Abstract
This study aimed to assess the applicability of eleven different products of solid-state fermentation of rapeseed cakes with commercial enzyme additives. Ground rapeseed cakes were mixed with water (1:2) and the enzymatic preparation (0.1%) according to the different variants and fermented at 25 °C for 24 h under anaerobic conditions. For fermentation, different enzymes were used: (1) α-amylase, (2) endo 1,4-β-xylanase, (3) endo-1,3(4)β-glucanase, (4) endo 1,4-β-xylanase, endo-1,3(4)β-glucanase, (5) α-amylase, endo-1,3(4)β-glucanase, (6) 6-phytase, (7) RONOZYME HiPhos 6-phytase, (8) liquid exogenous-6-phytase; and mixed combinations: (9) endo 1,4-β-xylanase, endo-1,3(4)β-glucanase, liquid exogenous-6-phytase, (10) α-amylase, endo-1,3(4)β-glucanase, liquid exogenous-6-phytase, and (11) α-amylase, endo 1,4-β-xylanase, endo-1,3(4)β-glucanase, liquid exogenous-6-phytase. After fermentation, the crude protein content in the products was similar, but the true protein content was significantly higher than in nonfermented rapeseed cakes (p < 0.05). Fermentation significantly reduced (p < 0.05) the levels of phytate phosphorous, raffinose family oligosaccharides, and glucosinolates in the products. In the next step, the most advantageous fermentation product obtained using liquid exogenous-6-phytase was selected in terms of nutritional value, produced on the technical scale, and incorporated into the diets for broilers. Six diets containing 12, 17, or 22% raw or fermented rapeseed cakes, respectively, were prepared. Up to 22% fermented rapeseed cakes did not adversely affect broiler production rates. Replacing raw rapeseed cakes with fermented products improved body weight gain in the grower phase, lowered feed intake in the finisher phase, and lowered the feed conversion ratio in all periods. A level of 22% of naturally fermented rapeseed cakes with liquid exogenous-6-phytase in the diet of broilers could be recommended.
1. Introduction
In modern poultry nutrition, the nutritional value of diets is essential to meet genetic potential. However, the feed protein deficit has become increasingly severe worldwide [1], so it is necessary to search for other functional native components, such as rapeseed feed. Rape products after oil removal contain approximately 35% protein with a high proportion of sulfur amino acids and several functional properties so that it can be most commonly used as a protein feed source [2,3]. Rapeseeds and products contain different antinutritional factors, such as oligosaccharides, phytate, and glucosinolates, and a high fiber content, which reduces their use in the unprocessed form in the diets of young animals [4,5,6]. Various processing methods are used to resolve the contradiction between the relatively high nutritional value and the low applicability of raw rapeseed cakes (RRC). One is solid-state fermentation (SSF) using microorganisms such as bacteria, yeast culture, and fungi, which can effectively hydrolyze rapeseed proteins and reduce antinutrient content [7,8,9,10]. SSF has been reported as an effective method of lowering oligosaccharides, phytate, and fiber in rape products [11]. Fermented rapeseed cakes (FRC) are highly digestible and contain essential nutrients, including peptides, microelements, and vitamins [12]. Some data indicate that replacing raw RRC with FRC in animal diets increases body weight and significantly improves the feed conversion ratio (FCR) [13,14,15]. Fermented rapeseed products can be used in poultry nutrition; however, their efficacy in broiler nutrition must be better recognized [16], so the current study will try to fill this gap. Moreover, fermentation significantly increases some natural enzyme activity as an effect of microorganisms’ growth, but it requires more time.
To the best of our knowledge, our studies provided the first reports on the impact of different exogenous enzyme preparations containing carbohydrates such as α-amylase, β-xylanase or β-glucanase, and/or phytase on the chemical composition of the product obtained by natural SSF of rapeseed cakes and used them in practice. Enzymatic deamidation using commercial products and fermentation, if successful, could also significantly impact the feed protein processing industry. Carbohydrates may favor protein extraction by breaking polysaccharides present in the meal, but they have been occasionally used for the enzymatic processing of rapeseed protein [17]. The current study concentrated on enzyme additives reducing phytate-P, oligosaccharides of the raffinose family, and fiber, which could significantly negatively impact birds’ digestive tract and metabolism [18,19,20].
The aim of the current study was to: (1) determine the effect of solid-state fermentation of rapeseed cakes with different exogenous enzymes on the chemical composition of the obtained products; (2) choose the most valuable product to produce modified feed components with exceptional functional properties; and (3) investigate the effect of different doses of raw or fermented rapeseed cake in diets on the growth, feed intake, and feed efficiency of broiler chickens.
2. Materials and Methods
2.1. Fermentation Process
The rapeseed cakes were purchased from a commercial manufacturing plant (ZT Bielmar, Poland). It was ground (22 mm sieve). Fermentations were performed according to Scheme 1 using eight commercial mono- and poly-enzyme formulations in the powdered form to achieve 11 different combinations according to Table 1. The products used in variants 1–7 were obtained from DSM Nutritional Products, Mszczonów Poland, and the enzyme used in variant 8 was obtained from Huvepharma N.V., Belgium. All fermentation processes were carried out under patent-pending procedures No. PL429784 and PL429785. After chemical analysis, product No. 8 was produced on a technical scale as a feed component for broilers. This technology has been patented by the Patent Office of the Republic of Poland Pat. No. PL237574).
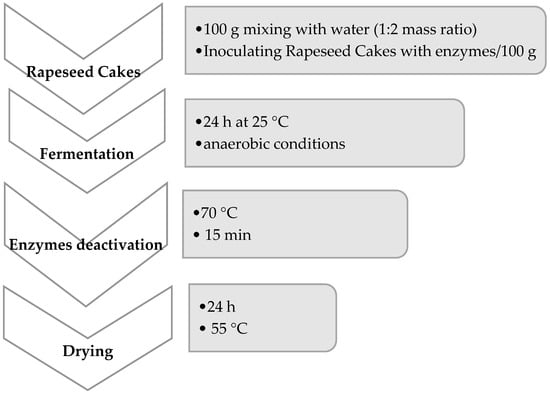
Scheme 1.
Fermentation process of rapeseed cakes.

Table 1.
Enzymes used in the fermentation and product variants.
2.2. Chemical Analysis
For chemical analysis, all the samples were ground to pass through a 0.5 mm sieve. The raw and fermented products were analyzed in duplicate (n = 6) for dry matter (DM), crude protein (CP), crude fiber (CF), and phosphorous using methods 934.01, 976.05, 978.10, and 965.17, respectively, according to AOAC [21]. True protein digestibility was analyzed using the method described by Hsu et al. [22], where a multienzyme in vitro system consisting of trypsin, chymotrypsin, and peptidase was used. Phytate-P was assayed using the method described by Haug and Lantzsch [23], where phytic acid was precipitated with an acidic iron-III-solution of known iron content. The decreased iron content in the supernatant correlates with the phytic acid content. The solutions were analyzed using a UV spectrophotometer, and absorbance was measured at 519 nm (Spektrofotometr Marcel Media, Marcel S.A., Zielonka, Poland). The composition and contents of soluble carbohydrates were analyzed by the high-resolution gas chromatography method, as described earlier [24], with some modifications. Carbohydrates were extracted from pulverized material (40–45 mg each, in 3 replications) with 900 L of 50% aqueous ethanol solution containing xylitol (100 µg) as an internal standard. After heating at 90 °C for 30 min (with continuous shaking), samples were centrifuged (21,000× g for 30 min at 4 °C), and 400 µL of the homogenate was transferred to a centrifuge filter (with PVDF membrane, 0.2 mm porosity, Thermo Fischer Scientific, Waltham, Massachusetts, USA) and centrifuged (21,000× g for 15 min). A portion of the filtrate (200 µL) was concentrated in a rotary evaporator until dry. Dry residues were derivatized with a 200 µL mixture of TMSI (trimethylsilyl-imidazole) and pyridine (1:1, v/v) at 80 °C for 45 min. TMS derivatives of carbohydrates were separated in an Rtx®-1 capillary column (15 m length, 0.25 mm diameter, 0.1 µm thickness, Restek Corp., Bellefonte, PA, USA) in a GC 2010 gas chromatograph (Shimadzu, Kioto, Japan). The temperatures of the injector and detector (flame ionization detector) were 325 °C and 350 °C, respectively. The column was heated from 150 °C to 335 °C (at different rates of temperature increase), and helium was used as a carrier gas. Chromatograms were analyzed with an integrator in the CHROMA 3.2 application (Pol-Lab, Wilkowice, Poland). Carbohydrates were quantified using authentic standards (sugars, polyols, cyclitols, oligosaccharides, and galactinol) purchased from Sigma-Aldrich (Saint Louis, MO, USA). The content of the analyzed carbohydrates was calculated using the standard internal method. The contents of unidentified compounds were calculated according to the regression coefficients for the known carbohydrate standards with the closest retention time. The glucosinolates in rapeseed materials were determined by gas–liquid chromatography of trimethylsilyl derivatives of desulfated glucosinolates according to the Raney and McGregor method [25]. The technique was standardized with BC 190 reference material and gave results comparable to the effects of the high-pressure liquid chromatography method.
2.3. Broiler Experiment
According to the Act on the Protection of Animals Used for Scientific or Educational Purposes in Poland, adopted on 15 January 2015, and according to earlier regulations, the study described in this manuscript did not require the permission of the Local Ethical Commission for Investigations on Animals. The study was performed on birds in the standard production system. The experiment was conducted in an experimental unit (Piast, Olszowa Experimental Unit, no. 0161, Olszowa, Poland). All efforts were made to minimize animal suffering. In this experiment, 600 1-day-old male broiler chicks of the ROSS 308 line (initial BW 45.5 ± 0.10 g) were weighed and distributed randomly to 1 of 6 dietary treatments in a completely randomized design. Ten replicate pens were assigned to each of the 6 treatments, with 10 birds per replicate pen. The pens of each treatment were distributed in such a way that they were located in the front, center, and back of the house. The housing conditions were in accordance with the Aviagen recommendations. To simulate commercial production, the experimental pens were surrounded by a commercial broiler flock of 9000 birds of the same origin as those used in the experiments. The birds were given 23 h of light and 1 h without light for the first week, followed by 19 h of light and 5 h without light from 7 to 21 days of life. From 22 to 35 days of life, the lighting system was similar to the first week. The birds were kept in floor pens (1.00 × 1.00 m) for 35 days. All birds had ad libitum access to water and feed for the experiment.
Fermented rapeseed cakes (variant 8) obtained using liquid exogenous-6-phytase (5000 OUT/g, Huvepharma N.V., Antwerpen, Belgium) expressed in Pichia pastoris were selected for the experiment on broilers based on the results of the chemical analyses of the fermentation products, ease of application, and price of additive. The birds were offered a starter diet from 1 to 9 days, a grower from 10 to 19 days, and a finisher from 20 to 35 days. The arrangement, level of mixture of the FRC or RRC, and the names of the groups are shown in Table 2. For each feeding period, all the diets were formulated to meet or exceed the nutrient requirements recommended by the NRC [26] for broiler chickens (Table 3, Table 4 and Table 5). The diets were produced in the Piast Pasze feed mill (Lewkowiec, Poland) according to the ISO 9001:2008 procedures. The feed was prepared on a laboratory-scale line equipped with a horizontal double band mixer (Zuptor, Gostyn, Poland) with roller mills (Skiold, Saby, Denmark). On days 10, 19, and 35, the body weight gain (BWG) and feed intake (FI) were recorded using animal scales, and based on these two parameters, the FCR was calculated in every period. During the experiment, the health of the birds was observed, and mortalities and diseases were noted.

Table 2.
The arrangement and names of the groups in the broiler experiment. RRC—raw rapeseed cakes; FRC—fermented rapeseed cakes.

Table 3.
Composition and calculated analysis of the experimental diets (Starter).

Table 4.
Composition and calculated analysis of the experimental diets (Grower).

Table 5.
Composition and calculated analysis of the experimental diets (Finisher).
2.4. Statistical Analysis
Statistical calculations were performed using SAS ver. 5.0. software package (SAS Institute Inc., Cary, NC, USA). Mean values as well as the standard error of the mean (SEM) were calculated for all traits. The optimization process of fermentation was investigated with a one-way ANOVA. p values < 0.05 were statistically significant, and p < 0.01 was highly statistically significant in process fermentation. Differences among treatments in the bird experiment were determined by employing the two-way linear model of ANOVA:
where:
Yijk = αj + βk + (αβ)jk + eijk
- Yijk is the value of the analyzed trait,
- αj is the constant effect of ith dose (i = 12, 17, 22%),
- βk is the constant effect of jth type feed (j = RRC, FRC),
- (αβ)jk is the interaction between α and β,
- eijk is the effect of experimental error.
For all traits, the significance of differences between groups of broilers was verified using Duncan’s test. Student’s t test determined differences between types of feed within groups.
3. Results
3.1. Optimization of the Fermentation
The analyzed products were characterized by a variable share of all the nutrients. There were no significant differences in the contents of CP and CF between the raw material and fermented products; however, significant differences were found among the obtained fermentation products (Table 6). The true protein content in dry matter was significantly higher in all the fermented products than in the raw material. Products (7) and (8) were characterized by a higher (p < 0.05) protein content than products (4) and (5), and products (7), (8), (9), (10), and (11) contained more true protein than RRC, and products (2), (4), and (5). A significantly lower (p < 0.05) content of crude fiber was recorded in product (11); however, it did not differ from that of the original material. The total phosphorus content was significantly higher in RRC than in products (4), (5), (6), (9), and (11), but the lowest content was found in the fermented product (4), which was significantly lower than in (1), (2), (7), and (8). Each of the products obtained after fermentation was characterized by a significantly lower content of phytate-P than in RRC. In the case of products (6), (7), (8), (9), (10), and (11), when different phytases were used, no phytate-P was detected.

Table 6.
The basic composition and total and phytic contents of RRC and FRC (g/kg in DM).
A significant (p < 0.05) increase in the share of galactose, d-chiro-inositol, myo-inositol, mannitol, and digalactosyl glycerol (DGG) in all the fermented products was found in comparison with unprocessed material (Table 7). Saccharose, galactinol, raffinose, stachyose, and total carbohydrate levels decreased significantly (p < 0.05). Glucose and fructose levels were significantly higher in products (1), (2), and (3), where α-amylase, endo 1,4-β-xylanase, and endo-1,3(4) β-glucanase were individually used, than in RRC, whereas in the remaining products, glucose content was significantly lower (p < 0.05). The levels of maltose and maltotriose were lower (p < 0.01) in all the fermented products compared to raw rapeseed cakes, except for fermentation variants (1) and (11). The percentage of 1-kestose was significantly higher in variants (1), (3), (4), and (5), where different carboxylases were used, than in RRC (p < 0.05). In the variants from (4) to (11), the content of raffinose oligosaccharides was significantly lower than that in RRC (p < 0.05).

Table 7.
The composition and contents of soluble carbohydrates, including RFOs (mg/g in DM), in RRC and FRC.
In all the fermented products, the content of individual and total glucosinolates in DM was significantly lower than in the raw cakes (Table 8). At the same time, napoleiferyne and 4-OH-glucobrassisin were not detected after fermentation. The lowest levels of progoitrin, total glucosinolate, and total glucosinolate alkene were found in fermented products (8), but they were not different from those in products (1), (4), and (6).

Table 8.
The composition and contents of glucosinolates (µmol/g in DM) in RRC and FRC.
Taking into consideration the chemical changes in the fermented rapeseed cakes, the most valuable nutritional value characterized products are: from (6) to (11) because of the higher protein content and the lack of phytate; from (4) to (11) because of the lowest RFO content; and products (6), (8), and (11), because of the highest reduction of glucosinolate. Therefore, variants (6), (8), and (11) were the most optimistic, but variant (11) was removed because of the higher price of the enzyme mixture than one enzyme additive. Variants (6) and (8) contained phytase only, but variant (8) also had significantly higher myo-inositol content than (6) and was easily applicable because of its liquid form. The exogenous 6-phytase used in variant (8) proved the most valuable in direct solid-state fermentation of rapeseed cakes.
3.2. Broiler Experiment
During the experiment, broilers were in good health with no increased mortalities or visible disease symptoms. Their growth performance results are shown in Table 9.

Table 9.
The effect of the type and dose of RRC and FRC on the growth performance of broilers.
In the starter phase, where 3.5% RRC or FRC was offered in the diet, no differences between groups were found. In the grower phase, the broilers from the 12 and 22% FRC groups had better growth than those in the other groups. Fermented products in the diet increased BWG and reduced FCR compared to unprocessed material. Birds from groups offered 12 and 22% rapeseed cakes had higher BWG than 17%; also, at the highest content of rapeseed cakes, reduced FCR was found (p < 0.05). In the finisher period, no impact of diet on BWG and FI was found, but animals offered fermented products in the diet consumed less feed (p < 0.05) and had lower FCR in comparison with unfermented feed. There was also a type-by-dose interaction for FCR in the finisher period. In the total period, broilers offered fermented products in the diet had lower FCR (p < 0.05).
4. Discussion
Fermentation is a process that can effectively improve food and feed quality by activating microorganisms and enzymes present in the fermented mass. In this study, we performed fermentation, but we also added some active enzyme preparation. We used one-enzyme carbohydrases—variant (1—amylase), (2—xylanase), and (3—beta-glucanase); two-enzyme carbohydrases—variant (4—xylanase + beta-glucanase), (5—amylase + beta-glucanase), phytases—variants (6), (7), (8), and the mixture of carbohydrases and phytase (9—xylanase + beta-glucanase + phytase), (10—amylase + beta-glucanase + phytase), and (11—amylase + xylanase + beta-glucanase + phytase).
All the fermentation variants were characterized by a slight increase in crude protein but a significant increase in true protein content compared with raw rapeseed cakes, which is consistent with the findings of other authors [10,11]. Chiang et al. [7] considered these changes to be due to different dry matter contents. Ashayerizadeh et al. [9] and Hu et al. [27] observed that an increase in crude protein content was associated with a decrease in nonstructural and total carbohydrates in feed, which was confirmed in this study. In the current work, we expected a reduction in structural carbohydrates in the variants with carbohydrates, but no reduction in crude fiber was found in these fermented products. This could be because fiber fractions are generally more resistant to fermentation, and the time of 24 h was too short for decomposing plant cell walls. Moreover, the insufficient water content in the substrate limits the enzyme’s activity. Recently, Hsiao et al. [28] have proven that only after 48 h, sugar reduction increased through the action of carbohydrates, which provided the microorganisms to continue producing lactic acid. On the other hand, in the current study, glucose and fructose levels were significantly higher in products where α-amylase, endo 1,4-β-xylanase, and endo-1,3(4) β-glucanase were individually used, which can be a result of partial carbohydrate changes. Additionally, a significant increase in the share of galactose, d-chiro-inositol, myo-inositol, mannitol, and DGG in all the fermented products was noted, whereas saccharose, galactinol, raffinose, stachyose, and total carbohydrate levels decreased significantly. This proves intensive changes in the structure of carbohydrates, probably caused by the activity of native microorganisms that use non-starch polysaccharides and simple sugars to produce their protein. Some studies found that fermentation of rapeseed meal with bacteria and yeasts (Rhizopus oligosporus, or cultures of Lactobacillus fermentum, Enterococcus faecium, Saccharomyces cerevisiae, and Bacillus subtilis) may reduce the content of oligosaccharides (by 73%), lignin and NDF (by 30%), glucosinolates (by 97.3%), and phytates (by 67%), depending on the inoculant composition and process conditions [9,29].
Despite the high protein level, rapeseed usage and its by-products in broiler nutrition are limited due to antinutrients such as oligosaccharides, glucosinolates, and phytate-P [9,30]. Antinutritive substances are relatively stable under heat treatment but can be efficiently removed by fermentation [7,13]. A significant reduction in raffinose oligosaccharides and glucosinolate was found in all the variants. The raffinose family oligosaccharides were reduced by approximately 45–60%. RFOs are destroyed mainly through different microorganisms, primarily yeast, to simple sugars [8,9]. Lücke et al. [31] carried out SSF fermentation of rapeseeds using Rhizopus oligosporus and showed that fermentation degraded polyphenols, glucosinolates, and some polysaccharides in rapeseed products. In our previous study [8], yeast fermentation of lupine seeds totally reduced oligosaccharides. It is probable that in the used rapeseed cakes, native yeast strains are present, giving a similar reduction of oligosaccharides from the raffinose family in all the products. The reduced level of glucosinolate was also identical in all the ferments, and it is rather connected with the natural myrosinase content in the raw material. Myrosinase spontaneously degrades the S-glycosidic bond in glucosinolates [32] and may be activated during fermentation, especially at high temperatures. In water, myrosinase cleaves off the glucose group from a glucosinolate. The remaining molecule then quickly converts to various products, depending on physiological conditions such as pH and the presence of specific cofactors. Many authors have found that glucosinolate degradation depends on enzyme type and concentration, temperature, pH, and reaction time [11,17,30,33]. In a study by Rakariyatham and Sakorn [33], complete degradation of glucosinolates in rapeseed meal occurred only after 48 h of fermentation at 30 °C. The SSF of rapeseed meal using Rhizopus oligosporus and Aspergillus sp. at 25 °C under aerobic conditions for 10 days also reduced total glucosinolates [11]. Chiang et al. [7] showed that isothiocyanates were reduced from 119.6 to 14.7 mmol/kg in a 30-day fermentation. Therefore, in the current study, the fermentation time was probably too short, and the temperature was too low for the total degradation of glucosinolates. In all the types of fermented products, an intensive reduction in phytate-P content was found. The total reduction was found in variants 8 to 11, where phytase was added directly. Native phytase is present in almost all plant materials, but its effectiveness is connected with activity, pH, and temperature. The differences in enzyme efficiency were due to the different physicochemical properties of phytases. Phytase of plant origin hydrolyses the phosphate group at the C3 position first, whereas phytase of microbial origin acts first at the C6 position [34]. The optimum plant phytase activity (6-phytase) appears at temperatures between 45–55 °C and at a pH of approximately 5 [35], while microbial phytase has an optimum at 55–65 °C and two pH optima: one at pH 2.5 and the other at pH 5.5. In this study, the fermentation temperature was lower (25 °C), but during this process, the pH is commonly reduced to 4–5, which means that native enzymes may effectively and significantly reduce the phytate-P content in these conditions. The reduction in phytate-P in this experiment also agrees with the findings of El-Batal and Karem [36], who reported that phytase causes phytate-P breakdown during fermentation. Thus, reducing antinutrients may increase rapeseed components in animal diets. Moreover, in addition to expanding the pool of available phosphorus, the action of phytase made it possible to release the most significant pool of myoinositol, a compound commonly found in plant cells in all the fermented variants but especially in (8). Its role has not been fully clarified; however, it seems essential for the normal functioning of cells. It has also been suggested that some of the beneficial effects of microbial phytase in poultry may be derived from the generation of myo-inositol [37].
Our study shows that natural solid-state fermentation with the direct addition of different types of enzymes induced changes in the chemical composition of products. Generally, in variants (1) to (5), where carbohydrases were added, no effect on the content of crude protein and crude fiber and slightly on the true protein was noted, although in these ferments, significantly reduced raffinose oligosaccharides and phytate contents were found. In variants (6), (7), and (8), where single phytases were used, a significant increase in true protein content, a significant reduction in raffinose oligosaccharides, and a total reduction in phytate content were found. In the case of enzyme mixtures (9, 10, 11), the results were similar to the case of phytases. The comparison of different variants in the context of their effectivity in the reduction of antinutrients, ability to improve the nutritional value of rapeseed cakes, application, and price of the enzyme, has allowed for the selection of variant (8) developed by the addition of exogenous-6-phytase as the most valuable in direct solid-state fermentation of rapeseed cakes. It is also crucial that this is produced in liquid form and is accessible to dosing to the feed in practice. Additionally, adding enzymes as liquids avoids this loss of enzyme activity, resulting in a more stable product and flexibility in dosing.
The fermentation technique of high-protein feed using various microorganisms, such as bacteria or yeast, has been frequently studied and utilized in formulated animal mixtures in recent years [7,8,14,15]. To date, there are limited data on the evaluation of directly fermented feed with exogenous enzymes in animal nutrition [10,28] because most fermentation mechanisms that reduce antinutrient contents are uncharacterized [38], except for phytate [39]. Many studies have shown that high dietary inclusion of raffinose family sugars in feeds containing high levels of crude fiber and other antinutrients could lead to decreased feed intake and BW gains and increased mortality [9,20]. In the current study, the fermentation of rapeseed cakes did not negatively affect the feed intake of birds. Broiler chickens fed fermented rapeseed cakes achieved a lower final FCR and better growth rate in the grower period. Antinutrients are commonly known to negatively affect the growth parameters of young animals with a digestive tract that is not fully adapted to digestion [7,8,10,32]. In this research, fermented rapeseed cakes were characterized by a significantly reduced antinutrient content, which probably positively affected nutrient digestibility and utilization and partially lowered the FCR. On the other hand, these positive changes had no influence on BWG or FI in the finisher phase and the whole experiment. Conversely, a fermented product with exogenous phytase (variant 8) incorporated at levels from 12 to 22% into the poultry diets did not negatively affect growth performance compared to RRC.
5. Conclusions
The direct solid-state fermentation of rapeseed cakes with exogenous enzymes significantly reduced phytate, raffinose oligosaccharides, and glucosinolate. In the presence of a phytase additive, phytate was completely eliminated from the material. Enzymatic deamidation using commercial products and fermentation positively impacts feed protein processing. Replacing raw rapeseed cakes with fermented rapeseed cakes with liquid exogenous-6-phytase from 12 and 22% in diets for broiler chickens improved the growth of animals, especially in the grower phase. The level of 22% fermented rapeseed cakes in the diet of broilers could be recommended; however, further research in this direction should be conducted, taking into account the economics of production.
6. Patents
All fermentation processes were carried out under patent-pending procedures No. PL429784 and PL429785. FRC was produced on a technical scale and was added as a feed component for the broiler mixture. This technology has been patented by the Patent Office of the Republic of Poland Pat. No. PL237574.
Author Contributions
Conceptualization, A.Z.-Z. and D.J. methodology, A.Z.-Z., M.K.-P. and D.J.; software, A.Z.-Z.; validation, M.K.-P., D.J. and B.K.; formal analysis, A.Z.-Z. and B.K. investigation, M.K.-P. and D.J.; resources, A.Z.-Z. and D.J.; data curation, A.Z.-Z. and B.K.; writing—original draft preparation, A.Z.-Z. and M.K.-P. writing—review and editing, A.Z.-Z. and M.K.-P. visualization, B.K. and D.J. supervision, D.J.; project administration, A.Z.-Z. and D.J.; funding acquisition, A.Z.-Z. and D.J. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by Programme: BIOSTRATEG1/267659/NCBR/2015 “GUTFEED—INNOVATIVE NUTRITION FOR SUSTAINABLE POULTRY PRODUCTION” and a subsidy from the Ministry of Science and Higher Education of Poland and by Poznan University of Life Sciences (Poland) as the research program “First grant”, no. 1/2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available at a reasonable request to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- De Ron, A.M.; Sparvoli, F.; Pueyo, J.J.; Bazile, D. Protein crops: Food and feed for the future. Front. Plant Sci. 2017, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Ghodsvali, A.; Khodaparast, M.H.; Vosoughi, M.; Diosady, L.L. Preparation of canola protein materials using membrane technology and evaluation of meals functional properties. Food Res. Int. 2005, 38, 223–231. [Google Scholar] [CrossRef]
- Gerzhova, A.; Mondor, M.; Benali, M.; Aider, M. Study of the functional properties of canola protein concentrates and isolates extracted by electroactivated solutions as noninvasive extraction method. Food Biosci. 2015, 12, 128–138. [Google Scholar] [CrossRef]
- Kocher, A.; Choct, M.; Porter, M.D.; Broz, J. The effects of enzyme addition to broiler diets containing high concentrations of canola or sunflower meal. Poult. Sci. 2000, 79, 1767–1774. [Google Scholar] [CrossRef]
- Schoène, F.; Leiterer, M.; Hartung, H.; Jahreis, G.; Tischendorf, F. Rapeseed glucosinolates and iodine in sows affect the milk iodine concentration and the iodine status of piglets. Br. J. Nutr. 2001, 85, 659–670. [Google Scholar] [CrossRef]
- Khajali, F.; Slominski, B.A. Factors that affect the nutritive value of canola. Poult. Sci. 2012, 91, 2564–2575. [Google Scholar] [CrossRef]
- Chiang, G.; Lu, W.Q.; Piao, X.S.; Hu, J.K.; Gong, L.M.; Thacker, P.A. Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian-Australas. J. Anim. Sci. 2010, 23, 263–271. [Google Scholar] [CrossRef]
- Zaworska, A.; Frankiewicz, A.; Kasprowicz-Potocka, M. The influence of narrow-leafed lupin seed fermentation on their chemical composition and ileal digestibility and microbiota in growing pigs. Arch. Anim. Nutr. 2017, 71, 285–296. [Google Scholar] [CrossRef]
- Ashayerizadeh, A.; Dastar, B.; Shargh, M.S.; Mahoonak, A.S.; Zerehdaran, S. Effects of feeding fermented rapeseed meal on growth performance, gastrointestinal microflora population, blood metabolites, meat quality, and lipid metabolism in broiler chickens. Livest. Sci. 2018, 216, 183–190. [Google Scholar] [CrossRef]
- Drażbo, A.; Ognik, K.; Zaworska, A.; Ferenc, K.; Jankowski, J. The effect of raw and fermented rapeseed cake on the metabolic parameters, immune status, and intestinal morphology of turkeys. Poult. Sci. 2018, 97, 3910–3920. [Google Scholar] [CrossRef]
- Vig, A.P.; Walia, A. Beneficial effects of Rhizopus oligosporus fermentation on reduction of glucosinolates, fiber and phytic acid in rapeseed (Brassica napus) meal. Bioresour. Technol. 2001, 78, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Marczak, E.D.; Usui, H.; Fujita, H.; Yang, Y.J.; Yokoo, M.; Lipkowski, A.W.; Yoshikawa, M. New antihypertensive peptides isolated from rapeseed. Peptides 2003, 24, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Drażbo, A.; Kozłowski, K.; Ognik, K.; Zaworska, A.; Jankowski, J. The effect of raw and fermented rapeseed cake on growth performance, carcass traits, and breast meat quality in turkey. Poult. Sci. 2019, 98, 6161–6169. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, Y.; Cheng, Q.; Xv, J.; Hou, Y.; Wu, X.; Du, E.; Ding, B. Partial substitution of fermented soybean meal for soybean meal influences the carcass traits and meat quality of broiler chickens. Animals 2020, 10, 225. [Google Scholar] [CrossRef]
- Li, Y.; Guo, B.; Wu, Z.; Wang, W.; Li, C.; Liu, G.; Cai, H. Effects of fermented soybean meal supplementation on the growth performance and cecal microbiota community of broiler chickens. Animals 2020, 10, 1098. [Google Scholar] [CrossRef]
- Gao, M.; Cieślak, A.; Kierończyk, B.; Huang, H.; Yanza, Y.R.; Zaworska-Zakrzewska, A.; Józefiak, D.; Szumacher-Strabel, M. Effects of Raw and Fermented Rapeseed Cake on Growth Performance, Methane Production, and Breast Meat Fatty Acid Composition in Broiler Chickens. Animals 2020, 10, 2250. [Google Scholar] [CrossRef]
- Mahajan, A.; Dua, S. Role of enzymatic treatments in modifying the functional properties of rapeseed (Brassica campestris var.toria) meal. Int. J. Food Sci. Nutr. 1998, 49, 435–440. [Google Scholar] [CrossRef]
- O’Shea, C.J.; Mc Alpine, P.O.; Solan, P.; Curran, T.; Varley, P.F.; Walsh, A.M.; Doherty, J.V.O. The effect of protease and xylanase enzymes on growth performance, nutrient digestibility, and manure odor in grower–finisher pigs. Anim. Feed Sci. Technol. 2014, 189, 88–97. [Google Scholar] [CrossRef]
- Humer, E.; Schwarz, C.; Schedle, K. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 605–625. [Google Scholar] [CrossRef]
- Kaczmarek, S.A.; Hejdysz, M.; Kubis, M.; Kasprowicz-Potocka, M.; Rutkowski, A. The nutritional value of yellow lupin (Lupinus luteus L.) for broilers. Anim. Feed Sci. Technol. 2016, 222, 43–53. [Google Scholar] [CrossRef]
- AOAC-Association of Official Analytical Chemists. Official Methods of Analysis, Agricultural Chemicals, 19th ed.; Gaithersburg: Maryland, VA, USA, 2007; pp. 46–48. [Google Scholar]
- Hsu, H.W.; Vavak, D.L.; Satterlee, L.; Miller, G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Haug, W.; Lantzsch, H.J. Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 1983, 34, 1423–1426. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Ciak, M.; Rybiński, W.; Bocianowski, J.; Börner, A. Diversity of the composition and content of soluble carbohydrates in seeds of the genus Vicia (Leguminosae). Genet. Resour. Crop Evol. 2018, 65, 541–554. [Google Scholar] [CrossRef]
- Raney, J.P.; McGregor, D.I. Determination of glucosinolate content by gas liquid chromatography of trimethylsilyl derivatives of desulfatedglucosinolates. In Oil Crops: Brassica Subnetwork; Proceedings of the Third Workshop, Quality Training, and Chinese Project Reports, Held in Shanghai, People’s Republic of China, 21–24 April 1990; IDRC: Ottawa, ON, Canada, 1993; pp. 14–19. [Google Scholar]
- National Research Council (N.R.C.). Nutrient Requirements of Poultry, 9th ed.; The National Academies Press: Washington, DC, USA, 1994; Volume 27, p. 63. [Google Scholar]
- Hu, Y.; Wang, Y.; Li, A.; Wang, Z.; Zhang, X.; Yun, T.; Qiu, L.; Yin, Y. Effects of fermented rapeseed meal on antioxidant functions, serum biochemical parameters and intestinal morphology in broilers. Food Agric. Immunol. 2016, 27, 182–193. [Google Scholar] [CrossRef]
- Hsiao, F.S.H.; Artdita, C.A.; Lin, S.Y.; Yu, Y.H.; Cheng, Y.H. Mixed Solid-State Fermentation of Okara and Copra Meal by Probiotics with Non-Starch Polysaccharide Enzymes and Its Effects on the Growth Performance and Ileal Microbiota in Broilers. Fermentation 2022, 8, 478. [Google Scholar] [CrossRef]
- Bau, H.M.; Villaume, C.; Lin, C.F.; Evrard, J.; Quemener, B.; Nicolas, J.P.; Mejean, L. Effect of a solid-state fermentation using Rhizopus oligosporus sp. T-3 on elimination of antinutritional substances and modification of biochemical constituents of defatted rapeseed meal. J. Sci. Food Agric. 1994, 65, 315–322. [Google Scholar] [CrossRef]
- Rabie, M.H.; El-Maaty, H.M.A.; El-Gogary, M.R.; Abdo, M.S. Nutritional and physiological effects of different levels of Canola Meal in broiler chick diets. Asian J. Anim. Vet. Adv. 2015, 10, 161–172. [Google Scholar] [CrossRef]
- Lücke, F.K.; Fritz, V.; Tannhäuser, K.; Arya, A. Controlled fermentation of rapeseed presscake by Rhizopus, and its effect on some components with relevance to human nutrition. Food Res. Int. 2019, 120, 726–732. [Google Scholar] [CrossRef]
- Misra, A.K.; Mishra, A.S.; Tripathi, M.K.; Prasad, R.; Vaithiyanathan, S.; Jakhmola, R.C. Optimization of solid-state fermentation of mustard (Brassica campestris) straw for production of animal feed by white rot fungi (Ganoderma lucidum). Asian-Australas. J. Anim. Sci. 2006, 20, 208–213. [Google Scholar] [CrossRef]
- Rakariyatham, N.; Sakorn, P. Biodegradation of glucosinolates in brown mustrad meal (Brassica juncea) by Aspergillus sp. NR-4201 in liquide and solid culture. Biodegradation 2002, 3, 395–409. [Google Scholar] [CrossRef]
- Weremko, D.; Fandrejewski, H.; Skiba, G. Enzymatic efficiency of plant and microbial phytase in cereal-rapeseed diets for growing pigs. J. Anim. Feed Sci. 2001, 10, 649–660. [Google Scholar] [CrossRef]
- Kies, K. Phytase—Mode of action. In Phytase in Animal Nutrition and Waste Management; Coelho, M.B., Kornegay, E.T., Eds.; BASF Corporation: Ludwigshafen, Germany, 1996; pp. 205–212. [Google Scholar]
- El-Batal, A.I.; Karem, H.A. Phytase production and phytic acid reduction in rapeseed meal by Aspergillus niger during solid-state fermentation. Food Res. Int. 2001, 34, 715–720. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Ptak, A.; Maćkowiak, P.; Sassek, M.; Pruszyńska-Oszmałek, E.; Żyła, K.; Świątkiewicz, S.; Kaczmarek, S.; Józefiak, D. The effect of microbial phytase and myo-inositol on performance and blood biochemistry of broiler chickens fed wheat/corn-based diets. Poult. Sci. 2013, 92, 124–2134. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, S.; Ranjitkar, S. Recent advances in fermented feeds toward improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Anim. Nutr. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sokrab, A.M.; Mohamed Ahmed, I.A.; Babiker, E.E. Effect of malting and fermentation on antinutrients, and total and extractable minerals of high and low phytate corn genotypes. Int. J. Food Sci. Technol. 2012, 47, 1037–1043. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).