [9].
Recent studies explored the potential antiviral activity of the plant. A defatted extract (DEAG) from it was standardised to saponins and tested in vitro alone and in combination with acyclovir against human herpes simplex virus alpha types 1 (acyclovir sensitive) and 2 (acyclovir resistant). When used in a concentration of 0.6 mg/mL, the extract reached between 60% and 70% protection against both viral strains. DEAG was used to prepare tablets which fulfilled the requirements of the pharmacopoeia for disintegration and friability [1]. The potential antiviral activity of the species was further proven when three saponins (17(R),20(R)-3β,6α,16β-trihydroxycycloartanyl-23-carboxylic acid 16-lactone 3-O-β-D-glucopyranoside; 3-O-[α-L-rhamnopyranosyl-(1→2)-β-D-xylopyranosyl]-24-O-α-L-arabinopyranosyl-3β,6α,16β,24,25-pentahydroxy-20R,24R-cycloartane; and astrachrysoside A), a purified saponin mixture, and the DEAG were tested on the replication of Human Coronavirus 229E with the saponin mixture and DEAG showing 100% inhibition [1].
Even though a great number of pharmacological activities have been reported for saponins, the degree of their effect is crucially linked to their cellular uptake. Generally, triterpenoid saponins are characterised with substantial molecular mass (700–2500 Da) which affect negatively their cell permeability [10]. Given their high polarity, due to the sugar residues [11], there is yet another problem for cell membrane permeation. The application of nanotechnology is a known and extensively developed approach for bioavailability amelioration [12]. A recent study indicates that the formation of micelle-like complexes between the triblock copolymer Pluronic F127 and a saponin from Glycyrrhiza glabra roots could be a valuable approach for potential nanotechnological agents [13].
Given this background, as a continuation of our research on A. glycyphyllos, the aim was to develop and validate a quantitative LC-MS method for the determination of a recently isolated cycloartane saponin. In addition, the saponin was loaded in copolymeric micelles based on triblock copolymers of polyethylene oxide and polypropylene oxide (Pluronics), and they were further tested for possible pharmacological effects (at concentrations of 1, 5, 10, 25, 50, and 100 µg/mL) on isolated murine macrophages and lymphocytes in vitro.
2. Materials and Methods
2.1. Plant Material and Saponin Isolation
A. glycyphyllos herbs were collected from Vitosha Mountain, Bulgaria, in June 2020. The species was identified by Dr. D. Pavlova from the Faculty of Biology, Sofia University, where a voucher specimen was deposited (SO-107613). The air-dried plant material (200 g) was powdered (3 mm) and then defatted with dichloromethane. The defatted plant substance was exhaustively extracted with 80% MeOH (72 L) via percolation. The obtained methanol extract was concentrated and then lyophilized to produce a dry extract (42 g). The extract was separated over a Diaion HP-20 (4.7 × 45 cm) column (H2O/MeOH gradient). Seven main fractions were collected (A-G), and after a TLC analysis, fractions F and G were found to be rich in saponins. Fraction F was chromatographed (CH2Cl2/MeOH/H2O step gradient) on a silica gel cartridge by flash chromatography on Reveleris® X2 (Buchi, Flawil, Switzerland), and 21 subfractions were obtained. Subfraction 13, which contained a main compound (TLC), was further separated (CH2Cl2/MeOH/H2O step gradient) on a silica gel cartridge (flash chromatography), yielding compound AGOS3 (10 mg).
2.2. Formulation of Saponin-Loaded Copolymeric Micelles
Pluronic micelles loaded with the saponin were prepared via two methods, in particular, film hydration and solvent evaporation. For the film hydration method, the saponin (1 mg) and 10 mg of the copolymers (Pluronic P123: Pluronic F127 in a ratio 1:1 wt/wt) were dissolved in 2 mL of methanol. The solution was stirred at room temperature (700 rpm) until the complete evaporation of the solvent. The resulting film was dispersed in 4 mL of purified water and filtered through a membrane filter (0.2 µm). The second method included the dissolution of both copolymers in purified water and the dropwise addition of methanol solution of the saponin (1 mg/mL). The mixture was stirred for the evaporation of methanol (700 rpm) and filtered after that as described above.
The amount of the non-loaded saponin in both procedures was determined into the filter fractions by the newly developed UHPLC-MS method. The amount of saponin loading into the micelles (SL) was calculated following this Equation [14]:
SL = (Initial amount of saponin − Non-loaded saponin)/Volume of the loaded micellar dispersion
The average diameter and polydispersity index of the micelles were determined by dynamic light scattering (DLS) using Zetasizer NanoBrook 90Plus PALS (Brookhaven Instruments Corporation, Holtsville, NY, USA), equipped with a 35 mW red diode laser (λ = 640 nm) at a scattering angle of 90°.
2.3. Pharmacological Evaluation
For the study, male healthy mice (18–20 g, aged 6–8 weeks, obtained from the National Breeding Centre in Sofia, Bulgaria) were kept in controlled conditions with a temperature range of 20 °C ± 5 °C. They were provided with a standard pellet diet and had unrestricted access to water. All experimental protocols were sanctioned by the Institutional Animal Care and Use Committee at the Medical University of Sofia, Bulgaria (protocol code 621, date 3 May 2024). All procedures on animals were further approved by The Bulgarian Food Safety Agency at the Ministry of Agriculture and Foods with Permit number 342, which is valid until 2028. The animals were humanely euthanized via cervical dislocation, followed by the removal of the abdominal skin under sterile conditions. Peritoneal macrophages were obtained through peritoneal lavage using 10 mL of sterile RPMI solution. Afterward, cell suspension underwent two washes in RPMI-1640 medium and was quantified using a haematocytometer. Subsequently, cells were seeded in 96-well microplates at a concentration of 3 × 104 cells per well using RPMI supplemented with 10% FBS. Spleens were excised and placed in tissue culture dishes containing approximately 5 mL of RPMI cell culture medium. They were finely minced with scissors, filtered to obtain tissue fragments, and the resulting cell suspension was subjected to a histopaque density gradient centrifugation at 2000 rpm for 20 min at 4 °C. The lymphocyte layer at the interface was collected using a Pasteur pipette, washed twice with the RPMI-1640 medium, and centrifuged at 2000 rpm for 10 min at 4 °C. Finally, the cells were resuspended in RPMI-1640 medium at a concentration of 4 × 105 cells/mL.
The cell vitality assessment was performed using MTT-dye reduction assay. This quantitative measurement provides a linear relationship between cell metabolic activity and absorbance, allowing researchers to assess cell proliferation and growth rates. The proliferation of isolated murine lymphocytes and macrophages refers to the process by which these cells divide and multiply. The MTT assay provides a reliable measure of cell proliferation by quantifying the metabolic activity of living cells. Since cell proliferation generally correlates with higher metabolic activity, the amount of formazan produced in the assay reflects the growth rate and number of cells [15]. Suspensions of peritoneal macrophages and spleen lymphocytes were individually seeded in 96-well microplates at concentrations of 3 × 104 cells/well for macrophages and 4 × 104 cells/well for lymphocytes. Subsequently, varying concentrations (1, 5, 10, 25, 50, and 100 µg/mL) of pure AGOS3, the saponin loaded in corresponding concentrations in micelles (micelles/AGOS3) and 2,10, 20 50, 100, and 200 µg/mL of empty micelles were introduced to the cells, followed by an incubation period for 72 h at 37 °C, 5% CO2, and 95% humidity. After that, aliquots of the MTT solution (5 mg/mL in PBS) were added to each well. Following an additional 3 h incubation, the formazan crystals formed intracellularly were dissolved in DMSO. Absorbance readings were taken using a plate reader (Synergy 2, BioTek Instruments, Inc. Highland Park, Winooski, VT, USA) at 570 nm, with background absorbance readings recorded at 690 nm. The intensity of the colour, measured by absorbance using a spectrophotometer, is directly proportional to the number of viable, metabolically active cells [16].
The results are expressed as the mean ± SD (standard deviation). Statistical significance was assessed by one-way ANOVA (analysis of variance) followed by Dunnett’s post-test using GraphPad Prism software (version 6, GraphPad Software, La Jolla, CA, USA). Significance levels were denoted as * p < 0.05, ** p < 0.01, and *** p < 0.001, indicating values considered statistically significant.
2.4. LC-MS Analysis
Each sample from micelles (for the study of loading capacity and encapsulation effectiveness) was precipitated with MeCN-MeOH (1:1, v/v) by sonication at 37 kHz for 30 min, and then centrifuged at 15,200× g, and the supernatant was filtered through a PVDF syringe filter (0.22 µm). It was diluted 1:10 with MeOH and then sealed in an auto sampler vial. The reference 3-O-[α-L-rhamnopyranosyl-(1→2)-β-D-xylopyranosyl]-24-O-α-L-arabinopyranosyl-3β,6α,16β,24(R),25-pentahydroxy-20R-cycloartane (AGOS3) was used. The saponin was isolated (99.8% purity) and structurally elucidated by MS analyses as reported before [5]. A stock solution of the compound was prepared in MeOH (1 mg/mL), and after subsequent serial dilutions with the same solvent, work solutions (10, 50, 102, 612, and 1020 ng/mL) were obtained. An aliquot of 1 µL of each sample or reference was injected to the ultra-high-performance liquid chromatography (UHPLC) system. A QExactive Plus Orbitrap mass spectrometer with a heated electrospray ionisation (HESI) ion source (ThermoFisher Scientific, Bremen, Germany) coupled with a UHPLC system (Dionex UltiMate 3000 RSLC, ThermoFisher Scientific, Bremen, Germany) was used. The full scan MS was set at resolution 70,000 (at m/z 200), AGC target 3e6, max IT 100 ms, scan range 250 to 1700 m/z. The MS2 conditions were as follows: resolution 17,500 (at m/z 200), AGC target 1e5, max IT 50 ms, mass range m/z 250 to 1750, isolation window 2.0 m/z, and (N)CE 20. The ionisation device (HESI source) was operating at the following: −2.5 kV spray voltage and 320 °C capillary and probe temperature, 38 arbitrary units (a.u., as set by the Extactive Tune software, v. 2.12.) of sheath gas and 12 a.u. of auxiliary gas (both N2); S-Lens RF level 50.0. UHPLC separations were performed on a Kromasil C18 column (1.9 μm, 2.1 × 50 mm, Akzo Nobel, Malmö, Sweden) at 40 °C. The mobile phase was (A) H2O + 0.1% HCOOH and (B) MeCN + 0.1% HCOOH; the flow rate was 0.3 mL/min. The elution programme was as follows: 10% B for 1.5 min, increase to 30% B for 1 min, isocratic with 30% B for 0.5 min, increase to 95% B for 9 min, isocratic with 95% B for 2 min, return to 10% B for 0.1 min. The software Xcalibur v. 4.2 (Thermo Scientific, Bremen, Germany) was used for data collection, calibration cure construction, statistical analysis, and data processing.
All the used materials and reagents were of analytical or of UHPLC grade quality and provided by Fisher Scientific (Loughborough, UK).
3. Results and Discussion
Three parts of the study were completed—the isolation of the saponin (AGOS3), its incorporation into copolymeric micelles, and effect evaluation in two types of isolated immune system cells—peritoneal macrophages and lymphocytes. The first task was to isolate the saponin AGOS3 and to develop and validate a LC-MS method for its quantitation. The compound was isolated from the overground parts of A. glycyphyllos for the second time in a relatively sufficient quantity (10 mg). The compound’s structure was confirmed by UHPLC-MS analysis and comparison to a previously isolated reference [5] (Figure 1).
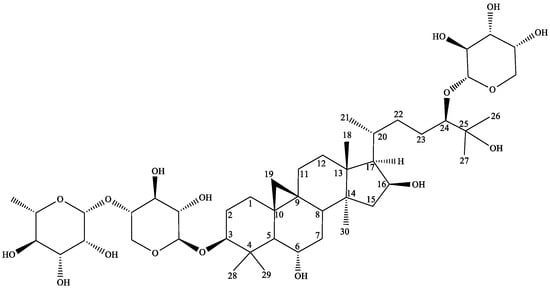
Figure 1.
Structure of AGOS3.
The repeatability of the chromatographic separation proves that the saponin was isolated in its native form, and the obtained compound was neither hydrolysed nor chemically modified in another way (methylation and oxidation) during preparative chromatography [17]. These problems occur relatively frequently when it comes to bis- or trisdesmosides, or tri-, tertra- or pentasaccharides [18,19]. The sufficient quantity of the isolated saponin allowed us to proceed with further experiments. The chromatographic purity of the compound was 99.8% (UHPLC-MS), which was used both as a reference for the quantitation, to prepare micelles, and to perform pharmacological evaluation.
A novel UHPLC-MS method for the quantitation of AGOS3 was developed and validated, according to the approved ICH Q2 (R1) [20] guideline, in order to determine the amount of saponin in the micelles. Under the conditions used (see Section 2), AGOS3 had a retention time of 5.82 ± 0.01 min (Figure 2a). The MS spectrum confirmed that the deprotonated saponin [M − H]− (m/z 901.5162) forms a formate adduct during electrospray (−) ionisation [M + HCOO]− (m/z 947.5218) (Figure 2b). This flinging was in accordance to the literature [5]. Although rather expensive, MS detection is considered the “golden standard” when it comes to plant-derived secondary metabolites, especially those lacking a chromophore (as in the case of triterpenoid saponins, the chromophore is weak) [21].
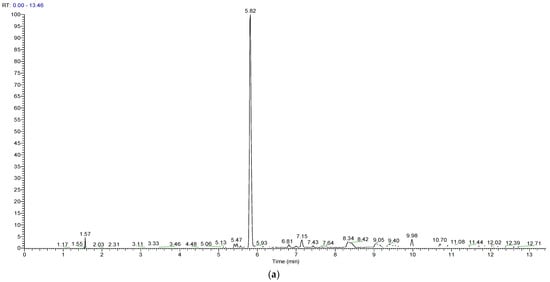
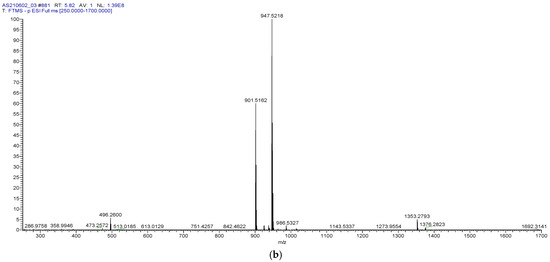
Figure 2.
Base peak chromatogram (a) and HRESIMS spectrum (b) of AGOS3.
During the method validation procedure, we examined the suitability of the system, the precision, the linearity, the accuracy, and the selectivity. The system was found suitable. The UHPLC used provided a minimal dwell volume, ensuring rapid gradient change and minimising the effect of the tubing as in HPLC systems [22]. No peaks interfering at tR (AGOS3) with AUC above 0.04% were detected in the blank baseline. A 0.11%, the AGOS3 solution in MeOH showed a signal-to-noise (S/N) ratio equivalent to or more than ten. The agreement of this solution was ≤1.6%. The factor of resolution (peaks neighbouring) was determined as ≥1.7 (Table 1).

Table 1.
System suitability.
Two injections had a difference of ≤1.4%. Specificity was examined using a blank (MeOH). Peaks in with AUC >0.1% interfering at tR (AGOS3) were not found. No evidence of co-elution was found after peak purity analysis. A SIM mode was used to detect the compound (m/z 947.52), which makes the chance of the misidentification of the saponin statistically impossible [21]. Limits of detection and quantitation were calculated using the S/N from the response’s slope and standard deviation (SD). The limit of detection (LOD) is 0.001 ng/mL, and the limit of quantification (LOQ) is 0.01 ng/mL. These parameters allowed the quantitation of the biologically active compound in nanosized micelles, which are often a challenging object [23]. The linearity of the method was examined from 22% to 175% of the known concentration of the compound, using model solutions, prepared in MeOH. The calibration data were processed by linear regression. A correlation coefficient (r2) of 0.9990 was calculated after the linear least-square analysis (Table 2).

Table 2.
Regression analysis of calibration curves (linearity parameters), n = 3.
This is indicative of the correlation [24] between the concentration and the peaks’ AUC in the interval studied (Figure 3).
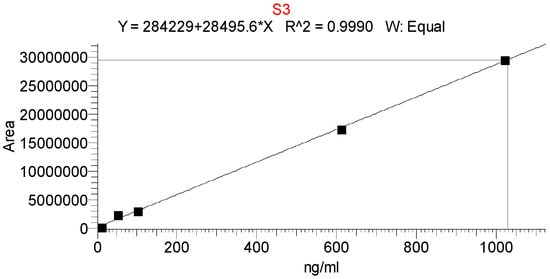
Figure 3.
Calibration curve of AGOS3 with equation and correlation coefficient.
There was no significant bias, and quantitation can be performed, as deduced from the y-intercept (≤0.5%) of the 100% level. Accuracy was in accepted levels [20] (Table 3).

Table 3.
Accuracy of the method.
The method was examined for precision. It was found that the coincidence between the singular data intraday and inter-day (Table 4) is in the accepted levels [20,24].

Table 4.
Precision.
The robustness of the method was investigated when the column thermostat temperature was lowered by increments of five °C. It was found that the separation was better at the accepted temperature—40 °C, but the lowering had no statistically significant effect (Table 5).

Table 5.
Robustness of the method.
The chemical stability of the standard solution of AGOS3 was investigated for one-month storage at +4 °C and at room temperature (25 °C). After this period, the solutions were analysed multiple times to establish the mean concentration of the saponin (Table 6).

Table 6.
Standard solution stability.
According to ICH guidelines [20], the developed method for AGOS3 was found linear, accurate, and precise.
Copolymeric micelles, loaded with AGOS3, were prepared by two different techniques, and technological parameters were measured. It is suggested that the poor permeability, due to the high polarity and gut microflora-induced hydrolysis of saponins, could limit their bioavailability [25]. In this view, the encapsulation of the saponin in polymeric micelles could overcome these limitations. AGOS3 was inserted in micelles based on two triblock copolymers of polyethylene oxide and polypropylene oxide, particularly Pluronic P123 and Pluronic F127 (1:1 wt/wt). The micelles were prepared applying film hydration or solvent evaporation method. The developed UHPLC-MS method was used to quantify the loading degree of the saponin into the micelles formulated by both methods. The results showed that the incorporation of the saponin into micelles was more efficient when the film hydration method was performed (Figure 4a). In this case, the encapsulation efficiency reached 82% vs. 65% by the solvent evaporation method. The latter was probably because the saponin and Pluronics interacted more efficiently in pure organic phase (methanol) compared to the interaction in the mixed phase (methanol and water). Our previous studies with Pluronic micelles showed that the resulting micelles are spherical in shape [14]. In the present study, we measured the size and polydispersity of the micelles by DLS. The mean diameter of the micelles prepared via both methods was less than 50 nm (Figure 4b). As seen, there was no difference between the micelles prepared by both methods. The values for polydispersity index of the micelles prepared by both methods were similar (0.278 and 0.283), and they suggested a narrow size distribution. Thus, considering the higher encapsulation efficiency achieved with the film hydration method, further studies were performed with these micelles.
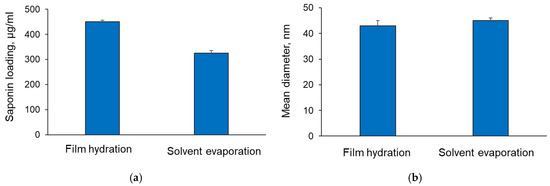
Figure 4.
Saponin loading into the micelles (SL) (a) and mean diameter (b) achieved via film hydration and solvent evaporation method.
The MTT assay is a widely used colorimetric assay that assesses cell metabolic activity and is, by extension, a reliable indicator of cell proliferation and viability. It is particularly valued for its quantitative and sensitive detection of cell proliferation, making it a fundamental tool in various areas of biological and medical research. The assay determines metabolically active cells. Mitochondrial enzymes catalyse this process, primarily succinate dehydrogenase, which are active only in living cells [15]. Cells with higher metabolic activity convert more MTT to formazan, resulting in a deeper purple colour. As cells proliferate and their numbers increase, there is a corresponding increase in MTT’s conversion to formazan. Higher absorbance values indicate greater cell activity, which is interpreted as increased cell proliferation [26].The pure saponin (AGOS3) at different concentrations (1, 5, 10, 25, 50, and 100 µg/mL) and AGOS3 loaded into micelles (in the corresponding concentrations), alongside empty micelles, were tested for its proliferation modulatory activity in isolated mouse lymphocytes and macrophages. First, the effects of empty micelles on isolated lymphocytes and macrophages were evaluated. The results indicated that, following a 72 h incubation period, empty nanosized micelles at concentrations from 2 to 200 µg/mL did not induce the proliferation of mouse lymphocytes and macrophages (Figure 5A and Figure 6A). When administered alone, the saponin exhibited a mild stimulatory effect on lymphocyte proliferation, reaching statistical significance only at the highest concentration of 100 µg/mL (Figure 5B). Conversely, it stimulated macrophage proliferation at concentrations exceeding 25 µg/mL (by 17% at concentration 25 µg/mL, by 29% at concentration 50 µg/mL, and by 34% at concentration 100 µg/mL) (Figure 6B). The encapsulated AGOS3 demonstrated a capacity to stimulate lymphocyte proliferation at lower concentrations (10 and 25 µg/mL) compared to its free form (Figure 6C).
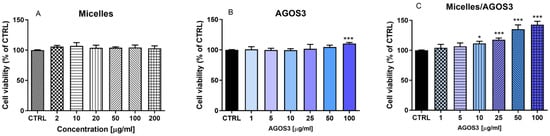
Figure 5.
Effects of empty micelles, pure AGOS3, and AGOS3 loaded in micelles (micelles/AGOS3), on the proliferation of isolated mouse lymphocytes. The results are expressed as means ± SD of triplicate assays (n = 3). All groups were compared statistically vs. untreated controls by one-way Anova with Dunnet’s post-test. * p < 0.05, *** p < 0.001 vs. control.
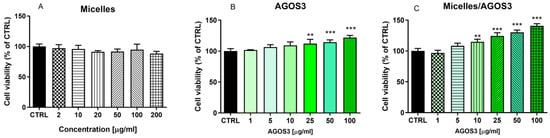
Figure 6.
Effects of empty micelles, pure AGOS3, and AGOS3 loaded in micelles (micelles/AGOS3), on the proliferation of isolated mouse macrophages. The results are expressed as means ± SD of triplicate assays (n = 3). All groups were compared statistically vs. untreated controls by one-way Anova with Dunnet’s post-test; ** p < 0.01, *** p < 0.001 vs. control.
It should be noted that the proliferation-stimulating effects of AGOS3 loaded in micelles were more pronounced than those of the pure saponin. This effect was even more pronounced in the isolated murine macrophages. For instance, at the highest concentration of 200 µg/mL, AGOS3 increased proliferation by 22%, whereas micelles/AGOS3 increased it by 43%.
Isolated lymphocytes and macrophages represent a suitable in vitro model for detecting the direct effects of the test substances on the proliferation of immune system cells [27]. There are many existing data on triterpenoid saponins acting as immune modulators. In a recent study, triterpenoid saponins from the roots of Camellia sinensis showed in vitro immunomodulatory activity against Th1 and Th17cells at a concentration of 10 μM [28]. In another study, the effect of a total extract of Ginseng Fibrous root (GRS), containing dammarane-type saponins, was tested on peripheral blood haematology parameters and on serum cytokine levels in mice. GRS showed improvement in white and red blood cells and neutrophil counts, as well as elevations in the serum levels of IL-2, IFN-γ, TNF-α, and IL-1ß [29].
The Astragalus species are reported to possess immunomodulatory activities that are attributed both to the polysaccharides and to the saponins contained therein. Studies indicate that A. mongholicus roots’ polysaccharide can promote the activities of macrophages, natural killer cells, dendritic cells, T lymphocytes, B lymphocytes, and microglia and induce the expression of a variety of cytokines and chemokines [30]. It has been reported that A. mongholicus extracts, containing saponins, increase resistance to the immunosuppressive effects of chemotherapy drugs while stimulating macrophages to produce interleukin-6 and tumour necrosis factor (TNF) [31]. Different studies showed that a purified saponin fraction had a protective effect against the invasiveness of bone marrow carcinoma [32]. Additionally, when the hamsters were administered with saponins mixture, an increased mitogenic response to phytohemagglutinin and lipopolysaccharides was observed, i.e., saponins have an immunostimulating effect [9]. Recently, there is growing evidence that triterpenoid saponins from the species can also act as potent immunomodulatory agents [33]. The rare cycloartane-type tetracyclic triterpenoid saponins are specific phytochemicals for the genus, which have a proved immunomodulatory effect [34]. The effects of Astragaloside IV (the main cycloartane saponin in A. mongholicus roots) on immune regulation were evaluated in RAW264.7 cells. The saponin showed improvements in the immune functions of these cells by modulating the mRNA and/or protein expression of pro-inflammatory and anti-inflammatory cytokines, NO, surface-stimulating factor, and cell cycle [35]. The structural similarity between Astragaloside IV and AGOS3 provided the basis for this immunomodulatory investigation. In previous research on A. glycyphyllos, the immunomodulation properties of another cycloartane-type triterpenoid saponin in vitro (at concentrations, 10, 20, 60, 100, and 200 µmol/L) on murine spleen lymphocytes and peritoneal macrophages were evaluated. The compound showed notable dose-dependent stimulation on both cell types at a concentration higher than 10 µmol/L [9].
The results of the present study are consistent with our previous reports on in vitro immunostimulatory activity of different sources of saponins, as well as with existing literature data [36]. The functionality of the body’s immune response can be influenced by immune cells, specifically macrophages and lymphocytes, along with their respective receptors [37]. These cells represent a suitable in vitro model for detecting the direct effects of the test substances on the proliferation of immune system cells [27]. Typically, these cells remain in a resting state under normal conditions. However, when exposed to various stimuli, spleen lymphocytes and peritoneal macrophages increase in size, enabling them to engulf foreign molecules and release cytokines [38]. One approach to improve and preserve the biological activity of various biologically active substances is to include them in nanosized drug delivery systems. Varieties of polymeric micelles are currently being studied in animal models and clinical trials for discovering their potential. Targeting specific tissues and prolonged circulation in the body compartments are one of the advantages of these novel forms as cancer treatment alternatives. Tumour growth is connected to the body’s immune system response and capabilities, so modifying these through immune modulation is one of the key treatment options currently [39]. In our study, the isolated saponin was incorporated into micelles prepared from two triblock copolymers, Pluronic P123 and Pluronic F127 (1:1 ratio). The main advantage of the formulated micelles is their small diameter that could further improve cellular uptake. The studies on two types of isolated immune system cells showed that the saponin loaded in the nanosized micelles stimulates the proliferation of lymphocytes at lower concentrations than the free saponin in vitro. In the treatment of murine macrophages, the results indicate an improvement in the effects concerning the stimulation of the immune system cells. These findings suggest that the newly developed nanosized drug delivery system represents a potential approach to improving the pharmacological properties of the isolated saponin. The presented findings on saponin’s stimulation of isolated cells proliferation are interesting and might be a basis of future in vitro and in vivo study on the mechanisms of immune response modulation.
4. Conclusions
This study reported a validated method for the quantitative determination of a cycloartane saponin from Astragalus glycyphyllos. The loading of the saponin in nanosized micelles enhanced the proliferation of isolated murine immune cells in vitro. The effects were more evident on isolated lymphocytes, where, at lower substance concentrations, we found a better stimulation of cell proliferation of encapsulated vs. non-encapsulated saponins. These findings suggest that the encapsulation represents a potential approach for improving the pharmacological properties of this saponin.
Author Contributions
Conceptualization, I.K., K.Y. and V.T.; methodology, A.S., K.Y., V.T. and D.S.; software, A.S. and D.S.; validation, A.S. and D.S.; formal analysis, A.S., D.S., I.S., K.Y., V.T. and I.K.; investigation, A.S., I.S., D.S. and K.Y.; resources, I.K., K.Y. and V.T.; data curation, A.S., I.S., D.S. and K.Y.; writing—original draft preparation, A.S., D.S., I.S., K.Y., V.T. and I.K.; writing—review and editing, A.S., D.S., I.S., K.Y., V.T. and I.K.; visualisation, A.S., D.S. and K.Y.; supervision, I.K., K.Y. and V.T.; project administration, I.K.; funding acquisition, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study is financed by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No BG-RRP-2.004-0004-C01.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee at the Medical University of Sofia, Bulgaria (protocol code: 621; date: 3 May 2024). The Bulgarian Food Safety Agency at the Ministry of Agriculture and Foods further approved all procedures on animals (Permit number 342, valid until 2028).
Data Availability Statement
All data connected with this research are included in this manuscript. Further information is available from the corresponding author upon reasonable written request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stambolov, I.; Shkondrov, A.; Krasteva, I. Astragalus Glycyphyllos L.: Phytochemical Constituents, Pharmacology, and Biotechnology. Pharmacia 2023, 70, 635–641. [Google Scholar] [CrossRef]
- Linnek, J.; Mitaine-Offer, A.-C.; Miyamoto, T.; Tanaka, C.; Paululat, T.; Avunduk, S.; Alankuş-Çalişkan, Ö.; Lacaille-Dubois, M.-A. Cycloartane Glycosides from Three Species of Astragalus (Fabaceae). Helv. Chim. Acta 2011, 94, 230–237. [Google Scholar] [CrossRef]
- Shkondrov, A.; Krasteva, I.; Bucar, F.; Kunert, O.; Kondeva-Burdina, M.; Ionkova, I. Flavonoids and Saponins from Two Bulgarian Astragalus Species and Their Neuroprotective Activity. Phytochem. Lett. 2018, 26, 44–49. [Google Scholar] [CrossRef]
- Shkondrov, A.; Krasteva, I.; Bucar, F.; Kunert, O.; Kondeva-Burdina, M.; Ionkova, I. A New Tetracyclic Saponin from Astragalus glycyphyllos L. and Its Neuroprotective and HMAO-B Inhibiting Activity. Nat. Prod. Res. 2020, 34, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Stambolov, I.; Shkondrov, A.; Kunert, O.; Bucar, F.; Kondeva-Burdina, M.; Krasteva, I. Cycloartane Saponins from Astragalus glycyphyllos and Their In Vitro Neuroprotective, Antioxidant, and HMAO-B-Inhibiting Effects. Metabolites 2023, 13, 857. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Liu, Y.; Zhang, Z.-P.; Wu, J.-T.; Wang, J.; Pan, J.; Guan, W.; Kuang, H.; Yang, B. Seven New Triterpenoid Saponins from Astragalus membranaceus Var. Mongholicus and the Inhibition of High-Glucose Induced SV40 MES 13 Cells. New J. Chem. 2023, 47, 8776–8784. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.-A. Saponins as Immunoadjuvants and Immunostimulants. In Immunomodulatory Agents from Plants; Wagner, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 243–272. ISBN 3764358483. [Google Scholar]
- Wang, P. Natural and Synthetic Saponins as Vaccine Adjuvants. Vaccines 2021, 9, 222. [Google Scholar] [CrossRef]
- Ionkova, I.; Shkondrov, A.; Zarev, Y.; Kozuharova, E.; Krasteva, I. Anticancer Secondary Metabolites: From Ethnopharmacology and Identification in Native Complexes to Biotechnological Studies in Species of Genus Astragalus L. and Gloriosa L. Curr. Issues Mol. Biol. 2022, 44, 3884–3904. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Piazzini, V.; Reglero, G.; Martin, D.; Bergonzi, M.C. In Vitro Permeability of Saponins and Sapogenins from Seed Extracts by the Parallel Artificial Membrane Permeability Assay: Effect of in Vitro Gastrointestinal Digestion. J. Agric. Food Chem. 2020, 68, 1297–1305. [Google Scholar] [CrossRef]
- Ikram, M.; Jo, M.H.; Choe, K.; Khan, A.; Ahmad, S.; Saeed, K.; Kim, M.W.; Kim, M.O. Cycloastragenol, a Triterpenoid Saponin, Regulates Oxidative Stress, Neurotrophic Dysfunctions, Neuroinflammation and Apoptotic Cell Death in Neurodegenerative Conditions. Cells 2021, 10, 2719. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative Preparation of Curcumin for Improved Oral Bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- de Oliveira, R.S.S.; Marín Huachaca, N.S.; Lemos, M.; Santos, N.F.; Feitosa, E.; Salay, L.C. Molecular Interactions between Pluronic F127 and Saponin in Aqueous Solution. Colloid Polym. Sci. 2020, 298, 113–122. [Google Scholar] [CrossRef]
- Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tzankova, V.; Yoncheva, K. Double-Loaded Doxorubicin/Resveratrol Polymeric Micelles Providing Low Toxicity on Cardiac Cells and Enhanced Cytotoxicity on Lymphoma Cells. Pharmaceutics 2023, 15, 1287. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for Cell Viability Assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tava, A.; Biazzi, E.; Mella, M.; Quadrelli, P.; Avato, P. Artefact Formation during Acid Hydrolysis of Saponins from Medicago spp. Phytochemistry 2017, 138, 116–127. [Google Scholar] [CrossRef]
- Oleszek, W.A. Chromatographic Determination of Plant Saponins. J. Chromatogr. A 2002, 967, 147–162. [Google Scholar] [CrossRef]
- Abhijith, B.; Natakkakath Kaliyathan Raveena, M.V.R.; Lankalapalli, R.S. Artifacts from the Methanolic Extract of Solanum Nigrum Linn. Nat. Prod. Res. 2023, 38, 2896–2900. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2 (R1); International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: Geneva, Switzerland, 2005; pp. 11–12. [Google Scholar]
- Savarino, P.; Demeyer, M.; Decroo, C.; Colson, E.; Gerbaux, P. Mass Spectrometry Analysis of Saponins. Mass Spectrom. Rev. 2023, 42, 954–983. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kord, A. UHPLC Method Development. In Ultra-High Performance Liquid Chromatography and Its Applications; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2013; pp. 1–30. ISBN 9781118533956. [Google Scholar]
- Figueiras, A.; Domingues, C.; Jarak, I.; Santos, A.I.; Parra, A.; Pais, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Kabanov, A.; Cabral, H.; et al. New Advances in Biomedical Application of Polymeric Micelles. Pharmaceutics 2022, 14, 1700. [Google Scholar] [CrossRef]
- Swartz, M.E.; Krull, I.S. Analytical Method Development and Validation; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Gao, S.; Basu, S.; Yang, Z.; Deb, A.; Hu, M. Bioavailability Challenges Associated with Development of Saponins as Therapeutic and Chemopreventive Agents. Curr. Drug Targets 2012, 13, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Liu, J.-Y.; Liu, Z.-Q.; Xiao, D.; Chen, J. The Role of ADCY1 in Regulating the Sensitivity of Platinum-Based Chemotherapy in NSCLC. Pharmaceuticals 2024, 17, 1118. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Basara, G.; Ellis, B.W.; Ren, X.; Zorlutuna, P. Breast Cancer Models: Engineering the Tumor Microenvironment. Acta Biomater. 2020, 106, 1–21. [Google Scholar] [CrossRef]
- Lee, J.; Lim, J.-H.; Jung, G.-Y.; Kang, J.; Jo, I.; Kang, K.; Kim, J.-H.; Kim, B.-S.; Yang, H. Triterpenoid Saponins from Camellia sinensis Roots with Cytotoxic and Immunomodulatory Effects. Phytochemistry 2023, 212, 113688. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, D.; Ma, C.; Wang, R.; Wang, W. Free Radical Scavenging Effect and Immunomodulatory Activity of Total Saponins Extract of Ginseng Fibrous Roots. Molecules 2024, 29, 2770. [Google Scholar] [CrossRef]
- Li, C.-X.; Liu, Y.; Zhang, Y.-Z.; Li, J.-C.; Lai, J. Astragalus Polysaccharide: A Review of Its Immunomodulatory Effect. Arch. Pharm. Res. 2022, 45, 367–389. [Google Scholar] [CrossRef]
- Ding, G.; Gong, Q.; Ma, J.; Liu, X.; Wang, Y.; Cheng, X. Immunosuppressive Activity Is Attenuated by Astragalus polysaccharides through Remodeling the Gut Microenvironment in Melanoma Mice. Cancer Sci. 2021, 112, 4050–4063. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Liu, Y.-Q.; Liu, D.; Zhang, L.; Qin, J.; Zhang, Z.; Su, Y.; Yan, C.; Luo, Y.-L.; Li, J.; et al. The Effects of Astragalus Polysaccharide on Bone Marrow-Derived Mesenchymal Stem Cell Proliferation and Morphology Induced by A549 Lung Cancer Cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 4110–4121. [Google Scholar] [CrossRef]
- Dong, M.; Li, J.; Yang, D.; Li, M.; Wei, J. Biosynthesis and Pharmacological Activities of Flavonoids, Triterpene Saponins and Polysaccharides Derived from Astragalus Membranaceus. Molecules 2023, 28, 5018. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, L.; Gao, C.; Chen, W.; Vong, C.T.; Yao, P.; Yang, Y.; Li, X.; Tang, X.; Wang, S.; et al. Astragali Radix (Huangqi): A Promising Edible Immunomodulatory Herbal Medicine. J. Ethnopharmacol. 2020, 258, 112895. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, T.; Hao, N.; Tao, H.; Zou, S.; Li, M.; Ming, P.; Ding, H.; Dong, J.; Feng, S.; et al. Immune Regulation Mechanism of Astragaloside IV on RAW264.7 Cells through Activating the NF-ΚB/MAPK Signaling Pathway. Int. Immunopharmacol. 2017, 49, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, I.N.; Toshkova, R.A.; Nikolov, S.D. Protective Effect of Astragalus corniculatus Saponins against Myeloid Graffi Tumour in Hamsters. Phyther. Res. 2004, 18, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Anang, V.; Kumari, K.; Kottarath, S.K.; Verma, C. Chapter Eleven—Role of Lymphocytes, Macrophages and Immune Receptors in Suppression of Tumor Immunity. In Receptor Endocytosis and Signalling in Health and Disease—Part A; Mani, I., Singh, V., Eds.; Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2023; Volume 194, pp. 269–310. [Google Scholar]
- Sam-Yellowe, T.Y.; Sam-Yellowe, T.Y.; Sam-Yellowe, T. Immunology: Overview and Laboratory Manual; Springer: Basel, Switzerland, 2021. [Google Scholar]
- Wang, Q.; Atluri, K.; Tiwari, A.K.; Babu, R.J. Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine. Pharmaceuticals 2023, 16, 433. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).