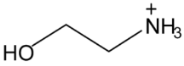
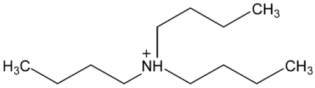
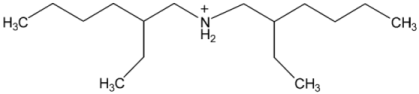
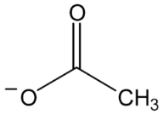
Abstract
Ionic liquids, which are classified as new solvents, have been identified to be potential solvents in the application of CO2 capture. In this work, six ammonium-based protic ionic liquids, containing ethanolammonium [EtOHA], tributylammonium [TBA], bis(2-ethylhexyl)ammonium [BEHA] cations, and acetate [AC] and butyrate [BA] anions, were synthesized and characterized. The thermophysical properties of the ammonium-based protic ionic liquids were measured. Density, ρ, and dynamic viscosity, η, were determined at temperatures between 293.15 K and 363.15 K. The density and viscosity values were correlated using empirical correlations and the thermal coefficient expansion, αp, and molecular volume, Vm, were estimated using density values. The thermal stability of the ammonium-based protic ionic liquids was investigated using thermogravimetric analyzer (TGA) at a heating rate of 10 °C·min‒1. The CO2 absorption of the ammonium-based ionic liquids were measured up to 20 bar at 298.15 K. From the experimental results, [BEHA][BA] had the highest affinity towards CO2 with the mol fraction of CO2 absorbed approaching 0.5 at 20 bar. Generally, ionic liquids with butyrate anions have better CO2 absorption than that of acetate anions while [BEHA] ionic liquids have higher affinity towards CO2 followed by [TBA] and [EtOHA] ionic liquids.
1. Introduction
Natural gas consists mainly of methane as well as other higher alkanes in varied amounts. It is mainly used as a fuel and as a raw material in petrochemical industries [1]. While natural gas is principally a mixture of combustible hydrocarbons, many natural gases also contain impurities, such as carbon dioxide, CO2, hydrogen sulfide, H2S, and water. Refining processes are required to remove all of these unwanted impurities from natural gas. Besides water and higher-molecular-weight hydrocarbons, one of the most crucial parts of gas processing is the elimination of CO2 and this process is normally done by means of chemical absorption techniques using alkanolamine solutions. Despite the successful practice of using alkanolamines for CO2 removal, several disadvantages have been identified, such as solvent loss and degradation as well as corrosion issues [2]. In view of these issues, there is a need to develop new alternative, yet effective solvents for the same purposes. Ionic liquids have emerged as new solvents that have potential to be used for CO2 removal due to their special features, namely non-detectable vapor pressure, wide liquid range, and remarkable thermal stability. Ionic liquids are low melting salts with typical melting points of below 100 °C. Furthermore, there are countless possible combinations of cations and anions that can yield ionic liquids and this flexibility is utilized to design ionic liquids based on the application. Ionic liquids are used in many applications, such as in chemical reactions and separation processes [3,4,5,6]. In the field of CO2 capture using ionic liquids, initial work on the CO2-ionic liquid system was done in 2001 [7] and, following this discovery, extensive works have been done to explore the absorption of CO2 in ionic liquids under various operating conditions [8,9,10,11,12,13,14,15]. Imidazolium-based ionic liquids were used in most of the study of CO2 absorption. They are highly unsymmetrical and therefore have low melting points. Recently, protic ionic liquids have been used in the study of CO2 capture [16,17]. Protic ionic liquids are formed by a proton transfer between an equimolar amount of a Brønsted acid and a Brønsted base. The modern type of protic ionic liquids have been described by Ohno and co-workers [18] and an extensive review on the properties and applications of protic ionic liquids has been provided by Greaves and Drummond [19] who also noted the ability of protic ionic liquids to support amphiphile self-assembly [20,21]. The interest in the ionicity of protic ionic liquids has come to light due to the unlikeliness of complete proton transfer between the acid and base contributing to the presence of a neutral acid and base mixture [19,22,23]. MacFarlane and Seddon [24] proposed a limit of a 1% neutral species presence in an ionic liquid to be called ‘pure ionic liquid’. The term pseudo-protic ionic liquid was suggested by Doi et al. [25] after they discovered that a mixture of N-methylimidazole and acetic acid exhibited electrical conductivity behavior even though the mixture was mostly dominated by neutral species rather than ions when inspected using Raman spectroscopy.
Nevertheless, the simple synthesis pathway of protic ionic liquids, i.e., a one-step neutralization reaction, and their proven ability to absorb CO2 motivated us to explore this type of ionic liquids in the field of CO2 capture. Nonetheless, physical properties, such as density, viscosity, and thermal stability of ionic liquids, are very important prior to using these new solvents in any applications. Precise understanding on the thermophysical properties is important as it is required to evaluate the suitability of ionic liquids to be used at an industrial scale [26]. For instance, viscosity is an important property for the design of industrial processes involving heat and mass transfer and dissolution of compounds in fluids [27]. Therefore, the aim of our work was to synthesize several new ammonium-based protic ionic liquids using a 1-step neutralization reaction, measure their thermophysical properties, and, lastly, test their ability to capture CO2. In this work, six ammonium-based protic ionic liquids, containing acetate and butyrate anions, were synthesized using solvent-free, 1-step neutralization reaction. The density, dynamic viscosity, and thermal stability of these ionic liquids were determined. The density values enable the estimation of thermal expansion coefficient and the molecular volume of the ionic liquids. To assess the capability of these ionic liquids towards CO2, absorption of CO2 was done using a solubility cell and the screening was done in the CO2 pressure range up to 20 bar and at 298.15 K. Results showed that the ammonium-based protic ionic liquids synthesized in this study have the potential to absorb CO2.
2. Materials and Methods
2.1. Chemicals
Three amines and two organic acids from Merck Sdn. Bhd. were used in the production of the ammonium-based protic ionic liquids. All chemicals were of analytical grade. The amines and acids CAS numbers, abbreviations, and grades are as follows: ethanolamine (141–43–5, 99%), bis(2–ethylhexyl)amine (106–20–7, 99%), tributylamine (102–82–9, 99%), acetic acid (64–19–7, 99.8%), and butyric acid (107–92–6, 99%).
2.2. Synthesis
For the synthesis of each of the ammonium-based protic ionic liquids, an equimolar amount of the acid was added dropwise to the amine at ambient conditions and the mixture was consistently stirred for 24 h to facilitate mixing. The resulting solution was dried under vacuum at 65 °C for 6 h to remove remaining reactants. The final product was kept in a seal container until further use. The combinations of two acids and three amines produce six ammonium-based protic ionic liquids. Table 1 shows the structures and the abbreviation used for the ionic liquids. All ionic liquids exist as liquids except [BEHA][AC], which exists as a solid compound at room temperature.

Table 1.
Structures of cations and anions, names and abbreviations.
2.3. NMR and Water Content
The structure of the ammonium-based protic ionic liquids was analyzed and confirmed via nuclear magnetic resonance (NMR) spectroscopy. About 5 mg sample of ionic liquid was dissolved in 6 mL deuterated solvent and the sample’s purity was determined using 500 MHz Bruker NMR Oxford Instrument. Coulometric Karl Fischer autotitrator DL39 from Mettler was used to determine the water content of the ionic liquids.
2.4. Thermophysical Characterization
The viscosity and density of the ammonium-based protic ionic liquids were determined simultaneously using Anton Parr Stabinger Viscometer SVM3000 in the temperature range of 293.15 K to 363.15 K. The temperature measurement‘s accuracy was within 0.02 K while the reproducibility of the viscosity and density measurements were 0.35% and ±5.10−4 g·cm−3, respectively [28]. The decomposition temperatures of the ionic liquids were examined by means of thermogravimetric analyzer, TGA Perkin Elmer STA 6000. About 10 mg of sample was loaded into a platinum pan and the sample was heated at a heating rate of 10 °C·min‒1 under nitrogen flow.
2.5. CO2 Absorption Measurement
The ability of the ammonium-based protic ionic liquids to absorb CO2 was investigated based on a pressure drop technique using a solubility cell as described in our previous publication [29]. The solubility cell consists of an equilibrium cell and a gas vessel immersed in a thermostatic bath. In a pressure drop method, the gas with a known pressure at constant volume is allowed to be in contact with the ionic liquid in the equilibrium cell and the pressure drop is monitored as the gas absorbs into the ionic liquid until equilibrium is attained. In a typical experiment, the equilibrium cell was loaded with a pre-weighed amount of the ionic liquid and the equilibrium cell was evacuated to remove any gases. In the gas vessel, CO2 was allowed to stabilize before being quickly charged into the equilibrium cell. The CO2-ionic liquid system was assumed to achieve equilibrium when the pressure attained a constant value. The system was maintained in that conditions for an additional two hours to ensure equilibration. Equation (1) was used to calculate the amount of CO2 absorbed in the ionic liquid, n2 [30]:
where Pini and Tini are the initial pressure and temperature of the system, Peq and Teq are the pressure and temperature of the system at equilibrium, Vtotal is the volume of the equilibrium cell, Vliq is the volume of ionic liquid, R is the gas constant, and Z2 represents the compressibility factor of the gas. Z2 can be calculated using Soave–Redlich–Kwong equation of state [31]. The mole fraction of CO2 absorbed in the ionic liquid (x2) was calculated using Equation (2):
where n2liq represents the mole of dissolved CO2 and n1liq is the mole of the ionic liquid.
3. Results and Discussion
In this work, six ammonium-based protic ionic liquids—ethanolammonium acetate [EtOHA][AC], ethanolammonium butyrate [EtOHA][BA], tributylammonium acetate [TBA][AC], tributylammonium butyrate [TBA][BA], bis(2-ethylhexyl)ammonium acetate [BEHA][AC], and bis(2-ethylhexyl)ammonium butyrate [BEHA][BA]—were synthesized and characterized. All the ammonium-based protic ionic liquids exist as liquids at room temperature except [BEHA][AC], which is a solid. The NMR and water content of each ionic liquids are presented as follows:
- [EtOHA][AC]: 1H NMR (500 MHz, D2O): δ 3.613 [t, 2H, H2(-OH)], 2.922 [t, 2H, CH2(-NH2)], 1.726 [s, 3H, CH3]. Water content: 2.93%
- [EtOHA][BA]: 1H NMR (500 MHz, D2O): δ 3.669 [t, 2H, CH2(-OH)], 2.988 [t, 2H, CH2(-NH2)], 2.010 [t, 2H, CH2(-COOH)], 1.375–1.448 [m, 2H, CH2], 0.747 [t, 3H, CH3]. Water content: 2.06%
- [BEHA][AC]: 1H NMR (500 MHz, D2O): δ 2.861 [d, 4H, CH2(-NH)], 1.805 [s, 3H, CH3], 1.613–1.677 [m, 2H, CH], 1.181–1.309 [m, 16H, CH2], 0.786 [t, 12H, CH3]. Solid
- [BEHA][BA]: 1H NMR (500 MHz, D2O): δ 2.856 [d, 4H, CH2(-NH)], 2.062 [t, 2H, CH2(-COOH)], 1.582–1.660 [m, 2H, CH], 1.412–1.486 [m, 2H, CH2(AC)], 1.236–1.339 [m, 16H, CH2], 1.214-1.229 [t,t 15H, CH3]. Water content: 0.15%
- [TBA][AC]: 1H NMR (500 MHz, D2O): δ 3.002 [t, 4H, CH2(-NH)], 1.788 [s, 3H, CH3], 1.508–10571 [m, 6H, CH2], 1.207–1.282 [m, 6H, CH2], 0.805 [t, 9H, CH3]. Water content: 0.47%
- [TBA][BA]: 1H NMR (500 MHz, D2O): δ 3.003 [t, 6H, CH2(-NH)], 2.031 [t, 2H, CH2(-COOH)], 1.510–1.573 [m, 6H, CH2], 1.394–1.468 [m, 2H, CH2(AC)], 1.211-1.285 [m, 6H, CH2], 0.809 [t, 9H, CH3], 0.768 [t, 3H, CH3(AC)]. Water content: 0.23%
3.1. Thermophysical Properties
The experimental density and dynamic viscosity values for all liquid samples of synthesized ammonium-based protic ionic liquids are presented in Table 2 and Table 3. The experimental densities of [EtOHA][AC], [EtOHA][BA], [BEHA][BA], [TBA][AC], and [TBA][BA] as a function of temperature are shown in Figure 1, Figure 2 and Figure 3. [BEHA][AC] is not included as it exists as solid. As can be seen from Figure 1, the density of all five ammonium-based protic ionic liquids decreased gradually and linearly with increasing temperature over the range of temperature studied. An increase in temperature caused higher mobility of the ions which, in turn, weakens the intermolecular forces between the constituent ions and correspondingly increases the unit volume for these ions [32]. The density of these ammonium-based protic ionic liquids was slightly affected by the length of the alkyl chain of the anion in which the density of ionic liquids with the [AC] anion was higher than of ionic liquids with the [BA] anion for a fixed cation, as shown in Figure 2a,b. This observation is consistent with the literature in which it has been shown that the density value drops as the alkyl chain gets longer [28,32,33,34,35,36,37]. Our experimental density value of [EtOHA][AC] is in good agreement with Kurnia et al. [35] and Hosseini et al. [38] with the value differences of less than 0.2% and 0.8%, respectively. Generally, effective arrangement of ions in a liquid can increase the density of the liquid due to a greater number of ions available in a unit volume [39]. Based on our experimental results, as shown in Figure 3, ionic liquids with the [EtOHA] cation have higher density values compared to the rest, suggesting a better packing of the ions due to the small size of the cation. [TBA][BA] had the lowest density values at all temperatures due to the combined effects of branching of the cation, which increases the asymmetricity and steric hindrance of the ionic liquid [32], along with the large size of the alkyl chain of the [BA] anion.

Table 2.
Density (ρ) values of ionic liquids from 293.15 K to 363.15 K.

Table 3.
Dynamic viscosity (η) values of ionic liquids from 293.15 K to 363.15 K.
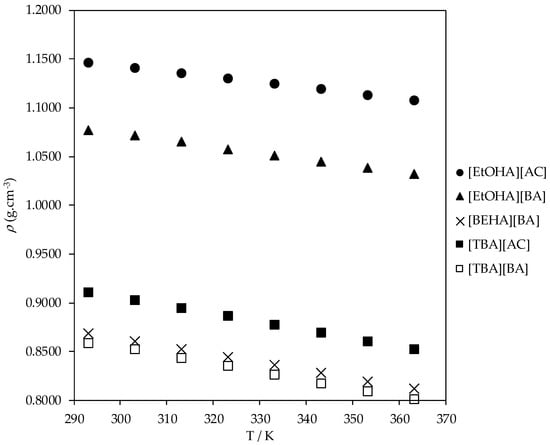
Figure 1.
Plot of experimental density (ρ) values of ammonium-based protic ionic liquids.
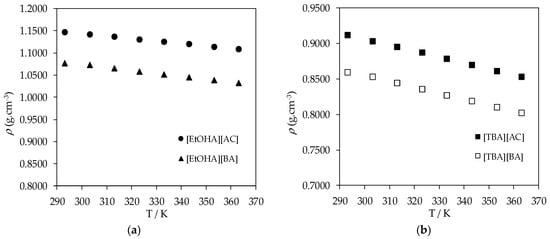
Figure 2.
(a) Plot of experimental density (ρ) values of [EtOHA][AC] and [EtOHA][BA] and (b) plot of experimental density (ρ) values of [TBA][AC] and [TBA][BA] as a function of temperature.
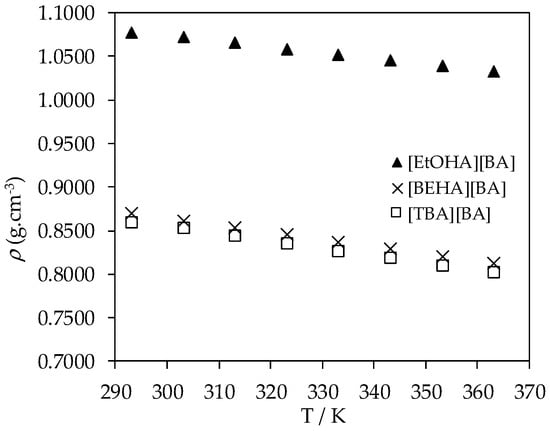
Figure 3.
Plot of experimental density (ρ) values of [EtOHA][BA], [BEHA][BA], and [TBA][BA] as a function of temperature.
The dynamic viscosity of the ammonium-based protic ionic liquids, presented in Figure 4, dropped significantly as the temperature increased and the viscosity of ionic liquids with a [BA] anion was higher than that of ionic liquids with an [AC] anion for each type of cation studied in this work, as shown in Figure 5. The longer the alkyl chain in the ionic liquid structure, the higher the viscosity of the ionic liquids due to the increase in van der Waals attraction between the aliphatic alkyl chains [35]. On the other hand, [EtOHA] ionic liquids have remarkable high viscosity values compared to [BEHA] and [TBA] ionic liquids due to the presence of the hydroxyl group which enables a hydrogen bonding interaction between the structures of the ions.
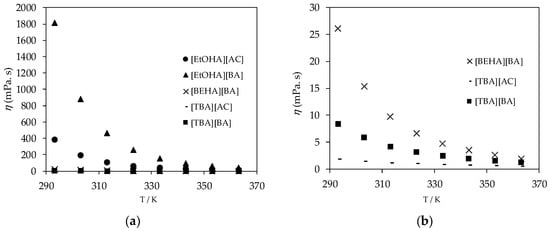
Figure 4.
(a) Plot of experimental viscosity (η) values of ammonium based ionic liquids and (b) plot of experimental viscosity (η) values of [BEHA][BA], [TBA][AC], and [TBA][BA].
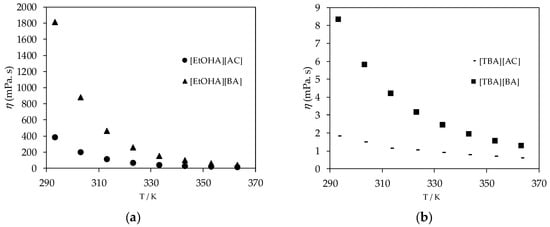
Figure 5.
(a) plot of experimental viscosity (η) values of [EtOHA][AC] and [EtOHA][BA] ionic liquids and (b) plot of experimental viscosity (η) values of [TBA][AC] and [TBA][BA].
The values of density, ρ and dynamic viscosity, η were fitted using Equations (3) and (4) [28]:
where ρ is the density, η is the dynamic viscosity of the ionic liquids, T is temperature in K, and A0, A1, A2, and A3 are correlation coefficients determined using the method of least squares. The calculated correlation coefficients together with standard deviations, SD are presented in Table 4 and Table 5. The standard deviations, SD, were calculated using Equation (5) in which Zexpt and Zcalc are experimental and calculated values, respectively, while nDAT is the number of experimental points:
ρ = A0 + A1T,
lg η = A2 + A3/T,

Table 4.
Fitting parameters of Equation (3) for density (ρ) correlation and standard deviation (SD) from Equation (5).

Table 5.
Fitting parameters of Equation (4) for dynamic viscosity (η) correlation and standard deviation (SD) from Equation (5).
The thermal expansion coefficient, αp, for the ammonium-based protic ionic liquids can be calculated using Equation (6) [28] while the molecular volume, Vm can be estimated from Equation (7) in which M is the molar mass of the ionic liquid and NA represents the Avogadro’s number [32,36,37]:
αp = ‒1/ρ · (∂ρ/∂T)p = ‒ (A1)/(A0 + A1T) and
Vm = M/NA · ρ.
The calculated thermal expansion coefficients and molecular volume values of the ammonium-based protic ionic liquids are presented in Table 6 and Table 7. The calculated values lie in the range of (5.3 to 9.8)·10‒4 K‒1 for all five ionic liquids. The thermal expansion coefficients were found to be quite consistent over the temperature range studied and therefore are considered to be temperature independent. The pattern of the results is consistent with other types of ionic liquids [27,28,36,40,41]. The molecular volume, Vm, of the [BA] ionic liquid was greater than that of [AC] for a fixed cation and this may be attributed to the presence of additional CH2 groups [36,37]. In this work, the Vm decreased in the sequence of [BEHA] > [TBA] > [EtOHA] for a fixed anion, i.e., [BA].

Table 6.
Thermal expansion coefficients (αp) of the ionic liquids calculated using Equation (6).

Table 7.
Molecular volume (Vm) of the ionic liquids calculated using Equation (7).
The thermal decomposition (Td) of the ammonium-based protic ionic liquids were measured at a heating rate of 10 °C·min‒1. The Td was approximately determined by the intersection of the baseline weight from the beginning of the measurement and the tangent of the weight against the temperature curve as the decomposition process occurs. The Td of the ionic liquids are presented in Table 8 and the thermal decomposition curves for [EtOHA][BA] and [BEHA][BA] are given in Figure 6. The thermal stability of the ammonium-based protic ionic liquids in this study varied with the ion combination. The Td of [BA] ionic liquid was higher than that of [AC] ionic liquid for every type of cation studied while [EtOHA] ionic liquids displayed the highest Td followed by [BEHA] and [TBA]. However, ammonium-based protic ionic liquids in this work and from the literature [36] tend to possess lower thermal stability compared to other ionic liquids, such as imidazolium and pyridinium ionic liquids [28,41,42]. However, generalization must not be made as the thermal stability depends largely on the combination of the cation and anion of the ionic liquids.

Table 8.
Thermal decomposition (Td) temperature of the ionic liquids.
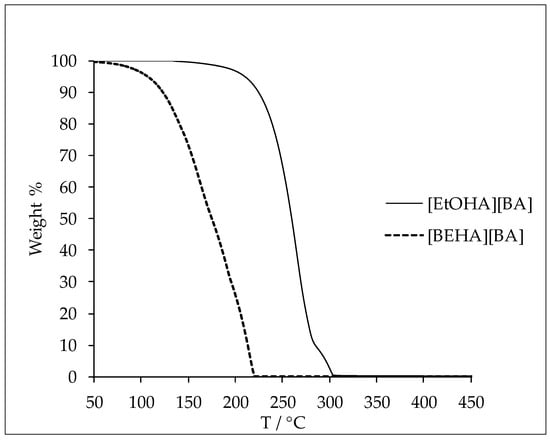
Figure 6.
Plot of thermal decomposition of [EtOHA][BA] and [BEHA][BA].
3.2. CO2 Absorption
The experimental results of CO2 absorption in the ammonium-based protic ionic liquids are shown in Figure 7. Generally, the CO2 absorption in these ammonium-based protic ionic liquids increased with pressure following Henry’s law; the solubility of a gas in a liquid is proportional to the partial pressure of the gas above the surface of the liquid. The mol fraction of CO2 absorbed in the ammonium-based protic ionic liquids was in the range of about 0.02 to 0.48 and up to 20 bar at 298.15 K. The effects of cation structure on the CO2 absorption in the ionic liquids are shown in Figure 8. For a fixed anion, the solubility of CO2 increased in the sequence of [EtOHA] < [TBA] < [BEHA] where the mol fraction of CO2 absorbed in [BEHA][BA] was 0.486 in comparison to 0.328 and 0.307 in [TBA][BA] and [EtOHA][BA], respectively. Meanwhile, Figure 9 indicates a slight increase in the CO2 solubility when the anion of a common cation was changed from [AC] to [BA]. Based on our experimental results, there is a relationship between absorption of CO2 with the density and the molecular volume of the ionic liquids. As the density decreases and molecular volume increases, the fractional free volume increases and, thus, the solubility of CO2 increases [43,44]. By using a common anion, the CO2 absorption in [BEHA][AC] was compared with 1-ethyl-3-methylimidazolium acetate, [EMIM][AC] [45] and 1-butyl-3-methylimidazolium acetate, [BMIM][AC] [11]. At about 20 bar and 298.15 K, there was only a marginal difference between the CO2 solubility in [BEHA][AC] compared to that of [EMIM][AC] and [BMIM][AC]. This result shows a positive indication that our newly synthesized [BEHA][AC] and [BEHA][BA] ionic liquids have comparable performance towards CO2 capture when compared to more established type of ionic liquids. However, more experimental investigation and data are needed to further evaluate the potential ability of our ammonium-based protic ionic liquids in the application of CO2 capture.
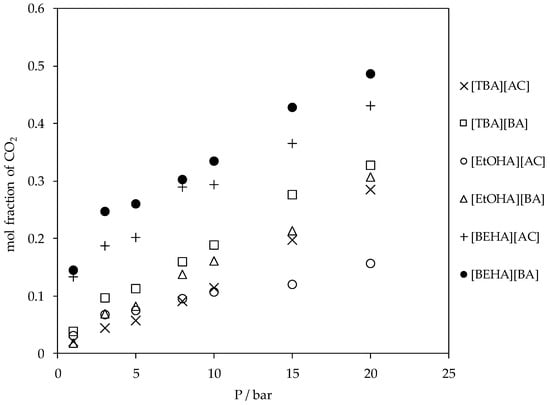
Figure 7.
Plot of CO2 absorption in ammonium-based protic ionic liquids at 298.15 K.
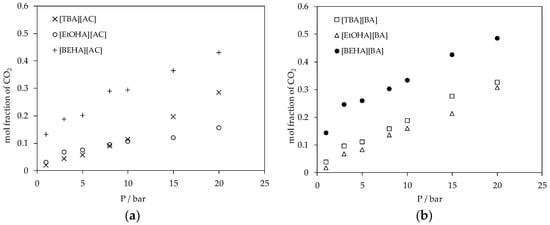
Figure 8.
(a) Plot of CO2 absorption in ammonium-based protic ionic liquids with [AC] anion and (b) plot of CO2 absorption in ammonium-based protic ionic liquids with [BA] anion at 298.15 K.
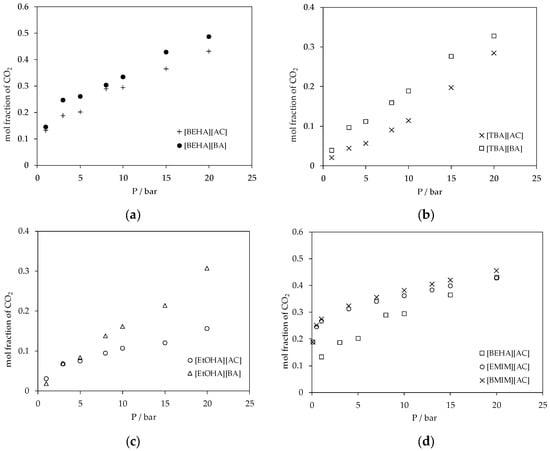
Figure 9.
(a) Plot of CO2 absorption in ammonium-based protic ionic liquids with the [BEHA] cation; (b) plot of CO2 absorption in ammonium-based protic ionic liquids with the [TBA] cation; (c) plot of CO2 absorption in ammonium-based protic ionic liquids with the [EtOHA] cation and; and (d) plot of CO2 absorption in [BEHA][AC], [EMIM][AC] [45] and [BMIM][AC] [11] at 298.15 K.
4. Conclusions
Six ammonium-based protic ionic liquids were successfully synthesized via solvent-free 1-step neutralization reaction. The density, viscosity, and decomposition temperature were measured. The thermal expansion coefficient and the molecular volume were calculated using the density values. The density and viscosity values were inversely proportional with temperature in the range of temperature studied at atmospheric pressure. The density decreased when the alkyl chain of the anion increased, while the viscosity increased with the alkyl chain of the anion. The decomposition temperature of the ammonium-based protic ionic liquids was affected by the combination of cation and anion and [EtOHA] ionic liquids had the highest thermal stability when compared to the other ionic liquids. The absorption of CO2 in the six ammonium-based protic ionic liquids was measured at 298.15 K and up to a pressure of 20 bar. The CO2 absorption values in the ammonium-based protic ionic liquids increased with pressure and both the cation and anion affected the solubility of CO2 in the ionic liquids. The amount of CO2 absorbed was affected by the length of the alkyl chain of the anion while [BEHA] ionic liquids displayed higher CO2 absorption capacity compared to [TBA] and [EtOHA] ionic liquids. Results indicate the potential of the ammonium-based protic ionic liquids to be used as solvents for CO2 capture.
Author Contributions
Conceptualization, N.M.Y. and N.H.H.; methodology, N.H.H. and N.M.Y.; validation, N.H.H.; formal analysis, T.M.; resources, C.D.W. and T.M.; data curation, N.M.Y. and T.M.; writing—original draft preparation, N.M.Y. and N.H.H.; writing—review and editing, J.W.L. and P.L.S.; supervision, N.M.Y.; project administration, N.M.Y. and C.D.W.; funding acquisition, N.M.Y.
Funding
This research was funded by the Ministry of Higher Education (MOHE), Government of Malaysia, under the Fundamental Research Grant Scheme, [FRGS No: FRGS/1/2016/STG01/UTP/03/1] entitled The impact of cations and anions structures of ionic liquids on the CO2 absorption from natural gas and also YUTP grant (cost centre 015LC0-054).
Acknowledgments
Financial assistance and support from Universiti Teknologi PETRONAS and Center of Research in Ionic Liquids (CORIL), UTP are greatly acknowledged.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Mokhatab, S.; Poe, W.A.; Speight, J.G. Handbook of Natural Gas Transmission and Processing; Gulf Processing Publishing, Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Davis, J.; Rochelle, G. Thermal degradation of monoethanolamine at stripper conditions. Energy Procedia 2009, 1, 327–333. [Google Scholar] [CrossRef]
- Jin, X.; Feng, J.; Li, S.; Song, H.; Yu, C.; Zhao, K.; Kong, F. A novel homogeneous catalysis-liquid/solid separation system for highly effective recycling of homogeneous catalyst based on a phosphine-functionalized polyether guanidium ionic liquid. Mol. Catal. 2019, 475, 110503. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, Z.; Cui, Y.; Xie, G.; Jin, Y.; Guo, L.; Xu, Y.; Zhang, Q.; Li, X. Catalytic performance of ionic liquid for dehydrochlorination reaction: Excellent activity and unparallel stability. Appl. Catal. B 2019, 255, 117757. [Google Scholar] [CrossRef]
- Abejón, R.; Rabadán, J.; Lanza, S.; Abejón, A.; Garea, A.; Irabien, A. Supported ionic liquid membranes for separation of lignin aqueous solutions. Processes 2018, 6, 143. [Google Scholar] [CrossRef]
- Baicha, Z.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Hernández-Fernández, F.J.; de los Ríos, A.P.; Maqueda Marín, D.P.; Collado, J.A.; Tomás-Alonso, F.; El Mahi, M. On the selective transport of nutrients through polymer inclusion membranes based on ionic liquids. Processes 2019, 7, 544. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Gu, Z.; Brennecke, J.F. High-pressure phase behavior of ionic liquid/CO2 systems. J. Phys. Chem. B 2001, 105, 2437–2444. [Google Scholar] [CrossRef]
- Anthony, J.L.; Maginn, E.J.; Brennecke, J.F. Solubilities and thermodynamic properties of gases in the ionic liquid 1-n-butyl-3-methylimidazolium hexafluorophosphate. J. Phys. Chem. B 2002, 106, 7315–7320. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, W.Y.; Jang, J.H.; Yoo, K.-P.; Lee, C.S. Solubility measurement and prediction of carbon dioxide in ionic liquids. Fluid Phase Equilib. 2005, 228–229, 439–445. [Google Scholar] [CrossRef]
- Muldoon, M.J.; Aki, S.N.V.K.; Anderson, J.L.; Dixon, J.K.; Brennecke, J.F. Improving carbon dioxide solubility in ionic liquids. J. Phys. Chem. B 2007, 111, 9001–9009. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Kasprzak, D.J.; Junk, C.P.; Yokozeki, A. Phase behavior of {carbon dioxide + [bmim][Ac]} mixtures. J. Chem. Thermodyn. 2008, 40, 25–31. [Google Scholar] [CrossRef]
- Gurkan, B.E.; de la Fuente, J.C.; Mindrup, E.M.; Ficke, L.E.; Goodrich, B.F.; Price, E.A.; Schneider, W.F.; Brennecke, J.F. Equimolar CO2 absorption by anion-functionalized ionic liquids. J. Am. Chem. Soc. 2010, 132, 2116–2117. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Park, S.D.; Baek, I.H.; Park, K.T.; Yoon, Y.I.; Jeong, S.K. Effects of anions on absorption capacity of carbon dioxide in acid functionalized ionic liquids. Fuel Process. Technol. 2012, 100, 55–62. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Khanna, A. Carbon dioxide absorption studies using amine-functionalized ionic liquids. J. Ind. Eng. Chem. 2014, 20, 2497–2509. [Google Scholar] [CrossRef]
- Zoubeik, M.; Mohamedali, M.; Henni, A. Experimental solubility and thermodynamic modeling of CO2 in four new imidazolium and pyridinium-based ionic liquids. Fluid Phase Equilib. 2016, 419, 67–74. [Google Scholar] [CrossRef]
- Zhu, X.; Song, M.; Xu, Y. DBU-Based protic ionic liquids for CO2 capture. ACS Sustian. Chem. Eng. 2017, 5, 8192–8198. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; Oncsik, T.; Mitschke, B.; MacFarlane, D.R. Base-rich diamino protic ionic liquid mixtures for enhanced CO2 capture. Sep. Purif. Technol. 2018, 196, 27–31. [Google Scholar] [CrossRef]
- Hirao, M.; Sugimoto, H.; Ohno, H. Preparation of novel room-temperature molten salts by neutralization of amines. J. Electrochem. Soc. 2000, 147, 4168–4172. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Properties and applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Ionic liquids as amphiphile self-assembly media. Chem. Soc. Rev. 2008, 37, 1706–1726. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Solvent nanostructure, the solvophobic effect and amphiphile self-assembly in ionic liquids. Chem. Soc. Rev. 2013, 42, 833–1412. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Izgorodina, E.I.; Abbott, A.P.; Annat, G.; Fraser, K. On the concept of ionicity in ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 4962–4967. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zhang, Y.; Chen, K.; Che, S.; Yao, J.; Li, H. Ionicity if protic ionic liquid: Quantitative measurement by spectroscopic methods. J. Phys. Chem. B 2017, 121, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.R.; Seddon, K.R. Ionic liquids–Progress on the fundamental issues. Aust. J. Chem. 2007, 60, 3–5. [Google Scholar] [CrossRef]
- Doi, H.; Song, X.; Minofar, B.; Kanzaki, R.; Takamuku, T.; Umebayashi, Y. A new proton conductive liquid with no ions: Pseudo-protic ionic liquids. Chem. Eur. J. 2013, 19, 11522–11526. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, S.; Atilhan, M.; Karadas, F. Thermophysical properties of pure ionic liquids: Review of present situation. Ind. Eng. Chem. Res. 2010, 49, 9580–9595. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Lopes-da-Silva, J.A.; Freire, M.G.; Coutinho, J.A.P.; Carvalho, P.J. Thermophysical properties of phosphonium-based ionic liquids. Fluid Phase Equilib. 2015, 400, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Yunus, N.M.; Abdul Mutalib, M.I.; Man, Z.; Bustam, M.A.; Murugesan, T. Thermophysical properties of 1-alkylpyridinium bis(trifluoromethylsulfonyl)imide ionic liquids. J. Chem. Thermodyn. 2010, 42, 491–495. [Google Scholar] [CrossRef]
- Yunus, N.M.; Abdul Ghani, M.A.; Nik Mohamad Kamil, R. Synthesis, characterization and CO2 solubility of [him][Tf2N] and [hmim][Ac] ionic liquids. AIP Conf. Proc. 2014, 1621, 284–289. [Google Scholar]
- Jacquemin, J.; Husson, P.; Majer, V.; Costa Gomes, M.F. Low-pressure solubilities and thermodynamics of solvation of eight gases in 1-butyl-3-methylimidazolium hexafluorophosphate. Fluid Phase Equilib. 2006, 240, 87–95. [Google Scholar] [CrossRef]
- Soave, G. Equilibrium constants from a modified Redlich-Kwong equation of state. Chem. Eng. Sci. 1972, 2, 1197–1203. [Google Scholar] [CrossRef]
- Gusain, R.; Panda, S.; Bakshi, P.S.; Gardas, R.L.; Khatri, O.P. Thermophysical properties of trioctylalkylammonium bis(salicylato)borate ionic liquids: Effect of alkyl chain length. J. Mol. Liq. 2018, 269, 540–546. [Google Scholar] [CrossRef]
- Pinkert, A.; Ang, K.L.; Marsh, K.N.; Pang, S. Density, viscosity and electrical conductivity of protic alkanolammonium ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 5136–5143. [Google Scholar] [CrossRef] [PubMed]
- Kurnia, K.A.; Wilfred, C.D.; Murugesan, T. Thermophysical properties of hydroxyl ammonium ionic liquids. J. Chem. Thermodyn. 2009, 41, 517–521. [Google Scholar] [CrossRef]
- Machanová, K.; Boisset, A.; Sedláková, Z.; Anouti, M.; Bendová, M.; Jacquemin, J. Thermophysical properties of ammonium-based bis{(trifluoromethyl)sulfonyl}imide ionic liquids: Volumetric and transport properties. J. Chem. Eng. Data 2012, 57, 2227–2235. [Google Scholar] [CrossRef]
- Chhotaray, P.K.; Gardas, R.L. Thermophysical properties of ammonium and hydroxylammonium protic ionic liquids. J. Chem. Thermodyn. 2014, 72, 117–124. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Bolívar, C.L.; Guillermo Díaz Baños, F.; Víllora, G. Effect of temperature, anion, and alkyl chain length on the density and refractive index of 1-alkyl-3-methylimidazolium-based ionic liquids. J. Chem. Eng. Data 2015, 60, 1986–1996. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hosseinian, A.; Aparicio, S. An experimental and theoretical study on 2-hydroxyethylammonium acetate ionic liquid. J. Mol. Liq. 2019, 284, 271–281. [Google Scholar] [CrossRef]
- Rios-Vera, R.M.; Sirieix-Plénet, J.; Gaillon, L.; Rizzi, C.; Ávila-Rodríguez, M.; Cote, G.; Chagnes, A. Physicochemical properties of novel cholinium ionic liquids for the recovery of silver from nitrate media. RSC Adv. 2015, 5, 78268–78277. [Google Scholar] [CrossRef]
- Sarkar, A.; Sharma, G.; Singh, D.; Gardas, R.L. Effect of anion on thermophysical properties of N, N-diethanolammonium based protic ionic liquids. J. Mol. Liq. 2017, 242, 249–254. [Google Scholar] [CrossRef]
- Vieira, N.S.M.; Luís, A.; Reis, P.M.; Carvalho, P.J.; Lopes-da-Silva, J.A.; Esperança, J.M.S.S.; Araújo, J.M.M.; Rebelo, L.P.N.; Freire, M.G.; Pereiro, A.B. Fluorination effects on the thermodynamic, thermophysical and surface properties of ionic liquids. J. Chem. Thermodyn. 2016, 97, 354–361. [Google Scholar] [CrossRef]
- Shanchez-Ramirez, N.; Martins, V.L.; Ando, R.A.; Camilo, F.F.; Urahata, S.M.; Ribeiro, M.C.C.; Torresi, R.M. Physicochemical properties of three ionic liquids containing a tetracyanoborate anion and their lithium salt mixtures. J. Phys. Chem. B 2014, 118, 8772–8781. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.M. Tetracyanoborate based ionic liquids for CO2 capture: From ab initio calculations to molecular simulations. Fluid Phase Equilib. 2016, 415, 34–41. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Karkhanechi, H.; Kamio, E.; Yoshioka, T.; Matsuyama, H. Quantum mechanical and molecular dynamics simulations of dual-amino-acid ionic liquids for CO2 capture. J. Phys. Chem. C 2016, 120, 27734–27745. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Yokozeki, A. Phase behavior of carbon dioxide in ionic liquids: [emim][acetate], [emim][trifluoroacetate], and [emim][acetate] + [emim][trifluoroacetate] mixtures. J. Chem. Eng. Data 2009, 54, 108–114. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).