Abstract
Rice husk biomass was investigated under O2/CO2 oxy-fuel conditions using Thermogravimetric analysis (TG)-derivative thermogravimetry (DTG)-mass spectrometry (MS) experiments, iso-conversional kinetic analysis, and ReaxFF reactive molecular dynamics simulations. Oxy-fuel combustion significantly enhanced combustion performance compared with air combustion. At 10 °C·min−1, the ignition and burnout temperatures decreased to 235 °C and 435 °C under 70%O2/30%CO2, while the maximum mass loss rate increased more than fivefold and the comprehensive combustion index increased markedly. Online MS analysis showed concentrated CO2 formation and O2 consumption within 280–330 °C, accompanied by markedly suppressed NOx and SO2 emissions. Kinetic analysis revealed high apparent activation energies (525–548 kJ·mol−1) at α ≈ 0.5; these values are conversion-dependent and sensitive to the iso-conversional method employed and therefore reflect relative kinetic trends rather than intrinsic Arrhenius parameters, indicating a transition from chemical control to diffusion–structure-coupled control. Molecular dynamics simulations further confirmed that moderate oxygen enrichment promotes organic backbone cleavage, whereas excessive oxygen leads to a carbon-limited regime. These results provide mechanistic insights into biomass oxy-fuel combustion and its optimization for CO2 capture applications.
1. Introduction
Biomass combustion is recognized as an important pathway for renewable energy utilization, playing a significant role in reducing greenhouse gas emissions, optimizing energy structures, and promoting the achievement of carbon neutrality targets [1,2]. During its growth, biomass fixes atmospheric carbon dioxide through photosynthesis, and the CO2 released during combustion can be reabsorbed by subsequent biomass growth, thereby being generally regarded as a nearly carbon-neutral energy source [3,4,5]. Among various biomass resources, rice husk, as a typical agricultural residue, is widely available and produced in large quantities annually, exhibiting considerable potential for energy utilization [6,7]. However, rice husk is characterized by high ash content, complex mineral composition, and a dense structure, which often result in ignition difficulty, unstable combustion behavior, and complicated ash-related phenomena, thereby limiting its large-scale application in efficient and clean combustion systems [7].
Oxy-fuel combustion is an advanced combustion technology in which high-purity oxygen is used as an oxidizer, while CO2 replaces N2 as the primary diluent. In recent years, this approach has attracted considerable attention due to its inherent compatibility with carbon capture and storage (CCUS) systems [8,9]. Compared with conventional air combustion, oxy-fuel combustion can significantly increase the volumetric concentration of CO2 in flue gas, thereby reducing the cost of downstream separation and capture processes. In addition, by adjusting the oxygen concentration, combustion reactions can be intensified, and combustion stability can be improved to a certain extent. Studies on coal and other fossil fuels have demonstrated that oxygen-enriched conditions can markedly affect ignition characteristics, reaction rates, and pollutant formation pathways [10,11,12]. However, in contrast to coal combustion, biomass combustion is inherently more complex, as its reaction behavior is jointly governed by volatile release, char oxidation, mineral matter interactions, and gas-solid mass transfer processes [13]. Consequently, the combustion mechanisms of biomass under O2/CO2 oxy-fuel atmospheres remain insufficiently understood and lack systematic investigation.
Existing studies have predominantly employed thermogravimetric analysis (TG) to systematically characterize the combustion behavior of biomass under oxygen-enriched atmospheres, generally reporting that increasing oxygen concentration lowers the ignition temperature, accelerates mass loss rates, and shortens the overall combustion interval [14]. However, information derived solely from TG and derivative thermogravimetry (DTG) curves mainly reflects macroscopic mass variation and is insufficient to directly elucidate the reaction control mechanisms at different conversion stages or the corresponding evolution of gaseous products [15,16]. This limitation is particularly pronounced under oxy-fuel conditions, where volatile release, gas-phase oxidation, and char reactions are often strongly coupled. In the absence of simultaneous detection of gaseous products, it remains difficult to accurately identify the reaction pathways and kinetic implications underlying the observed mass loss behavior, thereby constraining a deeper understanding of oxy-fuel combustion mechanisms [17].
To address this issue, TG has been increasingly coupled with online mass spectrometry (MS) to investigate the temperature-dependent release characteristics of gaseous products during biomass combustion under oxygen-enriched atmospheres. For example, Guo et al. [18] examined the co-combustion behavior of biomass and calcium-rich oil shale under air (21%O2/79%N2) and oxy-fuel conditions (30%O2/70%CO2). Their TG-MS results revealed pronounced synergistic effects during the stages of volatile release and light hydrocarbon combustion, while the release of CO2 and H2O became more concentrated under oxygen-enriched conditions.
Building upon macroscopic insights into oxy-fuel combustion behavior of fuels such as biomass and coal obtained from thermogravimetric and gaseous product analyses, further clarification of reaction control mechanisms at different conversion stages requires the introduction of kinetic analysis methods [19,20]. Iso-conversional kinetic approaches, which do not require a priori assumptions regarding reaction mechanisms, have been widely applied to biomass combustion and pyrolysis to characterize the evolution of apparent activation energy as a function of conversion degree, among which the Kissinger-Akahira-Sunose (KAS) and Flynn-Wall-Ozawa (FWO) methods are the most commonly used [21,22]. However, existing kinetic studies on biomass oxy-fuel combustion have largely focused on the calculation and comparison of apparent activation energy values, leading to considerable discrepancies among reported results. This inconsistency primarily arises from the fact that reaction control mechanisms vary with conversion progress, while conventional kinetic analyses often fail to distinguish the respective contributions of parallel processes such as volatile release, gas-phase oxidation, and char reactions. Therefore, it is essential to integrate the evolution of kinetic parameters with fuel structural changes and reaction pathway transitions in order to elucidate the physicochemical origins of activation energy variations from a mechanistic perspective.
Fundamentally, biomass combustion is a complex reaction process governed by the cleavage, rearrangement, and oxidative transformation of macromolecular structures such as cellulose, hemicellulose, and lignin under high-temperature conditions. In recent years, reactive molecular dynamics (MD) simulations based on the Reactive force field (ReaxFF) have emerged as a powerful tool for elucidating the molecular-scale mechanisms of biomass thermochemical conversion [23,24]. This approach enables real-time tracking of chemical bond evolution, radical formation, and product generation at the atomic level, and has been successfully applied to the pyrolysis and combustion of individual biomass components [25,26]. However, studies that systematically integrate molecular dynamics simulations with experimental observations to elucidate reaction pathways under O2/CO2 oxy-fuel combustion conditions, and to clarify their impacts on macroscopic combustion behavior and kinetic characteristics, remain scarce. Such integrated investigations, by coupling molecular-scale reaction pathways with experimental combustion behavior and kinetic parameters, can provide fundamental insights into the reaction control mechanisms of biomass combustion under oxygen-enriched conditions and offer a physicochemical basis for interpreting macroscopic kinetic phenomena.
Based on the above background, this study focuses on rice husk biomass and systematically investigates its combustion behavior and underlying mechanisms under O2/CO2 oxy-fuel atmospheres. TG-DTG-MS experiments were conducted to examine the combustion characteristics and gaseous product evolution of rice husk in air as well as in 30%O2/70%CO2, 50%O2/50% CO2, and 70%O2/30% CO2 atmospheres at different heating rates (10, 20, and 30 °C/min). Iso-conversional kinetic analyses using the KAS and FWO methods were employed to quantitatively evaluate the kinetic characteristics of the combustion process. Furthermore, MD simulations based on ReaxFF were performed to elucidate the evolution of key chemical bonds, transitions in reaction pathways, and product formation mechanisms at the molecular scale. Unlike most existing studies that rely mainly on TG curves or kinetic parameter fitting, this work integrates TG–DTG–MS experiments, iso-conversional kinetic analysis, and ReaxFF molecular dynamics simulations within a unified multiscale framework. By coupling experimental observations, kinetic evolution, and molecular-scale bond cleavage and reaction pathway regulation, the present study establishes intrinsic links between macroscopic combustion behavior and microscopic reaction mechanisms under O2/CO2 oxy-fuel conditions. This mmultiscaleintegration provides mechanistic insights into rice husk oxy-fuel combustion that cannot be captured by conventional TG-based analyses alone, thereby offering theoretical support for the development of biomass oxy-fuel combustion technologies and their integration with carbon capture systems.
2. Materials and Methods
2.1. Materials
The rice husk used in this study was sourced from Northeast China. The raw rice husk was oven-dried at 105 °C to a constant weight. The dried samples were subsequently ground and sieved, and particles with a size smaller than 0.2 mm were selected for thermogravimetric experiments to minimize the influence of particle size variation on heat and mass transfer processes. The proximate analysis, ultimate analysis, higher heating value, and lignocellulosic composition of the rice husk are summarized in Table 1. As shown in Table 1, rice husk is a typical biomass characterized by high volatile matter and high ash content, with the elevated ash content mainly attributed to the enrichment of SiO2. During combustion, this mineral component tends to form an ash layer structure, which can significantly affect the reaction kinetics and mass transfer behavior during the char combustion stage [27,28]. However, detailed ash composition analysis was not performed, as the primary objective was to investigate the combustion behavior and reaction mechanisms of rice husk under oxy-fuel conditions rather than ash chemistry itself. Nevertheless, it should be noted that the presence of ash can influence the release of combustion-related pollutants. In addition, the formation and evolution of ash layers during combustion may contribute to diffusion-controlled or diffusion–structure-coupled reaction pathways. Given the high ash content and silica enrichment of rice husk, slagging and ash-related deposition may occur during combustion. In practical applications, such issues can be mitigated through fuel pretreatment, blending with low-alkali fuels, and operation under CO2-diluted oxy-fuel conditions, which can help regulate local temperatures and ash transformation behavior.

Table 1.
The fundamental properties of rice husk sample.
2.2. TG-DTG-MS Experiments
In this study, TG–DTG-MS experiments were carried out using a coupled system consisting of a Mettler-Toledo thermogravimetric analyzer and an OmniStar quadrupole mass spectrometer. Gaseous products released from the TG-DTG analyzer were transferred to the mass spectrometer through a heated transfer line maintained at 220 °C and introduced via a quartz capillary for online detection, thereby preventing condensation during gas transport.
Thermogravimetric measurements were conducted at heating rates of 10, 20, and 30 °C/min under different reaction atmospheres, including air, 30%O2/70%CO2, 50%O2/50%CO2, and 70%O2/30%CO2. During the experiments, the total flow rate of the reaction gas was fixed at 100 mL/min, while N2 was supplied as the protective gas at a flow rate of 50 mL/min. The initial temperature was set to 85 °C for the analysis of dry-basis samples to ensure stable baseline conditions prior to combustion, and approximately 10 mg (±0.1 mg) of biomass sample was loaded into the crucible. The thermogravimetric analyzer and the online mass spectrometer were operated simultaneously. The experiments were terminated after heating to 850 °C, and the corresponding TG–DTG and MS data were recorded and stored for subsequent analysis. Furthermore, the MS signals were used for qualitative analysis to compare the relative evolution trends of gaseous products as a function of temperature. Background correction was performed by subtracting baseline signals obtained under the same atmosphere without biomass. Duplicate experiments were conducted to verify the repeatability of the TG–DTG–MS measurements.
2.3. Kinetic Analysis of Combustion Reactions
Based on the TG data of rice husk obtained at different heating rates, the apparent activation energy was determined using two widely adopted iso-conversional, model-free methods, namely the KAS and FWO approaches. These methods enable the evaluation of activation energy as a function of conversion degree without assuming a specific reaction model. The kinetic analysis is based on the Arrhenius equation:
where α is the conversion degree, T is the reaction temperature, t is the reaction time, k(T) is the temperature-dependent reaction rate constant, and f(α) is the reaction model function. The conversion degree α is defined as:
where m0 and mf denote the initial mass of the sample and the final mass after completion of the reaction, respectively, and mt represents the sample mass at reaction time t. k(T) can be expressed as:
By substituting Equation (3) into Equation (1), the following expression can be obtained:
where E is the activation energy (kJ·mol−1), A is the pre-exponential factor (s−1), and R is the universal gas constant.
Under non-isothermal thermogravimetric conditions, the rice husk sample is heated at a constant heating rate β = dT/dt (K·min−1). Accordingly, Equation (4) can be transformed into:
By integrating both sides of Equation (5), the following expression is obtained:
where P(u) denotes the temperature integral and u is the apparent activation energy, which can be expressed as:
By combining Equations (6) and (7), the kinetic equations for the KAS method (Equation (8)) and the FWO method (Equation (9)) can be derived, respectively:
By analyzing the linear relationship between ln(β/T2) (for the KAS method) or ln β (for the FWO method) and 1/T, the activation energy of the reaction process can be determined. Moreover, it should be noted that the above equations are standard formulations widely used in iso-conversional kinetic analyses and are not proposed as original contributions of this study but are provided to facilitate understanding of the applied methods.
2.4. Molecular Dynamics Methodology for Combustion
2.4.1. Construction of the Biomass Model and Reaction Systems
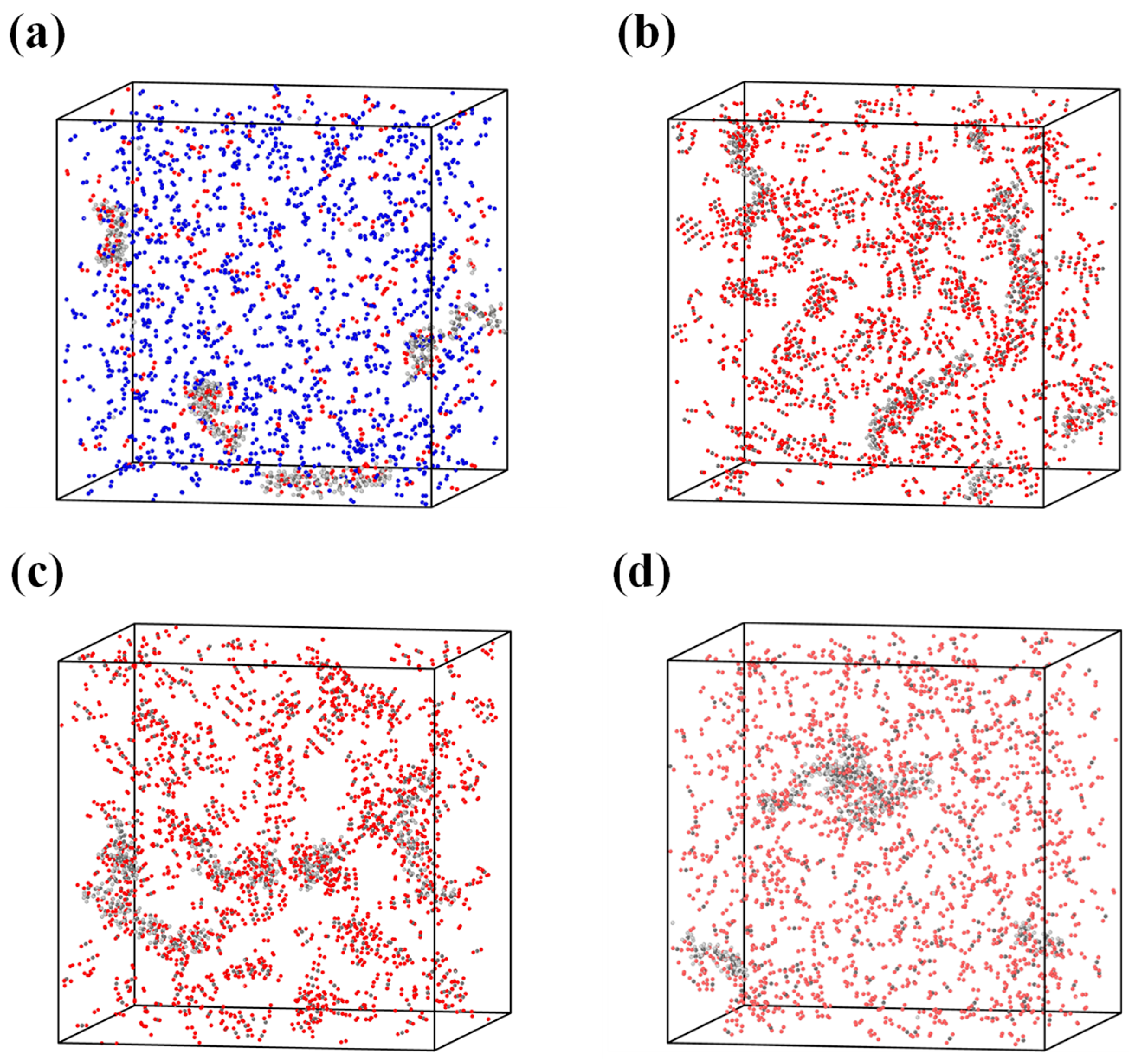
Cellulose, hemicellulose, and lignin were constructed using the polymer builder module. Glucose and xylose were employed as the monomeric units for cellulose and hemicellulose, respectively, and were linked through β-1,4-glycosidic bonds [29]. The lignin model was polymerized from β-O-4 lignin monomers [30]. In this study, biomass was assumed to consist solely of cellulose, hemicellulose, and lignin, while proteins and inorganic components were neglected for model simplification. Based on the contents of cellulose, hemicellulose, and lignin as well as the H/C and O/C atomic ratios obtained from Table 1, the degrees of polymerization and the numbers of polymer chains were adjusted to construct a representative molecular model of rice husk. Specifically, the rice husk molecular model used in the MD study is represented by an elemental formula of C178H278O88. For the molecular dynamics simulations, mineral components were not explicitly included in the ReaxFF models in order to focus on the intrinsic reaction pathways of the organic matrix and to maintain computational feasibility. Four combustion simulation systems were established in this work, all employing the same rice husk molecular model, while varying the composition and concentration of the surrounding reaction atmospheres. Consistent with the TG experiments, the reaction atmospheres in the molecular dynamics simulations included air, 30%O2/70%CO2, 50%O2/50%CO2, and 70%O2/30%CO2. The numbers and degrees of polymerization of the three biomass components, as well as the numbers of added O2, N2, and CO2 molecules in each simulation system, are summarized in Table 2. Furthermore, Figure 1 illustrates the visualization cells of the four combustion simulation systems, showing the spatial arrangement of the biomass molecules and the surrounding gas species.

Table 2.
Model and parameter settings for MD simulations of rice husk combustion.
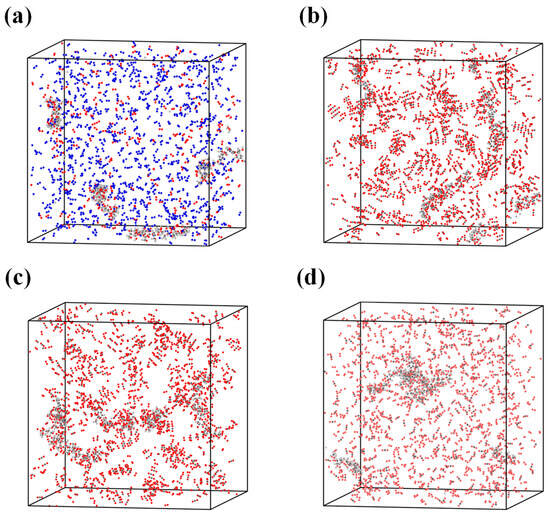
Figure 1.
Molecular models of the reaction systems under different atmospheres: (a) air; (b) 30%O2/70%CO2; (c) 50%O2/50%CO2; (d) 70%O2/30%CO2. The atomic species are color-coded as follows: C (gray), H (white), O (red), and N (blue).
2.4.2. MD Simulation Procedure and Parameter Settings
Reactive molecular dynamics simulations were performed using the LAMMPS (Version 1.7.0) package, with the ReaxFF reactive force field originally developed by van Duin et al [31]., which is well suited for modeling complex chemical reactions by dynamically describing interatomic interactions. Based on the continuous evolution of interatomic potentials and bond orders during the simulations, reaction pathways and intermediate species can be tracked as a function of time [32]. The total energy expression of the ReaxFF force field is given in Equation (10) [31]:
In Equation (10), Ebond represents the bond energy term; Eover and Eunder denote the energy corrections for over-coordinated and under-coordinated atoms, respectively; Eval and Epen correspond to the valence angle term and the associated penalty energy; Etors represents the torsional energy term; and Econj, Evwaals, and Ecoulomb account for the conjugation effects, van der Waals interactions, and Coulomb interactions, respectively.
The detailed molecular dynamics simulation procedure is described as follows. First, energy minimization was performed for all reaction systems using the conjugate gradient algorithm to eliminate unreasonable atomic overlaps and local stress concentrations in the initial configurations, ensuring that each system reached a stable local minimum in potential energy. Subsequently, molecular dynamics simulations were carried out under the NVT ensemble (constant number of particles, volume, and temperature) with three-dimensional periodic boundary conditions, thereby minimizing boundary effects and representing a bulk reaction environment. Temperature control was implemented using the Berendsen thermostat, with the characteristic damping time constant set to 0.1 ps, which allows for smooth temperature regulation while avoiding excessive thermal perturbations that could influence reaction kinetics. A time step of 0.1 fs was employed throughout the simulations, providing a reasonable compromise between computational accuracy and efficiency.
The MD simulations were conducted under a programmed heating scheme. The systems were initially equilibrated at 300 K and then heated to the target temperatures at a constant heating rate of 20 K/ps. The final temperatures were set to 1000, 1500, 2000, 2500, and 3000 K, respectively. After reaching the target temperature, each system was maintained under isothermal conditions for an additional 1000 ps to allow sufficient reaction evolution. It should be noted that the model size, heating rate, and simulation temperatures adopted in this study were deliberately selected as a compromise between computational feasibility and mechanistic representativeness. Although the temperatures and heating rates are significantly higher than those used in experiments, such settings are commonly employed in reactive molecular dynamics simulations to accelerate reaction events, thereby enabling the capture of dominant reaction pathways, bond evolution, and product formation trends during combustion within accessible computational timescales [33,34].
3. Results and Discussion
3.1. TG-DTG Characteristics Under Different Atmospheres
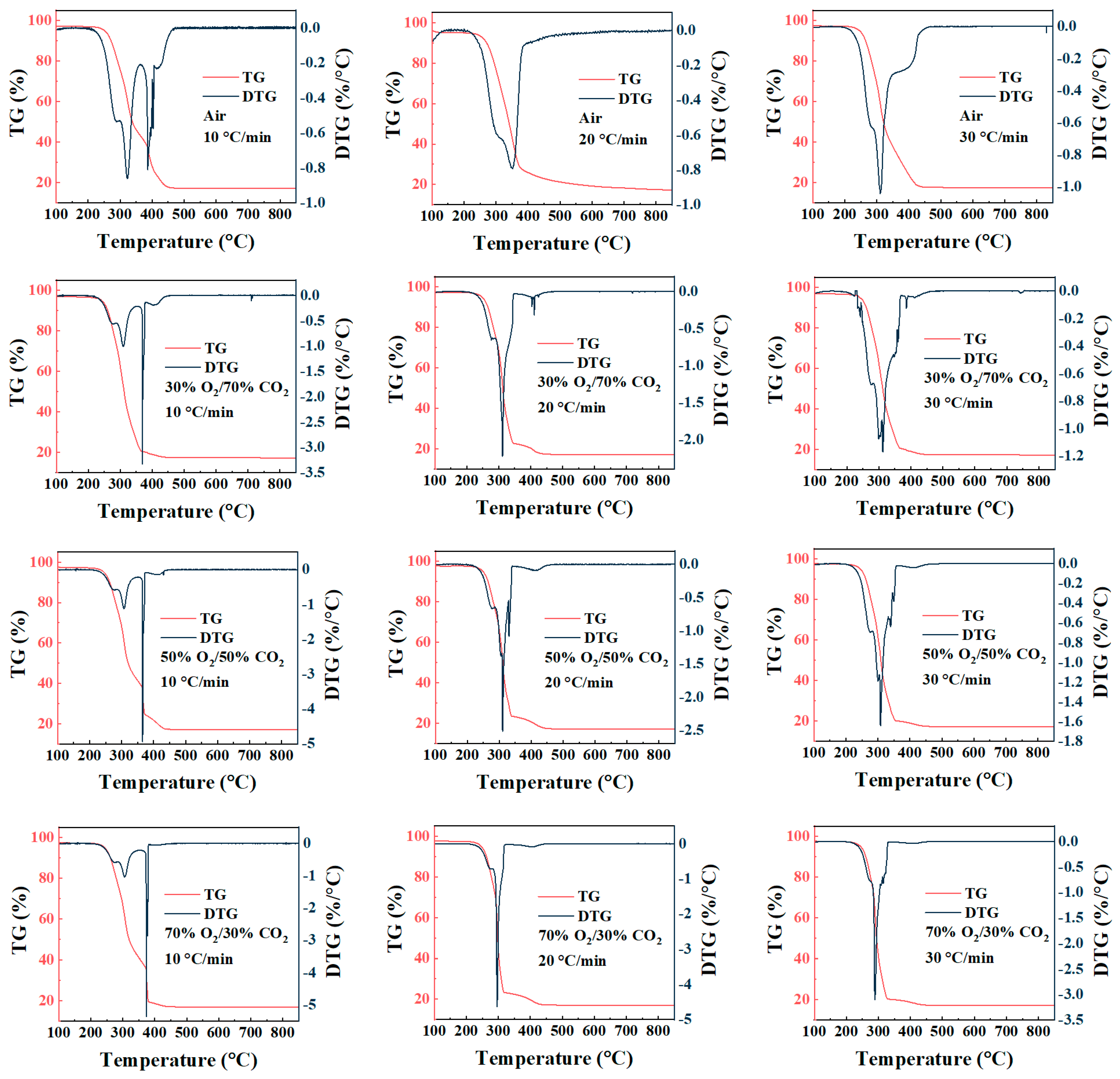
As shown in Figure 2, the TG curves of rice husk under different atmospheres and heating rates exhibit similar overall mass-loss profiles, with the final residual mass stabilizing at approximately 17%, reflecting the relatively high inorganic ash content of rice husk. The major mass loss occurs predominantly within the intermediate temperature range of about 200–380 °C, which corresponds to the most pronounced peak in the DTG curves, indicating that the combustion process is mainly governed by volatile release and its subsequent rapid oxidation. The DTG curves further reveal that the combustion reactions are highly concentrated within a specific temperature window. Similarly, Wang et al. [35] observed two distinct weight-loss peaks during the air combustion of rice husk, corresponding to the devolatilization stage and the char combustion stage, respectively. Although noticeable differences in DTG peak intensity and peak temperature are observed under different conditions, the residual mass at the end of the TG curves varies only slightly. This suggests that external conditions primarily influence the combustion rate and the temperature range over which reactions occur, while their effect on the final burnout degree remains relatively limited. It should be noted that the TG-DTG results discussed here represent apparent combustion behavior under thermogravimetric conditions, where intrinsic reaction kinetics, oxygen availability, and transport phenomena coexist and cannot be fully decoupled.
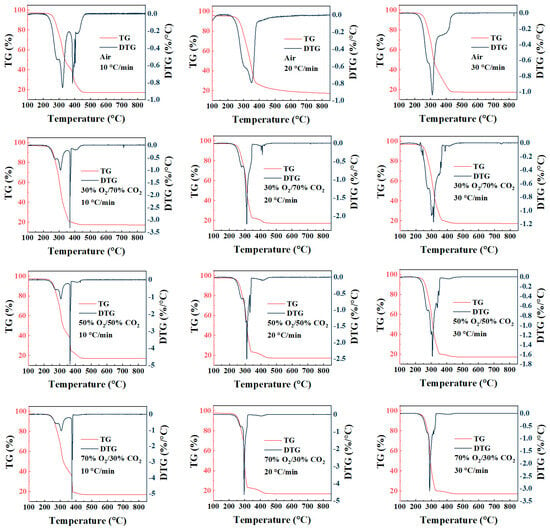
Figure 2.
TG-DTG curves of rice husk combustion under different atmospheres and heating rates.
Under conventional air combustion conditions, increasing the heating rate from 10 to 30 °C/min causes the main mass-loss region in the TG curves to shift toward higher temperatures, which can be attributed to the thermal lag effect commonly observed in non-isothermal combustion processes. Correspondingly, the main DTG peak exhibits a slight rightward shift with increasing heating rate, accompanied by a transition from a relatively broad profile to a sharper and more concentrated peak, indicating that mass loss tends to occur within a narrower temperature interval at higher heating rates. In contrast, this typical thermal lag behavior is markedly altered under oxygen-enriched conditions, particularly in the 70%O2/30%CO2 atmosphere. As the heating rate increases from 10 to 30 °C/min, the maximum DTG peak does not shift toward higher temperatures; instead, a noticeable advance of the peak position is observed. This trend is consistent with the results reported by Ma et al. [36]. They also reported that the relative importance of factors influencing the combustion behavior followed the order: heating rate > sample particle size > O2 concentration. This phenomenon suggests that, under high oxygen partial pressure, the mass-loss process is no longer governed solely by the overall heating history of the sample but is strongly influenced by the coupling between volatile release and rapid oxidation reactions. Under such conditions, volatiles can readily participate in oxidation immediately upon release, allowing the maximum mass-loss rate to be achieved at relatively lower temperatures. It should be noted that this behavior is predominantly observed under high oxygen concentrations and is not evident under air combustion conditions, further highlighting the decisive role of oxygen concentration in governing combustion reaction control characteristics.
At a given heating rate, the influence of atmosphere composition on rice husk combustion behavior is mainly reflected in the steepness of the TG curves and the variation in DTG peak characteristics. Compared with air combustion, the major mass-loss region under O2/CO2 oxy-fuel atmospheres shifts markedly toward lower temperatures, with the ignition temperature and the temperature corresponding to the maximum mass-loss rate decreasing by approximately 20–60 °C. This indicates that increasing the oxygen volume fraction effectively lowers the temperature threshold for combustion reactions. As the O2 concentration increases from 30% to 50% and 70%, the maximum DTG peak intensity increases significantly. In particular, under 70% O2 conditions, the absolute maximum mass-loss rate (DTGmax) exceeds that observed under air combustion by more than fivefold, accompanied by a pronounced advance of the peak temperature, reflecting a substantial enhancement of the combustion reaction rate. Meanwhile, the final residual mass derived from the TG curves shows only minor variations among different atmospheres, suggesting that elevated oxygen concentrations primarily modify combustion behavior by accelerating reaction rates and shortening the effective reaction temperature window, rather than by significantly altering the overall extent of conversion. Similar trends have also been reported in thermogravimetric kinetic studies of other biomass and biomass-coal systems. These studies indicate that under oxy-fuel combustion conditions, increasing the O2 concentration primarily promotes combustion by lowering the ignition temperature and enhancing reaction reactivity, while the combustion mechanism itself remains largely unchanged [37]. This is consistent with the TG-DTG characteristics observed for rice husk under O2/CO2 atmospheres in the present study.
3.2. Combustion Characteristic Parameters Under Different Atmospheres
Based on the TG–DTG curves of rice husk under different atmospheres and heating rates shown in Figure 2, a unified criterion was applied to determine key combustion characteristic parameters, including the ignition temperature (Tᵢ), DTG peak temperatures (Tp1 and Tp2), DTGmax, burnout temperature (Tb), main combustion interval width (ΔT), and the comprehensive combustion index (CCI) [38]. The calculated results are summarized in Table 3. These parameters enable a quantitative evaluation of the effects of different atmospheres on rice husk combustion behavior in terms of ignition characteristics, reaction rate, and overall combustion performance. The observed trends should therefore be interpreted as the combined effects of reaction characteristics and transport limitations inherent to TG experiments, rather than as purely intrinsic material responses.

Table 3.
Combustion characteristic parameters of rice husk under different atmospheres and heating rates.
From the perspective of ignition and burnout characteristics, the combustion behavior of rice husk is strongly regulated by the atmosphere composition. Compared with air combustion, the introduction of O2/CO2 oxy-fuel atmospheres leads to an overall decrease in Tᵢ, indicating that elevated oxygen concentrations effectively promote earlier ignition. At a heating rate of 10 °C/min, Tᵢ decreases from 246 °C in air to 244 °C under the 30%O2/70%CO2 atmosphere, and further declines to 242 °C and 235 °C at 50% and 70% O2, respectively.
Meanwhile, Tb is also noticeably reduced under oxy-fuel conditions, decreasing from 445 °C in air to approximately 435–447 °C as the oxygen concentration increases. This trend suggests that oxygen-enriched environments accelerate the late-stage char oxidation and burnout processes. Correspondingly, ΔT is narrowed from about 229 °C under air combustion to approximately 200 °C under oxy-fuel atmospheres, indicating a transition of the combustion process from a relatively dispersed regime to a more concentrated and intensified reaction mode. Similar trends have also been reported in previous studies. For example, Zhuo et al. [39] investigated the thermogravimetric combustion behavior of sludge and coal under N2/O2 and CO2/O2 atmospheres and found that increasing oxygen concentration markedly reduced both the ignition and burnout temperatures, leading to a significantly intensified combustion process. These results indicate that oxygen-enriched atmospheres exert a generally promoting effect on the combustion of solid fuels.
Variations in DTG peak characteristics and DTGmax under different atmospheres further reflect differences in combustion reaction rates. Under air conditions, rice husk exhibits a pronounced double-peak DTG profile at lower heating rates, corresponding to the staged combustion of volatiles and char. In contrast, under oxygen-enriched atmospheres or at higher heating rates, the secondary DTG peak gradually diminishes, and the combustion behavior evolves toward a single-peak profile, indicating a strong coupling between volatile release and char oxidation processes. Meanwhile, DTGmax increases markedly with increasing oxygen concentration. At a heating rate of 10 °C/min, DTGmax rises from 0.86%/°C under air combustion to 3.32, 4.73, and 4.97%/°C under 30%, 50%, and 70%O2 atmospheres, respectively, demonstrating that oxygen enrichment significantly enhances the combustion reaction rate. However, within the same oxygen-enriched atmosphere, DTGmax shows a decreasing trend with increasing heating rate, which can be attributed to the narrowing of the effective combustion temperature window as well as intensified limitations associated with heat transfer and gas diffusion.
The CCI value further quantifies the differences in overall combustion performance of rice husk under different atmospheres. Under air conditions, the CCI remains on the order of 10−6 (1.93–2.63 × 10−6), indicating relatively limited combustion activity of rice husk during conventional air combustion. In contrast, under oxygen-enriched atmospheres, the CCI increases significantly and exhibits a clear upward trend with increasing oxygen concentration. For instance, at a heating rate of 10 °C/min, the CCI increases from 2.29 × 10−6 in air to 9.67 × 10−6 under 30%O2 and further rises to 1.28 × 10−5 and 1.61 × 10−5 under 50% and 70% O2 conditions, respectively. The CCI is a composite parameter widely used to evaluate overall combustion performance by incorporating both reaction intensity and combustion concentration. In practice, CCI is strongly influenced by DTGmax, while the effective combustion temperature interval plays a secondary but synergistic role [38]. Therefore, an increase in CCI primarily reflects intensified and more concentrated combustion behavior. Hu et al. [40] also reported similar behavior in their studies on the oxy-fuel combustion of biomass such as straw and wood chips, where CCI increased from (1–3) × 10−6 under air conditions to values on the order of 10−5 or higher under oxygen-enriched atmospheres, indicating a pronounced enhancement in overall combustion performance with increasing oxygen concentration. These literature results are in good agreement with the present findings, in which the CCI of rice husk exhibits a continuous increase with oxygen concentration under oxy-fuel conditions. Overall, oxygen-enriched atmospheres markedly enhance the combustion intensity and concentration of rice husk by lowering Ti, increasing DTGmax, and narrowing ΔT, whereas the heating rate primarily plays a modulating role in the combustion process.
3.3. Mass Spectrometric Analysis of Combustion Products
To further elucidate the evolution characteristics and temperature-dependent behavior of gaseous products during rice husk combustion under different atmospheres, online MS analysis was conducted at a constant heating rate of 10 °C/min. Combustion experiments were performed under four representative atmospheres, and the corresponding MS ion intensity profiles of the major gaseous products as a function of combustion temperature were obtained.
3.3.1. CO2 Evolution Behavior During Combustion
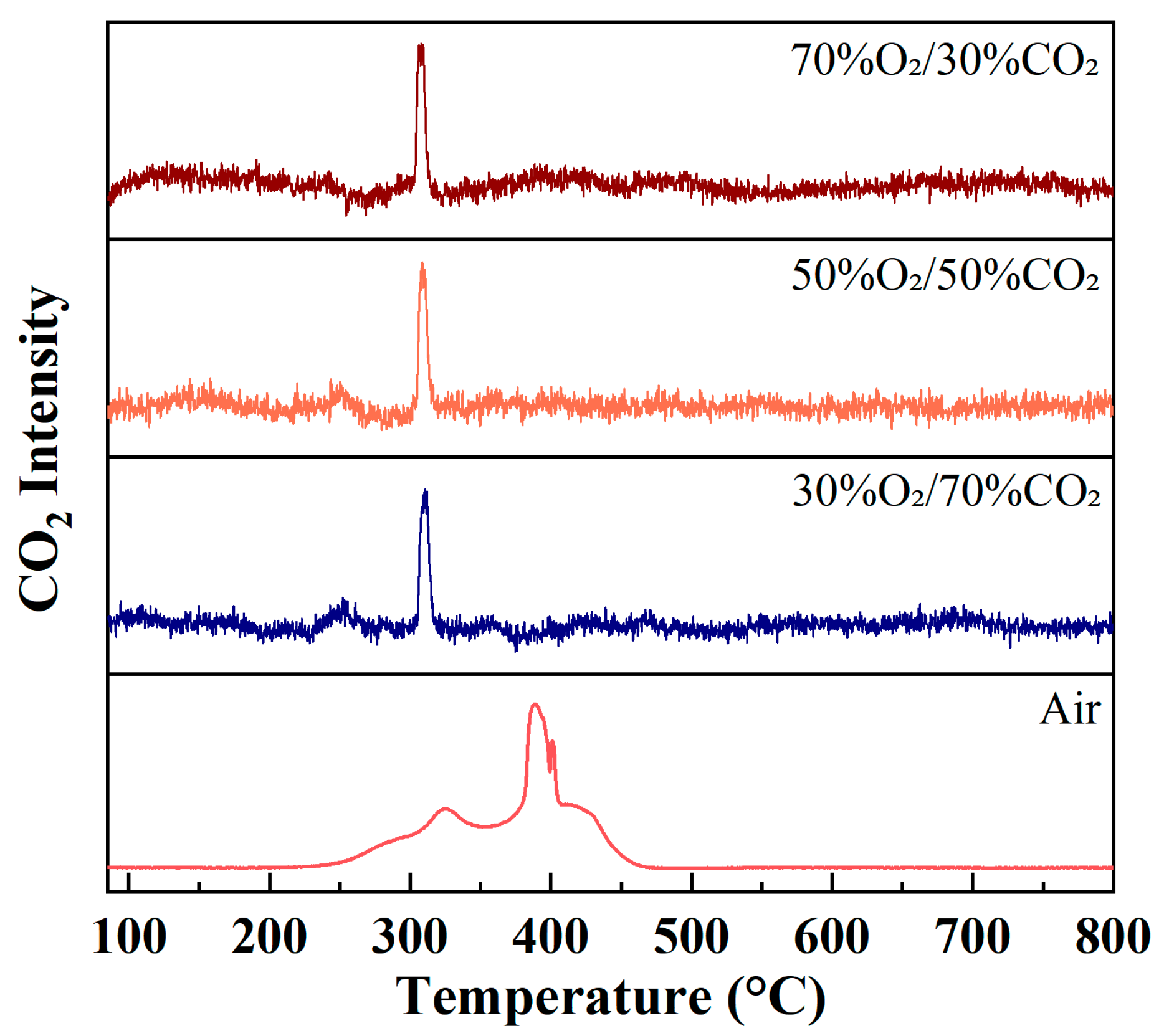
Figure 3 shows the evolution of CO2 (m/z = 44) ion intensity as a function of temperature during rice husk combustion under different atmospheres at a heating rate of 10 °C/min. Under air conditions, the CO2 signal exhibits a pronounced broad peak mainly distributed over the temperature range of approximately 300–450 °C, accompanied by a noticeable shoulder feature. This behavior is in good agreement with the DTG curve observed under air combustion conditions in Figure 2. These results indicate that, under air combustion, the volatile combustion and char oxidation stages overlap to a considerable extent, leading to a relatively dispersed CO2 generation process. Consequently, the combustion reactions proceed progressively over a wide temperature interval rather than being concentrated within a narrow temperature range.
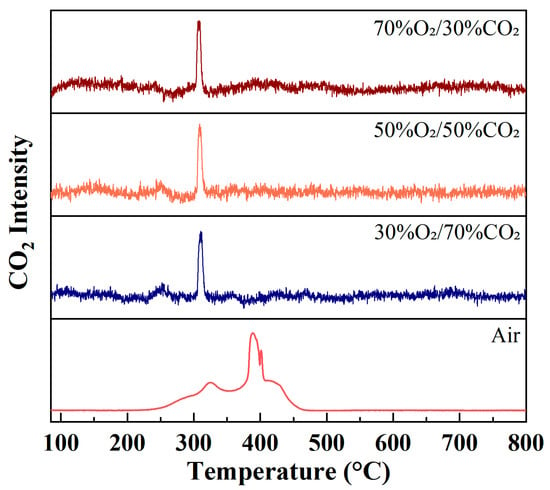
Figure 3.
Evolution of CO2 (m/z = 44) ion intensity during rice husk combustion under different atmospheres at a heating rate of 10 °C/min.
In contrast, under O2/CO2 oxy-fuel atmospheres, the CO2 ion intensity profiles display a narrow and sharp single-peak feature, with peak temperatures concentrated in the range of approximately 280–320 °C and significantly reduced peak widths compared to those observed under air conditions. This phenomenon suggests that rice husk combustion is markedly accelerated under oxygen-enriched conditions, where volatile release and subsequent oxidation reactions are rapidly completed within a much narrower temperature window, leading to highly concentrated CO2 formation. Moreover, as the oxygen concentration increases, the peak intensity of CO2 gradually rises, with the highest CO2 ion intensity observed under the 70%O2/30%CO2 atmosphere, reflecting that elevated oxygen partial pressure substantially enhances the oxidation rate of carbonaceous components.
3.3.2. O2 Evolution Behavior During Combustion
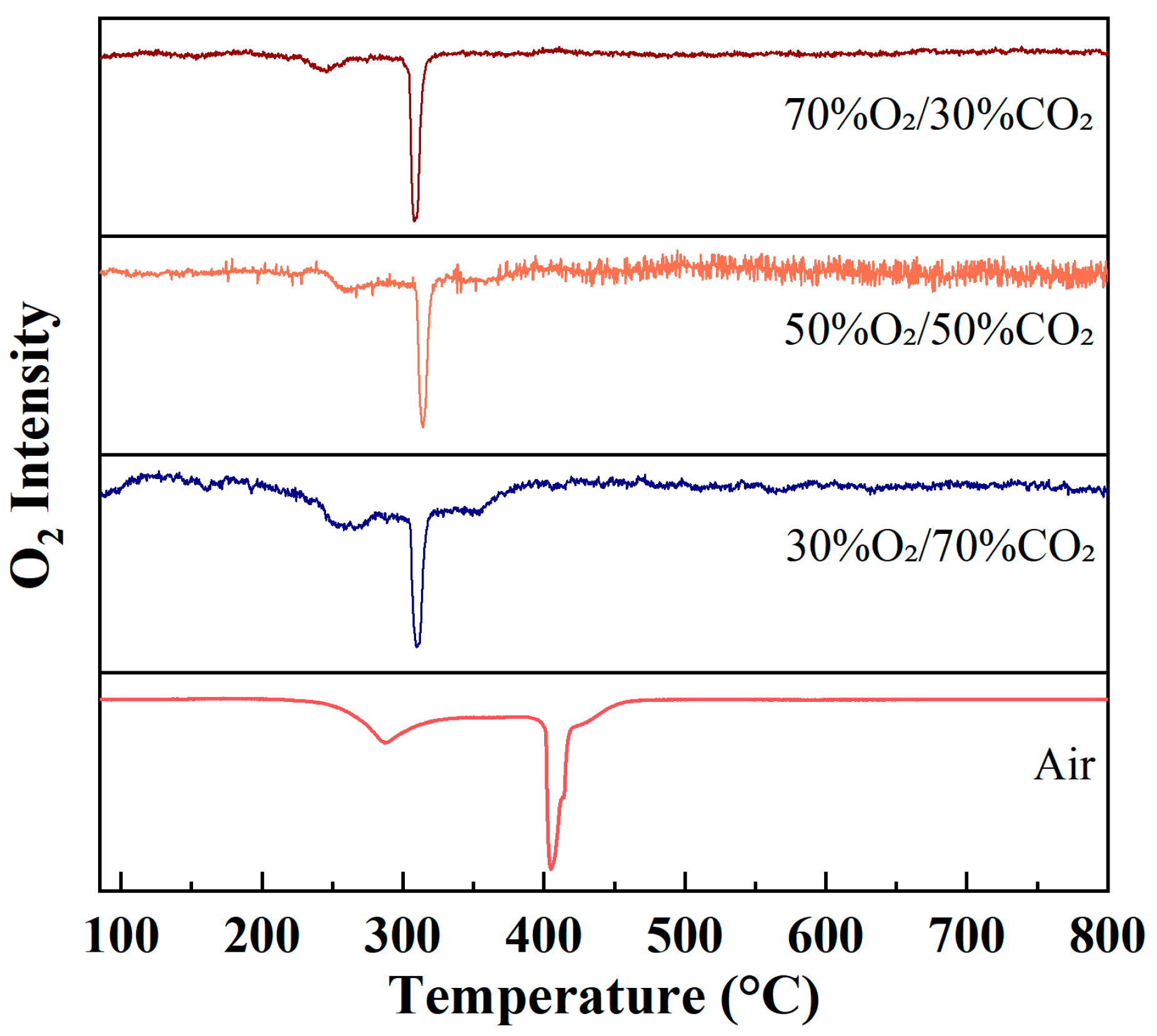
Figure 4 presents the evolution of O2 (m/z = 32) ion intensity as a function of temperature during rice husk combustion under different atmospheres at a heating rate of 10 °C/min. Unlike CO2, which serves as a combustion product and reflects carbon oxidation behavior, the O2 ion intensity profiles primarily represent the consumption of reactant oxygen during the combustion process. Therefore, variations in the O2 signal can be directly used to characterize the intensity of oxygen participation in combustion reactions as well as the temperature ranges over which these reactions occur.
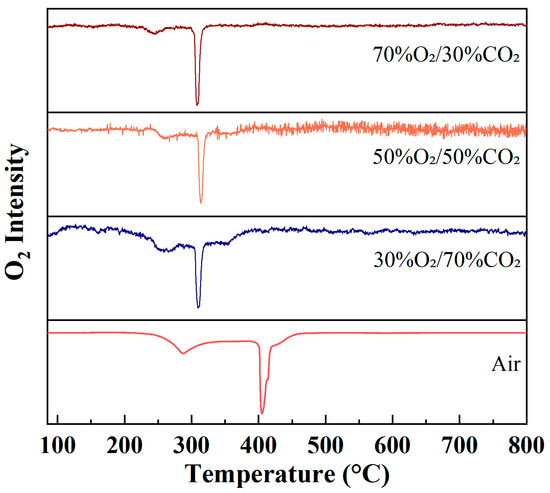
Figure 4.
Evolution of O2 (m/z = 32) ion intensity during rice husk combustion under different atmospheres at a heating rate of 10 °C/min.
Under air conditions, the O2 ion intensity gradually decreases with increasing temperature and exhibits a broad negative peak, mainly distributed over the temperature range of approximately 300–450 °C. This behavior indicates that oxygen consumption during rice husk combustion under air does not occur in a highly concentrated manner, but rather proceeds continuously as volatile combustion and char oxidation gradually evolve. Such a feature reflects an extended combustion process with pronounced stage overlap and diffusion-controlled characteristics.
In contrast, markedly different evolution patterns of O2 ion intensity are observed under O2/CO2 oxygen-enriched atmospheres. With increasing oxygen concentration, the O2 signal drops rapidly within the main combustion temperature region, forming sharp and concentrated negative peaks with peak positions mainly located in the range of 280–330 °C. In particular, under the 70%O2/30%CO2 atmosphere, the decrease in O2 ion intensity is the most pronounced, indicating a significantly enhanced combustion reaction rate and rapid oxygen consumption within a narrow temperature window under high oxygen partial pressure. This concentrated oxygen consumption behavior suggests that oxygen-enriched atmospheres substantially strengthen the chemical reaction-controlled regime of combustion, while diminishing the influence of diffusion limitations on the overall combustion process.
The O2 evolution profiles clearly reveal the differences in both the timing and intensity of oxygen participation in rice husk combustion under different atmospheres. Under air conditions, oxygen consumption is relatively dispersed and proceeds continuously over a wide temperature range, whereas under oxygen-enriched atmospheres, oxygen consumption becomes highly concentrated within the main combustion stage. From the perspective of reactant behavior, this observation further confirms the intensification and acceleration of the rice husk combustion process under oxygen-enriched conditions.
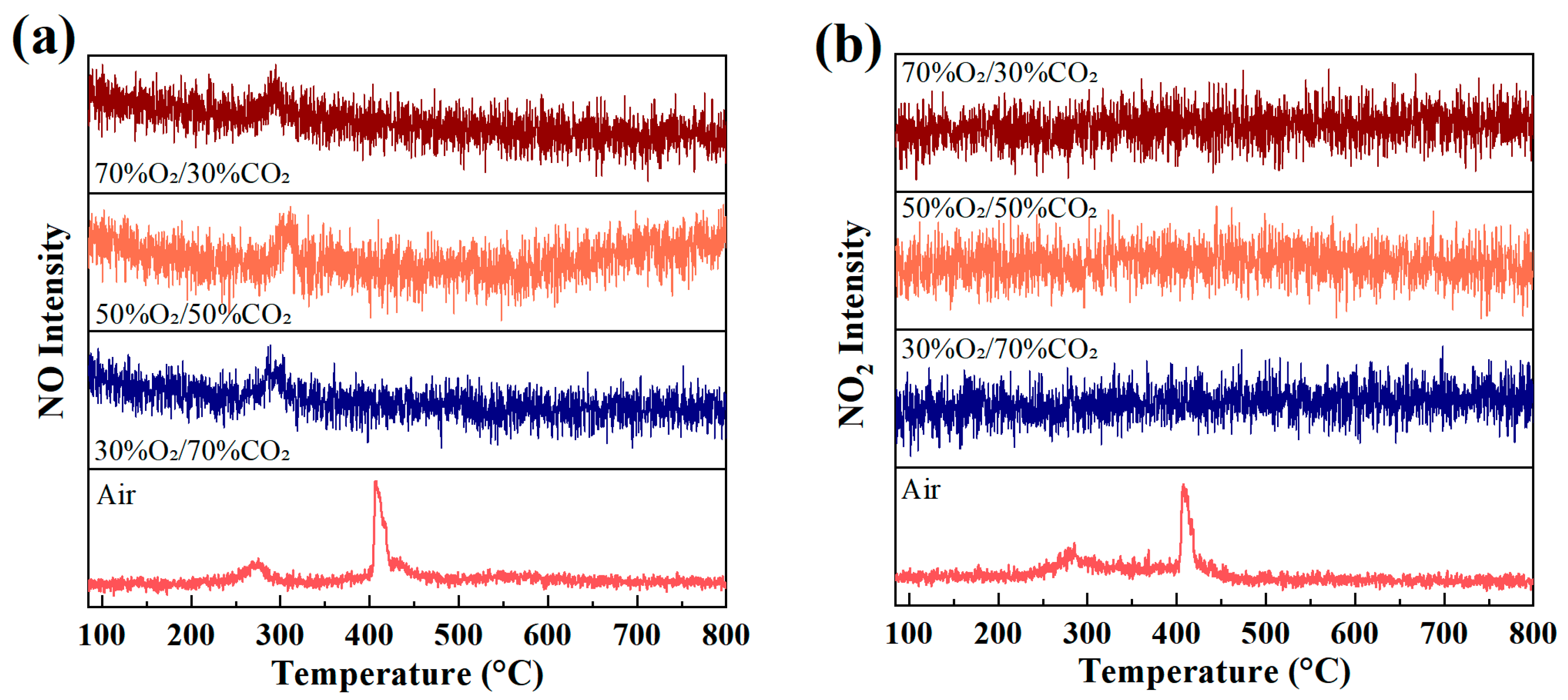
3.3.3. NOx Evolution Behavior During Combustion
Figure 5 presents the evolution of NO (m/z = 30) and NO2 (m/z = 46) ion intensities as a function of temperature during rice husk combustion under different atmospheres. Under air conditions, both NO and NO2 exhibit distinct double-peak profiles, with peak positions showing good correspondence to the volatile combustion peak and the char oxidation peak in the DTG curves. The first peak observed in the low-temperature range (approximately 250–350 °C) is mainly associated with the rapid conversion of fuel-bound nitrogen during volatile release and gas-phase combustion. As the temperature further increases, the second peak formed in the medium-to-high temperature range (approximately 380–430 °C) originates from the continuous release of fuel nitrogen during char oxidation, superimposed with the formation of thermal NOx resulting from the oxidation of atmospheric N2 under high-temperature conditions. Consequently, under air combustion, NOx formation is jointly governed by the stage-wise release of fuel nitrogen and the thermal NOx mechanism at elevated temperatures, leading to sustained NO and NO2 production over a broad temperature range and the characteristic double-peak distribution.
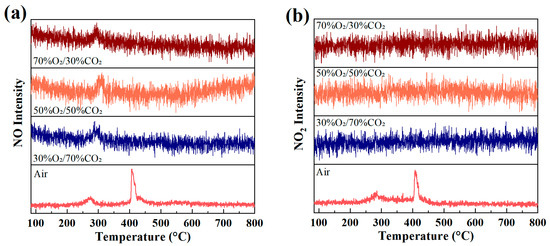
Figure 5.
Evolution of (a) NO (m/z = 30) and (b) NO2 (m/z = 46) ion intensities during rice husk combustion under different atmospheres at a heating rate of 10 °C/min.
In contrast, the formation behavior of NO and NO2 is markedly altered under O2/CO2 oxygen-enriched atmospheres. The overall NO signal intensity is significantly reduced and only a weak peak is observed within the main combustion temperature region, with its peak position still corresponding to the primary DTG mass-loss interval. This indicates that NO formation under oxygen-enriched conditions is predominantly associated with the limited conversion of fuel nitrogen under intensified combustion. Meanwhile, NO2 signals under all oxygen-enriched atmospheres do not exhibit distinct peaks and remain at a low background level, suggesting that the further oxidation of NO to NO2 is strongly suppressed in the CO2-diluted environment.
These differences reflect a fundamental shift in the NOx formation mechanisms under oxygen-enriched combustion conditions. In air combustion, the abundant presence of N2 facilitates thermal NOx formation at high temperatures, while fuel nitrogen is continuously released during different combustion stages, resulting in the double-peak behavior of both NO and NO2. In contrast, under O2/CO2 oxygen-enriched combustion, the absence of N2 effectively suppresses the thermal NOx pathway, and NOx formation is dominated by fuel nitrogen conversion. Moreover, the high heat capacity of CO2 lowers local peak temperatures and inhibits the oxidation of NO by reactive oxidizing intermediates, promoting the conversion of fuel nitrogen toward more stable nitrogen-containing products such as N2 via reduction pathways, thereby significantly suppressing NO2 formation. It should be noted that the proposed NOx suppression mechanisms are inferred from observed trends and established literature, and that direct identification of intermediate nitrogen-containing species is beyond the scope of the present study. In addition, Wang et al. [41] also reported that under oxygen-enriched combustion conditions, NOx emissions decrease markedly with increasing pressure. Their results indicate that pressurization effectively suppresses the release of volatile-bound nitrogen and enhances NO reduction via char-mediated heterogeneous reactions as well as reduction pathways involving nitrogenous intermediates such as NH3 and HCN, thereby promoting the conversion of NO to N2 and leading to an overall reduction in NOx emissions.
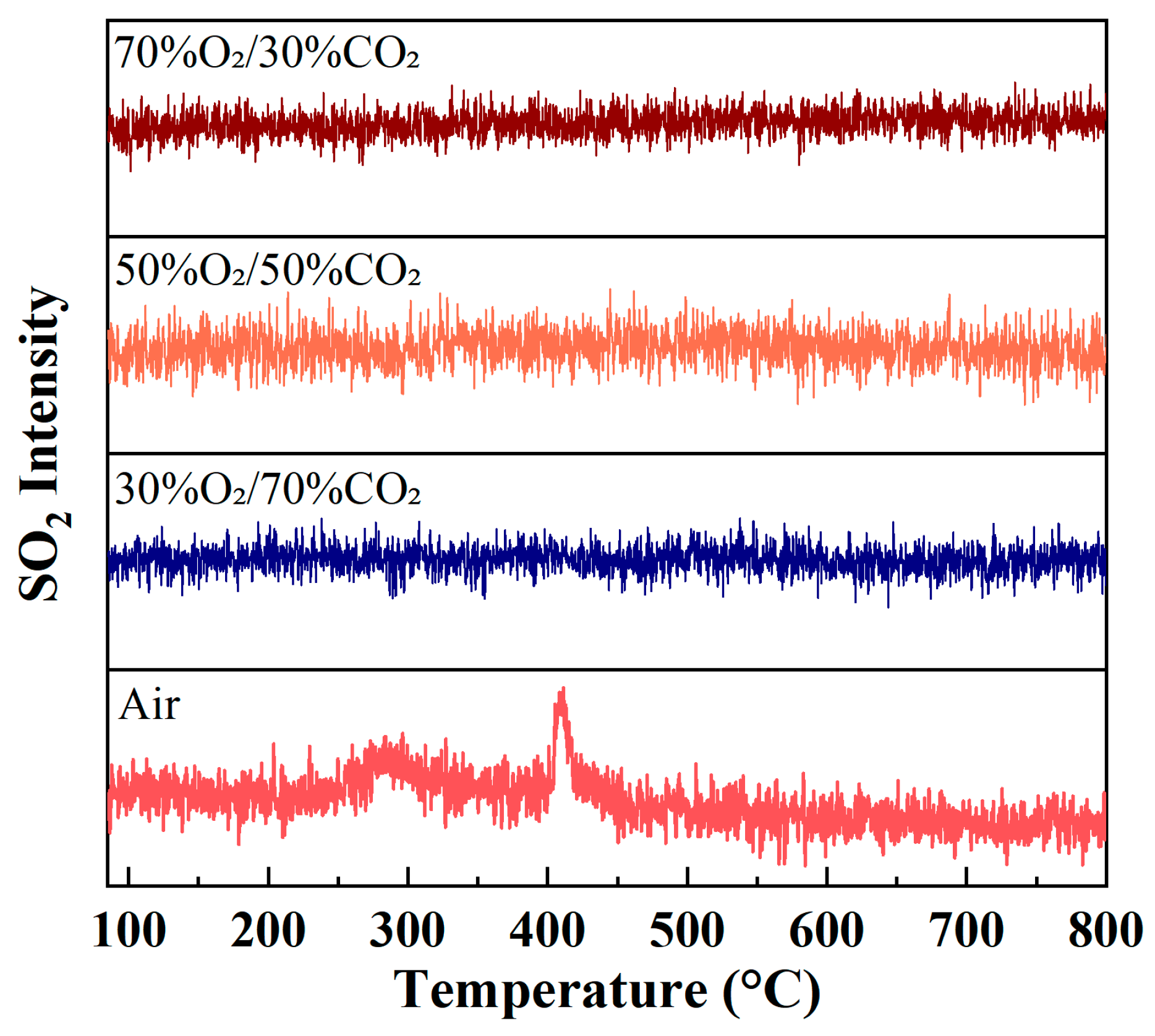
3.3.4. SO2 Evolution Behavior During Combustion
Figure 6 illustrates the evolution of SO2 ion intensity as a function of temperature during rice husk combustion under different atmospheres. Overall, the SO2 signal intensity remains relatively low under all atmospheric conditions, indicating that sulfur pollutant emissions during rice husk combustion are limited. This behavior is closely related to the inherently low sulfur content of rice husk, as well as the fact that sulfur in biomass predominantly exists in organic-bound or mineral-associated forms.
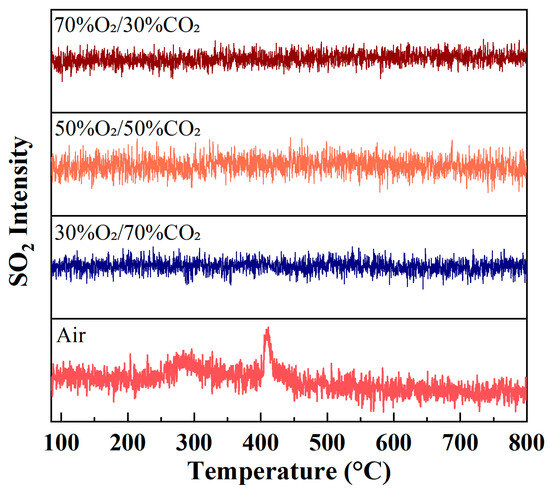
Figure 6.
Evolution of SO2 (m/z = 64) ion intensity during rice husk combustion under different atmospheres at a heating rate of 10 °C/min.
Under air conditions, the SO2 signal exhibits moderate fluctuations within the main combustion temperature region, accompanied by weak peak features. This suggests that sulfur contained in the fuel is gradually oxidized to SO2 during both the volatile release and char combustion stages. In contrast, under O2/CO2 oxygen-enriched atmospheres, the SO2 ion intensity is further reduced and shows a much smoother evolution profile, without any pronounced enhancement of peak intensity. Even at high oxygen concentrations, no significant increase in SO2 formation is observed.
These observations indicate that oxygen-enriched combustion does not significantly promote the conversion of sulfur in rice husk to SO2. On the one hand, the CO2 dilution effect reduces the effective local oxidation intensity within the reaction zone. On the other hand, the ash of rice husk is rich in alkaline mineral components, such as K and Ca, which can react with sulfur species through solid–gas or solid–solid interactions during combustion, thereby contributing to SO2 capture and fixation. Compared with air combustion, oxygen-enriched combustion proceeds in a more concentrated manner, while the CO2 dilution effect lowers local peak temperatures and prolongs the residence time of SO2 within the reaction zone, facilitating interactions between sulfur species and alkaline minerals such as K and Ca.
Lv et al. [42] systematically investigated ash deposition behavior during biomass/coal co-firing under O2/CO2 oxy-fuel atmospheres (O2 = 21–41%) and reported that increasing oxygen concentration markedly enhanced SO2 oxidation and the sulfation of KCl, leading to a pronounced increase in condensed K2SO4 and a corresponding reduction in gaseous sulfur species. As a result of these combined effects, rice husk exhibits a more pronounced low-SO2 emission characteristic under O2/CO2 oxygen-enriched combustion conditions.
3.4. Iso-Conversional Kinetic Analysis of Rice Husk Combustion
Kinetic parameters provide critical insights into the combustion behavior of rice husk under oxygen-enriched conditions. In this study, iso-conversional methods such as KAS and FWO are phenomenological approaches and do not identify specific reaction steps, but rather provide insight into changes in dominant rate-controlling processes during combustion. Table 4 and Figure 7 and Figure 8 present the apparent activation energy E(α) and the corresponding determination coefficients (R2) obtained from the KAS and FWO iso-conversional methods over the conversion range of 0.1 ≤ α ≤ 0.9. Both methods exhibit highly consistent trends in E(α), with satisfactory linear fitting quality, indicating that they are suitable for evaluating the combustion kinetics under all investigated atmospheres. Under practical combustion conditions, diffusion and heat transfer effects may play a more pronounced role; thus, the trends observed in TG measurements reflect apparent behaviors rather than fully isolated intrinsic kinetics. However, under practical combustion conditions, diffusion and heat transfer effects may play a more pronounced role; thus, the trends observed in TG measurements reflect apparent behaviors rather than fully isolated intrinsic kinetics.

Table 4.
Kinetic parameters of different samples using KAS and FWO method.
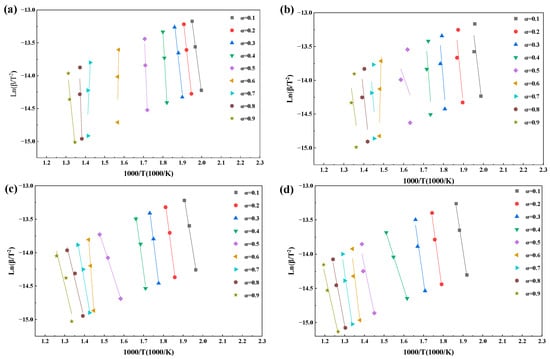
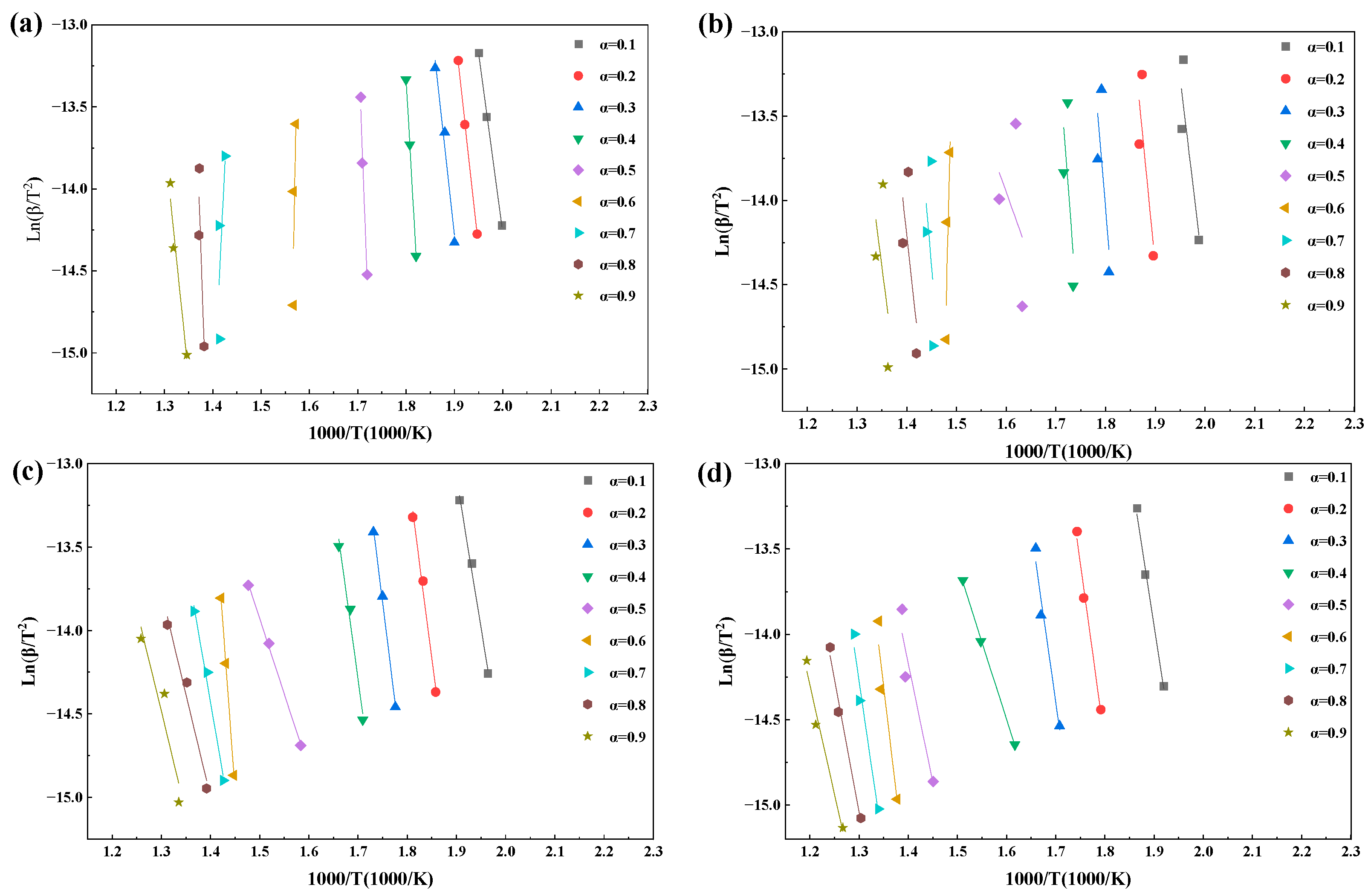
Figure 7.
The iso-conversional plots at 0.1 ≤ α ≤ 0.9 by KAS. (a) Air, (b) 30%O2/70%CO2, (c) 50%O2/50%CO2 and (d) 70%O2/30%CO2.
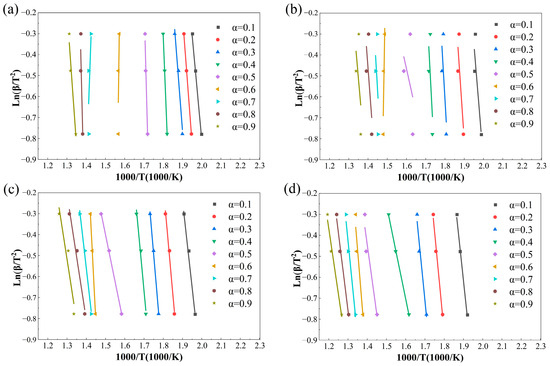
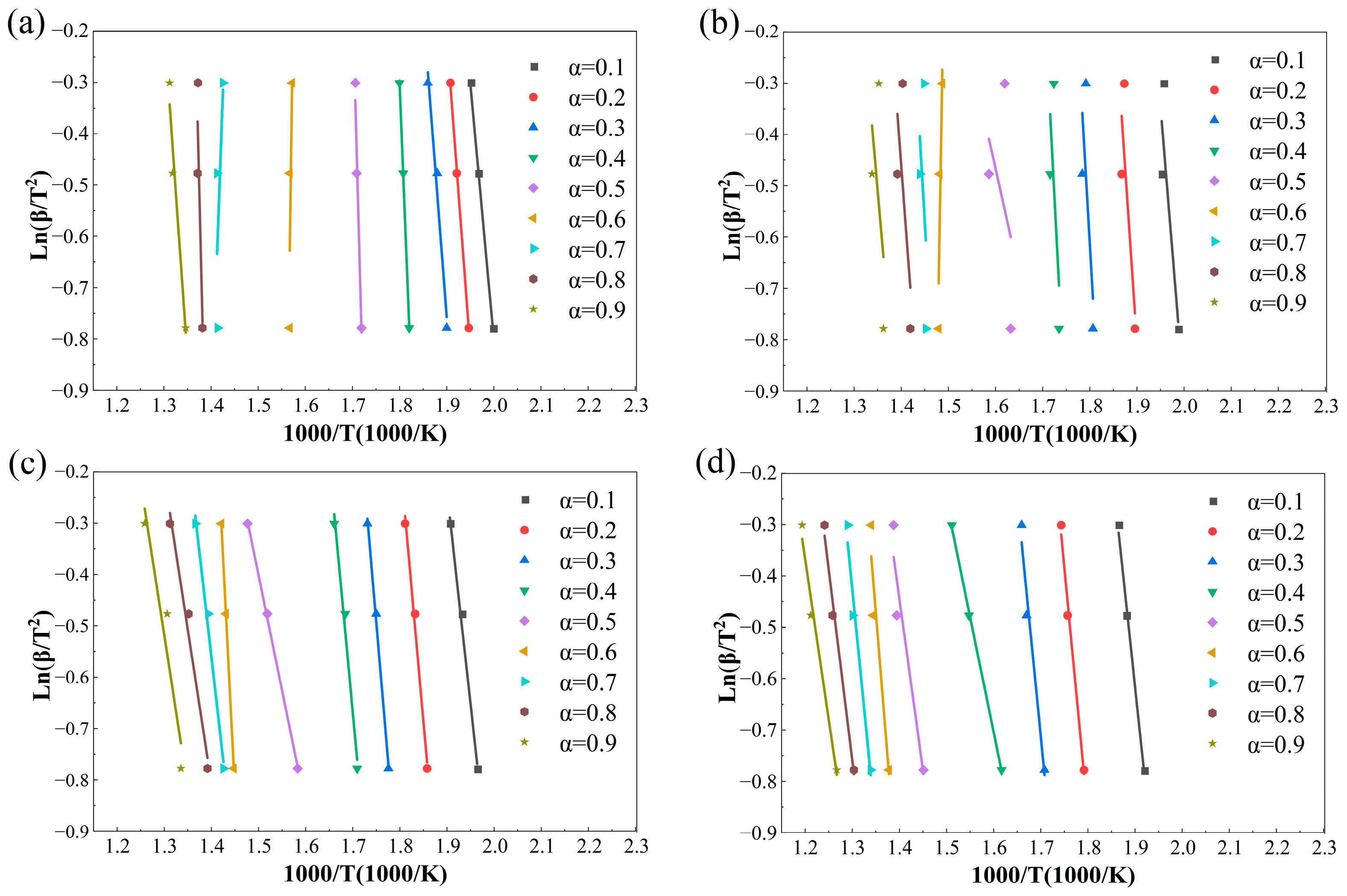
Figure 8.
The iso-conversional plots at 0.1 ≤ α ≤ 0.9 by FWO. (a) Air, (b) 30%O2/70%CO2, (c) 50%O2/50%CO2 and (d) 70%O2/30%CO2.
Under air conditions, the average apparent activation energies are 143.93 kJ·mol−1 (KAS) and 147.62 kJ·mol−1 (FWO). The E(α) profiles display a minimum around α ≈ 0.4, followed by an increase to a maximum near α ≈ 0.6. This behavior can be attributed to the rapid oxidation of volatiles and weak-bond structures at low conversion degrees, followed by a gradual transition toward char-dominated surface reactions accompanied by pore structure evolution. As combustion proceeds, the depletion of active sites and the progressive ordering of carbon structures lead to an increase in the apparent energy barrier. At higher conversion levels (0.8 ≤ α ≤ 0.9), the coupling between residual carbon and ash effects contributes to a subsequent decrease in E(α).
With the introduction of CO2-containing oxygen-enriched atmospheres, the magnitude of E(α) increases significantly. In particular, under the 70%O2/30%CO2 atmosphere, the average activation energy rises to 298.19 kJ·mol−1 (KAS) and 293.57 kJ·mol−1 (FWO), with E(α) reaching an extreme value of 525.17–547.54 kJ·mol−1 at α ≈ 0.5, followed by a pronounced decline. This behavior indicates that high oxygen partial pressure accelerates the early-stage reactions, leading to rapid consumption of active sites. As the reaction progresses, combustion becomes increasingly controlled by denser carbon structures and diffusion limitations associated with ash layer formation, resulting in a substantially elevated apparent energy barrier during the intermediate conversion stage [43].
Although the 50%O2/50%CO2 atmosphere also exhibits relatively high average activation energies (221.03–222.69 kJ·mol−1), the corresponding R2 values are consistently lower, suggesting reduced reliability of the extracted E(α). The pronounced fluctuations in E(α) under this condition are therefore more likely associated with the superposition of parallel reactions and the amplification of non-chemical control effects rather than a single dominant kinetic mechanism. Overall, the non-monotonic variation in E(α) with conversion degree confirms that rice husk combustion cannot be described by a single-step reaction mechanism. Under atmospheres with high fitting quality, the activation energies derived from the KAS and FWO methods provide reliable indicators for identifying reaction-control transitions, combustion stages, and the regulatory effects of atmosphere composition.
The iso-conversional linear fittings at different α values shown in Figure 7 and Figure 8 further reveal the restructuring of combustion control mechanisms induced by oxygen-enriched atmospheres. Under air and 30%O2/70%CO2 conditions, data points corresponding to each conversion degree are closely aligned with stable slopes, indicating that a single dominant control mechanism can reasonably describe the combustion process over a wide conversion range. The high R2 values reported in Table 4 support the statistical reliability of the corresponding E(α) values.
In contrast, under the 70%O2/30%CO2 atmosphere, good linearity is still observed in the range of 0.6 ≤ α ≤ 0.8, whereas noticeable data dispersion and reduced linearity emerge beyond this range. This suggests a shift in the rate-controlling mechanism from chemically controlled reactions toward structure-dominated evolution as combustion proceeds, thereby weakening the representativeness of a single slope for a unified mechanism. This interpretation is consistent with the occurrence of extremely high E(α) values around α ≈ 0.5 and the subsequent decline in R2. The 50%O2/50%CO2 condition exhibits the most pronounced data dispersion, with multiple conversion intervals failing to form consistent slopes, explaining the systematically lower R2 values and abnormal fluctuations in E(α). Therefore, oxygen-enriched atmospheres significantly modify the reaction environment and progression pathways, leading to enhanced stage dependence and pronounced atmosphere sensitivity in the combustion kinetics of rice husk.
Overall, the inflection behavior of E(α) observed under different oxy-fuel atmospheres provides clear evidence for a transition in the dominant rate-controlling regime. At lower conversion levels, the kinetic behavior is more sensitive to oxygen availability, whereas at higher conversions the reaction becomes increasingly carbon-limited due to char depletion and structural evolution. This transition, consistently reflected in the E(α) profiles summarized in Table 4, represents a key kinetic signature of oxy-fuel combustion of rice husk.
3.5. Reactive Molecular Dynamics of the Combustion Process
3.5.1. Chemical Bond Evolution and Reaction Pathway Regulation Under Different Atmospheres
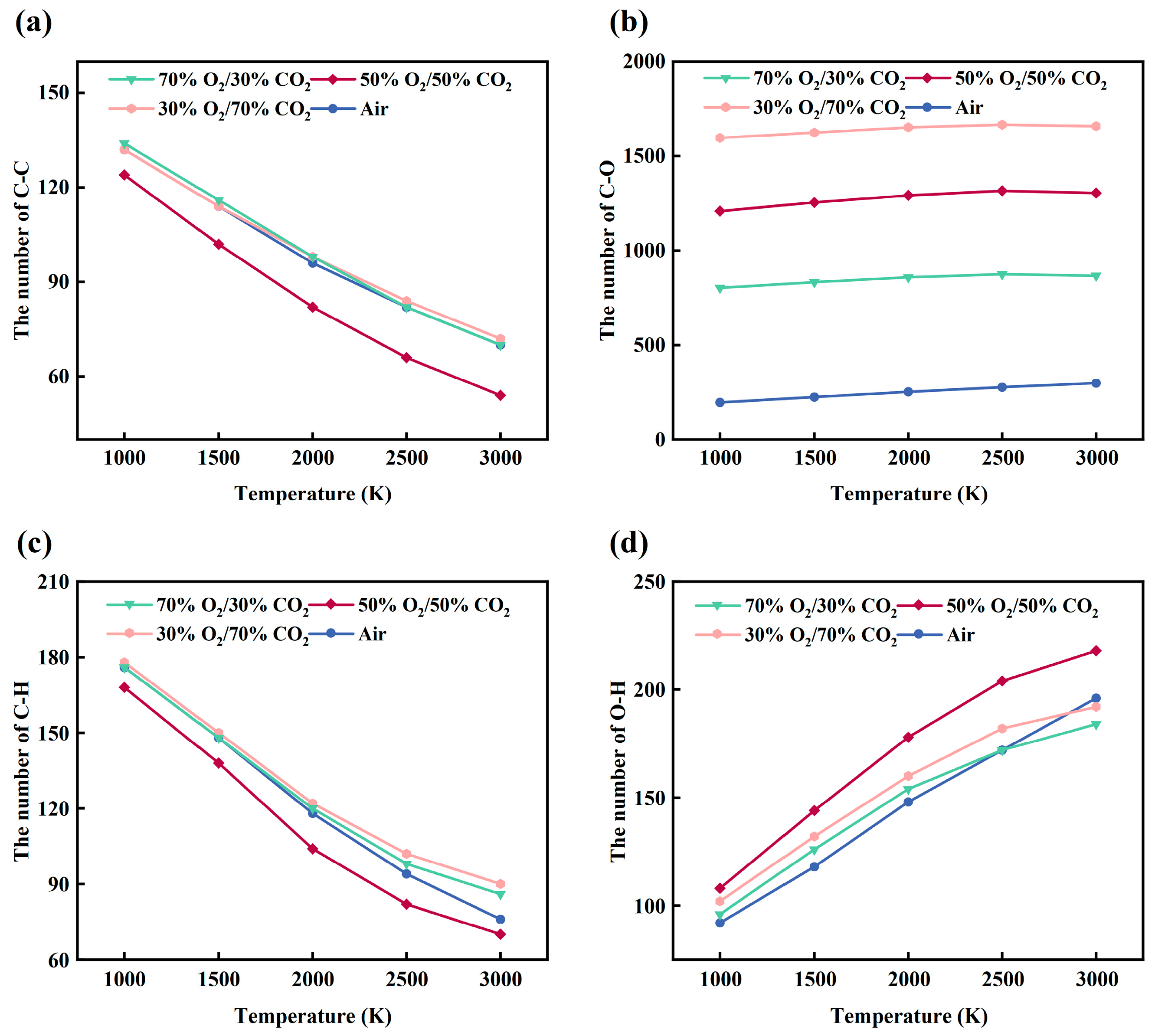
Based on ReaxFF-MD simulations, the chemical bond evolution of the rice husk model under different oxygen-enriched atmospheres (air, 30%O2/70%CO2, 50%O2/50%CO2, and 70%O2/30%CO2) was systematically analyzed, and the corresponding reaction pathways were discussed in combination with the formation of major gaseous products (CO, CO2, and H2O). Overall, as shown in Figure 9a–d, increasing the temperature from 1000 K to 3000 K leads to continuous cleavage of C-C and C-H bonds and a concurrent increase in C-O and O-H bonds under all investigated atmospheres, indicating progressive decomposition of the organic backbone of rice husk accompanied by oxidative reactions. Nevertheless, the reaction intensity and dominant pathways are strongly influenced by oxygen availability and the dilution effect of the surrounding atmosphere. It should be noted that the molecular dynamics simulations are designed to capture comparative reaction behaviors and molecular-scale mechanisms under different oxy-fuel conditions. While the simulations allow for relative comparison of reaction pathways and species evolution, they are not intended to directly reproduce experimental timescales or absolute kinetic parameters. Furthermore, ReaxFF-MD simulations were performed at elevated temperatures to accelerate reaction events and to enable relative comparison of mechanistic trends, rather than to directly reproduce experimental combustion temperatures or absolute reaction rates [44,45,46,47].
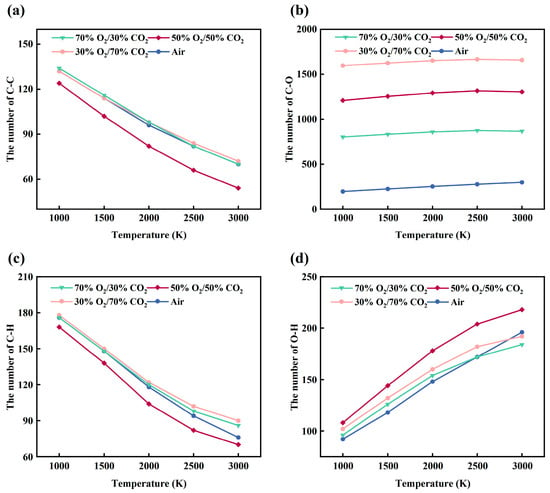
Figure 9.
Temperature-dependent evolution of major chemical bonds in the rice husk model under different atmospheres: (a) C–C; (b) C–O; (c) C–H; (d) O–H.
Under air conditions, the limited oxygen supply results in relatively moderate cleavage of C-C and C-H bonds, and the system mainly undergoes gradual pyrolysis coupled with mild oxidation. Upon introducing CO2 (30%O2/70%CO2), the oxidation intensity is enhanced, as evidenced by a pronounced increase in C-O and O-H bonds, reflecting more extensive formation of oxygen-containing functional groups and water molecules. However, at high temperatures (2500–3000 K), the number of C-O bonds tends to plateau, suggesting that CO2 participation and thermal decomposition of oxygenated groups begin to influence the reaction pathways.
In the 50%O2/50%CO2 atmosphere, the system exhibits the highest overall reactivity. The cleavage of C-C and C-H bonds is most pronounced, while C-O and O-H bonds increase rapidly in the intermediate-to-high temperature range (2000–3000 K), indicating maximized oxygen utilization efficiency. Nevertheless, the growth of C-O bonds no longer continues at the highest temperatures, implying that under strong oxidative conditions, reaction progression becomes constrained by the stability of oxygen-containing groups.
By contrast, under the highly oxygen-rich atmosphere (70%O2/30%CO2), despite the highest oxygen concentration, a substantial number of O-O bonds are retained throughout the entire temperature range, revealing a clear oxygen-excess feature. In this system, both the cleavage of C-C and C-H bonds and the formation of C-O and O-H bonds are suppressed, indicating a transition of the combustion regime from oxygen-limited to carbon-limited control. Overall, different oxygen-enriched atmospheres significantly modify the balance between oxygen supply and dilution effects, thereby altering the decomposition and oxidation pathways of the rice husk organic framework.
3.5.2. Evolution Characteristics of Combustion Products Under Different Atmospheres
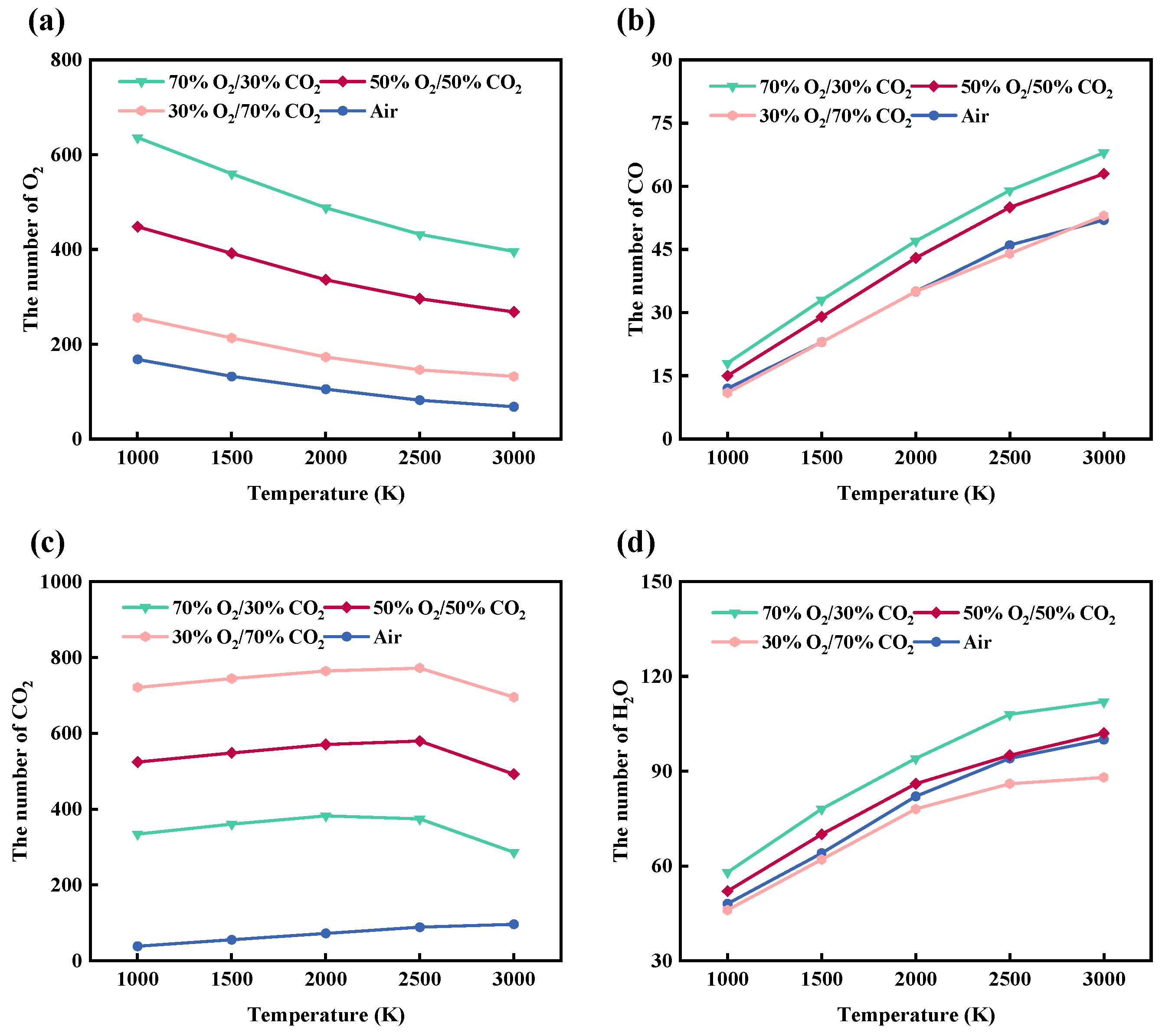
In the previous section, the differences in combustion reaction pathways of rice husk under various atmospheres were analyzed from the perspective of chemical bond evolution, with particular emphasis on the temperature-dependent cleavage of C-C and C-H bonds and the formation of C-O bonds. To further validate the proposed reaction mechanisms from the viewpoints of reactant consumption and product formation, this section focuses on the evolution behaviors of the reactant O2 and representative combustion products CO, CO2, and H2O under different atmospheres and temperatures.
As shown in Figure 10a–d, the evolution of O2 and major gaseous products during rice husk combustion exhibits pronounced differences under oxygen-enriched atmospheres, which are in good agreement with the previously discussed chemical bond evolution characteristics. From Figure 10a, it can be observed that the amount of O2 in all systems generally decreases with increasing temperature from 1000 K to 3000 K, with the most significant O2 consumption occurring in the temperature range of 1500–2500 K. This region corresponds to extensive cleavage of C-C and C-H bonds and the rapid progression of oxidation reactions. At the lower temperature stage (1000–2000 K), O2 consumption remains relatively limited, indicating that the combustion process is mainly constrained by the initial activation of chemical bonds within the organic framework. As the temperature exceeds 2000 K, the reaction rate increases markedly, and the differences among various atmospheres become increasingly pronounced. For systems with higher oxygen concentrations, a noticeable amount of unreacted O2 persists even at elevated temperatures, suggesting that the combustion regime gradually shifts from oxygen-limited control to carbon-limited control as the reaction proceeds.
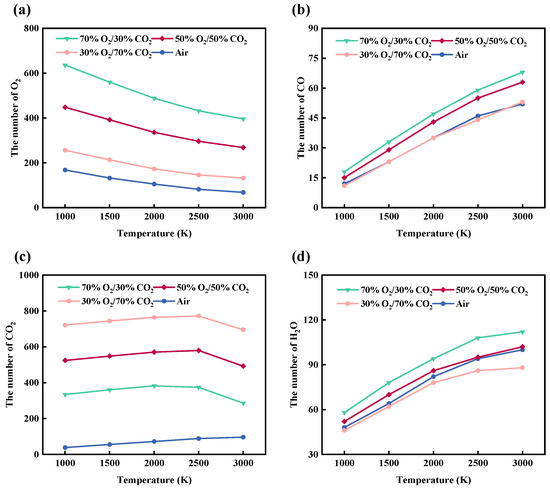
Figure 10.
Temperature-dependent evolution of (a) O2, (b) CO, (c) CO2, and (d) H2O during rice husk combustion under different atmospheres from ReaxFF molecular dynamics simulations.
As a key intermediate during combustion, the formation behavior of CO is closely associated with the partial oxidation of the carbon backbone. As shown in Figure 10b, the amount of CO increases monotonically with rising temperature; however, the growth rate gradually decreases beyond 2500 K, indicating an enhanced tendency for CO to undergo further oxidation into CO2 at elevated temperatures. This evolution behavior corresponds well to the pronounced increase in C-O bond formation observed below 2500 K, suggesting that CO generation shares a consistent reaction pathway with the formation of oxygen-containing intermediates. Compared with inert atmospheres, CO formation is more pronounced in CO2-containing oxygen-enriched systems, demonstrating that atmospheric composition plays a promotive role in regulating the evolution of combustion intermediates. This trend is consistent with previous mechanistic investigations of oxy-fuel combustion based on ReaxFF molecular dynamics simulations. Qiu et al. [48] reported that CO formation predominantly proceeds through the thermal decomposition of the carbon skeleton, followed by the formation of oxygen-containing intermediates and subsequent dehydrogenation-oxidation steps. Increasing temperature was found to markedly promote CO generation while simultaneously accelerating its further oxidation toward CO2. Moreover, their results demonstrated that a CO2-rich oxy-fuel atmosphere does not act as an inert diluent; instead, CO2 actively participates in reaction pathways and regulates the dynamic evolution and interconversion of CO and CO2.
As the reaction proceeds, CO2, serving as the most stable final product, continuously accumulates in all systems. As shown in Figure 10c, the amount of CO2 increases rapidly in the temperature range of 1000–2500 K. However, except for the air atmosphere, the other three oxygen-enriched atmospheres exhibit a noticeable decline in CO2 quantity beyond 2500 K. It should be noted that CO2 is present as an initial component in some reaction atmospheres, and the newly generated CO2 formed with increasing temperature still dominates the overall CO2 population. This observation indicates that CO2 not only acts as a diluent but also participates, to some extent, in regulating oxidation reaction pathways. Such behavior is consistent with the maintenance of relatively high C-O bond populations under high-temperature conditions, as discussed in the previous section. When the temperature exceeds 2500 K, the progressive depletion of oxygen and the pronounced enhancement of strongly endothermic gasification reactions, such as the Boudouard reaction (C + CO2 → 2CO), lead to a transition of CO2 from net formation to significant consumption, resulting in a decrease in its concentration. This inflection point indicates a transition in the dominant controlling mechanism, from oxygen-concentration-controlled combustion kinetics to gasification and reduction reactions governed by high-temperature thermodynamic effects.
The formation of H2O is primarily governed by the coupled processes of hydrogen release and oxidation reactions. As shown in Figure 10d, the amount of H2O increases continuously with rising temperature, with the most pronounced growth occurring in the range of 1000–2500 K. During this stage, extensive cleavage of C-H bonds provides an abundant hydrogen source for water formation, while the marked increase in O-H bonds directly reflects the continuous generation of H2O molecules. Under oxygen-rich atmospheres, H2O formation tends to stabilize at elevated temperatures, indicating that hydrogen gradually becomes the limiting reactant as the reaction proceeds. Overall, different atmospheres significantly influence oxygen consumption behavior as well as the formation pathways of CO, CO2, and H2O by regulating oxygen availability and the CO2 dilution effect. The evolution trends of these species show good consistency with the corresponding chemical bond evolution characteristics across the investigated temperature ranges.
4. Conclusions
This study systematically investigated the combustion behavior, kinetic characteristics, and multiscale reaction mechanisms of rice husk biomass under O2/CO2 oxy-fuel conditions by integrating TG–DTG–MS experiments, iso-conversional kinetic analysis, and ReaxFF reactive molecular dynamics simulations. The results demonstrate that oxygen enrichment markedly intensifies rice husk combustion, leading to reduced ignition and burnout temperatures, significantly enhanced mass loss rates, and a more concentrated combustion temperature window compared with air combustion. Oxy-fuel conditions also promote highly synchronized CO2 formation and O2 consumption while effectively suppressing NOx and SO2 emissions, highlighting the combined roles of CO2 dilution and reaction pathway regulation. Kinetic analysis reveals a pronounced non-monotonic evolution of apparent activation energy with conversion degree, particularly under high oxygen concentrations, indicating a transition in the rate-controlling mechanism from chemically controlled reactions to a regime governed by structural evolution and diffusion coupling. These findings are further supported by molecular dynamics simulations, which elucidate the oxygen-dependent regulation of chemical bond cleavage and product formation pathways at the molecular scale.
From an application perspective, the observed sensitivity of combustion intensity and reaction regime transitions to oxygen concentration provides useful guidance for oxy-fuel boiler tuning, particularly in balancing combustion stability, burnout efficiency, and pollutant suppression. Moreover, the highly concentrated CO2 release behavior and reduced NOx/SO2 emissions under O2/CO2 atmospheres highlight the strong compatibility of biomass oxy-fuel combustion with downstream carbon capture and purification systems. Importantly, the conversion-dependent evolution and inflection behavior of E(α) identified in this study suggest that apparent activation energy may serve as a diagnostic metric for identifying combustion regime transitions and optimizing operating conditions in oxy-fuel combustion systems.
Despite the systematic multiscale investigation of rice husk oxy-fuel combustion mechanisms in this study, several limitations remain. The experimental work was primarily conducted under thermogravimetric conditions with small sample sizes, which cannot fully represent the complex flow, heat transfer, and mass transfer processes in practical combustion systems. In addition, the high-temperature acceleration strategy adopted in reactive molecular dynamics simulations imposes certain limitations on the quantitative correspondence of reaction rates and timescales. Future studies should therefore incorporate fluidized-bed or pilot-scale combustion experiments to validate the oxy-fuel combustion behavior and pollutant formation characteristics and further refine molecular models and kinetic parameters through multiscale coupled modeling and experimental characterization, thereby providing more reliable theoretical and data support for the engineering application of biomass oxy-fuel combustion and its integration with CO2 capture processes.
Author Contributions
Conceptualization, D.L. and Q.W.; methodology, D.C.; software, Y.P. and D.C.; validation, Y.P., C.Y. and D.L.; formal analysis, D.L.; investigation, Y.P.; resources, X.Z.; data curation, Y.W.; writing—original draft preparation, D.L. and Y.W.; writing—review and editing, Q.W. and D.C.; visualization, S.W.; supervision, H.Z.; project administration, Y.P.; funding acquisition, Y.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “The major project of China Power Engineering Consulting Group Co., Ltd.: Research on CFB boiler technology based on biomass oxy-fuel combustion and CO2 purification, capture, and green methanol synthesis, grant number: DG3-J04-2024”.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
During the preparation of this manuscript, the authors used ChatGPT (OpenAI, GPT-5.2) for language polishing, restructuring of sentences, and assistance in improving the clarity and coherence of the text. The authors have reviewed, verified, and edited all AI-generated content and take full responsibility for the scientific accuracy and originality of this work.
Conflicts of Interest
Authors Dandan Li, Yufeng Pei, Xiuyan Zhang, Chang Yu and Hongpeng Zhao were employed by Northeast Electric Power Design Institute Co., Ltd. of China Power Engineering Consulting Group. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The Northeast Electric Power Design Institute Co., Ltd. of China Power Engineering Consulting Group had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Yin, L.; Yang, H.; Cao, X.; Bu, C. Iron-Based oxygen carrier aided oxy-fuel combustion of biomass in fluidized Beds: Effects of types of oxygen carriers and biomass ash. Fuel 2026, 408, 137708. [Google Scholar] [CrossRef]
- Magiera, T.; Górka-Kostrubiec, B.; Bućko, M.S.; Wawer-Liszka, M.; Lukkari, S. Magnetic properties of ash produced during combustion of various types of biomass pellets. J. Magn. Magn. Mater. 2025, 630, 173468. [Google Scholar] [CrossRef]
- Li, X.; Lin, H.; Wang, G.; Dai, G.; Chen, Y.; Luo, Y.; Liu, B.; Zhang, J.; Axelbaum, R.L.; Wang, X. Innovative performance evaluation and process simulation of a 550 MW staged, pressurized oxy-biomass combustion power plant for negative carbon emissions. Renew. Energy 2026, 256, 124534. [Google Scholar] [CrossRef]
- Amirante, R.; De Palma, P.; Distaso, E.; Pantaleo, A.M.; Tamburrano, P. Thermodynamic analysis of a small scale combined cycle for energy generation from carbon neutral biomass. Energy Procedia 2017, 129, 891–898. [Google Scholar] [CrossRef]
- Xin, B.; Gu, Y.; Lv, L.; Zhu, X.; Wang, S.; Dong, J.; Zhang, N. Advancing biomass power through policy: A dynamic perspective under China’s carbon goals. Energy 2026, 344, 139925. [Google Scholar] [CrossRef]
- Limmer, M.A.; Seyfferth, A.L. Rice husk biochar as a sustainable source of plant silicon: A 3-year study. Field Crops Res. 2026, 336, 110225. [Google Scholar] [CrossRef]
- Yu, W.; Tan, X.; Yi, W.; Wang, G.; Hu, T.; Su, H.; Liao, L. Investigation of rice husk oxy-fuel combustion: Kinetics and correlation analysis. Biomass Bioenergy 2026, 209, 108923. [Google Scholar] [CrossRef]
- Bu, C.; Liu, D.; Chen, X.; Pallarès, D.; Gómez-Barea, A. Ignition behavior of single coal particle in a fluidized bed under O2/CO2 and O2/N2 atmospheres: A combination of visual image and particle temperature. Appl. Energy 2014, 115, 301–308. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.a.; Luo, M.; Zhao, L.; Zhao, P.; Che, D. Effects of residual carbon on slag flow characteristics of Zhundong high-alkali coal under oxy-fuel condition. Fuel 2025, 385, 134181. [Google Scholar] [CrossRef]
- Raho, B.; Giangreco, M.; Colangelo, G.; Milanese, M.; de Risi, A. Technological, economic, and emission analysis of the oxy-combustion process. Appl. Energy 2025, 378, 124821. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, C.; Li, Y.; Li, H.; Gu, Z.; Deng, H.; Oppong, F.; Li, X. Enhancing ammonia combustion: Study of laminar burning velocity, flame instability, and NOx emissions under oxygen-enriched conditions. J. Energy Inst. 2026, 125, 102424. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Z.; Li, Y.; Yang, S.; Jiang, H.; Zhao, H.; Zhang, Y.; Lin, H. Unveiling the conversion mechanisms of NO and N2O in ammonia blending combustion under high pressure, oxygen enrichment, and H2O addition conditions. J. Energy Inst. 2026, 124, 102405. [Google Scholar] [CrossRef]
- Wang, M.; Pang, Z.; Wei, G.; Wang, J.; Wang, G.; Jia, G.; Zhang, L.; Guan, J. Research on oxy-fuel combustion characteristics of two typical chinese coals. Processes 2023, 11, 1933. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Q.; Wang, Z.; Di, H. Influence of CO2 substitution for N2 on the combustion characteristics and kinetics of oil shale under oxy-fuel conditions. RSC Adv. 2025, 15, 37937–37950. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Zhang, Y.; Zhou, J.; Deng, S.; Fu, Y. Study of the combustion behavior of wheat straw using TG–DSC under multiple heating rates. Biomass Bioenergy 2026, 205, 108507. [Google Scholar] [CrossRef]
- Ma, X.; Ning, H.; Zhang, X.; Zang, Z.; Xing, X. Co-combustion characteristics of coal slime and moso bamboo based on artificial neural network modeling and TG-FTIR. Energy 2024, 313, 133816. [Google Scholar] [CrossRef]
- Ni, Z.; Zhang, Y.; Liu, X.; Shi, H.; Yao, Y.; Tian, J.; Hu, P.; He, L.; Lin, Q.; Liu, L. Co-combustion of sewage sludge with corn stalk based on TG-MS and TG-DSC: Gas products, interaction mechanisms, and kinetic behavior. Energy 2024, 308, 132747. [Google Scholar] [CrossRef]
- Guo, D.; Feng, D.; Sun, H.; Zhao, Y.; Sun, S. Oxy-fuel combustion kinetics of biomass: Insights from multi-heating-rate experiments and kinetic analysis. Bioresour. Technol. 2026, 441, 133589. [Google Scholar] [CrossRef] [PubMed]
- Baqain, M.; Neshumayev, D.; Konist, A. TG-MS analysis and kinetic study of co-combustion of ca-rich oil shale with biomass in air and oxy-like conditions. Carbon Capture Sci. Technol. 2024, 10, 100162. [Google Scholar] [CrossRef]
- Yan, J.; Fang, M.; Lv, T.; Zhu, Y.; Cen, J.; Yu, Y.; Xia, Z.; Luo, Z. Thermal and kinetic analysis of pressurized oxy-fuel combustion of pulverized coal: An interpretation of combustion mechanism. Int. J. Greenh. Gas Control. 2022, 120, 103770. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Z.; Ma, X.; Long, J.; Peng, Y.; Hu, L.; Lu, Q. Oxy-fuel combustion characteristics and kinetics of microalgae Chlorella vulgaris by thermogravimetric analysis. Bioresour. Technol. 2013, 144, 563–571. [Google Scholar] [CrossRef]
- Barzegar, R.; Yozgatligil, A.; Olgun, H.; Atimtay, A.T. TGA and kinetic study of different torrefaction conditions of wood biomass under air and oxy-fuel combustion atmospheres. J. Energy Inst. 2020, 93, 889–898. [Google Scholar] [CrossRef]
- Cui, D.; Li, Y.; Zhang, X.; Wu, S.; Luk, H.M.; Han, S.; Pan, S.; Fattahi, M.; Wang, Q.; Zhang, X. Molecular dynamics insights into hydrogen production by biomass supercritical water gasification: A free radical reaction perspective. Energy 2025, 341, 139498. [Google Scholar] [CrossRef]
- Cui, D.; Zhou, X.; Wu, S.; Luk, H.M.; Lu, Q.; Bai, J.; Liu, B.; Xu, X.; Pan, S.; Wang, Q.; et al. Synergistic mechanism and radicals interaction of the Co-SCWG of cellulose and polystyrene based on ReaxFF-MD and DFT. J. Energy Inst. 2026, 125, 102441. [Google Scholar] [CrossRef]
- Wei, Z.; Li, Y.; Wang, Y.; He, Z. Mechanism investigations on co-pyrolysis of polyethylene and biomass using ReaxFF simulation and DFT computation. J. Environ. Chem. Eng. 2023, 11, 110808. [Google Scholar] [CrossRef]
- Zheng, M.; Li, X. Co-pyrolysis behaviors of biomass and polymer plastics by using reactive molecular dynamics simulation. Energy 2024, 296, 131165. [Google Scholar] [CrossRef]
- Xu, Q.; Lu, J.; Zhou, Z.; Jin, Y. FAU zeolites from coal/rice husk co-combustion ash (co-ash) for CO2 adsorption: Synthesis, characterization, adsorption and regeneration performance. Sep. Purif. Technol. 2025, 369, 133147. [Google Scholar] [CrossRef]
- Hirose Carlsen, M.-M.; Saito, Y. Phase diagram of SiO2 crystallization upon rice husk combustion to control silica ash quality. Waste Manag. 2024, 182, 55–62. [Google Scholar] [CrossRef]
- Yu, M.; Chen, C.; Xing, Z.; Jiang, X. ReaxFF molecular dynamics simulation of nickel catalysed gasification of cellulose in supercritical water. Int. J. Hydrogen Energy 2023, 48, 123–137. [Google Scholar] [CrossRef]
- Bai, M.; Huo, E.; Sun, Y.; Wang, S.; Zhao, Y.; Zhang, Q.; Wang, C.; Zou, R.; Qian, M.; Lei, H. Supercritical water co-gasification mechanism of lignin and low density polyethylene into syngas: ReaxFF molecular dynamic simulation and density functional theory calculation study. Fuel Process. Technol. 2023, 250, 107877. [Google Scholar] [CrossRef]
- van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Chenoweth, K.; van Duin, A.C.T.; Goddard, W.A. ReaxFF Reactive Force Field for Molecular Dynamics Simulations of Hydrocarbon Oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, C.; Yang, L.; Fan, X.; Chu, L. A study on co-pyrolysis mechanisms of biomass and polyethylene via ReaxFF molecular dynamic simulation and density functional theory. Process Saf. Environ. Prot. 2021, 150, 22–35. [Google Scholar] [CrossRef]
- Xuan, W.; Gao, J.; Ma, Z.; Cao, C.; Yan, S.; Wang, Q. Synergistic mechanism and radicals interaction of the co-pyrolysis of lignite and PE based on ReaxFF-MD and DFT. Energy 2024, 289, 129978. [Google Scholar] [CrossRef]
- Wang, T.; Hou, H.; Ye, Y.; Rong, H.; Li, J.; Xue, Y. Combustion behavior of refuse-derived fuel produced from sewage sludge and rice husk/wood sawdust using thermogravimetric and mass spectrometric analyses. J. Clean. Prod. 2019, 222, 1–11. [Google Scholar] [CrossRef]
- Ma, X.; Xing, X.; Ma, P. Research on combustion characteristics of typical biomass: Gas emissions, reaction mechanism and ANN application. Int. J. Hydrogen Energy 2026, 203, 153156. [Google Scholar] [CrossRef]
- Irfan, M.F.; Arami-Niya, A.; Chakrabarti, M.H.; Wan Daud, W.M.A.; Usman, M.R. Kinetics of gasification of coal, biomass and their blends in air (N2/O2) and different oxy-fuel (O2/CO2) atmospheres. Energy 2012, 37, 665–672. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, S.; Cui, D.; Pan, S.; Xu, F.; Xu, F.; Wang, Z.; Li, G. Co-hydrothermal carbonization of corn stover and food waste: Characterization of hydrochar, synergistic effects, and combustion characteristic analysis. J. Environ. Chem. Eng. 2022, 10, 108716. [Google Scholar] [CrossRef]
- Zhuo, Z.; Liu, J.; Sun, S.; Sun, J.; Kuo, J.; Chang, K.; Fu, J.; Wang, Y. Thermogravimetric characteristics of textile dyeing sludge, coal and their blend in N2/O2 and CO2/O2 atmospheres. Appl. Therm. Eng. 2017, 111, 87–94. [Google Scholar] [CrossRef]
- Hu, J.; Song, Y.; Liu, J.; Evrendilek, F.; Zhang, G.; Ren, M.; Xie, W.; Sun, S. Torrefaction-assisted oxy-fuel co-combustion of textile dyeing sludge and bamboo residues toward enhancing emission-to-ash desulfurization in full waste circularity. Fuel 2022, 318, 123603. [Google Scholar] [CrossRef]
- Li, X.; Dai, G.; Wang, G.; Luo, Y.; Lin, H.; Wang, P.; Zhang, Y.; Zhang, J.; Tan, H.; Wang, X. NOx emission of pressurized oxy-biomass combustion: Experimental and modeling study in a pressurized entrained flow reactor. Appl. Therm. Eng. 2025, 262, 125111. [Google Scholar] [CrossRef]
- Lv, Y.; Lei, Y.; Hui, S.e.; Li, Y.; Niu, Y. Research on the formation of ash deposition during biomass/coal co-firing in oxy-fuel atmospheres. Fuel 2025, 386, 134267. [Google Scholar] [CrossRef]
- Wang, Q.; Han, K.; Wang, P.; Li, S.; Zhang, M. Influence of additive on ash and combustion characteristics during biomass combustion under O2/CO2 atmosphere. Energy 2020, 195, 116987. [Google Scholar] [CrossRef]
- Yao, Y.; Li, H.; Wang, J.; Diao, S.; Wei, C.; Yu, M. Combustion behaviors and soot formation pathways of methane/hydrogen mixtures under an external electric field: A ReaxFF-MD simulation study. Int. J. Hydrogen Energy 2025, 193, 152422. [Google Scholar] [CrossRef]
- Guo, S.; Qi, G.; Zhao, D.; Gao, L.; Qu, H.; Li, X.; Song, D. Study on the combustion characteristics of NH3 and hydrochar mixture using ReaxFF MD: Oxygen equivalence ratio, ammonia co-combustion ratio, and combustion environment. J. Environ. Manag. 2025, 391, 126635. [Google Scholar] [CrossRef]
- Yang, Y.; Kai, R.; Watanabe, H. Exploring reaction mechanism and kinetics of acetone pyrolysis and combustion in O2/H2O/CO2 environments via ReaxFF MD simulations. Energy 2025, 335, 137999. [Google Scholar] [CrossRef]
- Monge-Palacios, M.; Grajales-González, E.; Sarathy, S.M. Methanol oxy-combustion and supercritical water oxidation: A ReaxFF molecular dynamics study. Energy 2023, 283, 129104. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhong, W.; Shao, Y.; Yu, A. Reactive force field molecular dynamics (ReaxFF MD) simulation of coal oxy-fuel combustion. Powder Technol. 2020, 361, 337–348. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.