Abstract
Metal hydrides have recently been proposed for not only hydrogen storage materials but also high-efficiency thermal storage materials. NaMgH3 contains a considerable theoretical thermal storage density of 2881 kJ/kg. However, its sluggish de/re-hydrogenation reaction kinetics and poor cycling stability exhibit unavailable energy efficiency. Doping with active catalyst into NaMgH3 is deemed to be a potential strategy to mitigate these disadvantages. In this work, the enhancement of de/re-hydrogenation kinetics and cycling properties of NaMgH3 is investigated by doping with lamellar-structure 2D carbon-based MXene, Ti3C2. Results shows that introducing 7 wt.% Ti3C2 is proved to perform excellent catalytic efficiency for NaMgH3, dramatically reducing the two-step hydrogen desorption peak temperatures (324.8 and 345.3 °C) and enhancing the de/re-hydrogenation kinetic properties with the hydrogen desorption capacity of 4.8 wt.% H2 within 15 min at 365 °C and absorption capacity of 3.5 wt.% H2 within 6 s. Further microstructure analyses reveal that the unique lamellar-structure of Ti3C2 can separate the agglomerated NaMgH3 particles homogeneously and decrease the energy barriers of two-step reaction of NaMgH3 (114.08 and 139.40 kJ/mol). Especially, lamellar-structure Ti3C2 can improve the reversibility of hydrogen storage of NaMgH3, rendering 4.6 wt.% H2 capacity remained after five cycles. The thermal storage density of the composite is determined to be 2562 kJ/kg through DSC profiles, which is suitable for thermal energy storage application.
1. Introduction
Sodium-based hydrides such as NaH, MgH2 (simple binary hydrides), NaBH4 (borohydride), NaAlH4 (alanates hydride), and NaMgH3 (perovskite hydride) have been researched as promising candidates for hydrogen storage or thermal energy storage during the past decades [1,2,3,4,5,6,7,8]. As the GdFeO3-type perovskite (space group Pnma) hydride, NaMgH3 has received considerable attention for its high gravimetric and volumetric hydrogen densities (6 wt.% and 88 kg/m3) and possesses a considerable theoretical thermal storage gravimetric density (2881 kJ/kg) with reversible two-step de/re-hydrogenation process [9,10,11,12,13,14,15]:
NaMgH3 → NaH + Mg + H2
NaH + Mg → Na + Mg + 1/2 H2
Especially, NaMgH3 is considered as a potential thermal energy medium for concentration solar power (CSP) because of the high thermodynamic stability [16]. In particular, as a suitable candidate for thermal energy storage (TES), NaMgH3 has several obvious advantages: high hydrogen storage capacity, low and flat hydrogen de/absorption pressure and plateau, negligible hysteresis, high thermal storage density, and low cost of raw materials [7]. In addition, de/re-hydrogenation kinetics performance and cycling properties are also important parameters to determine whether it is available to TES. However, the further applications to thermal energy storage are limited by sluggish de/re-hydrogenation kinetic performance and pronounced degradation of cycling properties of NaMgH3.
Much attention has been focused on improving de/re-hydrogenation kinetics performance and cycling properties of NaMgH3 during the last few decades [17,18,19,20,21]. It has been proved that adding Li or K into NaMgH3 would make Li or K partially take the place of the Na position and the formation of distorted perovskite structure would enhance the de-hydrogenation kinetics performance [17,18]. Li et al. [19] improved the cycling properties through in situ embedding of Mg2NiH4 and YH3 nanoparticles in NaMgH3. Except alkali metal and metal hydrides, the Ti-based catalysts have been found to possess remarkably high catalytic activity. Wang’s group [20] decreased the onset desorption temperature of NaMgH3 to 328 K with 153.47 kJ/mol by introduced K2TiF6 as a dopant. Moreover, it was confirmed that TiF3 can significantly reduce the activation energy of NaMgH3 to 104 kJ/mol [18].
As a new type of two-dimension materials, MXenes have been successfully synthesized through etching ternary carbides, nitrides, or carbonitrides (MAX) with HF or in situ HF [22,23,24,25]. The general formula of MXenes is Mn+1XnTx (n = 1–3), where M represents a transition metal, X is carbon and/or nitrogen, and Tx stands for the surface terminations [26]. The carbide transition metal MXene, such as Ti3C2, possesses a particularly lamellar structure, showing promising application as an anode material for Li-ion batteries [27,28]. Furthermore, much effort has been devoted to exploring the superior catalytic effect of Ti3C2 on de/re-hydrogenation reactions of hydrides [29,30,31,32,33,34]. Liu et al. [29,30] observed a strong catalytic effect of Ti3C2 in de/re-hydrogenation kinetics properties on both MgH2 and NaAlH4. In particular, Ti3C2 tremendously improved the cycling stability of NaAlH4, with the de/re-hydrogenation performance remaining nearly constant over 10 cycles. The superior catalytic efficiency of Ti3C2 has also been proven on LiNa2AlH6 and Li1.3Na1.7AlH6 [31,32]. Therefore, the superior catalytic efficiency of the lamellar-structure Ti3C2 is highly anticipated to expand to the NaMgH3.
There are indeed many other higher hydrogen storage capacity compounds, such as LiBH4 with 18.5 wt.%, MgH2 with 7.6 wt.% and NaAlH4 with 5.6 wt.% [35,36]. Although the reversible hydrogen storage capacity of NaMgH3 is about 6 wt.%, it exhibits other engineering feasibility in heat storage, accompanying the reversible hydrogen storage process [37]. From pressure–composition isotherm (PCI) measurements, the enthalpy, ΔH(des), and entropy, ΔS(des), of desorption for Equation (1) were determined as 86.6 kJ·mol−1 H2 and 132.2 J·mol−1·H2·K−1. The enthalpy and entropy of desorption for Equation (2) correspond to those for pure NaH (ΔH(des) = 116 kJ·mol−1·H2 and ΔS(des) = 168.2 J·mol−1·H2·K−1). The theoretical gravimetric heat storage capacity of Equations (1) and (2) is 2881 kJ·kg−1 (Equation (1) = 1721 kJ·kg−1, Equation (2) = 1160 kJ·kg−1). Therefore, NaMgH3 would be an ideal thermal storage medium during the hydrogen storage process. However, further applications for thermal energy storage are limited by sluggish de/re-hydrogenation kinetic performance and pronounced degradation of NaMgH3 cycling properties.
In this work, the 2D MXenes Ti3C2 with a unique lamellar-structure was introduced into NaMgH3 for enhancing its de/re-hydrogenation kinetics and cycling behaviors for the first time. Five composites including NaMgH3-x wt.% Ti3C2 (x = 0, 3, 5, 7, and 9) were synthesized via ball-milling. Among them, the 7 wt.% Ti3C2-containing NaMgH3 shows the optimal de/re-hydrogenation storage performance as it can desorb 4.8 wt.% H2 in 15 min at 365 °C and then can be easily hydrogenated at 300 °C under 80 bar H2. More importantly, the reversible hydrogen storage performance stays at a relatively high level after 5 cycles. It is confirmed that the 2D Ti3C2 can disperse the agglomerated NaMgH3 particles homogeneously, decrease the activation energy of NaMgH3, and prevent Na from separating Mg during the cycles. Moreover, the thermal storage density for NaMgH3-7 wt.% Ti3C2 is evaluated to be 2562 kJ/kg through DSC profile.
2. Materials and Methods
The lamellar-structure Ti3C2 was prepared via etching the Ti3AlC2 (98% purity, Laizhou Kai Xi Ceramic Materials Co., Ltd., Laizhou, China) with the HF, which was mixed by combining 15 mL HCl (12 M), 15 mL deionized water and 1.98 g LiF. Then 3 g Ti3AlC2 precursor was immersed into the in situ HF and transferred to a 100 mL Teflon-lined autoclave. The Ti3AlC2 was treated with a 48-hour heat treatment in an oil bath at 60 °C. After heat treatment, the precursor was washed with deionized water through centrifugation treatment. The final pH of this precursor shoul−d reach 6 and then further drying could be carried out. The drying process was operated at 80 °C for 24 h under vacuum.
The as-formed lamellar-structure Ti3C2 was doped into NaMgH3 as a catalyst to enhance de/re-hydrogenation kinetics and cycling properties. Five composites of NaMgH3-x wt.% Ti3C2 (x = 0, 3, 5, 7, and 9) were synthesized by milling with stainless steel balls of which the weight ratio to composites was 40:1 under 10 bar H2 for 12 h at 400 rpm using a planetary ball mill (QM-3SP4, Nanjing, China).
The phase transformations, structures and morphological features of Ti3C2 and NaMgH3-x wt.% Ti3C2 (x = 0, 3, 5, 7, and 9) composites were characterized via X-ray diffraction (XRD, PANalytical, The Netherlands, Cu Kα) and scanning electron microscopy (SEM, Hitachi FSEM, SU-70, Tokyo, Japan). During the XRD and SEM analyses, all samples were protected by a laboratory-made container filled with argon for preventing samples from reacting with oxygen and water. Additionally, the de/re-hydrogenation performance of NaMgH3-x wt.% Ti3C2 (x = 0, 3, 5, 7, and 9) composites were examined by a homemade Sieverts-type apparatus. The temperature-programmed desorption (TPD) analyses were tested with the heating rate of 5 °C/min from 25 to 450 °C under vacuum. The hydrogen desorption kinetics of all composites were examined at 365 and 350 °C with a base pressure of 3 × 10−2 bar H2. The hydrogen absorption kinetics were examined at 400, 350, and 300 °C under 80 bar H2 for 1 h. The cycling property tests were determined by a repeated de/re-hydrogenation procedure at 400 °C, but changing the back pressure from 3 × 10−2 to 60 bar H2. The gravimetric heat storage capacity and heat change of composites were determined using a differential scanning calorimeter (DSC).
3. Results and Discussion
First, the successful synthesis of lamellar-structure MXene Ti3C2 was confirmed through XRD and SEM analyses (Figure 1). It is obvious that the strongest and sharpest XRD diffraction peaks of Ti3AlC2 at 2θ = 39° totally disappeared after etching treatment, which were replaced by another curve, the XRD pattern of Ti3C2. The diffraction peaks of as-formed Ti3C2 belonging to (002) and (004) are significantly broadened and shift to lower angles. Especially, the diffraction (002) peak was much stronger than before. Furthermore, the insert SEM image obviously suggests that the as-formed Ti3C2 exhibited a 2D lamellar-structure morphology, which was etched by in situ HF treatment and effectively separated by LiF [22].
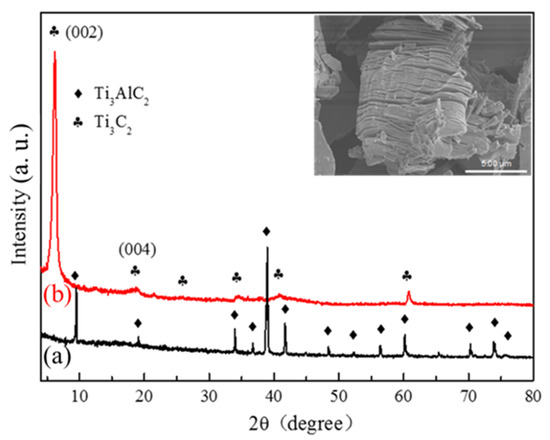
Figure 1.
XRD patterns of (a) Ti3AlC2 and (b) as-prepared Ti3C2. The insert is the SEM image of Ti3C2.
Five composites of NaMgH3-x wt.% Ti3C2 (x = 0, 3, 5, 7, and 9) were formed/synthesized by doping the as-prepared Ti3C2 into NaMgH3 by ball milling, and the phase components and structures were determined by XRD, as displayed in Figure 2. As can be seen from the XRD analyses, only NaMgH3 and MgO can be obviously identified in all five composites. The appearance of MgO phase is due to the impurity of Mg powders during the synthesis of pure NaMgH3. It is almost impossible to find the diffraction peaks belonging to lamellar-structure Ti3C2 in these five patterns due to the small amounts of Ti3C2 additive [34]. The other possibility is that the ball milling destroyed the multilayered structure of Ti3C2. Therefore, the XRD results indicate the successful synthesis of the five composites without any other impurity peaks.
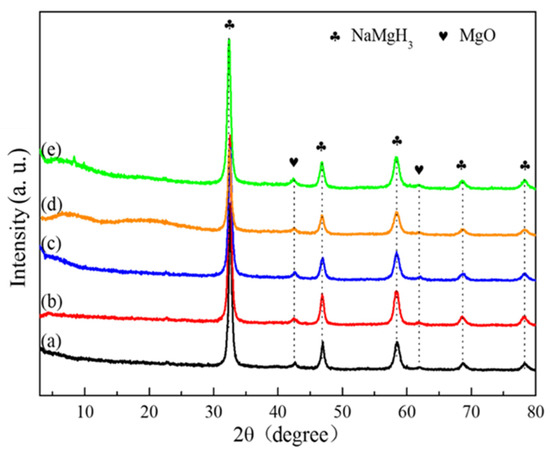
Figure 2.
XRD patterns of (a) pure NaMgH3, (b) NaMgH3-3 wt.% Ti3C2, (c) NaMgH3-5 wt.% Ti3C2, (d) NaMgH3-7 wt.% Ti3C2, and (e) NaMgH3-9 wt.% Ti3C2.
After assessing the phase components and morphology of NaMgH3-x wt.% Ti3C2 (x = 0, 3, 5, 7, and 9) samples, the hydrogen desorption kinetics of all samples were evaluated by DSC, TPD, and isothermal dehydrogenation measurements (Figure 3). As expected, doping a certain amount of Ti3C2 remarkably decreases the hydrogen desorption temperature in contrast to pure NaMgH3 (Figure 3a). The first- and second-step dehydrogenation temperatures of NaMgH3-3 wt.% Ti3C2 decreased from 372.3 and 380.3 °C to 344.5 and 358.4 °C, exhibiting 27.8 and 21.9 °C reduction, respectively. With increasing the Ti3C2 content to 5, 7, or 9 wt.%, the first-step dehydrogenation temperatures are further reduced to 327.5, 324.8 and 320.3 °C, respectively. Meanwhile, the second-step dehydrogenation temperatures of these samples declined to 341.3, 345.3, and 341.8 °C, respectively.
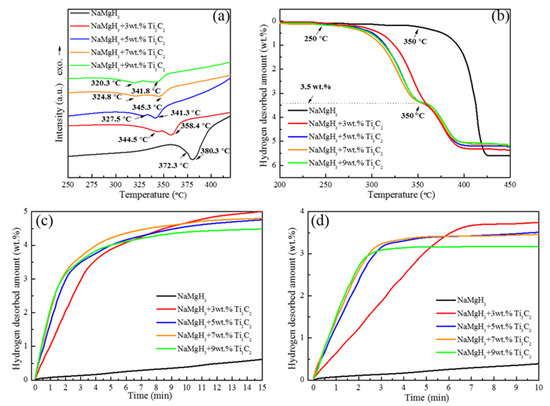
Figure 3.
(a) DSC, (b) TPD, and isothermal dehydrogenation curves at (c) 365 °C and (d) 350 °C for NaMgH3-x wt.% Ti3C2 (x = 0, 3, 5, 7, and 9) samples.
Combined with the TPD results (Figure 3b), more hydrogen desorption kinetic properties of NaMgH3 undoped/doped with Ti3C2 are revealed. First, doping with 3 wt.% Ti3C2 reduces the onset dehydrogenation temperature of pure NaMgH3 tremendously, from 350 °C to 250 °C. In particular, further increasing the content of Ti3C2 to 5, 7, or 9 wt.% allows around 3.5 wt.% H2 to release (first-step decomposition reaction) below 350 °C. Importantly, the Ti3C2 sample containing 7 wt.% performs faster dehydrogenation kinetics than that of others below 350 °C. Nevertheless, the dehydrogenation capacity suffers a small loss, from 5.6 wt.% H2 to 5.4, 5.3, 5.2, and 5.1 wt.% H2, for the NaMgH3-x wt.% Ti3C2 (x = 3, 5, 7, and 9) samples, respectively, which is mainly due to the dead weight of Ti3C2 and existence of MgO.
More specific dehydriding kinetic characterizations were carried out through the isothermal dehydrogenation measurements at 365 and 350 °C (Figure 3c,d). Just as shown by the earlier research, the sample containing 7 wt.% Ti3C2 exhibits the best dehydriding kinetic properties at 365 and 350 °C, desorbing 4.8 wt.% H2 in 15 min and 3.4 wt.% H2 in 5 min, respectively. However, for the pure NaMgH3, only 0.6 wt.% H2 is released in 15 min at 365 °C. As the temperature decreases to 350 °C, the pure NaMgH3 sample only desorbs 0.2 wt.% H2 in 5 min.
As a result, it is obvious that doping of 7 wt.% Ti3C2 leads to a tremendous improvement in the dehydriding kinetic properties of NaMgH3. Furthermore, considering the observed results, including the dehydrogenation temperatures, dehydrogenation kinetic performance, and dehydrogenation capacities, the NaMgH3-7 wt.% Ti3C2 composite exhibits the best energy efficiency and represents the optimal constituent.
To further understand the reason for such a huge dehydrogenation property gap between NaMgH3-7 wt.% Ti3C2 and pure NaMgH3, the DSC analyses were measured at different heating rates (1, 2, 5, and 8 °C/min) (Figure 4a,b). The activation energy (Ea) corresponding to the two-step dehydrogenation reactions of both two composites are quantitatively shown in Figure 4c and Table 1, determined by Kissinger fitting methods [38] (Equation (3)).
where β is the heating rate, Tp represents peak temperature, A is the pre-exponential factor, and R represents the gas constant.
ln(β/Tp2) = −Ea/RTp + ln(AR/Ea)
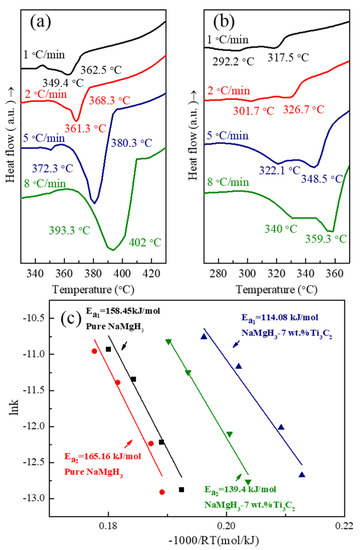
Figure 4.
DSC curves for (a) pure NaMgH3; (b) NaMgH3-7 wt.% Ti3C2 at different heating rates; and Kissinger plots of (c) first- and second-step dehydrogenation of both samples.

Table 1.
Activation energies (Ea) for the dehydrogenation of the NaMgH3 sample undoped and doped with 7 wt.% Ti3C2.
Based on these data, the values of Ea of the first-step hydrogen desorption reaction of NaMgH3 and NaMgH3-7 wt.% Ti3C2 are calculated to be 158.45 and 114.08 kJ/mol, respectively. For the second-step reaction, the value of Ea declines from 165.16 to 139.40 kJ/mol by doping with 7 wt.% Ti3C2. The reduction of Ea for both two reactions demonstrates that the improved dehydriding properties of NaMgH3 can be ascribed to the Ti3C2 dopant by reducing the second-step dehydrogenation energy barrier.
The role played by unique lamellar-structure Ti3C2 during the dehydrogenation reactions was researched and explored. Figure 5a,b shows the SEM images of the pure NaMgH3 sample, in which some small particles with average size of approximately 1 µm agglomerate together to form a larger cluster. In Figure 5c, the results of EDS analyses further confirm the results of the XRD measurements noted above, suggesting the successful synthesis of NaMgH3. In contrast, the large layer-structure (about 10 µm wide) of Ti3C2 is completely broken into smaller lamellar-structure (about 2 µm wide) during the ball-milling procedure, with some tiny NaMgH3 particles attached, as shown in Figure 5d,e. Furthermore, after 12 h ball milling, the agglomerated NaMgH3 particles were separated homogeneously by the lamellar-structure Ti3C2. The following EDS analyses (Figure 5f) prove the existence of NaMgH3 and Ti3C2. Based on the significant reduction of operating temperature and the superior enhancement of dehydrogenation kinetics properties in the Ti3C2 doped sample, it can be proposed that Ti3C2 plays the role of catalyst to improve the dehydrogenation kinetics properties of NaMgH3 hydride, which is in agreement with our previous papers in Ti3C2-doped Mg(BH4)2 [33] and sodium alanate systems [34]. Apparently, the exploration of the SEM and EDS results described above indicate that the significant reduction of operating temperature and the superior enhancement of dehydrogenation kinetic properties of the 7 wt.% Ti3C2 doped sample can be mainly due to the unique lamellar-structure. The detailed microstructure information of Ti3C2 could not be easily revealed after the re/dehydrogenation process due to the small amounts of Ti3C2 additive; thus, the catalytic mechanism remains undiscovered. We hope that the catalytic mechanism of NaMgH3-x wt.% Ti3C2 composite can be revealed in the future by a X-ray absorption near-edge structure (XANES) spectrum.
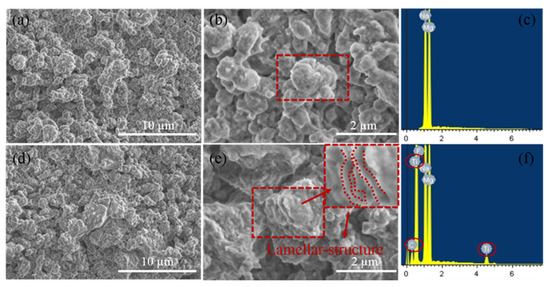
Figure 5.
SEM images of (a,b) NaMgH3 and (d,e) NaMgH3-7 wt.% Ti3C2; EDS results of (c) NaMgH3 and (f) NaMgH3-7 wt.% Ti3C2.
Figure 6 shows the isothermal hydrogenation curves of pure NaMgH3 and NaMgH3-7 wt.% Ti3C2 samples at different temperatures. It is obvious that the hydrogen absorption kinetics of NaMgH3 can be enhanced by doping with Ti3C2, resulting in the high capacity achieved at the same hydrogenation condition. Figure 6a shows the isothermal hydrogenation curves of pure NaMgH3 and NaMgH3-7 wt.% Ti3C2 samples at 300 °C. The 7 wt.% Ti3C2-containing composite can quickly absorb approximately 3.5 wt.% H2 within 6 s. However, the pure NaMgH3 displays unacceptably slow re-hydrogenation kinetics, storing 3.0 wt.% H2 in 15 min. This result suggests that only the first-step hydrogenation reaction is thermodynamically available at 300 °C. As the hydrogenation temperature increases to 350 °C, the hydrogenation kinetics of two dehydrogenated samples are improved, approximately 3.8 and 4.3 wt.% H2 stored for NaMgH3 and NaMgH3-7 wt.% Ti3C2 samples, respectively. The results reveal that first- and second-step hydrogenation reactions of the samples are feasible in thermodynamics, as the temperature increases to 350 °C. However, the second-step hydrogenation kinetics of both samples are too slow to desorb more hydrogen within 30 min. When the hydrogenation temperature rises to 400 °C, the Ti3C2-containing sample exhibits excellent hydrogenation kinetics, absorbing 4.9 wt.% H2 in 8 min, confirming that the first- and second-step hydrogen absorption kinetics have been further improved. The hydrogenation capacity of pure NaMgH3 is found to be 4.2 wt.% H2 in 15 min, with the second-step hydrogen absorption kinetics still being sluggish. In summary, Ti3C2 has obvious catalytic efficiency on the enhancement of both first- and second-step hydrogenation kinetic properties of NaMgH3 under 80 bar H2 at 300, 350, and 400 °C.
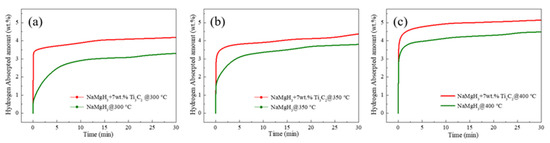
Figure 6.
Isothermal hydrogenation curves of pure NaMgH3 and NaMgH3-7 wt.% Ti3C2 at different temperatures. (a) 300 °C (b) 350 °C (c) 400 °C.
The reversible dehydrogenation properties of NaMgH3 are remarkably enhanced because of the superior catalytic activity of Ti3C2 with lamellar-structure. To obtain the cycling properties of NaMgH3 with or without dopant, the cycling isothermal dehydrogenation curves and hydrogen capacities of two samples are displayed in Figure 7. In the first cycle, pure NaMgH3 desorbs 5.5 wt.% H2 in 15 min at 400 °C. After hydrogenation at 400 °C under 60 bar H2, it releases only 2.6 wt.% H2 in 60 min, losing approximately half of its initial capacity. The dehydrogenation kinetic property of pure NaMgH3 also suffers tremendous degradation in the second cycle, especially in the latter 3rd, 4th and 5th cycles, 3.8, 4.0, and 3.75 wt.% H2 were desorbed in 20 min, respectively. The dehydrogenation kinetics and capacity of pure NaMgH3 in the latter three cycles are recovered from the poor second cycle. For the Ti3C2 doped NaMgH3, in the first cycle, about 5.1 wt.% H2 is released in 5 min. The difference is that the Ti3C2 doped sample does not suffer tremendous capacity loss in the second cycle. In the latter four cycles, the Ti3C2 doped sample desorbs 4.3, 4.4, 4.4, and 4.6 wt.% H2 in 20 min, respectively. In five cycles, pure NaMgH3 suffers a huge capacity loss from 5.5 to 4.1 wt.% H2, losing a quarter of its initial capacity. It is well known that a material with faster kinetics will release more hydrogen within a fixed time. Hence, Ti3C2 can relieve the degradation of dehydrogenation capacity to some extent due to its improved dehydrogenation kinetics. However, it is clear that the lamellar-structure Ti3C2 can effectively enhance the cycling stability of NaMgH3 in five cycles. The expectation of the dehydrogenation kinetics and cycling stability of NaMgH3 remaining unchanged is not achieved. Therefore, further investigation of enhancing the cycle stability of NaMgH3 should be explored.
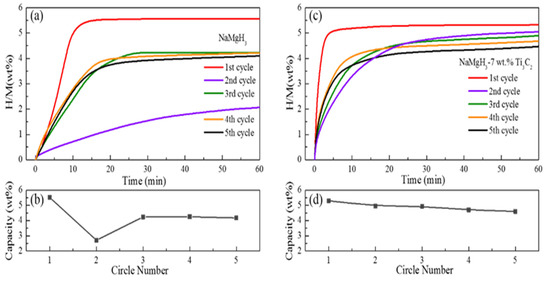
Figure 7.
Isothermal dehydrogenation cycle curves of (a) NaMgH3 and (c) NaMgH3-7 wt.% Ti3C2 at 400 °C; cycle capacity curves of (b) NaMgH3 and (d) NaMgH3-7 wt.% Ti3C2.
The pronounced degradation of dehydrogenation performance and hydrogen capacity in the second cycle for the pure NaMgH3 is abnormal. There should be some phase separations during hydrogenation after the first cycle dehydrogenation. Furthermore, it was found that pure NaMgH3 would segregate into separate NaH and Mg during the high-temperature cycling tests [39]. In order to confirm the phase transformation of Ti3C2 doped/undoped NaMgH3 samples during hydrogen storage process, XRD characterization was carried out for the re-hydrogenated sample. In Figure 8a,b, the NaMgH3, NaH, Mg, and MgO phases can be seen in the XRD patterns of the pure NaMgH3 sample after the second and third hydrogenations. The separation of NaH and Mg phases after hydrogenation can explain the reason why abnormal degradation occurs. Therefore, the detailed reaction process can be proposed as follows. When pure NaMgH3 dehydrogenates at 400 °C, both of the two step decomposition reactions occur quickly, forming molten Na and solid Mg. The drastic molten Na covers the outside surface of solid Mg particles to form the compact Na layer so that most Na and Mg are separated; the residual Na would be hydrogenated to form the solid NaH during the subsequent hydrogenation process, which hinders the further absorption of H2, making the formation of MgH2 and NaMgH3 much more difficult, thereby decreasing the hydrogen capacity of NaMgH3 [37]. Therefore, the formation of the NaH layer must be responsible for the pronounced degradation of dehydrogenation performance in the second cycle for the pure NaMgH3. This hypothesis can be proved by the strong diffraction peaks of NaH in the second hydrogenation sample (Figure 8). Moreover, the enhanced dehydrogenation performance of the pure NaMgH3 sample in the latter three cycles would be due to the distillation of molten Na making the NaH layer thinner [37]. In contrast, the XRD patterns of the hydrogenated NaMgH3-7 wt.% Ti3C2 sample displays the diffraction peaks of NaMgH3, MgH2, and MgO, without any diffraction peaks belonging to NaH (Figure 8c,d). The weak diffraction peaks of MgH2 are caused by the distillation of molten Na during the cycles. Meanwhile, to confirm the separation phenomenon of Na and Mg, the dehydrogenation processes at 400 °C can be observed through a homemade quartz tube. It can be observed that after hydrogenation the pure NaMgH3 appears as a light-yellow metal layer ingoting with metallic luster, corresponding to the existence of NaH (Figure S1a,b). However, the deposition of NaH disappears in the Ti3C2-doped NaMgH3 sample (Figure S1c,d). Herein, the XRD results and dehydrogenation observation indicate that the existence of lamellar-structure Ti3C2 can effectively prevent Na from separating Mg to form the insular NaH layer, largely preserving the cycling dehydrogenation properties of NaMgH3.
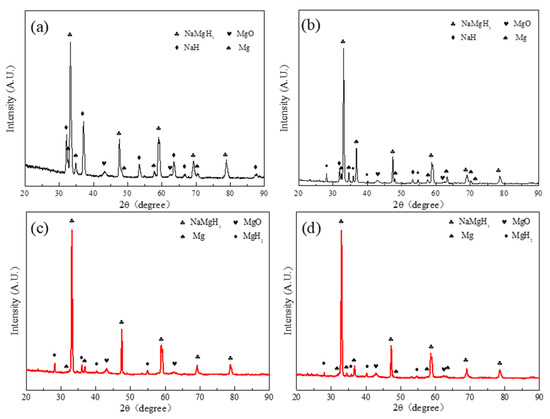
Figure 8.
XRD patterns of pure NaMgH3 before the (a) second and (b) third dehydrogenations at 400 °C. XRD patterns of NaMgH3-7 wt.% Ti3C2 before the (c) second and (d) third dehydrogenations at 400 °C.
In addition to its use as a hydrogen storage application, NaMgH3 can also be a potential candidate for thermal energy storage (TES) owing to the theoretical thermal storage density as high as 2881 kJ/kg [37]. In Figure S2a,b, the thermal storage densities of as-formed pure NaMgH3 and NaMgH3-7 wt.% Ti3C2 samples are calculated to be 2727 and 2562 kJ/kg through DSC profiles, respectively. The reduction of ~6.0% thermal storage density for the NaMgH3-7 wt.% Ti3C2 sample is mainly due to the dead weight of Ti3C2 and existence of MgO. Even with this reduction, as-formed NaMgH3-7 wt.% Ti3C2 sample exceeds the thermal storage properties of most other candidates (Table 2). Meanwhile, Ti3C2 dramatically enhances the de/re-hydrogenation kinetics and cycling performance of NaMgH3, making it more suitable for TES application [40].

Table 2.
Thermal energy storage densities of different thermochemical energy storage materials [41,42,43,44].
4. Conclusions
NaMgH3 is considered a potential candidate for hydrogen storage and thermal storage, but further practical applications are limited by its poor de/re-hydrogenation kinetics and cycling performance. An investigation of enhancing the re/dehydrogenation kinetics and cycling performance of NaMgH3 through doping with Ti3C2 was carried out by using XRD, SEM, TPD, DSC, isothermal de/re-hydrogenation measurements and cycling tests. The NaMgH3-7 wt.% Ti3C2 sample exhibits the optimal dehydrogenation performance, reducing the two-step desorption peak temperatures from 372.3 and 380.3 °C to 324.8 and 345.3 °C, respectively. The NaMgH3-7 wt.% Ti3C2 can release 4.8 wt.% H2 in 15 min and 3.4 wt.% H2 in 5 min at 365 °C and 350 °C, respectively. The unique lamellar-structure of Ti3C2 can separate the agglomerated NaMgH3 particles homogeneously and decrease the activation energy of NaMgH3 to 114.08 and 139.4 kJ/mol for the first- and second-step dehydrogenation reactions, respectively. The addition of Ti3C2 also dramatically enhances the hydrogen storage kinetics of NaMgH3 at 300, 350, and 400 °C. Approximately 3.5 wt.% H2 is absorbed in 6 s for the NaMgH3-7 wt.% Ti3C2 sample at 300 °C. The poor cycling properties of NaMgH3 are improved by Ti3C2, with 4.6 wt.% H2 capacity remaining after five cycles. Lamellar-structure Ti3C2 can homogeneously separate aggregated NaMgH3 particles and prevent Na from separating Mg after dehydrogenation. Furthermore, NaMgH3-7 wt.% Ti3C2 with its high thermal storage density of 2562 kJ/kg and fast kinetics can be a potential candidate for TES.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pr9101690/s1, Figure S1: Photographs of pure NaMgH3 and NaMgH3-7 wt.% Ti3C2 (a,c) before and (b,d) after dehydrogenation at 400 °C. Figure S2: DSC profiles of (a) pure NaMgH3 and (b) NaMgH3-7 wt.% Ti3C2 at a heating rate of 5 °C/min.
Author Contributions
The experimental work, original draft preparation, and modification, Z.H. (Zhouming Hang) and Z.H. (Zhencan Hu); methodology for experiments, manuscript review, and editing, X.X. and R.J.; conceptualization and data analysis, resources, project administration, and funding acquisition, X.X.; data analysis, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work is supported by the National Key Research and Development Program (2018YFB1502100), the National Natural Science Foundation of China (52171223), and the Zhejiang Provincial Natural Science Foundation of China (LZ21E010002).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santos, D.M.F.; Sequeira, C.A.C. Sodium borohydride as a fuel for the future. Renew. Sust. Energ. Rev. 2011, 15, 3980–4001. [Google Scholar] [CrossRef]
- Ahluwalia, R.K. Sodium alanate hydrogen storage system for automotive fuel cells. Int. J. Hydrogen Energy 2007, 32, 1251–1261. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Li, Z.; Gao, M.; Liu, Y.; Sun, W.; Pan, H. Enhanced hydrogen storage performance of MgH2 by the catalysis of a novel Intersected Y2O3/NiO hybrid. Processes 2021, 9, 892. [Google Scholar] [CrossRef]
- Hu, Z.C.; Qin, H.Y.; Xiao, X.Z.; Chen, M.; Liu, M.J.; Jiang, R.C.; Chen, L.X. Excellent catalysis of various TiO2 dopants with Na0.46TiO2 in situ formed on the enhanced dehydrogenation properties of NaMgH3. J. Phys. Chem. C 2019, 123, 22832–22841. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Ren, Z.; Zhang, X.; Hu, J.; Huang, Z.; Lu, Y.; Gao, M.; Pan, H. Realizing 6.7 wt% reversible storage of hydrogen at ambient temperature with non-confined ultrafine magnesium hydrides. Energy Environ. Sci. 2021, 14, 2302–2313. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, X.; He, Y.; Yao, Z.; Ye, X.; Kou, H.; Chen, C.; Huang, T.; Fan, X.; Chen, L. Probing an intermediate state by X-ray absorption near-edge structure in nickel-doped 2LiBH4–MgH2 reactive hydride composite at moderate temperature. Mater. Today Nano 2020, 12, 100090. [Google Scholar] [CrossRef]
- Poupin, L.; Humphries, T.D.; Paskevicius, M.; Buckley, C.E. A thermal energy storage prototype using sodium magnesium hydride. Sustain. Energy Fuels 2019, 3, 985–995. [Google Scholar] [CrossRef]
- Chen, W.; Xiao, X.; He, J.; Dong, Z.; Wang, X.; Chen, M.; Chen, L. A dandelion-like amorphous composite catalyst with outstanding performance for sodium borohydride hydrogen generation. Int. J. Hydrogen Energy 2021, 46, 10809–10818. [Google Scholar] [CrossRef]
- Reshak, A.H. NaMgH3 a perovskite-type hydride as advanced hydrogen storage systems: Electronic structure features. Int. J. Hydrogen Energy 2015, 40, 16383–16390. [Google Scholar] [CrossRef]
- Ikeda, K.; Kogure, Y.; Nakamori, Y.; Orimo, S. Reversible hydriding and dehydriding reactions of perovskite-type hydride NaMgH3. Scr. Mater. 2005, 53, 319–322. [Google Scholar] [CrossRef]
- Bouamrane, A.; Laval, J.P.; Soulie, J.P.; Bastide, J.P. Structural characterization of NaMgH2F and NaMgH3. Mater. Res. Bull. 2000, 35, 545–549. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Udovic, T.J.; Rush, J.J.; Yildirim, T. Crystal chemistry of perovskite-type hydride NaMgH3: Implications for hydrogen storage. Chem. Mater. 2008, 20, 2335–2342. [Google Scholar] [CrossRef]
- Pottmaier, D.; Pinatel, E.R.; Vitillo, J.G.; Garroni, S.; Orlova, M.; Baró, M.D.; Vaughan, G.B.M.; Fichtner, M.; Lohstroh, W.; Baricco, M. Structure and Thermodynamic Properties of the NaMgH3 Perovskite: A Comprehensive Study. Chem. Mater. 2011, 23, 2317–2326. [Google Scholar] [CrossRef]
- Bouhadda, Y.; Fenineche, N.; Boudouma, Y. Hydrogen storage: Lattice dynamics of orthorhombic NaMgH3. Phys. B Condens. Matter 2011, 406, 1000–1003. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, S.; Li, J.J.; Deng, J.Q.; Zhou, H.; Yao, Q. The Improvement of Dehydriding the Kinetics of NaMgH3 Hydride via Doping with Carbon Nanomaterials. Metals 2017, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, D.; Paskevicius, M.; Buckley, C.E. Thermodynamics of hydrogen desorption from NaMgH3 and its application as a solar heat storage medium. Chem. Mater. 2011, 23, 4298–4300. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.J.; Tao, S.; Deng, J.Q.; Zhou, H.; Yao, Q. Structure, thermal analysis and dehydriding kinetic properties of Na1-xLixMgH3 hydrides. J. Alloys Compd. 2015, 660, 402–406. [Google Scholar] [CrossRef]
- Tao, S.; Wang, Z.M.; Wan, Z.Z.; Deng, J.Q.; Zhou, H.; Yao, Q. Enhancing the dehydriding properties of perovskite-type NaMgH3 by introducing potassium as dopant. Int. J. Hydrogen Energy 2017, 42, 3716–3722. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Zhang, Q.; Fang, F.; Sun, D.; Li, K.; Wang, H.; Ouyang, L.; Zhu, M. In situ embedding of Mg2NiH4 and YH3 nanoparticles into bimetallic hydride NaMgH3 to inhibit phase segregation for enhanced hydrogen storage. J. Phys. Chem. C 2014, 118, 22635–23644. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, S.; Deng, J.; Zhou, H.; Yao, Q. Significant improvement in the dehydriding properties of perovskite hydrides, NaMgH3, by doping with K2TiF6. Int. J. Hydrogen Energy 2017, 42, 8554–8559. [Google Scholar] [CrossRef]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Dou, S.X. Improved hydrogen storage performance of MgH2–NaAlH4 composite by addition of TiF3. Int. J. Hydrogen Energy 2012, 37, 8395–8401. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of 2D titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Yang, J.; Chen, B.; Song, H.; Tang, H.; Li, C. Synthesis, characterization, and tribological properties of two-dimensional Ti3C2. Cryst. Res. Technol. 2014, 49, 926–932. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M. Two-Dimensional Nanocrystals: Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Wang, Y.; Gao, S.; Chen, Y.; Shi, J. Theranostic 2D tantalum carbide (MXene). Adv. Mater. 2017, 30, 1703284. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 1–17. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Lukatskaya, M.; Dyatkin, B.; Zhang, C.; Presser, V.; Gogotsi, Y.; Barsoum, M. One-step synthesis of nanocrystalline transition metal oxides on thin sheets of disordered graphitic carbon by oxidation of MXenes. Chem. Commun. 2014, 50, 7420–7423. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New two-dimensional niobium and vanadium carbides as promising materials for Li-ion batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Du, H.; Wang, Z.; Gao, M.; Pan, H.; Liu, Y. Remarkably improved hydrogen storage properties of NaAlH4 doped with 2D titanium carbide. J. Power Sources 2016, 327, 519–525. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Zhang, X.; Yang, Y.; Gao, M.; Pan, H. Superior catalytic activity derived from two-dimensional Ti3C2 precursor towards the hydrogen storage reaction of magnesium hydride. Chem. Commun. 2015, 52, 705–708. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Y.; Zhu, Y.; Guo, X.; Chen, J.; Li, L. Hydrogen storage performances and reaction mechanism of non-stoichiometric compound Li1.3Na1.7AlH6 doped with Ti3C2. Chem. Phys. 2018, 513, 135–140. [Google Scholar] [CrossRef]
- Cheng, H.; Li, K.; Fan, X.; Lou, H.; Liu, Y.; Qi, Q.; Zhang, J.; Liu, J.; Yan, K.; Zhang, Y. The enhanced de/re-hydrogenation performances of LiNa2AlH6 combined with two-dimension lamellar Ti3C2. Int. J. Hydrogen Energy 2017, 42, 25285–25293. [Google Scholar] [CrossRef]
- Zheng, J.G.; Cheng, H.; Xiao, X.Z.; Chen, M.; Chen, L.X. Enhanced low temperature hydrogen desorption properties and mechanism of Mg(BH4)2 composited with 2D MXene. Int. J. Hydrogen Energy 2019, 44, 24292–24300. [Google Scholar] [CrossRef]
- Jiang, R.; Xiao, X.; Zheng, J.; Chen, M.; Chen, L. Remarkable hydrogen absorption/desorption behaviors and mechanism of sodium alanates in-situ doped with Ti-based 2D MXene. Mater. Chem. Phys. 2020, 242, 122529. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; Bellosta von Colbe, J.M.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- He, T.; Pachfule, P.; Wu, H.; Xu, Q.; Chen, P. Hydrogen carriers. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Sheppard, D.A.; Humphries, T.D.; Buckley, C.E. Sodium-based hydrides for thermal energy applications. Appl. Phys. A 2016, 122, 406. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ward, P.A.; Corgnale, C.; Teprovich, J.A.; Motyka, T.; Hardy, B.; Peters, B.; Zidan, R. High performance metal hydride based thermal energy storage systems for concentrating solar power applications. J. Alloys Compd. 2015, 645, S374–S378. [Google Scholar] [CrossRef] [Green Version]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sust. Energ. Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Harries, D.; Paskevicius, M.; Sheppard, D.; Edward Cameron Price, T.; Buckley, C.E. Concentrating solar thermal heat storage using metal hydrides. Proc. IEEE 2012, 100, 539–549. [Google Scholar] [CrossRef]
- Fellet, M.; Buckley, C.E.; Paskevicius, M.; Sheppard, D.A. Research on metal hydrides revived for next-generation solutions to renewable energy storage. MRS Bull. 2013, 38, 1012–1013. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, D.A.; Corgnale, C.; Hardy, B.; Motyka, T.; Zidan, R.; Paskevicius, M.; Buckley, C.E. Hydriding characteristics of NaMgH2F with preliminary technical and cost evaluation of magnesium-based metal hydride materials for concentrating solar power thermal storage. RSC Adv. 2014, 4, 26552–26562. [Google Scholar] [CrossRef]
- Paskevicius, M.; Sheppard, D.A.; Williamson, K.; Buckley, C.E. Metal hydride thermal heat storage prototype for concentrating solar thermal power. Energy 2015, 88, 469–477. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).