Abstract
With the aim of reducing catalysts’ cost while maintaining high performance in water splitting, ZnO and RuO2 were combined into composites with ZnO to RuO2 mass ratios of 1:1, 2:1, and 10:1. The ZnO/RuO2 composites were prepared by microwave processing of a suspension containing Zn(OH)2 in situ precipitated onto RuO2 powder, and subsequently thermally modified at 600 °C to promote heterojunction formation and alter the defect chemistry. Phase composition, crystal structure, morphology, and optical properties were analyzed in detail employing XRD, TEM/HRTEM, HAADF-STEM with EDS, PL and XPS spectroscopy. The photoelectrocatalytic (PEC) activity of the composites toward the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) was evaluated by linear sweep voltammetry in alkaline electrolyte (0.1 M NaOH, pH 13), before and after one hour of electrochemical system illumination. The analysis focused on surface and bulk oxygen vacancies, which may have a crucial impact in PEC activity, by (1) promoting charge separation and increasing the number of active sites thus enhancing PEC activity, or (2) acting as electron–hole traps and recombination centers, reducing the lifetime of photo-induced charge carriers and thus deteriorating PEC activity. The presented results demonstrate that the combination of ZnO with RuO2 in a specific mass ratio, along with controlled defect structure, offers a worthwhile route for developing bifunctional, noble-metal-reduced catalysts for green hydrogen and oxygen production.
1. Introduction
Global climate change, driven by greenhouse gas emissions from fossil fuel use, has made the transition to sustainable and clean energy sources a major priority. Among the various energy alternatives, hydrogen is considered a clean fuel, as its use leads to no direct environmental pollution. However, conventional industrial hydrogen production methods still heavily rely on fossil fuel reforming, which causes high levels of CO2 emissions and thus impedes the aims of sustainable energy systems [1,2]. In this context, photoelectrochemical (PEC) water splitting offers a promising route for hydrogen production with minimal environmental impact, supporting the move toward renewable and environmentally friendly energy technologies [3,4].
Water splitting involves two half-reactions: the hydrogen evolution reaction (HER) at the cathode, and the oxygen evolution reaction (OER) at the anode. The thermodynamic requirement for water splitting under standard conditions is a potential difference of ∆E0 = 1.23 V [5]. However, practical systems require significantly higher voltages due to kinetic activation barriers at the electrode surfaces, electrolyte resistance, and interfacial resistance between the electrodes and the electrolyte. These factors result in overpotential and limit the overall performance of the process. Efficient electrocatalysts are essential for improving performance by accelerating reaction kinetics and enhancing HER and OER activity [6]. It is known that an ideal electrocatalyst should have high conductivity, an appropriate adsorption and desorption capacity, and high structural stability [7]. Noble metals and metal oxides are widely recognized as the most effective electrocatalysts for achieving high activity and stability in both reactions [8]. Pt/C is recognized as the best electrocatalyst for HER and ORR, while IrO2 and RuO2 are recognized as the best for OER [9]. Although noble metals are highly efficient, limited availability and a high cost significantly hinder their wide-ranging application in commercial devices [10]. Therefore, it is essential to develop new materials that can either replace them entirely or at least reduce their content, without compromising the efficiency of the overall process. A common strategy involves also combining noble metal oxides with affordable, multifunctional metal oxides to maintain high catalytic activity using reduced noble metal content [11,12,13,14,15,16,17]. Some novel approaches in the development of electrocatalysts with enhanced HER, OER, ORR or even bifunctional HER/OER activity include organic complexes, such as, e.g., ionic pentanuclear complex of the type [Ru(dmbpy)3]3[Fe(CN)6]2, where dmbpy is 4,4′-dimethyl-2,2′-bipyridine, which is used to impregnate highly reduced graphene oxide nanosheet-supported RuO2-Fe2O3 nanocomposite [13], or metal-free and metal phthalocyanine complex [18].
Due to their favorable opto-electronic properties such as a wide band gap, high exciton binding energy, and good electron mobility, ZnO-based materials have been widely studied as (photo)electrocatalysts for water splitting [19,20]. Previous studies have shown that ZnO-based particles processed by microwave irradiation exhibit enhanced (photo)electrocatalytic performance due to the rich defect chemistry induced by rapid crystallization during the synthesis [21]. The (photo)electrocatalytic properties can be further improved by tuning the ratio of surface-to-bulk defects [22], which has been shown to be effectively adjusted through the thermal treatment of ZnO-based composites with another semiconductor [23]. In addition, the formation of heterojunctions is considered an effective approach, as the interface between semiconductors with different band gaps can facilitate more efficient charge separation and slow down the recombination of photogenerated carriers, thereby improving overall catalytic efficiency [24,25,26].
In our previous work, we investigated the influence of thermal modification on the (photo)electrocatalytic activity of ZnO/RuO2 composites with ZnO:RuO2 mass ratios of 2:1 and 10:1 [26,27]. In the present study, a composite with a 1:1 mass ratio was synthesized using microwave processing of a precipitate to examine how the relative contents of ZnO and RuO2 affect the (photo)electrocatalytic activity toward HER and OER in an alkaline electrolyte. The composite was subsequently annealed at 600 °C to modify both the concentration of oxygen vacancies and the local symmetry within the crystal structure. Phase composition, crystal structure, morphology, and optical properties of the composites were analyzed in detail. The obtained physicochemical properties were then correlated with their (photo)electrocatalytic performance, highlighting the role of oxygen vacancies in determining catalytic activity.
2. Materials and Methods
2.1. Materials
Commercially available ruthenium oxide (RuO2, >99.9%, Sigma-Aldrich, Saint Louis, MO, USA), zinc chloride (ZnCl2, >99.5%, Lach-Ner, Neratovice, Czech Republic) and sodium hydroxide (NaOH, >98%, CARLO ERBA Reagents, Cornerado (MI), Italy) were used in synthesis procedures. Distilled water was used as the solvent and for rinsing the synthesized powders, while absolute ethanol (Zorka, Šabac, Serbia) was applied for final rinsing.
2.2. Synthesis of the Composite Catalysts
ZnO/RuO2 composites were synthesized by microwave processing of a precipitate method in different mass ratios, ZnO:RuO2 = 1:1, 2:1, and 10:1. The synthesis procedure was identical for all samples, with the only difference being the initial mass of commercial RuO2 powder, adjusted according to the targeted ratio. Specifically, 0.4400 g, 0.2200 g, and 0.0440 g of RuO2 were used for the 1:1, 2:1, and 10:1 mass ratios, respectively. In each case, the RuO2 powder was dispersed in 100 mL of distilled water under magnetic stirring for 5 min. Subsequently, 0.8976 g of ZnCl2 was added, and the synthesis was continued under the same conditions as previously described [26,27]. The synthesized ZnO/RuO2 composite powders were further thermally modified with the aim of tuning their crystallinity and oxygen vacancy concentration. Annealing was carried out at 600 °C in a tube furnace (Protherm Furnaces, Ankara, Turkey). The powders were heated in air at a rate of 10 °C min−1 to the target temperature and isothermally treated for 60 min. After thermal treatment, the samples were left to cool to room temperature inside the furnace. A schematic diagram of the step-by-step synthesis procedure used for the manufacture of ZnO/RuO2 composites is presented in Figure 1.
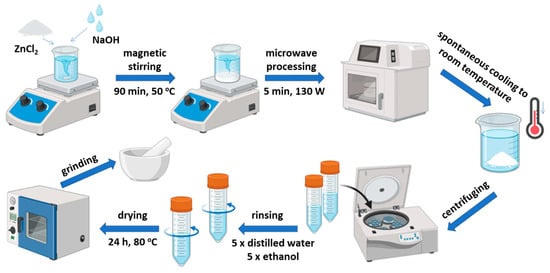
Figure 1.
Schematic diagram of the synthesis procedure.
The synthesized (photo)electrocatalysts were designated as follows: 1ZnO/1RuO2, 1ZnO/1RuO2-600, 2ZnO/1RuO2, 2ZnO/1RuO2-600, 10ZnO/1RuO2, and 10ZnO/1RuO2-600. The numbers 1, 2, and 10 represent the mass ratios of zinc oxide to ruthenium oxide, while 600 indicates the annealing temperature in °C.
2.3. Characterization
X-ray diffraction (XRD) patterns were recorded on a Philips PW 1050 diffractometer (Philips Analytical, Almelo, The Netherlands) with CuKα1,2 (λ = 1.54178 Å) Ni-filtrated radiation, operating at 40 kV and 20 mA. The data were collected over an angular range from 20 to 80° 2θ, with a step size of 0.05° and a dwell time of 5 s per step. The crystal phases were identified using Match!3 database software (Version 3.5) [28], with reference to the Crystallography Open Database (COD) patterns [29]. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) micrographs were recorded using an FEI Talos F200X microscope (Thermo Fisher Scientific, Waltham, MA, USA) operated at 200 kV. For sample preparation, the powders were first dispersed in ethanol using an ultrasonic bath, and a small amount of the suspension was drop-cast onto a carbon-coated copper grid. The grid was then left to dry in air. Elemental distribution and mapping were carried out on the same instrument using HAADF-STEM (High-Angle Annular Dark Field Scanning Transmission Electron Microscopy) and EDX (Energy Dispersive X-ray Spectroscopy) at 200 keV. The particle size distribution was estimated from the TEM images employing a SemAfore 5.21 digital slow scan image recording system. Photoluminescence (PL) spectra were recorded at room temperature using a Horiba Jobin Yvon Fluorolog FL3–22 spectrofluorometer (Tokyo, Japan), equipped with a xenon (Xe) lamp as the excitation source. Spectra were measured in the 350–700 nm range, with a step size of 1 nm and a slit width of 4 nm. Excitation was performed at a wavelength of 280 nm using a slit width of 6 nm. The integration time was set to 0.2 s. X-ray photoelectron spectroscopy (XPS) analysis was performed on SPECS Systems with a monochromatized Al Kα X-ray source (1486.74 eV), operating at 12.5 kV and 250 W. During the analysis, the samples were fixed onto an adhesive copper foil to provide strong mechanical attachment and good electrical contact. The measurements were taken in the fixed analyzer transmission mode (FAT) under a vacuum below 5 × 10−9 mbar. Survey spectra were recorded over a binding energy range from −5 to 1000 eV, with an energy step width of 0.5 eV, a dwell time of 0.2 s, and constant pass energy of 40 eV. Detailed XPS spectra were acquired at pass energy of 20 eV, an energy step of 0.1 eV, and a dwell time of 2 s. All spectra were collected by SpecsLab data analysis software Version 2.79-r33432 and analyzed using the CasaXPS software package Version 2.3.16Dev52. The spectral analysis was performed after Shirley-type background subtraction.
The (photo)electrocatalytic activity of the synthesized materials was evaluated using linear sweep voltammetry (LSV). All (photo)electrochemical measurements were performed using an Ivium VertexOne potentiostat/galvanostat (Eindhoven, The Netherlands). Experiments were conducted in a three-electrode quartz cell consisting of a glassy carbon electrode (GCE) as the working electrode (surface area ~0.3 cm2), a saturated calomel electrode (SCE) as the reference electrode, and a platinum foil as the counter electrode. The ink was prepared using the same procedure for all samples by dispersing 10 mg of the catalytic material in a solution containing 10 µL of Nafion (Sigma-Aldrich, Saint Louis, MO, USA, 5%), 50 µL of ethanol, and 50 µL of distilled water, using an ultrasonic bath for 45 min. A amount of 5 µL of the ink was coated onto the GCE as a thin film and dried under an infrared lamp Tungsram INFRASEC 250 W (Budapest, Hungary) for 45 s to evaporate the solvent.
The electrocatalytic activity was investigated in an alkaline electrolyte (0.1 M NaOH, p.a., Merck, Darmstadt, Germany; pH~13). Measurements were performed before and after 60 min of electrochemical cell illumination using an Osram Ultra-Vitalux 300 W lamp (Munich, Germany). The distance between the light source and the cell was approximately 15 cm, and the light intensity at the sample surface was estimated to be ~100 mW cm−2, based on measurements with a PeakTech 5165 Digital Lux Meter (Ahrensburg, Germany) and the conversion factor (1 mW cm−2 ≈ 126 lx). The potential was swept in the range from 0.2 to −1.9 V vs. SCE for HER and from 0.2 to 1.9 V vs. SCE for OER with a scan rate of 20 mV s−1. All measured potentials were converted to the reversible hydrogen electrode (RHE) scale using Equation (1):
For the purpose of comparison, the LSV of the bare GCE was recorded in the same experimental conditions as the modified electrodes.
2.4. Computational Procedure
The DFT calculations were carried out with the Vienna ab initio simulation package VASP [30,31] and we used several different supercells constructed with the experimental lattice parameters. In particular, the 2 × 2 × 2, 3 × 3 × 3, and 4 × 4 × 4 supercell geometries were employed, spanning the range of concentrations from 6.25 to 0.39 at. %. The charge state of the vacancy 2+, as known from the literature [32], was factored into the calculations. Geometry optimization of all the atoms was performed with a force criterion of 0.02 eV/Å and the cut-off energy was set to 500 eV. For the lattice relaxation, we used the Projector Augmented Wave (PAW) pseudopotentials in the PBE implementation [31,33], whereas for the band structure calculations in the optimized geometry, the modified Becke-Johnson (mBJ) functional was used [34]. For the geometry optimization, we have selected a 4 × 4 × 3 and 2 × 2 × 1 Monkhorst Pack [35] k-point mash in Brillouin-zone (BZ) of the 2 × 2 × 2 and 3 × 3 × 3 supercell models, respectively, and only the Γ-point was used in the largest supercell. One of the advantages of the mBJ functional is that it can be tailored to accurately reproduce the experimental ZnO band gap value (the c parameter of the mBJ potential was set to 1.64, as in Ref. [33]), which is important for considerations of the position and shape of the vacancy defect levels within the band gap.
3. Results
The crystal structure, element distribution and morphology of the ZnO/RuO2 composites were investigated using XRD, HAADF-STEM and TEM/HRTEM analyses. The diffraction patterns, HAADF-STEM and TEM images of the as-prepared and annealed ZnO/RuO2 composites are presented in Figure 2. The diffraction patterns of the as-prepared composites can be fully assigned by reflections characteristic for a wurtzite-type ZnO structure with a hexagonal phase (P63mc space group; COD no. 96-230-0113; patterns marked with the blue asterisk), and a rutile-type RuO2 structure with a tetragonal phase (P42/mnm space group; COD no. 96-900-7542; patterns marked with the red circle) (Figure 2a) [26,27,29]. According to the XRD patterns, the composites annealed at 600 °C show the same phase compositions as the as-prepared counterpart, with certainly increased crystallinity.
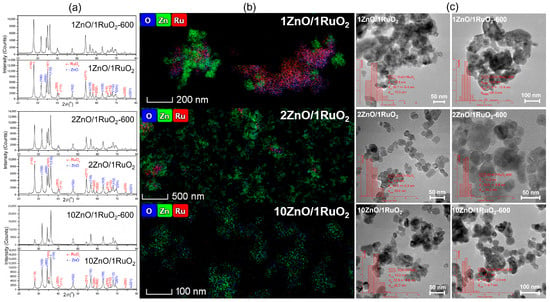
Figure 2.
(a) XRD patterns, (b) HAADF-STEM images with EDS maps (O, Zn, Ru), and (c) TEM micrographs with particle size distribution histograms of ZnO/RuO2 composites.
HAADF-STEM images, as presented in Figure 2b, show that Zn, Ru and O elements are uniformly distributed in the 2ZnO/1RuO2 and 10ZnO/1RuO2 composites, which is in contrast with the non-uniform distribution of these elements observed in the 1ZnO/1RuO2 powder. TEM images of the as-prepared 1ZnO/1RuO2, 2ZnO/1RuO2, and 10ZnO/1RuO2 composites predominantly show spheroidal nanoparticles, with notable agglomeration observed in the 1ZnO/1RuO2 sample. Upon annealing, the nanoparticles maintain their spheroidal morphology, although their size slightly increases due to further agglomeration (Figure 2c). The particle size distribution histograms determined from the TEM images are presented as insets in Figure 2c. The estimated average particle sizes are 26.7, 41.8, 21.2, 30.5, 22.8, and 38.5 nm, for 1ZnO/1RuO2, 1ZnO/1RuO2-600, 2ZnO/1RuO2, 2ZnO/1RuO2-600, 10ZnO/1RuO2, and 10ZnO/1RuO2-600, respectively.
Moreover, HRTEM images of the 1ZnO/1RuO2, 2ZnO/1RuO2 and 10ZnO/1RuO2 composites annealed at 600 °C revealed the formation of heterojunctions at the interfaces between grains of different phases (Figure 3). Heterojunction formation is a consequence of the homogeneous initial mixing of ZnO and RuO2 oxides, which enabled effective phase contact during thermal treatment. Accordingly, the HRTEM image of the 1ZnO/1RuO2-600 composite (Figure 3a’) shows a heterojunction created at the interface of two grains with a different phase composition: observed d spacing of 2.282 Å, corresponds to the (100) plane of ZnO, while 2.560 Å, corresponds to the (101) plane of RuO2. The overlap region, which indicates the heterojunction formation, is marked with a green dashed line. For the 2ZnO/1RuO2-600 composite (Figure 3b’), a heterojunction is observed at the interface of two grains with a d spacing of 2.282 Å, corresponding to the (100) plane of ZnO, and 3.176 Å, corresponding to the (110) plane of RuO2. Similarly, Figure 3c’ reveals that there are two classes of lattice fringes with a d spacing of 2.282 Å, belonging to (100) plane of ZnO, and 2.560 Å, belonging to (101) planes of RuO2 in 10ZnO/1RuO2-600 composite. Observed d values are in accordance with data reported for ZnO and RuO2 phases in COD: 96-230-0113 and 96-900-7542 [29].
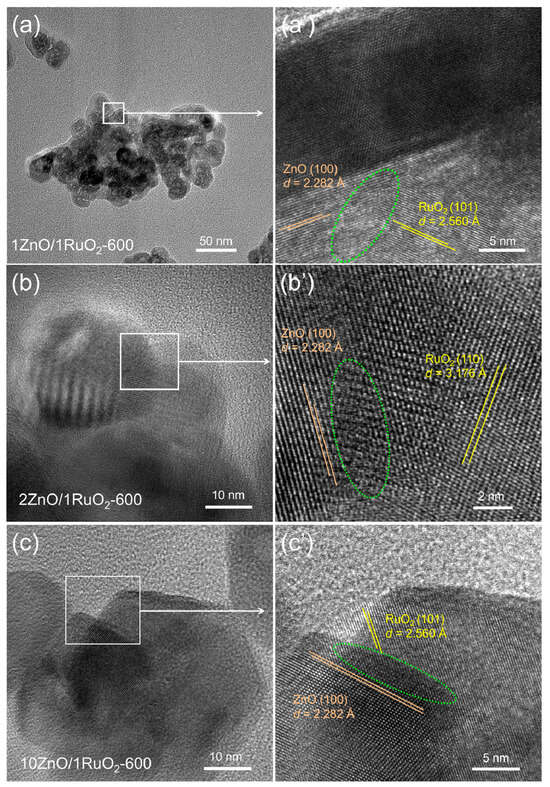
Figure 3.
TEM (a–c) and HRTEM (a’–c’) micrographs of the ZnO/RuO2-600 composites, showing the crystal planes and interplanar spacing (d) of ZnO and RuO2; the heterojunction regions are marked with a green dashed line.
Photoluminescence (PL) spectroscopy was employed to investigate how different mass ratios and thermal treatments affect the number of defects in the crystal structure, especially oxygen vacancies. PL spectra of the as-prepared ZnO/RuO2 composites (Figure 4a) exhibit broad emission bands of high intensity centered at around 630 nm, which are associated with bulk defects. In contrast, the low-intensity bands observed near 420 nm indicate a negligible amount of surface defects across all examined mass ratios (Figure 4b). PL spectra of the annealed ZnO/RuO2 composites, as presented in Figure 4c, indicate that thermal modification leads to a significant reduction in bulk defects accompanied by a slight reduction in surface defects compared with as-prepared composites. To reveal the position (nm) and integrated area (a.u.) of the emission bands, the PL spectra were mathematically deconvoluted using a Gaussian–Lorentzian area function. The deconvoluted spectra are presented in Figure 4d–i, while data obtained by deconvolution are listed in Table 1. Spectra components are centered near 380, 420, 570, 630 and 750 nm. UV emission near the band edge at 380 nm can be attributed to the recombination of charge carriers and violet-blue emission is attributed to the transition from surface defects to the valence band [22], while green-yellow, orange-red, and red emissions are defined as defect-related deep-level emission [36].
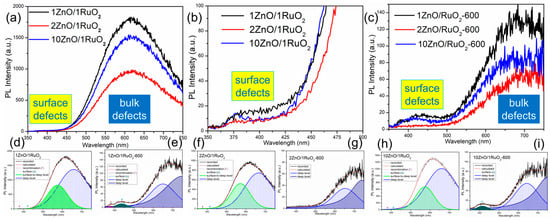
Figure 4.
PL spectra of ZnO/RuO2 catalysts with different ZnO:RuO2 mass ratios: (a) as-prepared powders; (b) zoomed view of (a) with surface defect region; (c) composites calcined at 600 C; (d–i) PL spectra deconvoluted with a Gaussian–Lorentzian area function.

Table 1.
Position and integrated area of emission bands in PL spectra obtained after deconvolution by a Gaussian–Lorentzian area function.
Integrated area obtained by the deconvolution of the PL spectra indicate that, among the as-prepared composites, 2ZnO/1RuO2 contains the smallest amount of surface and bulk defects while 1ZnO/1RuO2 has the biggest amount of both types of defects. Thermal treatment of as-prepared composites stimulates a significant reduction in bulk defects, accompanied by a slight decrease in surface defect amount, which, in terms of total yield, improved crystal structure order.
The PEC activity of the catalysts toward HER and OER in 0.1 M NaOH was examined using linear sweep voltammetry (LSV). The performance of the ZnO/RuO2 composites as catalysts was evaluated based on two key parameters: the onset potential taken as the potential at which the current density reached −10 Ag−1, and the current density measured at the end of the applied potential range. The LSV curves of the as-prepared and annealed 1ZnO/1RuO2, 2ZnO/1RuO2 and 10ZnO/1RuO2 composites before and after 60 min of system illumination are shown in Figure 5a–c. The LSV of the bare glassy carbon (GC) electrode, recorded in the same experimental conditions as the modified electrodes, is added in diagrams in Figure 5a–c for comparison, allowing us to evaluate the photoelectrocatalytic activity of ZnO/RuO2 composites.
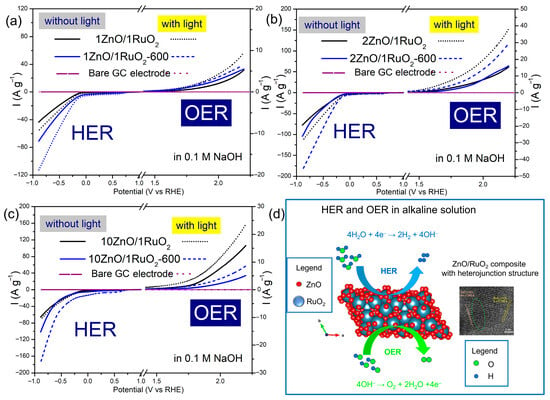
Figure 5.
LSV curves of ZnO/RuO2 catalysts in 0.1 M NaOH, recorded before and after 60 min of illumination: (a–c) effect of thermal treatment on the HER and OER activity of 1ZnO/1RuO2, 2ZnO/1RuO2, and 10ZnO/1RuO2, respectively; (d) illustration of HER and OER in alkaline solution in the presence of ZnO/RuO2 composite.
It can be seen that the electrocatalytic performance of the ZnO/RuO2 composites was notably influenced by the mass ratio of the components. The estimated onset potentials for hydrogen evolution, recorded before system illumination, were −0.322, −0.238, and –0.291 V vs. RHE for 1ZnO/1RuO2, 2ZnO/1RuO2 and 10ZnO/1RuO2, respectively. The corresponding current densities were –43.47, –78.01 and –65.61 A g−1, Table 2. The (photo)electrocatalytic activity of the composites improved after illumination of the electrochemical cell, as indicated by a shift of onset potentials to more negative values and an increase in current densities. Illumination of the system leads to the generation of electron–hole pairs within the (photo)electrocatalyst, which accelerates the reduction of water molecules in an alkaline electrolyte and promotes the formation of hydrogen molecules [7,37]. After 60 min of system illumination, the onset potentials shifted to −0.263, −0.164, and −0.251 V vs. RHE, while the current densities increased to −55.40, −115.41, and −71.56 Ag−1 for 1ZnO/1RuO2, 2ZnO/1RuO2, and 10ZnO/1RuO2, respectively. The values of the onset potential and the current density read from the graphs are listed in Table 2.

Table 2.
Onset potentials (V vs. RHE) and current densities (A g−1) of the tested catalysts toward HER and OER in alkaline media before and after 60 min of light irradiation.
Similarly, Ubaidullah et al. demonstrated that ZnO-based composites, such as ZnO@NMC, exhibit remarkable electrocatalytic properties, with HER onset potentials of –1.700 V vs. Ag/AgCl, which is comparable to the results of the present study [38]. After 60 min of illumination, all tested materials also displayed enhanced activity, suggesting that the generation of electron–hole pairs significantly promoted the reduction of adsorbed protons and facilitated the formation of hydrogen molecules [39].
The estimated onset potentials for oxygen evolution, recorded before system illumination, were 2.047, 1.737 and 1.824 V vs. RHE for 1ZnO/1RuO2, 2ZnO/1RuO2 and 10ZnO/1RuO2, respectively. The corresponding current densities were 5.26, 15.58 and 15.96 Ag−1. After 60 min of system illumination, a pronounced improvement was observed, as the onset potentials shifted to 1.879, 1.566 and 1.686 V vs. RHE, while the current densities significantly increased to 9.56, 38.22 and 23.56 Ag−1 for 1ZnO/1RuO2, 2ZnO/1RuO2, and 10ZnO/1RuO2, respectively. The 2ZnO/1RuO2 composite exhibited the best performance in the oxygen evolution reaction, similar to its behavior in HER, maintaining the lowest onset potential and highest current density both before and after system illumination.
Among the as-prepared samples, the 2ZnO/1RuO2 composite demonstrated the best activity toward the hydrogen evolution reaction and oxygen evolution reaction, both before and after system illumination, exhibiting the lowest onset potential and the highest current density. This suggests that a balanced proportion of ZnO and RuO2 facilitates more efficient charge transfer and enhances catalytic performance. The 1ZnO/1RuO2 composite, despite its higher RuO2 content, exhibited lower activity, likely due to inhomogeneous distribution of the components and increased particle agglomeration. In contrast, the 10ZnO/1RuO2 composite, with the lowest RuO2 content, also showed reduced activity, indicating that an insufficient amount of RuO2 limits the number of catalytically active sites. Our results are consistent with the findings of Jang et al., who reported similar trends while investigating the (photo)electrocatalytic activity of sol–gel synthesized ZnO-based materials in 0.1 M KOH [40].
All samples were annealed at 600 °C to elucidate the effect of thermal treatment on their electrochemical performance for HER and OER activity (Figure 5a–c). For the 1ZnO/1RuO2 composite, the estimated onset potentials were −0.226 V vs. RHE, with current densities of −71.04 A g−1 after annealing for HER. In the case of the 2ZnO/1RuO2 composite, onset potentials of −0.217 V vs. RHE were recorded, along with current densities of −104.11 A g−1 after annealing for HER. For the 10ZnO/1RuO2 composite, onset potentials were −0.250 V vs. RHE, and the corresponding current densities were −101.96 A g−1 after annealing for HER (Table 2).
Similar improved HER activity was observed for all annealed composites after illumination. For 1ZnO/1RuO2, the onset potentials shifted to −0.157 V vs. RHE, with current densities of −112.93 A g−1 after annealing. The 2ZnO/1RuO2 composite showed onset potentials of −0.138 V vs. RHE, and current densities of −185.41 A g−1. In the case of 10ZnO/1RuO2, the onset potentials were −0.198 V vs. RHE, with current densities of −175.26 A g−1. After illumination, enhanced OER activity was observed for all annealed composites. For 1ZnO/1RuO2, the onset potentials shifted to 1.958 V vs. RHE, with current densities of 6.62 A g−1 after annealing. The 2ZnO/1RuO2 composite showed onset potentials of 1.650 V vs. RHE, and current densities of 29.59 A g−1. In the case of 10ZnO/1RuO2, the onset potentials were 1.893 V vs. RHE, with current densities of 8.53 A g−1, respectively.
A significant enhancement in (photo)electrocatalytic activity was observed for all composites annealed at 600 °C. This improvement can be attributed to the reduced number of bulk oxygen vacancies and the formation of heterojunctions. The reduced number of bulk defects lowers the density of recombination centers, thereby extending the lifetime of photogenerated charge carriers. As a result, more electrons and holes remain separated and available to participate in catalytic processes, enhancing (photo)electrocatalytic efficiency. However, while a high concentration of oxygen vacancies tends to act as a recombination center and reduce the lifetime of charge carriers, an intermediate concentration of bulk defects can contribute to improved performance by facilitating charge separation and enhancing electron transport. Overall, optimizing the relative ratio between surface and bulk defects may significantly improve the overall photoelectrocatalytic activity of ZnO-based composites.
The 2ZnO/1RuO2-600 composite showed the best electrocatalytic (without employed light) activity toward HER. Among those analyzed in our research, this material possesses the lowest amount of surface oxygen vacancies (SOVs). Since SOVs can act as traps for electrons, which are crucial for the reduction of H2O to H2, their lower amount enables more efficient charge transfer and enhanced HER performance. The 2ZnO/1RuO2-600 composite is also the best photoelectrocatalyst for HER due to its relatively small number of bulk defects, as is explained above. According to Liu and coworkers, compared with HER, OER is a more complex and energy-intensive process in the water splitting reaction, with multiple proton-/electron-coupled steps [41]. In an alkaline solution, OER involves the oxidation of hydroxide ions (OH−) to produce oxygen (O2), as shown in the illustration in Figure 5d. The electron transfer and reaction kinetics will be faster if OH− has intimate contact with the catalyst’s surface; thus, OER prefers catalyst surfaces with positive active sites, which oxygen vacancies are. This fact explains why 2ZnO/1RuO2 composites have better activity than their thermally treated counterpart.
XPS was employed to comprehend the contribution of different surface oxygen species in 2ZnO/1RuO2 as the best PEC; recorded high-resolution XPS spectra of O 1s peaks are presented in Figure 6a,b. The O 1s peaks were deconvoluted by two Voigt area functions, represented in Figure 6 as O 1s (1) and O 1s (2). According to the literature data, low-energy peak, O 1s (1), positioned at 529.8 eV, can be attributed to O2 ions from the Zn–O bonds in the ZnO lattice [42], while the O 1s (2) peak at 531.3 eV can be related to oxygen vacancies in the ZnO lattice [36]. The relative concentrations of O 1s (1) and O 1s (2) gained by spectra deconvolution and fitting are 63.8 and 31.7% for 2ZnO/1RuO2 and 7.8.6 and 21.4% for 2ZnO/1RuO2-600; their ratios are presented in Figure 6c. A change in the O(1)/O(2) ratio from 2.15 for 2ZnO/1RuO2 to 3.67 for 2ZnO/1RuO2-600 suggests that surface oxygen vacancy defects in metal oxides can be healed by thermal treatment up 600 °C, even in an air atmosphere. This statement about crystal structure ordering by thermal treatment is in line with the finding of the XRD (Figure 2a) and PL (Figure 5) studies.
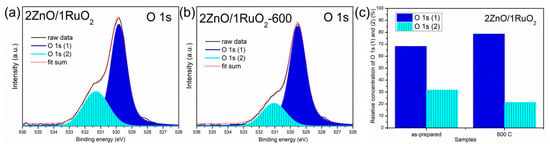
Figure 6.
(a,b) High-resolution XPS spectra of O 1s peaks deconvoluted with the Voigt area function, and (c) the O 1s (1) and O 1s (2) relative amount calculated from the XPS spectra.
Density Functional Theory was employed in order to explain the observed trends in the photocatalytic activity of the studied composites, calculating the electronic structure of oxygen vacancy (VO) in wurtzite-type ZnO as a function of vacancy concentration. Figure 7 shows the effective band structures [43,44] of four different concentrations of VO, unfolded onto the primitive ZnO cell. The defect-like state(s) are clearly visible within the band gap. For the supercell models with the two higher concentrations, the dispersion of the defect levels within the band gap is larger (Figure 7a,b), while as the concentration of VO is reduced, the levels become flatter and are shifted toward the bottom of the conduction band minimum (Figure 7c,d). The defect states inside the gap can act as electron–hole traps and recombination centers, reducing the lifetime of the photo-induced charge carriers, which brings about a decrease in the photocatalytic activity. However, if these states inside the gap are located in the lower or top third of the band gap, the lifetime of the charge carriers will be reduced but will not reach its minimum, as it would should they be located at the mid-band energy level. In the examined case of ZnO-based composites, that means that the sample with the defect states near the conduction band minimum would be the most PEC active, as was observed in the experiment.
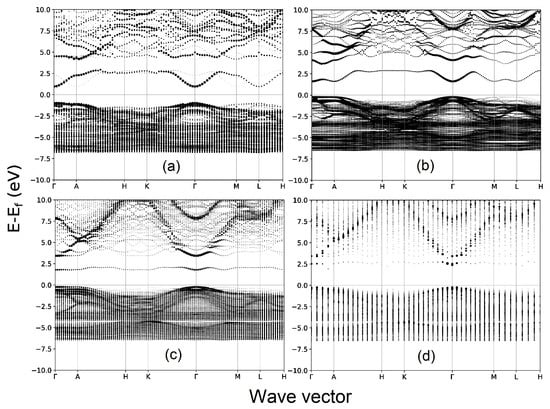
Figure 7.
Effective band structures of VO in ZnO: (a) 6.25 at.%-two VO in a 2 × 2 × 2 supercell, (b) 3.125 at.%-one VO in a 2 × 2 × 2 supercell, (c) ~0.926 at.%-one VO in a 3 × 3 × 3 supercell, and (d) ~0.39 at.%-one VO in a 4 × 4 × 4 supercell.
To gain deeper insight into the electronic structure of the studied composites, site-projected densities of states (PDOSs) were calculated and the results are displayed in Figure 8. As expected, Zn 3d and O 2p states mainly constitute the valence band (VB), while the main component in the conduction band (CB) is the Zn 4s state. The peaks corresponding to the defect states can be clearly seen between valence band maximum (VBM) and conduction band minimum (CBM). These states are mainly of O p character and are located about 2 eV above the VBM.
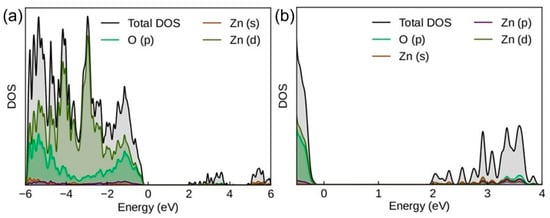
Figure 8.
Density of states for ZnO containing 6.25% of oxygen vacancies: (a) complete spectrum, and (b) enlarged part with the defect states.
4. Conclusions
The present study revealed the results of the phase composition, crystal structure ordering, ZnO, Ru, and O elemental distribution, morphology, and optical properties of ZnO/RuO2 composites with three different mass ratios (1:1, 2:1, and 10:1), as well as the potential for their application as photoelectrocatalysts for HER and OER in an alkaline solution. It was revealed that the increased amount of RuO2 in the composite with ZnO did not necessary increase PEC; thus, expensive RuO2 can be successfully replaced with affordable ZnO. The results confirmed that the thermal treatment of prepared composites at 600 °C provokes crystal structure changes, particularly a significant reduction in bulk defects and the formation of heterojunctions at the interfaces between grains of different crystal phases. Both a reduced number of bulk defects and heterojunctions improve the PEC of ZnO/RuO2 composites. The presence of heterojunctions in the ZnO/RuO2 composites led to a modification of their electronic structure, slowing down the recombination of charge carriers and prolonging the lifetime of photogenerated electrons and holes. As a result, the electron transfer process was accelerated, enhancing the electrocatalytic efficiency of the composites. Reducing the bulk defects, i.e., defects in the middle of the gap, which act as electron–hole traps and recombination centers, also prolongs the lifetime of the photo-induced charge carriers, which brings about an increase in the photocatalytic activity.
Applying strategies including the formation of heterojunction structures, optimization of total oxygen vacancy amount, and surface-to-bulk oxygen vacancy ratio can improve surface properties, electron transfer, and charge separation efficiency in ZnO/RuO2 composites, promoting them as an efficient photoelectrocatalysts for water splitting.
Author Contributions
K.A.: investigation, methodology, and writing—reviewing; I.S.S.: methodology, investigation, writing–reviewing and editing, and supervision; M.P.: investigation, writing; J.N.B.-Č.: investigation and writing; L.M.: investigation and writing—reviewing; S.M.: conceptualization, methodology, investigation, writing—reviewing and editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Contract Nos. 451-03-136/2025-03/200175, 451-03-137/2025-03/200146, and 451-03-136/2025-03/200017).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data supporting the findings of this study will be deposited in the repository of the Institute of Technical Sciences of SASA following publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- El-Adawy, M.; Dalha, I.B.; Ismael, M.A.; Al-Absi, Z.A.; Nemitallah, M.A. Review of Sustainable Hydrogen Energy Processes: Production, Storage, Transportation, and Color-Coded Classifications. Energy Fuels 2024, 38, 22686–22718. [Google Scholar] [CrossRef]
- Tian, J.; Yu, L.; Xue, R.; Zhuang, S.; Shan, Y. Global Low-Carbon Energy Transition in the Post-COVID-19 Era. Appl. Energy 2022, 307, 118205. [Google Scholar] [CrossRef]
- Jilani, A.; Ibrahim, H. Development in Photoelectrochemical Water Splitting Using Carbon-Based Materials: A Path to Sustainable Hydrogen Production. Energies 2025, 18, 1603. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Lai, T.-H.; Kuo, M.-Y.; Hsieh, P.-Y.; Hsu, Y.-J. Photoelectrochemical Cells for Solar Hydrogen Production: Challenges and Opportunities. APL Mater. 2019, 7, 080901. [Google Scholar] [CrossRef]
- Li, W.; Tian, H.; Ma, L.; Wang, Y.; Liu, X.; Gao, X. Low-Temperature Water Electrolysis: Fundamentals, Progress, and New Strategies. Mater. Adv. 2022, 3, 5598–5644. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, X.; Zhen, Y.; Liang, Y. Photogenerated Carrier-Assisted Electrocatalysts for Efficient Water Splitting. Catalysts 2023, 13, 712. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Feng, X. Support and Interface Effects in Water-Splitting Electrocatalysts. Adv. Mater. 2019, 31, 1808167. [Google Scholar] [CrossRef]
- Hanan, A.; Nazim Lakhan, M.; Shu, D.; Hussain, A.; Ahmed, M.; Soomro, I.A.; Kumar, V.; Cao, D. An efficient and durable bifunctional electrocatalyst based on PdO and Co2FeO4 for HER and OER. Int. J. Hydrog. Energy 2023, 48, 19494–19508. [Google Scholar] [CrossRef]
- Milikić, J.; Balčiūnaitė, A.; Sukackienė, Z.; Mladenović, D.; Santos, D.M.F.; Tamašauskaitė-Tamašiūnaitė, L.; Šljukić, B. Bimetallic Co-Based (CoM, M = Mo, Fe, Mn) Coatings for High-Efficiency Water Splitting. Materials 2021, 14, 92. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Hong, M.; Zhang, L.; Feng, X.; Shi, M.; Hu, W.; Mu, S. Defective RuO2/TiO2 Nano-Heterostructure Advances Hydrogen Production by Electrochemical Water Splitting. Chem. Eng. J. 2022, 431, 134072. [Google Scholar] [CrossRef]
- Jin, D.; Yoo, H.; Lee, Y.; Lee, C.; Kim, M.H. IrO2–ZnO Composite Nanorod Array as an Acid-Stable Electrocatalyst with Superior Activity for the Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5, 3810–3820. [Google Scholar] [CrossRef]
- Mosallaei, H.; Hadadzadeh, H.; Ensafi, A.A.; Mousaabadi, K.Z.; Weil, M.; Foelske, A.; Sauer, M. Evaluation of HER and OER Electrocatalytic Activity over RuO2–Fe2O3 Nanocomposite Deposited on HrGO Nanosheets. Int. J. Hydrog. Energy 2023, 48, 1813–1830. [Google Scholar] [CrossRef]
- Shekhawat, A.; Samanta, R.; Barman, S. MOF-Derived Porous Fe3O4/RuO2-C Composite for Efficient Alkaline Overall Water Splitting. ACS Appl. Energy Mater. 2022, 5, 6059–6069. [Google Scholar] [CrossRef]
- Tariq, M.; Wu, Y.; Ma, C.; Ali, M.; Zaman, W.Q.; Abbas, Z.; Ayub, K.S.; Zhou, J.; Wang, G.; Cao, L.; et al. Boosted up Stability and Activity of Oxygen Vacancy Enriched RuO2/MoO3 Mixed Oxide Composite for Oxygen Evolution Reaction. Int. J. Hydrog. Energy 2020, 45, 17287–17298. [Google Scholar] [CrossRef]
- Ren, F.; Xu, J.; Feng, L. An Effective Bimetallic Oxide Catalyst of RuO2-Co3O4 for Alkaline Overall Water Splitting. Nano Res. 2024, 17, 3785–3793. [Google Scholar] [CrossRef]
- Uzgören, İ.N.; Hüner, B.; Yıldırım, S.; Eren, O.; Özdoğan, E.; Süzen, Y.O.; Demir, N.; Kaya, M.F. Development of IrO2–WO3 Composite Catalysts from Waste WC–Co Wire Drawing Die for PEM Water Electrolyzers’ Oxygen Evolution Reactions. ACS Sustain. Chem. Eng. 2022, 10, 13100–13111. [Google Scholar] [CrossRef]
- Aralekallu, S.; Sannegowda, L.K.; Singh, V. Developments in electrocatalysts for electrocatalytic hydrogen evolution reaction with reference to bio-inspired phthalocyanines. Int. J. Hydrog. Energy 2023, 48, 16569–16592. [Google Scholar] [CrossRef]
- Kulmas, M.; Paterson, L.; Höflich, K.; Bashouti, M.Y.; Wu, Y.; Göbelt, M.; Ristein, J.; Bachmann, J.; Meyer, B.; Christiansen, S. Composite Nanostructures of TiO2 and ZnO for Water Splitting Application: Atomic Layer Deposition Growth and Density Functional Theory Investigation. Adv. Funct. Mater. 2016, 26, 4882–4889. [Google Scholar] [CrossRef]
- Ghorbani, M.; Abdizadeh, H.; Taheri, M.; Golobostanfard, M.R. Enhanced Photoelectrochemical Water Splitting in Hierarchical Porous ZnO/Reduced Graphene Oxide Nanocomposite Synthesized by Sol-Gel Method. Int. J. Hydrog. Energy 2018, 43, 7754–7763. [Google Scholar] [CrossRef]
- Rajić, V.; Simatović, I.S.; Veselinović, L.; Čavor, J.B.; Novaković, M.; Popović, M.; Škapin, S.D.; Mojović, M.; Stojadinović, S.; Rac, V.; et al. Bifunctional Catalytic Activity of Zn1-xFexO toward the OER/ORR: Seeking an Optimal Stoichiometry. Phys. Chem. Chem. Phys. 2020, 22, 22078–22095. [Google Scholar] [CrossRef]
- Marković, S.; Simatović, I.S.; Ahmetović, S.; Veselinović, L.; Stojadinović, S.; Rac, V.; Škapin, S.D.; Bogdanović, D.B.; Častvan, I.J.; Uskoković, D. Surfactant-Assisted Microwave Processing of ZnO Particles: A Simple Way for Designing the Surface-to-Bulk Defect Ratio and Improving Photo(Electro)Catalytic Properties. RSC Adv. 2019, 9, 17165–17178. [Google Scholar] [CrossRef]
- Marković, S.; Stanković, A.; Dostanić, J.; Veselinović, L.; Mančić, L.; Škapin, S.D.; Dražič, G.; Janković-Častvan, I.; Uskoković, D. Simultaneous Enhancement of Natural Sunlight- and Artificial UV-Driven Photocatalytic Activity of a Mechanically Activated ZnO/SnO2 Composite. RSC Adv. 2017, 7, 42725–42737. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, D.; Lian, J.; Ma, Q.; Yu, J.; Huang, H.; Zhong, J.; Li, J. Preparation of Efficient Visible-Light-Driven BiOBr/Bi2O3 Heterojunction Composite with Enhanced Photocatalytic Activities. J. Alloys Compd. 2015, 649, 474–482. [Google Scholar] [CrossRef]
- Hamrouni, A.; Lachheb, H.; Houas, A. Synthesis, Characterization and Photocatalytic Activity of ZnO–SnO2 Nanocomposites. Mater. Sci. Eng. B 2013, 178, 1371–1379. [Google Scholar] [CrossRef]
- Aleksić, K.; Stojković Simatović, I.; Stanković, A.; Veselinović, L.; Marković, S. Influence of Thermal Treatment on the Photoelectrocatalytic Activity of 2ZnO/1RuO2 Composites as Photoanode for Water Splitting. Sci. Sinter. 2025, in press. [Google Scholar] [CrossRef]
- Aleksić, K.; Stojković Simatović, I.; Stanković, A.; Veselinović, L.; Stojadinović, S.; Rac, V.; Radmilović, N.; Rajić, V.; Škapin, S.D.; Mančić, L.; et al. Enhancement of ZnO@RuO2 Bifunctional Photo-Electro Catalytic Activity toward Water Splitting. Front. Chem. 2023, 11, 1173910. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Match!—Phase Analysis Using Powder Diffraction; Version 3.5; Crystal Impact: Bonn, Germany, 2023. [Google Scholar]
- Crystallography Open Database. Open-Access Collection of Crystal Structures of Organic, Inorganic, Metal-Organic Compounds and Minerals, Excluding Biopolymers. Available online: http://www.crystallography.net/cod/ (accessed on 1 February 2023).
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 1169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Liu, L.; Mei, Z.; Tang, A.; Azarov, A.; Kuznetsov, A.; Xue, Q.-K.; Du, X. Oxygen vacancies: The origin of n-type conductivity in ZnO. Phys. Rev. B 2016, 93, 235305. [Google Scholar] [CrossRef]
- Perdew, P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Tran, F.; Blaha, V. Accurate band gaps of semiconductors and insulators with a semilocal exchange-correlation potential. Phys. Rev. Lett. 2009, 102, 226401. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Kumar, V.; Swart, H.C.; Ntwaeaborwa, O.M.; Kroon, R.E.; Terblans, J.J.; Shaat, S.K.K.; Yousif, A.; Duvenhage, M.M. Origin of the red emission in zinc oxide nanophosphors. Mater. Lett. 2013, 101, 57–60. [Google Scholar] [CrossRef]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of Interfaces in Two-Dimensional Photocatalyst for Water Splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Ubaidullah, M.; Al-Enizi, A.M.; Shaikh, S.; Ghanem, M.A.; Mane, R.S. Waste PET Plastic Derived ZnO@NMC Nanocomposite via MOF-5 Construction for Hydrogen and Oxygen Evolution Reactions. J. King Saud Univ.-Sci. 2020, 32, 2397–2405. [Google Scholar] [CrossRef]
- Biroju, R.K.; Pal, S.; Sharma, R.; Giri, P.K.; Narayanan, T.N. Stacking Sequence Dependent Photo-Electrocatalytic Performance of CVD Grown MoS2/Graphene van Der Waals Solids. Nanotechnology 2017, 28, 085101. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.M.; Kwak, I.H.; Kwon, E.L.; Jung, C.S.; Im, H.S.; Park, K.; Park, J. Transition Metal Doping of Oxide Nanocrystals for Enhanced Catalytic Oxygen Evolution. J. Phys. Chem. C 2015, 119, 1921–1927. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Gu, L.; Zhang, Y.; Li, G.-D.; Zou, X. Corrosion engineering towards efficient oxygen evolution electrodes with stable catalytic activity for over 6000 hours. Nat. Commun. 2018, 9, 2609. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Popescu, V.; Zunger, A. Effective Band Structure of Random Alloys. Phys. Rev. Lett. 2010, 104, 236403. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W.T. VASPKIT: A User-Friendly Interface Facilitating High-Throughput Computing and Analysis Using VASP Code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).