Experimental and Computational Evaluation of 1,2,4-Triazolium-Based Ionic Liquids for Carbon Dioxide Capture
Abstract
1. Introduction
2. Materials and Methods
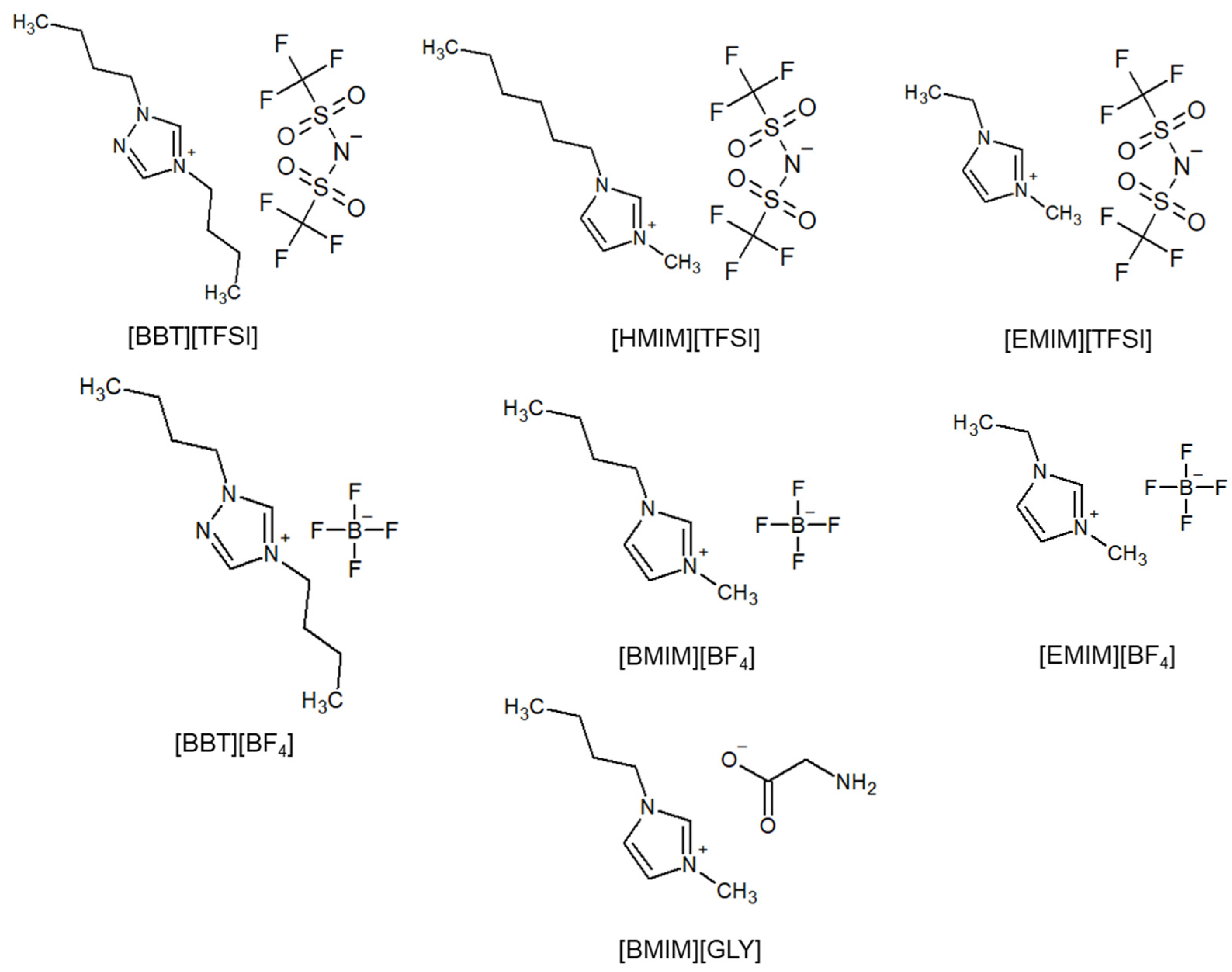
2.1. Materials
2.2. Density Measurement
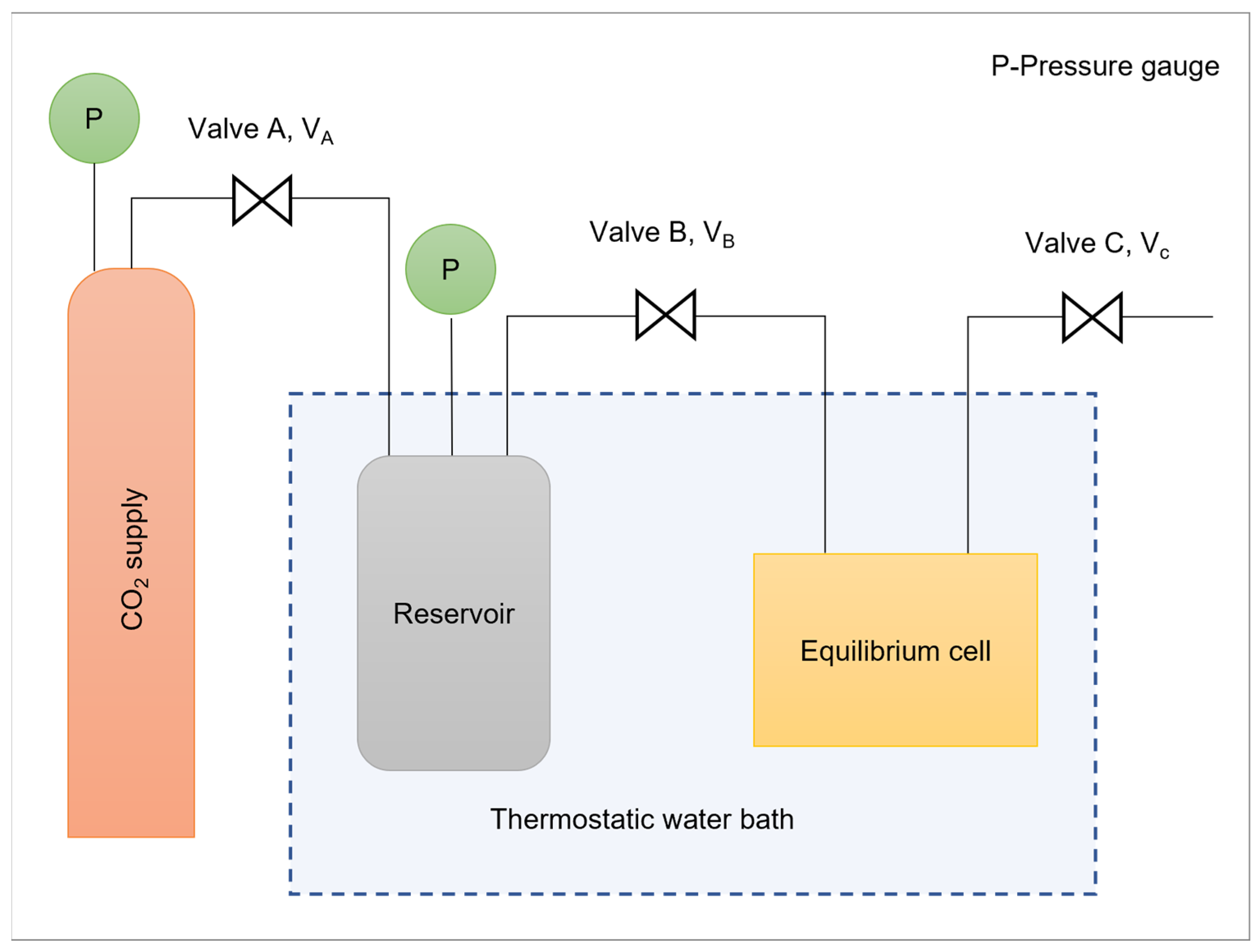
2.3. CO2 Absorption Capacity in ILs
2.4. Computational Methods
3. Results and Discussions
3.1. Density and CO2 Solubility of Different ILs
3.2. Role of the Anions on the ILs’ Capacity to Dissolve CO2
3.3. Role of the Cations on the ILs’ Capacity to Dissolve CO2
3.4. Effect of the Alkyl Chain Length on CO2 Solubility of the ILs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shama, V.M.; Swami, A.R.; Aniruddha, R.; Sreedhar, I.; Reddy, B.M. Process and engineering aspects of carbon capture by ionic liquids. J. CO2 Util. 2021, 48, 101507. [Google Scholar] [CrossRef]
- Cassia, R.; Nocioni, M.; Correa-Aragunde, N.; Lamattina, L. Climate Change and the Impact of Greenhouse Gasses: CO2 and NO, Friends and Foes of Plant Oxidative Stress. Front. Plant Sci. 2018, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J. Global carbon budget 2021. Earth Syst. Sci. Data 2022, 14, 1917–2005. [Google Scholar] [CrossRef]
- Franta, B. Early oil industry knowledge of CO2 and global warming. Nat. Clim. Chang. 2018, 8, 1024–1025. [Google Scholar] [CrossRef]
- Baker, H.S.; Millar, R.J.; Karoly, D.J.; Beyerle, U.; Guillod, B.P.; Mitchell, D.; Shiogama, H.; Sparrow, S.; Woollings, T.; Allen, M.R. Higher CO2 concentrations increase extreme event risk in a 1.5 °C world. Nat. Clim. Chang. 2018, 8, 604–608. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. Materials Science for Energy Technologies. Mater. Sci. Technol. 2019, 2, 607–623. [Google Scholar]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 2012, 5, 7281–7305. [Google Scholar] [CrossRef]
- Sinha, R.K.; Chaturvedi, N.D. A review on carbon emission reduction in industries and planning emission limits. Renew. Sustain. Energy Rev. 2019, 114, 109304. [Google Scholar] [CrossRef]
- Peter, S.C. Reduction of CO2 to Chemicals and Fuels: A Solution to Global Warming and Energy Crisis. ACS Energy Lett. 2018, 3, 1557–1561. [Google Scholar] [CrossRef]
- Benhelal, E.; Shamsaei, E.; Rashid, M.I. Challenges against CO2 abatement strategies in cement industry: A review. J. Environ. Sci. 2021, 104, 84–101. [Google Scholar] [CrossRef]
- Gür, T.M. Carbon dioxide emissions, capture, storage and utilization: Review of materials, processes and technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Langevin, J.; Harris, C.B.; Reyna, J.L. Assessing the potential to reduce US building CO2 emissions 80% by 2050. Joule 2019, 3, 2403–2424. [Google Scholar] [CrossRef]
- Hong, W.Y. A techno-economic review on carbon capture, utilisation and storage systems for achieving a net-zero CO2 emissions future. Carbon Capture Sci. Technol. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, N.; Wang, M.; Wu, T.; Wei, W.; Pang, C.H. The integration of hydrogenation and carbon capture utilisation and storage technology: A potential low-carbon approach to chemical synthesis in China. Int. J. Energy Res. 2021, 45, 19789–19818. [Google Scholar] [CrossRef]
- Ghiat, I.; Al-Ansari, T. A review of carbon capture and utilisation as a CO2 abatement opportunity within the EWF nexus. J. CO2 Util. 2021, 45, 101432. [Google Scholar] [CrossRef]
- Berstad, D.; Anantharaman, R.; Nekså, P. Low-temperature CO2 capture technologies–Applications and potential. Int. J. Refrig. 2013, 36, 1403–1416. [Google Scholar] [CrossRef]
- Timmerhaus, K.D.; Reed, R.P. Cryogenic Engineering: Fifty Years of Progress; Springer: New York, NY, USA, 2007. [Google Scholar]
- Font-Palma, C.; Cann, D.; Udemu, C. Review of cryogenic carbon capture innovations and their potential applications. C 2021, 7, 58. [Google Scholar] [CrossRef]
- Meng, F.; Meng, Y.; Ju, T.; Han, S.; Lin, L.; Jiang, J. Research progress of aqueous amine solution for CO2 capture: A review. Renew. Sustain. Energy Rev. 2022, 168, 112902. [Google Scholar] [CrossRef]
- Muldoon, M.J.; Aki, S.N.; Anderson, J.L.; Dixon, J.K.; Brennecke, J.F. Improving carbon dioxide solubility in ionic liquids. J. Phys. Chem. B 2007, 111, 9001–9009. [Google Scholar] [CrossRef]
- Sun, Y.; Schemann, A.; Held, C.; Lu, X.; Shen, G.; Ji, X. Modeling thermodynamic derivative properties and gas solubility of ionic liquids with ePC-SAFT. Ind. Eng. Chem. Res. 2019, 58, 8401–8417. [Google Scholar] [CrossRef]
- Dębski, B.; Hänel, A.; Aranowski, R.; Stolte, S.; Markiewicz, M.; Veltzke, T.; Cichowska-Kopczyńska, I. Thermodynamic interpretation and prediction of CO2 solubility in imidazolium ionic liquids based on regular solution theory. J. Mol. Liq. 2019, 291, 110477. [Google Scholar] [CrossRef]
- Moosanezhad-Kermani, H.; Rezaei, F.; Hemmati-Sarapardeh, A.; Band, S.S.; Mosavi, A. Modeling of carbon dioxide solubility in ionic liquids based on group method of data handling. Eng. Appl. Comput. Fluid Mech. 2021, 15, 23–42. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Lu, J.G.; Li, X.; Zhao, Y.X.; Ma, H.L.; Wang, L.F.; Wang, X.Y.; Yu, Y.F.; Shen, T.Y.; Xu, H.; Zhang, Y.T. CO2 capture by ionic liquid membrane absorption for reduction of emissions of greenhouse gas. Environ. Chem. Lett. 2019, 17, 1031–1038. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Palomar, J. Process analysis overview of ionic liquids on CO2 chemical capture. Chem. Eng. J. 2020, 390, 124509. [Google Scholar] [CrossRef]
- Ghandi, K. A review of ionic liquids, their limits and applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, E.; Li, Y.; Bernards, M.T.; He, Y.; Shi, Y. CO2 capture with polyamine-based protic ionic liquid functionalized mesoporous silica. J. CO2 Util. 2019, 34, 606–615. [Google Scholar] [CrossRef]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: An overview. J. Mol. Liq. 2017, 233, 403–414. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Zhang, Y.; Liu, X.; Han, J.; Li, X.; Liu, Z.; Liu, S.; Choy, W.C. Water-soluble triazolium ionic-liquid-induced surface self-assembly to enhance the stability and efficiency of perovskite solar cells. Adv. Funct. Mater. 2019, 29, 1900417. [Google Scholar] [CrossRef]
- Aghel, B.; Janati, S.; Wongwises, S.; Shadloo, M.S. Review on CO2 capture by blended amine solutions. Int. J. Greenh. Gas Control 2022, 119, 103715. [Google Scholar] [CrossRef]
- Solangi, N.H.; Hussin, F.; Anjum, A.; Sabzoi, N.; Mazari, S.A.; Mubarak, N.M.; Aroua, M.K.; Siddiqui, M.T.H.; Qureshi, S.S. A review of encapsulated ionic liquids for CO2 capture. J. Mol. Liq. 2023, 374, 121266. [Google Scholar] [CrossRef]
- Neumann, J.G.; Stassen, H. Anion effect on gas absorption in imidazolium-based ionic liquids. J. Chem. Inf. Model. 2020, 60, 661–666. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Zhang, Z.; Fei, W.; Luo, M.; Zhao, Y. Experimental and simulation study of CO2 and H2S solubility in propylene carbonate, imidazolium-based ionic liquids and their mixtures. J. Chem. Thermodyn. 2020, 142, 106017. [Google Scholar] [CrossRef]
- Yim, J.H.; Park, K.W.; Oh, B.K.; Lim, J.S. CO2 Solubility in 1,1,2,2-Tetrafluoroethanesulfonate Anion-Based Ionic Liquids: [EMIM][TFES], [BMIM][TFES], and [BNMIM][TFES]. J. Chem. Eng. Data 2020, 65, 617–627. [Google Scholar] [CrossRef]
- Darabi, M.; Pahlavanzadeh, H. Mathematical modeling of CO2 membrane absorption system using ionic liquid solutions. Chem. Eng. Process. 2020, 147, 107743. [Google Scholar] [CrossRef]
- Huang, Q.; Jing, G.; Zhou, X.; Lv, B.; Zhou, Z. A novel biphasic solvent of amino-functionalized ionic liquid for CO2 capture: High efficiency and regenerability. J. CO2 Util. 2018, 25, 22–30. [Google Scholar] [CrossRef]
- Song, Z.; Shi, H.; Zhang, X.; Zhou, T. Prediction of CO2 solubility in ionic liquids using machine learning methods. Chem. Eng. Sci. 2020, 223, 115752. [Google Scholar] [CrossRef]
- Venkatraman, V.; Alsberg, B.K. Predicting CO2 capture of ionic liquids using machine learning. J. CO2 Util. 2017, 21, 162–168. [Google Scholar] [CrossRef]
- Dashti, A.; Harami, H.R.; Rezakazemi, M.; Shirazian, S. Estimating CH4 and CO2 solubilities in ionic liquids using computational intelligence approaches. J. Mol. Liq. 2018, 271, 661–669. [Google Scholar] [CrossRef]
- Jiang, W.; Li, X.; Gao, G.; Wu, F.; Luo, C.; Zhang, L. Advances in applications of ionic liquids for phase change CO2 capture. Chem. Eng. J. 2022, 445, 136767. [Google Scholar] [CrossRef]
- Anthony, J.L.; Anderson, J.L.; Maginn, E.J.; Brennecke, J.F. Anion effects on gas solubility in ionic liquids. J. Phys. Chem. B 2005, 109, 6366–6374. [Google Scholar] [CrossRef] [PubMed]
- Almantariotis, D.; Stevanovic, S.; Fandino, O.; Pensado, A.S.; Padua, A.A.; Coxam, J.Y.; Costa Gomes, M.F. Absorption of Carbon Dioxide, Nitrous Oxide, Ethane and Nitrogen by 1-Alkyl-3-methylimidazolium (Cnmim, n = 2,4,6) Tris(pentafluoroethyl)trifluorophosphate Ionic Liquids (eFAP). J. Phys. Chem. B 2012, 116, 7728–7738. [Google Scholar] [CrossRef] [PubMed]
- Noorani, N.; Mehrdad, A. CO2 solubility in some amino acid-based ionic liquids: Measurement, correlation and DFT studies. Fluid Phase Equilib. 2020, 517, 112591. [Google Scholar] [CrossRef]
- Wei, L.; Guo, R.; Tang, Y.; Zhu, J.; Liu, M.; Chen, J.; Xu, Y. Properties of aqueous amine based protic ionic liquids and its application for CO2 quick capture. Sep. Purif. Technol. 2020, 239, 116531. [Google Scholar] [CrossRef]
- Aki, S.N.; Mellein, B.R.; Saurer, E.M.; Brennecke, J.F. High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids. J. Phys. Chem. B 2004, 108, 20355–20365. [Google Scholar] [CrossRef]
- Safavi, M.; Ghotbi, C.; Taghikhani, V.; Jalili, A.H.; Mehdizadeh, A. Study of the solubility of CO2, H2S and their mixture in the ionic liquid 1-octyl-3-methylimidazolium hexafluorophosphate: Experimental and modelling. J. Chem. Thermodyn. 2013, 65, 220–232. [Google Scholar] [CrossRef]
- Fukui, K. Theory of Orientation and Stereoselection; Springer: Berlin/Heidelberg, Germany, 1975. [Google Scholar]
- Liu, C.; Li, Y.; Takao, M.; Toyao, T.; Maeno, Z.; Kamachi, T.; Hinuma, Y.; Takigawa, I.; Shimizu, K.I. Frontier molecular orbital based analysis of solid–adsorbate interactions over group 13 metal oxide surfaces. J. Phys. Chem. C 2020, 124, 15355–15365. [Google Scholar] [CrossRef]
- Mohammed, S.A.S.; Yahya, W.Z.N.; Bustam, M.A.; Kibria, M.G.; Masri, A.N.; Kamonwel, N.D.M. Study of the ionic liquids’ electrochemical reduction using experimental and computational methods. J. Mol. Liq. 2022, 359, 119219. [Google Scholar] [CrossRef]
- Palgunadi, J.; Palgunadi, J.; Kang, J.E.; Cheong, M.S.; Kim, H.G.; Lee, H.J.; Kim, H.S. Fluorine-Free Imidazolium-Based Ionic Liquids with a Phosphorous-Containing Anion as Potential CO2 Absorbents. Bull. Korean Chem. Soc. 2009, 30, 1749–1754. [Google Scholar]
- Li, C.; Feng, S.; Xu, L.; Peng, X.; Liu, W. Solubilities of CO2, O2 and N2 in rocket propellant 5 under low pressure. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Deng, D. Solubilities and Thermodynamic Properties of CO2 in Four Azole-Based Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63, 2091–2096. [Google Scholar] [CrossRef]
- Jalili, A.H.; Shokouhi, M.; Maurer, G.; Zoghi, A.T.; Ahari, J.S.; Forsat, K. Measuring and modelling the absorption and volumetric properties of CO2 and H2S in the ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate. J. Chem. Thermodyn. 2019, 131, 544–556. [Google Scholar] [CrossRef]
- Leinweber, A.; Mu, K. Solubility of carbon dioxide, methane, and nitrogen in liquid dibenzyl toluene. J. Chem. Eng. Data 2018, 63, 3527–3533. [Google Scholar] [CrossRef]
- Losetty, V.; Matheswaran, P.; Wilfred, C.D. Synthesis, thermophysical properties and COSMO-RS study of DBU based protic ionic liquids. J. Chem. Thermodyn. 2017, 105, 151–158. [Google Scholar] [CrossRef]
- Shahrom, M.S.R.; Wilfred, C.D.; MacFarlane, D.R.; Vijayraghavan, R.; Chong, F.K. Amino acid based poly (ionic liquid) materials for CO2 capture: Effect of anion. J. Mol. Liq. 2019, 276, 644–652. [Google Scholar] [CrossRef]
- Khan, H.W.; Elgharbawy, A.A.; Bustam, A.; Moniruzzaman, M. Design and selection of ionic liquids via COSMO for pharmaceuticals and medicine. In Application of Ionic Liquids in Drug Delivery; Springer: Singapore, 2021; pp. 137–164. [Google Scholar]
- Qin, C.; Gao, H.; Liu, X.; Li, X.; Xie, Y.; Bai, Y.; Nie, Y. The dissolution of human hair using ionic liquids through COSMO-RS predication and experimental verification. J. Mol. Liq. 2022, 349, 118094. [Google Scholar] [CrossRef]
- Palomar, J.; Gonzalez-Miquel, M.; Polo, A.; Rodriguez, F. Understanding the Physical Absorption of CO2 in Ionic Liquids Using the COSMO-RS Method. Ind. Eng. Chem. Res. 2011, 50, 3452–3463. [Google Scholar] [CrossRef]
- Villarroel, E.; Olea, F.; Araya-López, C.; Merlet, G.; Cabezas, R.; Romero, J.; Quijada-Maldonado, E. COSMO-RS evaluation as a tool for prediction of solvents in dispersive liquid-phase microextraction: Evaluation of conventional solvents and ionic liquids as extractants. J. Mol. Liq. 2022, 354, 118861. [Google Scholar] [CrossRef]
- Balchandani, S.; Singh, R. COSMO-RS Analysis of CO2 Solubility in N-Methyldiethanolamine, Sulfolane, and 1-Butyl-3-methyl-imidazolium Acetate Activated by 2-Methylpiperazine for Postcombustion Carbon Capture. ACS Omega 2020, 6, 747–761. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Abranches, D.O.; Ferreira, A.M.; Mafra, M.R.; Coutinho, J.A. Using COSMO-RS to predict solvatochromic parameters for deep eutectic solvents. ACS Sustain. Chem. Eng. 2021, 9, 10240–10249. [Google Scholar] [CrossRef]
- Bououden, W.; Benguerba, Y.; Darwish, A.S.; Attoui, A.; Lemaoui, T.; Balsamo, M.; Erto, A.; Alnashef, I.M. Surface adsorption of Crizotinib on carbon and boron nitride nanotubes as Anti-Cancer drug Carriers: COSMO-RS and DFT molecular insights. Journal of Molecular Liquids. J. Mol. Liq. 2021, 338, 116666. [Google Scholar] [CrossRef]
- Sosa, J.E.; Santiago, R.; Redondo, A.E.; Avila, J.; Lepre, L.F.; Gomes, M.C.; Araújo, J.M.; Palomar, J.; Pereiro, A.B. Design of ionic liquids for fluorinated gas absorption: COSMO-RS selection and solubility experiments. Environ. Sci. Technol. 2022, 56, 5898–5909. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, Z.; Zhang, Y.; Liu, Y.; Wang, H. Screening of alternative solvent ionic liquids for artemisinin: COSMO-RS prediction and experimental verification. J. Mol. Liq. 2021, 338, 116778. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A. COSMOtherm; Release 19.0.1; COSMOlogic GmbH & Co. Kg.: Leverkusen, Germany, 2013. [Google Scholar]
- Klamt, A. COSMO-RS: From Quantum Chemistry to Fluid Phase Thermodynamics and Drug Design; Elsevier BV: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Klamt, A. Conductor-like screening model for real solvents: A new approach to the quantitative calculation of solvation phenomena. J. Phys. Chem. 1995, 99, 2224–2235. [Google Scholar] [CrossRef]
- Klamt, A.; Jonas, V.; Bürger, T.; Lohrenz, J.C. Refinement and parametrization of COSMO-RS. J. Phys. Chem A 1998, 102, 5074–5085. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, T.; Zhang, X.; Zhang, S.; Liang, X.; Gani, R.; Kontogeorgis, G.M. Application of COSMO-RS and UNIFAC for ionic liquids based gas separation. Chem. Eng. Sci. 2018, 192, 816–828. [Google Scholar] [CrossRef]
- Lei, Z.; Yuan, J.; Zhu, J. Solubility of CO2 in Propanone, 1-Ethyl-3-methylimidazolium Tetrafluoroborate, and Their Mixtures. J. Chem. Eng. Data 2010, 55, 4190–4194. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, W.Y.; Jang, J.H.; Yoo, K.P.; Lee, C.S. Solubility measurement and prediction of carbon dioxide in ionic liquids. Fluid Phase Equilib. 2005, 228, 439–445. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Khanna, A. CO2 absorption studies in amino acid-anion based ionic liquids. Chem. Eng. J. 2015, 273, 268–276. [Google Scholar] [CrossRef]
- Onofri, S.; Adenusi, H.; Le Donne, A.; Bodo, E. CO2 Capture in Ionic Liquids Based on Amino Acid Anions With Protic Side Chains: A Computational Assessment of Kinetically Efficient Reaction Mechanisms. ChemistryOpen 2020, 9, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.S.; Tedstone, J.M.; Danielsen, S.P.; Hindman, M.S.; Irvin, A.C.; Bara, J.E. Free volume as the basis of gas solubility and selectivity in imidazolium-based ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 5565–5576. [Google Scholar] [CrossRef]
- Greb, L. Lewis superacids: Classifications, candidates, and applications. Chem. Eur. J. 2018, 24, 17881–17896. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Jiang, Y.; Xiong, Y. Photocatalytic CO2 conversion: What can we learn from conventional CO x hydrogenation? Chem. Soc. Rev. 2020, 49, 6579–6591. [Google Scholar] [CrossRef] [PubMed]
- Klamt, A. COSMO-RS for aqueous solvation and interfaces. Fluid Phase Equilib 2016, 407, 152–158. [Google Scholar] [CrossRef]

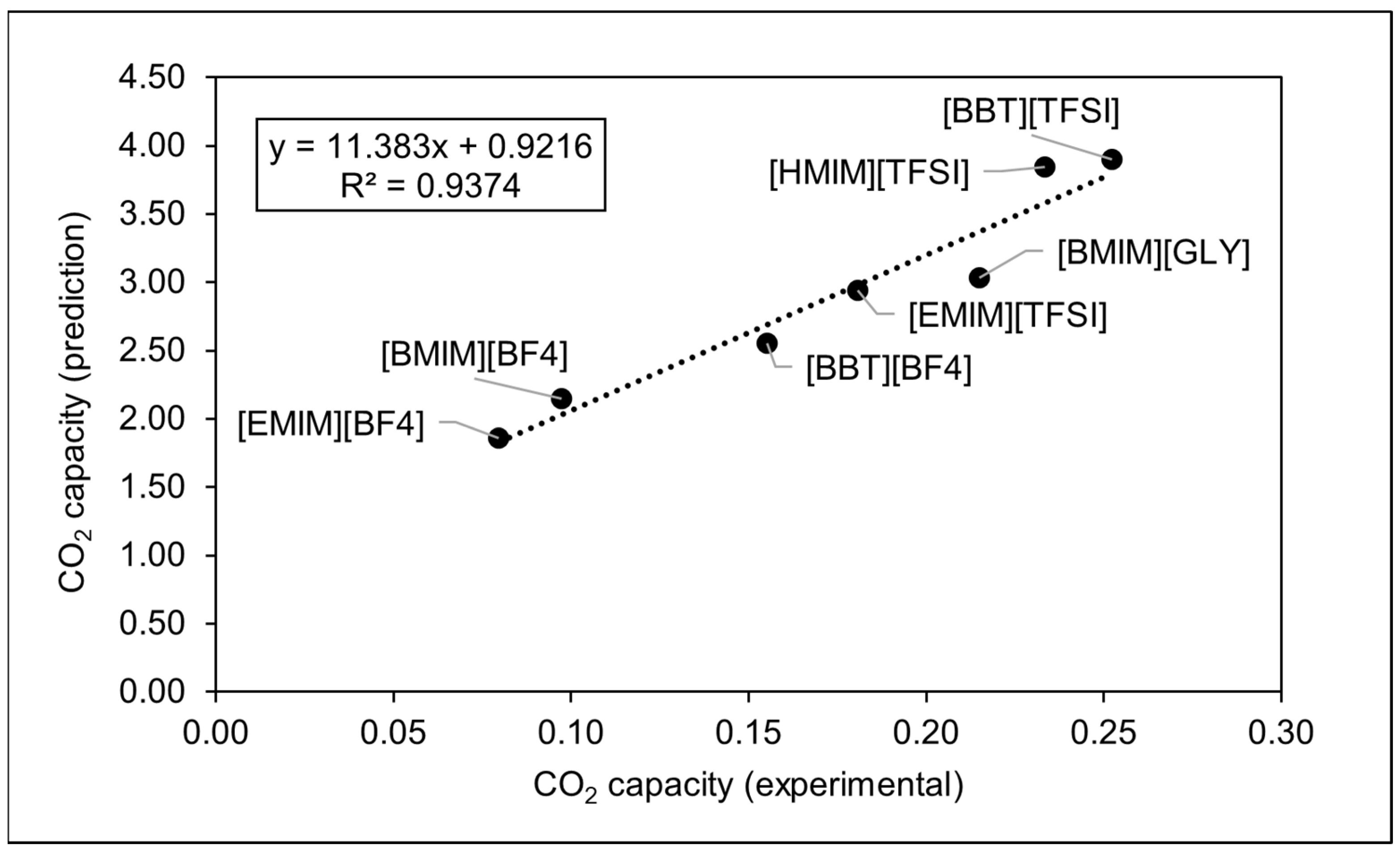
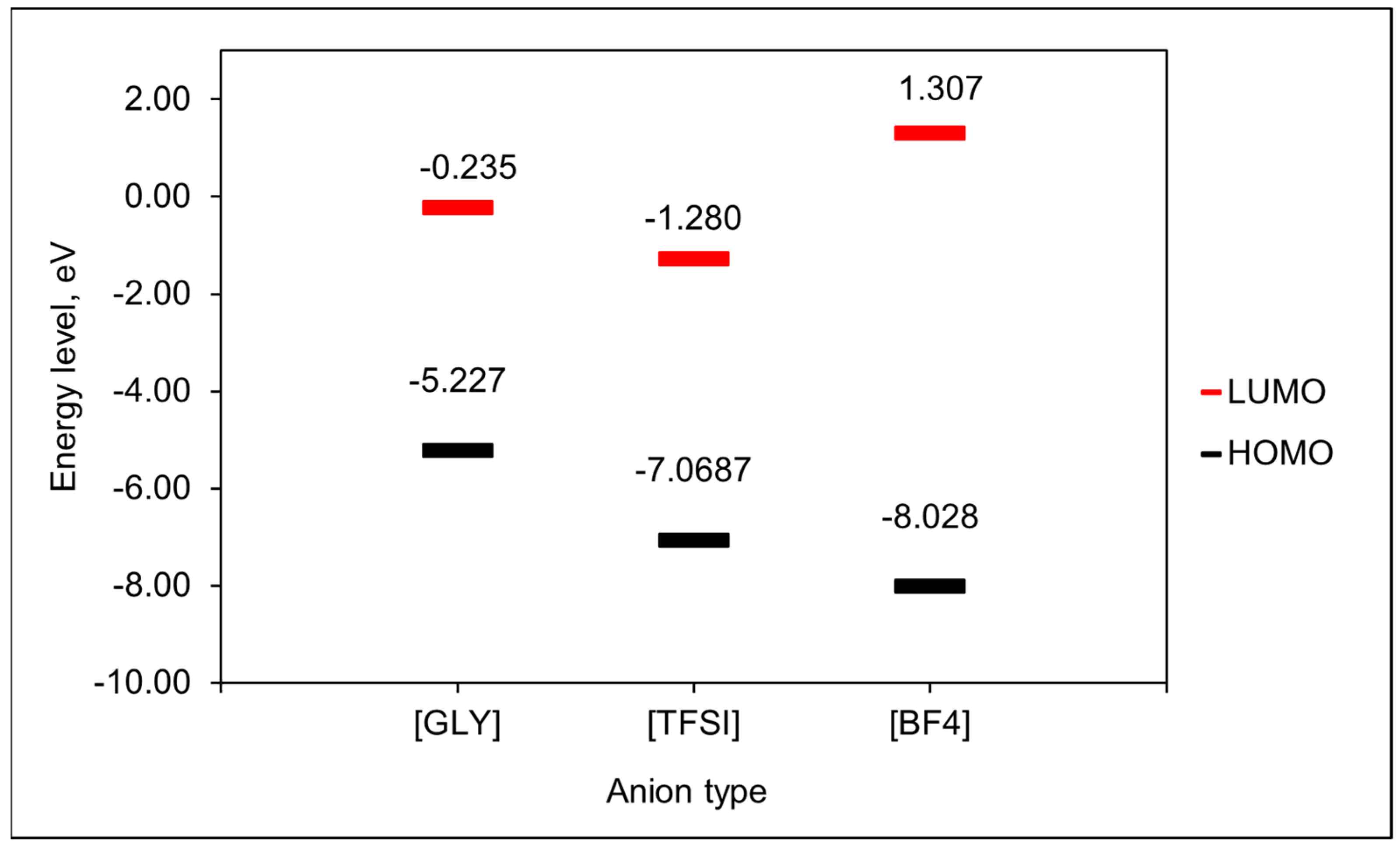
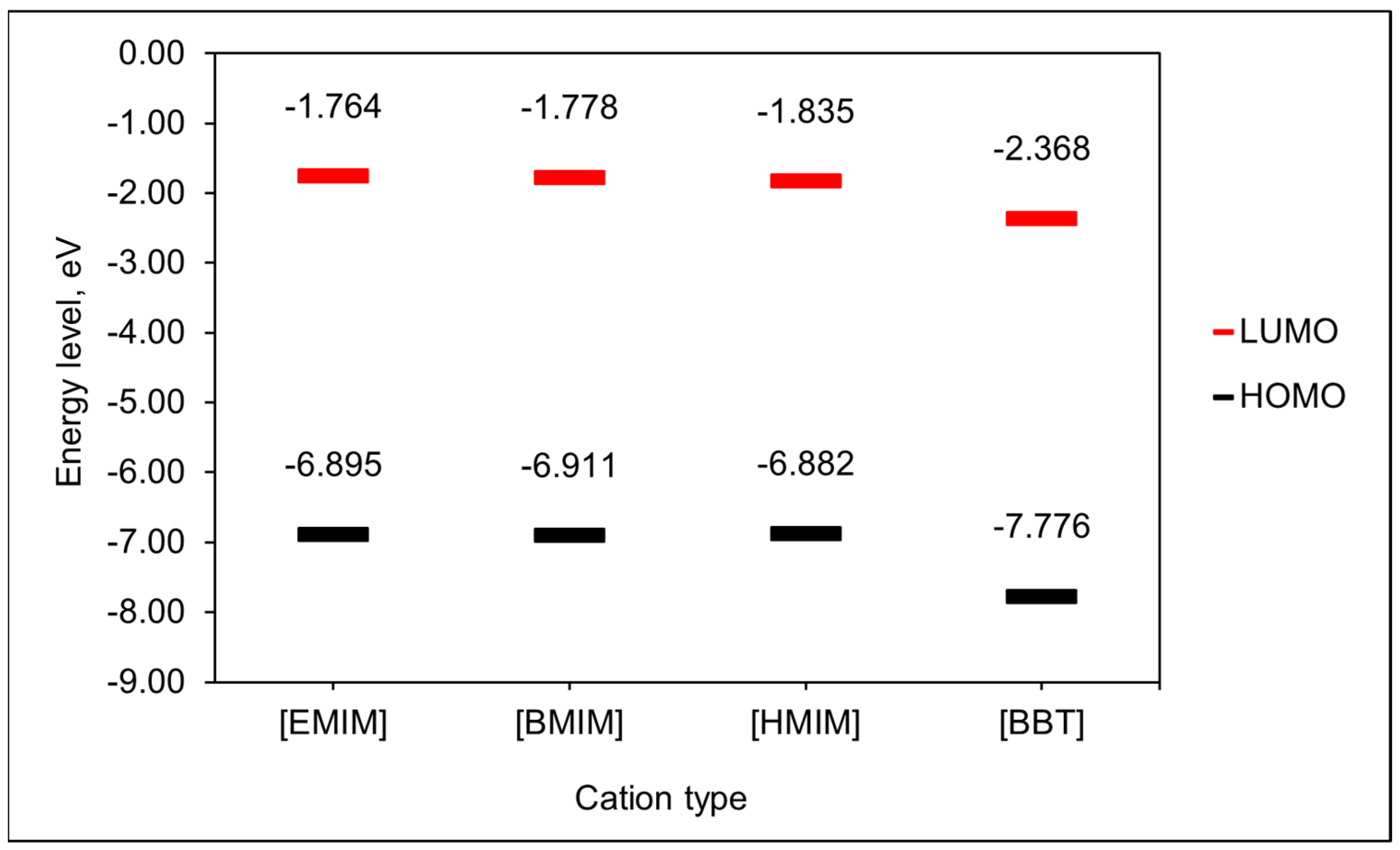
| Ionic Liquids | Density (g/cm3) | Molecular Weight, MW (g/mol) | Molar Volume (cm3/mol) | Maximum Capacity Values, x, (Mol (CO2 abs)/Mol (IL)) |
|---|---|---|---|---|
| [EMIM][BF4] | 1.280 | 197.97 | 154.63 | 0.0795 |
| [BMIM][BF4] | 1.222 | 226.02 | 185.03 | 0.0972 |
| [BMIM][GLY] | 1.108 | 213.29 | 192.48 | 0.2150 |
| [BBT][BF4] | 1.154 | 269.09 | 233.12 | 0.1553 |
| [EMIM][TFSI] | 1.517 | 391.3 | 257.96 | 0.1808 |
| [HMIM][TFSI] | 1.371 | 447.42 | 326.35 | 0.2335 |
| [BBT][TFSI] | 1.359 | 462.45 | 340.19 | 0.2523 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, S.A.S.; Yahya, W.Z.N.; Bustam, M.A.; Kibria, M.G. Experimental and Computational Evaluation of 1,2,4-Triazolium-Based Ionic Liquids for Carbon Dioxide Capture. Separations 2023, 10, 192. https://doi.org/10.3390/separations10030192
Mohammed SAS, Yahya WZN, Bustam MA, Kibria MG. Experimental and Computational Evaluation of 1,2,4-Triazolium-Based Ionic Liquids for Carbon Dioxide Capture. Separations. 2023; 10(3):192. https://doi.org/10.3390/separations10030192
Chicago/Turabian StyleMohammed, Sulafa Abdalmageed Saadaldeen, Wan Zaireen Nisa Yahya, Mohamad Azmi Bustam, and Md Golam Kibria. 2023. "Experimental and Computational Evaluation of 1,2,4-Triazolium-Based Ionic Liquids for Carbon Dioxide Capture" Separations 10, no. 3: 192. https://doi.org/10.3390/separations10030192
APA StyleMohammed, S. A. S., Yahya, W. Z. N., Bustam, M. A., & Kibria, M. G. (2023). Experimental and Computational Evaluation of 1,2,4-Triazolium-Based Ionic Liquids for Carbon Dioxide Capture. Separations, 10(3), 192. https://doi.org/10.3390/separations10030192