Abstract
The hydration layer has a significant effect on the adsorption behavior of reagents during the flotation process of low-rank coal. Understanding the effect of hydration layer on the adsorption of common collectors on low-rank coal is a prerequisite for proposing a new enhanced coal floatation method. In this study, a smooth low-rank coal surface model with a density of 1.2 g/cm3 was constructed and compared with the XPS results. Three different systems, coal-water, coal-collector, and coal-water-collector, were constructed. Molecular dynamics method was applied to study the adsorption behaviors of water and dodecane molecules. Simulation results revealed that a stable hydration layer with a thickness of about 5 Å was formed due to the strong attraction of coal surface. The negative value of interaction energy (IE) indicated that dodecane molecules could spontaneously adsorb on the coal surface. Dodecane molecules were successfully adsorbed on the coal surface when it was located inside the hydration layer. While the dodecane molecule was outside the hydration layer, it could not pass through the hydration layer on the surface of low-rank coal.
1. Introduction
Coal is China’s main energy source, and it occupies a dominant position in the energy consumption system. China’s thermal coal consumption accounts for more than 70% of the total coal consumption, and it is the largest coal species in China. Among them, low-rank coals such as lignite, long-flame coal, non-stick coal, and weak-stick coal account for about 40% of the discovered coal resources. After the separation of raw coal, much more fine slime is obtained. The most efficient method to separate coal slime is froth flotation. [1,2,3,4]. However, since a large number of hydrophilic oxygen-containing functional groups and pores exist on the low-rank coal, a stable hydration layer can be formed on the surface during the flotation process [5,6,7,8,9]. Therefore, the traditional oil flotation collector is difficult to adsorb on its surface, which ultimately leads to low flotation efficiency of low-rank coal [10,11,12,13,14,15,16]. With the increase in mining depth and mine service life, the raw coal quality is getting worse and worse, and the direct combustion of low-rank slime can no longer meet the current environmental protection situation and product quality requirements. In February 2017, the National Energy Administration issued the “Thirteenth Five-Year Plan for Demonstration of Coal Deep Processing Industry”, which clearly proposed the use of low-rank coal for quality and classification. The high-efficiency flotation and upgrading technology of low-rank coal slime has become a “stuck neck” problem that restricts clean and efficient production and utilization of low-rank coal.
In order to improve the flotation performance of low-rank coal, a great deal of research has been carried out in recent years [3,12,14,16,17,18]. In general, the research was mainly conducted through the following three aspects: pretreatment technology, such as microwave [19], compressing [6], etc., to improve the surface hydrophobicity of low-rank coal; adding surfactant to improve the adsorption of hydrocarbon collector on the surface of low-rank coal; designing and developing new agents to improve the interaction between agents and low-rank coal surface [13,14,17,20,21,22]. Lubricating oil was studied as a flotation collector for low-rank coal. The results indicated that the oxygenated functional groups in lubricating oil promoted its interaction with low-rank coal [12]. In another paper, they found that the yield of clean coal could be significantly improved by being pre-conditioned with dodecyl trimethyl ammonium bromides (DTAB). They proposed that dodecane molecules could hardly adsorb on the coal surface with many oxygen-containing groups [13]. However, they did not further study how these oxygen-containing functional groups affect the adsorption of reagents.
In recent years, with the rapid development of computer simulation technology, molecular simulation has become an effective means for investigating adsorption processes and the interfacial interaction in flotation [5,23,24,25,26]. Xia et al. carried out molecular dynamics simulations (MD) to study the adsorption process of dodecane on low-rank coal in the presence of DTAB. The simulation results were consistent with the experimental results [13]. Pores on low-rank coal surface also affect the adsorption process of water and collectors, which was studied in our previous work [1]. Molecular simulation technology can allow us to understand some phenomena which cannot be observed in experiments from the molecular level and can better explain some macroscopic experimental results. Besides, the results of molecular simulation can provide guidance for our experimental research.
Experts have conducted a great deal of excellent research on the flotation of low-rank coal. This article intends to study the effect of the hydration layer on the adsorption of the collector on the surface of low-rank coal from the molecular level by molecular dynamics simulation. The research results can provide new ideas for the flotation of low-rank coal.
2. Materials and Methods
2.1. Materials
The coal sample used in the experiment was obtained from Inner Mongolia. Clean coal with density of −1.2 g/cm3 was applied in X-ray photoelectron spectroscopy experiments. The basic analysis of the coal sample is shown as Table 1, where M, A, V, and FC stand for moisture, ash, volatile, and fixed carbon of the coal sample, respectively.

Table 1.
Basic analysis of coal sample (Air dried).
2.2. XPS Measurements
XPS (Shimadzu, Japan) was used to indicate the element composition of coal samples. The XPS test result was used to compare with the coal model constructed in Section 3.2. The C1s peak at 284.8 eV was used to correct the binding energy.
2.3. Molecular Dynamics Simulation
2.3.1. Simulation Model
In the past few decades, scholars have proposed many coal molecular models. In this paper, the Wender coal model was used to represent low-rank coal [27]. It contains the common oxygen-containing functional groups in coal. The Wender coal model is presented in Figure 1 and the Mulliken charges of each atom are given in Figure S1. The molecular model of coal surface constructed in past research is often irregular. In the later analysis of the simulation results, it is difficult to extract some parameters, such as the thickness of the water layer, the concentration distribution of water molecules in the direction perpendicular to the surface, etc. In order to construct a smooth coal surface structure, the following operations were performed. The constructed process of a basic rectangular coal cell with the density of 1.0 g/cm3 can be found in our previous study [1]. Then, graphite sheets were added on each side of the coal molecule along the Z-axis direction, as shown in Figure 2. Fixing the graphite atoms and minimizing the system using geometry optimization in Forcite module, coal molecules were gradually compressed due to the effect of graphite, forming a smooth surface. We repeated the above steps until the density of coal molecules reached 1.2 g/cm3. Finally, we removed the graphite sheet. The size of the simulation cell was 64 × 64 × 150 Å3 with the angles of 90° × 90° × 90°. As shown in Figure 2, there were 96 oxygen atoms, including carboxyl, hydroxyl, carbonyl, and ether groups, and 413 carbon atoms on the surface, indicating that the surface was strongly hydrophilic. The Wender coal molecule and the top view of the coal surface model used in this paper are shown in Figure 1. The Mulliken charge of each atom was calculated using density functional theory (DFT) method by Dmol3 module in MS 8.0, and the values are given in Figure 1a. The density of the coal molecules can be calculated according to Equation (1).
where M is the molar mass of single coal molecule; n is the number of coal molecules; NA is the Avogadro constant; V is the volume of the simulation cell; R is the relative concentration of the coal molecules, which can be obtained from Forcite analysis module.
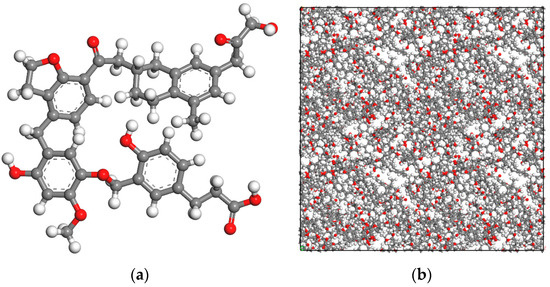
Figure 1.
Wender coal molecule (a) and top view of the coal surface (b) (C in gray, O in red, H in white).
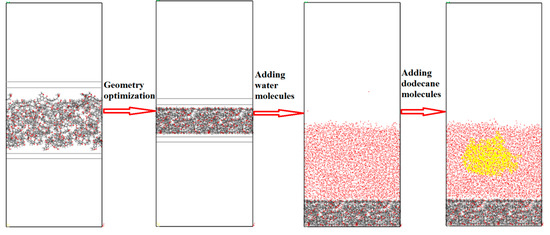
Figure 2.
Coal-water-collector system construction process (the thicknesses of coal seam and water layer were 16.8 Å and 55 Å).
To study the effect of water on the oil adsorption process, water slabs containing 8000 water molecules were constructed using an amorphous cell module. A dodecane droplet was placed at the top of the water slab. A vacuum section with a thickness of 80 Å was added at the top to avoid the effect of periodic boundary conditions (PBC). The coal-water-collector system (CWC) is presented in Figure 2. Energy minimization was applied by geometry optimization with smart algorithm method before each MD.
2.3.2. Simulation Method
Forcite modules in MS 8.0 were used for all the MD simulations. In all simulations, the polymer consistent force field (PCFF) and the Nosé thermostat method were applied [28,29]. The atom based method with a cutoff distance of 12.5 Å and the Ewald method were used for the calculation of van der Waals interactions and electrostatic interactions, respectively. NVT ensemble with a Nosé thermostat and a temperature of 298 K was applied. Initial velocities were set to random values from a temperature-dependent Gaussian distribution. A time step of 1.0 fs was applied to calculate the equations of motion [30,31]. Coal molecules were fixed in all MD simulations. The total simulation time was 1000 ps for each MD process, and the final 100 ps were used for further analysis.
3. Results and Discussion
3.1. XPS Measurements
XPS test results of the coal sample are presented in Figure 3. As shown in Figure 3, the coal surface mainly contained oxygen and carbon elements. By fitting the curve, the contents (in percentages) of C1s and O1s on the coal surface were 76.53 and 17.00, respectively. The results showed that there were many oxygen atoms on the surface of low-rank coal, which could promote the formation of hydrogen bonds of water molecules in an aqueous solution. Coincidentally, the number ratio of carbon atom to oxygen atom in the molecular model of the coal surface constructed in Figure 1 was 413:96, which was close to the XPS results, indicating that the model constructed was suitable.

Figure 3.
XPS wide-energy spectra of the coal samples.
3.2. Molecular Dynamics Simulation
In order to study the influence of the hydration layer on the adsorption of the oil collector on the surface of low-rank coal, the adsorption process of water molecules and oil collector on the surface of coal was simulated in three different systems, named coal-water (CW), coal-collector (CC), and coal-water-collector (CWC). The mean square displacement (MSD), the interaction energy (IE), and the relative concentration distribution (RCD) between collector and coal molecules were analyzed.
3.2.1. Coal-Water Adsorption System Simulation
The dynamic simulation results of CW are presented as Figure 4. As shown in Figure 4, water molecules were mainly located near the surface of coal molecules due to the attraction of coal molecules. The relative concentration distribution (RCD) of water molecules is shown in Figure 5. The distribution of water molecules in X and Y directions was uniform. However, in the vertical direction from the surface of the coal molecule, the RCD of the water molecule had three peaks. This indicated that the hydrophilic group in the coal molecule interacted with the water molecule, forming a hydration layer with a high concentration of water molecules. In addition, in the vertical direction, there was a peak value of RCD near 13 Å. Combined with the coal surface model, it was found that there were some pore structures at this position. Water molecules could enter these pores and promote the formation of the hydration layer on the coal surface [32,33].
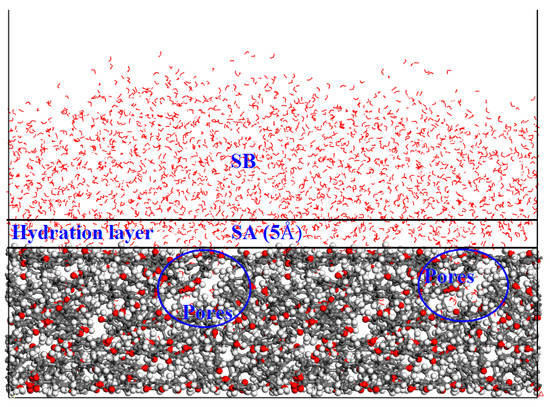
Figure 4.
Final configuration of the coal-water (CW) system.
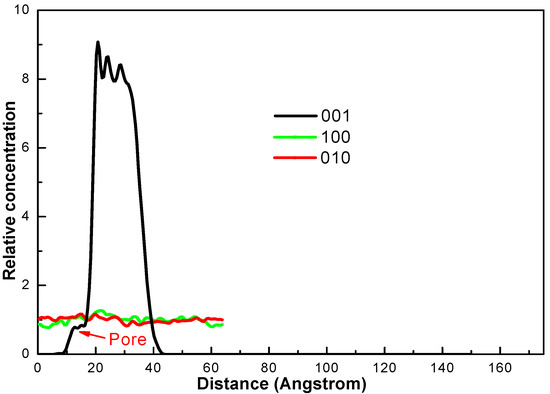
Figure 5.
Relative concentration distribution (RCD) of water molecules in the CW system.
MSD of the last 100 ps MD simulation was calculated to indicate the mobility of water molecules. The definitions of MSD is as follows:
The MSD–time curves of water molecules in SA and SB (Figure 4) are compared in Figure 6. The larger the slope of the curve was, the stronger the mobility of the water molecules was. As shown in Figure 6, the slope of SA was lower than that of SB, which revealed that the water molecules in the hydration layer were strongly attracted by the low-rank coal surface.
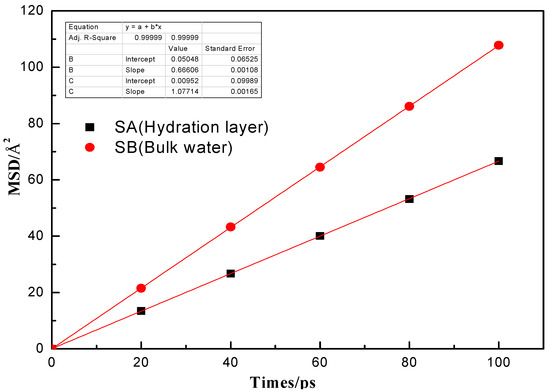
Figure 6.
Mean square displacement (MSD) of water molecules in SA (hydration layer) and SB (bulk water).
3.2.2. Coal-Collector Adsorption System Simulation
Initial and final configurations of the CC adsorption system are presented in Figure 7. Due to the strong attraction of coal molecules, dodecane droplets dispersed rapidly and evenly adsorbed on the coal surface. In addition, it can be found in Figure 7 that some dodecane molecules entered the pores of the coal surface, which was consistent with the previous studies [33]. This explained that the surface hydrophobicity of low-rank coal could be improved by the oil pre-condition method.
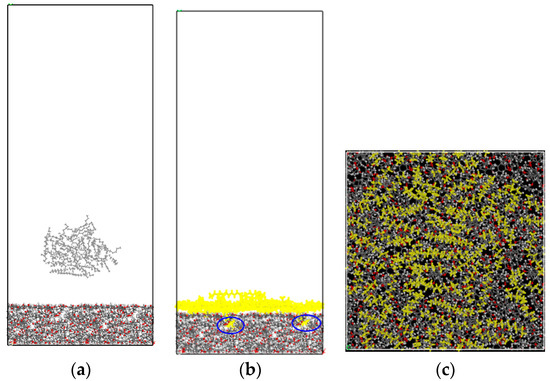
Figure 7.
Initial (a), final (b), and top view (c) configurations of the coal-collector (CC) adsorption system (the yellow molecules represent dodecane molecules).
The IE between dodecane and coal was −597 kcal/mol, while that between water and coal was −1401 kcal/mol. This indicated that dodecane could hardly pass through the hydration layer on the coal surface.
The IE between coal surface and dodecane molecules was −597 kcal/mol, which can be calculated from Equation (3):
where Etotal, Edodecane/water, and Ecoal represent the total energy of the CC adsorption system, the summation of single point energies of the free dodecane, and the coal surface, respectively. The energy calculation results are presented in Table 2 and Table 3.

Table 2.
Interaction energy (IE) between coal and dodecane, kcal/mol.

Table 3.
IE between coal and water, kcal/mol.
It can be seen from Table 2 that dodecane molecules were adsorbed on the coal surface mainly by van der Waals force. The negative value of IE indicated that dodecane molecules could spontaneously adsorb on the surface of low-rank coal.
3.2.3. Coal-Water-Collector Adsorption System Simulation
Initial and final configurations of the CWC adsorption system under different condition are presented in Figure 8 and Figure 9. It can be seen from Figure 8b that dodecane molecules could not be adsorbed on the coal surface, and the dodecane molecules were kept agglomerated together during the whole simulation process. This was because, in the initial state (Figure 8a), the dodecane molecules were about 35 Å away from the coal surface, and the dodecane molecules could not break through the hydration layer formed by the surface water molecules. However, it can be seen from Figure 9b that a large number of dodecane molecules adsorbed the coal surface. This was because, in the initial state, the collector molecule was close to the coal surface (less than 5 Å), the thickness of the hydration layer was 5 Å, and the collector molecule was already located in the inside of the hydration layer, thus it could be successfully adsorbed on the coal surface.
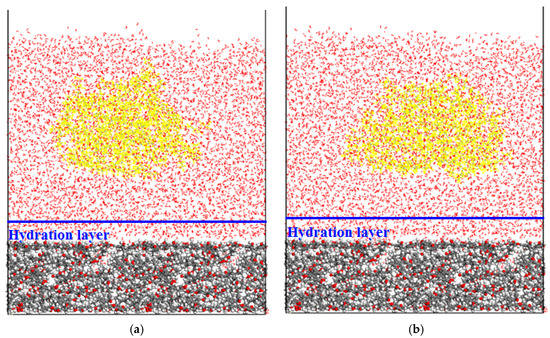
Figure 8.
Initial (a) and final (b) configurations of the coal-water-collector (CWC) adsorption system.
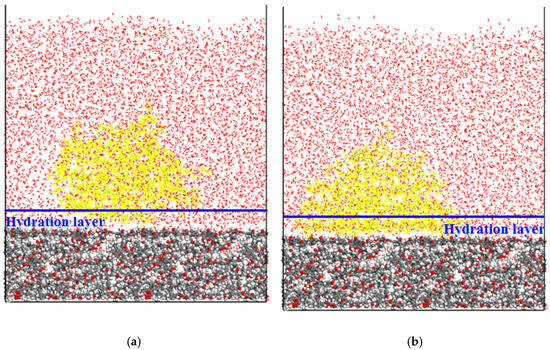
Figure 9.
Initial (a) and final (b) configurations of the CWC adsorption system.
The RCDs of water molecules as a function of simulation time in Figure 9b are presented in Figure 10.
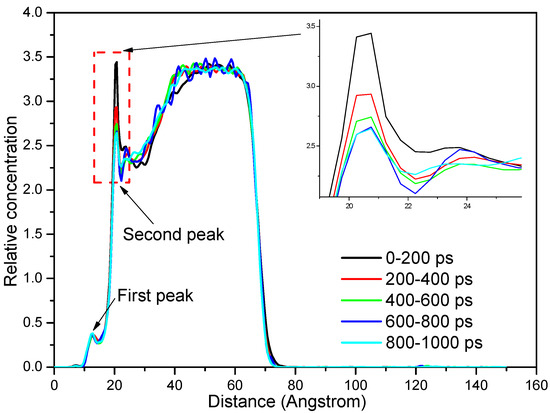
Figure 10.
Relative concentration distribution of water molecules as a function of simulation time.
It can be seen from Figure 10 that there were two peaks at 13 Å and 21.5 Å. The first peak value was caused by the water molecules adsorbed in the pores of coal, which was consistent with the results of Section 3.2.2, indicating that the addition of dodecane molecules would not affect the adsorption morphology of water molecules in pores. The second peak was due to the water molecules adsorbed on the coal surface (hydration layer). With the increase of simulation time, the collector gradually adsorbed on the coal surface and began to spread, pushing away the water molecules near the coal surface. As a result, the RCD of water molecules on the coal surface decreased gradually. In the initial 200 ps, the RCD of water molecules decreased rapidly. After 600 ps, the RCD of water molecules in the system remained stable, indicating that the system had reached the equilibrium state.
3.2.4. Discussion on Method to Improve Low-Rank Coal Flotation
This paper mainly studied the influence mechanism of the hydration layer on the adsorption of the collector on the surface of low-rank coal. Based on the research results of this paper, we think that the flotation effect of low-rank coal can be improved from the following aspects: (1) designing new reagents that can produce stronger interaction with low-rank coal molecules. There are a large number of oxygen-containing functional groups on the surface of low rank coal, thus a small amount of polar functional groups such as ether group or ammonium group can be introduced into the collector molecule to enhance the interaction between collector and low-rank coal surface. The reagent molecules may successfully pass the hydration layer and adsorb on the surface of low-rank coal; (2) using physical and chemical methods such as microwave pretreatment or hot pressing to change the surface hydrophobicity of low-rank coal, weakening the interaction between low-rank coal and water molecules and preventing the formation of hydration layer; (3) using auxiliary force fields such as ultrasonic wave and strong stirring to weaken the interaction between water molecules and low-rank coal surface and to reduce the stability of the hydration layer. According to Figure 8 and Figure 9, by increasing the collision velocity between the collector molecules and the coal particles, the collector molecules may pass the hydration layer and then adsorb on the coal surface; (4) based on the simulation results of Section 3.2.2, dodecane molecules can spontaneously adsorb on the surface of low-rank coal in the absence of water. Therefore, we can preferentially adsorb low-rank coal with the collector and then carry out conventional flotation to improve the flotation effect of low-rank coal.
4. Conclusions
In this paper, effects of a hydration layer on the adsorption of a dodecane collector on low-rank coal were studied by molecular dynamics simulation. This study revealed at the atomic/molecular level that a hydration layer with a thickness of about 5 Å was formed on the low-rank coal. In a vacuum, dodecane can spontaneously adsorb on the surface of low-rank coal. The hydration layer has an important influence on the adsorption of dodecane on the surface of low-rank coal. When the dodecane molecule is located inside the hydration layer, the dodecane molecule can be successfully adsorbed and spread on the surface of low-rank coal. While the dodecane molecule is outside the hydration layer, the dodecane molecule cannot pass through the hydration layer on the surface of low-rank coal. The results revealed the influence of the hydration layer on the adsorption of reagents on the surface of low-rank coal from the molecular level, which has important theoretical significance for flotation and utilization of low-rank coal.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9717/8/10/1207/s1, Figure S1: Wender coal molecule (The numbers are the Mulliken charges of each atom).
Author Contributions
Conceptualization, L.S. and F.M.; Investigation, L.L. and C.X.; Methodology, F.M.; Validation, L.L.; Writing—original draft, L.S.; Writing—review & editing, C.X. and E.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Anhui Province, grant number 1808085QE120, National Natural Science Foundation of China, grant number 51874011 and 11872001, Postdoctoral Research Foundation of China, grant number 2019M652163, Postdoctoral Science Foundation of Anhui Province, grant number 2019B338, Excellent Youth Foundation of Anhui Scientific Committee, grant number 1808085J30.
Acknowledgments
The authors would like to thank Zhang Mingxu at Anhui University of Science and Technology for his help with this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shen, L.; Wang, C.; Min, F.; Liu, L.; Xue, C. Effect of pores on the flotation of low-rank coal: An experiment and simulation study. Fuel 2020, 271, 117557. [Google Scholar] [CrossRef]
- Shen, L.; Min, F.; Liu, L.; Zhu, J.; Xue, C.; Cai, C.; Zhou, W.; Wang, C. Application of gaseous pyrolysis products of the waste cooking oil as coal flotation collector. Fuel 2019, 239, 446–451. [Google Scholar] [CrossRef]
- Shen, L.; Min, F.; Liu, L.; Xue, C.; Zhu, J. Improving Coal Flotation by Gaseous Collector Pretreatment Method and its Potential Application in Preparing Coal Water Slurry. Processes 2019, 7, 500. [Google Scholar] [CrossRef]
- Wang, C.; Xing, Y.; Xia, Y.; Chen, P.; Chen, W.; Tan, J.; Zhang, C.; Gui, X. Effect of vacancy defects on electronic properties and wettability of coal surface. Appl. Surf. Sci. 2020, 511, 145546. [Google Scholar] [CrossRef]
- Zhang, R.; Xing, Y.; Xia, Y.; Luo, J.; Tan, J.; Rong, G.; Gui, X. New insight into surface wetting of coal with varying coalification degree: An experimental and molecular dynamics simulation study. Appl. Surf. Sci. 2020, 511, 145610. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, Y.; Li, M.; Ma, Z.; Xing, Y.; Gui, X. Effects of pore compression pretreatment on the flotation of low-rank coal. Fuel 2019, 239, 63–69. [Google Scholar] [CrossRef]
- Nie, B.; Liu, X.; Yang, L.; Meng, J.; Li, X. Pore structure characterization of different rank coals using gas adsorption and scanning electron microscopy. Fuel 2015, 158, 908–917. [Google Scholar] [CrossRef]
- Lu, F.; Liu, L.; Min, F.; Chen, J.; Zhang, M. Density Functional Theory Analysis of the Adsorption Interactions of Carbon Impurities in Coal-associated Kaolinite. Processes 2019, 7, 782. [Google Scholar] [CrossRef]
- Xia, W.; Niu, C.; Ren, C. Enhancement in floatability of sub-bituminous coal by low-temperature pyrolysis and its potential application in coal cleaning. J. Clean. Prod. 2017, 168, 1032–1038. [Google Scholar] [CrossRef]
- Xia, W.; Li, Y.; Nguyen, A.V. Improving coal flotation using the mixture of candle soot and hydrocarbon oil as a novel flotation collector. J. Clean. Prod. 2018, 195, 1183–1189. [Google Scholar] [CrossRef]
- Wen, B.; Xia, W.; Sokolovic, J.M. Recent advances in effective collectors for enhancing the flotation of low rank/oxidized coals. Powder Technol. 2017, 319, 1–11. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhang, R.; Xing, Y.; Gui, X. Performance of used lubricating oil as flotation collector for the recovery of clean low-rank coal. Fuel 2019, 239, 717–725. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, R.; Xing, Y.; Gui, X. Improving the adsorption of oily collector on the surface of low-rank coal during flotation using a cationic surfactant: An experimental and molecular dynamics simulation study. Fuel 2019, 235, 687–695. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Cao, Y.; Wang, D.; Zhang, H. Clean low-rank-coal purification technique combining cyclonic-static microbubble flotation column with collector emulsification. J. Clean. Prod. 2017, 153, 657–672. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Qu, J.; Liu, Q.; Tang, L.; Tao, X.; Fan, H. Oily bubble flotation technology combining modeling and optimization of parameters for enhancement of flotation of low-flame coal. Powder Technol. 2018, 335, 171–185. [Google Scholar] [CrossRef]
- Xu, M.; Xing, Y.; Cao, Y.; Gui, X. Waste colza oil used as renewable collector for low rank coal flotation. Powder Technol. 2019, 344, 611–616. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S.; Li, L.; Qu, J.; Tao, X.; He, H. Exploration on the mechanism of enhancing low-rank coal flotation with cationic surfactant in the presence of oily collector. Fuel 2018, 227, 190–198. [Google Scholar] [CrossRef]
- Wang, S.; Tao, X. Comparison of flotation performances of low rank coal in air and oily bubble processes. Powder Technol. 2017, 320, 37–42. [Google Scholar] [CrossRef]
- Xia, W.; Yang, J.; Liang, C. Effect of microwave pretreatment on oxidized coal flotation. Powder Technol. 2013, 233, 186–189. [Google Scholar] [CrossRef]
- Wang, S.; Tang, L.; Tao, X. Investigation of effect of surfactants on the hydrophobicity of low rank coal by sliding time measurements. Fuel 2018, 212, 326–331. [Google Scholar] [CrossRef]
- Dey, S. Enhancement in hydrophobicity of low rank coal by surfactants—A critical overview. Fuel Process. Technol. 2012, 94, 151–158. [Google Scholar] [CrossRef]
- Wang, S.; Albijanic, B.; Tao, X.; Fan, H. Thin liquid film drainage mechanism between air bubbles and low-rank coal particles in the presence of surfactant. Fuel Process. Technol. 2019, 186, 18–24. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, R.; Cao, Y.; Xing, Y.; Gui, X. Role of molecular simulation in understanding the mechanism of low-rank coal flotation: A review. Fuel 2020, 262, 116535. [Google Scholar] [CrossRef]
- Chen, J.; Min, F.; Liu, L.-Y.; Liu, C.-F. Mechanism research on surface hydration of kaolinite, insights from DFT and MD simulations. Appl. Surf. Sci. 2019, 476, 6–15. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Fan, M.; Zhang, L. The effect of ethylene oxide groups in dodecyl ethoxyl ethers on low rank coal flotation: An experimental study and simulation. Powder Technol. 2019, 344, 684–692. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Guo, J.; Zhang, L. Interaction between low rank coal and kaolinite particles: A DFT simulation. Appl. Surf. Sci. 2018, 456, 215–220. [Google Scholar] [CrossRef]
- Wender, I. Catalytic Synthesis of Chemicals from Coal. Catal. Rev. 1976, 14, 97–129. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Yan, K. Adsorption of collectors on model surface of Wiser bituminous coal: A molecular dynamics simulation study. Miner. Eng. 2015, 79, 31–39. [Google Scholar] [CrossRef]
- Peng, C.; Min, F.; Liu, L. Effect of pH on the adsorption of dodecylamine on montmorillonite: Insights from experiments and molecular dynamics simulations. Appl. Surf. Sci. 2017, 425, 996–1005. [Google Scholar] [CrossRef]
- Liang, Y.H.; Wang, F.; Zhang, H.; Wang, J.P.; Li, Y.; Li, G.Y. A ReaxFF molecular dynamics study on the mechanism of organic sulfur transformation in the hydropyrolysis process of lignite. Fuel Process. Technol. 2016, 147, 32–40. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, X.; Liu, X.; Yang, K.; Zhou, H. Surface Wettability of Basal Surfaces of Clay Minerals: Insights from Molecular Dynamics Simulation. Energy Fuels 2016, 30, 149–160. [Google Scholar] [CrossRef]
- Xia, W.; Zhou, C.; Peng, Y. Enhancing flotation cleaning of intruded coal dry-ground with heavy oil. J. Clean. Prod. 2017, 161, 591–597. [Google Scholar] [CrossRef]
- Xia, W.; Yang, J. Reverse Flotation of Taixi Oxidized Coal. Energy Fuels 2013, 27, 7324–7329. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).