1. Introduction
In modern poultry nutrition, the nutritional value of diets is essential to meet genetic potential. However, the feed protein deficit has become increasingly severe worldwide [
1], so it is necessary to search for other functional native components, such as rapeseed feed. Rape products after oil removal contain approximately 35% protein with a high proportion of sulfur amino acids and several functional properties so that it can be most commonly used as a protein feed source [
2,
3]. Rapeseeds and products contain different antinutritional factors, such as oligosaccharides, phytate, and glucosinolates, and a high fiber content, which reduces their use in the unprocessed form in the diets of young animals [
4,
5,
6]. Various processing methods are used to resolve the contradiction between the relatively high nutritional value and the low applicability of raw rapeseed cakes (RRC). One is solid-state fermentation (SSF) using microorganisms such as bacteria, yeast culture, and fungi, which can effectively hydrolyze rapeseed proteins and reduce antinutrient content [
7,
8,
9,
10]. SSF has been reported as an effective method of lowering oligosaccharides, phytate, and fiber in rape products [
11]. Fermented rapeseed cakes (FRC) are highly digestible and contain essential nutrients, including peptides, microelements, and vitamins [
12]. Some data indicate that replacing raw RRC with FRC in animal diets increases body weight and significantly improves the feed conversion ratio (FCR) [
13,
14,
15]. Fermented rapeseed products can be used in poultry nutrition; however, their efficacy in broiler nutrition must be better recognized [
16], so the current study will try to fill this gap. Moreover, fermentation significantly increases some natural enzyme activity as an effect of microorganisms’ growth, but it requires more time.
To the best of our knowledge, our studies provided the first reports on the impact of different exogenous enzyme preparations containing carbohydrates such as α-amylase, β-xylanase or β-glucanase, and/or phytase on the chemical composition of the product obtained by natural SSF of rapeseed cakes and used them in practice. Enzymatic deamidation using commercial products and fermentation, if successful, could also significantly impact the feed protein processing industry. Carbohydrates may favor protein extraction by breaking polysaccharides present in the meal, but they have been occasionally used for the enzymatic processing of rapeseed protein [
17]. The current study concentrated on enzyme additives reducing phytate-P, oligosaccharides of the raffinose family, and fiber, which could significantly negatively impact birds’ digestive tract and metabolism [
18,
19,
20].
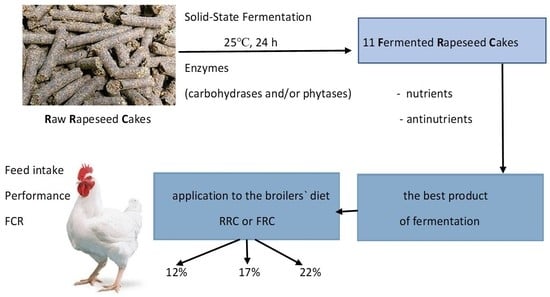
The aim of the current study was to: (1) determine the effect of solid-state fermentation of rapeseed cakes with different exogenous enzymes on the chemical composition of the obtained products; (2) choose the most valuable product to produce modified feed components with exceptional functional properties; and (3) investigate the effect of different doses of raw or fermented rapeseed cake in diets on the growth, feed intake, and feed efficiency of broiler chickens.
4. Discussion
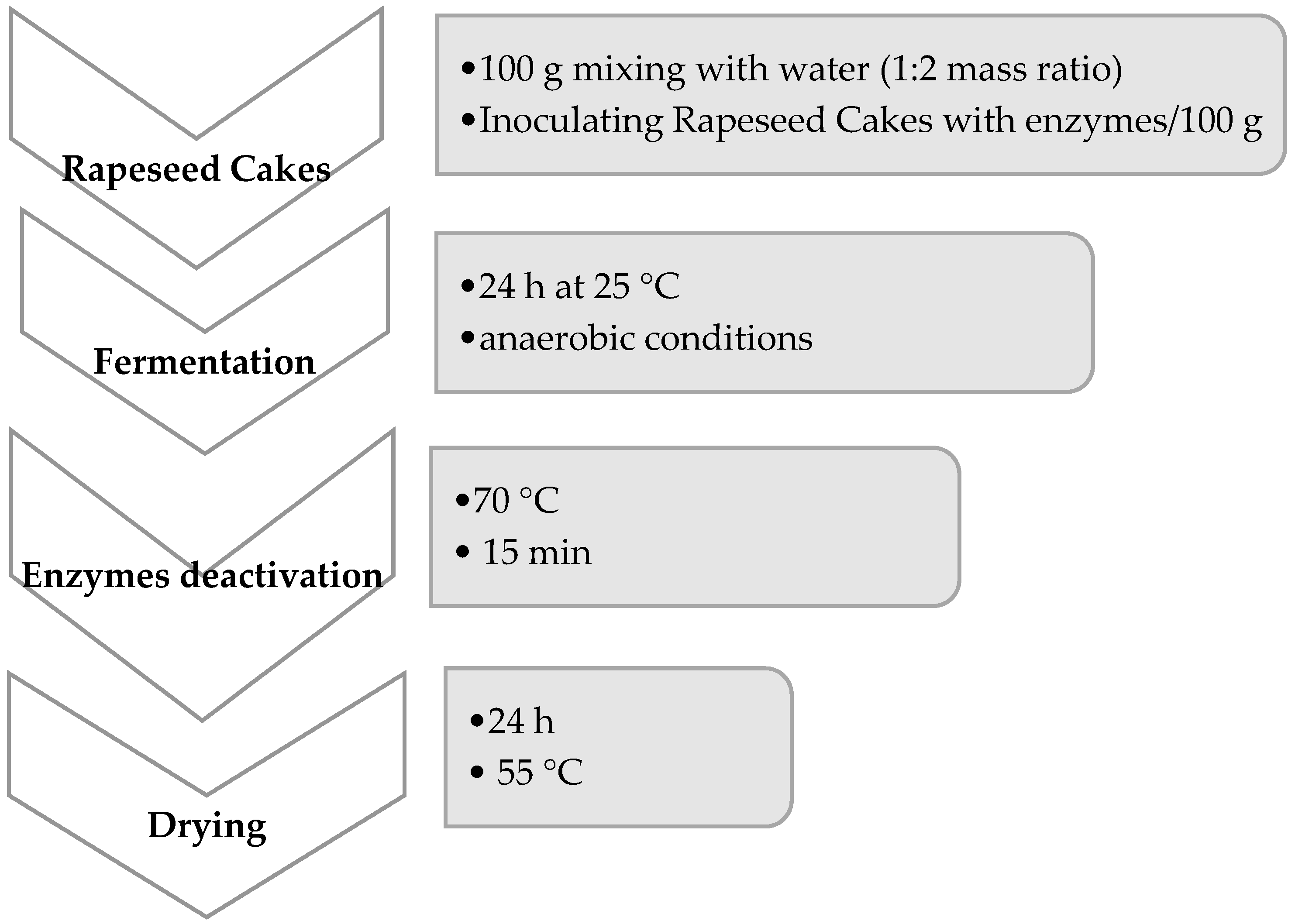
Fermentation is a process that can effectively improve food and feed quality by activating microorganisms and enzymes present in the fermented mass. In this study, we performed fermentation, but we also added some active enzyme preparation. We used one-enzyme carbohydrases—variant (1—amylase), (2—xylanase), and (3—beta-glucanase); two-enzyme carbohydrases—variant (4—xylanase + beta-glucanase), (5—amylase + beta-glucanase), phytases—variants (6), (7), (8), and the mixture of carbohydrases and phytase (9—xylanase + beta-glucanase + phytase), (10—amylase + beta-glucanase + phytase), and (11—amylase + xylanase + beta-glucanase + phytase).
All the fermentation variants were characterized by a slight increase in crude protein but a significant increase in true protein content compared with raw rapeseed cakes, which is consistent with the findings of other authors [
10,
11]. Chiang et al. [
7] considered these changes to be due to different dry matter contents. Ashayerizadeh et al. [
9] and Hu et al. [
27] observed that an increase in crude protein content was associated with a decrease in nonstructural and total carbohydrates in feed, which was confirmed in this study. In the current work, we expected a reduction in structural carbohydrates in the variants with carbohydrates, but no reduction in crude fiber was found in these fermented products. This could be because fiber fractions are generally more resistant to fermentation, and the time of 24 h was too short for decomposing plant cell walls. Moreover, the insufficient water content in the substrate limits the enzyme’s activity. Recently, Hsiao et al. [
28] have proven that only after 48 h, sugar reduction increased through the action of carbohydrates, which provided the microorganisms to continue producing lactic acid. On the other hand, in the current study, glucose and fructose levels were significantly higher in products where α-amylase, endo 1,4-β-xylanase, and endo-1,3(4) β-glucanase were individually used, which can be a result of partial carbohydrate changes. Additionally, a significant increase in the share of galactose, d-chiro-inositol, myo-inositol, mannitol, and DGG in all the fermented products was noted, whereas saccharose, galactinol, raffinose, stachyose, and total carbohydrate levels decreased significantly. This proves intensive changes in the structure of carbohydrates, probably caused by the activity of native microorganisms that use non-starch polysaccharides and simple sugars to produce their protein. Some studies found that fermentation of rapeseed meal with bacteria and yeasts (
Rhizopus oligosporus, or cultures of
Lactobacillus fermentum, Enterococcus faecium,
Saccharomyces cerevisiae, and
Bacillus subtilis) may reduce the content of oligosaccharides (by 73%), lignin and NDF (by 30%), glucosinolates (by 97.3%), and phytates (by 67%), depending on the inoculant composition and process conditions [
9,
29].
Despite the high protein level, rapeseed usage and its by-products in broiler nutrition are limited due to antinutrients such as oligosaccharides, glucosinolates, and phytate-P [
9,
30]. Antinutritive substances are relatively stable under heat treatment but can be efficiently removed by fermentation [
7,
13]. A significant reduction in raffinose oligosaccharides and glucosinolate was found in all the variants. The raffinose family oligosaccharides were reduced by approximately 45–60%. RFOs are destroyed mainly through different microorganisms, primarily yeast, to simple sugars [
8,
9]. Lücke et al. [
31] carried out SSF fermentation of rapeseeds using
Rhizopus oligosporus and showed that fermentation degraded polyphenols, glucosinolates, and some polysaccharides in rapeseed products. In our previous study [
8], yeast fermentation of lupine seeds totally reduced oligosaccharides. It is probable that in the used rapeseed cakes, native yeast strains are present, giving a similar reduction of oligosaccharides from the raffinose family in all the products. The reduced level of glucosinolate was also identical in all the ferments, and it is rather connected with the natural myrosinase content in the raw material. Myrosinase spontaneously degrades the
S-glycosidic bond in glucosinolates [
32] and may be activated during fermentation, especially at high temperatures. In water, myrosinase cleaves off the glucose group from a glucosinolate. The remaining molecule then quickly converts to various products, depending on physiological conditions such as pH and the presence of specific cofactors. Many authors have found that glucosinolate degradation depends on enzyme type and concentration, temperature, pH, and reaction time [
11,
17,
30,
33]. In a study by Rakariyatham and Sakorn [
33], complete degradation of glucosinolates in rapeseed meal occurred only after 48 h of fermentation at 30 °C. The SSF of rapeseed meal using
Rhizopus oligosporus and
Aspergillus sp. at 25 °C under aerobic conditions for 10 days also reduced total glucosinolates [
11]. Chiang et al. [
7] showed that isothiocyanates were reduced from 119.6 to 14.7 mmol/kg in a 30-day fermentation. Therefore, in the current study, the fermentation time was probably too short, and the temperature was too low for the total degradation of glucosinolates. In all the types of fermented products, an intensive reduction in phytate-P content was found. The total reduction was found in variants 8 to 11, where phytase was added directly. Native phytase is present in almost all plant materials, but its effectiveness is connected with activity, pH, and temperature. The differences in enzyme efficiency were due to the different physicochemical properties of phytases. Phytase of plant origin hydrolyses the phosphate group at the C3 position first, whereas phytase of microbial origin acts first at the C6 position [
34]. The optimum plant phytase activity (6-phytase) appears at temperatures between 45–55 °C and at a pH of approximately 5 [
35], while microbial phytase has an optimum at 55–65 °C and two pH optima: one at pH 2.5 and the other at pH 5.5. In this study, the fermentation temperature was lower (25 °C), but during this process, the pH is commonly reduced to 4–5, which means that native enzymes may effectively and significantly reduce the phytate-P content in these conditions. The reduction in phytate-P in this experiment also agrees with the findings of El-Batal and Karem [
36], who reported that phytase causes phytate-P breakdown during fermentation. Thus, reducing antinutrients may increase rapeseed components in animal diets. Moreover, in addition to expanding the pool of available phosphorus, the action of phytase made it possible to release the most significant pool of myoinositol, a compound commonly found in plant cells in all the fermented variants but especially in (8). Its role has not been fully clarified; however, it seems essential for the normal functioning of cells. It has also been suggested that some of the beneficial effects of microbial phytase in poultry may be derived from the generation of myo-inositol [
37].
Our study shows that natural solid-state fermentation with the direct addition of different types of enzymes induced changes in the chemical composition of products. Generally, in variants (1) to (5), where carbohydrases were added, no effect on the content of crude protein and crude fiber and slightly on the true protein was noted, although in these ferments, significantly reduced raffinose oligosaccharides and phytate contents were found. In variants (6), (7), and (8), where single phytases were used, a significant increase in true protein content, a significant reduction in raffinose oligosaccharides, and a total reduction in phytate content were found. In the case of enzyme mixtures (9, 10, 11), the results were similar to the case of phytases. The comparison of different variants in the context of their effectivity in the reduction of antinutrients, ability to improve the nutritional value of rapeseed cakes, application, and price of the enzyme, has allowed for the selection of variant (8) developed by the addition of exogenous-6-phytase as the most valuable in direct solid-state fermentation of rapeseed cakes. It is also crucial that this is produced in liquid form and is accessible to dosing to the feed in practice. Additionally, adding enzymes as liquids avoids this loss of enzyme activity, resulting in a more stable product and flexibility in dosing.
The fermentation technique of high-protein feed using various microorganisms, such as bacteria or yeast, has been frequently studied and utilized in formulated animal mixtures in recent years [
7,
8,
14,
15]. To date, there are limited data on the evaluation of directly fermented feed with exogenous enzymes in animal nutrition [
10,
28] because most fermentation mechanisms that reduce antinutrient contents are uncharacterized [
38], except for phytate [
39]. Many studies have shown that high dietary inclusion of raffinose family sugars in feeds containing high levels of crude fiber and other antinutrients could lead to decreased feed intake and BW gains and increased mortality [
9,
20]. In the current study, the fermentation of rapeseed cakes did not negatively affect the feed intake of birds. Broiler chickens fed fermented rapeseed cakes achieved a lower final FCR and better growth rate in the grower period. Antinutrients are commonly known to negatively affect the growth parameters of young animals with a digestive tract that is not fully adapted to digestion [
7,
8,
10,
32]. In this research, fermented rapeseed cakes were characterized by a significantly reduced antinutrient content, which probably positively affected nutrient digestibility and utilization and partially lowered the FCR. On the other hand, these positive changes had no influence on BWG or FI in the finisher phase and the whole experiment. Conversely, a fermented product with exogenous phytase (variant 8) incorporated at levels from 12 to 22% into the poultry diets did not negatively affect growth performance compared to RRC.