Abstract
The recovery of difficult-to-float coal using traditional nonpolar hydrocarbon oil collectors can be challenging, particularly for low-rank or oxidized coal. Thus, there is a need for more efficient flotation agents. Nanoparticle flotation collector technology has become increasingly popular in the field of mineral processing, and the presence of various ions in the slurry can significantly affect the interaction between collectors and mineral surfaces. In this study, cationic polystyrene (PS) nanoparticles were prepared using an emulsion polymerization method, and the effects of Na+ ion concentration on the in situ adsorption and desorption processes, adsorption layer configuration, and adsorption kinetics of PS particles on amorphous carbon (coal model) and SiO2 sensors (quartz mineral model) were analyzed using the quartz crystal microbalance with dissipation (QCM-D) technique. Our results showed that the hydrophobic PS nanoparticles irreversibly adsorbed onto both amorphous carbon and SiO2 sensors under different environmental conditions, and their adsorption capacity decreased gradually with increasing Na+ ion concentration. Increasing Na+ ion concentration from 0 M to 1.0 M resulted in a 24.4% and 30.9% decrease in equilibrium adsorption capacities of PS nanoparticles onto amorphous carbon and SiO2 surfaces, respectively. The adsorption rate of PS nanoparticles onto the SiO2 surface was much greater than that on the amorphous carbon surface. The adsorption rate constant of PS nanoparticles onto SiO2 surfaces was 0.782 at 0.1 M Na+ ion concentration, while its adsorption rate constant onto amorphous carbon surfaces was only 0.060. Moreover, the adsorption process was found to be more in line with the quasi-primary kinetic model. These findings suggest that PS nanoparticles may serve as promising flotation collectors for the recovery of difficult-to-float coal, and highlight the importance of considering the effect of dissolved ions on the adsorption properties of flotation collectors.
1. Introduction
The coal flotation process is a complex and dynamic process that involves various physicochemical aspects, and its final outcome is influenced by multiple factors, such as feed mineral properties, concentration, process flow, equipment performance, and agent regime [1,2,3,4,5,6]. The use of flotation agents can significantly modify the nature of the solid–liquid–gas phase of the system, thereby improving the hydrophobicity of fine-grained coal and enhancing the flotation effect [7]. Therefore, the design of a reasonable agent system plays a crucial role in achieving an optimal flotation outcome.
Nonpolar hydrocarbon oils are the collectors most commonly used in coal flotation systems. Many coal processing plants currently use hydrocarbon oil products that are refined and processed from petroleum, with diesel and kerosene being typical representatives. However, due to the rising cost of petroleum and the limited effectiveness of traditional nonpolar hydrocarbon oils in recovering low-grade coal, oxidized coal, and other challenging-to-float coals, synthetic collectors have gained increasing attention from researchers and are being applied in actual production processes.
The use of emerging nanomaterials has garnered significant attention from researchers in the flotation field due to their unique size advantages, which allow for improved physical and chemical properties compared to conventional materials [8,9,10,11]. Yang et al. [12,13,14,15,16] were among the first to propose the use of hydrophobic nanoparticles as collectors in mineral flotation, and they experimentally validated their approach using glass beads as a pure mineral flotation model. Their findings showcased how nanoparticles can effectively adsorb onto the surface of target minerals to alter their hydrophobicity, thereby facilitating mutual adhesion between mineral particles and air bubbles. Since then, nanoparticle collectors have garnered increasing attention from researchers, and studies have reported their successful application in the flotation of coal, chalcopyrite, malachite, quartz, and pyrite. However, despite the growing interest in nanoparticles as flotation collectors, research on their interaction with mineral surfaces is still in its nascent stages, with Yang et al.’s work in 2011 marking the first report on the topic.
An et al. [8] successfully obtained a clean concentrate from high-ash coal using cationic hydrophobic polystyrene (PS) nanoparticles as a coal flotation collector, which significantly improved the combustible recovery of the coal. Dong et al. [17] utilized hydrophobic cationic polystyrene nanoparticles as collectors for flotation experiments on hydrophilic glass beads of 43 µm diameter, and observed the irreversible abrasion of the nanoparticles, explaining the effectiveness of smaller polystyrene particles over larger ones. In a separate study, Dong et al. [18] found that PS-PB (cationic polystyrene core/poly(n-butyl methacrylate) shell) nanoparticles exhibited significantly higher pull-off force and adhesion work on glass compared to harder PS particles in colloidal probe atomic force microscopy measurements. In laboratory flotation experiments, the recovery of glass beads was found to increase dramatically with the thickness of the soft PB shell on the core/shell nanoparticles. Mabudi et al. [19] explored the effect of polystyrene nanoparticles with different surface coverage on glass surface wettability using molecular dynamics simulations. They found that glass surfaces partially covered with cationic polystyrene nanoparticles up to 10% could reduce the surface tension to a hydrophobic level favorable for flotation. Lastly, Hajati et al. [20] used natural hydrophobic talc nanoparticles as a collector in the quartz flotation process and observed that talc could be adsorbed onto quartz particles, with a significant reduction in the amount of collector required when the size of talc nanoparticles was decreased.
Coal typically contains large amounts of soluble salts, which can accumulate in circulating water during processing. The metal ions from these salts can affect the foaming performance of frothers and alter the interaction between collectors and mineral surfaces during flotation. By applying nanotechnology to the traditional mineral processing industry, researchers have the potential to make significant advancements in both theoretical research and practical production applications. However, many questions remain unanswered regarding the microstructure and principles of nanoparticle collectors. Traditional methods for studying collector adsorption behavior, such as infrared spectroscopy, ultraviolet spectroscopy, fluorescence spectroscopy, ultraviolet spectrophotometry, combustion, and total organic carbon (TOC) analysis, can provide a clear analysis of adsorption modes but struggle to accurately determine the amount of collector adsorbed onto the mineral surface. These indirect measurement methods lead to overly complicated experimental procedures, and their reliability and accuracy are often low. Thus, traditional research methods have significant limitations. To address these limitations, the quartz crystal microbalance with dissipation (QCM-D), which uses the piezoelectric effect to study the adsorption and desorption behavior of agents and different mineral surfaces, has emerged as a more advanced option [21,22,23,24,25,26]. QCM-D provides real-time dynamic observations of collector adsorption behavior on mineral surfaces, including the adsorption amount and desorption in case of liquid flow disturbance, offering unique advantages for studying the adsorption mechanism of collectors.
This study aims to investigate the interaction mechanism between metal ions and nanoparticle collectors on mineral surfaces, providing valuable theoretical support for the future widespread application of nanoparticles as novel mineral collectors. PS nanoparticle coal flotation collectors were synthesized using emulsion polymerization, with amorphous carbon and SiO2 sensors used as models for coal particles and the typical quartz mineral in coal, respectively. The QCM-D technique was utilized to examine the effect of NaCl solution concentration on the in situ adsorption behavior of PS nanoparticles at the solid–liquid interface. By exploring the adsorption behavior of PS nanoparticles in coal flotation, this study provides a critical theoretical basis for the field’s advancement. Applying nanotechnology to the mineral flotation industry offers numerous opportunities, especially when dealing with poor-quality minerals at microfine particle sizes.
2. Experimental Materials and Methods
2.1. Preparation and Particle Size Characterization of PS Nanoparticles
The PS nanoparticle collector used in this study was synthesized using the emulsion polymerization method. A fixed amount of the emulsifier cetyltrimethylammonium bromide (CTAB) was added to deionized water, which was then fully dissolved before adding styrene (St). The mixture was continuously stirred for 24 h at room temperature to achieve full emulsification. Nitrogen was then injected, and the initiator 2,2′-azobis[2-methylpropionamidine] dihydrochloride (V50) was added under the protection of a nitrogen atmosphere. The temperature was increased to a constant temperature of 75 ± 0.5 °C and turned off after 0.5 h. The emulsion was continuously stirred for another 24 h until synthesis completion, after which it was left to cool down to room temperature. The specific formulation used in this synthesis is outlined in Table 1.

Table 1.
Formulation and conditions of nanoparticle emulsion.
The particle size distribution of the synthesized nanoparticles was measured using a Malvern Zetasizer nanoZS laser particle sizer. The instrument was set to a wavelength of 633.0 nm with the light source angle adjusted to 90° and powered by a He–Ne laser at 22 mW. To visualize the morphology of the dried emulsion, a Zeiss Merlin Compact scanning electron microscope (SEM) was used, with the prepared sample placed on a conductive film-coated sample table for analysis.
2.2. QCM-D Test Mechanism
Q-Sense has developed a patented technology for surface analysis, called quartz crystal microbalance with dissipation monitoring (QCM-D). This technique enables real-time, label-free measurement of molecular adsorption onto and/or interactions with a variety of surfaces. QCM-D not only measures the adsorption mass (with ng/cm2 sensitivity) as a function of changes in the frequency (f) of the quartz crystal, but also the dissipation parameter (D), which provides information about the structural properties (viscoelasticity) of the adsorbed layer. Dissipation measurements allow qualitative analysis of the structural properties of the adsorbed molecular layers. Furthermore, the QCM-D technique allows quantitative analysis of the thickness, shear elastic modulus, and viscosity of the adsorbed films.
Compared to traditional adsorption testing methods, QCM-D testing has several advantages. Real-time monitoring: QCM-D testing can monitor the process of the formation of the adsorption layer on the crystal surface in real-time, as well as its evolution over time. This greatly improves the sensitivity and accuracy of the testing. Controllable experimental conditions: Experimental conditions such as temperature, pressure, and flow rate can be controlled in QCM-D testing, which better simulates the actual application of materials in different environments, improving the reliability and repeatability of the testing results. Richer information: QCM-D testing not only provides changes in the quality of the material surface adsorption layer but also analyzes the physical and chemical characteristics of the layer such as elasticity and viscosity. Therefore, it can provide more comprehensive information to help people better understand the properties of the material surface adsorption layer.
Raw data obtained from the QCM-D instrument were processed using the Q-tool software [27], and Δf and ΔD at different overtones were analyzed with various models. In QCM-D testing, Δf refers to the change in the oscillation frequency of the crystal, while ΔD refers to the change in the dissipation the crystal. These two parameters are usually related to material mass, hardness, viscosity, etc., and can be used to study surface adsorption phenomena, film thickness, and biomolecular interactions. The adsorption calculations provided clearer and simpler-to-interpret results. When fitting the adsorption of viscoelastic substances, the Sauerbrey model often resulted in lower-than-expected values, while the Voigt model produced more accurate results. The Sauerbrey model is applicable to cases where the mass of the adsorbed layer is small, the thickness of the adsorbed layer is much smaller than the thickness of the crystal, and the adsorbed layer is rigid. The Voigt model is widely used to analyze parameters such as viscosity and elastic modulus of viscoelastic adsorbed layers.
2.3. Adsorption Tests of the Nanoparticles onto Mineral Surfaces
The QCM-D test procedure for the PS nanoparticles on the surface of amorphous carbon and SiO2 sensors at different concentrations of inorganic salts (NaCl) was similar, and the adsorption test of nanoparticles onto the surface of the SiO2 sensor is illustrated here. After the SiO2 sensor was aligned in the sensor recess and assembled, it was placed in the QCM-D test tank, the power was turned on, and the Q-tool software was opened to test the sensor in an air environment for 30 min to make the sensor vibrate fully. When the test was formally started, ultrapure water (UP) was introduced to fully wet the sensor, and then, when the frequency and dissipation changes stabilized, the equilibrium curve under a water environment was obtained. Next, different concentrations of NaCl solutions were passed through to observe the adsorption onto the surface of the sensor, and the equilibrium state was reached when the curve change stabilized. Afterward, the emulsion containing PS nanoparticles was passed through, and the flow of the emulsion caused an instantaneous change in f and D when it touched the sensor surface. After the change became stable, the same concentrations of NaCl solution and ultrapure water were passed through again to observe the desorption of nanoparticles, and the test was stopped when the curves of f and D no longer changed, being regarded as equilibrium. During the test and data fitting process, all conditions were kept the same as above.
Due to the complexity of preparing coal nanoparticles and their highly variable surface properties, processing and fabricating them into sensors is challenging. Since coal is considered to be naturally occurring amorphous carbon, using an amorphous carbon sensor as a coal model is appropriate. Similarly, the main component of quartz is SiO2, making SiO2 an appropriate representation of the quartz mineral surface. Typically, the third or fifth overtone has the most stable and sensitive data variation; therefore, they are preferred for data analysis. The test results presented in this paper were generated under the third overtone.
3. Results and Discussion
3.1. PS Nanoparticle Size Analysis
Figure 1 presents the SEM results of PS nanoparticles, which demonstrated a regular spherical structure. Although a limited number of particles had diameters exceeding 100 nm, they likely formed due to the aggregation of multiple nanoparticles during synthesis. Most of the particles had diameters around 100 nm, displaying a narrow size distribution.

Figure 1.
SEM analysis of polystyrene nanoparticles.
Figure 2 depicts the results of laser particle size analysis of PS nanoparticles. The percentage of particles smaller than 100 nm was negligible, with most nanoparticles having diameters around 100 nm, and a small number of particles exceeded 100 nm in diameter. Remarkably, the results of both SEM and laser particle sizing tests were highly consistent.
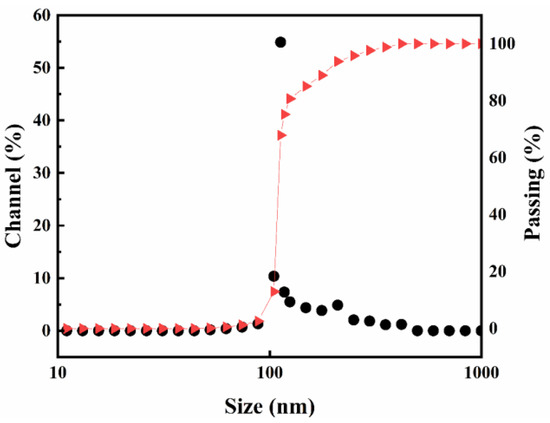
Figure 2.
Nanoparticle laser particle sizing analysis.
3.2. Adsorption of the PS Nanoparticles onto Amorphous Carbon and SiO2 Surfaces in Pure Water
The QCM-D test results of nanoparticles on the amorphous carbon surface are presented in Figure 3. According to Figure 3a, the frequency initially plummeted rapidly upon the introduction of PS nanoparticles, with the rapid decrease stage lasting approximately 100 min. Subsequently, the frequency continued to decrease slowly, and it took an additional 100 min to achieve adsorption equilibrium, suggesting that large amounts of PS nanoparticles adhered to the amorphous carbon surface during the fast-decreasing stage. Following this stage, the rate of PS nanoparticle adsorption gradually declined as the number of effective adsorption sites on the surface decreased. Nevertheless, the adsorption capacity still grew slowly since the first PS molecular layer attached to the surface could further absorb PS nanoparticles from the solution and form a multilayer adsorbate structure. Hence, the equilibrium state of adsorption was reached gradually after 200 min.
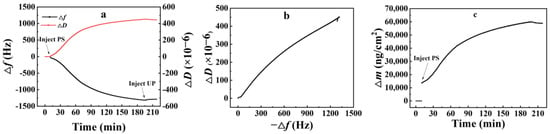
Figure 3.
QCM-D test results of nanoparticle adsorption onto amorphous carbon surface. (a), change of frequency with time; (b), change of adsorption layer configuration; (c), change of adsorption amount with time.
No significant rebound of Δf and ΔD was observed after the addition of ultrapure water for cleaning, indicating that the adsorption of PS nanoparticles was almost irreversible. It was also obvious from the corresponding −Δf and ΔD curve (Figure 3b) that the slope of the curve was slowly decreasing, i.e., the rate of increase of ΔD became smaller as −Δf increased, indicating that the PS nanoparticle adsorption layer configuration changed with the increase in adsorption capacity. With the increase in adsorption time, the adsorption layer of nanoparticles on the surface of amorphous carbon became denser, probably due to the hydrophobic attraction between the hydrophobic PS nanoparticles and the surface of amorphous carbon, which made the nanoparticles gradually move toward the carbon surface.
Regarding adsorption capacity, the results indicated that it increased to a certain extent following the addition of PS nanoparticles, as shown in Figure 3c. The capacity then continued to increase gradually during the slow adsorption process. After reaching equilibrium, the adsorption capacity stabilized at 59,379 ng/cm2. Subsequent cleaning with ultrapure water did not noticeably decrease the adsorption capacity.
The QCM-D test results of nanoparticles on the SiO2 surface are presented in Figure 4a. Upon introduction of the nanoparticle emulsion, Δf initially decreased rapidly, with the duration of this stage being around 10 min. Subsequently, the adsorption rate of PS nanoparticles decelerated significantly for 10–20 min, with little change in Δf thereafter. This observation implied that a substantial quantity of PS nanoparticles adhered to the SiO2 surface within a brief interval, after which their adsorption rate declined quickly due to the limited availability of effective adsorption sites on the surface. Eventually, Δf approached an equilibrium state. After cleaning with ultrapure water, neither Δf nor ΔD changed substantially, suggesting that the adsorption of PS nanoparticles onto the SiO2 surface was nearly irreversible.
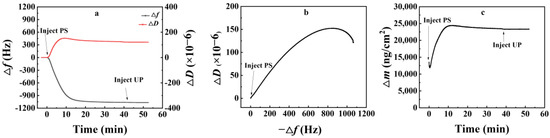
Figure 4.
QCM-D test results of nanoparticle adsorption onto SiO2 surface. (a), change of frequency with time; (b), change of adsorption layer configuration; (c), change of adsorption amount with time.
Figure 4b illustrates the relationship between −Δf and ΔD. As the adsorption layer became denser, the slope of the curve gradually diminished. This phenomenon could be attributed to the rapid stacking of numerous PS nanoparticles on the SiO2 surface in a short duration, forming a multilayer adsorption structure that achieved its maximum adsorption capacity. The arrangement of the adsorption layer then thickened as the outer-layer nanoparticles slowly filled the interstices between those in the inner layer. At equilibrium, the adsorption capacity was 23,325 ng/cm2.
In summary, PS nanoparticles could be adsorbed onto both amorphous carbon and SiO2 surfaces, and the nanoparticles basically did not desorb under the rinsing effect of ultrapure water. This result also confirms that PS nanoparticles can be used as a collector for coal flotation. From the perspective of equilibrium adsorption capacity, the adsorption capacity of nanoparticles onto the surface of amorphous carbon was much larger than their adsorption capacity onto the surface of SiO2. The results of this study are in good agreement with the findings reported in the literature, where An et al. [8] successfully used PS nanoparticles as a coal collector to obtain flotation concentrate. The adsorption of PS nanoparticles onto the surfaces of both the concentrate and the tailings was observed by scanning electron microscopy, while the amount of adsorption onto the concentrate product was greater than that on the tailings.
In conclusion, PS nanoparticles could readily adsorb onto both amorphous carbon and SiO2 surfaces, with little desorption observed even after ultrapure water rinsing. These findings affirm that PS nanoparticles can be used as a collector for coal flotation. According to the equilibrium adsorption capacity, the nanoparticles appeared to exhibit higher adsorption capacity onto the surface of amorphous carbon than on the SiO2 surface. These results are consistent with previous studies; for instance, An et al. [8] utilized PS nanoparticles as a coal collector and obtained flotation concentrate. Scanning electron microscopy revealed that PS nanoparticle adsorption occurred onto both concentrate and tailing surfaces, yet adsorption was greater onto the concentrate product than onto the tailings. The adsorption mechanism of PS nanoparticles onto the surface of SiO2 may involve electrostatic forces. Electrostatic forces are caused by the cationic surfactant CTAB added to the synthesis process of polystyrene nanoparticles, which is adsorbed onto the surface of the nanoparticles. As a result, the surface of PS nanoparticles is positively charged. When in contact with the surface of SiO2, opposite charges between the two produce attractive forces. The adsorption mechanism of PS nanoparticles onto amorphous carbon surfaces may involve π–π stacking. Specifically, there is a mutual interaction between the aromatic ring system on the polystyrene nanoparticles and the amorphous carbon surface because they are both composed of molecules containing benzene rings and carbon–carbon double bonds. In addition, both PS nanoparticles and amorphous carbon are hydrophobic, resulting in a long-range hydrophobic interaction between them.
It is worth noting that the adsorption rate of PS nanoparticles onto the amorphous carbon surface was substantially lower than that on the SiO2 surface. The initial fast adsorption stage of PS nanoparticles onto the amorphous carbon surface persisted for 100 min, while, for the SiO2 surface, this period was reduced to roughly 10 min. For instance, at an adsorption time of 10 min, the adsorption of PS nanoparticles onto the amorphous carbon surface was about 12,000 ng/cm2, whereas their adsorption onto the SiO2 surface reached about 24,000 ng/cm2. Consequently, in coal flotation, if the amount of nanoparticle collection is insufficient, the coal particles will be at a disadvantage in the process of competing with the adsorption of agents onto the surface of gangue minerals, which will affect the effectiveness of coal flotation.
3.3. Effect of Different Concentrations of Na+ on the Adsorption Behavior of PS Nanoparticles onto Amorphous Carbon Surface
The QCM-D test results for PS nanoparticles on the amorphous carbon surface at different NaCl concentrations are presented in Figure 5. At a NaCl concentration of 0.1 M, Δf declined markedly after the addition of PS nanoparticles, and the rate of Δf decrease declined after reaching 1000 Hz (Figure 5a). This finding suggests that most PS nanoparticles can adsorb onto the amorphous carbon surface within a brief period at this concentration. Nevertheless, a small number of nanoparticles could continue to adhere to the surface over time. Following cleaning with a solution of the same NaCl concentration, Δf and ΔD remained relatively unchanged, indicating irreversible adsorption of nanoparticles onto the amorphous carbon surface in the NaCl solution. Subsequently, washing with water resulted in a minor increase in Δf and a decrease in ΔD, suggesting limited desorption of some nanoparticles from the surface.
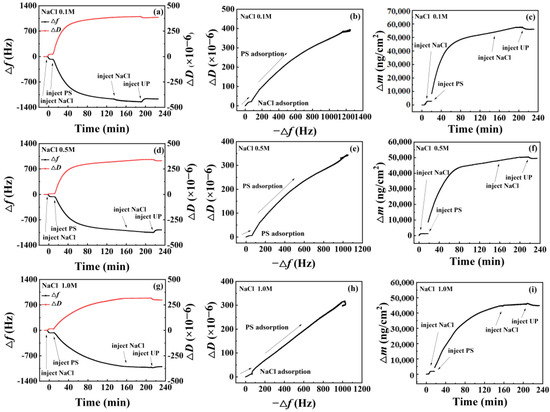
Figure 5.
QCM-D test results of PS nanoparticles adsorption onto the amorphous carbon surface at different concentrations of Na+ ions. (a,d,g): change of frequency with time; (b,e,h): change of adsorption layer configuration; (c,f,i): change of adsorption amount with time.
The curves of −Δf versus ΔD depicted in Figure 5b displayed a gradual decrease in slope, indicating densification of the nanoparticle adsorption layer with increasing adsorption. The change in adsorption amount (Figure 5c) revealed that PS nanoparticle adsorption capacity initially increased rapidly upon addition, then gradually slowed down, followed by a minor decline after water washing.
At a NaCl concentration of 0.5 M, Δf declined rapidly upon PS nanoparticle addition (Figure 5d). After decreasing up to 800 Hz, Δf decreased gradually, reaching adsorption equilibrium after 180 min. This outcome indicates that most PS nanoparticles could adsorb onto the amorphous carbon surface in a brief period. However, reaching adsorption equilibrium required an extended interval. After cleaning with a solution of the same NaCl concentration, Δf and ΔD remained largely unchanged, indicating irreversible nanoparticle adsorption onto the amorphous carbon surface in the 0.5 M NaCl solution. Washing with ultrapure water resulted in a minor trend toward the baseline for Δf and ΔD, implying that numerous PS nanoparticles still adhered to the surface, with only a small amount of desorption. The curve of −Δf versus ΔD slope (Figure 5e) also gradually decreased at a concentration of 0.5 M NaCl, indicating densification of the nanoparticle adsorption layer with adsorption, consistent with the findings at 0.1 M NaCl. Regarding the relationship between adsorption capacity and time (Figure 5f), PS nanoparticle adsorption capacity increased rapidly upon addition and gradually slowed down as adsorption continued, finally stabilizing at approximately 50,000 ng/cm2.
At a NaCl concentration of 1.0 M (Figure 5g), PS nanoparticle adsorption behaved differently than at the lower concentrations. The overall adsorption process was slow upon PS nanoparticle addition, with minimal initial adsorption. Following water washing, Δf and ΔD displayed only minor changes, suggesting a steadier nanoparticle adsorption process at higher NaCl solution concentrations. The −Δf versus ΔD relationship at 1.0 M NaCl (Figure 5h) was a straight line, indicating that the nanoparticle adsorption layer configuration did not change with adsorption. Moreover, the relationship between adsorption capacity and time (Figure 5i) displayed a slowly increasing curve, eventually stabilizing at approximately 45,000 ng/cm2.
3.4. Effect of Different Concentrations of Na+ on the Adsorption Behavior of PS Nanoparticles onto the Surface of SiO2
PS nanoparticle adsorption patterns onto the SiO2 sensor surface at different NaCl concentrations were similar to those on the amorphous carbon surface. Figure 6a displays that, upon adding PS emulsion at an NaCl concentration of 0.1 M, Δf decreased rapidly to approximately 900 Hz, slowing down afterward. After 30 min, Δf was smaller, and adsorption equilibrium was attained at 50 min. This outcome indicates that most PS nanoparticles could adsorb onto the SiO2 surface within a short period at a concentration of 0.1 M NaCl, reaching equilibrium state. After cleaning with a solution of the same NaCl concentration, neither Δf nor ΔD exhibited significant changes. Washing with pure water revealed minimal changes in both Δf and ΔD. This suggests irreversible PS nanoparticle adsorption onto the SiO2 surface, with neither NaCl solution nor pure water rinsing sufficient for desorption, thereby maintaining the adsorption state.
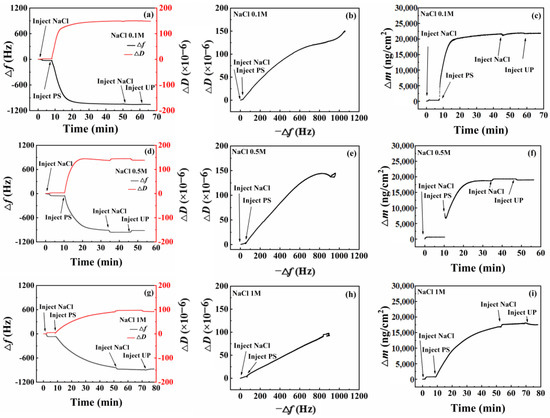
Figure 6.
QCM-D test results of nanoparticle adsorption onto the SiO2 surface at different concentrations of Na+ ions. (a,d,g): change of frequency with time; (b,e,h): change of adsorption layer configuration; (c,f,i): change of adsorption amount with time.
At NaCl concentrations of 0.5 M and 1.0 M (Figure 6d and Figure 6g, respectively), Δf and ΔD exhibited slower changes after PS emulsion addition. Cleaning with NaCl solution resulted in increased Δf and ΔD magnitudes, attributed to NaCl adsorption. Subsequently, water washing returned Δf and ΔD to their original magnitudes, indicating desorption of newly adsorbed NaCl from the surface.
The −Δf versus ΔD curves shown in Figure 6b,e,h are divided into two distinct segments. The first segment is attributed to NaCl adsorption, displaying a linear curve and suggesting an unchanged adsorption layer configuration. The second segment is caused by PS nanoparticle adsorption, with Figure 6b,e showing a section of decreasing slope. This outcome implies densification of the adsorption layer with increased adsorption capacity, potentially due to nanoparticle self-adhesion. Figure 6h displays a straight-line segment, indicating greater nanoparticle stability under a higher ion concentration environment and an unchanging adsorption layer configuration.
Figure 6c, Figure 6f, and Figure 6i display adsorption capacities of 21,364 ng/cm2, 18,130 ng/cm2, and 16,114 ng/cm2, respectively, after reaching equilibrium in NaCl environments at concentrations of 0.1 M, 0.5 M, and 1.0 M, respectively. This outcome indicates that higher adsorption capacities could be achieved at low NaCl concentrations upon reaching adsorption equilibrium. However, PS nanoparticle adsorption onto the SiO2 surface was hindered at high NaCl concentrations. This may have been caused by increased Na+ ion adsorption onto the SiO2 surface, leading to gradual changes from negative to positive electrical properties. As a result, the electrostatic attraction between the SiO2 surface and cationic PS nanoparticles decreased gradually.
Contrary to our experimental results, Liu et al. [28] found that increasing the calcium ion concentration increased the adsorption of the anionic surfactant alcohol alkoxy sulfate (AAS). They concluded that the bridging effect of calcium ions has an important influence on the adsorption of anionic surfactant AAS onto calcite surface. This is understandable because, in this paper, a positively charged metal ion (Ca2+) was also used, and AAS is an anionic surfactant; hence, the opposite charge increased the electrostatic interaction, leading to an increase in adsorption.
3.5. Adsorption Kinetic Analysis
Figure 7 compares −Δf variations induced by PS nanoparticle adsorption under different Na+ ion concentrations. The impact of different Na+ ion concentrations on PS nanoparticle adsorption behavior onto amorphous carbon (Figure 7a) and SiO2 (Figure 7b) surfaces was similar. With increasing Na+ ion concentration, the curves with time displayed smaller slopes, indicating slower PS nanoparticle adsorption rates onto both amorphous carbon and SiO2 surfaces after Na+ ion adsorption.
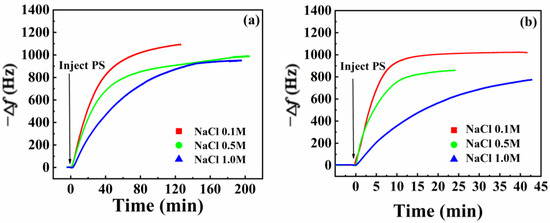
Figure 7.
Frequency variation of PS nanoparticles adsorption onto the surfaces of amorphous carbon (a) and SiO2 (b) at different concentrations of Na+ ions.
Table 2 displays PS nanoparticle equilibrium adsorption capacities onto amorphous carbon and SiO2 surfaces at NaCl concentrations of 0 M, 0.1 M, 0.5 M, and 1.0 M. For amorphous carbon, the capacities were 59,379 ng/cm2, 56,155 ng/cm2, 49,552 ng/cm2, and 44,900 ng/cm2, respectively. For SiO2, the capacities were 23,325 ng/cm2, 21,364 ng/cm2, 18,130 ng/cm2, and 16,114 ng/cm2, respectively. The equilibrium adsorption capacities decreased with increasing Na+ ion concentration onto both sensors’ surfaces. Upon increasing Na+ ion concentration from 0 M to 1.0 M, the equilibrium adsorption capacities onto amorphous carbon and SiO2 surfaces decreased by 24.4% and 30.9%, respectively. These results indicate that higher Na+ ion concentrations hindered PS nanoparticle adsorption onto both amorphous carbon and SiO2 surfaces, resulting in lower adsorption capacities and slower adsorption rates.

Table 2.
Adsorption capacity of PS nanoparticles onto amorphous carbon and SiO2 surfaces at different concentrations of Na+ ions.
To understand the adsorption mechanism of PS nanoparticles onto amorphous carbon and SiO2 surfaces, quasi-primary and quasi-secondary adsorption kinetic equations were linearly fit to the adsorption process. Figure 8 and Table 3 display the results. The correlation coefficients (R2) obtained from the quasi-primary kinetic equation were higher than those of the quasi-secondary kinetic equation at different Na+ ion concentrations. The R2 value was obtained by calculating the ratio of the sum of squares of the difference between the measured data and the corresponding fitted data to the total variance. The theoretical equilibrium adsorption capacities (qe) from the quasi-primary equation closely matched experimental equilibrium adsorption capacities.
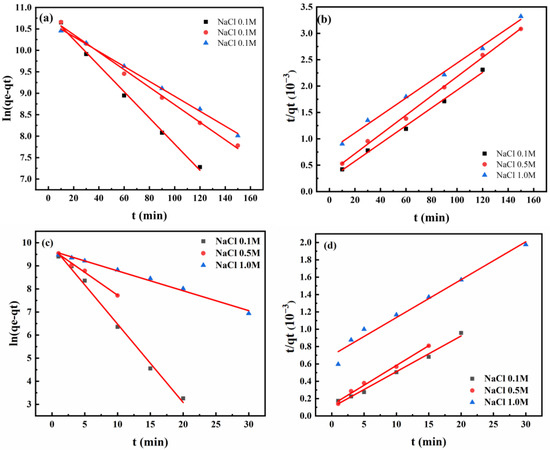
Figure 8.
Results of fitting the adsorption kinetics of PS nanoparticles onto the surfaces of amorphous carbon (a,b) and SiO2 (c,d) at different concentrations of Na+ ions: (a,c) quasi-primary kinetics; (b,d) quasi-secondary kinetics.

Table 3.
Adsorption kinetic parameters of PS nanoparticles onto amorphous carbon and SiO2 surfaces at different concentrations of Na+ ions.
The results suggest that the adsorption process of PS nanoparticles onto amorphous carbon and SiO2 surfaces was predominantly controlled by physical adsorption. The adsorption rate constant k1 decreased with increasing Na+ ion concentration (Table 3). For instance, on amorphous carbon surfaces, k1 decreased from 0.060 to 0.049 and 0.038 with increasing Na+ ion concentration. Additionally, Table 3 indicates that the adsorption rate constants of PS nanoparticles onto SiO2 surfaces were significantly higher than those on amorphous carbon surfaces at identical Na+ ion concentrations. This conclusion aligns with Section 2.2. Specifically, PS nanoparticle adsorption onto amorphous carbon surfaces was slower in ultrapure water and in the presence of Na+ ions. For example, the adsorption rate constant of PS nanoparticles onto SiO2 surfaces was 0.782 at 0.1 M Na+ ion concentration, while its adsorption rate constant onto amorphous carbon surfaces was only 0.060.
4. Conclusions
Dissolved metal ions in pulp can adsorb onto mineral particle surfaces, potentially affecting mineral surface properties and subsequent collector–mineral interactions. This study utilized QCM-D to assess PS nanoparticle collector adsorption and desorption behavior onto amorphous carbon and SiO2 surfaces at varying Na+ ion concentrations. Our study yielded the following key conclusions:
(1) PS nanoparticles synthesized via emulsion polymerization were predominantly regular, spherical in shape with an average diameter around 100 nm. The hydrophobic nature of the PS nanoparticles resulted in significant adsorption onto the amorphous carbon sensor’s surface, suggesting their potential use as coal flotation collectors. However, their adsorption onto the SiO2 surface also indicated poor selectivity as flotation collectors.
(2) Upon reaching equilibrium, the adsorption capacity of PS nanoparticles onto amorphous carbon and SiO2 sensors did not significantly change upon rinsing with ultrapure water, implying that irreversible adsorption occurred. Furthermore, the adsorption mass of nanoparticles onto amorphous carbon surfaces was greater than onto SiO2 surfaces under ultrapure water conditions. It should be noted, however, that the adsorption rate of PS nanoparticles onto amorphous carbon surfaces was slower than that onto SiO2 surfaces.
(3) Similar trends were observed for PS nanoparticle adsorption onto amorphous carbon and SiO2 surfaces with increasing Na+ ion concentrations. Specifically, adsorption capacity gradually decreased, and adsorption rates also decreased. Increasing Na+ ion concentration from 0 M to 1.0 M resulted in 24.4% and 30.9% decreases in equilibrium adsorption capacities of PS nanoparticles onto amorphous carbon and SiO2 surfaces, respectively. This clearly suggests that Na+ ions hindered PS nanoparticle adsorption onto both amorphous carbon and SiO2 surfaces.
(4) The adsorption rate of PS nanoparticles onto amorphous carbon surfaces was significantly slower than onto SiO2 surfaces, both in pure water and in the presence of Na+ ions. The experimental data align more closely with the quasi-primary kinetic model for PS particle adsorption onto amorphous carbon and SiO2 surfaces. Additionally, adsorption rate constants decreased with increasing Na+ ion concentrations.
We have the following suggestions for future research: investigation of the effect of other solution conditions, such as pH and temperature, on the adsorption behavior of PS nanoparticles onto amorphous carbon and SiO2 surfaces; further investigation of other kinetics models to gain a deeper understanding of the adsorption behavior of PS nanoparticles. In this way, the adsorption behavior of nanoparticles as a flotation collector can be further understood to improve selectivity in the target minerals and to promote their industrial application in the field of flotation.
Author Contributions
Y.S., conceptualization, methodology, formal analysis, funding acquisition, and supervision; N.J., data curation and writing—original draft; X.D., supervision, funding acquisition, and writing—review and editing; Y.F., data acquisition; M.Y., methodology and writing—review and editing; P.X., writing—review and editing; Y.C., funding acquisition, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (52204275, 51820105006) and Natural Science Foundation of Henan (232300421320). This study was also funded by Open Foundation of State Key Laboratory of Mineral Processing (BGRIMM-KJSKL-2023-07) and research project supported by Shanxi Scholarship Council of China (2022-059). And The APC was funded by the National Natural Science Foundation of China (52204275).
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (52204275, 51820105006) and the Natural Science Foundation of Henan (232300421320). This study was also funded by Open Foundation of State Key Laboratory of Mineral Processing (BGRIMM-KJSKL-2023-07) and a research project supported by the Shanxi Scholarship Council of China (2022-059).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gomez-Flores, A.; Heyes, G.W.; Ilyas, S.; Kim, H. Prediction of Grade and Recovery in Flotation from Physicochemical and Operational Aspects Using Machine Learning Models. Miner. Eng. 2022, 183, 107627. [Google Scholar] [CrossRef]
- Sun, Y.; Xie, G.; Peng, Y.; Chen, Y.; Ma, G. How Does High Intensity Conditioning Affect Flotation Performance? Int. J. Coal Prep. Util. 2019, 39, 302–316. [Google Scholar] [CrossRef]
- Hu, X.; Tong, Z.; Sha, J.; Bilal, M.; Sun, Y.; Gu, R.; Ni, C.; Li, C.; Deng, Y. Effects of Flotation Reagents on Flotation Kinetics of Aphanitic (Microcrystalline) Graphite. Separations 2022, 9, 416. [Google Scholar] [CrossRef]
- Zhou, S.; Bu, X.; Wang, X.; Ni, C.; Ma, G.; Sun, Y.; Xie, G.; Bilal, M.; Alheshibri, M.; Hassanzadeh, A.; et al. Effects of Surface Roughness on the Hydrophilic Particles-Air Bubble Attachment. J. Mater. Res. Technol. 2022, 18, 3884–3893. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Dong, X.; Bu, X.; Drelich, J.W. Spreading and Adhesion Forces for Water Droplets on Methylated Glass Surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124562. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH-dependent surface charging and the points of zero charge. J. Colloid Interface Sci. 2002, 253, 77–87. [Google Scholar] [CrossRef]
- Sahasrabudhe, G.; DeIuliis, G.; Galvin, K. Hydrophobization of minerals by sorbitan mono oleate (Span® 80): Selectivity of a novel agglomeration process. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127460. [Google Scholar] [CrossRef]
- An, M.; Liao, Y.; Gui, X.; Zhao, Y.; He, Y.; Liu, Z.; Lai, Q. An Investigation of Coal Flotation Using Nanoparticles as a Collector. Int. J. Coal Prep. Util. 2020, 40, 679–690. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, M.; Li, Y.; Lau, E. Improving Micro-Fine Mineral Flotation via Micro/Nano Technologies. Sep. Sci. Technol. 2023, 58, 520–537. [Google Scholar] [CrossRef]
- Kim, H.; You, J.; Gomez-Flores, A.; Solongo, S.K.; Hwang, G.; Zhao, H.; Lee, B.; Choi, J. Malachite Flotation Using Carbon Black Nanoparticles as Collectors: Negative Impact of Suspended Nanoparticle Aggregates. Miner. Eng. 2019, 137, 19–26. [Google Scholar] [CrossRef]
- Nasirimoghaddam, S.; Mohebbi, A.; Karimi, M.; Reza Yarahmadi, M. Assessment of PH-Responsive Nanoparticles Performance on Laboratory Column Flotation Cell Applying a Real Ore Feed. Int. J. Min. Sci. Technol. 2020, 30, 197–205. [Google Scholar] [CrossRef]
- Yang, S.; Pelton, R. Nanoparticle Flotation Collectors II: The Role of Nanoparticle Hydrophobicity. Langmuir 2011, 27, 11409–11415. [Google Scholar] [CrossRef]
- Yang, S.; Pelton, R.; Abarca, C.; Dai, Z.; Montgomery, M.; Xu, M.; Bos, J.-A. Towards Nanoparticle Flotation Collectors for Pentlandite Separation. Int. J. Miner. Process. 2013, 123, 137–144. [Google Scholar] [CrossRef]
- Yang, S.; Pelton, R.; Montgomery, M.; Cui, Y. Nanoparticle Flotation Collectors III: The Role of Nanoparticle Diameter. ACS Appl. Mater. Interfaces 2012, 4, 4882–4890. [Google Scholar] [CrossRef]
- Yang, S.; Pelton, R.; Raegen, A.; Montgomery, M.; Dalnoki-Veress, K. Nanoparticle Flotation Collectors: Mechanisms Behind a New Technology. Langmuir 2011, 27, 10438–10446. [Google Scholar] [CrossRef]
- Yang, S.; Razavizadeh, B.B.M.; Pelton, R.; Bruin, G. Nanoparticle Flotation Collectors—The Influence of Particle Softness. ACS Appl. Mater. Interfaces 2013, 5, 4836–4842. [Google Scholar] [CrossRef]
- Dong, X.; Price, M.; Dai, Z.; Xu, M.; Pelton, R. Mineral-Mineral Particle Collisions during Flotation Remove Adsorbed Nanoparticle Flotation Collectors. J. Colloid Interface Sci. 2017, 504, 178–185. [Google Scholar] [CrossRef]
- Dong, X.; Marway, H.S.; Cranston, E.D.; Pelton, R.H. Relating Nanoparticle Shape and Adhesiveness to Performance as Flotation Collectors. Ind. Eng. Chem. Res. 2016, 55, 9633–9638. [Google Scholar] [CrossRef]
- Mabudi, A.; Noaparast, M.; Gharabaghi, M.; Vasquez, V.R. Polystyrene Nanoparticles as a Flotation Collector: A Molecular Dynamics Study. J. Mol. Liq. 2019, 275, 554–566. [Google Scholar] [CrossRef]
- Hajati, A.; Shafaei, S.Z.; Noaparast, M.; Farrokhpay, S.; Aslani, S. Novel Application of Talc Nanoparticles as Collector in Flotation. RSC Adv. 2016, 6, 98096–98103. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, S.; Liu, Q.; Masliyah, J.; Xu, Z. QCM-D Study of Nanoparticle Interactions. Adv. Colloid Interface Sci. 2016, 233, 94–114. [Google Scholar] [CrossRef]
- Hou, Y.; Sobhy, A.; Wang, Y. Significance of Reagents Addition Sequence on Iron Anionic Reverse Flotation and Their Adsorption Characteristics Using QCM-D. Physicochem. Probl. Miner. Process. 2021, 57, 284–293. [Google Scholar] [CrossRef]
- Kou, J.; Tao, D.; Xu, G. Fatty Acid Collectors for Phosphate Flotation and Their Adsorption Behavior Using QCM-D. Int. J. Miner. Process. 2010, 95, 1–9. [Google Scholar] [CrossRef]
- Kou, J.; Xu, S. In Situ Kinetics and Conformation Studies of Dodecylamine Adsorption onto Zinc Sulfide Using a Quartz Crystal Microbalance with Dissipation (QCM-D). Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 110–120. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Sui, H.; He, L. Understanding Desorption of Oil Fractions from Mineral Surfaces. Fuel 2018, 232, 257–266. [Google Scholar] [CrossRef]
- Molaei, N.; Bashir Wani, O.; Bobicki, E.R. A Comparative Study of Biopolymer Adsorption on Model Anisotropic Clay Surfaces Using Quartz Crystal Microbalance with Dissipation (QCM-D). J. Colloid Interface Sci. 2022, 615, 543–553. [Google Scholar] [CrossRef]
- Liu, S.; Kim, J. Application of Kevin—Voigt model in quantifying whey protein adsorption on polyethersulfone using QCM-D. JALA J. Assoc. Lab. Autom. 2009, 14, 213–220. [Google Scholar] [CrossRef]
- Liu, Z.; Hedayati, P.; Ghatkesar, M.K.; Sun, W.; Onay, H.; Groenendijk, D.; Sudhölter, E.J. Reducing anionic surfactant adsorption using polyacrylate as sacrificial agent investigated by QCM-D. J. Colloid Interface Sci. 2021, 585, 1–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).