Abstract
Sewage sludge production is increasing rapidly, yet current sludge treatment capacity and technology remain insufficient. The thermochemical process has been widely adopted for sewage sludge disposal; its solid product (bio/hydro-char) shows considerable potential to improve soil quality by enriching soil nutrient contents. However, limited heavy metals are volatilized during the thermochemical process, and the majority is concentrated in the derived bio/hydro-char. Therefore, it is essential to ensure the environmental safety of sewage sludge-derived bio/hydro-char and avoid heavy metal risk, and thus appropriate heavy metal removal technology is required prior to land application. This review provides an overview of the major sewage sludge treatment approaches focusing on the heavy metal removal and phosphorus recovery, along with emerging challenges and future perspectives for the sustainable utilization of sewage sludge. Notably, the combination of electrokinetic treatment with thermochemical treatments emerges as a promising strategy to simultaneously treat sewage sludge and achieve P reclamation.
1. Introduction
Recently, the accelerated pace of global economic development and urbanization has triggered a substantial surge in municipal wastewater production. As the primary byproduct of wastewater treatment plants, sewage sludge has witnessed a corresponding and remarkable increase in its generation [1]. On a global scale, the annual output of dry sewage sludge has reached 75–100 million Mt, and is expected to grow at an annual rate of approximately 3.75% [2,3]. In China, the generation of dry sludge has exhibited an even more pronounced upward trend, rising from 6.25 million tons in 2011 to 15.05 million tons in 2023 [4]. This dramatic growth trajectory underscores the urgency of addressing sludge-related issues in China’s pursuit of sustainable urban development. However, a striking disparity exists between the soaring sludge production and the current state of sludge treatment. The existing sludge treatment capacities and technologies remain significantly inadequate to cope with the escalating demand.
The complexity of sewage sludge compositions leads to difficulty in sustainable sludge management. Sewage sludge is inherently a complex multiphase mixture with valuable nutrient elements (N, P, and K) that hold great potential for resource utilization [5], while simultaneously containing harmful substances, especially hazardous heavy metals that pose significant risks to ecological environments and human health [6]. The hazardous nature restricts the sustainable management and high-value utilization of sewage sludge [7]. Therefore, the exploration and development of appropriate sludge treatment technologies in terms of realizing its high value and safe utilization has emerged as an urgent and critical issue worldwide.
Heavy metals, due to their non-biodegradable characteristic, tend to be accumulated in the environment and food chain [8]. Zn, Pb, Ni, As, Cu, Hg, and Cd metals are considered priority control pollutants by the USEPA. Prolonged exposure to these heavy elements can have negative impacts on the human central nervous system [9]. Thus, heavy metal control of sewage sludge is crucial for public health and the environment. Many approaches have been developed to remove heavy metals from sewage sludge, such as bioleaching [10], chemical extraction [11], and membrane separation [12], etc. These cost-effective and benefit-focused processes may entail notable drawbacks and limitations, such as incomplete removal of heavy metals, high energy demands, and the generation of toxic sludge [13]. Makowska et al. found that pyrolysis significantly reduced the environmental risk of heavy metals, from high (PERI 1158) in the sewage sludge feedstock to low (PERI 50) in biochar obtained at temperatures above 600 °C [14]. Czerwińska et al. indicated that from 92 to almost 100% of heavy metals were transferred to the hydrochar, but the acidic environment (pH = 2) favored a higher amount of mobile heavy metals in hydrochar. [15]. Electrokinetic-based techniques achieve efficient de-metallization of sewage sludge by applying an electric field; the removal rate of Cu, Ni, and Zn can reach up to 90–96% [16]. Beyrami et al. introduced chelating agents during electrokinetic remediation and found it can facilitate the transformation of heavy metals into more exchangeable forms; however, without acidification, these agents may raise the pH, inhibiting metal solubilization [17]. To systematically summarize the mainstream heavy metal removal technologies and thereby promote the enhanced recovery of phosphorus (P) from sewage sludge, this review presents a detailed discussion of electrokinetic treatment processes alongside a comparative assessment with traditional treatment approaches.
The availability of P represents a critical and non-negotiable factor underpinning both human survival and global food security. Notably, over 60% of the P entering municipal wastewater treatment plants is retained and concentrated in sewage sludge, making sewage sludge an excellent resource for P recycling [18]. Sewage sludge typically contains P with concentrations ranging from 7.74 to 48.81 mg g−1 [19]. Its P content is the highest among common solid wastes [20]. Several techniques have been applied to reclaim P from sewage sludge, including direct land application, chemical precipitation, and thermal treatment [21]. Among them, thermochemical conversion (e.g., pyrolysis and hydrothermal carbonization) is considered as an effective process, as the P recovery efficiency approximately reached 90% after pyrolysis treatment [22]. Organic contaminants were decomposed during pyrolysis; meanwhile, P is concentrated in the solid product (biochar), resulting in a higher P content than in the feedstock [23]. Hydrochar has been proven to be an effective soil amendment, as it has the ability to retain nutrients (especially P) and pH buffering capacity [24]. Filho et al. noted that pyrolysis converted organic P into inorganic forms, and apatite P increased with the temperature [25]. Figueiredo et al. also showed that pyrolysis temperature differently affected the biochar P solubility; therefore, the solubility of P should be considered in the recommendations for biochars as an alternative phosphate fertilizer [26]. Phosphorus recovery in hydrochars was most sensitive to extremes of pH, and can increase wheat biomass with >5-fold higher P uptake than controls [27]. Although bio/hydro-char has good potential to improve soil quality by increasing the contents of soil nutrients [28], a limited number of heavy metals are evaporated during the thermochemical process [29], and heavy metals are largely enriched in bio/hydro-char [30]. For example, catalytic hydrothermal carbonization concentrated Cu (1151.4 mg kg−1) and Zn (1569.5 mg kg−1) in hydrochar [31], and pyrolysis concentrated heavy metals in biochar with Zn (497 mg/kg), Cr (157 mg/kg), and Ni (327 mg kg−1), respectively [32], but enhanced stability reduced their leachate levels. Therefore, to ensure the environmental safety of sewage sludge-derived bio/hydro-char and improve the utilization efficiency of P in bio/hydro-char, it is necessary to clarify the speciation mechanisms of P during pyrolysis and the hydrothermal carbonization treatment of sewage sludge.
Several reviews have been published focusing on the heavy metal removal or P conversion principles during the sewage sludge thermal treatment technologies, including pyrolysis [33], incineration [34], gasification [7], and hydrothermal carbonization [35]. They also emphasize the stabilization mechanisms of additives [36] and pay attention to the recovery of P from sewage sludge ash [37]. In addition, the influence of process parameters such as temperature and pH on the fraction transformation of heavy metals [24,38] and P during sludge heat treatment is also discussed [39,40,41,42]. Unlike previous reviews that separately discuss heavy metal removal or P recovery, this work centers on the co-speciation mechanism governing both P availability and heavy metal immobilization in sewage sludge treatment, filling the gap of disconnected analyses. Additionally, this review integrates the electrokinetic treatment with the thermochemical process, while existing reviews rarely cover electrokinetic treatment for sludge valorization. This review comprehensively analyzes electrokinetic treatment as a pre-treatment to enhance heavy metal separation, followed by pyrolysis/hydrothermal carbonization for P enrichment—providing a complete technical chain for safe and efficient sludge resource utilization. Therefore, the objectives of this review are to (1) clarify the pathways of sewage sludge treatment processes in the perspective of heavy metal removal and P recovery, and (2) discuss the emerging challenges and perspectives of sustainable utilization development of sewage sludge. This review is structured as follows: Section 2 critically assesses conventional and electrokinetic technologies for heavy metal removal; Section 3 delves into the production, characterization, and agricultural application of P-enriched bio/hydrochar; finally, Section 4 presents the challenges and future perspectives, with a focus on the promising integration of electrokinetic and thermochemical treatments.
2. Heavy Metal Removal Processes
2.1. Characteristics of Sewage Sludge
Sewage sludge is the main byproduct of wastewater treatment plants; it is a heterogeneous material composed of a complex mixture of different components [43].
As shown in Figure 1, the main composition of sewage sludge mainly depends on the source and wastewater treatment process [44]. Elemental and proximate composition determines the thermal chemical characteristics during the pyrolysis and hydrothermal process [45], which affects the heavy metal removal rate and the phosphorus recovery efficiency.
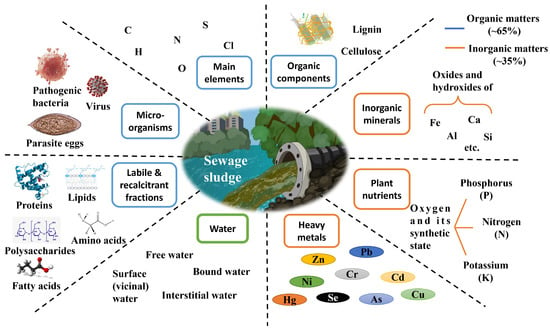
Figure 1.
The basic composition of sewage sludge.
The average heavy metal contents in the sewage sludge of China ranked the accumulation rate in the following order: Zn > Cu > Cr > Ni > Pb > As > Hg > Cd [35]. A summary of heavy metal concentration in the representative cities of China is listed in Table 1.

Table 1.
Heavy metal contents (mg kg−1) in representative cities of China.
Several techniques have been applied to remove heavy metals from sewage sludge. In the following section, the conventional technologies, including pyrolysis, hydrothermal carbonization, chemical extraction, bioleaching, membrane separation, electrokinetic treatment, and enhanced electrokinetic processes, are described. Table 2 highlights the metal removal performance of the above-mentioned techniques. Specifically, considering the practical requirements of subsequent P application, the pyrolysis, hydrothermal carbonization, and electrokinetic treatment have been emphasized as the core research focus.

Table 2.
Heavy metal removal performance (%) of selected processes [12,16,56,57].
2.2. Conventional Technologies
2.2.1. Pyrolysis
Sewage sludge pyrolysis technology refers to the thermal conversion process (300~900 °C) carried out in an inert atmosphere (N2, Ar, etc.). The solid byproduct, biochar, exhibits substantial potential in enhancing soil quality through augmenting levels of soil nutrients, soil microbial biomass, and soil pH.
However, a limited quantity of heavy metals undergoes evaporation during the pyrolysis process, while inorganic salts and heavy metals are predominantly converted into biochar. Heavy metals exhibit enrichment within the biochar matrix, with their concentrations potentially escalating at elevated pyrolysis temperatures and heating rates. Hossain et al. demonstrated that Pb, Ni, Cu, and Zn were enriched in the sewage sludge-derived biochars under 500 °C or less [58]. However, at 600 °C, Ca compounds were found to be volatilized, and complete volatilization of Hg occurred at 350 °C. Gong et al. observed that the heavy metal concentration of derived biochar was 2.5–6.0 times higher than that in sewage sludge [59]. But, compared to incineration ash and the hydrochar, the leachable heavy metal contents of the biochar are lower [60]. Zhu et al. pointed out that movable metal forms were reduced during the pyrolysis process, which facilitates the agricultural use of biochar [61]. Although heavy metal transformations have been extensively investigated, the variability in heavy metal contents within raw sewage sludge and differences in operating conditions pose challenges to reaching a unified conclusion. Consequently, direct application of sewage sludge-derived biochar as a P source in agriculture is discouraged due to these uncertainties.
2.2.2. Hydrothermal Carbonization
Hydrothermal carbonization treatment facilitates the utilization of wet wastes, particularly sewage sludge containing 70% to 80% moisture. It is recognized as a process that converts sewage sludge into carbonaceous products known as hydrochar, achieved by subjecting it to temperatures between 180 °C and 260 °C under autogenously saturated pressures within the subcritical region for several hours. The high hydrophobicity and homogeneous properties of hydrochar facilitate its separation from suspension [62]. Temperature, pressure, water medium, and residence time are the main characteristics affecting the hydrothermal carbonization process [63].
Hydrothermal carbonization is typically conducted at temperatures below 300 °C, resulting in the concentration of heavy metals, primarily in hydrochars [64]. Liu et al. reported that the levels of Ni, Cu, Zn, Cr, and Hg in hydrochar derived from sewage sludge exceeded the land application standards set by China, Japan, Canada, the US, and the EU [65]. During the hydrothermal carbonization process, a significant immobilization of heavy metals occurs in hydrochars, leading to a reduced environmental risk. Shi et al. reported that the leachable Cu, Cd, Cr, and Ni decreased by 72–97% after hydrothermal carbonization and decreased with the increase in the reaction temperature [66]. It should be noted that the substantial presence of metal ions in sewage sludge can impact dehydration, decomposition, and carbohydrate hydrolysis. Unsuccessful removal or reduction in heavy metal contents from sewage sludge limits the practical applications of hydrochars [67]. Therefore, researchers have added chemicals (HCl, FeCl3, Al(OH)3, etc.) to improve the heavy metal removal of hydrochars. Xu et al. illustrated that importing FeCl3 and Al(OH)3 during the hydrothermal carbonization process could transfer the exchangeable state of heavy metals into residual states, which decreased the leaching risk of hydrochars [68]. However, the removal of heavy metals was not ideal; alternative processes or electrokinetic treatment should be developed to optimize nutrient recovery and reduce contaminant accumulation.
2.2.3. Traditional Processes
Conventional heavy metal removal treatments have various advantages, but some concerns are hindering the application of these techniques [69].
The strengths and limitations of these technologies are summarized in Table 3. The chemical extraction process consumes large numbers of chemical treatments, which negatively impact its further utilization, and the membrane technology requires stringent application conditions [70]. The bioleaching technique offers various advantages, including low production of waste material, easy operation, regeneration capabilities of the bio–sorbents, and high pollutant removal efficiencies, which have attracted increasing interest among researchers. However, drawbacks including high temperature, pH dependence, high maintenance, and energy requirements are unavoidable [71]. Most processes are currently performed at the laboratory scale, with the exception of electrokinetic remediation, which has been applied at the pilot scale level. The electrokinetic technique has shown promise in removing heavy metals and organic pollutants from soil [72], making it an innovative technology for effluent treatment to date. In the following section, a detailed discussion is conducted focusing on the electrokinetic treatment.

Table 3.
Comparison of different heavy metal removal processes.
2.3. Electrokinetic Treatment
2.3.1. Electrokinetic Technology
The electrokinetic technology is a promising remediation process with high removal efficiency [57]. It utilizes low-level direct current to pass through sewage sludge, facilitating the migration of contaminants by electrophoresis, electromigration, and electro-osmosis [43].
As shown in Figure 2, during electrokinetic treatment, water oxidation takes place in the anode chamber, leading to the generation of hydrogen (H+) ions (Equation (1)), while water is reduced in the cathode chamber, resulting in hydroxyl (OH−) ion (Equation (2)) production. When an electric field (e.g., input voltage of 20–50 V) is applied across sewage sludge, H+ ions drift toward the cathode and interact with cationic metals on the surface, causing desorbed metal ions to move toward the cathode along with H+ ions [73]. Heavy metal removal can be achieved through precipitation, electrodeposition, or ion exchange during their migration process. The pH value of sewage sludge is affected by the migration rate and distribution of H+ and OH−, which is gradually decreased from the cathode to the anode [74]. Heavy metal removal efficiency of the electrokinetic technique is influenced by the pH value of sewage sludge, electrolyte concentration, voltage gradient, current density, and electrode material [75].
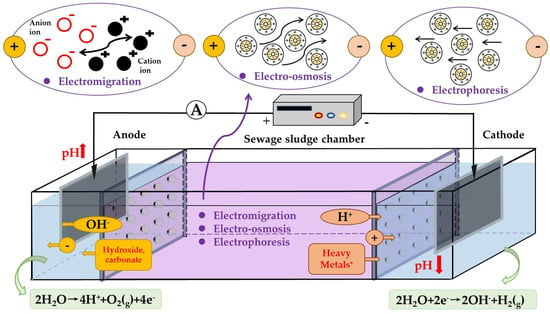
Figure 2.
Schematic diagram of the mass transport mechanism in the electrokinetic process.
The core mechanism of electrokinetic treatment can be illustrated in Figure 2, including electromigration, electro-osmosis, and electrophoresis. The electromigration mechanism refers to the transportation of ions and complexes in solution when negatively charged particles are placed in an electrical field. Each species moves according to its ionic mobility, determined by the ionic charge number. Electro-osmosis occurs at the solid/liquid interface, where the movement of ions in the liquid is induced by a stationary charged surface. Within a pore, electro-osmosis arises from the interaction between the bulk liquid and a thin layer of charged fluid near the pore wall that migrates under an electric field. Charged thin-layer fluids appeared because surface charges should be balanced by fluids with opposite charges to maintain charge neutrality [76]. Electrophoresis is the reciprocal phenomenon of electro-osmosis, where a charged particle undergoes relative motion with respect to a stationary liquid [77]. Upon application of an electric field on heavy metal ions, the charge on these particles interacts with the field and propels them towards an electrode carrying opposite charge [78].
The performance of the heavy metal removal rate and energy consumption is quantified by voltage gradient (1.0–1.5 V·cm−1), sludge pH, and heavy metal speciation. At 1.25 V·cm−1, Cu/Zn removal rate reaches 45–60% with an energy consumption of 0.8–1.2 kW·h·kg−1 [79]. Initial pH 4–6 of sludge maximizes metal mobility, while alkaline sludge (pH > 8) requires pre-acidification [80]. Exchangeable fractions (e.g., exchangeable Pb) achieve removal rates > 70%, while residual fractions (e.g., residual Cr) show removal rates < 20% [81].
Researchers applied various chemicals to reduce electrolyte pH variation, which confirmed the feasibility of electrokinetic treatment with considerable heavy metal removal rates (19–34%) [82,83]. The electrokinetic method exhibits minimal secondary pollution and holds promise due to its high efficiency in removing heavy metals within a short treatment duration. However, the electrokinetic remediation method is limited by its economic (high investment and electrode stability) and energy consumption [43]. The low concentration of resources in the sludge source restricts its efficacy. Therefore, enhanced electrokinetic treatment has attracted increasing interest by coupling it with other techniques (for example, chemical extraction) to improve heavy metal removal and reduce consumption.
2.3.2. Enhanced Electrokinetic Technology
The electrokinetic process exhibits minimal secondary pollution [84] and high efficiency [85]. However, the electrokinetic remediation process is limited by its energy consumption [86], which may restrict its industrial application [87]. Therefore, enhanced electrokinetic treatment has attracted increasing interest by coupling it with other techniques (for example, chemical extraction) to improve heavy metal removal and reduce consumption [88]. In this section, enhanced electrokinetic technology with processes of adding electrodes and additives is discussed.
- Effects of electrodes on electrokinetic technology
The selection of appropriate electrolytes (anolytes and catholytes) is crucial for the electrokinetic process, as the chemical properties of electrolytes can significantly impact metal speciation, pH values, electrical conductivity, Zeta potential, and electro-osmotic flow in sewage sludge. It has been reported that a lower pH level can enhance the removal efficiency of heavy metals by dissolving and transforming stable heavy metals into exchangeable forms [89]. However, the pH values are affected by hydrolysis during the electrokinetic treatment; thus, researchers have applied various chemicals as electrolytes to maintain the pH values [73]. Inorganic acid, organic acid, chelant, and alkali solutions are used for electrolytes.
Liu et al. developed electrokinetic equipment with two and three sewage sludge chambers and indicated optimal heavy metal removal efficiency with Cu (~51.5%) and Ni (~46.6%) with 0.2 mol·L−1 HNO3 as electrolytes [75]. Almeira O. et al. confirmed that the addition of HNO3 as electrolytes enhanced the Cd removal of Cd from kaolin by 63% [89]. Wu et al. applied citric acid as an electrolyte, which decreased the pH to lower than 1.2 [90]. They indicated Cr concentration in the contaminated soil decreased by 45.04–138.13 mg·kg−1 after the electrokinetic treatment. The electro-osmotic flow direction in sewage sludge is influenced by its low pH value, and the electromigration efficiency can be enhanced by employing an appropriate alkaline solution as an anolyte to adjust the pH value. Alkaline soluble salts, such as NaHCO3 and NaOH [91], are widely accepted for maintaining a favorable electro-osmotic flow direction in electrokinetic treatment due to their cost-effectiveness and environmentally friendly properties. Tang et al. reported the high removal efficiency of Cu, Zn, Cr, Pb, and Ni (~60.40%) by applying biodegradable ethylene diamine disuccinic acid (EDDS) as electrolytes to acidify sewage sludge and reduce pH values [92]. The introduction of electrolytes enhances the mobilization of heavy metal ions through electrokinetic treatment.
- Effects of additives on electrokinetic technology
The electrokinetic technique encounters challenges due to the adsorption of dissolved heavy metal ions on sewage sludge particles, resulting in the formation of precipitates with carbonates, hydroxides, and other compounds near the cathode (high pH region), thereby obstructing the electrokinetic process. Moreover, highly mobile heavy metals can be readily eliminated from sewage sludge through electrokinetic treatment. To enhance overall removal efficiency, chemical agents such as acids (citric acid, HNO3), chelating agents (ethylene diaminete traacetic acid (EDTA), nitrilotriacetic acid (NTA), and dicarboxymethyl glutamic acid (GLDA)), surfactants (such as saponin, sophorolipid, and rhamnolipid), and inorganic salts (NaCl, FeCl3) are frequently employed for solubilizing heavy metals [83].
Adding HNO3 in the sewage sludge chamber, and adjusting pH = 2.0, 68–95% of heavy metals can be removed by the electrokinetic treatment [93]. The utilization of citric acid as an additive exhibits superior efficacy in heavy metal removal compared to deionized water and sodium dodecyl sulfate [94]. Conventional chelating agents, including EDTA [95], EDDS, and NTA [96], could solubilize the heavy metals and improve the electro-osmotic flow rate, thereby confirming the viability of electrokinetic treatment with significant removal efficiencies (19–34%) for heavy metals [97].
A comparison of different conditions on electrokinetic treatment is listed in Table 4. Chemical addition has the potential to enhance heavy metal removal efficiency through chelation and pH regulation; however, conventional agents such as EDTA and EDDS are non-biodegradable, leading to secondary pollution and posing environmental risks [82]. Hence, selecting more environmentally friendly, efficient, and biodegradable chelating agents is of great importance. Recently, biosurfactants have been investigated due to their low toxicity, ecological safety, and exceptional biodegradability [98]. The high removal efficiencies of Zn (>70%), Cr, and Ni (>60%) were observed when adding rhamnolipid in the electrokinetic process [99]. Chen et al. added GLDA for pretreating flue gas desulfurization-derived sewage sludge in an electrokinetic treatment [97]. Their findings demonstrated a significant 22.48% increase in Cr removal efficiency compared to the unenhanced electrokinetic process. Sophorolipids and saponins as novel surfactants have also been reported to enhance the remediation efficiencies of the electrokinetic treatment [100].

Table 4.
Comparison of different conditions on electrokinetic treatment [43,93,101,102,103,104].
The heavy metal removal efficiency of the unenhanced electrokinetic process is limited, and the energy consumption restricts its further industrial application. In addition, the hydrolysis under the electric field affects the pH values in the sludge chamber, which may cause heavy metal accumulation during the electrokinetic process. Therefore, agent-assisted electrokinetic treatment holds great promise for sewage sludge disposal as it has the potential to overcome the aforementioned challenges. Citric acid has been utilized to enhance heavy metal removal efficiencies by pH adjustment, while ammonium hydroxide can induce a transformation of stable heavy metals into unstable states [105]. FeCl3 has been reported for its ability to increase ion concentration in the sewage sludge solution during heavy metal removal [106]. However, the underlying mechanism of biosurfactants in electrokinetic treatment remains unknown. Since different agents operate through distinct mechanisms, resulting in varying effects on heavy metal removal efficiency, further study is required to understand the effectiveness of agent-assisted electrokinetic treatment.
3. P-Enriched Bio/Hydrochar Derived from Sewage Sludge
3.1. Characterization and Characteristics of Sewage Sludge and the Derived Bio/Hydrochar
3.1.1. Methods for P Characterization
The speciation of P, defined by its chemical state and physical distribution, primarily controls its bioavailability, mobility, and solubility [107]. P exists in diverse molecular forms, including phosphonates, orthophosphates, polyphosphates, and organophosphates [108]. Several analytical methods are employed to determine P speciation, including 31P nuclear magnetic resonance (31P NMR) spectroscopy [109], sequential extraction [107], and P K-edge X-ray absorption spectroscopy (P K-edge XANES) [110].
Fristak et al. found the P content of biochar increased by 2–3 times after the pyrolysis treatment of sewage sludge [111]. Most P is present in inorganic forms, accounting for 60% to 90% of total P. Inorganic P species primarily occur as phosphate compounds bound to metal cations, such as Al, Ca, Mg, and Fe [112]. Among these, apatite inorganic phosphorus (AP) mainly contains Ca–P and Mg–P, which account for 35–40% of inorganic P [113]. While non-apatite inorganic phosphorus (NAIP) is the most prevalent form, P is bound with the oxides/hydroxides of Al, Fe, and Mn [42]. To examine changes in P speciation and their associated metals, researchers selected the standard measurement and testing (SMT) protocol and XANES spectroscopy [114].
Most organic P and inorganic P in sewage sludge is non-bioavailable, and sequential chemical extraction effectively differentiates major P pools. (1) Water-soluble P (e.g., H2PO4−, HPO42−) can be directly absorbed by plants with the highest bioavailability [115], but accounts for <5%. (2) NaHCO3-extractable P consists of weakly adsorbed P combined with Ca and Mg, and part of easily mineralizable P in microorganisms, which is highly bioavailable in neutral or weakly alkaline soil [116]. (3) NaOH-extractable P (Fe/Al oxides/hydroxides) exhibits moderate bioavailability. (4) HCl-extractable P (stable Ca/Mg-bound) [117] with high stability and can only be slowly dissolved in strongly acidic soils. NaOH and HCl-soluble P are the dominant fractions (~60–90% of the total P) [118] in sewage sludge-derived bio/hydro-char. (5) Residual P(accounts for <10%) is tightly bound to insoluble minerals, has extremely low bioavailability, and can be regarded as a long-term potential P pool [119].
Liquid 31P NMR is also employed to characterize P fractions extracted from feedstock and the derived bio/hydro-chars, which offers insights into relatively labile P fractions [117]. Extensive existing studies have demonstrated that the orthophosphate, which is associated with Al, Ca, and Fe metals and their minerals, is the main fraction in the sewage sludge [120].
The content of available P in the soil can be estimated by a variety of methods. Compared with standard chemical extraction tests, the diffusive gradients in thin films (DGT) technology has a higher correlation with plant-available P in the soil [115,121]. Vogel et al. used DGT technology to determine the available P content in fertilized soil in pot experiments, and the correlation between P content and the P absorption of corn was much higher than that of traditional extraction methods, such as Olsen [122]. Six et al. also found that the correlation (R2 = 0.84) between the soil-available P concentration measured by DGT technology and corn yield was significantly higher than that of traditional extracts such as Olsen, Colwell, Brray–1, Mehlich–3, ammonium oxalate, and resin (R2 < 0.53) [123]. At the same time, DGT, as an in situ sampling technology, can eliminate the risk of P form changes during sample transportation, storage, and analysis. At present, this technology has been widely used to analyze the distribution and migration law of available P in water bodies and soil [124]. Combining traditional P extraction methods with DGT technology can more accurately evaluate the availability of P in biochar.
3.1.2. P Speciation in Raw Sewage Sludge
Before exploring P transformation during biochar/hydrochars production, it is critical to understand the initial speciation of P in raw sewage sludge. Inorganic P typically accounts for 50–80% of total P in sewage sludge, and Ca-bound, Al-bound, and Fe-bound P species are the predominant forms [125]. These inorganic phosphate forms are relatively stable under normal conditions, although their bioavailability can be affected by the pH value of the environment [126].
Organic P in sewage sludge mainly derives from the residual organic matter in wastewater, such as phospholipids, nucleic acids, and humic acid-bound P, accounting for around 20–50% of total P [127]. The stability and bioavailability of organic P are closely related to its molecular structure [128]. For example, phospholipids and nucleic acids are relatively labile and can be decomposed into orthophosphate via microbial activity, whereas humic acid-bound P exhibits high stability and limited biological availability [129]. Shober et al. pointed out that the distribution of these forms of P varies significantly depending on the source of the raw wastewater and the sludge stabilization techniques employed [130].
3.2. Migration and Transformation of P During the Bio/Hydrochar Production Processes
3.2.1. Pyrolysis
During pyrolysis, P remains mostly in the solid product, biochar [131]. It drives P transformation via thermal decomposition, redox reactions, and mineral transitions across three stages: dehydration (<200 °C), devolatilization (200–500 °C), and carbonization (>500 °C) [120]. The key transformation mechanism could be summarized as (1) the decomposition of organic phosphorus, (2) the conversion of inorganic P fractions, and (3) the migration of P in the biochar matrix [132]. At below 300 °C, labile organic P (e.g., phospholipids) undergoes hydrolysis, releasing soluble inorganic P [133]. At above 300 °C, recalcitrant organic P (humic-bound) degrades progressively, resulting in a reduction in the organic P fraction from approximately 30% of total P in raw sewage sludge to less than 10% at 600 °C [134]. P volatilization is negligible (<5% of total P) due to its high boiling point, ensuring that the majority of P is retained in the biochar matrix [40].
As for the conversion of inorganic P fractions, at temperatures around 300–500 °C, labile inorganic P desorbs and reacts with Fe/Al oxides originating from flocculants to form stable Fe/Al–P compounds (NAIP) [135]. This transformation is facilitated by the enhanced crystallinity and adsorption capacity of metal oxides under thermal treatment. While at temperatures > 500 °C, elevated temperatures promote the dissolution of Fe/Al oxides, releasing ions that subsequently react with Ca2+ and PO43− to form apatite-like minerals (e.g., hydroxyapatite) [136]. XRD confirms that AP becomes the dominant P species (accounting for 40–60% of total P) at temperatures ≥ 600 °C [137]. Simultaneously, a portion of Fe/Al–P is converted into oxyhydroxide–bound P (e.g., FePO4·2H2O) through redox-driven reactions.
The migration of P in the biochar matrix is also affected by the reaction temperature [14]. At <400 °C, P accumulates on the surface of biochar as amorphous organic and inorganic byproducts [138]. At temperatures above 500 °C, P migrates into the pores and precipitates as crystalline AP and Fe/Al–P phases, thereby reducing its leaching potential [25]. SEM–EDS reveals the uniform distribution and embedding of P in high-temperature biochars (700–800 °C) [139], in contrast to the surface-concentrated localization observed in low-temperature biochars [140].
- Effects of process parameters
The migration and transformation behavior of P during the pyrolysis process is strongly influenced by temperature [141], residence time [14], heating rate [142], and the physicochemical properties of raw sewage sludge [143].
Among them, the reaction temperature is the most critical factor, and P exhibits a distinct pattern as the temperature increases [144]. At 300–400 °C, labile organic P decomposes into orthophosphate [145], as shown in Figure 3a which forms more stable Fe–P, Al–P, and Ca–P [146]; therefore, the NAIP is the main fraction [147]. During this phase, the decomposition of organic P is incomplete, leading to high bioavailability of P, but may elevate the leaching risk [148]. At 500–700 °C, complete organic matter decomposition drives amorphous Fe–P and Al–P into crystalline FePO4 and AlPO4, which exhibit greater stability [149]. Ca–P may react with SiO2, forming Ca3(PO4)2·CaSiO3, which is characterized as lower solubility [133]. The volatilization loss of P is typically less than 5% [150]. At temperatures above 800 °C, marked P volatilization due to the decomposition of stable phosphate minerals (e.g., Ca3(PO4)2) [40]. Researcher [151] indicates that, at 900 °C, the volatilization loss of P can reach 10–15%. Therefore, to minimize P loss and produce sludge-derived biochar with high P retention, the recommended pyrolysis temperature range for biochar production is typically maintained between 400 °C and 700 °C.
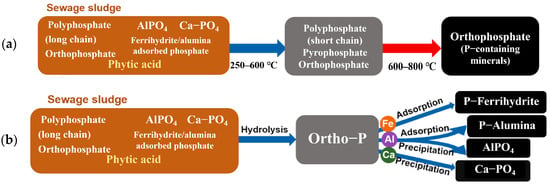
Figure 3.
The potential P transformation pathways during (a) pyrolysis and (b) hydrothermal carbonization of sewage sludge [14].
Extending residence time (from 30 to 90 min) enhances the complete decomposition of organic P (15–20% reduction) [152] and facilitates a more thorough transformation of amorphous inorganic P into crystalline forms (crystalline Fe–P and Al–P increase by 10–15%) [153]. Equilibrium is reached after 120 min [154].
The heating rate influences the pyrolysis process by modulating the decomposition kinetics of organic matter and the characteristics of biochar. A low heating rate (5–10 °C min−1) favors stable P species but increases energy consumption [155]. In contrast, a high heating rate (50–100 °C min−1) can accelerate the decomposition of organic matter, resulting in the rapid release of volatile substances [156]. Currently, a medium heating rate (20–30 °C min−1) is recommended for the preparation of biochars [8], as it achieves an optimal compromise between the decomposition of organic matter, efficient P transformation, and acceptable production efficiency.
3.2.2. Hydrothermal Carbonization
Firstly, aqueous conditions facilitate the hydrolysis of organic P through acid-base catalysis. Macromolecular organic P compounds (e.g., phytate) are decomposed into smaller organic P molecules (e.g., orthophosphate monoesters) and subsequently mineralized into inorganic P, as shown in Figure 3b. The mineralization rate of organic P during hydrothermal carbonization (50–80%) is lower than that in pyrolysis (>90%), primarily due to the lower reaction temperature, which restricts complete degradation [157].
Furthermore, the transformation of P during the hydrothermal carbonization can also be characterized by the solubilization and re-adsorption of inorganic P species. In the derived hydrochar, inorganic P is mainly present as Fe/Al-bound P (NaOH-extractable P) and loosely bound P (NaHCO3-extractable P). In the presence of Ca2+ ions, part of the dissolved P in the process water reprecipitates as Ca-bound P (HCl-extractable P) and is adsorbed on the surface of hydrochar [18]. In contrast to pyrolysis, the hydrothermal carbonization process does not lead to the formation of residual P, as the relatively low temperature is insufficient to incorporate P into the carbon matrix. The hydrochar is enriched with carboxyl (–COOH) and hydroxyl (–OH) functional groups, which facilitate the adsorption of soluble inorganic P through electrostatic attraction and cation complexation (e.g., Ca2+, Fe3+). The adsorption capacity increases with rising reaction temperature, attributable to an enhanced specific surface area and the greater availability of surface functional groups [158].
- Effects of process parameters
Shi et al. indicated that various factors including reaction temperature, duration, sludge-to-water ratio, the presence of metallic metals such as Ca, Mg, Al, and Fe in raw sewage sludge, and pH exert varying degrees of influence on the transformation of different P species [159]. Among these, reaction temperature plays a key role in governing both the hydrothermal carbonization process and the migration and transformation behavior of P [160]. At 180–220 °C, the organic matter in sewage sludge mainly undergoes hydrolysis and dehydration reactions, resulting in limited decomposition of organic P [161]. Inorganic P content increases slightly, with Fe–P, Al–P, and Ca–P remaining the dominant forms [162]. At 230–260 °C, organic P is mineralized into orthophosphate, which subsequently reacts with metal ions (e.g., Fe, Al, and Ca) to form new inorganic P compounds [163]. When the reaction temperature exceeds 270 °C, the organic matter is further carbonized to form hydrochars with elevated carbon content. But certain inorganic P species may react with other sludge components to form more stable phosphate minerals [164]. In addition, a minor amount of P may dissolve into the aqueous phase, resulting in a slight reduction in the total P content of the derived hydrochars. Therefore, the optimal temperature range is generally considered to be 230–260 °C.
At a specific temperature, prolonging the residence time promotes the decomposition of organic P and facilitates the thorough conversion of orthophosphate into inorganic P [127]. When the residence time exceeds 120 min, the transformation process tends to stabilize, and metal ions approach equilibrium. Extended residence time can also promote the crystallization of inorganic P phases, increased energy consumption, and higher production costs [165]. Hence, the hydrothermal residence time is usually controlled between 60 and 120 min.
3.2.3. Comparison of P Migration and Transformation Between Pyrolysis and Hydrothermal Carbonization
The hydrothermal carbonization process is conducted in a liquid medium, driving hydrolysis, and solvation rather than thermal decomposition. In addition, lower temperature and higher pressure compared to pyrolysis treatment lead to distinct P behavior [120]. The main difference is summarized in Table 5.

Table 5.
Difference in P migration and transformation between pyrolysis and hydrothermal carbonization [166].
3.3. Applications of Sewage Sludge-Derived Bio/Hydrochar as P Fertilizer in Agricultural Soils
Bio/hydro-char is applied in many research fields, including soil improvement, carbon sequestration, pollutant removal, and energy production. Bio/hydro-char can increase the overall surface area of soils and improve water retention capacity [167]. The use of bio/hydro-char in land application also contributes to the mitigation of global warming through carbon sequestration [168]. Among them, sewage sludge-derived bio/hydro-chars can serve as a soil amendment [169] due to their enhanced nutrient, low density, and porous structure [170]. Particularly, bio/hydro-chars, by retaining all or part of the mineral elements of the original feedstock (N, P, K, and S), constitute a stable source for the mineral nutrition of plants in the long term [150]. Numerous studies have reported that sewage sludge-derived bio/hydro-char exhibits substantial potential in enhancing soil quality through augmenting levels of soil nutrients, soil microbial biomass, and soil pH [171]. The advantages, mechanisms, influencing factors, and existing challenges of applying sewage sludge-derived bio/hydrochar P Fertilizer in agricultural soils are summarized in this section.
3.3.1. Advantages for Serving as Agricultural P Fertilizer
The risk of heavy metal contamination by sewage sludge was efficiently reduced through the pyrolysis and hydrothermal carbonization process. The leaching potential evaluated by the toxicity characteristic leaching procedure (TCLP) was all under the threshold and decreased with the hydrothermal carbonization temperature [172]. Thus, compared with sewage sludge, the derived bio/hydro-char has lower heavy metal toxicity, which is more beneficial to land applications.
Table 6 presents the total P amount and TCLP-derived leaching rates of heavy metals in sewage sludge and the derived bio/hydrochars. However, considering that the bioaccumulation of heavy metals is a significant concern during the practical application of bio/hydrochar, it is essential to establish control standards for heavy metals in bio/hydrochar. Additionally, toxicity analysis of specific bio/hydrochars is recommended before large-scale practical application.

Table 6.
The total P amount and TCLP-derived leaching rates of heavy metals in sewage sludge and the derived bio/hydrochars [52,173,174].
The total P content of sewage sludge-derived bio/hydro-chars ranges from 28.6 to 106.0 mg·g−1 [19], significantly higher than that of biochars derived from common biomass (e.g., wood and crop residues), which only have a total P content of 0.1–16.6 mg·g−1 [175]. As discussed in Section 3.2, 21–26% of water-soluble P in sewage sludge is partially converted into moderately labile P during the pyrolysis process [65]. This conversion not only avoids the P loss risk associated with direct sludge application but also ensures continuous P supply for crops, balancing ‘immediate availability’ and ‘long-term sustainability’. Brar et al. reported that biochars significantly reduce nutrient leaching in soils [176]. Wang et al. showed that bio/hydro-char derived from sewage sludge exhibited a slower release rate of P compared to animal manure, thereby reducing the risk of P loss [177]. Compared to sewage sludge-derived biochars, the moderately labile P (e.g., citric acid-soluble P) in hydrochars accounts for 30–55% of total P, which is higher than that (20–40%) in biochars [178]. Thus, avoiding P leaching risks from excessive water-soluble P better meets short-term crop P demands. Therefore, sewage sludge-derived bio/hydro-char shows great potential for improving soil quality through nutrient retention and its capacity to store carbon in the soil for long periods.
A large number of field experiments and pot experiments have shown that applying biochar to soil can improve the growth rate and yield of crops (such as kidney beans, cucumbers, strawberries, tomatoes, corn, watermelons, and black peppers) [179]. Zhang et al. conducted a six-year experiment on biochar soil improvement in paddy fields and found that, compared with traditional chemical fertilizers, the application of biochar increased crop yields by 10–16% [180]. Organic acid generation during the hydrothermal carbonization process renders the derived hydrochars weakly acidic (pH 4.5–6.0), in contrast to the alkaline nature of sewage sludge-derived biochars (pH 8.0–10.0) due to high-temperature decarboxylation [181]. This trait enables the derived hydrochars to activate Fe/Al-bound P in alkaline soils and mitigate Ca–P fixation, addressing the limitation of low P availability when the derived biochars are applied in alkaline soils [182]. In addition, the mild hydrothermal carbonization conditions (low temperature, moist environment) better preserve mineral elements (N and K) in sludge. Thus, the total N content of the derived hydrochar reaches 1.5–3.0%, higher than that (1.0–2.0%) in the derived biochars, enabling the synergistic supply of ‘P–N–K’ and enabling soil nutrient imbalance.
- Heavy metal mobility and plant uptake
The long-term application of bio/hydrochar (10–40 t/hm2) reduced the exchangeable fraction of Cd, Pb, and As in contaminated soils by 32–67% (average: 48%) after 5 years, with the reduction efficiency positively correlated with the char’s specific surface area and functional group content [183]. For example, a 10-year trial in Cd-contaminated paddy soil showed that wheat straw biochar application (20 t/hm2) decreased exchangeable Cd by 59% [184], while converting it to Fe/Al oxide-bound and residual fractions (accounting for 62% of total Cd after 8 years) [185].
For food crops (rice, wheat, maize), long-term bio/hydrochar application reduced grain Cd/Pb accumulation by 21–53% (average: 34%) [186], with the effect more pronounced in acidic soils (pH < 6.0) than alkaline soils [187]. Leafy vegetables (e.g., spinach, lettuce) showed a higher reduction in heavy metal uptake (37–61%) due to their stronger reliance on soil solution nutrients [188]. Notably, the uptake reduction efficiency tended to stabilize after 3–5 years, as the dynamic balance between char aging (functional group loss) and soil remediation (microbial community optimization) was established [189].
3.3.2. Mechanisms of Soil Quality Enhancement
- Biochar
Sewage sludge-derived biochars exert their P fertilizer effect in soils through a dual pathway combining direct P supply and indirect efficiency enhancement, and there are three potential impact mechanisms [190]: (1) Bochar, as a phosphorus source, can provide soluble and exchangeable P to the soil. The P contained in the derived biochars (including moderately labile P retained after pyrolysis) is slowly released into plant-available P under the action of soil moisture and microorganisms. Its release rate is slower than that of animal manure, which reduces P leaching loss [33]. (2) Biochar enhances the availability of soil-endogenous P by affecting phosphorus-related complexation and metabolic reactions. Biochars weaken the fixation of P by Fe/Al–(hydr)oxides by altering soil pH (especially in acidic soils) and releasing dissolved organic matter [191]. Simultaneously, it adsorbs metal cations (e.g., Fe3+, Al3+) bound to P in soils, releasing fixed native P and improving the overall P availability of soils [192]. (3) Biochar directly or indirectly affects P leaching by adsorbing P, improving the soil’s retention of P, and promoting the assimilation of P by plants. Biochar promotes the reproduction of P-solubilizing microorganisms (e.g., Pseudomonas putida, Lysinibacillus) and increases soil phosphatase activity [193]. This accelerates the conversion of organic P to available P in soils, establishing a positive loop characterized by ‘P supply promotion of conversion’.
- Hydrochar
Similarly to biochar (Figure 4), the mechanism of the sewage sludge-derived hydrochars on soil quality enhancement also contains three main pathways: (1) the direct release of endogenous P, (2) soil microenvironment regulation for P transformation, and (3) reduction in P leaching loss. P in the derived hydrochars is predominantly weakly bound and released under the influence of soil moisture and microbial activity. This results in a 15–30% faster release rate than that of the derived biochars, with a sustained release period of 6–8 months, well matching the P requirements of most crop growth cycles [194]. The acidity of hydrochars reduces the pH of alkaline soils by 1–1.5 units, weakening Ca-mediated P fixation. The porous structure of hydrochars, characterized by a specific surface area of 20–80 m2 g−1, enriches P-solubilizing microorganisms, which increases soil phosphatase activity by 25–40% and accelerates the transformation of organic P to available forms. Oxygen-containing functional groups (–COOH, –OH) on the surface of hydrochars electrostatically adsorb PO43− in soil, reducing P leaching by 30–50% [195]. The adsorption capacity of hydrochars is approximately 20% higher than that of biochars [39].
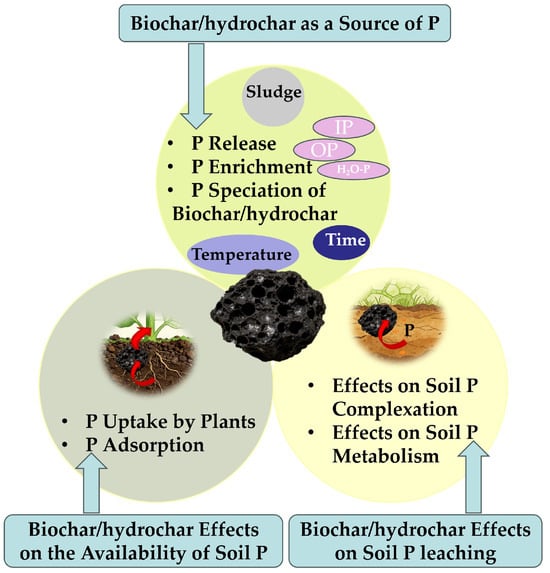
Figure 4.
Potential effects of bio/hydrochar on P behavior in soil.
The main difference in the agricultural application of the biochars and hydrochars is listed in Table 7. The P supply ability of biochar to soil depended not only on the content and form of P in biochar, but also on the interaction between biochar and soil, which was affected by soil properties such as pH, cation concentration, P activity, and microbial community [190]. Xu et al. [196] applied DGT technology to evaluate the agricultural application potential of the biochar by measuring the changes in available P concentration in soil after applying it to the soil. The DGT–P concentration of soils with the additions of sewage sludge and the derived biochars (326.90–1314.63 μg L−1) was much higher than that of the control soil (142.18 μg L−1) [173], demonstrating that adding sludge and the derived biochars could enhance the available P content in the soil. The available P can promote the growth and development of plants, and thus increase their yield [197]. Compared with the control soil (103.19 µg L−1), the DGT–P concentration of the mixed samples was significantly improved (970.8 µg L−1), suggesting that the addition of sewage sludge-derived hydrochars would raise the available P content in soil [174]. Li et al. reported that the application of chemical fertilizers increased the soil-available P concentration by 2.1–4.8 times, implying that the application of bio/hydro-chars could reduce the reliance on chemical fertilizers by improving soil fertility [198].

Table 7.
Agricultural application effects of biochars and hydrochars [199,200].
3.3.3. Key Influencing Factors
- Reaction conditions
The pyrolysis and hydrothermal carbonization temperature determined the speciation and availability of P. At low/medium pyrolysis temperature (~300 °C), the derived biochar exhibits a higher proportion of available P. Similarly, under hydrothermal carbonization conditions at 180–220 °C, moderately labile P accounts for 50–55% of total P, corresponding to the highest short-term P release rate [201]. This temperature range also sustains high crop yield during the residual application phase, thereby representing the optimal condition for P availability and agronomic performance [25]. At high pyrolysis temperature (>500 °C), P is predominantly converted into stable forms (e.g., hydroxyapatite) [202], which is suitable for soils requiring long-term fertility enhancement. Meanwhile, when the hydrothermal carbonization temperature exceeds 230 °C, partial moderately labile P is transformed to stable P species (e.g., AP), reducing the immediate P supply capacity [203].
Retention time is another key influence parameter. A retention time of 2–4 h is optimal for hydrothermal carbonization preparation; shorter reaction time leads to incomplete carbonization and unstable P speciation, while longer time increases energy consumption without significant improvement in P retention rate [197]. In addition, adding calcium and organic additives can also affect the speciation of P in the hydrothermal carbonization reaction. Specifically, adding calcium salts facilitates the formation of moderately labile Ca-bound P, thereby stabilizing P supply [204]; in contrast, organic acids enhance the dissolution of Fe/Al-bound P in sewage sludge, which in turn increases the proportion of available P in the derived hydrochars [205].
- Soil Physicochemical Properties
As the most critical factor, soil pH determines the adaptability of the derived biochars and hydrochars in agricultural soils. In acidic soils (pH < 6.5), the alkalinity of biochars neutralizes the acidity of soil, reducing the fixation of Fe/Al-bound P. Notably, this effect enhances soil-available P by 5.1-fold compared to the control soil, representing the optimal adaptability of the biochars [206]. Conversely, the weak acidity of hydrochars may exacerbate soil acidification, requiring combined application with lime (1–2 t ha−1) to adjust pH levels [207]. In neutral soils (6.5 < pH < 7.5), applying biochars increases soil-available P by 2.4-fold [208], though this improvement is less pronounced than that observed in acidic soils. For hydrochars, their application in neutral soils promotes the activity of soil microorganisms, thereby improving P use efficiency by 20–30% [209]. In alkaline soils (pH > 7.5), the alkalinity of biochars intensifies the fixation of Ca-bound P. To mobilize Fe/Al-bound P, acidic soil amendments or sewage sludge-derived hydrochar should be applied in combination. The inherent acidity of hydrochars helps neutralize soil alkalinity, reducing Ca–P fixation and increasing P availability by 40–60% [210]. Regarding soil texture, in sandy soils, the porous structure of hydrochars enhances water and nutrient retention, reducing P leaching losses by 35–50% [211]. In clay soils, the derived hydrochars improve soil aggregation, enhance soil aeration, and accelerate P diffusion to crop roots, therefore increasing P uptake rates by 15–25% [212].
- Application Strategies
The application rate of sewage sludge-derived biochars and hydrochars should be adjusted according to the total P content of soil and crop nutrient demand. The excessive application of biochars or hydrochars may easily cause P leaching, while insufficient application fails to satisfy the P requirements of crops. In practical agricultural scenarios, biochars or hydrochars can be combined with a small proportion of chemical P fertilizer. This combination not only ensures rapid P supply but also reduces the chemical P loss through the retention capacity of biochars and hydrochars, thereby improving the overall P utilization efficiency [213]. In addition, the application rate also varies by the biochar or hydrochar type and soil pH. The biochars are primarily applied in acidic (pH < 6.5) or neutral (6.5 < pH < 7.5) soils. In acidic soils with low available P content (<5 mg kg−1), the optimal application rate of biochar is 15–25 t·ha−1, which increases soil-available P by 3.2–4.5-fold and avoids excessive alkalinity-induced nutrient imbalance [214]. Exceeding 30 t ha−1 may cause P leaching and soil pH over-elevation, while rates below 10 t ha−1 fail to meet crop P requirements [215]. In contrast, the hydrochars are mostly used in alkaline (pH > 7.5) or neutral soils. In alkaline soils with low P content (<10 mg kg−1), the optimal application rate of hydrochar is 20–30 t ha−1, enhancing soil-available P by 7.4–8.5-fold and crop yield by 15–18% [216]. Rates > 40 t ha−1 may pose nitrogen accumulation risk and elevate heavy metal bioavailability, whereas rates < 10 t ha−1 may be insufficient for crop P supply [217]. Moreover, both biochar and hydrochar exhibit improved performance when combined with other amendments, including chemical P fertilizer, livestock manure, potassium, P-solubilizing microbial agent, or mineral, though the optimal combinations differ by material. Notably, when applying bio/hydrochars, the P2O5 content of the bio/hydrochars should be carefully tested to ensure it remains within permissible limits, which vary depending on soil and plant type. Otherwise, it may lead to nutrient imbalance, reduced uptake of micronutrients such as zinc and iron, and inhibited plant root development.
Exploring the short-term disturbance and long-term recovery trajectory of plant communities is crucial to improving the comprehensiveness of sludge-derived bio/hydrochar’s long-term sustainability assessment [218]. The potential influence of sewage sludge-derived bio/hydro-char application on human health and the soil environment is unclear [219]. Some studies found that sewage sludge-derived bio/hydro-char inhibits plant growth and changes soil bacteria after applying it to soil due to the content of potentially toxic elements [220]. Hence, necessary procedures should be conducted to ensure the environmental friendliness of sewage sludge-derived bio/hydro-char, especially to reduce their heavy metal toxicity.
4. Perspectives and Challenges
Coupling electrokinetic with thermochemical treatments may be optimal to mitigate the eco-toxic risk of sewage sludge while maximizing P recovery. Although our preliminary research has verified the effectiveness of this coupling approach in immobilizing heavy metals and reducing bio/hydrochar toxicity, critical knowledge gaps remain that require targeted investigation [52,173,174]. To address these gaps, corresponding research is suggested to be carried out with the following aspects:
- The potential impact of electrokinetic treatment on the separation and transformation of heavy metals in sludge and the derived bio/hydro-chars remains unexplored. Adopting the BCR (Community Bureau of Reference) sequential extraction for the whole coupling treatment is suggested to enhance the rigor of heavy metal speciation characterization.
- It will be highly beneficial to explore the synergistic effect between electrokinetic treatment and thermochemical conversion treatment on P reclamation and heavy metal removal. This can be achieved by integrating batch experiments and advanced spectroscopic analyses, combined with big data modeling calculations.
- Whether the soil properties, including water holding capacity, pH value, P bioavailability, and heavy metal stability, would alter after applying these bio/hydrochars in soil, long-term (2–10 years) field experiments can be further investigated.
- Other combined treatment processes are also a significant research prospect, for example, electrokinetic-coupled catalyst pyrolysis [221], electro-Fenton treatment [222], and joint SVM–GIS pollution mapping linking mineral exploration [223], sludge remediation, and soil amendment.
5. Conclusions
Sewage sludge, a nutrient-rich yet heavy metal-contaminated wastewater byproduct, demands sustainable treatment balancing resource recovery and environmental safety. Conventional methods like pyrolysis and hydrothermal carbonization effectively concentrate P in bio/hydrochar but accumulate heavy metals, limiting direct application. Electrokinetic treatment, especially when enhanced with electrolytes or additives, achieves efficient heavy metal removal (up to 96% for certain metals) and emerges as a complementary solution. P speciation transformation during thermochemical processes is critical for bioavailability, with optimal conditions identified as 400–700 °C for pyrolysis and 230–260 °C for hydrothermal carbonization. Sewage sludge-derived bio/hydrochar exhibits significant agricultural potential as a P fertilizer, improving soil quality and crop yields. The integration of electrokinetic and thermochemical treatments stands out as a promising strategy to mitigate eco-toxic risks and enhance resource recovery. Future research is suggested to address knowledge gaps in their synergistic mechanisms, investigating the long-term soil impacts of bio/hydrochar application, and exploring the effects of electrokinetic treatment on heavy metal transformation. This holistic approach will advance the sustainable utilization of sewage sludge, balancing environmental safety and resource reclamation.
Author Contributions
X.W.: Conceptualization, writing—original draft preparation, and writing—review and editing; H.L.: Supervision and investigation; J.W.: Methodology and data curation; F.Y.: Visualization and formal analysis; G.C.: Project administration and writing—review and editing; B.Y.: Supervision and validation; G.D.: Visualization and investigation; X.C.: Writing—review and editing, writing—original draft preparation, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2025YFE0109000).
Data Availability Statement
No datasets were generated or analyzed during the current study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SS | Sewage sludge |
| P | Phosphorus |
| EDDS | Ethylene diamine disuccinic acid |
| EDTA | Ethylene diamine tetraacetic acid |
| NTA | Nitrilotriacetic acid |
| GLDA | Dicarboxymethyl glutamic acid |
| PASP | Polyaspartic acid |
| Na2EDTA | Ethylenedinitrilotetraacetic acid disodium salt dihydrate |
| 31P NMR | 31P nuclear magnetic resonance |
| P K-edge XANES | P K-edge X-ray absorption spectroscopy |
| AP | Apatite inorganic phosphorus |
| NAIP | Non-apatite inorganic phosphorus |
| SMT | Standard measurement and testing |
| DGT | Diffusive gradients in thin films |
| TCLP | Toxicity characteristic leaching procedure |
References
- Katsoulea, A.; Zafeirakou, A. Sewage sludge pyrolysis and gasification for biochar production in circular economy. Sustain. Futur. 2025, 10, 101067. [Google Scholar] [CrossRef]
- Moussa, O.; Makkawi, Y.; Masek, O.; Mohamed, B. Pyrolysis of anaerobically digested and undigested sewage sludge: Comparative assessment of product quality, emissions, and carbon sequestration. Energy Convers. Manag. X 2025, 27, 101213. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Liu, H.; Mou, J.; Khan, S.J.; Lin, C.S.K.; Wang, Q. Evaluating energy balance and environmental footprint of sludge management in BRICS countries. Water Res. X 2024, 25, 100255. [Google Scholar] [CrossRef] [PubMed]
- China, Ministry of Housing and Urban-Rural Development. Constrution Statistical Yearbook of 2023. Available online: https://www.mohurd.gov.cn/cms_files/filemanager/1150240553/attach/202411/DFCB889EC2BF48242085D2C9460A147F.xls?fileName=2023%E5%B9%B4%E5%9F%8E%E5%B8%82%E5%BB%BA%E8%AE%BE%E7%BB%9F%E8%AE%A1%E5%B9%B4%E9%89%B4 (accessed on 1 November 2025).
- Li, H.; Gao, Y.; Chen, H.; Zhou, H.; Qin, Y.; Niu, S. Acidic Fe(III)-Driven hydrothermal co-transformation of phosphorus and arsenic in sewage sludge: Vivianite crystallization coupled with amorphous ferric arsenate formation for phosphorus-enriched hydrochar. Sep. Purif. Technol. 2025, 373, 133617. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, L.; Li, P.; Fang, W.; Zhang, P. Mechanisms of heavy metal migration and removal in sewage sludge by Fe2+/Ca(ClO)2/tannic acid conditioning and risk assessment. J. Water Process Eng. 2025, 77, 108584. [Google Scholar] [CrossRef]
- Brandstätter, G.; Fürsatz, K.; Long, A.; Hannl, T.K.; Schubert, T. Exploring the potential of sewage sludge for gasification and resource recovery: A review. Environ. Technol. Innov. 2025, 40, 104346. [Google Scholar] [CrossRef]
- Subbiah, G.; Singh, R.P.; Deepak, K.; Nayak, P.P.; venkadeshwaran, K.; Tiwari, A.; Sahoo, J.; Priya, K.K. Continuous pyrolysis of rice husk for sustainable biochar production and carbon sequestration: Recent advances and techno-economic perspectives. Results Eng. 2025, 27, 106991. [Google Scholar] [CrossRef]
- Khanam, R.; Kumar, A.; Nayak, A.K.; Shahid, M.; Tripathi, R.; Vijayakumar, S.; Bhaduri, D.; Kumar, U.; Mohanty, S.; Panneerselvam, P.; et al. Metal(loid)s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health. Sci. Total Environ. 2019, 699, 134330. [Google Scholar] [CrossRef]
- Pooja. Concurrent bioleaching of the selected metals and nutrients from dewatered sewage sludge: Addressing post-acidification challenges using nature-based material. J. Environ. Chem. Eng. 2025, 13, 116800. [Google Scholar] [CrossRef]
- Nee Kew, S.Y.; Lau, S.Y. Review of electrochemical reactors for the efficient removal of heavy metals from wastewater. J. Ind. Eng. Chem. 2025, 152, 18–32. [Google Scholar] [CrossRef]
- Azmi, L.S.; Jabit, N.A.; Ismail, S.; Ku Ishak, K.E.H.; Abdullah, T.K. Membrane filtration technologies for sustainable industrial wastewater treatment: A review of heavy metal removal. Desalin. Water Treat. 2025, 323, 101321. [Google Scholar] [CrossRef]
- Geng, H.; Xu, Y.; Zheng, L.; Gong, H.; Dai, L.; Dai, X. An overview of removing heavy metals from sewage sludge: Achievements and perspectives. Environ. Pollut. 2020, 266, 115375. [Google Scholar] [CrossRef] [PubMed]
- Makowska, D.; Pandiyan, M.; Kapusta, K.; Kolarz, K.; Stypka, Z.; Boehman, A.L.; Wooldridge, M.S. Effects of pyrolysis parameters on biochar derived from sewage sludge including environmental risk assessment of heavy metals. J. Environ. Manag. 2025, 395, 127888. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska, K.; Wierońska-Wiśniewska, F.; Bytnar, K.; Mikusińska, J.; Śliz, M.; Wilk, M. The effect of an acidic environment during the hydrothermal carbonization of sewage sludge on solid and liquid products: The fate of heavy metals, phosphorus and other compounds. J. Environ. Manag. 2024, 365, 121637. [Google Scholar] [CrossRef]
- Molaey, R.; Appels, L.; Yesil, H.; Tugtas, A.E.; Calli, B. Sustainable heavy metal removal from sewage sludge: A review of bioleaching and other emerging technologies. Sci. Total Environ. 2024, 955, 177020. [Google Scholar] [CrossRef]
- Beyrami, H. Effect of different treatments on electrokinetic remediation of Zn, Pb and Cd from a contaminated calcareous soil. Chin. J. Chem. Eng. 2021, 38, 255–265. [Google Scholar] [CrossRef]
- Huang, R.; Tang, Y. Speciation Dynamics of Phosphorus during (Hydro)Thermal Treatments of Sewage Sludge. Environ. Sci. Technol. 2015, 49, 14466–14474. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Yu, F.; Wang, X.; Xiao, H.; Yan, B.; Cui, X. A review on the production of P-enriched hydro/bio-char from solid waste: Transformation of P and applications of hydro/bio-char. Chemosphere 2022, 301, 134646. [Google Scholar] [CrossRef]
- Cui, X.; Li, X.; Wang, J.; Wang, X.; Yu, F.; Yang, G.; Xu, S.; Cheng, Z.; Yang, Q.; Yan, B.; et al. A review on the production of nutrient-enriched biochar: Insights from the evolution of nitrogen, phosphorus, and potassium. Crit. Rev. Environ. Sci. Technol. 2025, 55, 885–903. [Google Scholar] [CrossRef]
- Zhang, S.; Du, Q.; Cheng, K.; Antonietti, M.; Yang, F. Efficient phosphorus recycling and heavy metal removal from wastewater sludge by a novel hydrothermal humification-technique. Chem. Eng. J. 2020, 394, 124832. [Google Scholar] [CrossRef]
- Qian, T.; Wang, L.; Le, C.; Zhou, Y. Low-temperature-steam activation of phosphorus in biochar derived from enhanced biological phosphorus removal (EBPR) sludge. Water Res. 2019, 161, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, S.; Chen, M.; Liu, J.; Ding, X. A sustainable option: Biochar addition can improve soil phosphorus retention and rice yield in a saline–alkaline soil. Environ. Technol. Innov. 2021, 24, 102070. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, H.; Wu, C.; Sun, W.; Sun, X.; He, C. Phosphorus recovery from sewage sludge-derived hydrochar: Balancing phosphorus recovery, heavy metal concomitant leaching and residual hydrochar utilization. J. Clean. Prod. 2025, 491, 144756. [Google Scholar] [CrossRef]
- Lustosa Filho, J.F.; Viana, R.d.S.R.; Melo, L.C.A.; de Figueiredo, C.C. Changes in phosphorus due to pyrolysis and in the soil-plant system amended with sewage sludge biochar compared to conventional P fertilizers: A global meta-analysis. Chemosphere 2025, 371, 144055. [Google Scholar] [CrossRef]
- Figueiredo, C.C.d.; Reis, A.d.S.P.J.; Araujo, A.S.d.; Blum, L.E.B.; Shah, K.; Paz-Ferreiro, J. Assessing the potential of sewage sludge-derived biochar as a novel phosphorus fertilizer: Influence of extractant solutions and pyrolysis temperatures. Waste Manag. 2021, 124, 144–153. [Google Scholar] [CrossRef]
- McIntosh, S.; Padilla, R.V.; Rose, T.; Rose, A.L.; Boukaka, E.; Erler, D. Crop fertilisation potential of phosphorus in hydrochars produced from sewage sludge. Sci. Total Environ. 2022, 817, 153023. [Google Scholar] [CrossRef]
- Hedayati Marzbali, M.; Hakeem, I.G.; Ngo, T.; Balu, R.; Jena, M.K.; Vuppaladadiyam, A.; Sharma, A.; Choudhury, N.R.; Batstone, D.J.; Shah, K. A critical review on emerging industrial applications of chars from thermal treatment of biosolids. J. Environ. Manag. 2024, 369, 122341. [Google Scholar] [CrossRef]
- Murtaza, G.; Rizwan, M.; Zeeshan, A.; Usman, M.; Khan, I.; Kashif Irshad, M. 17-Effective heavy metals removal from wastewater: Exploring the efficacy of various biochar-based adsorbents. In Composites and Biocomposites for Heavy Metal Adsorption; Birniwa, A.H., Jagaba, A.H., Noor, A., Mohammad, R.E.A., Lawal, I.M., Eds.; Woodhead Publishing: Delhi, India, 2026; pp. 313–334. [Google Scholar]
- Paudel, P.P.; Kafle, S.; Park, S.; Kim, S.J.; Cho, L.; Kim, D.H. Advancements in sustainable thermochemical conversion of agricultural crop residues: A systematic review of technical progress, applications, perspectives, and challenges. Renew. Sustain. Energy Rev. 2024, 202, 114723. [Google Scholar] [CrossRef]
- Nahar, K.; Hakeem, I.G.; Chiang, K.; Ball, A.S.; Shah, K. Catalytic and co-hydrothermal treatment of sewage sludge: Hydrochar properties and fate of heavy metals and per and polyfluoroalkyl substances (PFAS). J. Water Process Eng. 2025, 72, 107605. [Google Scholar] [CrossRef]
- Embaye, T.M.; Zhou, A.; Li, R.; Ahmed, M.B.; Ruan, R.; Wu, D.; Deng, N.; Wang, X. Assessment of heavy metals distribution and environmental risks in biochar from co-pyrolysis of sewage sludge and mixed municipal waste. Process Saf. Environ. Prot. 2025, 193, 1332–1342. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Tariq, R.; Shahbaz, M.; Naqvi, M.; Aslam, M.; Khan, Z.; Mackey, H.; McKay, G.; Al-Ansari, T. Recent developments on sewage sludge pyrolysis and its kinetics: Resources recovery, thermogravimetric platforms, and innovative prospects. Comput. Chem. Eng. 2021, 150, 107325. [Google Scholar] [CrossRef]
- Chen, M.; Oshita, K.; Takaoka, M.; Shiota, K. Co-incineration effect of sewage sludge and municipal solid waste on the behavior of heavy metals by phosphorus. Waste Manag. 2022, 152, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chang, Y.; Liu, Q. Fate and distribution of nutrients and heavy metals during hydrothermal carbonization of sewage sludge with implication to land application. J. Clean. Prod. 2019, 225, 972–983. [Google Scholar] [CrossRef]
- Matheri, A.N.; Ntuli, F.; Ngila, J.C. Sludge to energy recovery dosed with selected trace metals additives in anaerobic digestion processes. Biomass Bioenergy 2021, 144, 105869. [Google Scholar] [CrossRef]
- Abdolrezayi, A.; Puricelli, S.; Dolci, G.; Turolla, A.; Canziani, R.; Rigamonti, L. Phosphorus recovery from sewage sludge ash: Life cycle inventory and critical review of LCA case studies. J. Environ. Manag. 2025, 389, 125620. [Google Scholar] [CrossRef]
- Li, D.; Shan, R.; Jiang, L.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y. A review on the migration and transformation of heavy metals in the process of sludge pyrolysis. Resour. Conserv. Recycl. 2022, 185, 106452. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Wang, J.; He, C.; Zhang, J. Recovery of nitrogen and phosphorus in sewage sludge treatment technologies. Chin. Chem. Lett. 2025, 37, 111431. [Google Scholar] [CrossRef]
- Fei, Y.H.; Zhao, D.; Cao, Y.; Huot, H.; Tang, Y.T.; Zhang, H.; Xiao, T. Phosphorous Retention and Release by Sludge-Derived Hydrochar for Potential Use as a Soil Amendment. J. Environ. Qual. 2019, 48, 502–509. [Google Scholar] [CrossRef]
- Kwapinski, W.; Kolinovic, I.; Leahy, J.J. Sewage Sludge Thermal Treatment Technologies with a Focus on Phosphorus Recovery: A Review. Waste Biomass Valorization 2021, 12, 5837–5852. [Google Scholar] [CrossRef]
- Yu, B.; Luo, J.; Xie, H.; Yang, H.; Chen, S.; Liu, J.; Zhang, R.; Li, Y.Y. Species, fractions, and characterization of phosphorus in sewage sludge: A critical review from the perspective of recovery. Sci. Total Environ. 2021, 786, 147437. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, H.; Tan, Y.; Chen, G.; Hao, T. Emerging electrochemistry-based process for sludge treatment and resources recovery: A review. Water Res. 2021, 209, 117939. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, G.; Tian, H. Current state of sewage treatment in China. Water Res. 2014, 66, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Feng, J.; Qin, Y.-H.; Li, W.-Y. Prediction of elemental composition of coal using proximate analysis. Fuel 2017, 193, 315–321. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Ye, Z.; Yang, C. Effect of manganese oxide-modified biochar addition on methane production and heavy metal speciation during the anaerobic digestion of sewage sludge. J. Environ. Sci. 2019, 76, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, R.; Guo, X.; Wu, W.; Guo, Q.; Zhang, Y.; Yan, B. Comparative evaluation on municipal sewage sludge utilization processes for sustainable management in Tibet. Sci. Total Environ. 2021, 765, 142676. [Google Scholar] [CrossRef]
- Chen, G.; Yang, R.; Cheng, Z.; Yan, B.; Ma, W. Nitric oxide formation during corn straw/sewage sludge co-pyrolysis/gasification. J. Clean. Prod. 2018, 197, 97–105. [Google Scholar] [CrossRef]
- Kavehei, E.; Jenkins, G.A.; Adame, M.F.; Lemckert, C. Carbon sequestration potential for mitigating the carbon footprint of green stormwater infrastructure. Renew. Sustain. Energy Rev. 2018, 94, 1179–1191. [Google Scholar] [CrossRef]
- Gao, N.; Li, J.; Qi, B.; Li, A.; Duan, Y.; Wang, Z. Thermal analysis and products distribution of dried sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2014, 105, 43–48. [Google Scholar] [CrossRef]
- Sun, S.; Huang, X.; Lin, J.; Ma, R.; Fang, L.; Zhang, P.; Qu, J.; Zhang, X.; Liu, Y. Study on the effects of catalysts on the immobilization efficiency and mechanism of heavy metals during the microwave pyrolysis of sludge. Waste Manag. 2018, 77, 131–139. [Google Scholar] [CrossRef]
- Wang, X.; Cui, X.; Fang, C.; Yu, F.; Zhi, J.A.; Mašek, O.; Yan, B.; Chen, G.; Dan, Z. Agent-assisted Electrokinetic treatment of Sewage Sludge: Heavy Metal Removal Effectiveness and Nutrient Content Characteristics. Water Res. 2022, 224, 119016. [Google Scholar] [CrossRef]
- Yang, T.; Huang, H.-J.; Lai, F.-Y. Pollution hazards of heavy metals in sewage sludge from four wastewater treatment plants in Nanchang, China. Trans. Nonferrous Met. Soc. China 2017, 27, 2249–2259. [Google Scholar] [CrossRef]
- GB 4284-2018; Control Standards of Pollutants in Sludge for Agricultural Use. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- GB 15618-2018; Soil Environmental Quality-Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment: Beijing, China, 2018.
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Yesil, H.; Tugtas, A.E. Removal of heavy metals from leaching effluents of sewage sludge via supported liquid membranes. Sci. Total Environ. 2019, 693, 133608. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef]
- Gong, H.; Zhao, L.; Rui, X.; Hu, J.; Zhu, N. A review of pristine and modified biochar immobilizing typical heavy metals in soil: Applications and challenges. J. Hazard. Mater. 2022, 432, 128668. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhai, Y.; Li, S.; Liu, X.; Wang, B.; Liu, X.; Fan, Y.; Shi, H.; Li, C.; Zhu, Y. Thermal treatment of sewage sludge: A comparative review of the conversion principle, recovery methods and bioavailability-predicting of phosphorus. Chemosphere 2022, 291, 133053. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Zielinska, B.; Felix, L. Hydrothermal carbonization (HTC) of selected woody and herbaceous biomass feedstocks. Biomass Convers. Biorefinery 2013, 3, 113–126. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S.; Balasubramani, S.R.; Parthasarathy, V.; Deehen, A.D.; Jananie, E.; Lokesh, V.; Shivani, S.; Keerthana, S.; Shivani, I.; et al. Engineering properties of hydrochar fabricated from hydrothermal carbonization of lignocellulosic biomass: Practice as adsorbent and catalyst in water treatment. J. Water Process Eng. 2025, 74, 107739. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q.; Sun, G.; Wang, K.; Yang, Y.-P.; Chen, J.; Chen, G.; Zheng, R. Valorization of dual waste: Heavy metal mitigation and carbon/nutrient regulation in hydrochar via co-hydrothermal carbonization of sewage sludge and sawdust. Bioresour. Technol. 2025, 436, 133038. [Google Scholar] [CrossRef]
- Liu, H.; Basar, I.A.; Nzihou, A.; Eskicioglu, C. Hydrochar derived from municipal sludge through hydrothermal processing: A critical review on its formation, characterization, and valorization. Water Res. 2021, 199, 117186. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, C.; Ding, D.; Lei, Z.; Yang, Y.; Feng, C.; Zhang, Z. Immobilization of heavy metals in sewage sludge by using subcritical water technology. Bioresour. Technol. 2013, 137, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Huang, H.J.; Yuan, X.Z. The migration and transformation behaviors of heavy metals during the hydrothermal treatment of sewage sludge. Bioresour. Technol. 2016, 200, 991–998. [Google Scholar] [CrossRef]
- Yusuf, B.O.; Aliyu, M.; Azeez, M.O.; Taialla, O.A.; Lateef, S.; Sulaimon, R.; Akinpelu, A.A.; Ganiyu, S.A. Comprehensive technologies for heavy metal remediation: Adsorption, membrane processes, photocatalysis, and AI-driven design. Desalination 2025, 615, 119261. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Pathak, A.; Dastidar, M.G.; Sreekrishnan, T.R. Bioleaching of heavy metals from sewage sludge: A review. J. Environ. Manag. 2009, 90, 2343–2353. [Google Scholar] [CrossRef]
- Gao, J.; Luo, Q.S.; Zhu, J.; Zhang, C.B.; Li, B.Z. Effects of electrokinetic treatment of contaminated sludge on migration and transformation of Cd, Ni and Zn in various bonding states. Chemosphere 2013, 93, 2869–2876. [Google Scholar] [CrossRef]
- Peng, G.; Tian, G. Using electrode electrolytes to enhance electrokinetic removal of heavy metals from electroplating sludge. Chem. Eng. J. 2010, 165, 388–394. [Google Scholar] [CrossRef]
- Fu, R.; Wen, D.; Xia, X.; Zhang, W.; Gu, Y. Electrokinetic remediation of chromium (Cr)-contaminated soil with citric acid (CA) and polyaspartic acid (PASP) as electrolytes. Chem. Eng. J. 2017, 316, 601–608. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Cai, Z.; Chen, R.; Sun, Q.; Sun, M. Removal of copper and nickel from municipal sludge using an improved electrokinetic process. Chem. Eng. J. 2017, 307, 1008–1016. [Google Scholar] [CrossRef]
- Vocciante, M.; Dovì, V.; Ferro, S. Sustainability in ElectroKinetic Remediation Processes: A Critical Analysis. Sustainability 2021, 13, 770. [Google Scholar] [CrossRef]
- Vocciante, M.; Caretta, A.; Bua, L.; Bagatin, R.; Ferro, S. Enhancements in ElectroKinetic Remediation Technology: Environmental assessment in comparison with other configurations and consolidated solutions. Chem. Eng. J. 2016, 289, 123–134. [Google Scholar] [CrossRef]
- Ryu, B.-G.; Park, G.-Y.; Yang, J.-W.; Baek, K. Electrolyte conditioning for electrokinetic remediation of As, Cu, and Pb-contaminated soil. Sep. Purif. Technol. 2011, 79, 170–176. [Google Scholar] [CrossRef]
- Jensen, P.E.; Kirkelund, G.M.; Fritt-Rasmussen, J.; Ottosen, L.M. Electrodialytic Remediation of Toxic Elements and P Recovery from Sediments of Eutrophic Fresh-Waters in 3-Compartment Batch and Stack Setup. Waste Biomass Valorization 2022, 13, 4085–4098. [Google Scholar] [CrossRef]
- Gao, J.; Luo, Q.; Zhang, C.; Li, B.; Meng, L. Enhanced electrokinetic removal of cadmium from sludge using a coupled catholyte circulation system with multilayer of anion exchange resin. Chem. Eng. J. 2013, 234, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; Zhao, M.; Rong, H.; Zhang, K.; Chen, Q. Comparison of bioleaching and electrokinetic remediation processes for removal of heavy metals from wastewater treatment sludge. Chemosphere 2017, 168, 1152–1157. [Google Scholar] [CrossRef]
- Tang, J.; He, J.; Tang, H.; Wang, H.; Sima, W.; Liang, C.; Qiu, Z. Heavy metal removal effectiveness, flow direction and speciation variations in the sludge during the biosurfactant-enhanced electrokinetic remediation. Sep. Purif. Technol. 2020, 246, 116918. [Google Scholar] [CrossRef]
- Acar, Y.B.; Alshawabkeh, A.N. Principles of electrokinetic remediation. Environ. Sci. Technol. 2002, 27, 2638–2647. [Google Scholar] [CrossRef]
- Adhikary, A.; Nath, A.; Das, S.; Pal, S.; Ghosh, S. A novel hybrid constructed Wetland–Electrokinetic system for simultaneous removal of aqueous lead and naphthalene with in-situ bed rejuvenation. J. Environ. Manag. 2026, 397, 128234. [Google Scholar] [CrossRef]
- Barkhordari, M.S.; Zhou, N.; Li, K.; Qi, C. Interpretable machine learning for predicting heavy metal removal efficiency in electrokinetic soil remediation. J. Environ. Chem. Eng. 2024, 12, 114330. [Google Scholar] [CrossRef]
- Suanon, F.; Tomètin, L.A.S.; Zveushe, O.K.; de Dios, V.R.; Han, Y.; Ifon, B.E.; Atakpa, E.O.; Yete, P.; Sesu, F.; Li, J.; et al. Electrokinetic remediation of chromium-contaminated soils: The potential for advanced materials in three-dimensional EKR approaches. J. Environ. Chem. Eng. 2025, 13, 116774. [Google Scholar] [CrossRef]
- Abou-Shady, A. Explore the potential for improving phosphorus availability in calcareous soil through electrokinetic methods. Soil Tillage Res. 2025, 250, 106525. [Google Scholar] [CrossRef]
- Fardin, A.B.; Jamshidi-Zanjani, A.; Saeedi, M. A comprehensive review of soil remediation contaminated by persistent organic pollutants using electrokinetic: Challenging enhancement techniques. J. Environ. Manag. 2025, 373, 123587. [Google Scholar] [CrossRef]
- Almeira, O.J.; Peng, C.-S.; Abou-Shady, A. Simultaneous removal of cadmium from kaolin and catholyte during soil electrokinetic remediation. Desalination 2012, 300, 1–11. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Xiao, C. Focus on factors affecting pH, flow of Cr and transformation between Cr(VI) and Cr(III) in the soil with different electrolytes. Electrochim. Acta 2016, 211, 652–662. [Google Scholar] [CrossRef]
- Chen, B.; Li, H.; Qu, G.; Yang, J.; Jin, C.; Wu, F.; Ren, Y.; Liu, Y.; Liu, X.; Qin, J.; et al. Aluminium sulfate synergistic electrokinetic separation of soluble components from phosphorus slag and simultaneous stabilization of fluoride. J. Environ. Manag. 2023, 328, 116942. [Google Scholar] [CrossRef]
- Tang, J.; Qiu, Z.; Tang, H.; Wang, H.; Sima, W.; Liang, C.; Liao, Y.; Li, Z.; Wan, S.; Dong, J. Coupled with EDDS and approaching anode technique enhanced electrokinetic remediation removal heavy metal from sludge. Environ. Pollut. 2021, 272, 115975. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Zhang, D.-S.; Stabnikova, O.; Tay, J.-H. Evaluation of electrokinetic removal of heavy metals from sewage sludge. J. Hazard. Mater. 2005, 124, 139–146. [Google Scholar] [CrossRef]
- Yuan, C.; Weng, C.-H. Electrokinetic enhancement removal of heavy metals from industrial wastewater sludge. Chemosphere 2006, 65, 88–96. [Google Scholar] [CrossRef]
- Ge, X.; Song, X.; Xie, J.; Huang, W.; Wang, Y.; Xu, Z.; Wang, Y.; Hou, X.; Cao, X. Comparison of the influence of EDTA, nitrilotriacetic acid, diethylenetriamine pentaacetic acid, chitosan, fulvic acid and pine needle extract on the removal of multiple heavy metal by electrokinetic remediation. Int. J. Electrochem. Sci. 2022, 17, 221012. [Google Scholar] [CrossRef]
- Salom, D.; Fernández-Verdejo, D.; Isidro, J.; Vicent, T.; Blánquez, P. Coupling electrokinetic soil flushing with bioremediation for the removal of chlorinated benzenes and hexachlorocyclohexane. J. Hazard. Mater. 2025, 496, 139397. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Han, K.; Liu, C.; Yan, B. Quantitative research on heavy metal removal of flue gas desulfurization-derived wastewater sludge by electrokinetic treatment. J. Hazard. Mater. 2021, 414, 125561. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; He, J.; Liu, T.; Xin, X.; Hu, H. Removal of heavy metal from sludge by the combined application of a biodegradable biosurfactant and complexing agent in enhanced electrokinetic treatment. Chemosphere 2017, 189, 599–608. [Google Scholar] [CrossRef]
- Abou-Shady, A.; Ismail, S.; Yossif, T.M.H.; Yassin, S.A.; Ali, M.E.A.; Habib, A.A.M.; Khalil, A.K.A.; Tag-Elden, M.A.; Emam, T.M.; Mahmoud, A.A.; et al. Comprehensive review of progress made in soil electrokinetic research during 1993–2020, part II. No.1: Materials additives for enhancing the intensification process during 2017–2020. S. Afr. J. Chem. Eng. 2023, 45, 182–200. [Google Scholar] [CrossRef]
- Tang, J.; He, J.; Xin, X.; Hu, H.; Liu, T. Biosurfactants enhanced heavy metals removal from sludge in the electrokinetic treatment. Chem. Eng. J. 2018, 334, 2579–2592. [Google Scholar] [CrossRef]
- Ebbers, B.; Ottosen, L.M.; Jensen, P.E. Electrodialytic treatment of municipal wastewater and sludge for the removal of heavy metals and recovery of phosphorus. Electrochim. Acta 2015, 181, 90–99. [Google Scholar] [CrossRef]
- Mahmoud, A.; Olivier, J.; Vaxelaire, J.; Hoadley, A.F. Electrical field: A historical review of its application and contributions in wastewater sludge dewatering. Water Res. 2010, 44, 2381–2407. [Google Scholar] [CrossRef]
- Babel, S.; del Mundo Dacera, D. Heavy metal removal from contaminated sludge for land application: A review. Waste Manag. 2006, 26, 988–1004. [Google Scholar] [CrossRef]
- Kou, Y.; Zhao, Q.; Cheng, Y.; Wu, Y.; Dou, W.; Ren, X. Removal of heavy metals in sludge via joint EDTA-acid treatment: Effects on seed germination. Sci. Total Environ. 2020, 707, 135866. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.; Cui, C. Remediation of heavy metal-contaminated soils by electrokinetic technology: Mechanisms and applicability. Chemosphere 2021, 265, 129071. [Google Scholar] [CrossRef]
- Moon, D.H.; Chang, Y.Y.; Lee, M.; Koutsospyros, A.; Koh, I.H.; Ji, W.H.; Park, J.H. Assessment of soil washing for heavy metal contaminated paddy soil using FeCl3 washing solutions. Environ. Geochem. Health 2021, 43, 3343–3350. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Tang, Y. Evolution of phosphorus complexation and mineralogy during (hydro)thermal treatments of activated and anaerobically digested sludge: Insights from sequential extraction and P K-edge XANES. Water Res. 2016, 100, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Du, Y.; Xu, Y.; Li, Y.; Wu, X.; Wang, D. Phosphorus recovery from sewage sludge: Progress, challenges, policies, and future directions. Chem. Eng. J. 2025, 520, 165735. [Google Scholar] [CrossRef]
- Han, X.; Wang, F.; Zhou, B.; Chen, H.; Yuan, R.; Liu, S.; Zhou, X.; Gao, L.; Lu, Y.; Zhang, R. Phosphorus complexation of sewage sludge during thermal hydrolysis with different reaction temperature and reaction time by P K-edge XANES and 31P NMR. Sci. Total Environ. 2019, 688, 1–9. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Chang, R.; Zhan, Y.; Wei, D.; Zhang, L.; Chen, Q. Composting with biochar or woody peat addition reduces phosphorus bioavailability. Sci. Total Environ. 2021, 764, 142841. [Google Scholar] [CrossRef]
- Frišták, V.; Pipíška, M.; Soja, G. Pyrolysis treatment of sewage sludge: A promising way to produce phosphorus fertilizer. J. Clean. Prod. 2018, 172, 1772–1778. [Google Scholar] [CrossRef]
- Feng, H.; Zhou, S.; Guan, W.; Zhu, L.; Li, R.; Wang, S. Enhancement of apatite-phosphorus-rich biochar production from sewage sludge pyrolysis assisted by carbide slag. Process Saf. Environ. Prot. 2024, 192, 26–35. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, Z.; Liu, Y.; Liu, Y.; Xu, Y.; Ruan, X.; Zhang, X.; Cao, W. Phosphorus speciation and bioavailability of sewage sludge derived biochar amended with CaO. Waste Manag. 2019, 87, 71–77. [Google Scholar] [CrossRef]
- Werner, F.; Prietzel, J. Standard Protocol and Quality Assessment of Soil Phosphorus Speciation by P K-Edge XANES Spectroscopy. Environ. Sci. Technol. 2015, 49, 10521–10528. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Zhang, H.; Stutter, M.; Giles, C.D.; Darch, T.; George, T.S.; Shand, C.; Lumsdon, D.; Blackwell, M.; Wearing, C.; et al. A Holistic Approach to Understanding the Desorption of Phosphorus in Soils. Environ. Sci. Technol. 2016, 50, 3371–3381. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Sun, H.; Luo, L.; Wang, J.; Wang, D.; Huang, R.; Ma, C.; Zhu, Y.G.; Liu, Z. Speciation Evolution of Phosphorus and Sulfur Derived from Sewage Sludge Biochar in Soil: Ageing Effects. Environ. Sci. Technol. 2022, 56, 6639–6646. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Liu, H.; He, Z.; Yao, H. Intensified Enrichment and Leaching Strategy of Phosphorus in Sewage Sludge via Species-Targeted Conversion. Environ. Sci. Technol. 2025, 59, 9285–9297. [Google Scholar] [CrossRef] [PubMed]
- Petzet, S.; Peplinski, B.; Cornel, P. On wet chemical phosphorus recovery from sewage sludge ash by acidic or alkaline leaching and an optimized combination of both. Water Res. 2012, 46, 3769–3780. [Google Scholar] [CrossRef]
- Huang, R.; Fang, C.; Lu, X.; Jiang, R.; Tang, Y. Transformation of Phosphorus during (Hydro)thermal Treatments of Solid Biowastes: Reaction Mechanisms and Implications for P Reclamation and Recycling. Environ. Sci. Technol. 2017, 51, 10284–10298. [Google Scholar] [CrossRef]
- Mackay, J.E.; Cavagnaro, T.R.; Jakobsen, I.; Macdonald, L.M.; Grønlund, M.; Thomsen, T.P.; Müller-Stöver, D.S. Evaluation of phosphorus in thermally converted sewage sludge: P pools and availability to wheat. Plant Soil 2017, 418, 307–317. [Google Scholar] [CrossRef]
- Vogel, C.; Sekine, R.; Steckenmesser, D.; Lombi, E.; Herzel, H.; Zuin, L.; Wang, D.; Félix, R.; Adam, C. Combining diffusive gradients in thin films (DGT) and spectroscopic techniques for the determination of phosphorus species in soils. Anal. Chim. Acta 2019, 1057, 80–87. [Google Scholar] [CrossRef]
- Six, L.; Smolders, E.; Merckx, R. The performance of DGT versus conventional soil phosphorus tests in tropical soils—Maize and rice responses to P application. Plant Soil 2013, 366, 49–66. [Google Scholar] [CrossRef]
- Ren, M.; Ding, S.; Shi, D.; Zhong, Z.; Cao, J.; Yang, L.; Tsang, D.C.W.; Wang, D.; Zhao, D.; Wang, Y. A new DGT technique comprised in a hybrid sensor for the simultaneous measurement of ammonium, nitrate, phosphorus and dissolved oxygen. Sci. Total Environ. 2020, 725, 138447. [Google Scholar] [CrossRef]
- Zheng, X.; Jiang, Z.; Ying, Z.; Ye, Y.; Chen, W.; Wang, B.; Dou, B. Migration and Transformation of Phosphorus During Hydrothermal Carbonization of Sewage Sludge: Focusing on the Role of pH and Calcium Additive and the Transformation Mechanism. ACS Sustain. Chem. Eng. 2020, 8, 7806–7814. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Shao, H.; Sun, J. Pyrolysis temperature affects phosphorus transformation in biochar: Chemical fractionation and 31P NMR analysis. Sci. Total Environ. 2016, 569-570, 65–72. [Google Scholar] [CrossRef]
- Li, S.; Zeng, W.; Jia, Z.; Wu, G.; Xu, H.; Peng, Y. Phosphorus species transformation and recovery without apatite in FeCl3-assisted sewage sludge hydrothermal treatment. Chem. Eng. J. 2020, 399, 125735. [Google Scholar] [CrossRef]
- Garcia Jimenez, D.; Ermondi, G.; Jandova, Z.; Vallaro, M.; Caron, G.; Arnhof, H. Linker Methylation as a Strategy to Enhance PROTAC Oral Bioavailability: Insights from Molecular Properties and Conformational Analysis. J. Med. Chem. 2025, 68, 16666–16677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, B.; Zhang, X.; Xu, H.; Cheng, N.; Feng, Q.; Zhao, R.; Gao, Y.; Wei, M. Release characteristics of phosphate from ball-milled biochar and its potential effects on plant growth. Sci. Total Environ. 2022, 821, 153256. [Google Scholar] [CrossRef] [PubMed]
- Shober, A.L.; Hesterberg, D.L.; Sims, J.T.; Gardner, S. Characterization of phosphorus species in biosolids and manures using XANES spectroscopy. J. Environ. Qual. 2006, 35, 1983–1993. [Google Scholar] [CrossRef] [PubMed]
- Montazerharzand, M.; Paya, H.; Taghizadeh, A.; Hosseinkhani, A.; Ramin, M. Effects of walnut shell biochar feed additive on rumen fermentation, nutrient utilization, and performance in fattening lambs. Vet. Anim. Sci. 2025, 30, 100515. [Google Scholar] [CrossRef]
- Abioye, K.J.; Falua, K.J.; Rezaee, M.; Zamiri, M.A.; Zou, F.; Acharya, B. Global insights into biomass pyrolysis mechanisms: A scientometric and mechanistic approach. Results Eng. 2025, 28, 107123. [Google Scholar] [CrossRef]
- Knijnenburg, J.T.N.; Suwanree, S.; Macquarrie, D.; Kasemsiri, P.; Jetsrisuparb, K. Phosphorus recovery from animal manures through pyrolysis: Phosphorus transformations, release mechanisms, and applications of manure biochars in agriculture. RSC Sustain. 2024, 3, 1084–1101. [Google Scholar] [CrossRef]
- Michalska, J.; Turek-Szytow, J.; Dudło, A.; Surmacz-Górska, J. Characterization of humic substances recovered from the sewage sludge and validity of their removal from this waste. EFB Bioeconomy J. 2022, 2, 100026. [Google Scholar] [CrossRef]
- Adhikari, S.; Gasco, G.; Mendez, A.; Surapaneni, A.; Jegatheesan, V.; Shah, K.; Paz-Ferreiro, J. Influence of pyrolysis parameters on phosphorus fractions of biosolids derived biochar. Sci. Total Environ. 2019, 695, 133846. [Google Scholar] [CrossRef] [PubMed]
- Cheremisina, O.V.; Gorbacheva, A.A.; Balandinsky, D.A.; Adhikari, M.K.; Ojha, A.M.; Bhattarai, A.; Chakraborty, G. Physicochemical criteria for selection of reagents on phosphate flotation: Recent advances and future approaches. Miner. Eng. 2026, 235, 109863. [Google Scholar] [CrossRef]
- Luo, X.; Ding, W.; Sheng, Y.; Ma, Z.; Tan, L.; Wu, Z.; Gao, X.; Wang, N.; Luo, Z.; Zhou, Y.; et al. Different phosphorus reclamation strategies of solid biowastes after thermal treatment: Insights into phosphorus bioavailability and metal species. Chem. Eng. J. 2025, 524, 169417. [Google Scholar] [CrossRef]
- Gupta, A.; Ramachandran, S.; Mayilswamy, N.; Nighojkar, A.; Kandasubramanian, B. Dye-laden sludge-derived biochar for wastewater remediation: A review on pyrolytic engineering, adsorptive interactions, and environmental prospects. Sustain. Chem. Environ. 2025, 11, 100271. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Chan, W.P.; Veksha, A.; Lisak, G.; Lim, T.-T. Advancing insights into phosphorus transformation during thermochemical treatment of sewage sludge. J. Environ. Manag. 2025, 391, 126505. [Google Scholar] [CrossRef]
- Meng, X.; Huang, Q.; Gao, H.; Tay, K.; Yan, J. Improved utilization of phosphorous from sewage sludge (as Fertilizer) after treatment by Low-Temperature combustion. Waste Manag. 2018, 80, 349–358. [Google Scholar] [CrossRef]
- Ponnusamy, P.; Loganathan, M.; Krishnasamy, S. Sewage sludge pyrolysis: Aspen plus simulation and AI-assisted optimization for biochar yield and properties. Sustain. Chem. Pharm. 2025, 48, 102241. [Google Scholar] [CrossRef]
- Kamesh, M.R.; Kumar, P.S.; Vijayalakshmi, A.; Dubey, S.; Madhu, P.; Sowmya Dhanalakshmi, C.; Vadivel, A.; Yadav, A.S.; Subramani, N. Interaction between sewage sludge and biomass components for enhanced biofuel production via pyrolysis based on studies of the mild acid pre-treatment process. J. Energy Inst. 2025, 121, 102167. [Google Scholar] [CrossRef]
- Mustapha, S.I.; Isa, Y.M. Catalytic co-pyrolysis of sewage sludge and softwood using CuO/CoO/CeO2/HZSM-5 hybrid catalysts: Influence of catalyst synthesis techniques on bio-oil product yields and quality. J. Environ. Chem. Eng. 2025, 13, 119621. [Google Scholar] [CrossRef]
- Mutsvene, B.; Chetty, M.; Bux, F.; Kumari, S. Waste to wealth transformation: Maximising resource and energy recovery through hydrothermal treatment of sewage sludge—A technoeconomic evaluation. Case Stud. Chem. Environ. Eng. 2025, 11, 101182. [Google Scholar] [CrossRef]
- Mbasabire, P.; Murindangabo, Y.T.; Frouz, J.; Brom, J. Characterization, fractionation and untapped potential of phosphate-amended sewage sludge biochar in soil-plant systems. Chemosphere 2024, 367, 143565. [Google Scholar] [CrossRef] [PubMed]
- Naciri, R.; Chtouki, M.; El Maalam, L.; Hirt, H.; Belkachach, D.; Oukarroum, A. Phosphate-modified biochar attenuates cadmium availability in contaminated soil and reduces its transfer to tomato fruits. Environ. Res. 2025, 279, 121775. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, Y.; Wang, Y.; An, W.; Jin, J.; Sun, K.; Wang, X. Effects of biochar addition on the abundance, speciation, availability, and leaching loss of soil phosphorus. Sci. Total Environ. 2021, 758, 143657. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Shi, R.; Liu, W.; Khan, S.; Baig, A.M.; Iqbal, H.; Tariq, H.; Zhao, Y.; Sun, Y. A critical review of tire wear particles aging and ecotoxicological consequences in terrestrial environments: Insights into environmentally persistent free radicals. J. Hazard. Mater. 2026, 501, 140641. [Google Scholar] [CrossRef]
- Lv, M.; Zhao, T.; Chen, J.; Tong, L.; Ni, Z.; Lin, Q.; Ruan, Z.; Qiu, R. Distribution and transformation behavior of heavy metals and phosphorus during sewage sludge pyrolysis. Environ. Technol. Innov. 2025, 37, 104049. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Liu, F.; Zhang, X.; Song, M.; Li, R. Pyrolysis of sewage sludge to biochar: Transformation mechanism of phosphorus. J. Anal. Appl. Pyrolysis 2023, 173, 106065. [Google Scholar] [CrossRef]
- Xiao, Y.; Yan, T.; Yao, P.; Xiang, W.; Wu, Y.; Li, J. Co-pyrolysis of sewage sludge and phosphate tailings: Synergistically enhancing heavy metal immobilization and phosphorus availability. Waste Manag. 2024, 181, 44–56. [Google Scholar] [CrossRef]
- Irewale, A.T.; Elemike, E.E.; Aikpokpodion, P.E.; Thangavelu, R.M.; Dimkpa, C.O.; Oguzie, E.E. Morphological and chemical profiling of biochar derived from invasive aquatic weed towards bio-nanofertilizer development. RSC Sustain. 2025, 3, 3947–3963. [Google Scholar] [CrossRef]
- Mainali, K.; Sarker, M.I.; Mullen, C.A.; Sharma, B.K.; Yadav, M.P.; Ngo, H.; Garcia-Perez, M. Thermal decomposition kinetics of dairy manure hydrochars. J. Energy Inst. 2025, 120, 102088. [Google Scholar] [CrossRef]
- Zhao, C.; Liang, S.; Wu, Z.; Huang, Y.; Peng, D.; Huang, T. Mechanically activated steel slag induces Ca-P crystallization to recover phosphates from phosphogypsum leachate: Adsorption and crystallization coupled processes. Colloids Surf. A Physicochem. Eng. Asp. 2025, 713, 136535. [Google Scholar] [CrossRef]
- Yang, W.; Hu, W.; Liu, H.; Wang, P.; Li, P.; Li, H.; Wang, Z.; Yang, S.; Li, Z.; Zhu, Y. Exploring the effects of calcium bicarbonate on endogenous phosphorus conversion and pyrolysis product distributions of oil crop residues. J. Anal. Appl. Pyrolysis 2025, 192, 107287. [Google Scholar] [CrossRef]
- Dahiru, U.H.; Saleem, F.; Rehman, A.; Zhang, K.; Harvey, A.P. A comprehensive review of non-catalytic plasma-assisted decomposition of volatile organic compounds. Total Environ. Eng. 2025, 5, 100050. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, K.; Yu, T.; Bai, S.; Pei, D.; Bai, T.; Zhang, Q.; Yin, L.; Hu, Y.; Chen, D. Phosphorus Transformation in Hydrothermal Pretreatment and Steam Gasification of Sewage Sludge. Energy Fuels 2018, 32, 8545–8551. [Google Scholar] [CrossRef]
- Meng, W.; Zheng, L.; Cheng, S.; Li, Z. Unlocking sustainable opportunities for sludge-derived aqueous phase via hydrothermal carbonization. Renew. Sustain. Energy Rev. 2025, 224, 116084. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, G.; Rao, Y.; Chen, H.; Zhang, S. Hydrothermal conversion of dewatered sewage sludge: Focusing on the transformation mechanism and recovery of phosphorus. Chemosphere 2019, 228, 619–628. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Pozarlik, A.K.; Bramer, E.A.; Niedzwiecki, L.; Pawlak-Kruczek, H.; Brem, G. Hydrothermal carbonization of wet biomass from nitrogen and phosphorus approach: A review. Renew. Energy 2021, 171, 401–415. [Google Scholar] [CrossRef]
- Sangué Djandja, O.; Duan, B.; Yin, L.-X.; Cao, C.; Shan, Y.; Duo, J.; Yao, G.; Duan, P.-G. Ammonia-assisted thermal hydrolysis of sewage sludge: Solid and liquid phases characterization. Sustain. Energy Technol. Assess. 2022, 53, 102693. [Google Scholar] [CrossRef]
- Huang, W.; Cai, W.; Huang, H.; Lei, Z.; Zhang, Z.; Tay, J.H.; Lee, D.J. Identification of inorganic and organic species of phosphorus and its bio-availability in nitrifying aerobic granular sludge. Water Res. 2015, 68, 423–431. [Google Scholar] [CrossRef]
- Khiar, H.; Barka, N.; Puga, A. Metal phosphates for the design of advanced heterogeneous photocatalysts. Coord. Chem. Rev. 2024, 510, 215814. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, F.; Li, Y.; Li, B.; Yang, T.; Li, R. Influence of microwave-assisted pyrolysis parameters and additives on phosphorus speciation and transformation in phosphorus-enriched biochar derived from municipal sewage sludge. J. Clean. Prod. 2021, 287, 125550. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, M.; Ying, Z.; Feng, Y.; Wang, B.; Dou, B. Correlating phosphorus transformation with process water during hydrothermal carbonization of sewage sludge via experimental study and mathematical modelling. Sci. Total Environ. 2022, 807, 150750. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Z.; Wang, J.; Zhao, X.; Zhao, Y.; Qian, J.; Wang, T. Pyrolysis and hydrothermal carbonization of biowaste: A comparative review on the conversion pathways and potential applications of char product. Sustain. Chem. Pharm. 2023, 33, 101106. [Google Scholar] [CrossRef]
- Manya, J.J. Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Xu, Z.; Li, J.; Chai, X. Removal of water nutrients by different aquatic plant species: An alternative way to remediate polluted rural rivers. Ecol. Eng. 2018, 110, 18–26. [Google Scholar] [CrossRef]
- Shahbeig, H.; Nosrati, M. Pyrolysis of municipal sewage sludge for bioenergy production: Thermo-kinetic studies, evolved gas analysis, and techno-socio-economic assessment. Renew. Sustain. Energy Rev. 2020, 119, 109567. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Tang, X.; Guan, Z.; Reid, B.J.; Rajapaksha, A.U.; Ok, Y.S.; Sun, H. Biochar increased water holding capacity but accelerated organic carbon leaching from a sloping farmland soil in China. Environ. Sci. Pollut. Res. 2016, 23, 995–1006. [Google Scholar] [CrossRef]
- Mašek, O.; Brownsort, P.; Cross, A.; Sohi, S. Influence of production conditions on the yield and environmental stability of biochar. Fuel 2013, 103, 151–155. [Google Scholar] [CrossRef]
- Fei, Y.H.; Zhao, D.; Liu, Y.; Zhang, W.; Tang, Y.Y.; Huang, X.; Wu, Q.; Wang, Y.X.; Xiao, T.; Liu, C. Feasibility of sewage sludge derived hydrochars for agricultural application: Nutrients (N, P, K) and potentially toxic elements (Zn, Cu, Pb, Ni, Cd). Chemosphere 2019, 236, 124841. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Wang, J.; Buss, W.; Bogush, A.; Masek, O.; Zhang, Y.; Yu, F.; Yan, B.; Cheng, Z.; et al. Reclamation of available phosphorus and separation of heavy metals from sewage sludge via FeCl3-assisted electrokinetic treatment and pyrolysis. J. Environ. Manag. 2025, 387, 125882. [Google Scholar] [CrossRef]
- Wang, X.; Mašek, O.; Li, H.; Yu, F.; Wurzer, C.; Wang, J.; Yan, B.; Cui, X.; Chen, G.; Hou, L.a. Coupling electrokinetic technique with hydrothermal carbonization for phosphorus-enriched hydrochar production and heavy metal separation from sewage sludge. Chem. Eng. J. 2024, 481, 148144. [Google Scholar] [CrossRef]
- Rojas, M.; Manrique, R.; Hornung, U.; Funke, A.; Mullen, C.A.; Chejne, F.; Maya, J.C. Advances and challenges on hydrothermal processes for biomass conversion: Feedstock flexibility, products, and modeling approaches. Biomass Bioenergy 2025, 194, 107621. [Google Scholar] [CrossRef]
- Brar, G.S.; Malhotra, K.; Kumar, R.; Lamba, J.; Way, T.R.; Prasad, R.; Adhikari, S. Elucidating the impact of broiler litter and pinewood biochar application method on metal retention and leaching in undisturbed soil columns using simulated rainfall. J. Hazard. Mater. Adv. 2025, 20, 100860. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Chiu, P.C.; Imhoff, P.T.; Guo, M. Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci. Total Environ. 2015, 512–513, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, Q.; Guan, C.-Y.; Yang, X.; Jiang, X.; Wei, M.; Tan, J.; Li, X. Metal oxide modified biochars for fertile soil management: Effects on soil phosphorus transformation, enzyme activity, microbe community, and plant growth. Environ. Res. 2023, 231, 116258. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, D.; Zhao, J.; Zhang, Q.; Li, X.; Zhao, Y.; Zheng, W.; Zhang, B.; Liu, Z. Biochar application does not improve crop growth and yield in a semi-humid region in the HuangHuaiHai Plain of China: A 7-year consecutive field experiment. Soil Tillage Res. 2025, 247, 106367. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, Y.; Wu, Z.; Yan, X.; Gunina, A.; Kuzyakov, Y.; Xiong, Z. Effects of six-year biochar amendment on soil aggregation, crop growth, and nitrogen and phosphorus use efficiencies in a rice-wheat rotation. J. Clean. Prod. 2020, 242, 118435. [Google Scholar] [CrossRef]
- Taran, J.; Bhar, R.; Jha, H.; Kuila, S.K.; Samal, B.; Pradhan, R.; Dubey, B.K. Synthetic coalification of microalgae through hydrothermal carbonization: Strategies for enhanced hydrochar characteristics and technological advancements. Bioresour. Technol. 2025, 429, 132542. [Google Scholar] [CrossRef]
- Martinez-Sanchez, L.; Maestro-Gaitán, I.; de la Rubia, M.A.; Reguera, M.; Mohedano, A.F.; Tobajas, M. Hydrothermal carbonization and pyrolysis of sewage sludge: Plant growth effects of hydrochar and biochar. Biomass Bioenergy 2026, 204, 108424. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Jaya Prasanna Kumar, D.; Narula, A.; Minnat Chistie, S.; Ullhas Naik, S. Production and beneficial impact of biochar for environmental application: A review on types of feedstocks, chemical compositions, operating parameters, techno-economic study, and life cycle assessment. Fuel 2023, 343, 127968. [Google Scholar] [CrossRef]
- Lu, H.; Li, K.; Shi, Y.; Nkoh, J.N.; Hong, Z.; Xu, R.; Jiang, J. Combining biochar and lime amendments for remediation of Cd-contaminated paddy soil around e-waste dismantling site: A study from laboratory to field. J. Environ. Manag. 2025, 394, 127490. [Google Scholar] [CrossRef]
- Gao, Y.; Tong, H.; Zhao, Z.; Cheng, N.; Wu, P. Effects of Fe oxides and their redox cycling on Cd activity in paddy soils: A review. J. Hazard. Mater. 2023, 456, 131665. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.R.; Jahan, I.; Mou, S.J.; Hasin, M.F.; Angon, P.B.; Sultana, R.; Mazumder, B.; Sakil, M.A. Function of Biochar: Alleviation of Heat Stress in Plants and Improvement of Soil Microbial Communities. Phyton-Int. J. Exp. Bot. 2025, 94, 1177–1210. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Antoniadis, V.; Shahid, M.; Yang, Y.; Abdelrahman, H.; Zhang, T.; Hassan, N.E.E.; Bibi, I.; Niazi, N.K.; Younis, S.A.; et al. Sustainable applications of rice feedstock in agro-environmental and construction sectors: A global perspective. Renew. Sustain. Energy Rev. 2022, 153, 111791. [Google Scholar] [CrossRef]
- Acevedo-De-los-Ríos, A.; Dyson, A.; Claeys, D.; Cardenas-Mamani, U. Environmental performance of urban agriculture in the global south: A comprehensive literature review and life cycle analysis approach. Environ. Impact Assess. Rev. 2025, 115, 108040. [Google Scholar] [CrossRef]
- Tabakh, M.; Attarzadeh, R.; Yarahmadi, R.; Amanati, M.; Momeny, M.; Maleki, H.; Najafi, A.M.; Zandifar, A.; Mukhitdinov, O.; Jumanazarov, D. From waste to functional materials: A review on innovations in waste Management for Environmental Remediation Applications. Coord. Chem. Rev. 2026, 549, 217379. [Google Scholar] [CrossRef]
- Ghodszad, L.; Reyhanitabar, A.; Maghsoodi, M.R.; Asgari Lajayer, B.; Chang, S.X. Biochar affects the fate of phosphorus in soil and water: A critical review. Chemosphere 2021, 283, 131176. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Liu, H.; Mao, W.; Zhang, J.; Wang, B.; Yang, L.; Wang, S.; Zhou, H.; Zeng, P.; et al. Phosphorus-loaded magnetic biochar for remediation of cadmium contaminated paddy soil: Efficacy and identification of limiting factors. J. Hazard. Mater. 2025, 492, 138162. [Google Scholar] [CrossRef]
- Yuan, X.; Li, S.; Yang, F.; Wang, S.; Bie, S.; Wang, Z.; Zhang, H.; Liu, J.; Zhou, J.; Wang, X.; et al. A review on As-contaminated soil remediation using waste biomass feedstock-based biochar and metal-modified biochar. Ecotoxicol. Environ. Saf. 2025, 292, 117927. [Google Scholar] [CrossRef]
- Ma, P.; Rosen, C. Land application of sewage sludge incinerator ash for phosphorus recovery: A review. Chemosphere 2021, 274, 129609. [Google Scholar] [CrossRef]
- Chu, Q.N.; Lyu, T.; Xue, L.H.; Yang, L.Z.; Feng, Y.F.; Sha, Z.M.; Yue, B.; Mortimer, R.J.G.; Cooper, M.; Pan, G. Hydrothermal carbonization of microalgae for phosphorus recycling from wastewater to crop-soil systems as slow-release fertilizers. J. Clean. Prod. 2021, 283, 124627. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, C.; Zhao, B.; Di, D.; Xiao, J.; Chen, G. One-pot synthesis of high-efficiency hydroxyapatite-hydrochar composites derived from phytoremediation biomass and bone meal: Heavy metal stabilization, adsorption performance and mechanism. J. Alloys Compd. 2025, 1022, 179859. [Google Scholar] [CrossRef]
- Xu, X.; Zou, Z.; Guo, X.; Liang, S.; Yang, F.; Chen, S.; Yu, W.; Duan, H.; Yuan, S.; Yang, J. Comprehensive evaluation of bioavailable phosphorus in biochar synthesized by co-pyrolysis of sewage sludge and straw ash. Sci. Total Environ. 2024, 954, 176679. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: A meta-analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Li, W.T.; Liu, M.; Jiang, C.Y.; Wu, M.; Chen, X.F.; Ma, X.Y.; Li, Z.P. Changes in soil aggregate-associated enzyme activities and nutrients under long-term chemical fertilizer applications in a phosphorus-limited paddy soil. Soil Use Manag. 2017, 33, 25–33. [Google Scholar] [CrossRef]
- Cognigni, P.; Leonelli, C.; Berrettoni, M. A bibliographic study of biochar and hydrochar: Differences and similarities. J. Anal. Appl. Pyrolysis 2025, 187, 106985. [Google Scholar] [CrossRef]
- Kwapińska, M.; Burke, N.; Leahy, J.J. Hydrothermal carbonization and pyrolysis of dairy processing sludge for improved nutrient management in agriculture: Current state-of-the-art. Chem. Eng. J. Adv. 2025, 24, 100888. [Google Scholar] [CrossRef]
- Figueiredo, C.C.; Pinheiro, T.D.; de Oliveira, L.E.Z.; de Araujo, A.S.; Coser, T.R.; Paz-Ferreiro, J. Direct and residual effect of biochar derived from biosolids on soil phosphorus pools: A four-year field assessment. Sci. Total Environ. 2020, 739, 140013. [Google Scholar] [CrossRef]
- Ge, Q.; Dong, C.; Wang, G.; Zhang, J.; Hou, R. Production, characterization and environmental remediation application of emerging phosphorus-rich biochar/hydrochar: A comprehensive review. RSC Adv. 2024, 14, 33649–33665. [Google Scholar] [CrossRef]
- Leghari, A.; Xiao, Y.; Ding, L.; Raheem, A.; Ryzhkov, A.; Yu, G. Research advancements in nutrients and heavy metals, its speciation and behavior during hydrothermal carbonization of sludge—A critical review. Fuel 2023, 352, 129082. [Google Scholar] [CrossRef]
- Dou, R.; Gao, F.; Tan, Y.; Xiong, H.-R.; Xu, Z.-X.; Osman, S.M.; Zheng, L.-J.; Luque, R. Phosphorus species transformation and recovery in deep eutectic solvent-assisted hydrothermal carbonization of sewage sludge. Sustain. Chem. Pharm. 2024, 37, 101408. [Google Scholar] [CrossRef]
- Gou, L.; Ping, Z.; Dai, L.; Wang, Y. Influence of organic acids with varying chain lengths on the physicochemical properties and combustion performance of hydrochar from sewage sludge. J. Environ. Chem. Eng. 2025, 13, 118983. [Google Scholar] [CrossRef]
- Kooij, J.; Yang, P.-T.; Bruun, S.; Magid, J.; Gro Nielsen, U.; Theil Kuhn, L.; Müller-Stöver, D. Phosphorus speciation in different sewage sludges and their biochars and its implications for movement of labile phosphate in two soils. J. Environ. Manag. 2024, 370, 122565. [Google Scholar] [CrossRef]
- Emamverdian, A.; Khalofah, A.; Pehlivan, N.; Ghorbani, A. Utilizing nano-biochar and biochar for sustainable heavy metal remediation and enhanced crop tolerance: Innovative approaches in nano-biosensing and environmental health. Ind. Crop. Prod. 2025, 234, 121462. [Google Scholar] [CrossRef]
- Abdo, A.I.; Li, Y.; Shi, Z.; El-Saadony, M.T.; Alkahtani, A.M.; Chen, Y.; Wang, X.; Zhang, J.; Wei, H. Biochar of invasive plants alleviated impact of acid rain on soil microbial community structure and functionality better than liming. Ecotoxicol. Environ. Saf. 2024, 282, 116726. [Google Scholar] [CrossRef]
- Wang, K.; Xu, J.; Chen, P.; Liao, L.; Fan, J.; Wang, H.; Sleutel, S. Balancing energy inputs and carbon outcomes in hydrochar applications: Temperature-Dependent effects on soil carbon sequestration. Biomass Bioenergy 2026, 204, 108382. [Google Scholar] [CrossRef]
- Djandja, O.S.; Liew, R.K.; Liu, C.; Liang, J.; Yuan, H.; He, W.; Feng, Y.; Lougou, B.G.; Duan, P.-G.; Lu, X.; et al. Catalytic hydrothermal carbonization of wet organic solid waste: A review. Sci. Total Environ. 2023, 873, 162119. [Google Scholar] [CrossRef]
- Isakovski, M.K.; Jevrosimov, I.; Tamindžija, D.; Apostolović, T.; Knicker, H.; de la Rosa, J.M.; Rončević, S.; Maletić, S. Enhanced retention of hydrophobic pesticides in subsurface soils using organic amendments. J. Hazard. Mater. 2024, 480, 135738. [Google Scholar] [CrossRef]
- Khosravi, A.; Zheng, H.; Liu, Q.; Hashemi, M.; Tang, Y.; Xing, B. Production and characterization of hydrochars and their application in soil improvement and environmental remediation. Chem. Eng. J. 2022, 430, 133142. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, S.; Yan, P.; Wei, Z.; Wang, H.; Zhang, H.; Niu, X.; Aurangzeib, M.; Tao, G. Biochar application strategies mediating the soil temperature, moisture and salinity during the crop seedling stage in Mollisols. Sci. Total Environ. 2025, 958, 178098. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, C.; Chen, X.; Jiang, F.; Hopkins, I.; Zhang, X.; Han, Z.; Billy, G.; Benavides, J. Soil acidity, available phosphorus content, and optimal biochar and nitrogen fertilizer application rates: A five-year field trial in upland red soil, China. Field Crop. Res. 2019, 232, 77–87. [Google Scholar] [CrossRef]
- Höyhtyä, I.; Ronkanen, A.-K.; Liimatainen, M.; Hyvärinen, M.; Kløve, B.; Marttila, H. Soil chemical properties to retain phosphorus in managed boreal peatlands in northern Finland. Soil Tillage Res. 2025, 248, 106452. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Ohoro, C.R.; Ojukwu, V.E.; Oniye, M.; Shaikh, W.A.; Biswas, J.K.; Seth, C.S.; Mohan, G.B.M.; Chandran, S.A.; Rangabhashiyam, S. Biochar for ameliorating soil fertility and microbial diversity: From production to action of the black gold. iScience 2025, 28, 111524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, M.; Yang, C.; Sun, R.; Li, H.; Zhao, Y.; Wang, L. Biochar and Phosphogypsum in agricultural soils: Individual roles, combined potentials, and future prospects. Pedosphere 2025. [Google Scholar] [CrossRef]
- Zhang, E.; Meng, C.; Qu, J.; Zhu, Z.; Niu, J.; Wang, L.; Song, N.; Yin, Z. Dual effects of Caragana korshinskii introduction on herbaceous vegetation in Chinese desert areas: Short-term degradation and long-term recovery. Plant Soil 2025. [Google Scholar] [CrossRef]
- Yin, X.; Xi, M.; Li, Y.; Kong, F.; Jiang, Z. Improvements in physicochemical and nutrient properties of sewage sludge biochar by the co-pyrolysis with organic additives. Sci. Total Environ. 2021, 779, 146565. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Effects of sewage sludge amendment on heavy metal accumulation and consequent responses of Beta vulgaris plants. Chemosphere 2007, 67, 2229–2240. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.G.; Sha, H.Y.; Wang, Y.R.; Wang, H.Y.; Zhu, G.C.; Lu, Y.Z. Lanthanum-quaternized chitosan-modified zeolite for long-lasting operation of constructed wetland: A bifunctional strategy for simultaneous phosphorus removal and microbial clogging mitigation. Water Res. 2026, 288, 124688. [Google Scholar] [CrossRef]
- Yu, N.; Zeng, X.; Ma, X.; Sun, Y.; Wang, Y.; Chen, B.; Luo, Z.; Dong, C.; Shen, K.; Wu, J. Preparation of Ce-Fe2O3/Al2O3 catalyst for simultaneous degradation of benzodiacetone and reduction of Cr(VI) by electro-Fenton process: Performance, mechanism, degradation pathways. J. Alloys Compd. 2025, 1045, 184745. [Google Scholar] [CrossRef]
- Xi, W.; Ping, Y.; Tao, J.; Ye, X.; Fu, M.; Zhang, Y.; Xie, M. Enhanced prediction of copper-polymetallic deposits in the Kalatag mining district using integrated SVM and GIS technology. Earth Sci. Inform. 2024, 18, 11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.