Abstract
(1) Background: Diet and statins are commonly used to treat high cholesterol (CHOL) levels. (2) Aim: To compare adherence to Mediterranean diet (Med-D), orthorexia nervosa (ON), and musculoskeletal pain in individuals in treatment with statins metabolized by CYP3A4, not metabolized by CYP3A4 or red yeast rice (RYR, containing monacolin K: MON-K). (3) Methods: starting from 80 individuals, after the exclusion of those with other causes of possible pain, 56 individuals were selected and divided into three groups according to the type of statin (CYP3A4, NO-CYP3A4 and MON-K). Adherence to the Med-D was evaluated with the MEDScore and a sub-score was calculated for fruit and vegetables consumption (MEDScore-FV). ON and musculoskeletal pain were assessed with the ORTO-15 and with the Nordic Musculoskeletal questionnaires, respectively. A retrospective analysis of CHOL decrease after treatment was conducted. (4) Results: CHOL levels were lower in CYP3A4 and NO-CYP3A4 after treatment (182.4 ± 6.3 and 177.0 ± 7.8 mg/dL, respectively), compared with MON-K (204.2 ± 7.1 mg/dL, p < 0.05). MON-K and CYP3A4 groups had a high prevalence of reported knee pain (33.3% and 18.8%, respectively) than NO-CYP3A4 group (0%, p < 0.05). A high percentage of individuals in MON-K take supplements and nutraceuticals (87.5%), whereas MEDScore-FV was higher in CYP3A4 (9.4 ± 0.2) compared to NO-CYP3A4 (7.6 ± 0.5, p < 0.05). (5) Conclusions: This study suggests that individuals receiving treatment with statins and RYR should be monitored from the perspective of plant foods’ consumption and nutraceutical use, to prevent musculoskeletal pain.
1. Introduction
The additive cholesterol (CHOL)-lowering effects of statins’ treatment and Mediterranean diet (Med-D), rich in fruits, vegetables, monounsaturated and omega 3 fatty acids, are known since 2002 [1]. Although in the ATTICA study [2] a high adherence to Med-D reduced cardiovascular disease (CVD) risk independently of statin use, in the Moli-sani study [3] the association of Med-D and statin decreased the mortality more than Med-D or statin use alone in patients at risk of CVD. Besides, Med-D in combination with red yeast rice (RYR) reduced low-density lipoproteins (LDL) in individuals with statin intolerance [4]. RYR, obtained by the traditional fermentation of cooked rice kernels with a Monascaceae mold, Monascus purpureus [5], by solid-state culture method [6] or by liquid-state culture method [6], contains monacolin K, a weak reversible inhibitor of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, the rate-limiting enzyme of the mevalonate pathway of cholesterol synthesis [7]. Although citrinin (CIT), a nephrotoxic and hepatotoxic mycotoxin, is produced by Monascus spp., it has been reported that dietary supplements contained CIT below the established limit of detection [8]. On the other hand, monacolin K is often associated with berberine in commercial nutraceutical, but scarce standardization and discrepancy between the doses of bioactive molecules reported by the manufacturers and the amounts resulting from analysis of the same products were reported [9]. The European Food Safety Authority (EFSA) Panel on Food Additives and Nutrient Sources added to Food (ANS) in 2018 concluded that exposure to monacolin K from RYR could lead to severe adverse effects on musculoskeletal system [10]. Authorities stated that food supplements containing RYR were of significant safety concern at the dosage of 10 mg/day and that individual cases of severe adverse reactions have been reported for monacolins from RYR at intake levels as low as 3 mg/day [10]. However, Cicero et al. recently concluded that the risk related to 3 to 10 mg/day monacolin K is minimal (mild myalgia in previously severely statin-intolerant subjects) [7]. Despite a meta-analysis suggested an improvement of moderate hypercholesterolemia by RYR without increased incidence of muscular adverse effects [11], in clinical practice experience, 1/18 patients with statin-associated muscle symptoms had side effects after nutraceutical treatment [12]. Recently, most reported adverse drug reactions for RYR were labelled musculoskeletal and connective tissue disorders (n = 64 among 94 case reports) [13]. Monacolin K is structurally identical to lovastatin [11], therefore it is metabolized by cytochrome P450 (CYP)3A4 isoenzyme, such as lovastatin, atorvastatin and simvastatin, whereas rosuvastatin and pravastatin (not susceptible to CYP inhibition) could be less prone to food-drug interactions [14,15]. However, organic anion transporters (OAT)-mediated interactions on rosuvastatin pharmacokinetic have been reported for honey flavonoids [16] and epigallocatechin gallate [17]. Vaquero et al. [15] pointed out that studies on the effect of statins in patients consuming a Med-D are necessary to assure the correct prescription. Relationships between adherence to Med-D and orthorexic behavior [18] and between orthorexia nervosa (ON) and a high level of physical activity [19] have been reported. Despite statins having a safety profile, many patients reported muscle symptoms which contribute to drug discontinuation and reducing physical activity [20]. In this context, a recent study found that sitting time was associated with musculoskeletal symptoms [21]. From that, our hypothesis was that patients in treatment with statins metabolized by CYP3A4 or mon-K could report more pain when consuming plant food potential interfering with phase I metabolism and can be more aware of their food consumption, more adherent to Med-D, and sedentary. Therefore, in this study we aimed to compare adherence to Med-D, ON [22] and self-reported musculoskeletal pain in statin and RYR users. Moreover, the reduction in CHOL levels (retrospectively) and potential confounders, including use of other drugs, physical activity and sitting time, were evaluated.
2. Materials and Methods
This retrospective observational study was approved by the Ethics Committee for Human Non-Clinical Research of the Department of Physiology and Pharmacology “V. Erspamer”, “Sapienza” University of Rome (Approval Date: 18 April 2018) and all procedures involving human subjects, complied with the Declaration of Helsinki as revised in 2000. Adult individuals were recruited (April 2019–February 2020) by pharmacies and verbal disclosures.
During the selection of volunteers, the following inclusion criteria were applied:
- statins or RYR use;
- consent to furnish data from questionnaire;
- consent to furnish analysis report.
A total of 80 volunteers were enrolled and all participants provided a signed informed consent. Information submitted through the questionnaires were used to characterize the subjects and included body mass (BM), height, medical history, postmenopausal state and previous pregnancy and/or spontaneous abortion (for women), use of drugs and supplements, smoking habits and consumption of alcoholic beverages, cocoa, coffee and tea. The body mass index (BMI) was calculated dividing BM (in kg) by squared height (in meter). Primary outcomes were the degree of adherence to the Med-D, ON and the musculoskeletal symptoms. Adherence to the Med-D was calculated through the MEDScore proposed by Panagiotakos [23]. Besides a sub-score for fruit and vegetables was calculated (MEDScore-FV: range 0–10). The ORTO-15 score was assessed according to Donini et al. [22] and also ORTO 12, ORTO-11, ORTO-9 and ORTO-7 were calculated [24].
The musculoskeletal pain was assessed through the Nordic Musculoskeletal Questionnaire (NMQ) [25], previously used to evaluate the effects of statins [26] and musculoskeletal symptoms in workers [21].
Daily sitting time (minutes/day), as well as weekly time spent in walking and in moderate and high intensity activities, were self-reported by the respondents when answering specific items in the International Physical Activity Questionnaire (IPAQ) as previously described [21].
The individuals enrolled in the study were asked to provide diagnostic reports (pre- and post-treatment) and the reduction in CHOL levels was calculated based on the blood test results provided by the volunteers.
Data of the 80 enrolled individuals allowed us to divide the sample into three groups: CYP3A4 (in treatment with lovastatin, atorvastatin or simvastatin), NO-CYP3A4 (in treatment with rosuvastatin or pravastatin) and MON-K (in treatment with RYR). After the exclusion of individuals with other causes of possible pain (Figure 1), 56 individuals were analyzed.
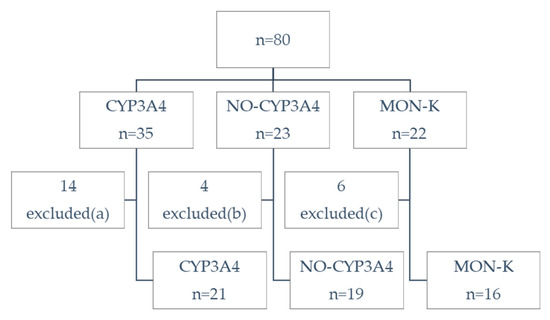
Figure 1.
Flow diagram. CYP3A4: volunteers in treatment with lovastatin, atorvastatin or simvastatin; NO-CYP3A4: volunteers in treatment with rosuvastatin or pravastatin; MON-K: volunteers in treatment with monacolin K (red yeast rice, RYR). Excluded for potential confounders (other causes of pain): (a) 9 injury in the last 12 months, 4 arthritis, 1 in treatment with both atorvastatin and rosuvastatin (b) 4 injury, (c) 5 injury, 1 arthritis.
Statistical Methods
Categorical variables were expressed as percentages and significance assessed by the χ2 test. Continuous variables were expressed as means and standard error of the mean (SEM). Results passing Equal Variance or Normality test (Shapiro–Wilk) were analyzed by analysis of variance (ANOVA) and Student Newman–Keuls method, others by Kruskal–Wallis one-way analysis of variance on ranks and Dunn’s method.
3. Results
Table 1 shows the characteristics of the study sample according to statins or RYR use. Compared to RYR (MON-K) users, statins (CYP3A4 and NO-CYP3A4) users were older (p < 0.001). There were no statistically significant differences by gender and approximately two-thirds of the subjects were women in the group CYP3A4 and MON-K, while in the NO-CYP3A4 users were close to one-third. Menopausal state was present in 71.4% of CYP3A4 women, 85.7% of the NO-CYP3A4 and 45.4% of MON-K.

Table 1.
Characteristics of individuals.
No statistically significant differences were found between the three groups in terms of smoking habits, BMI, blood pressure and heart rate (Table 1), in any case a high prevalence of the subjects declared to take antihypertensive (Table 1).
A higher dose and a longer duration of treatment has been observed in CYP3A4 and NO-CYP3A4 users compared with MON-K (Table 1).
Accordingly, the retrospective analysis of CHOL levels revealed that MON-K was less efficacious than statins (Table 1).
The results related to the use of other drugs show that MON-K users take fewer drugs (as they are younger than CYP3A4 and NO-CYP3A4), but more nutraceuticals (Table 1). In particular, 81% of the products with RYR also contain other lipid-lowering nutraceuticals like policosanols, berberine and polyunsaturated ω-3 fatty acids, at the same time containing vitamins and minerals (56% and 38% of the products, respectively). On the other hand, non-significant difference was found in the prevalence of the use of anti-inflammatory/analgesic drugs (Table 1).
Table 2 shows the self-reported musculoskeletal pain in different anatomical regions for statin users compared to RYR users during the last 7 days. The prevalence of pain was higher among patients in treatment with statins metabolized by CYP3A4 compared with the other groups (NO-CYP3A4 and MON-K), but also individuals in the MON-K group reported lower extremity pain (Table 2).

Table 2.
Musculoskeletal pain.
Few subjects practiced a sport especially in group NO-CYP3A4, while 31% of the subjects in group MON-K practiced at least one sport (Table 3). However, differences in sport practice, physical activity, walking and sitting time did not reach significance (Table 3).

Table 3.
Physical activity and dietary habits.
Table 3 presents the results relating to the dietary habits of the sample. There were no statistically significant differences for adherence to Med-D (MEDScore), whereas individuals in the CYP3A4 group had higher MEDScore-FV compared to NO-CYP3A4.
We observed a non-significant higher prevalence of consumers of fruit juice and tea in MON-K group (Table 3). On the other hand, there were no statistically significant differences for tea, chocolate, coffee, and alcoholic beverages consumption (Table 3). Furthermore, no significant differences in ON were observed among groups (Table 3).
4. Discussion
The prevalence of pain in any region was 66.7%, 42.1% and 68.8% for CYP3A4, NO-CYP3A4 and MON-K, respectively. This is in agreement with our hypothesis and with the conclusion of EFSA Panel [10] and with previous case reports [12,13]. The body regions with the higher prevalence of symptoms in the CYP3A4 group were wrist/hand, knees and ankle/feet. Although these body parts are not the mainly affected muscles from statin-induced myopathy, a case of statin-induced bilateral foot myopathy has been reported [28] and it has been proposed that the decrease in lower extremity muscle-strengthening activities could be related to the increase in muscle pain associated with statin therapy [29]. Knee pains were also reported by MON-K users with a high prevalence compared to individuals in the NO-CYP3A4 group. However, low extremity pain can be due to other causes. A higher percentage of construction workers who practice moderate or vigorous leisure-time physical activity (LTPA), including walking, bicycling, hockey, weight lifting and gardening, reported lower extremity pain (i.e., ankle, knee) compared with those who did not engage in either LTPA (57% and 21%, respectively) [30]. Whereas increasing steps per day reduced pain in adults with fibromyalgia [31] and with musculoskeletal diseases [32], the association of statins and exercise is controversial [33,34,35,36,37] and a case of rhabdomyolysis-induced by atorvastatin and strenuous exercise has been reported [38]. The development of statin-related myopathy may be enhanced by acute and chronic physical exercises. According to the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS), individuals affected by dyslipidemias should be encouraged to practice regular (30 min/day) moderate physical activity, but they have to must to pay attention to myopathy and creatine kinase (CK) elevation related to statin treatment [39]. It has been suggested that obsessive features of sport activities (guilt over skipping training, counting calories during training) play an important role in ON [40]. ON was often seen in men in exercise science studies in combination with a high level of physical activity and a high degree of self-reported pain [19]. Others suggested that obsessive features of sport activities (guilt over skipping training, counting calories during training) play an important role in ON [40]. On the other hand, ON has a bidimensional nature, including the pathological preoccupation for food and the healthy orthorexia, which could possibly be seen as a protective behaviour [41,42,43]. For example, ON symptoms were positively correlated to physical activity, fruit and vegetable consumption, body appreciation, and life satisfaction, in Chinese elderly [44]. In the present study prevalence of ON was about double when cut-off of 40 was applied, compared to cut-off of 35, as assessed with ORTO-15, with no differences among groups and there were no significant differences also for sub-scores ORTO-12, ORTO-11, ORTO-9 and ORTO-7. In other studies ON was associated with the use of dietary supplements [45,46]. Although no significant differences in ON were observed, individuals in the MON-K group used more nutraceuticals than other groups. In this context, omega-3 polyunsaturated fatty acids (omega-3 PUFA) supplementation has been suggested for preventing or treating statin myopathy [47] and for inflammation-mediated pain in sarcopenic elderlies [48]. Although a meta-analysis found that supplementation with omega-3 PUFA did not result in a clinically relevant reduction of muscle soreness after eccentric exercise [49], omega-3 PUFA are among the antinociceptive and analgesic natural compounds [50]. The analgesic effect can mask the reported pain due to the statin-induced myopathy. In the present study, the use of nutraceuticals is statistically significant, with a prevalence of 81.3% in MON-K users. MON-K group includes more individuals who take omega 3 fatty acids, and this could be a confounding factor for self-reported pain. On the other hand, the prevalence of oral analgesic use, in CYP3A4 (42.8%) and NO-CYP3A4 (42.1%), was similar to that previously reported in individuals with musculoskeletal disorders with lipid-lowering drug use (41.0%) [26]. On the contrary, in the MON-K group the prevalence of analgesic use (18.8%) was comparable to that found in the subjects with lipid-lowering drug use without musculoskeletal disorders (19.0%) [26].
However, a limitation of this study is that volunteers in the MON-K group were younger compared to those in the other groups. On the other hand, MEDScore-FV was higher in the CYP3A4 group, compared to the NO-CYP3A4. This result prevented us from investigating the potential interactions between flavonoid-rich foods and statins [7].
In addition to fruit juices, particularly grapefruit juice known to inhibit CYP3A4 by reducing the metabolism of simvastatin, lovastatin and atorvastatin [14,15], tea flavanols can interfere with the pharmacokinetics of drugs in humans, by interaction with CYP3A4 and with phase III transporters of the drug detoxifying system, mainly the multidrug resistance 1 (MDR1) [51], OAT [17,51], and organic cation transporters (OCT) [51]. However, the consumption of fruits and vegetables [52,53], rich in antioxidant flavonoids, as well as Med-D and physical activity [53], can reduce musculoskeletal pain. Med-D can reduce chronic pain [54], and a high adherence to the Med-D was associated with pain improvement in older adults recruited in the Seniors-ENRICA-1 and Seniors-ENRICA-2 cohorts [55]. Furthermore, the health effects of Med-D are well documented [1,2,3,4] and relationships between adherence to Med-D and ON [18] have been reported. However, in this pilot study we did not observe the hypothesized differences among groups in the adherence to Med-D and in ON. Besides, the hypothesized differences among groups in sport practice, physical activity, walking and sitting time did not reach significance. The major limitation of our study is the relatively limited sample size and further investigation would be desirable. Moreover, non-probabilistic sampling makes our preliminary results not applicable to general population.
5. Conclusions
In line with the recommendation of other authors [15], this pilot study suggested that individuals receiving treatment with statins and RYR should be monitored from the perspective of plant foods’ consumption and the use of nutraceuticals that can reduce pain or interfere with statins’ pharmacokinetic, to prevent musculoskeletal pain. Moreover, self-reported pain should be evaluated in conjunction with physical activity level [20,21]. Despite statins and RYR having a safety profile [11], our results confirm the needs of further investigation to finally describe the safety profile of these drugs metabolized by CYP3A4. Appropriate information about the potential adverse effects of RYR on musculoskeletal system should be provided to clinicians and patients also in order to submit suspected adverse effects to agencies and continuous monitoring the safety of “natural” dietary supplements.
Author Contributions
Conceptualization, I.P.; investigation, A.R., E.T., M.P., M.M.A.-D. and I.P.; writing, A.R., E.T., M.P., M.M.A.-D. and I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the by the Ethics Committee for Human Non-Clinical Research of the Department of Physiology and Pharmacology “V. Erspamer”, “Sapienza” University of Rome.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Individuals’ data availability is restricted by the Ethics Committee to protect privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jula, A.; Marniemi, J.; Huupponen, R.; Virtanen, A.; Rastas, M.; Ronnemaa, T. Effects of diet and simvastatin on serum lipids, insulin, and antioxidants in hypercholesterolemic men: A randomized controlled trial. JAMA 2002, 287, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Panagiotakos, D.B.; Georgousopoulou, E.N.; Georgiopoulos, G.A.; Pitsavos, C.; Chrysohoou, C.; Skoumas, I.; Ntertimani, M.; Laskaris, A.; Papadimitriou, L.; Tousoulis, D.; et al. Adherence to Mediterranean Diet Offers an Additive Protection Over the Use of Statin Therapy: Results from the ATTICA Study (2002–2012). Curr. Vasc. Pharmacol. 2015, 13, 778–787. [Google Scholar] [CrossRef]
- Bonaccio, M.; di Castelnuovo, A.; Costanzo, S.; Persichillo, M.; de Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Interaction between Mediterranean diet and statins on mortality risk in patients with cardiovascular disease: Findings from the Moli-sani Study. Int. J. Cardiol. 2019, 276, 248–254. [Google Scholar] [CrossRef]
- Sartore, G.; Burlina, S.; Ragazzi, E.; Ferraresso, S.; Valentini, R.; Lapolla, A. Mediterranean Diet and Red Yeast Rice Supplementation for the Management of Hyperlipidemia in Statin-Intolerant Patients with or without Type 2 Diabetes. Evid. Based Complement. Alternat. Med. 2013, 2013, 743473. [Google Scholar] [CrossRef]
- Zhu, B.; Qi, F.; Wu, J.; Yin, G.; Hua, J.; Zhang, Q.; Qin, L. Red Yeast Rice: A Systematic Review of the Traditional Uses, Chemistry, Pharmacology, and Quality Control of an Important Chinese Folk Medicine. Front. Pharmacol. 2019, 10, 1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukami, H.; Higa, Y.; Hisano, T.; Asano, K.; Hirata, T.; Nishibe, S. A Review of Red Yeast Rice, a Traditional Fermented Food in Japan and East Asia: Its Characteristic Ingredients and Application in the Maintenance and Improvement of Health in Lipid Metabolism and the Circulatory System. Molecules 2021, 26, 1619. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Zambon, A. Red Yeast Rice for Hypercholesterolemia: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 620–628. [Google Scholar] [CrossRef]
- Twaruzek, M.; Altyn, I.; Kosicki, R. Dietary Supplements Based on Red Yeast Rice-A Source of Citrinin? Toxins 2021, 13, 497. [Google Scholar] [CrossRef] [PubMed]
- Marcheluzzo, S.; Faggian, M.; Zancato, M.; Peron, G. Analysis of Monacolins and Berberine in Food Supplements for Lipid Control: An Overview of Products Sold on the Italian Market. Molecules 2021, 26, 2222. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of monacolins in red yeast rice. EFSA J. 2018, 16, e05368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fogacci, F.; Banach, M.; Mikhailidis, D.P.; Bruckert, E.; Toth, P.P.; Watts, G.F.; Reiner, Z.; Mancini, J.; Rizzo, M.; Mitchenko, O.D.; et al. Blood Pressure Meta-analysis Collaboration, and P. International Lipid Expert, Safety of red yeast rice supplementation: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 143, 1–16. [Google Scholar] [CrossRef]
- Ward, N.C.; Pang, J.; Ryan, J.D.M.; Watts, G.F. Nutraceuticals in the management of patients with statin-associated muscle symptoms, with a note on real-world experience. Clin. Cardiol. 2018, 41, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Vrolijk, M.F.; van de Koppel, S.; van Hunsel, F. Red yeast rice (Monascus purpureus) supplements: Case series assessment of spontaneously reported cases to The Netherlands Pharmacovigilance Centre Lareb. Br. J. Clin. Pharmacol. 2021, 87, 2146–2151. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Palmery, M.; Serafini, M. Association of flavonoid-rich foods and statins in the management of hypercholesterolemia: A dangerous or helpful combination? Curr. Drug Metab. 2015, 16, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, M.P.; Muniz, F.J.S.; Redondo, S.J.; Olivan, P.P.; Higueras, F.J.; Bastida, S. Major diet-drug interactions affecting the kinetic characteristics and hypolipidaemic properties of statins. Nutr. Hosp. 2010, 25, 193–206. [Google Scholar]
- Navratilova, L.; Mandikova, J.R.; Pavek, P.; Mladenka, P.; Trejtnar, F. Honey flavonoids inhibit hOATP2B1 and hOATP1A2 transporters and hOATP-mediated rosuvastatin cell uptake in vitro. Xenobiotica 2018, 48, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xu, Q.; Liu, L.; Bian, Y.; Zhang, S.; Huang, C.; Miao, L. Effect of Green Tea and (-)-Epigallocatechin Gallate on the Pharmacokinetics of Rosuvastatin. Curr. Drug Metab. 2020, 21, 471–478. [Google Scholar] [CrossRef]
- Strahler, J.; Hermann, A.; Walter, B.; Stark, R. Orthorexia nervosa: A behavioral complex or a psychological condition? J. Behav. Addict. 2018, 7, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Malmborg, J.; Bremander, A.; Olsson, M.C.; Bergman, S. Health status, physical activity, and orthorexia nervosa: A comparison between exercise science students and business students. Appetite 2017, 109, 137–143. [Google Scholar] [CrossRef]
- Pedro-Botet, J.; Climent, E.; Benaiges, D. Muscle and statins: From toxicity to the nocebo effect. Expert Opin. Drug Saf. 2019, 18, 573–579. [Google Scholar] [CrossRef]
- Moreira-Silva, I.; Azevedo, J.; Rodrigues, S.; Seixas, A.; Jorge, M. Predicting musculoskeletal symptoms in workers of a manufacturing company. Int. J. Occup. Saf. Ergon. 2019, 1–9, ahead of print. [Google Scholar] [CrossRef]
- Donini, L.M.; Marsili, D.; Graziani, M.P.; Imbriale, M.; Cannella, C. Orthorexia nervosa: Validation of a diagnosis questionnaire. Eat. Weight Disord. 2005, 10, e28–e32. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Milias, G.A.; Pitsavos, C.; Stefanadis, C. MedDietScore: A computer program that evaluates the adherence to the Mediterranean dietary pattern and its relation to cardiovascular disease risk. Comput. Methods Programs Biomed. 2006, 83, 73–77. [Google Scholar] [CrossRef]
- Toti, E.; Cavedon, V.; Raguzzini, A.; Fedullo, A.L.; Milanese, C.; Bernardi, E.; Bellito, S.; Bernardi, M.; Sciarra, T.; Peluso, I. Dietary Intakes and Food Habits of Wheelchair Basketball Athletes compared to Gym Attendees and Individuals who do not Practice Sport Activity. Endocr. Metab. Immune Disord. Drug Targets 2021. ahead of print. [Google Scholar] [CrossRef]
- Gobba, F.G.R.; Martinelli, S.; Richeldi, A.; Clerici, P.; Grazioli, P. Traduzione in lingua italiana e validazione del questionario standardizzato Nordic IRSST per la rilevazione di disturbi muscoloscheletrici. Med. Lav. 2008, 99, 424–443. [Google Scholar] [PubMed]
- Mosshammer, D.; Schwarz, J.; Meznaric, S.; Muche, R.; Lorenz, G.; Morike, K. Analgesic drug use associated with statin prescription--a cross-sectional study in primary care settings. Curr. Drug Saf. 2012, 7, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Babeau, C.; le Chevanton, T.; Julien-Sweerts, S.; Brochenin, A.; Donini, L.M.; Fouques, D. Structural validation of the ORTO-12-FR questionnaire among a French sample as a first attempt to assess orthorexia nervosa in France. Eat. Weight Disord. 2019, 25, 1771–1778. [Google Scholar] [CrossRef]
- Baggett, M.C.; Nykamp, D. Statin-Associated Bilateral Foot Myopathy. J. Pharm. Pract. 2019, 33, 899–902. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Loprinzi, P.D. Statin use may reduce lower extremity peak force via reduced engagement in muscle-strengthening activities. Clin. Physiol. Funct. Imaging 2018, 38, 151–154. [Google Scholar] [CrossRef]
- Caban-Martinez, A.J.; Lowe, K.A.; Herrick, R.; Kenwood, C.; Gagne, J.J.; Becker, J.F.; Schneider, S.P.; Dennerlein, J.T.; Sorensen, G. Construction workers working in musculoskeletal pain and engaging in leisure-time physical activity: Findings from a mixed-methods pilot study. Am. J. Ind. Med. 2014, 57, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Kaleth, A.S.; Slaven, J.E.; Ang, D.C. Does increasing steps per day predict improvement in physical function and pain interference in adults with fibromyalgia? Arthritis Care Res. 2014, 66, 1887–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansi, S.; Milosavljevic, S.; Baxter, G.D.; Tumilty, S.; Hendrick, P. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskelet. Disord. 2014, 15, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansi, I.; Frei, C.R.; Pugh, M.J.; Makris, U.; Mortensen, E.M. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern. Med. 2013, 173, 1318–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, H.B. Statin-induced Myopathy in Skeletal Muscle: The Role of Exercise. J. Lifestyle Med. 2014, 4, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, B.A.; Thompson, P.D. Effect of statins on skeletal muscle: Exercise, myopathy, and muscle outcomes. Exerc. Sport Sci. Rev. 2012, 40, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfim, M.R.; Oliveira, A.S.; Amaral, S.L.d.; Monteiro, H.L. Treatment of dyslipidemia with statins and physical exercises: Recent findings of skeletal muscle responses. Arq. Bras. Cardiol. 2015, 104, 324–331. [Google Scholar] [CrossRef]
- Parker, B.A.; Capizzi, J.A.; Grimaldi, A.S.; Clarkson, P.M.; Cole, S.M.; Keadle, J.; Chipkin, S.; Pescatello, L.S.; Simpson, K.; White, C.M.; et al. Effect of statins on skeletal muscle function. Circulation 2013, 127, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Dunphy, L.; Morhij, R.; Tucker, S. Rhabdomyolysis-induced compartment syndrome secondary to atorvastatin and strenuous exercise. BMJ Case Rep. 2017, 2017, bcr2016218942. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Bajraktari, G.; Miserez, A.R.; Cicero, A.F.G.; Bruckert, E.; Serban, M.C.; Mirrakhimov, E.; Alnouri, F.; Reiner, Z.; et al. Statin therapy in athletes and patients performing regular intense exercise—Position paper from the International Lipid Expert Panel (ILEP). Pharmacol. Res. 2020, 155, 104719. [Google Scholar] [CrossRef]
- Kiss-Leizer, M.; Toth-Kiraly, I.; Rigo, A. How the obsession to eat healthy food meets with the willingness to do sports: The motivational background of orthorexia nervosa. Eat. Weight Disord. 2019, 24, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Barthels, F.; Barrada, J.R.; Roncero, M. Orthorexia nervosa and healthy orthorexia as new eating styles. PLoS ONE 2019, 14, e0219609. [Google Scholar] [CrossRef] [Green Version]
- Depa, J.; Barrada, J.R.; Roncero, M. Are the Motives for Food Choices Different in Orthorexia Nervosa and Healthy Orthorexia? Nutrients 2019, 11, 697. [Google Scholar] [CrossRef] [Green Version]
- Strahler, J. Trait mindfulness differentiates the interest in healthy diet from orthorexia nervosa. Eat. Weight Disord. 2021, 26, 993–998. [Google Scholar] [CrossRef]
- He, J.; Zhao, Y.; Zhang, H.; Lin, Z. Orthorexia nervosa is associated with positive body image and life satisfaction in Chinese elderly: Evidence for a positive psychology perspective. Int. J. Eat. Disord. 2021, 54, 212–221. [Google Scholar] [CrossRef]
- Oberle, C.D.; Klare, D.L.; Patyk, K.C. Health beliefs, behaviors, and symptoms associated with orthorexia nervosa. Eat. Weight Disord. 2019, 24, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Tari Selcuk, K.; Cevik, C. Use of dietary supplements among nursing students in Turkey in the last 12 months and its relation with orthorexia nervosa. Perspect. Psychiatr. Care 2020, 56, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, S.A.; Popp, O.; Haafke, S.; Jedraszczak, N.; Grieben, U.; Saar, K.; Patone, G.; Kress, W.; Steinhagen-Thiessen, E.; Dittmar, G.; et al. Statin-induced myopathic changes in primary human muscle cells and reversal by a prostaglandin F2 alpha analogue. Sci. Rep. 2020, 10, 2158. [Google Scholar] [CrossRef] [Green Version]
- Perna, S.; Alalwan, T.A.; Al-Thawadi, S.; Negro, M.; Parimbelli, M.; Cerullo, G.; Gasparri, C.; Guerriero, F.; Infantino, V.; Diana, M.; et al. Evidence-Based Role of Nutrients and Antioxidants for Chronic Pain Management in Musculoskeletal Frailty and Sarcopenia in Aging. Geriatrics 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Z.-T.; Zhang, J.-M.; Zhu, W.-T. Omega-3 Polyunsaturated Fatty Acid Supplementation for Reducing Muscle Soreness after Eccentric Exercise: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomed. Res. Int. 2020, 2020, 8062017. [Google Scholar] [CrossRef] [Green Version]
- Bjorklund, G.; Aaseth, J.; Dosa, M.D.; Pivina, L.; Dadar, M.; Pen, J.J.; Chirumbolo, S. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition 2019, 66, 153–165. [Google Scholar] [CrossRef]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendonca, C.R.; Noll, M.; Castro, M.C.R.; Silveira, E.A. Effects of Nutritional Interventions in the Control of Musculoskeletal Pain: An Integrative Review. Nutrients 2020, 12, 3075. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, J.K.; Blafoss, R.; Sundstrup, E.; Bay, H.; Pastre, C.M.; Andersen, L.L. Association between lifestyle and musculoskeletal pain: Cross-sectional study among 10,000 adults from the general working population. BMC Musculoskelet. Disord. 2019, 20, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, A.S.; Strath, L.J.; Sorge, R.E. Dietary Interventions for Treatment of Chronic Pain: Oxidative Stress and Inflammation. Pain Ther. 2020, 9, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Ortola, R.; Garcia-Esquinas, E.; Sotos-Prieto, M.; Struijk, E.A.; Caballero, F.F.; Lopez-Garcia, E.; Rodriguez-Artalejo, F. Mediterranean diet and changes in frequency, severity and localization of pain in older adults: The Seniors-ENRICA cohorts. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, glab109, ahead of print. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).