Abstract
Flavonoids exhibit many biological properties, so it is very important to find an efficient and green method to extract them from plant materials. In this paper, DES-MAE (deep eutectic solvent-based microwave-assisted extraction) technique was developed to extract the seven major active flavonoids from Ribes mandshuricum leaves, namely, trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol. After the completion of the extraction process, macroporous adsorption resin was used for the purification of seven flavonoids. The BBD (Box–Behnken design) method combined with RSM (response surface methodology) was applied to acquire the optimal operating conditions of DES-MAE. The optimal parameters were: temperature: 54 °C, time: 10 min, extraction solvent: choline chloride/lactic acid with a 1:2 mass ratio, water content: 25%, and liquid/solid ratio: 27 mL/g. The yields of the seven target flavonoids were 4.78, 2.57, 1.25, 1.15, 0.34, 0.32, and 0.093 mg/g DW (dry weight), respectively. The direct purification of trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol in DES-MAE solution was achieved by using macroporous resin X-5. The recoveries were 87.02%, 81.37%, 79.64%, 87.13%, 97.36%, 88.08%, and 99.39%, respectively. The results showed that DES-MAE followed by MRCC (macroporous resin column chromatography) represents a promising approach to extracting and separating active components from plants.
1. Introduction
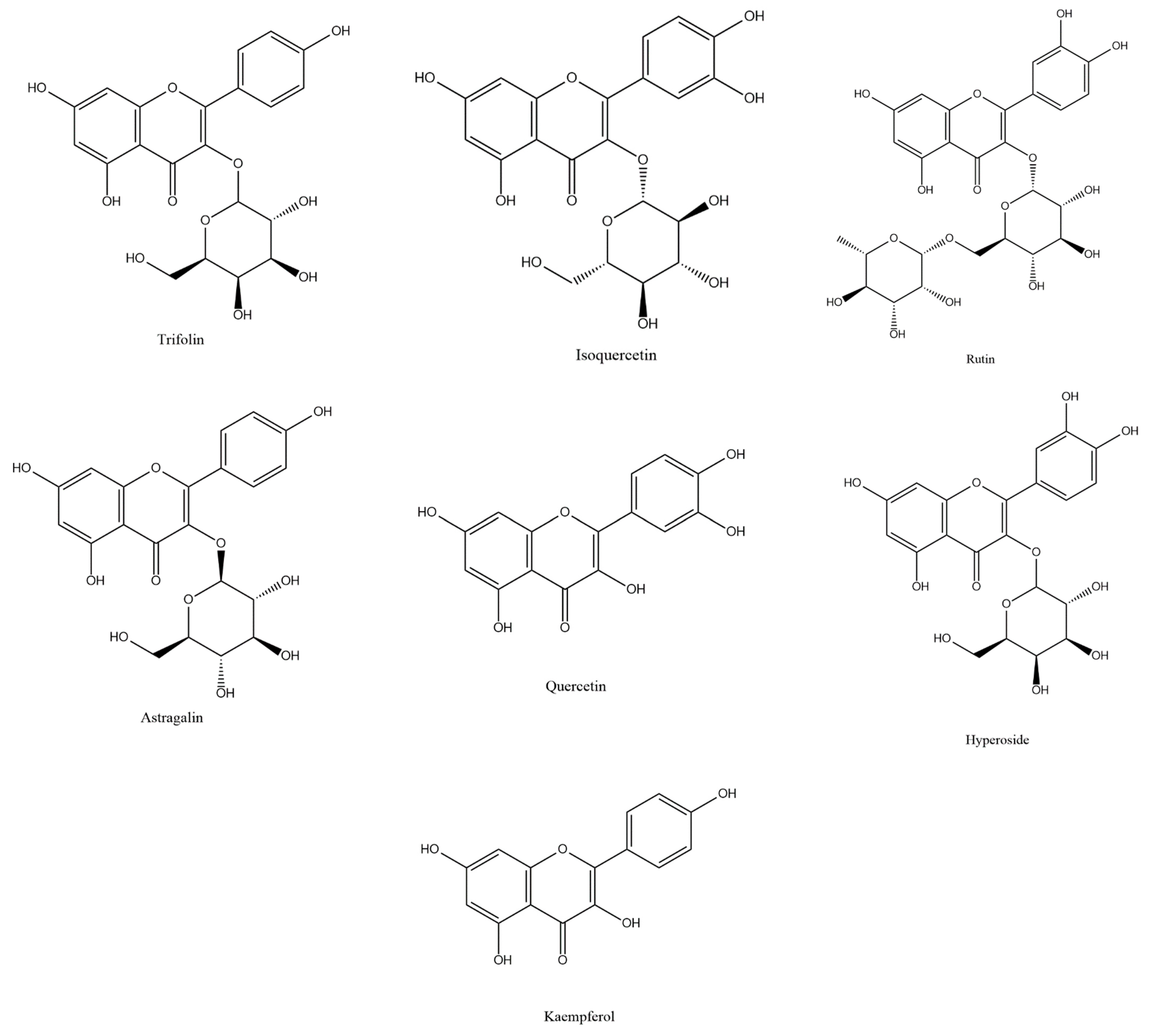
Ribes mandshuricum (Maxim.) Kom. belongs to the Grossulariaceae family, which belongs to the Saxifragales order. It is also known as Manchurian currant and is one kind of deciduous shrub [1]. It is widely distributed in China, Russia, and North Korea and is widely planted in the north of China as a landscape tree. Its berries can traditionally be used to create jellies, jams, juices, and teas and can also be used as a TCM (traditional Chinese medicine), known as Denglong-guo or Shan-Yingtao. Its berries contain abundant bioactive compounds such as flavonoids, phenolic acids, anthocyanins, and vitamins. However, as by-products, the leaves have not been fully utilized. A previous chemical study showed that in Ribes mandshuricum leaves, flavonoids were the main bioactive compounds [2]. Because of their multiple biological activities, the major flavonoids such as trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol (Figure 1) attracted more and more interest. The seven components possess lipid free radical scavenging activity, anti-viral activity, anti-inflammatory activity, hepatoprotective effects, vasodilatation, analgesic activity, peroxidation inhibitory activity, antagonistic of PAF (platelet-activating factor), antidepressant activity, etc. [2,3,4,5,6,7,8,9,10,11,12,13,14,15]. In order to study and fully utilize Ribes mandshuricum leaves’ potential medicinal values, we need an efficient method to extract and separate them from Ribes mandshuricum leaves.
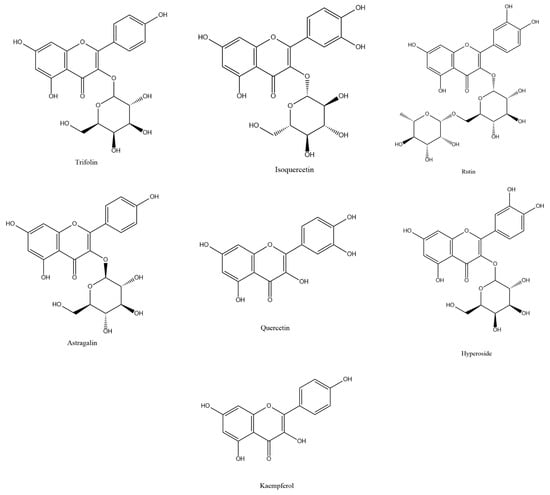
Figure 1.
Structures of trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol in Ribes mandshuricum leaves.
In recent years, “green extraction” has become the focus of attention of the scientific and industrial communities with the strengthening of human environmental protection consciousness [16,17,18]. With the development of chemistry techniques, environmentally friendly extraction techniques have caught more attention. Choline chloride/urea mixtures are one such green solvent. Abbott et al. (2003) first discovered that choline chloride and urea can be used as a solvent liquid through hydrogen bonds, with choline chloride as the hydrogen bond receptor and urea as the hydrogen bond donor. Abbott named the mixtures eutectic mixtures and put them forward as a concept, opening the way for the application of the eutectic solvent [19]. During the past decade, the application of deep eutectic solvents has developed in a variety of directions. Recently, the deep eutectic solvent has been regarded as a promising new green solvent for foodstuffs, cosmetics, and pharmaceuticals [16,20]. Studies showed that eutectic solvents can be recycled and recovered with extremely low toxicities [21]. In general, regular extraction procedures such as soxhlet extraction, maceration, and heating reflux extraction are conventionally used to extract natural products from plants. However, the shortcomings of these methods are obvious; for example, time-consuming and high energy input with unsatisfactory efficiency [22]. Compared with the above methods, microwave-assisted extraction (MAE) has a higher amount of anti-free radical components and higher yields in plant extracts [23,24,25,26,27]. Thus, it can be seen that MAE is more effective than other conventional extraction procedures. Therefore, DES-MAE is a promising method to extract the main flavonoids from Ribes mandshuricum leaves.
In this research, our goal is to exploit a green and efficient DES-MAE approach to extract the seven main bioactive flavonoids from Ribes mandshuricum leaves. In addition, we used macroporous resin, attempting the purification and separation of seven target flavonoids in extraction [28,29,30,31].
2. Materials and Methods
2.1. Plant Materials
Ribes mandshuricum leaves were collected from Heilongjiang Academy of Agricultural Sciences, P.R. China, on 6 July 2016, which was identified by Professor Bai-Lin Wang from Heilongjiang Academy of Agricultural Sciences, P.R. China. Then, the air-dried materials were pulverized by a pulverizer (HX-200A, Yongkang Hardware and Medical Instrument Plant, Yongkang, China) and thereafter passed through an 80~100 mesh sieve and stored in an airtight dryer before use.
2.2. Chemicals and Reagents
We purchased choline chloride (>98.0%) from Aladdin Chemistry Co., Ltd. (Shanghai, China) and bought citric acid (>98.0%), lactic acid (85.0–90.0%), ethylene glycol (>99.5%), 1, 4-butanediol (>99.0%), glucose (>99.0%), and ethanol from Fuchen Chemical Reagents Co. (Tianjin, China). Trifolin (>98.0%) and rutin (>98.0%) were purchased from Shanghai Yuanye Reagent Company (Shanghai, P.R. China). Astragalin (>98.0%), kaempferol (>98.0%), hyperoside (>98.0%), isoquercetin (>98.0%), and quercetin (>98.0%) were purchased from Nanjing Zelang Biological Technology Co., Ltd. (Jiangsu, China). Phosphoric acid and acetonitrile were obtained from J & K Chemical Ltd. (Beijing, China). We obtained deionized water by using a Unique-R20 water-purification system from Research Scientific Instruments Co. (Xiamen, China). In this study, all solvents were HPLC grade, and all extracts and standard solutions were filtered through 0.45 μm nylon membranes (Millipore, Burlington, MA, USA) before use.
2.3. HPLC Analysis
We analyzed the seven flavonoids in samples using an Agilent 1260 series liquid chromatography system (Agilent, Palo Alto, CA, USA) equipped with a G1316A temperature column compartment, a G1311C quaternary pump, a G1328C manual injector, and a G1314F VWD UV detector. Then, we detected the samples with a Phenomenex Curosil-PFP reverse-phase column (250 × 4.6 mm i.d., 5 μm, Phenomenex, NJ, USA). Gradient elution was carried out to analyze the samples, solvent A: phosphoric acid/water (0.5:99.5, v/v) and solvent B: acetonitrile. The gradient conditions were as follows: 10–15% (B), 0–7 min; 15–17% (B), 7–10 min; 17–17% (B), 10–25 min; 17–18% (B), 25–27 min; 18–19% (B), 27–31 min; 19–19% (B), 31–34 min; 19–26% (B), 34–38 min; 26–26% (B), 38–42 min; 26–50% (B), 42–55 min; 50–10% (B), 55–60 min. The mobile phase flow rate was 0.8 mL/min. Meanwhile, we retained the column temperature at 40 °C, and the injection volume was 20 μL. Finally, we set the detection wavelength at 360 nm.
This section validated the simultaneous detection of seven major flavonoids in Ribes mandshuricum leaves. The linearity, sensitivity, precision, and recovery of the method were tested.
2.4. Preparation of DESs
In this study, we prepared DESs by heating different compositions of mixtures to 70–80 °C with stirring in a magnetic stirrer for a certain time until a uniform liquid was formed. Table 1 showed the five different DESs systems prepared.

Table 1.
Different types of DESs (deep eutectic solvents) studied.
2.5. Sample Extraction Procedure
2.5.1. Microwave Assisted Extraction (MAE)
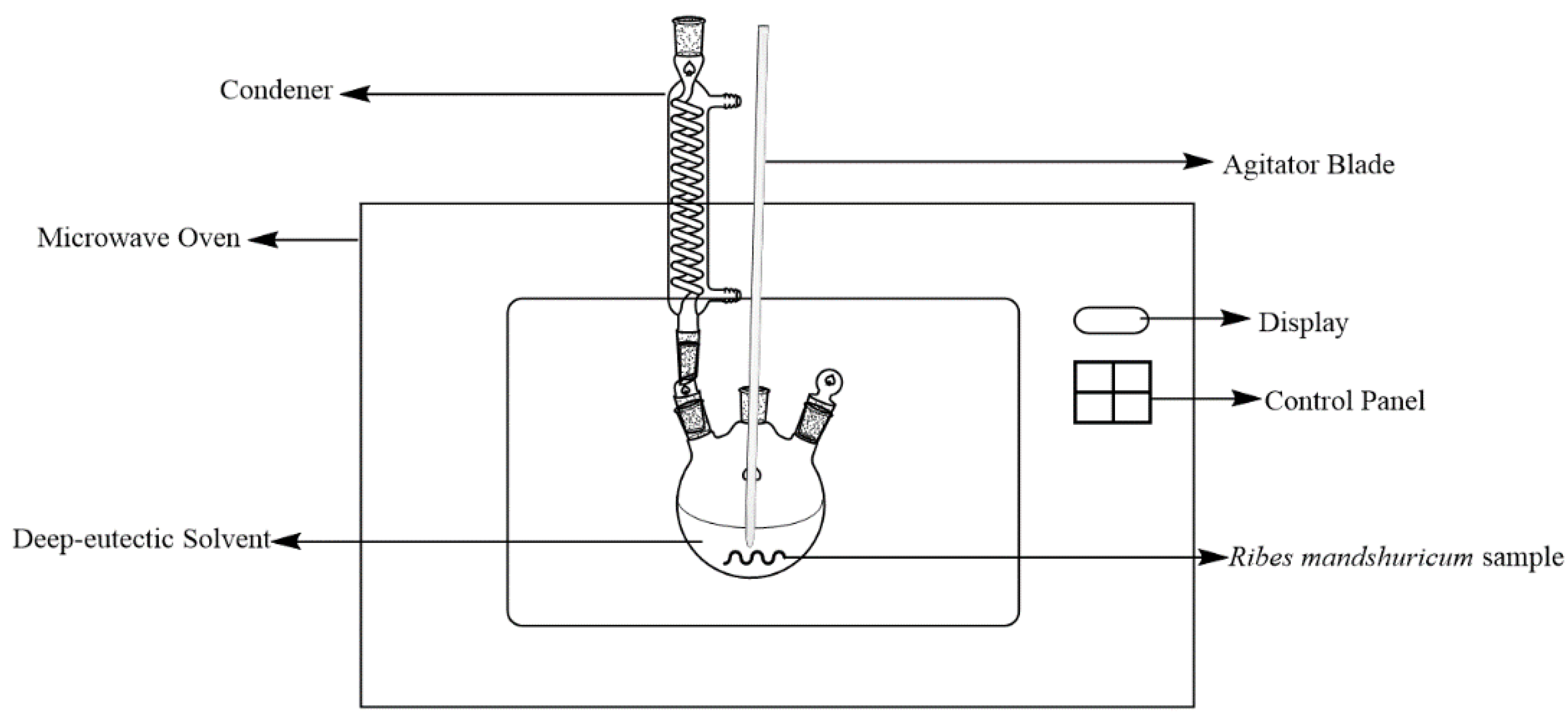
For microwave-assisted extraction, we used a modified MAS-II microwave oven, which was from Sineo Microwave Chemistry Technology CO., Ltd. (Shanghai, China). To mount a reflux condenser onto the oven, we drilled a hole in the outer casing, and to mount an agitator blade into the flask, we drilled another hole in the outer casing. Figure 2 showed a schematic figure of the MAE experimental setup.
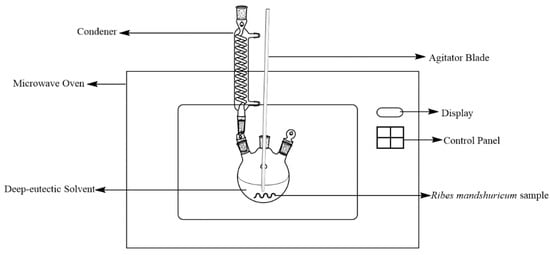
Figure 2.
Schematic figure of experimental setup for MAE.
We added a pulverized leaves sample (1.0 g) to a certain volume of deionized water and certain volumes of different kinds of DESs in a 100 mL three-neck round-bottom flask. Then, we set the flask into the microwave oven and set the microwave radiation power at 600 W. After a certain time extraction, we collected the solutions, centrifuged them, and defined the volume with deionized water to 50 mL. We filtered all the samples through a 0.45 μm nylon filter before detection by HPLC. We executed all the experiments in triplicate.
2.5.2. Common Extraction Methods
In order to compare with the MAE method, the following two conventional extraction methods were adopted. Each sample was analyzed in triplicate.
HRE (heat refluxing extraction): 1.00 g Ribes mandshuricum leaf sample was weighed precisely, then we accurately put 27 mL DES into a round-bottom flask. Afterwards, we placed the round-bottom flask into the water bath kettle with a reflux unit at 54 °C for 120 min. We filtered all the samples through a 0.45 μm nylon filter before HPLC detection.
UAE (ultrasound-assisted extraction): 1.00 g Ribes mandshuricum leaf sample was weighed precisely, then we accurately put 27 mL DES into a round-bottom flask. Thereafter, we placed the flask into an ultrasonic bath at 54 °C for 40 min. We filtered all the samples through a 0.45 μm nylon filter before HPLC detection.
2.6. Experimental Design
In this study, in order to obtain appropriate extraction conditions for the seven major flavonoids in Ribes mandshuricum leaves, the parameters of DES-MAE were examined statistically. For the single-factor experiments, 1:1, 1:2, 1:3, 1:4, and 1:5 M ratios of ChCl/lactic acid (choline chloride/lactic acid) and 10%, 25%, 40%, 55%, and 70% water contents in ChCl/lactic acid were investigated. Moreover, a 33 factorial portion BBD (Box–Behnken design) method combined with RSM (response surface methodology) was applied to acquire the optimal combination of the liquid/solid (L/S) ratio (X1), temperature (X2), and extraction time (X3); the response variable of the seven major flavonoids’ yields is expressed as Y. To match the model, we employed seventeen tests and three replicates at the center in the experiment. The average values were matched with a quadratic equation model as given below:
where Xi and Xj were the independent variables; Y was the response variable; β0, βi, βii, and βij were the intercept coefficient, linear coefficient, quadratic coefficient, and interaction coefficient, respectively.
Table 2 showed the coded and actual levels of the variables.

Table 2.
Experimental values of Box–Behnken design (BBD) for the extraction of rutin, hyperoside, isoquercetin, trifolin, astragalin, quercetin, and kaempferol.
2.7. Purification and Separation of Seven Major Flavonoids Extracted by DES-MAE
A column (26 mm × 400 mm) wet-packed with 10 g X-5 macroporous resin was used for the separation and purification of seven major flavonoids extracted by DES-MAE. Briefly, 25 mL of DES-MAE extract solution acquired under the optimum extraction conditions flowed through the X-5 macroporous resin column at 3 BV/h (bed volume/hour) flow velocity. We used 210 mL of deionized water to wash the adsorbate-laden column, and then 70% aqueous ethanol (v/v, 250 mL) at 6 BV/h flow velocity to elute. Then, we collected the elute and detected it by HPLC as mentioned above. Thereafter, we concentrated and dried the elute by vacuum-rotary evaporation and counted the recoveries of seven target flavonoids determined by HPLC. We executed all the experiments in triplicate.
2.8. Statistical Analysis
Statistica 8.0 was used to analyze the response surface contour plots of the independent variables, and Origin 9.0 was used to analyze other experimental data statistically. Moreover, we formulated experimental results in tables and figures as average ± SD (standard deviation).
3. Result and Discussion
3.1. HPLC Analysis
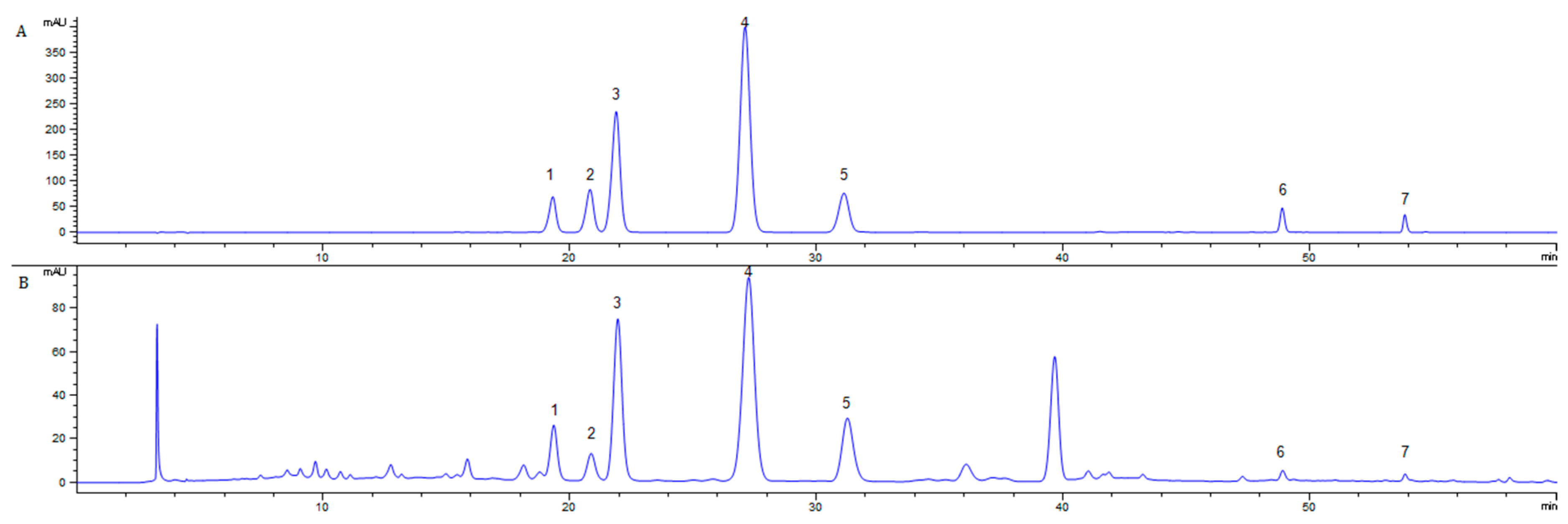
The HPLC chromatograms of the seven-target standard substance mixture (Figure 3A) and DES-MAE extraction solutions (Figure 3B) were shown in Figure 3. This result revealed that all seven target flavonoids were separated in both the standard substances mixture and Ribes mandshuricum leaf-extracting solutions.
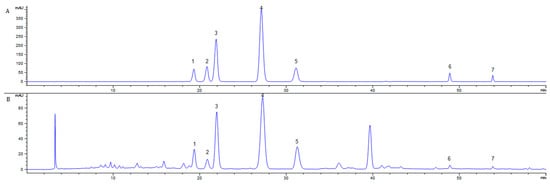
Figure 3.
HPLC chromatograms of standard mixture (A) and extracts by DES-MAE in Ribes mandshuricum leaves (B) for (1) Rutin, (2) Hyperoside, (3) Isoquercetin, (4) Trifolin, (5) Astragalin, (6) Quercetin, and (7) Kaempferol.
The measuring of the seven flavonoids in Ribes mandshuricum leaves by HPLC was validated in this portion. The performance research regulated includes: linearity, sensitivity, precision, and recovery.
3.1.1. Linearity and Sensitivity
The HPLC gradient elution method’s linearity was tested based on a matrix collocated correction model through linear fitting the data at seven concentrations by intercepts and a 1/x weighing factor. We executed the seven target flavonoids’ linear regression analyses by adopting the external standard method. The average of three replicates was regarded as the peak area value. Then, we gathered the data of calibration in Table 3. Each analyte’s linearity of the standard was well with correlation coefficients being more than 0.995. Moreover, we appraised the gradient elution method’s sensitivity using the parameters of limit of detection (LOD) and limit of quantification (LOQ), defined as the target analytes’ minimum concentrations that can be accurately measured and quantified. The LOD and LOQ were calculated based on a signal-to-noise ratio (SNR) of 3 and 10. Table 3 summarized the results. The thresholds of LOD and LOQ were extremely low, which showed that the analytical method was of a very high level of sensitivity.

Table 3.
Regression data and sensitivity for rutin, hyperoside, isoquercetin, trifolin, astragalin, quercetin, and kaempferol.
3.1.2. Precision and Recovery
The intra-day and inter-day precisions, that is, the precision of the analytical method, were evaluated according to RSDs (corresponding relative standard deviations). To determine the precision of intra-day, we analyzed the samples six times within 24 h, whereas for the precision of inter-day, we analyzed the samples three times for six consecutive days. The recovery was determined by using spiked samples of the Ribes mandshuricum leaves matrix. The results (Table 4) showed that the RSD values of intra-day and inter-day variations for RT (retention time) were in the range of 0.06–−0.67% and 0.06–−0.62%, respectively. The RSD values of the intra-day and inter-day variations for the peak area (PA) ranged from 1.81 to 3.41% and 2.45 to 3.13%. The seven target flavonoids’ average recoveries were 97.99 to 99.21% with RSD values less than 4.47% (Table 4). All the above results demonstrated that the analytical method was appropriate for the determination of trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol in Ribes mandshuricum leaves.

Table 4.
Precision and recovery of seven flavonoids (n = 6).
3.2. Screening of DESs
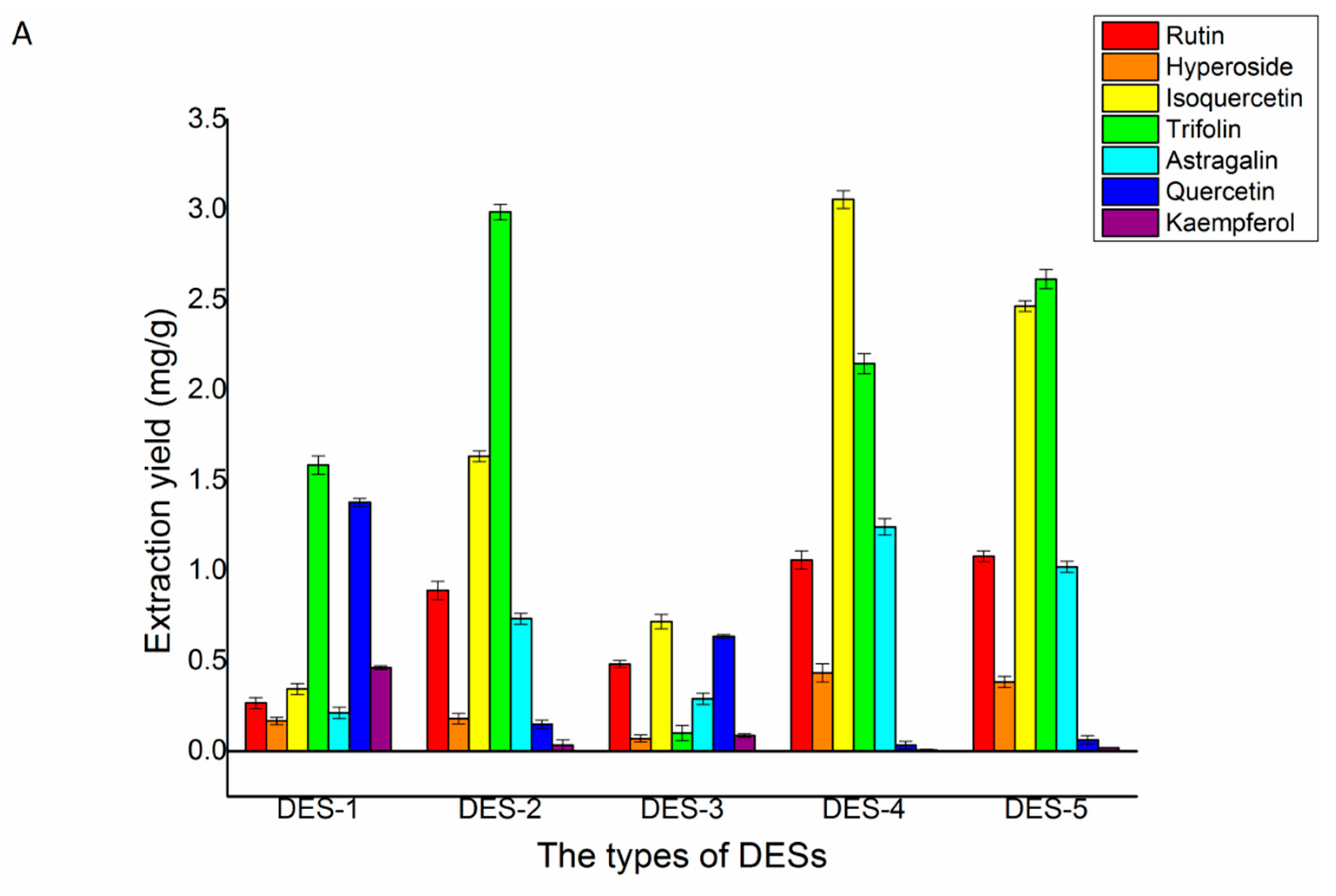
The different structures of DES determined they have different physicochemical properties. Consequently, different types of DES had different extraction yields of the seven target flavonoids. Thus, five different types of ChCl-based DESs were selected. Figure 4A depicted the extraction yields of the seven flavonoids. Compared with the other two solvents, using DES-2, DES-4, and DES-5 as solvents obtained higher concentrations of the flavonoids. Although the yield of isoquercetin in the DES-2 solvent was much lower than DES-4 and DES-5, the yields of trifolin, quercetin, and kaempferol in the DES-2 solvent were higher than DES-4 and DES-5. The different structures of DESs determined they have different physicochemical properties. The hydrogen donor of five DESs was different. The hydrogen donor of DES-1 and DES-2 was carboxylic acid. The hydrogen donor of DES-3 and DES-4 was polyalcohol. The hydrogen donor of DES-5 was sugar. Their viscosity, polarity, acidity or alkalinity, and ionic conductivity were different. The viscosity of DES-1 and DES-5 were larger than other DESs, the acidity of DES-2 was stronger than other DESs, and due to the polarity of quercetin and kaempferol, it was weaker than the other five flavonoids. The extraction yields of quercetin and kaempferol by lactic acid formed by DESs were higher than those formed by polyalcohol and sugar. In addition, the preferable extract yields of the seven flavonoids using DES-2 as a solvent may be due to their strong multi-interactions, including hydrogen bonding, π–π, and ionic/charge-charge with these flavonoids [32]. After all these comprehensive considerations, DES-2 was chosen as the extraction solvent for the extraction of the seven major flavonoids in Ribes mandshuricum leaves.
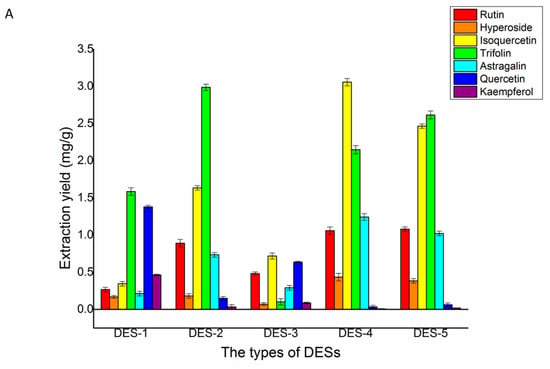
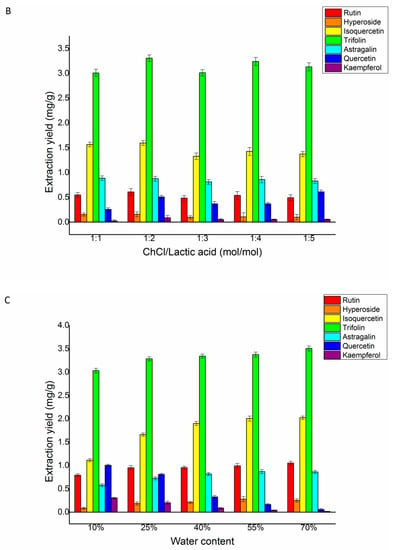
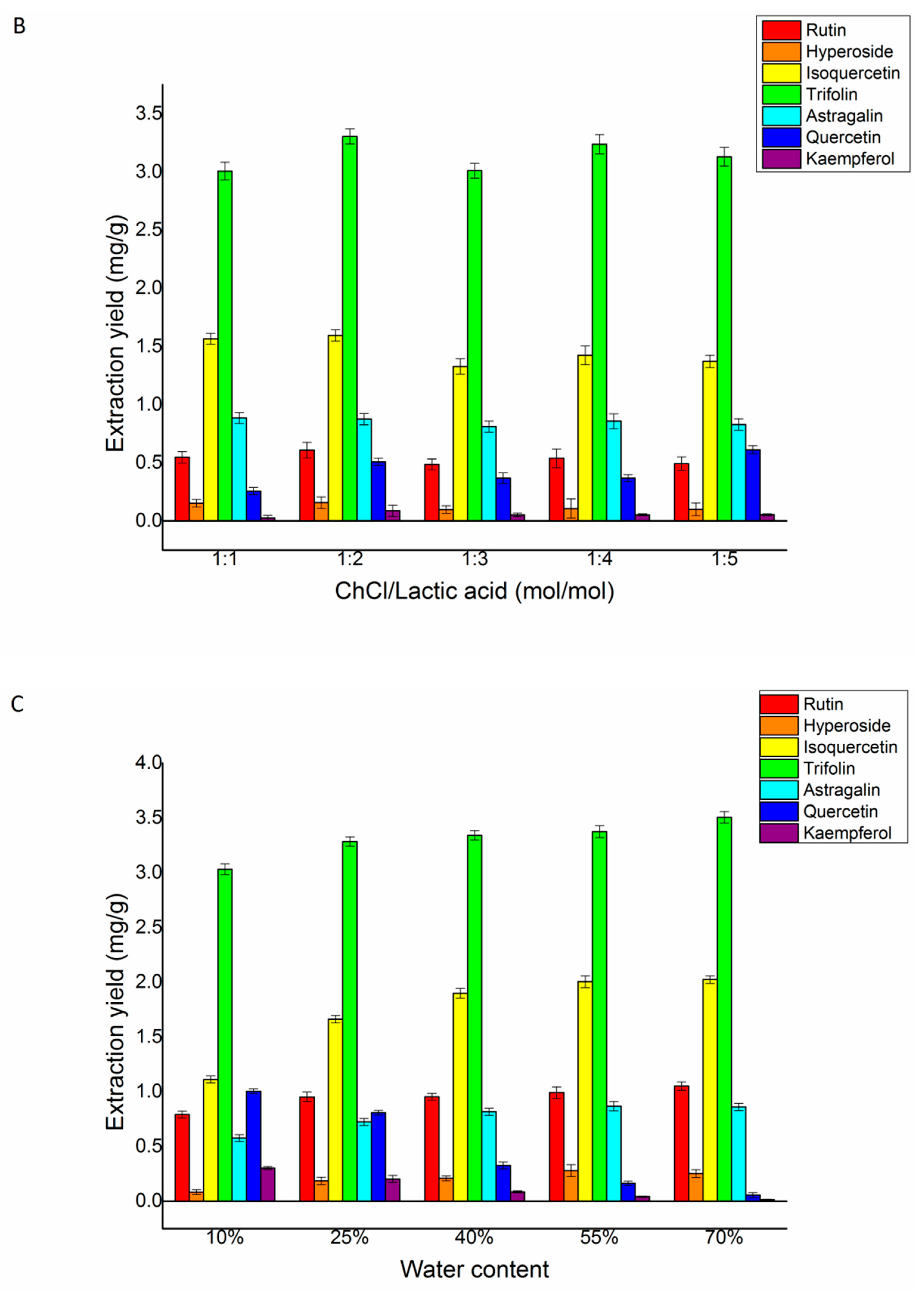
Figure 4.
Effects of different DESs (A), choline chloride/lactic acid ratio (B), and water content of DESs (C) on the extraction yield of rutin, hyperoside, isoquercetin, trifolin, astragalin, quercetin, and kaempferol in Ribes mandshuricum leaves.
3.3. Single-Factor Experiments
3.3.1. The Effect of ChCl/Lactic Acid Ratio
The effect of the ChCl/lactic acid ratio was investigated. A range of different ratios of ChCl/lactic acid (Figure 4B) was examined for extracting yields of trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol. The extraction yields of the seven target flavonoids were found to increase at first and decrease subsequently. With the increase in the ratio of the ChCl/lactic acid rate from 1:1 to 1:2 (mol/mol), the seven target flavonoids’ yields reached a stable level. Therefore, for further research, we selected DES-2 (ChCl/lactic acid) with a ratio of 1:2 (mol/mol) as the extraction solvent.
3.3.2. The Effect of Water Content of ChCl/Lactic Acid DES
Figure 4C showed the effect of the water content on the flavonoid yields. The seven target flavonoids were extracted with different water contents from 10% to 70% (v/v) in ChCl/lactic acid. Rutin and trifolin’s yields reached the highest when the water content was 25% (v/v). The yields of hyperoside, isoquercetin, and astragalin reached the highest when the content of water was 55% (v/v). The yields of quercetin and kaempferol reached a peak when the water content was 10% (v/v). In comprehensive consideration, the total yields of the seven flavonoids were maximal when the water content was 25% (v/v). Therefore, 25% (v/v) water in ChCl/lactic acid was chosen in further experiments to optimize the parameters of the L/S ratio, extraction time, and temperature.
3.4. Optimization of DES-MAE Using BBD
BBD combined with RSM was used to optimize the L/S ratio, temperature, and extraction time conditions in DES-MAE. Table 2 showed the experiment results. The mathematical regression model for the seven major flavonoids is shown below in accordance with the coded levels:
where Y was the target flavonoid’s yield (mg/g DW), X1 was the L/S ratio (mL/g), X2 was the extraction temperature (°C), and X3 was the extraction time (min).
Ytrifolin = 4.72 + 0.42X1 − 0.17X2 − 0.039X3 + 0.045X1X2 − 0.16X1X3 + 0.19X2X3 − 0.36X12 − 0.34X22 − 0.32X32
Yisoquercetin = 2.54 + 0.21X1 − 0.15X2 − 0.042X3 + 0.026X1X2 − 0.089X1X3 + 0.049X2X3 − 0.18X12 − 0.24X22 − 0.22X32
Yrutin = 1.25 + 0.078X1 − 0.044X2 − 4.51 × 10−3X3 + 8.09 × 10−3X1X2 − 0.043X1X3 + 0.050X2X3 − 0.10X12 − 0.093X22 − 0.089X32
Yastragalin = 1.15 + 0.095X1 − 0.049X2 − 0.012X3 + 3.89 × 10−3X1X2 − 0.040X1X3 + 0.037X2X3 − 0.098X12 − 0.11X22 − 0.10X32
Yquercetin = 0.38 − 0.026X1 + 0.10X2 + 0.053X3 − 0.038X1X2 − 0.013X1X3 + 0.061X2X3 − 0.17X12 − 0.10X22 − 0.12X32
Yhyperoside = 0.34 + 0.012X1 − 0.014X2 − 8.27 × 10−3X3 + 5.44 × 10−3X1X2 − 0.026X1X3 + 0.014X2X3 − 0.034X12 − 0.016X22 − 0.033X32
Ykaempferol = 0.11 + 5.02 × 10−6X1 + 0.020X2 + 0.011X3 + 4.28 × 10−4X1X2 + 1.26 × 10−4X1X3 + 0.013X2X3 − 0.057X12 − 0.036X22 − 0.039X32
Table 5 showed the ANOVA results in which the determination coefficient (R2) values of the seven major flavonoids were more than 0.90, and the p-values for the seven quadratic models were significant (p < 0.01), indicating that these quadratic models were suitable for describing the research response related to the seven target flavonoids. In addition, the t-test and p-value were used to determine the significance of each coefficient. In all seven quadratic models, the linear terms of X1, X2, and X3 and the quadratic terms of X12 and X22 were significant (Table 5).

Table 5.
ANOVA statistics of the model for extraction yields of rutin, hyperoside, isoquercetin, trifolin, astragalin, quercetin, and kaempferol.
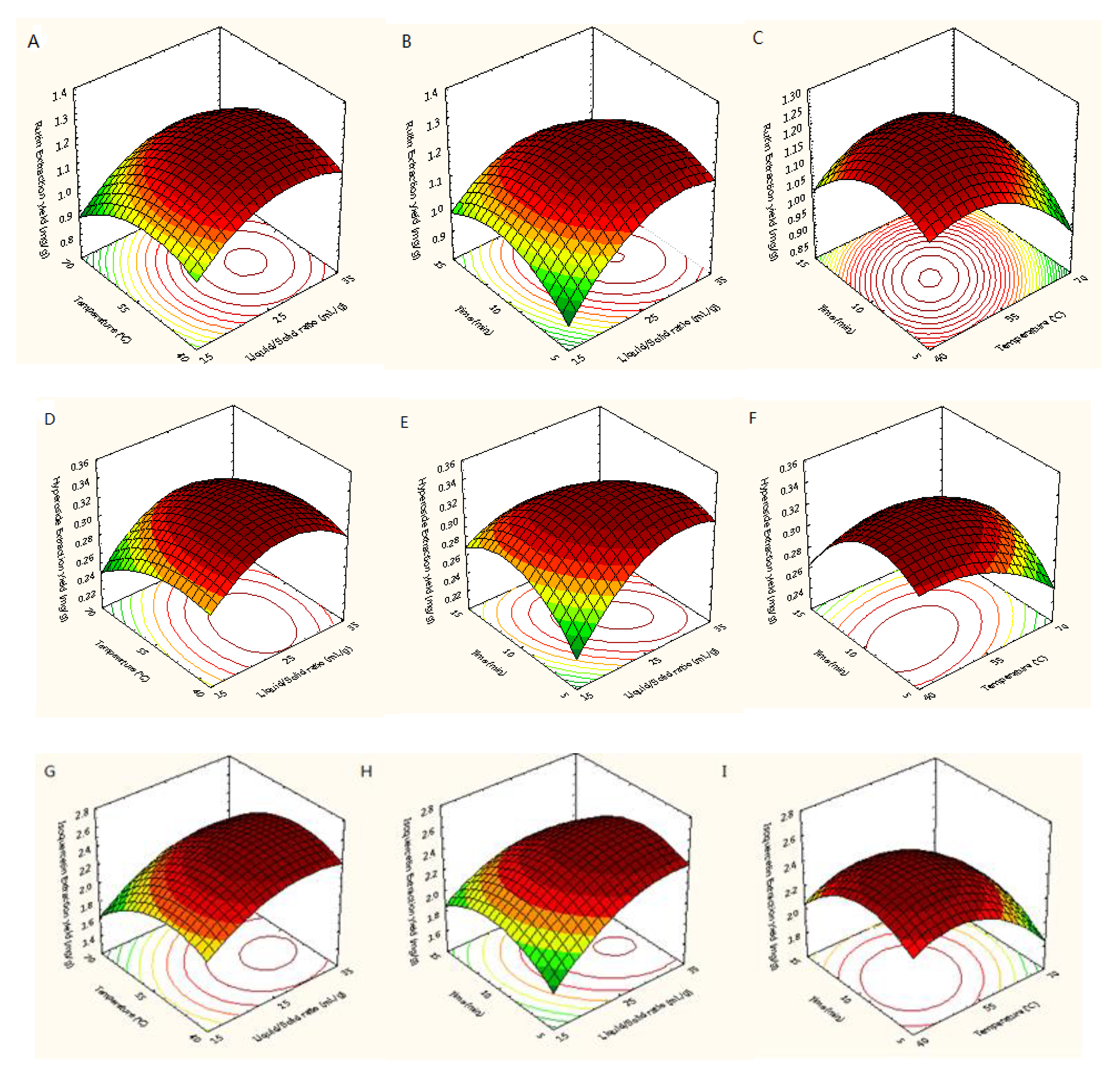
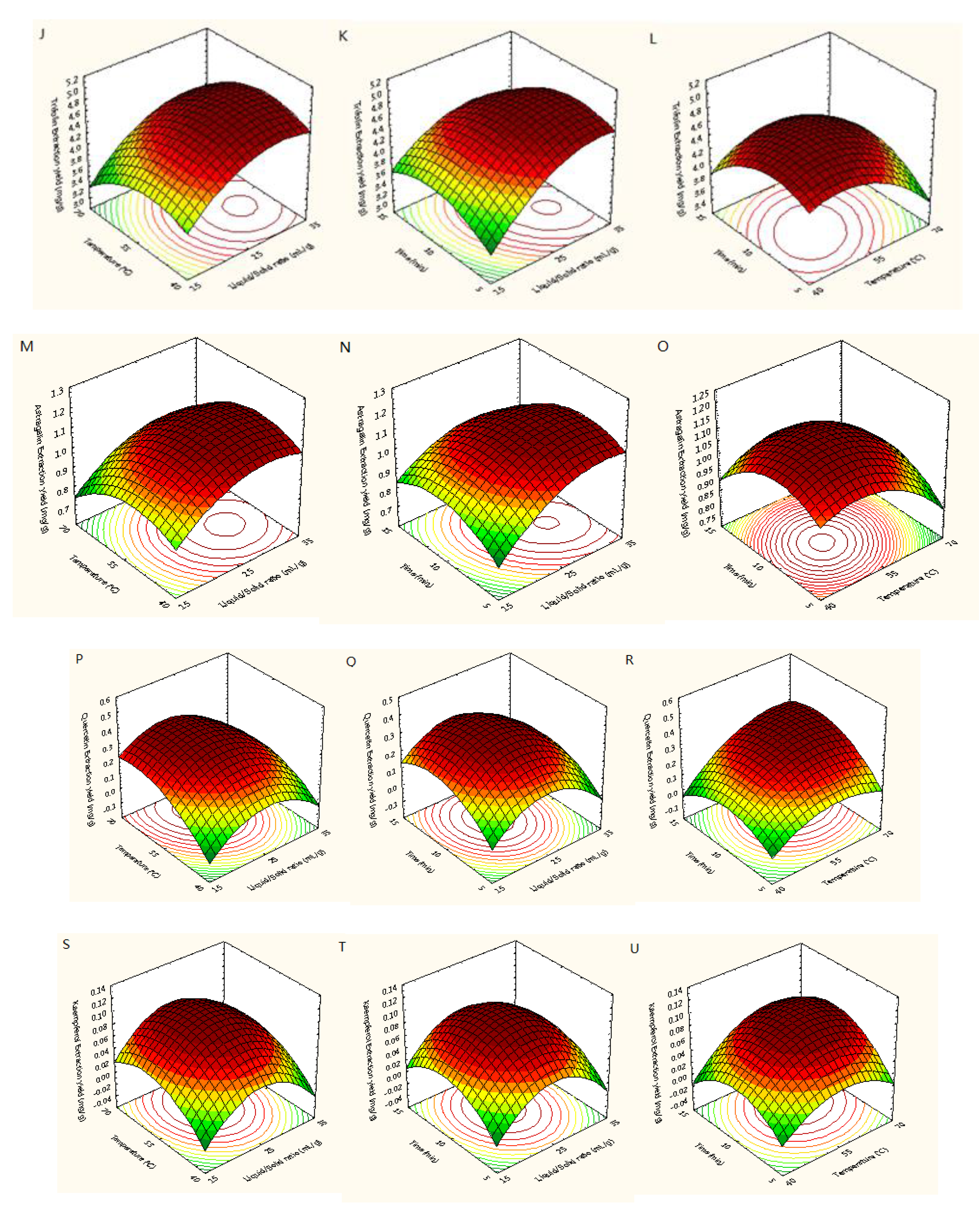
Figure 5 showed the three-dimensional (3D) response surfaces exploited from the fitted quadratic polynomial equation. In order to express the interactions between the operating variables and the responses, one independent variable remained at an intermediate level, whereas other variables changed within the defined ranges. Figure 5A,D,G,J,M,P,S showed 3D diagrams of the response surface for the yields of trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol as connected with X1 (L/S ratio) and X2 (temperature), respectively. In those plots, it was found that the yields of quercetin and kaempferol increased with increasing the L/S ratio and extraction temperature, and the extraction yields of rutin, hyperoside, trifolin, and astragalin increased with increasing the L/S ratio and decreasing temperature. Raising the L/S ratio from 15 to 32 mL/g with temperatures from 40 to 65 °C improved the extraction yields of the seven target flavonoids, but their extraction yields did not continuously improve when the L/S ratio and temperature exceeded 32 mL/g and 65 °C. As shown in Figure 5B,E,H,K,N,Q,T, the interaction between the L/S ratio and the extraction time was similar to that between the L/S ratio and the extraction temperature. The yields of trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol first increased and then decreased with increasing the L/S ratio and extraction time. Adding the extraction time from 5 to 12 min improved the extraction yields of the seven flavonoids, whereas their extraction yields did not continuously improve when the extraction time exceeded 12 min. Figure 5C, F, I, L, O, R, U showed the extraction yields of rutin, hyperoside, isoquercetin, trifolin, astragalin, quercetin, and kaempferol as related to X2 (temperature) and X3 (time), respectively. Its results were similar to that of the above.
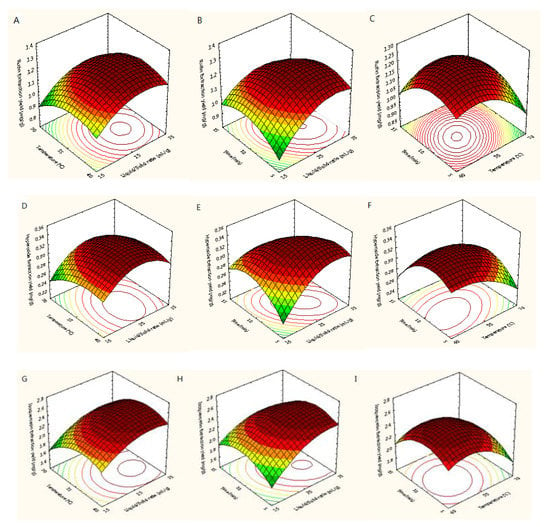
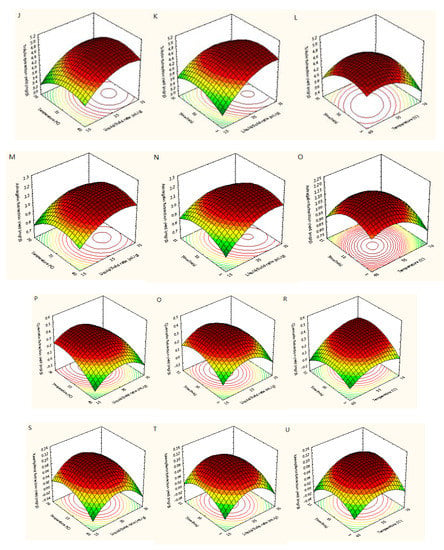
Figure 5.
Response surfaces for extraction yields of rutin (A–C), hyperoside (D–F), isoquercetin (G–I), trifolin (J–L), astragalin (M–O), quercetin (P–R), and kaempferol (S–U) from Ribes mandshuricum leaves.
In summary, by performing parameter optimization based on the model above, the optimal conditions for the simultaneous extraction of seven flavonoids from Ribes mandshuricum leaves using DES-MAE were: an L/S ratio of 27.34 mL/g, a temperature of 54.08 °C, and a time of 9.79 min. Under these optimal parameters, the predicted extraction yields of trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol were 4.81, 2.59, 1.27, 1.17, 0.36, 0.34, and 0.11 mg/g DW, respectively. Due to the parameters being difficult to execute in actual research, an L/S ratio of 27 mL/g, an extraction temperature of 54 °C, and an extraction time of 10 min were selected. In the circumstances, the predicted extraction yields for trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol were 4.81, 2.59, 1.26, 1.16, 0.35, 0.33, and 0.10 mg/g DW, respectively, which were approximate to those under optimal conditions. In conclusion, the optimal conditions for the simultaneous extraction of seven flavonoids from Ribes mandshuricum leaves using DES-MAE were: an L/S ratio of 27 mL/g, an extraction temperature of 54 °C, and an extraction time of 10 min.
3.5. Verification of the Models
In this paper, in order to validate the reliability of the predicted response values, three sequential experiments were carried out under optimal conditions. In the circumstances, the real extraction yields for trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol were 4.78, 2.57, 1.25, 1.15, 0.34, 0.32, and 0.093 mg/g DW, respectively, which were approximate to those predicted values. The RSD values between the predicted extraction yields of seven target flavonoids and the actual yields were 1.58%, 1.42%, 1.13%, 1.08%, 1.64%, 1.33%, and 1.02%, respectively. This revealed that the optimal extraction conditions obtained were practical and reliable. Therefore, it also showed that the established quadratic models were rational and reliable.
3.6. Contrast of Conventional Extraction Methods
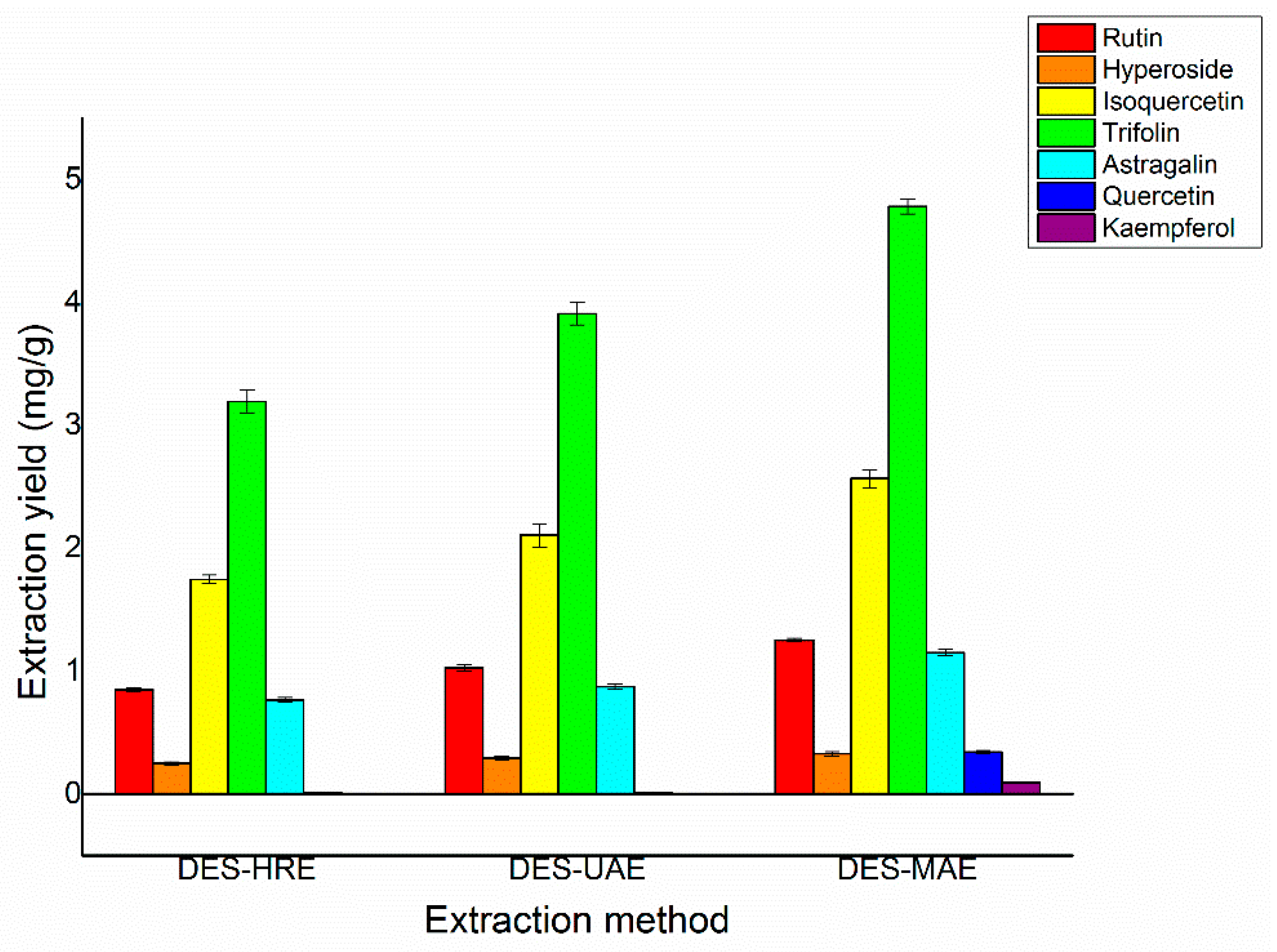
In order to further prove the yields of the seven target flavonoids by using MAE, a contrast was performed between the proposed DES-MAE method and the conventional DES-UAE and DES-HRE methods regarding their performances of trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol extraction from Ribes mandshuricum leaves (Figure 6). It showed that the extraction yields of trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol by the DES-MAE method were much better than the DES-UAE and DES-HRE methods. The obtained extraction yields of trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol were 4.78, 2.57, 1.25, 1.15, 0.34, 0.32, and 0.093 mg/g DW, respectively, which were higher than those of the DES-HRE method (3.20, 1.75, 0.85, 0.77, 0.25, 0.014, and 0.0030 mg/g DW, respectively) and the DES-UAE method (3.91, 2.11, 1.03, 0.87, 0.29, 0.011, and 0.0020 mg/g DW, respectively). In addition, compared with the conventional extraction methods, DES-MAE takes away only a small fraction of environmentally friendly extraction solvent. The proposed DES-MAE approach is a green and effective way for the extraction of trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol from Ribes mandshuricum leaves.
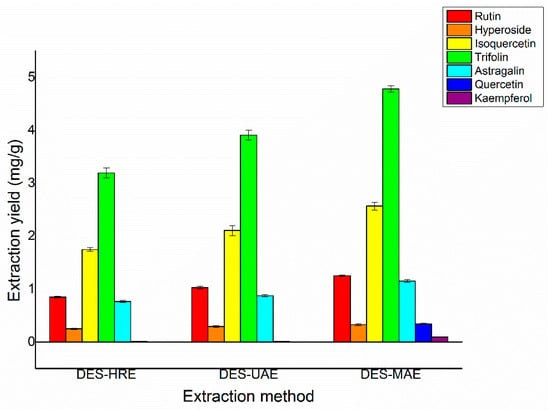
Figure 6.
Comparison of different extraction methods on the extraction yields of rutin, hyperoside, isoquercetin, trifolin, astragalin, quercetin, and kaempferol.
3.7. Purification and Separation of Seven Major Flavonoids from DES-MAE Extraction Solution
In this experiment, the direct separation of trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol from DES solution was executed by making use of macroporous resin X-5. After one run treatment with X-5 macroporous resin, the contents of trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol reached 2.85%, 1.43%, 0.69%, 0.68%, 0.23%, 0.19%, and 0.06%, respectively. The mass of the extract was 97.23 mg/g DW. Their contents were 4.30%, 2.16%, 1.04%, 1.03%, 0.34%, 0.30%, and 0.10%, respectively. The recovery yields were 87.02%, 81.37%, 79.64%, 87.13%, 97.36%, 88.08%, and 99.39%, respectively. In a word, X-5 macroporous resin could effectively purify the seven flavonoids from the extraction solution, whereas the DES solution could be removed with deionized water, and then the seven target flavonoids could be eluted with ethanol. In brief, the purification and separation of the seven major flavonoids in the DES-MAE solution were efficiently and practicably achieved by using X-5 macroporous resin.
4. Conclusions
In this paper, seven flavonoids were first detected in Ribes mandshuricum leaves. A green and efficient DES-MAE (deep eutectic solvents-based microwave-assisted extraction) technique was developed to extract the seven major active flavonoids trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol from Ribes mandshuricum leaves. The ChCl/lactic acid system turned out to be the optimal DES extraction solvent for the extraction of seven target flavonoids from Ribes mandshuricum leaves. The optimal yields were obtained under an extraction temperature of 54 °C, an extraction time of 10 min, an extraction solvent of ChCl/lactic acid with a 1:2 M ratio, a water content of 25%, and an L/S ratio of 27 mL/g. The seven target flavonoids’ yields reached 4.78, 2.57, 1.25, 1.15, 0.34, 0.32, and 0.093 mg/g DW, respectively. Based on the experimental results, the DES-MAE method obtained higher yields and took a shorter time than the common methods (HRE and UAE). Meanwhile, it was also testified that trifolin, isoquercetin, rutin, astragalin, quercetin, hyperoside, and kaempferol in a DES extract could be directly enriched and separated by X-5 resin. DES-MAE followed by macroporous resin column chromatography represents a promising approach to extracting and separating active compounds from natural plants for capable application.
Author Contributions
Supervision; validation, W.W.; writing—review and editing, S.-Q.X.; writing—original draft, L.-Y.L.; writing—review and editing, Q.-Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2022YFD2200604) and the Scientific Research Funds of Huaqiao University.
Data Availability Statement
All data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tomaszewski, D.; Zieliński, J. Epicuticular wax structures on stems and comparison between stems and leaves—A survey. Flora Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 215–232. [Google Scholar] [CrossRef]
- Nowak, A.; Czyzowska, A.; Efenberger, M.; Krala, L. Polyphenolic extracts of cherry (Prunus cerasus L.) and blackcurrant (Ribes nigrum L.) leaves as natural preservatives in meat products. Food Microbiol. 2016, 59, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, H.; Zhang, Z.; Zhang, Y.; Qiu, C.; Zhang, L.; Huang, P.; Li, F. Kaempferol slows intervertebral disc degeneration by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammation response in BMSCs. Int. Immunopharmacol. 2017, 43, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, H.; Wang, Y.; Song, F.; Yuan, Y. Inhibitory effects of quercetin on the progression of liver fibrosis through the regulation of NF-κB/IκBα, p38 MAPK, and Bcl-2/Bax signaling. Int. Immunopharmacol. 2017, 47, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Soromou, L.W.; Chen, N.; Jiang, L.; Huo, M.; Wei, M.; Chu, X.; Millimouno, F.M.; Feng, H.; Sidime, Y.; Deng, X. Astragalin attenuates lipopolysaccharide-induced inflammatory responses by down-regulating NF-κB signaling pathway. Biochem. Biophys. Res. Commun. 2012, 419, 256–261. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. Antioxidant capacity, phenolic compounds and minerals content of blackcurrant (Ribes nigrum L.) leaves as influenced by harvesting date and extraction method. Ind. Crops Prod. 2014, 53, 133–139. [Google Scholar] [CrossRef]
- Ma, Z.; Piao, T.; Wang, Y.; Liu, J. Astragalin inhibits IL-1β-induced inflammatory mediators production in human osteoarthritis chondrocyte by inhibiting NF-κB and MAPK activation. Int. Immunopharmacol. 2015, 25, 83–87. [Google Scholar] [CrossRef]
- Lian, J.-J.; Cheng, B.-F.; Gao, Y.-X.; Xue, H.; Wang, L.; Wang, M.; Yang, H.-J.; Feng, Z.-W. Protective effect of kaempferol, a flavonoid widely present in varieties of edible plants, on IL-1β-induced inflammatory response via inhibiting MAPK, Akt, and NF-κB signalling in SW982 cells. J. Funct. Foods 2016, 27, 214–222. [Google Scholar] [CrossRef]
- Li, F.; Liang, D.; Yang, Z.; Wang, T.; Wang, W.; Song, X.; Guo, M.; Zhou, E.; Li, D.; Cao, Y.; et al. Astragalin suppresses inflammatory responses via down-regulation of NF-κB signaling pathway in lipopolysaccharide-induced mastitis in a murine model. Int. Immunopharmacol. 2013, 17, 478–482. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kwon, S.-B.; Kim, M.-S.; Jin, S.W.; Ryu, H.W.; Oh, S.-R.; Yoon, D.-Y. Trifolin induces apoptosis via extrinsic and intrinsic pathways in the NCI-H460 human non-small cell lung-cancer cell line. Phytomedicine 2016, 23, 998–1004. [Google Scholar] [CrossRef]
- Kashafi, E.; Moradzadeh, M.; Mohamadkhani, A.; Erfanian, S. Kaempferol increases apoptosis in human cervical cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 89, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Guan, H.; Liu, D.; Wu, X.; Fan, M.; Han, J. Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through the MAPK and NF-κB pathways. Food Funct. 2017, 8, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Iskender, H.; Dokumacioglu, E.; Sen, T.M.; Ince, I.; Kanbay, Y.; Saral, S. The effect of hesperidin and quercetin on oxidative stress, NF-κB and SIRT1 levels in a STZ-induced experimental diabetes model. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 90, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Derksen, A.; Kühn, J.; Hafezi, W.; Sendker, J.; Ehrhardt, C.; Ludwig, S.; Hensel, A. Antiviral activity of hydroalcoholic extract from Eupatorium perfoliatum L. against the attachment of influenza A virus. J. Ethnopharmacol. 2016, 188, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Yang, J.; Fu, Y.-F.; Meng, X.-N.; Zhao, W.-D.; Hu, T.-J. Effect of total flavonoids of Spatholobus suberectus Dunn on PCV2 induced oxidative stress in RAW264.7 cells. BMC Complement. Altern. Med. 2017, 17, 244. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef]
- Zhuang, B.; Dou, L.-L.; Li, P.; Liu, E.-H. Deep eutectic solvents as green media for extraction of flavonoid glycosides and aglycones from Platycladi Cacumen. J. Pharm. Biomed. Anal. 2017, 134, 214–219. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef]
- Cui, Q.; Peng, X.; Yao, X.-H.; Wei, Z.-F.; Luo, M.; Wang, W.; Zhao, C.-J.; Fu, Y.-J.; Zu, Y.-G. Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep. Purif. Technol. 2015, 150, 63–72. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Liu, W.; Fu, Y.; Zu, Y.; Kong, Y.; Zhang, L.; Zu, B.; Efferth, T. Negative-pressure cavitation extraction for the determination of flavonoids in pigeon pea leaves by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 3841–3850. [Google Scholar] [CrossRef]
- Magnusson, M.; Yuen, A.K.; Zhang, R.; Wright, J.T.; Taylor, R.B.; Maschmeyer, T.; de Nys, R. A comparative assessment of microwave assisted (MAE) and conventional solid-liquid (SLE) techniques for the extraction of phloroglucinol from brown seaweed. Algal Res. 2017, 23, 28–36. [Google Scholar] [CrossRef]
- Dang, Y.Y.; Zhang, H.; Xiu, Z.L. Microwave-assisted aqueous two-phase extraction of phenolics from grape (Vitis vinifera) seed. J. Chem. Technol. Biotechnol. 2014, 89, 1576–1581. [Google Scholar] [CrossRef]
- Liang, H.; Wang, W.; Xu, J.; Zhang, Q.; Shen, Z.; Zeng, Z.; Li, Q. Optimization of ionic liquid-based microwave-assisted extraction technique for curcuminoids from Curcuma longa L. Food Bioprod. Process. 2017, 104, 57–65. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Huang, Y.; Wang, W. Extraction and antioxidant activity of total flavonoids from citronella. For. Eng. 2022, 38, 106–114. [Google Scholar] [CrossRef]
- Li, X.X.; Wang, F.; Cui, X.S.; Yang, F.J. Optimization of extraction technology of flavonoids from Perilla frutescens leaves by response surface methodology. For. Eng. 2019, 35, 48–54. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Gong, G.; Li, F.; Ren, H.; Liu, Y. Adsorption and desorption properties of macroporous resins for flavonoids from the extract of Chinese wolfberry (Lycium barbarum L.). Food Bioprod. Process. 2015, 93, 148–155. [Google Scholar] [CrossRef]
- Wei, Z.-F.; Wang, X.-Q.; Peng, X.; Wang, W.; Zhao, C.-J.; Zu, Y.-G.; Fu, Y.-J. Fast and green extraction and separation of main bioactive flavonoids from Radix scutellariae. Ind. Crops Prod. 2015, 63, 175–181. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Yi, Y.; Miao, Q.; Liu, S.; Zhao, F.; Cong, W.; Wang, C.; Xia, C. Purification of quercetin-3-O-sophoroside and isoquercitrin from Poacynum hendersonii leaves using macroporous resins followed by Sephadex LH-20 column chromatography. J. Chromatogr. B 2017, 1048, 56–63. [Google Scholar] [CrossRef]
- Fu, B.; Liu, J.; Li, H.; Li, L.; Lee, F.S.; Wang, X. The application of macroporous resins in the separation of licorice flavonoids and glycyrrhizic acid. J. Chromatogr. A 2005, 1089, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).