Abstract
This paper investigates aquathermolysis of heavy oil in carbonate reservoir rocks from Boca de Jaruco, which is developed by the cyclic steam stimulation method. The nickel-based catalyst precursor was introduced in order to intensify the conversion processes of heavy oil components. The active form of such catalysts—nickel sulfides—are achieved after steam treatment of crude oil at reservoir conditions. The experiments were carried out on a rock sample extracted from the depth of 1900 m. Changes in composition and structure of heavy oil after the conversion were identified using SARA-analysis, Gas Chromatography-Mass Spectroscopy of saturated fractions, FTIR spectroscopy of saturated fractions, and MALDI of resins. It is revealed that catalyst particles provide a reduction in the content of resins and asphaltenes due to the destruction of carbon-heteroatom bonds. Moreover, the destruction of C=Carom. bonds and interactions with aromatic rings are heightened. In contrast, the results of experiments in the absence of catalysts exposed polymerization and condensation of aromatic rings. The most remarkable result to emerge from the thermo-catalytic influence is the irreversible viscosity reduction of produced crude oil enhancing the oil recovery factor. Moreover, the introduction of catalysts increases the gas factor due to additional gas generation as a result of aquathermolysis reactions. The yield of methane gas is significantly high in the experimental runs with oil-saturated rocks rather than crude oil experiments. The gas factor reaches 45 m3/ton.
1. Introduction
The shortage of conventional hydrocarbon reserves and gradual increase in global energy demand has motivated the energy industry to seek sustainable alternative energy sources. In this regard, unconventional heavy hydrocarbon resources such as heavy oil, natural bitumen, and shale oil are attracting considerable interest due to the significant amount of reserves worldwide [1]. The underestimated total amount of heavy oil and natural bitumen resources in the world are 0.8–1 trillion tons, only 123 billion tons of which are industrially recoverable reserves [2]. However, heavy oil and natural bitumen have drawbacks of high viscosity in reservoir conditions, and significant amounts of high-molecular components such as resins, asphaltenes, and heteroatoms. These are the reasons for heavy crude oil immobility through reservoir rocks, and the main issues in pipeline transportation and refinery. As the viscosity of crude oil is very sensitive to heat, thermal recovery methods play a crucial role in producing hydrocarbons. Presently, various methods based on steam injection techniques are widely applied in an industrial scale. Unfortunately, heat delivered from the steam leads to only a temporal decrease in heavy oil viscosity. Chemical processes such as cracking, desulfurization, hydration, and dehydration are required in order to reach deep conversion. Therefore, it is important to modify existing thermal recovery methods.
One of the most challenging approaches for deep conversion of high-molecular components of oil during steam injection is the introduction of oil-soluble catalysts into the reservoir rocks that could accelerate aquathermolysis reactions in situ. Application of such catalysts during Cyclic Steam Stimulation (CSS) or Steam Assisted Gravity Drainage (SAGD) provides deep conversion of heavy oil by reducing the content of resins and asphaltenes, as well as in-situ removal of sulfur from heavy crude oil. Thus, in situ partial upgrading of heavy crude oil lessens the load on downstream facilities [3,4]. Moreover, the catalyst particles participate in acts of breaking high-molecular bonds multiple times, as they absorb the porous matrix and become active after cyclic steam and H-donor solvent stimulation. The absorption of catalyst particles on the rock surface releases operators from regeneration of catalysts or its recovery from crude oil and is a crucial point in terms of the feasibility of the given process. Some authors, including us, have published experimental results that justify the absorption of catalyst particles on the reservoir porous rock surface [5,6].
Many studies have been published on the application of catalysts during steam treatment of heavy hydrocarbons. Research results show that catalysts stimulate hydrogenation, hydrogenolysis, hydrolysis, and cracking reactions, which result in the enhancement of the physical and chemical, as well as rheological, properties of crude oil [7,8,9]. The authors imply that in the presence of catalysts the content of saturates and aromatics were increased, while the content of solid n-alkanes, resins, and asphaltenes were reduced. It is well known that even a small destruction of resins and asphaltenes can significantly decrease the viscosity and increase the mobility of crude oil.
Commonly used in-situ upgrading catalysts are water soluble, oil soluble, and dispersed catalysts. However, in terms of suitability with proposed thermal recovery methods based on steam injection, it is crucial and relevant to design catalysts in solution form. The active form of such catalyst precursors is obtained in-situ under certain temperatures [8]. Our research group has developed such catalyst precursors, which are based on transition metal carboxylates. The obtained active form of catalysts under reservoir conditions were identified as metal oxides and sulfides. Furthermore, contributions of metal sulfides and oxides to the conversion processes of heavy oil components were investigated [10,11,12]. It has been found that transition metal oxides and sulfides intensify mostly the cleavage of the least stable C-S bonds. Consequently, detachment of peripheral fragments from resins and asphaltenes occurs. Moreover, the content of light fractions—saturates and aromatics—increases due to the destruction of resins and asphaltenes [13]. Other work has shown the high performance of Co-based catalysts in the destruction of resins and asphaltenes [14]. Experiments were carried out on rock samples of the Ashal’cha reservoir. The hydrothermal stimulation results revealed that the content of resins and asphaltenes were reduced by 38% and 26%, respectively, in the presence of catalysts, where the temperature of aquathermolysis reaction was 180 °C.
This study focuses on the application of oil-soluble nickel carboxylate dissolved in hydrogen donor source (organic solvent) as a catalytic complex for in-situ upgrading of heavy crude oil of Boca De Jaruco (Cuba) via an aquathermolysis process. The group composition—SARA-analysis of extracts, viscosity measurement, elemental analysis, FTIR, and GC analysis—were used to determine the effect of nickel oxides and sulfides, as well as hydrogen donor on a deep conversion of heavy oil components.
2. Experimental Procedures
The laboratory simulation of the aquathermolysis process was carried out using a 300 mL volume high-pressure reactor with a stirrer—Parr Instruments 4560 (Parr Instruments, USA). The model system consisted of an oil and water mixture with a 70:30 mass ratio. This oil-in-water emulsion was exposed to the temperature treatment (250 °C) under catalytic and non-catalytic-processes conditions. The oil-soluble nickel carboxylates obtained by means of an exchange reaction between sodium salt of tall oil and non-organic salts of nickel was used as the catalyst [15]. The catalyst precursor (1.0 wt% by oil) was mixed with the emulsion. The initial pressure was fixed at the level of 0.3 MPa. The duration of the experiment was six hours. After the completion of the process, oil was initially separated from water by precipitation for 16 h, followed by the centrifugation on a laboratory centrifuge Eppendorf 5804 r at 3000 rpm for 1 h. Then, the definition of the water quantity in all test specimens by the Dean–Stark method was performed.
The SARA-analysis method was applied to determine the group composition of crude oil. The molecular mass of asphaltenes precipitated from the crude oil was investigated by the matrix-activated MALDI laser desorption/ionization method. The studies were carried out on the mass spectrometer—UltraFlex III TOF/TOF (Bruker) with the time-of-flight analyzer on the 2.5-dihydroxybenzoic acid matrix.
The saturate fractions of bitumoids were studied by the chromatomass-spectrometric system, including gas chromatography Chromatec-Crystal 5000 (Crystal, Russia) with a mass selective detector ISQ and software application—Xcalibur—for processing results. The chromatography is equipped with a capillary column, which is 30 m in length and 0.25 mm in diameter. The rate of the gas carrier (helium) phase and the temperature of the injector is 1 mL/min and 310 °C, respectively. Moreover, the temperature is adjustable from 100 °C up to 300 °C with a speed of 3 °C/min, and the process is isothermal through the whole analysis. The potential of the ion source is 70 W, and the temperature is 250 °C. Compounds are identified by a digital library of National Institute of Standards and Technology (NIST).
The structural-group composition was determined by FTIR spectroscopy with Vector 22 IR spectrometer (Bruker) in the range of 4000–400 cm−1 with a resolution of 4 cm−1. IR absorption spectra were compared in terms of optical density at maximums of corresponding characteristic absorption bands: paraffin entities at 720 cm−1 (methylene groups CH2 > 4), 1380 cm−1 and 1465 cm−1 (-CH3 methyl and -CH2CH3 methylene groups); aromatic entities at 1600 cm−1 (C = C bonds); oxygen compounds at 1710 (carbonyl groups in acids), 1740 cm−1 (ester carboxyl groups), and 1030 cm−1 (sulfoxide groups) [16]. For comparison of the products, the following spectral coefficients associated with the structural-group composition were used: C1 = D1600/D720 (aromaticity), C2 = D1710/D1465 (oxidation), C4 = (D720 + D1380)/D1600 (paraffinicity) and C5 = D1030/D1465 (sulfurization).
The elemental compositions of asphaltenes (the content of carbon, hydrogen, nitrogen, and sulfur) were determined using a combustion method in a semiautomatic CHN analyzer at 1000 °C.
3. Results and Discussion
The crude oil of the Boca de Jaruco reservoir is characterized by a high degree of biodegradation—a low content of saturated hydrocarbons and high content of resins and asphaltenes (60 wt%). A carbon preference index for the given crude oil sample was calculated in our previous works, which is equal to 0.61 [17].
The content of extracted oil from the rock samples after the thermal treatment decreases by 8%–14%. This is explained by the formation of gases and light volatile hydrocarbons after aquathermolysis of heavy oil. Moreover, thermal cracking of asphaltenes could form coke-like compounds and they could be adsorbed on the rock surface. In contrast to previous experimental results with crude oil [18], the conversion degree of heavy oil in the presence of rock minerals is much higher. This is due to several reasons: the native state of the crude oil, its discontinuity in pore volumes, and the presence of natural catalytically active compounds.
After 12 h thermo-catalytic upgrading, the content of resins and asphaltenes were reduced by 27% and 40%, respectively (Table 1) [19,20]. The increase in the light fractions of oil is due to the breakage of such high-molecular hydrocarbons. The results suggest that the cleavage of C-S bonds is the primary reaction causing the destruction of resins and asphaltenes. Moreover, the results of elemental analysis and FTIR analysis point to upgrading via C-C bond cleavage and hydrogenation. The destruction of asphaltenes is followed by free radical reactions occurring during the process, resulting in the active chains’ polymerization. The catalytic complex is able to prevent the ring growth leading to formation of higher molecular weight organics. Moreover, the catalyst plays an important role in the production of H2 by water-gas shift reaction, which in turn serves to close the free radicals and hydrogenate the heavy oil. Overall, the catalyst acts on conjugated π-bonds and the relative weaker C-C and C=C bonds, causing pyrolysis, depolymerization, hydrogenation, isomerization, ring opening, alcoholizaiton, and esterification reactions during the entire catalytic aquathermolysis process.

Table 1.
SARA-analysis of extracts from reservoir rock.
The results of the elemental composition of rock extracts after thermal treatment are demonstrated in Table 2. The results of elemental composition of crude oil and initial rock extracts are close in terms of H/Cat ratio. However, the content of sulfur and oxygen are slightly different. The influence of hydrothermal treatment upon the conversion of light hydrocarbons into a gas phase made a decrease in the content of carbon and hydrogen proportionally. This resulted in the increase in the sulfur and oxygen content of heavy oil components.

Table 2.
Elemental composition of rock extracts.
The combination of a nickel-based catalyst with a hydrogen donor during the aquathermolysis process shows a significant increase in the hydrogen content at a temperature of 250 °C and higher. This is the main reason for increasing the H/Cat ratio.
As the content of saturated hydrocarbons increases due to the detachment of alkyl substitutes from resins and asphaltenes after the upgrading of heavy oil in the presence of a nickel-based catalyst, the ratio of H/Cat for resins and asphaltenes declines (Table 2). Generally, for a nickel-based catalyst, the H/Cat ratio is improved because radicals formed after aquathermolysis reactions are deactivated by hydrogen.
The content of light hydrocarbons in saturated fraction was increased after catalytic hydrothermal treatment (Figure 1) due to detachment of alkyl substitutes in resins and asphaltenes.
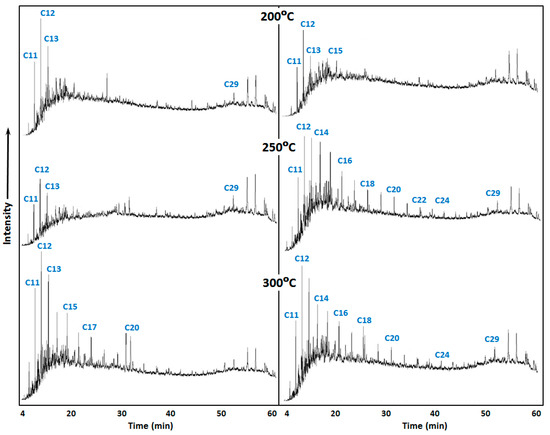
Figure 1.
Gas chromatograms (GC) of saturated fractions of rock extracts under various influence temperatures in the presence (right) and absence (left) of a catalyst.
The efficiency of a catalyst on the destruction of resins and asphaltenes is significant with increasing experiment duration. Light alkane hydrocarbons are observed in the destruction products. The catalytic effect accumulates over time. The content of alkanes C10-C15 increases from 45% to 65%. The content of light alkanes in the initial rock extracts is almost two times lower than the produced crude oil (that was the object of the physical simulation in the first part of investigation). The hydrothermal treatment provides some compositional changes. However, the catalyst provides more intensive destruction of resins and asphaltenes (Figure 2).
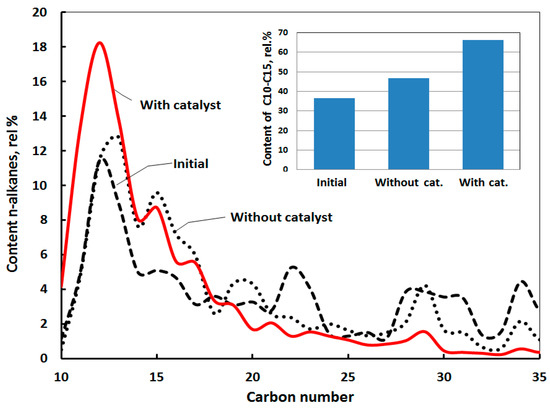
Figure 2.
The distribution of n-alkanes in saturated fractions of initial rock extracts, after hydrothermal and thermo-catalytic treatment samples (24 h, 300 °C).
The spectral coefficients of initial rock extracts and rock extracts after hydrothermal and thermo-catalytic influences, depending on the temperature under pressure of 90 bar, are presented in Table 3.

Table 3.
FTIR spectral coefficients of rock extracts.
According to the results of FTIR spectral coefficients, thermo-catalytic treatment of rock samples decreases the content of aromatic structures as the reaction temperature increases from 200 °C to 300 °C. However, oxidation and branching coefficients vary slightly. Moreover, the aliphatic coefficient increases due to the decrease in aromaticity. The sulfurization coefficient also decreases after thermo-catalytic influences due to the reduction of sulfoxides to sulfides and hydrogen sulfide.
The results of MALDI (Figure 3) of resins after thermo-catalytic treatment at 200 °C show the shifting of a maximum of molecular mass distribution from 300 to 360 units due to the destruction of asphaltene molecules and their conversion into the resin fraction. At higher temperatures, part of the carbon-heteroatom bonds in resins breaks apart, the chemical force of which is higher due to the lower molecule size of the resins in contrast to the asphaltenes. For the rock extracts after thermo-catalytic treatment, the average molecular mass of resins decreases from 360 to 320 as the reaction temperature increases from 200 to 300 °C (Figure 3).
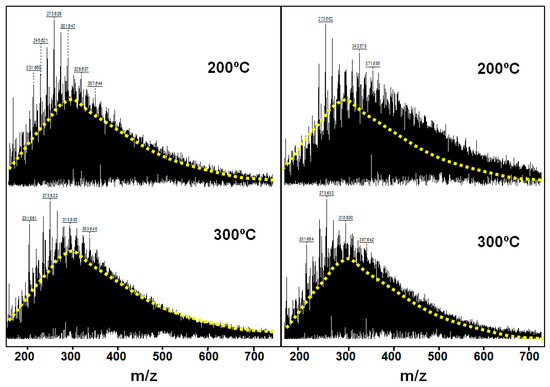
Figure 3.
MALDI spectra of resins from rock extracts under various influence temperatures for 12 h in the absence (left) and presence (right) of a catalyst. Punctuated lines indicate the distribution of molecular masses with respect to specters of experiments at 200 °C without a catalyst.
The conversion degree of asphaltenes increases as the temperature rises and with the addition of a catalyst (Figure 4). For the given rock extracts, the content of the asphaltenes decreases at a comparatively lower temperature due to thermal destruction independent from the catalyst presence. The nickel-based catalyst shows its activity at 300 °C.
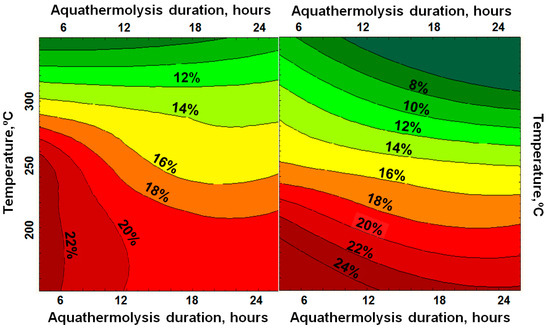
Figure 4.
The influence of various reaction conditions (aquathermolysis duration and temperature of experiments) on the content of asphaltenes (wt%) in the presence (right) and absence (left) of a nickel-based catalyst.
The rheology measurements at room temperature show that the viscosity of the given crude oil reduces upon the increase in hydrothermal treatment time (Figure 5). In contrast to the viscosity of the initial crude oil, the viscosity reduction rate after catalytic aquathermolysis treatment approaches 75%. This value is comparable with the results obtained by other groups of scientists [1,21,22]. Yusuf et al. synthesized oil soluble catalyst NiMo-oleate and applied it together with glycerol to upgrade Omani heavy oil by catalytic aquathermolysis. They also achieved maximum viscosity reduction of 69% at a 277 °C reaction temperature and 30 h of reaction [23].
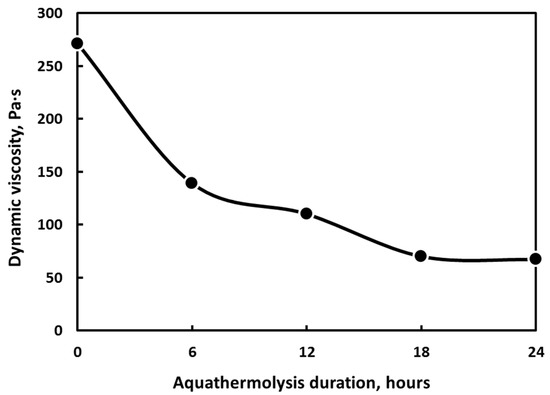
Figure 5.
The relation between the viscosity of crude oil and the duration of the aquathermolysis process in the presence of a nickel-based catalyst (5 s−1).
4. Conclusions
The hydrothermal treatment of rock samples from a depth interval of 633.1–634.0 m of the Boca de Jaruco reservoir was carried out at various temperatures. The active form of catalyst—nickel sulfide—forms after the hydrothermal treatment of oil in reservoir conditions. The catalyst based on nickel metal provides intensification of aquathermolysis processes with further upgrading of heavy oil. The viscosity reduces three times after the thermo-catalytic treatment. The amount of saturated and aromatic hydrocarbons increases, while the content of resins and asphaltenes decreases. After the catalytic aquathermolysis, the reduction in the molecular mass of resins was observed. This process is a result of carbon-heteroatom bond cleavage. The experimental results of hydrothermal treatment in the absence of a catalyst showed the behavior of aromatic ring condensation. Thermo-catalytic influence provides irreversible reduction of produced oil viscosity and enhances oil recovery.
Author Contributions
Conceptualization, A.V.V.; methodology, S.A.S.; investigation, I.I.M., F.A.A., A.V.S., and O.V.P.; data curation, I.S.A.; writing—original draft preparation, S.I.K. and I.S.A.; writing—review and editing, D.K.N. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare no competing financial interest.
Acknowledgments
The work is performed according to the Russian Government Program of Competitive Growth of Kazan Federal University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maity, S.K.; Ancheyta, J.; Marroquín, G. Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: A review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Meyer, R.F.; Attanasi, E.D.; Freeman, P.A. Heavy oil and natural bitumen resources in geological basins of the world: Map showing klemme basin classification of sedimentary provinces reporting heavy oil or natural bitumen. US Geol. Surv. Open-File Rep. 2007, 2007, 1084. [Google Scholar]
- Jiang, S.; Liu, X.; Liu, Y.; Zhong, L. In situ upgrading heavy oil by aquathermolytic treatment under steam injection conditions. In SPE International Symposium on Oilfield Chemistry; Society of Petroleum Engineers: The Woodlands, TX, USA, 2005. [Google Scholar]
- Wu, C.; Su, J.; Zhang, R.; Lei, G.; Cao, Y. The use of a nano-nickel catalyst for upgrading extra-heavy oil by an aquathermolysis treatment under steam injection conditions. Pet. Sci. Technol. 2013, 31, 2211–2218. [Google Scholar] [CrossRef]
- Elahi, S.M.; Scott, C.E.; Chen, Z.; Pereira-Almao, P. In-situ upgrading and enhanced recovery of heavy oil from carbonate reservoirs using nano-catalysts: Upgrading reactions analysis. Fuel 2019, 252, 262–271. [Google Scholar] [CrossRef]
- Kadyrov, R.; Sitnov, S.; Gareev, B.; Batalin, G. Modeling of cobalt-based catalyst use during CSS for low-temperature heavy oil upgrading. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Sitnov, S.A.; Mukhamatdinov, I.I.; Shmeleva, E.I.; Aliev, F.A.; Vakhin, A.V. Influence of nanosized iron oxides (II, III) on conversion of biodegradated oil. Pet. Sci. Technol. 2019, 37, 971–976. [Google Scholar] [CrossRef]
- Cao, Y.B.; Zhang, L.L.; Xia, D.H. Catalytic aquathermolysis of Shengli heavy crude oil with an amphiphilic cobalt catalyst. Pet. Sci. 2016, 13, 463–475. [Google Scholar] [CrossRef]
- Baygildin, E.R.; Sitnov, S.A.; Vakhin, A.V.; Sharifullin, A.V.; Amerkhanov, M.I.; Garifullina, E.I. Aquathermolysis of heavy oil in the presence of bimetallic catalyst that form in-situ from the mixture of oil-soluble iron and cobalt precursors. Georesursy 2019, 21, 62–67. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Onishchenko, Y.V.; Feoktistov, D.A. Aquathermolysis of High-Viscosity Oil in the Presence of an Oil-Soluble Iron-Based Catalyst. Chem. Technol. Fuels Oils 2017, 53, 666–674. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Foss, L.E.; Feoktistov, D.A.; Vakhin, A.V.; Petrukhina, N.N.; Romanov, G.V. Transformations of hydrocarbons of Ashal’hinskoe heavy oil under catalytic aquathermolysis conditions. Pet. Chem. 2017, 57, 657–665. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Tang, X.; Sun, Y.; Liu, H.; Deng, L.; Wei, Y. Upgrading heavy and extra-heavy crude oil for transportation by use an iron oil-soluble catalyst. Pet. Sci. Technol. 2017, 35, 1203–1208. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Khaidarova, A.R.; Zaripova, R.D.; Mukhamatdinova, R.E.; Sitnov, S.A.; Vakhin, A.V. The composition and structure of ultra-dispersed mixed oxide (Ii, iii) particles and their influence on in-situ conversion of heavy oil. Catalysts 2020, 10, 114. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Salih, I.S.S.; Rakhmatullin, I.Z.; Sitnov, S.A.; Laikov, A.V.; Klochkov, V.V.; Vakhin, A.V. Influence of Co-based catalyst on subfractional composition of heavy oil asphaltenes during aquathermolysis. J. Pet. Sci. Eng. 2020, 186, 106721. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Petrovnina, M.S.; Feoktistov, D.A.; Isakov, D.R.; Nurgaliev, D.K.; Amerkhanov, M.I. Intensification of thermal steam methods of production of heavy oil using a catalyst based on cobalt. Neft. Khozyaystvo-Oil Ind. 2016, 11, 106–108. [Google Scholar]
- Yusupova, T.N.; Ganeeva, Y.M.; Romanov, G.V.; Barskaya, E.E.; Morozov, V.I.; Okhotnikova, E.S.; Vakhin, A.V. Change in the structural-group composition of bitumen asphaltenes upon thermal bitumen recovery. Pet. Chem. 2017, 57, 198–202. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part I–changes in composition of saturated hydrocarbons. Pet. Sci. Technol. 2018, 36, 1829–1836. [Google Scholar] [CrossRef]
- Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Vakhin, A.V.; Sitnov, S.A.; Akhmadiayrov, A.A.; Varfolomeev, M.A.; Nurgaliev, D.K. Catalytic heavy oil upgrading by steam injection with using of transition metals catalysts. Neft. Khozyaystvo-Oil Ind. 2017, 8, 30–34. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Mukhamatdinov, I.I.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Sitnov, S.A.; Chemodanov, A.E.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part II–changes in composition of aromatic hydrocarbons. Pet. Sci. Technol. 2018, 36, 1850–1856. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part III—Changes in composition resins and asphaltenes. Pet. Sci. Technol. 2018, 36, 1857–1863. [Google Scholar] [CrossRef]
- Hamedi Shokrlu, Y.; Babadagli, T. Transportation and interaction of nano and micro size metal particles injected to improve thermal recovery of heavy-oil. In SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers: Denver, CO, USA, 2011. [Google Scholar]
- Hashemi, R.; Pereira, P. Experimental study of simultaneous athabasca bitumen recovery and upgrading using ultradispersed catalysts injection. In Proceedings of the Society of Petroleum Engineers—Canadian Unconventional Resources Conference, Calgary, AB, Canada, 15–17 November 2011. [Google Scholar]
- Yusuf, A.; Al-Hajri, R.S.; Al-Waheibi, Y.M.; Jibril, B.Y. In-situ upgrading of Omani heavy oil with catalyst and hydrogen donor. J. Anal. Appl. Pyrolysis 2016, 121, 102–112. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).