Modification of the Fermentation Process and Papain Enzymes in The Manufacture of Virgin Coconut Oil Using Optimization of Response Surface Methodology, Central Composite Design
Abstract
1. Introduction
2. Materials and Methods
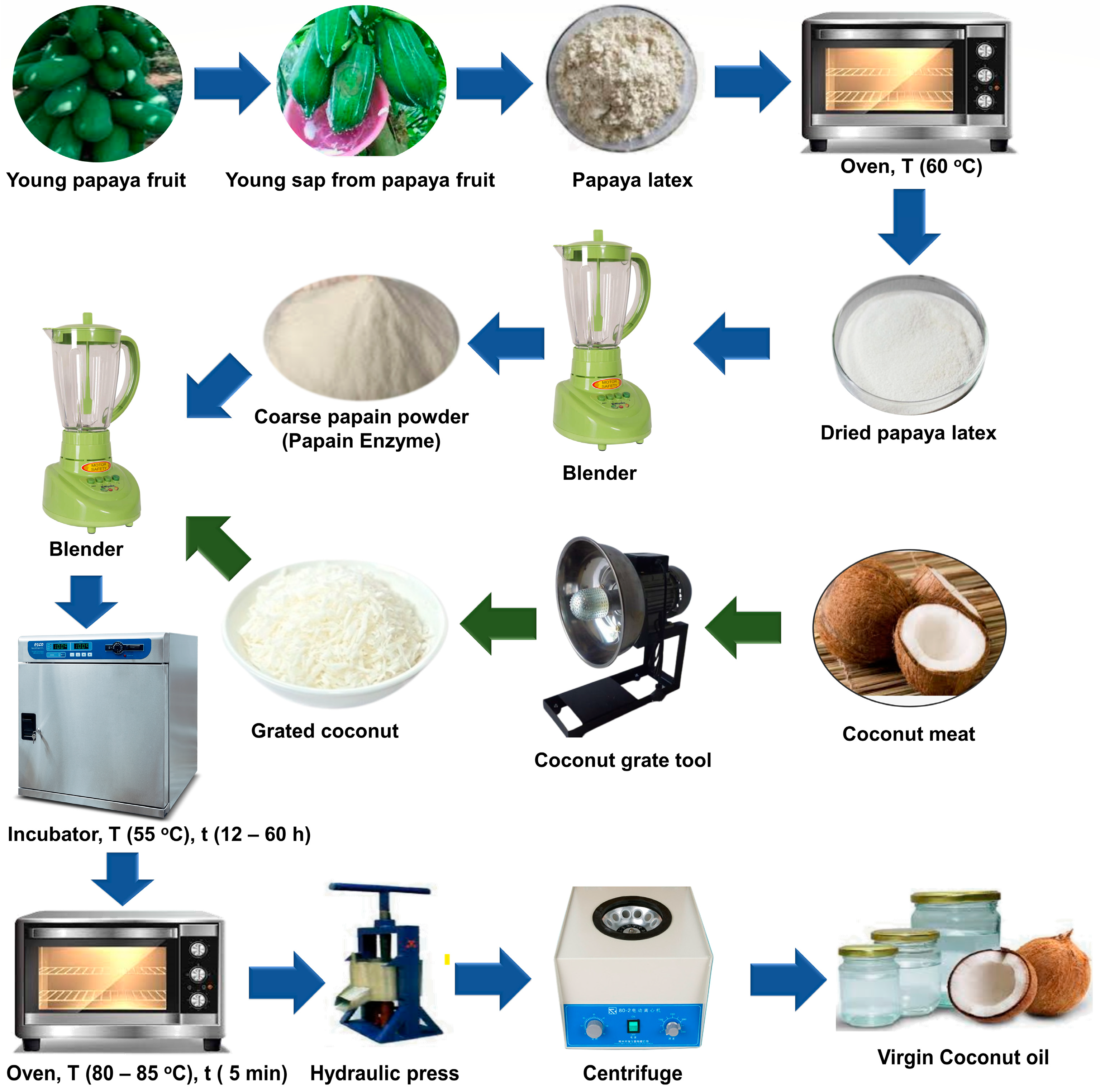
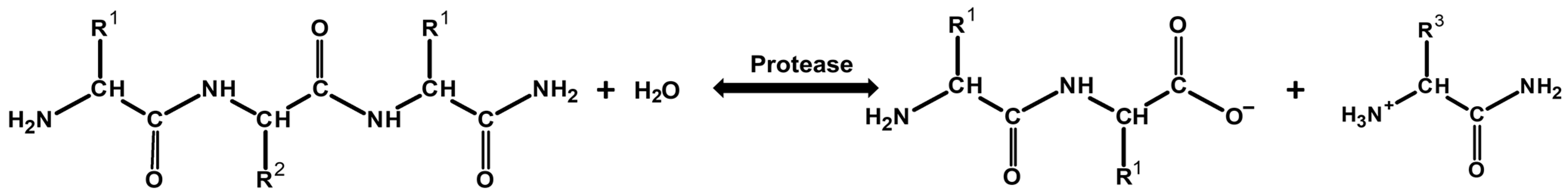
2.1. Production of Coarse Papain Enzymes
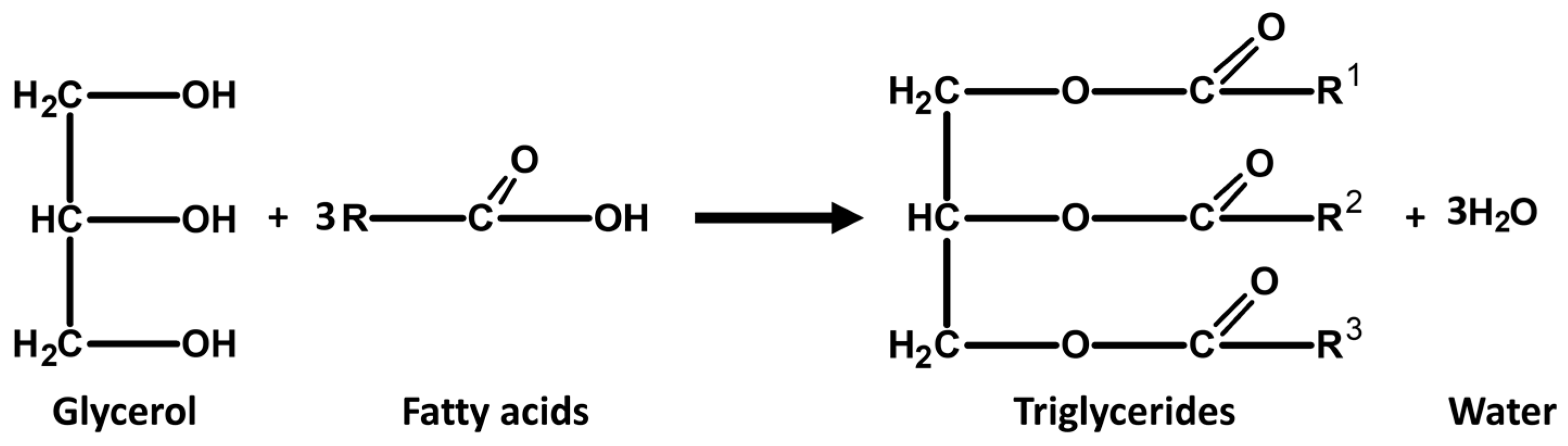
2.2. Virgin Coconut Oil Synthesis
2.3. Oil Quality Test
2.3.1. Moisture Content
2.3.2. Free Fatty Acid
2.3.3. Peroxide Number
2.4. Experimental Design
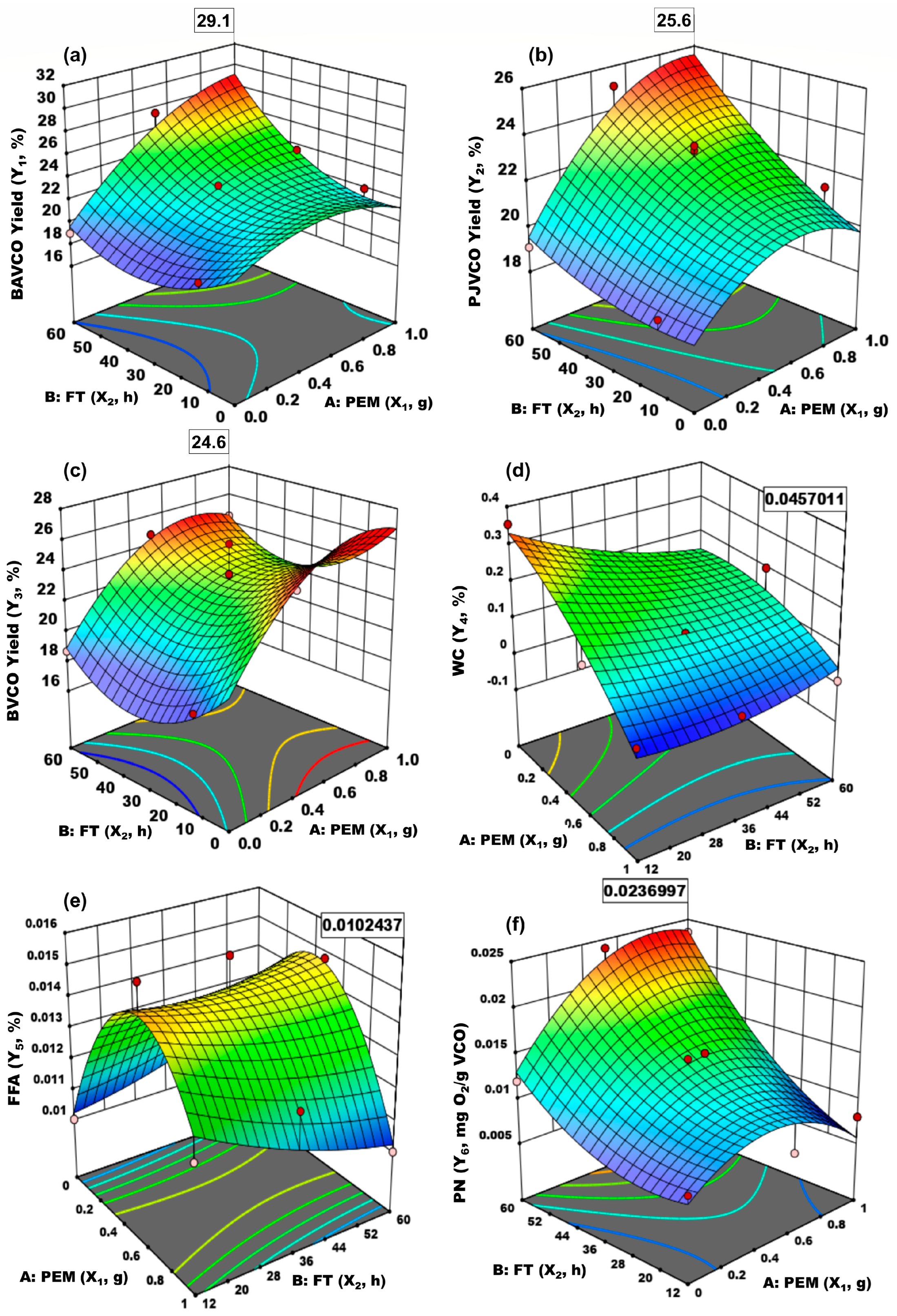
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BAVCO | Banda Aceh virgin coconut oil |
| BVCO | Bireun virgin coconut oil |
| CCD | Central composite design |
| CPE | Crude papain enzyme |
| FFA | Free fatty acid |
| FT | Fermentation time |
| PEM | Papain enzyme mass |
| PJVCO | Pidie Jaya virgin coconut oil |
| RSM | Response surface methodology |
| VCO | Virgin coconut oil |
| WC | Water content |
| PN | Peroxide number |
References
- Divya, P.M.; Roopa, B.S.; Manusha, C.; Balannara, P. A Concise Review on Oil Extraction Methods, Nutritional and Therapeutic Role of Coconut Products. J. Food Sci. Technol. 2023, 60, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Handayani, R.; Sulistyo, J.; Rahayu, R.D. Extraction of Coconut Oil (Cocos nucifera L.) through Fermentation System. Biodiversitas 2008, 10. [Google Scholar] [CrossRef]
- Hussain, M.S.; Al-Alaq, F.T.; Al-Khafaji, N.S.; Al-Dahmoshi, H.O.M. Antibacterial Effect of Virgin and Refined Coconut Oils on Pathogenic Bacteria: A Review. Indian J. Forensic Med. Toxicol. 2020, 14, 6426–6429. [Google Scholar] [CrossRef]
- Srivastava, Y.; Semwal, A.D.; Sharma, G.K. Studies on Storage Stability of Hot Extracted (HEVCO) and Cold Extracted Virgin Coconut Oil (CEVCO) in Different Flexible and Rigid Packaging System. Int. Food Res. J. 2013, 20, 1971–1976. [Google Scholar]
- Ihromilala, H. The Effect of Crude Papain Enzyme Extract Concentration from Papaya Leaves and Ripening Time to Coconut Oil Yield. J. Chem. Inf. Model. 2016, 53, 176–184. [Google Scholar]
- Konno, K.; Hirayama, C.; Nakamura, M.; Tateishi, K.; Tamura, Y.; Hattori, M.; Kohno, K. Papain Protects Papaya Trees from Herbivorous Insects: Role of Cysteine Proteases in Latex. Plant J. 2004, 37, 370–378. [Google Scholar] [CrossRef]
- Nitsawang, S.; Hatti-Kaul, R.; Kanasawud, P. Purification of Papain from Carica papaya Latex: Aqueous Two-Phase Extraction versus Two-Step Salt Precipitation. Enzym. Microb. Technol. 2006, 39, 1103–1107. [Google Scholar] [CrossRef]
- Nwinyi, O.C.; Anthonia, A.B. Antifungal Effects of Pawpaw Seed Extracts and Papain on Post Harvest Carica papaya L. Fruit Rot. Afr. J. Agric. Res. 2010, 5, 1531–1535. [Google Scholar]
- Ng, Y.J.; Tham, P.E.; Khoo, K.S.; Cheng, C.K.; Chew, K.W.; Show, P.L. A Comprehensive Review on the Techniques for Coconut Oil Extraction and Its Application. Bioprocess Biosyst. Eng. 2021, 44, 1807–1818. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Samir, Z.T.; Jassim, M.A.; Saeed, S.K. Effect of Different Extraction Methods on Physicochemical Properties, Antioxidant Activity, of Virgin Coconut Oil. Mater. Today Proc. 2021, 42, 2000–2005. [Google Scholar] [CrossRef]
- Kamalkumar, R.; Amutha, R.; Muthulaksmi, S.; Mareeswari, P.; Rani, W.B.; Nadu, T. Screening of Dioecious Papaya Hybrids for Papain Yield and Enzyme Activity. Res. J. Agric. Biol. Sci. 2007, 3, 447–449. [Google Scholar]
- Liggieri, C.; Obregón, W.; Trejo, S.; Priolo, N. Biochemical Analysis of a Papain-like Protease Isolated from the Latex of Asclepias curassavica L. Acta Biochim. Biophys. Sin. 2009, 41, 154–162. [Google Scholar] [CrossRef]
- Macalood, J.S.; Vicente, H.J.; Boniao, R.D.; Gorospe, J.G.; Roa, E.C. Chemical Analysis of Carica papaya L. Crude Latex. Am. J. Plant Sci. 2013, 4, 1941–1948. [Google Scholar] [CrossRef]
- Mansor, T.S.T.; Che Man, Y.B.; Shuhaimi, M.; Abdul Afiq, M.J.; Ku Nurul, F.K.M. Physicochemical Properties of Virgin Coconut Oil Extracted from Different Processing Methods. Int. Food Res. J. 2012, 19, 837–845. [Google Scholar]
- Benucci, I.; Esti, M.; Liburdi, K. Effect of Wine Inhibitors on the Proteolytic Activity of Papain from Carica papaya L. Latex. Biotechnol. Prog. 2015, 31, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Amri, E.; Mamboya, F. Papain, a Plant Enzyme of Biological Importance: A Review. Am. J. Biochem. Biotechnol. 2012, 8, 99–104. [Google Scholar]
- Roxas, M. The Role of Enzyme Supplementation in Digestive Disorders. Altern. Med. Rev. 2008, 13, 307–314. [Google Scholar]
- Bosco, S.J.D. Optimization of Aqueous Extraction of Virgin Coconut Oil Using Response Surface Methodology. Cord 2013, 29, 11. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Livinalli, N.F.; Baldasso, C.; Tessaro, I.C. Pervaporation in the Separation of Essential Oil Components: A Review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Bawalan, D.D.; Chapman, K.R. Virgin Coconut Oil: Production Manual for Micro- and Village-Scale Processing; FAO: Bangkok, Thailand, 2006. [Google Scholar]
- Wong, Y.C.; Hartina, H. Virgin Coconut Oil Production by Centrifugation Method. Orient. J. Chem. 2014, 30, 237–245. [Google Scholar] [CrossRef]
- Xu, D.H.; Bao, X.Q.; Wu, X.W.; Xing, Y.; Tan, C.Y. Metabolic Engineering Study on Biosynthesis of 4-Hydroxybenzyl Alcohol from L-Tyrosine in Escherichia coli. Zhongguo Zhongyao Zazhi 2022, 47, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.A.; Rofiee, M.S.; Somchit, M.N.; Zuraini, A.; Sulaiman, M.R.; Teh, L.K.; Salleh, M.Z.; Long, K. Hepatoprotective Activity of Dried- and Fermented-Processed Virgin Coconut Oil. Evid.-Based Complement. Altern. Med. 2011, 2011, 142739. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, B.P.M.; Freitas, S.P.; Coelho, M.A.Z. Enzymatic Aqueous Technology for Simultaneous Coconut Protein and Oil Extraction. Grasas Aceites 2003, 54, 77–80. [Google Scholar] [CrossRef]
- Alouw, J.C.; Wulandari, S. Present Status and Outlook of Coconut Development in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2020, 418, 012035. [Google Scholar] [CrossRef]
- Rasoulzadeh, H.; Dehghani, M.H.; Mohammadi, A.S.; Karri, R.R.; Nabizadeh, R.; Nazmara, S.; Kim, K.H.; Sahu, J.N. Parametric Modelling of Pb(II) Adsorption onto Chitosan-Coated Fe3O4 Particles through RSM and DE Hybrid Evolutionary Optimization Framework. J. Mol. Liq. 2020, 297, 111893. [Google Scholar] [CrossRef]
- Jahanbakhshi, A.; Abbaspour-Gilandeh, Y.; Heidarbeigi, K.; Momeny, M. Detection of Fraud in Ginger Powder Using an Automatic Sorting System Based on Image Processing Technique and Deep Learning. Comput. Biol. Med. 2021, 136, 104764. [Google Scholar] [CrossRef]
- Agarwal, R.K. Extraction Processes of Virgin Coconut Oil. MOJ Food Process. Technol. 2017, 4, 54–56. [Google Scholar] [CrossRef]
- Tano-Debrah, K.; Ohta, Y. Aqueous Extraction of Coconut Oil by an Enzyme-Assisted Process. J. Sci. Food Agric. 1997, 74, 497–502. [Google Scholar] [CrossRef]
- Shen, Y.; Du, Z.; Wu, X.; Li, Y. Modulating Molecular Interactions in Pea Protein to Improve Its Functional Properties. J. Agric. Food Res. 2022, 8, 100313. [Google Scholar] [CrossRef]
- Gomes, M.H.G.; Kurozawa, L.E. Improvement of the Functional and Antioxidant Properties of Rice Protein by Enzymatic Hydrolysis for the Microencapsulation of Linseed Oil. J. Food Eng. 2020, 267, 109761. [Google Scholar] [CrossRef]
- Sharma, B.; Shekhar, S.; Sharma, S.; Jain, P. The Paradigm in Conversion of Plastic Waste into Value Added Materials. Clean. Eng. Technol. 2021, 4, 100254. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- Diether, N.E.; Willing, B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet–Microbe–Host Interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Amit; Jamwal, R.; Kumari, S.; Dhaulaniya, A.S.; Balan, B.; Kelly, S.; Cannavan, A.; Singh, D.K. Utilizing ATR-FTIR Spectroscopy Combined with Multivariate Chemometric Modelling for the Swift Detection of Mustard Oil Adulteration in Virgin Coconut Oil. Vib. Spectrosc. 2020, 109, 103066. [Google Scholar] [CrossRef]
- Amit; Jamwal, R.; Kumari, S.; Kelly, S.; Cannavan, A.; Singh, D.K. Assessment of Geographical Origin of Virgin Coconut Oil Using Inductively Coupled Plasma Mass Spectrometry along with Multivariate Chemometrics. Curr. Res. Food Sci. 2022, 5, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Maini, Z.A.; Lopez, C.M. Transitions in Bacterial Communities across Two Fermentation-Based Virgin Coconut Oil (VCO) Production Processes. Heliyon 2022, 8, e10154. [Google Scholar] [CrossRef]
- Ng, S.P.; Khor, Y.P.; Lim, H.K.; Lai, O.M.; Wang, Y.; Wang, Y.; Nehdi, I.A.; Tan, C.P. In-Depth Characterization of Palm-Based Diacylglycerol-Virgin Coconut Oil Blends with Enhanced Techno-Functional Properties. LWT 2021, 145, 111327. [Google Scholar] [CrossRef]

| Run | F1 | F2 | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|---|---|
| A: X1 | B: X2 | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | |
| (g) | (h) | (%) | (%) | (%) | (%) | (%) | (mg O2/g VCO) | |
| 1 | 0.6 | 36 | 23 | 23.1 | 25.1 | 0.124 | 0.014 | 0.014 |
| 2 | 1 | 12 | 22 | 21.3 | 25 | 0.026 | 0.012 | 0.008 |
| 3 | 0.6 | 36 | 23 | 22.4 | 23.1 | 0.124 | 0.014 | 0.014 |
| 4 | 0.6 | 12 | 21 | 20.1 | 23.5 | 0.126 | 0.016 | 0.007 |
| 5 | 0.5 | 60 | 27.5 | 25.1 | 24.8 | 0.201 | 0.015 | 0.024 |
| 6 | 0.6 | 36 | 23 | 22.5 | 23.1 | 0.124 | 0.014 | 0.014 |
| 7 | 0 | 60 | 19 | 19.1 | 18.7 | 0.124 | 0.012 | 0.012 |
| 8 | 0.6 | 36 | 23 | 23.1 | 23.1 | 0.124 | 0.014 | 0.014 |
| 9 | 1 | 60 | 27.7 | 25.2 | 24.5 | 0.011 | 0.01 | 0.024 |
| 10 | 0.6 | 36 | 23 | 22.9 | 21.5 | 0.124 | 0.014 | 0.015 |
| 11 | 0 | 12 | 19.6 | 18.5 | 18.3 | 0.354 | 0.01 | 0.008 |
| 12 | 1 | 36 | 23.6 | 22.5 | 21.8 | 0.011 | 0.012 | 0.01 |
| 13 | 0.5 | 36 | 22.8 | 21.8 | 20.8 | 0.1242 | 0.016 | 0.015 |
| R1 | Source | SS | df | MS | F-v | p-v | R2 | SS | df | MS | F-v | p-v | R3 | SS | df | MS | F-v | p-v |
| BAVCO Yield (Y1, %) | Model | 69.5 | 5 | 13.9 | 16.9 | 0.0009 | PJVCO Yield (Y2, %) | 44.5 | 5 | 8.9 | 16.2 | 0.001 | BVCO Yield (Y3, %) | 44.5 | 5 | 8.9 | 16.2 | 0.001 |
| X1-PEM | 33.9 | 1 | 33.9 | 41.4 | 0.0004 | 20.6 | 1 | 20.6 | 37.5 | 0.0005 | 20.6 | 1 | 20.6 | 37.5 | 0.0005 | |||
| X2-FT | 22.9 | 1 | 22.9 | 27.9 | 0.001 | 15.6 | 1 | 15.6 | 28.4 | 0.001 | 15.6 | 1 | 15.6 | 28.4 | 0.001 | |||
| X1X2 | 10.8 | 1 | 10.8 | 13.2 | 0.008 | 3.1 | 1 | 3.1 | 5.7 | 0.048 | 3.1 | 1 | 3.1 | 5.7 | 0.048 | |||
| X12 | 7.9 | 1 | 7.9 | 9.7 | 0.017 | 5.6 | 1 | 5.6 | 10.3 | 0.015 | 5.6 | 1 | 5.6 | 10.3 | 0.015 | |||
| X22 | 4.8 | 1 | 4.8 | 5.8 | 0.047 | 0.3 | 1 | 0.3 | 0.5 | 0.506 | 0.3 | 1 | 0.3 | 0.5 | 0.506 | |||
| Residual | 5.7 | 7 | 0.82 | 3.9 | 7 | 0.5 | 3.9 | 7 | 0.5 | |||||||||
| R4 | Source | SS | df | MS | F-v | p-v | R5 | SS | df | MS | F-v | p-v | R6 | SS | df | MS | F-v | p-v |
| WC (Y4, %) | Model | 0.084 | 5 | 0.017 | 12.1 | 0.002 | FFA (Y5, %) | 0 | 5 | 7 × 10−6 | 8.6 | 0.006 | PN (Y6, mg O2/g VCO) | 0.0003 | 5 | 0.0001 | 18.3 | 0.0007 |
| X1-PEM | 0.053 | 1 | 0.053 | 38 | 0.0005 | 7 × 10−8 | 1 | 7 × 10−8 | 0.084 | 0.78 | 0 | 1 | 0 | 7.7 | 0.028 | |||
| X2-FT | 0.007 | 1 | 0.007 | 5 | 0.05 | 7.7 × 10−8 | 1 | 7.7 × 10−8 | 0.091 | 0.772 | 0.0002 | 1 | 0.0002 | 65.2 | <0.0001 | |||
| X1X2 | 0.013 | 1 | 0.013 | 9.1 | 0.02 | 5 × 10−6 | 1 | 5 × 10−6 | 6.3 | 0.04 | 0 | 1 | 0 | 9.9 | 0.016 | |||
| X12 | 0.004 | 1 | 0.004 | 2.5 | 0.156 | 0 | 1 | 0 | 27.4 | 0.001 | 0 | 1 | 0 | 11.3 | 0.012 | |||
| X22 | 0.002 | 1 | 0.002 | 1.2 | 0.309 | 2 × 10−7 | 1 | 2 × 10−7 | 0.211 | 0.66 | 0 | 1 | 0 | 4.6 | 0.068 | |||
| Residual | 0.01 | 7 | 0.001 | 6 × 10−6 | 7 | 8.5 × 10−6 | 0 | 7 | 3 × 10−6 |
| Name | Intercept | X1 | X2 | X1X2 | X12 | X22 | R2 |
|---|---|---|---|---|---|---|---|
| BAVCO Yield (Y1, %) | 22.6 | 2.7 | 1.9 | 1.6 | −1.9 | 1.5 | 0.92 |
| p-values | 0.0004 | 0.001 | 0.008 | 0.017 | 0.047 | ||
| PJVCO Yield (Y2, %) | 22.3 | 2.1 | 1.6 | 0.884 | −1.7 | 0.354 | 0.92 |
| p-values | 0.0005 | 0.001 | 0.048 | 0.015 | 0.506 | ||
| BVCO Yield (Y3, %) | 22.3 | 2.1 | 1 | 0.996 | −3.2 | 1.5 | 0.89 |
| p-values | 0.002 | 0.024 | 0.059 | 0.001 | 0.044 | ||
| WC (Y4, %) | 0.146 | −0.108 | −0.034 | 0.056 | −0.042 | 0.028 | 0.90 |
| p-values | 0.0005 | 0.06 | 0.019 | 0.156 | 0.309 | ||
| FFA (Y5, %) | 0.014 | 0.0001 | −0.0001 | −0.001 | −0.003 | 0.0003 | 0.86 |
| p-values | 0.78 | 0.772 | 0.04 | 0.001 | 0.659 | ||
| PN (Y6, mg O2/g s) | 0.014 | 0.002 | 0.006 | 0.003 | −0.004 | 0.003 | 0.93 |
| p-values | 0.028 | < 0.0001 | 0.016 | 0.012 | 0.068 |
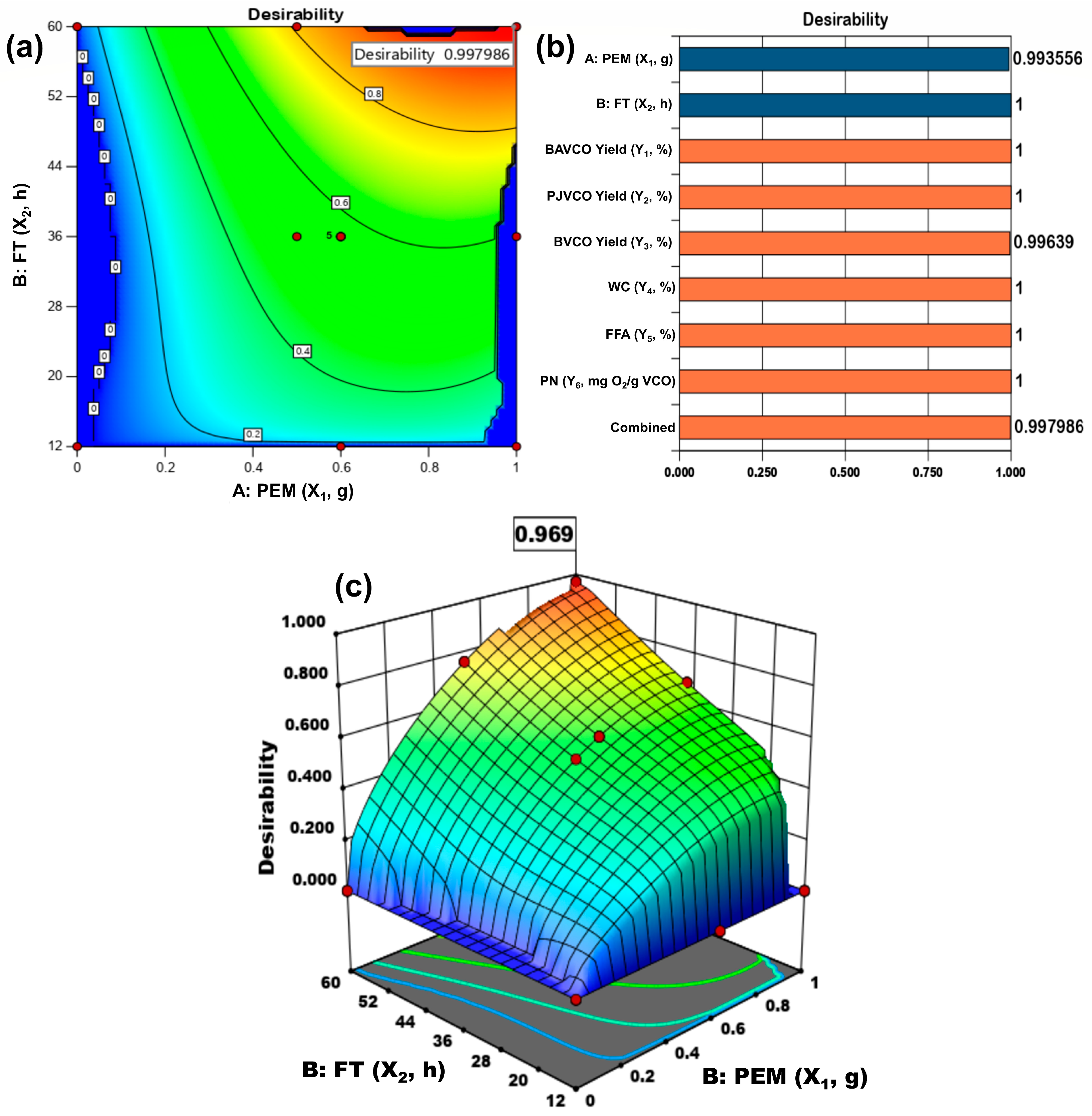
| Name | Goal | Lower Limit | Upper Limit | Lower Weight | Upper Weight | Importance |
|---|---|---|---|---|---|---|
| A: PEM (X1, g) | maximize | 0 | 1 | 1 | 1 | 3 |
| B: FT (X2, h) | maximize | 12 | 60 | 1 | 1 | 3 |
| BAVCO Yield (Y1, %) | maximize | 19 | 27.7 | 1 | 1 | 3 |
| PJVCO Yield (Y2, %) | maximize | 18.5 | 25.18 | 1 | 1 | 3 |
| BVCO Yield (Y3, %) | maximize | 18.32 | 24.75 | 1 | 1 | 3 |
| WC (Y4, %) | minimize | 0.111 | 0.354 | 1 | 1 | 3 |
| FFA (Y5, %) | is in range | 0.92 | 1.48 | 1 | 1 | 3 |
| PN (Y6, mg O2/g VCO) | is in range | 0.07 | 0.24 | 1 | 1 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakfar; Husin, H.; Pontas, K.; Mamat, R.; Salleh, M.R.; Zulrika, M.; Ahmadi. Modification of the Fermentation Process and Papain Enzymes in The Manufacture of Virgin Coconut Oil Using Optimization of Response Surface Methodology, Central Composite Design. Fermentation 2023, 9, 434. https://doi.org/10.3390/fermentation9050434
Jakfar, Husin H, Pontas K, Mamat R, Salleh MR, Zulrika M, Ahmadi. Modification of the Fermentation Process and Papain Enzymes in The Manufacture of Virgin Coconut Oil Using Optimization of Response Surface Methodology, Central Composite Design. Fermentation. 2023; 9(5):434. https://doi.org/10.3390/fermentation9050434
Chicago/Turabian StyleJakfar, Husni Husin, Komala Pontas, Rizalman Mamat, Mohd Rosdi Salleh, Mirna Zulrika, and Ahmadi. 2023. "Modification of the Fermentation Process and Papain Enzymes in The Manufacture of Virgin Coconut Oil Using Optimization of Response Surface Methodology, Central Composite Design" Fermentation 9, no. 5: 434. https://doi.org/10.3390/fermentation9050434
APA StyleJakfar, Husin, H., Pontas, K., Mamat, R., Salleh, M. R., Zulrika, M., & Ahmadi. (2023). Modification of the Fermentation Process and Papain Enzymes in The Manufacture of Virgin Coconut Oil Using Optimization of Response Surface Methodology, Central Composite Design. Fermentation, 9(5), 434. https://doi.org/10.3390/fermentation9050434






