Abstract
H2S detection is critical for personal and industrial safety. Generally, metal oxide-based H2S sensors exhibit no response at room temperature (RT). In this study, CuO/ZnWO4 (C-ZWO) nanocomposites were prepared via a two-step hydrothermal process and applied to RT H2S sensing. The results show that the C-ZWO sensors exhibit an elevated response value at RT and balanced gas-sensing properties at 100 °C. Significantly, the response value of a 10% C-ZWO sensor to 25 ppm of H2S at RT is 651.6 with a response time of 78 s, which is 310.3 times that of the ZnWO4 sensor (2.1). The systemic characterization results suggest that the enhanced RT H2S-sensing properties are ascribed to the synergistic effects of the growth-specific surface area and oxygen vacancy occupancy, the enhanced oxygen reduction ability, and the formation of the p–n heterojunction structure between CuO and ZnWO4. The C-ZWO nanocomposites possess added active sites for H2S adsorption and dissociation, with the p–n heterojunction giving rise to higher electrical resistance, and thus, the follow-up produces a high response value even at RT.
1. Introduction
H2S, a significant chemical, is used in metal refining, pesticides, pharmaceuticals, and catalyst regeneration [1]. However, H2S is a flammable, hazardous gas, i.e., it can form an explosive mixture with air, and lead to combustion and explosion with an open flame, high heat, or an electric spark [2]. Also, H2S is incredibly toxic, and human inhalation of H2S gas can cause headaches, anxiety, memory loss, olfactory paralysis, respiratory failure, and even death [2,3]. The Immediately Dangerous To Life or Health (IDLH) concentration of H2S is 100 ppm. The industry-recommended exposure limit for H2S gas is 10 ppm [4,5]. As a hazardous gas, H2S is generated in large amounts during various industrial production processes. Additionally, H2S is present in environments such as mines, ship holds, and septic tanks [6]. Therefore, real-time detection of H2S leaks and concentrations is necessary to ensure personal safety and reduce property damage. The most commonly used methods for detecting H2S include fluorescent probes [7,8], indicator paper [9], etc. Their drawbacks are the complexity of the detection method, the long detection time, and the inaccurate detection of concentration values. Therefore, developing low-cost and straightforward H2S sensors is imperative.
Gas sensors have broad applications in industrial production and daily life, including the detection of explosives [10] and toxic gases [11], volatile chemicals [12], medical detection [13,14], and environmental pollution monitoring [15,16]. Among a wide variety of materials, metal oxide semiconductors (MOSs) are considered an ideal candidate for gas-sensing materials due to their unique strengths, such as fast response, good stability, simplicity, lightness, low cost, and simple integration [17,18]. CuO is a p-type semiconductor (bandgap, Eg~1.2–1.5 eV) [19], which has been reported to have excellent selectivity for H2S, but it has some limitations in its development and application due to its drawbacks, such as slow response [20] and low sensitivity [21]. However, it was found that the H2S sensitivity of the material can be increased substantially by doping CuO in MOSs with a wide bandgap to construct heterojunctions, and it has excellent selectivity for H2S. Zhang et al. [22] prepared Cu-doped In2O3 hollow nanofibers via electrospinning. The response value to 50 ppm H2S at 250 °C increased by more than 20 times, reaching 350.7. Of note, the response value (S) is the ratio of the resistance of the sensor in the air (Rg) to the resistance in the target gas (Rg); S = Ra/Rg [10,12,23,24]. A high response value indicates a good gas-sensing property. Wang et al. [25] synthesized CuO/WO3 hollow microspheres; the response value to 10 ppm H2S at 70 °C increased by more than 103 times compared with that of WO3. The materials doped with CuO exhibit excellent selectivity, in addition to a substantial increase in sensitivity; one of the reasons for this is related to the formation of the metallic phase of CuS upon contact between CuO and H2S [10,22,25].
ZnWO4, a scheelite n-type semiconductor with a unique ABO4 structure [26,27,28], is a metallic tungstate with a low crystallographic symmetry monoclinic wolframite structure (Eg~3.37 eV) that poses a considerable obstacle to electron mobility [29]. However, because of its low cost, high chemical stability, non-toxicity, relatively high catalytic activity, scintillation, and magnetic and luminescent properties [30], ZnWO4 has been widely reported in the last few years in the fields of photocatalysis [31], photoelectrodes [32], capacitors [33], and electrochemistry [34]. The reported ZnWO4 sensors have only been used for the detection of acetone [30] and triethylamine [35,36]. Zang et al. [37] synthesized the ZnO@ZnWO4 heterojunction H2S sensor, exhibiting a response of 127.31 to 5 ppm H2S at 200 °C. Hu et al. [38] reported the ZnWO4/WO3 heterojunction for trace H2S detection with 0.0783 ppm at 310 °C. However, a high operating temperature poses an extra risk for the stability of H2S-sensing materials and sensors. There are few reports on the construction of p–n anisotype junctions (heterostructures) of CuO and ZnWO4 for H2S detection, especially at RT, which holds promise for research and application prospects. Herein, a combined hydrothermal-calcination process was carried out to construct CuO and ZnWO4 heterostructures, and the RT and low-temperature H2S gas-sensing properties and H2S-sensing mechanisms of CuO-ZnWO4 (C-ZWO) nanocomposites were studied for the first time. The results show that the construction of CuO-ZnWO4 heterojunctions can significantly improve H2S-sensing performances, and the selectivity is effectively improved.
2. Materials and Methods
2.1. Material
All chemical reagents (analytical-grade purity) involved in the experiments were used directly without further purification. CuO-ZnWO4 composite materials were prepared by a combined hydrothermal-calcination method, the details of which are provided in the Supplementary Materials. The four materials with specific molar percentages (0, 5, 10, and 15 mol%) of CuO were named 0% C-ZWO, 5% C-ZWO, 10% C-ZWO, and 15% C-ZWO, respectively (Table S1).
2.2. Characterization
The samples were characterized by X-ray diffraction (XRD), Scanning electron microscopy (SEM), Energy-dispersive X-ray spectroscopy (EDS), Transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), Ultraviolet photoelectron spectroscopy (UPS), the Micromeritics ASAP 2460 specific surface and porosity analyzer, and Oxygen Reduction Reaction (ORR) measurements. The details are provided in the Supplementary Materials.
2.3. Fabrication and Measurement of the C-ZWO Gas Sensors
The details of the preparation and measurement of the C-ZWO H2S sensors are provided in the Supplementary Materials, as reported in our previous work [39]. The sensor response value is defined as the ratio of the resistance of the sensor in air to the resistance in the target gas (S = Ra/Rg) [10,12,23,24], which corresponds to the output voltage (Uout) changes in the H2S-sensing measurement process (Figure 1). The response time and recovery time are defined as the time taken by the sensor to reach 90% of the total resistance change [40,41] in the case of adsorption and desorption, respectively. The working temperature is defined and obtained by adjusting the heating voltage (Uh) (Figure 1).
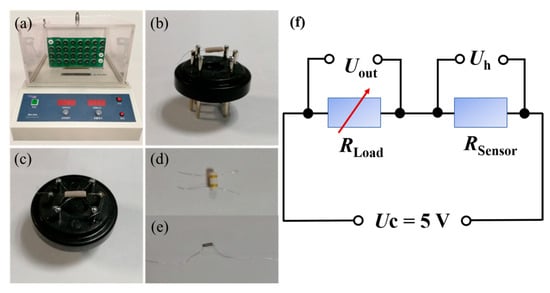
Figure 1.
(a) WS-30A gas sensor test system instrument; (b,c) photos of the gas sensor; (d,e) the gas-sensitive elements: (d) four platinum wires fixed on the gold electrode of the alumina ceramic tube as electrical contacts, (e) Ni-Cr alloy coil; and (f) equivalent circuit diagram of WS-30A gas sensor test system [39].
3. Results and Discussion
3.1. Structural and Morphological Analysis
3.1.1. Phase and Microstructure Analysis
Figure 2a shows the XRD patterns of the prepared C-ZWO composites and the CuO sample. The diffraction peaks at 18.90°, 23.81°, 24.52°, 30.45°, 30.70°, 48.74°, 53.64°, and 64.76° correspond to the (100), (011), (110), (−111), (111), (022), (−202), and (−132) crystal planes of monoclinic ZnWO4 (JCPDS card No. 89-0447) [31,42], respectively. The other weak peaks between 42° and 52° can also be indexed to ZnWO4, compared with the standard diffraction peaks of ZnWO4 (JCPDS card No. 89-0447). Generally, the firm diffraction peaks suggest that the prepared ZnWO4 and CuO-ZnWO4 samples are highly crystalline [29,36,43]. Meanwhile, the diffraction peaks of the obtained CuO sample correspond to CuO (JCPDS card No. 80-0076). It was proved that CuO was successfully prepared by this experimental process, as provided in the Supplementary Materials. However, the indeterminate peaks belonging to CuO within the C-ZWO composites may be attributed to the low amount and low diffraction intensity of CuO.
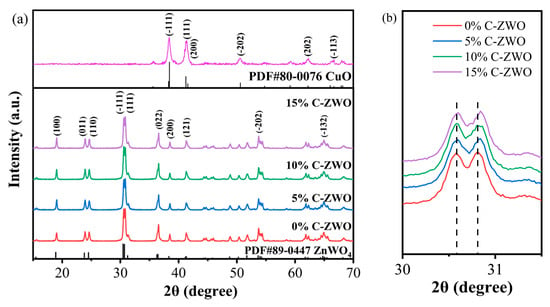
Figure 2.
(a) XRD patterns of 0%, 5%, 10%, and15% C-ZWO and CuO samples, (b) the (−111) and (111) peaks.
However, as Figure 2b shows, with an increase in the amount of CuO, the (111) peaks shift slightly to the right compared with 0% C-ZWO. One possible reason is that the ionic radius of Cu (0.72 Å (Cu2+)) is slightly smaller than that of Zn (0.74 Å (Zn2+)). Thus, the crystal interplanar spacing will be smaller when Cu2+ is mixed into the lattice of Zn2+, leading to the right shift of the diffraction angle based on the Bragg Equation [44,45]. Basically, the shift of (111) peaks indicates that the compound degree of the Cu-based compound and ZnWO4 changes, which may be due to the impurities in the C-ZWO composites. In addition, compared with the 0% C-ZWO sample, the peaks between 60° and 70° of the 5%, 10%, and 15% C-ZWO composites broaden slightly, which may be due to the impurities in the C-ZWO composites mentioned above. Further analysis needs to follow to distinguish the existence form of the Cu-based compound.
As can be observed in the SEM and TEM images in Figure S1 and Figure 3a, the synthesized samples are mainly composed of irregular particles with particle sizes less than 50 nm, and the smallest can reach 20–30 nm. Basically, a smaller grain size implies a larger surface area and, thus, more active sites for H2S adsorption, which improves the sensitivity [10]. The HRTEM images (Figure 3b and insets) exhibit the crystal plane spacings of 0.293 and 0.228 nm, attributed to the (−111) crystal plane of ZnWO4 and the (200) crystal plane of CuO, respectively, confirming the CuO and ZnWO4 in the 10% C-ZWO sample.
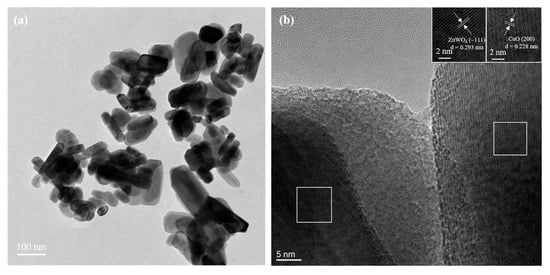
Figure 3.
(a) TEM and (b) HRTEM images of the 10% C-ZWO sample; insets in (b) exhibit the crystal plane spacings of 0.293 and 0.228 nm, corresponding to the left and right white squares in (b), respectively.
3.1.2. Elemental and Valence Analysis
The elemental and valence were analyzed by EDS and XPS measurements. The atomic number ratio obtained for Zn and W is close to 1:1 according to the EDS results (Figure S2 and Table S2). Meanwhile, the elemental mapping analysis of 10% C-ZWO from Figure S2b,f confirms that the elements of W, O, Zn, and Cu are homogeneously distributed in the material. Combined with the HRTEM results, it can be preliminarily confirmed that we successfully synthesized pure ZnWO4 and CuO-ZnWO4 composites via the hydrothermal-calcination method.
As shown in Figure 4a, the full XPS spectra exhibit that Zn, W, and O existed in the 0%C-ZWO sample, while Zn, W, O, and Cu existed in the 10%C-ZWO sample. The representative peaks at 1018 and 1041 eV correspond to Zn 2p3/2 and Zn 2p1/2 [31], respectively (Figure 4b). Two binding energy peaks appear at 32.9 and 34.8 eV for W 4f7/2 and W 4f5/2, respectively (Figure 4c), which determines the existence of W [46]. The two peaks located at 931.0 and 951.1 eV are related to Cu 2p3/2 and Cu 2p1/2 (Figure 4d), respectively, indicating the Cu (II) oxidation state of CuO [10,47].
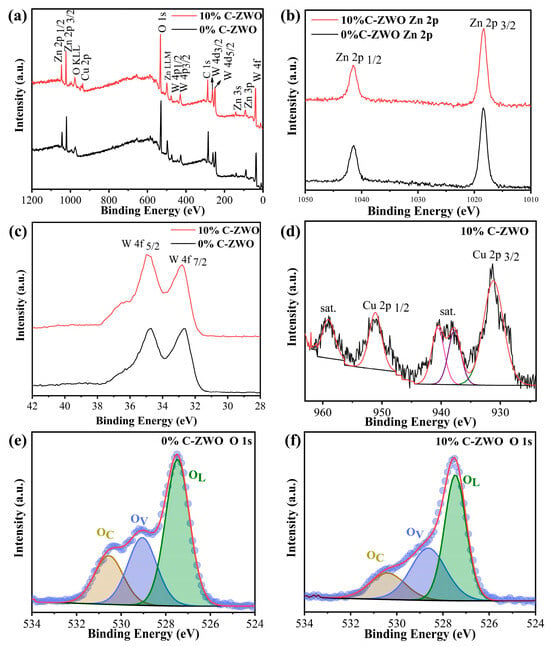
Figure 4.
XPS spectra of 0% and 10% C-ZWO samples: (a) survey, (b) Zn 2p, (c) W 4f, (d) Cu 2p, and (e,f) O 1s.
As exhibited in Figure 4e,f, the peaks of the 0% and 10% C-ZWO samples located at 527.5, 529.0 ± 0.1, and 530.6 ± 0.1 eV of O 1s correspond to lattice oxygen (OL) (light brown in Figure 4e,f), oxygen vacancies (OV) (light blue in Figure 4e,f), and chemisorbed oxygen species (OC) (light green in Figure 4e,f) [48], correspondingly. The relative percentages of OL, OV, and OC in the 0% C-ZWO were 51.0%, 28.5%, and 20.5%, respectively, while the relative percentages were 49.6%, 33.5%, and 19.9% in 10% C-ZWO. Notably, the increased OV of the 10% C-ZWO sample implies growing potential sites for gas adsorption and chemical reactions, thus enhancing its sensing performance [49].
3.1.3. Specific Surface Areas and Porosity Analysis
The significant effects of the porous structures on the H2S-sensing property based on CuO-ZnWO4 nanocomposites were studied, as exhibited in Figure 5. When the relative pressure varies from 0.8 to 1.0, the hysteresis loops are associated with typical IV-type curves with mesoporous structures [50]. All the samples show a broad pore size distribution, ranging from 1 to 70 nm. The average pore width of 0%, 5%, 10%, and 15% C-ZWO was 33.6, 32.2, 33.4, and 32.1 nm, respectively. The specific surface areas (SBET) calculated from the BET method were 12.08, 13.47, 12.41, and 12.27 m2g−1, respectively. The results show an increase in the SBET of the prepared nanoparticle composites compared to 0% C-ZWO after introducing CuO. Moreover, the large SBET and porous nanostructure may facilitate the adsorption and transport of the reaction medium [51], which is consistent with the increased OV results in the XPS analysis.
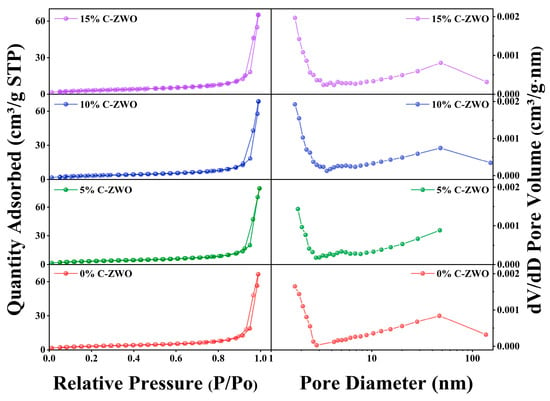
Figure 5.
Nitrogen adsorption–desorption isotherms and corresponding BJH pore size distributions of 0%, 5%, 10%, and 15%C-ZWO samples.
3.2. H2S-Sensing Properties
3.2.1. Effects of Temperature and Composite Ratios on H2S-Sensing Response Properties
Figure 6 shows the response of the C-ZWO sensors tested at temperatures ranging from 25 °C to 100 °C. The response of the 0% C-ZWO sensor to 25 ppm H2S at 25 °C is 2.1, while the responses of the 5%, 10%, and 15% C-ZWO sensors are 426.9, 651.6, and 161.6, respectively. It is demonstrated that ZnWO4 substantially improves the gas-sensing response of ZnWO4 for H2S after being combined with CuO. The enhancement in the response may be due to the increase in the active sites of the C-ZWO composite materials and the construction of the p-n heterojunction of CuO with ZnWO4. With the increasing CuO contents, the response of the C-ZWO composites increased dramatically, and the response of the material to H2S reached a maximum of 310.3 times that of the pristine ZnWO4 when the molar proportion of CuO was 10%. However, when the molar proportion of CuO was 15%, the response of the C-ZWO composite to H2S became lower, which might be the result of the excess CuO covering the H2S active sites on the surface of the C-ZWO composites. More importantly, H2S can interact with CuO, producing CuS in accordance with the reaction CuO + H2S = H2O + CuS, with the excess consumption of CuO leading to the lower response of the 15% C-ZWO sensor.
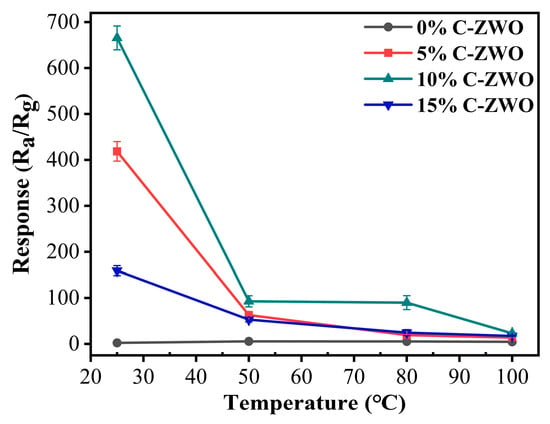
Figure 6.
Response of sensors to 25 ppm H2S at different operating temperatures.
When the temperature increased from 25 °C to 100 °C, the response of the three composite C-ZWO sensors showed a significant decrease. The responses of the 0%, 5%, 10% and 15% C-ZWO sensors are 3.5, 13.5, 22.9, and 17.7, respectively. This is attributed to the fact that the adsorption capacity of the H2S molecules was lower than the desorption capacity at high temperatures, resulting in reduced amounts of test molecules involved in the surface reaction. Although the sensitivity of the 0% C-ZWO sensor increases slightly, it is still lower than the sensitivity of the C-ZWO composite sensors. The excellent room temperature sensing performances of the C-ZWO composite sensors, especially the 10% C-ZWO sample, indicate their significant application potential in the field of H2S room temperature sensing.
3.2.2. Effects of Temperature and Composite Ratios on Gas-Sensing Response and Recovery Performances
The dynamic sensing transients of materials towards H2S detection are further investigated to verify the response and recovery times. Typical response–recovery curves of the composite gas sensors towards 25 ppm H2S at RT and 100 °C are shown in Figure 7 and Figure 8 (the shaded area is their response time or recovery time), respectively. The response time of 0%, 5%, 10% and 15% C-ZWO sensors at RT is 82, 114, 78, and 62 s, respectively. The response time of 0%, 5%, 10% and 15% C-ZWO sensors at 100 °C is 91, 80, 60, and 23 s, respectively. The C-ZWO composite sensors at RT and 100 °C both exhibit a faster response with the increase in the CuO contents.
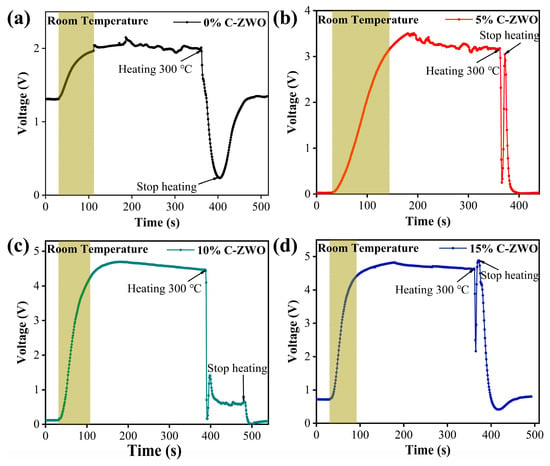
Figure 7.
The response curves of the (a) 0% C-ZWO, (b) 5% C-ZWO, (c) 10% C-ZWO, and (d) 10% C-ZWO sensors to 25 ppm H2S at RT. By adjusting the Uh to 4.58 V, the sensors reached 300 °C and fully recovered in a short period, as indicated by the arrows in Figures (c,d).
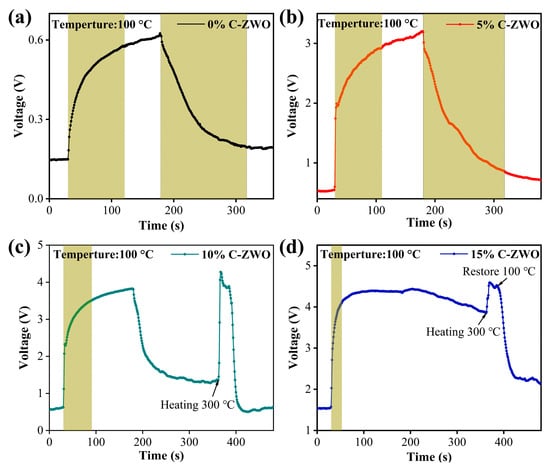
Figure 8.
The response curves of (a) 0% C-ZWO, (b) 5% C-ZWO, (c) 10% C-ZWO, and (d) 10% C-ZWO sensors to 25 ppm H2S at 100 °C. By adjusting the Uh to 4.58 V, the sensors reached 300 °C and fully recovered in a short period, as indicated by the arrows in Figures (c,d).
However, most of the sensors at both RT and 100 °C recover slowly (Figure 7 and Figure 8). Notably, at 100 °C, the 0% C-ZWO and 5% C-ZWO sensing recovered 90% in 180 and 138 s (Figure 8a,b), which fulfils the sensor recovery time criteria. The poor recovery of the sensors is mainly due to the low temperature and slow desorption of the gas. To solve the problem of the recoverability of C-ZWO sensors, a method of autonomous recovery and then heating was adopted in the testing process (Figure 7a–d and Figure 8c,d). As shown in Figure 1, the heating voltage (Uh) works as the working heating power supply of the sensing measure system. By adjusting the Uh as 4.58 V, the sensors reached 300 °C and fully recovered in a short period (as indicated by the arrows in Figure 7a–d and Figure 8c,d). We concluded that in the actual application of the process, we applied a 4.58 V short voltage pulse to recover the sensor. The reason for the fluctuation under the voltage/heating pulse may need further study in the future.
In summary, the 10% C-ZWO sensor at RT exhibits a balanced response of 651.6 and a response time of 78 s, while the 5% C-ZWO sensor at 100 °C shows potential with a response of 13.5 and a response and recovery time of 80 and 138 s, respectively. Notably, applying a 4.58 V short voltage pulse, i.e., heating it at 300 °C, could help to recover the other sensors quickly in potential applications (Table S3).
3.2.3. Effects of H2S Concentration on Gas-Sensing Properties at 100 °C
The effects of H2S concentration on H2S-sensing properties operating in a continuous cycle at 100 °C are shown in Figure 9 and Figure 10, respectively. As shown in Figure 8, compared with the 0% C-ZWO sensor, the three C-ZWO composite sensors all show increased responses as the gas concentration increases. It is noted that the 10% C-ZWO sample exhibits the highest response. The sensors also exhibited good recovery at low H2S concentrations (10 ppm and lower), as exhibited in Figure 9.
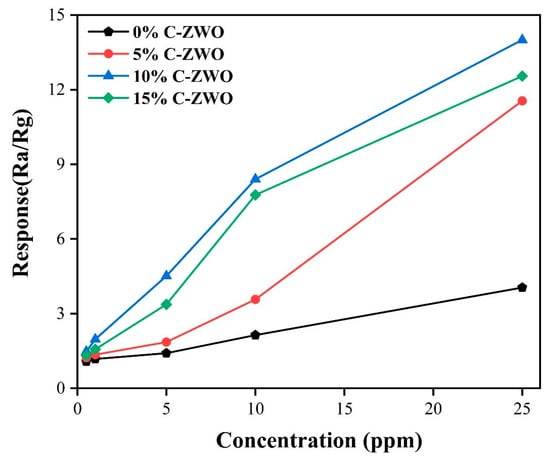
Figure 9.
Responses of the sensors versus H2S concentrations operating at 100 °C.
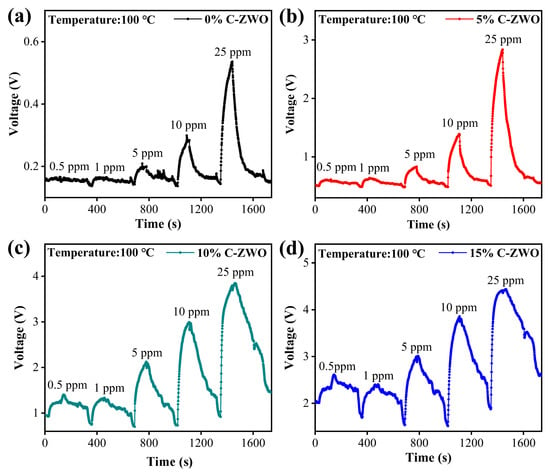
Figure 10.
Dynamic responses of (a) 0% C-ZWO, (b) 5% C-ZWO, (c) 10% C-ZWO, and (d) 10% C-ZWO sensors to various concentrations of H2S.
Notably, the sensitivity of the sensors does not increase by a factor of the H2S concentration, stemming from the fact that the active sites on the surface of gas-sensitive materials are limited. Thus, the adsorption and decomposition of H2S on the surface of the C-ZWO composites are also limited. As shown in Figure 10b, the 5% C-ZWO sensor exhibits the best stability and recovery. The 10% C-ZWO sensor, despite slightly fluctuating, exhibits the best response to different concentrations of H2S, as shown in Figure 10c, and its lowest limit detection is 1 ppm.
3.2.4. The Stability and Repeatability of the Sensors at 100 °C
To explore the stability and repeatability of the sensors, the response curves for five consecutive cycles to 25 ppm H2S at 100 °C were tested. As exhibited in Figure 11, the 0%, 5%, and 10% C-ZWO sensors show smooth dynamic responses. In particular, the 5% C-ZWO sensor has the best stability and recovery, making its application at 100 °C promising. Meanwhile, the recovery of the sensors deteriorates with the increase in CuO content within the same recovery time, and the repeated curve of the 15% C-ZWO sensor shows noticeable fluctuation and attenuation, which may primarily be because it takes a long time for the CuS to recover to CuO in the air at 100 °C [52].
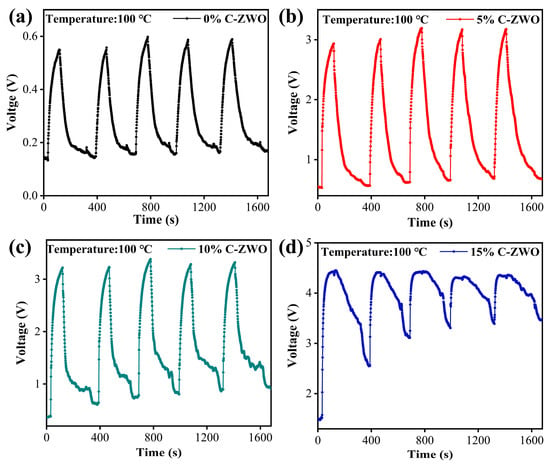
Figure 11.
Repeatability response test of (a) 0% C-ZWO, (b) 5% C-ZWO, (c) 10% C-ZWO, and (d) 10% C-ZWO sensors to 25 ppm H2S at 100 °C.
In addition to the short-term multiple response tests, we also tested the sensor performance over four weeks. A set of results was measured every 7 days (Figure 12). After four weeks (at optimum operation temperature), the sensors based on the 5% C-ZWO and 10% C-ZWO samples have good, acceptable long-term stability, which shows good potential for further applications.
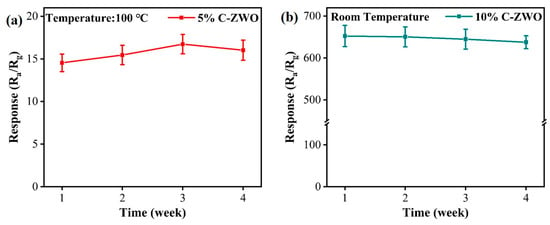
Figure 12.
The response for 4 weeks of (a) the 10%wt C-ZWO to 25 ppm H2S at RT and (b) the 5%wt C-ZWO to 25 ppm H2S at 100 °C.
3.2.5. The Selectivity of the Typical C-ZWO Sensors
The selectivity study was performed using typical C-ZWO sensors with optimal H2S-sensing performance at RT and 100 °C, respectively. To evaluate the selectivity performance of the 10% C-ZWO, we tested its response to five gases or vapors in addition to H2S, i.e., NH3, ethylene glycol, ethanol, methanol, and acetone (Figure 13). As exhibited in Figure 12a, the response of 10% C-ZWO to 25 ppm H2S gas is 651.61, while the response to 100 ppm NH3, ethylene glycol, ethanol, methanol, and acetone gas is 13.32, 3.22, 1.91, 1.50, and 1.40, respectively. The 10% C-ZWO sensor has a far smaller response to other gases than to H2S gas. Meanwhile, as exhibited in Figure 12b, the response of 5% C-ZWO to 25 ppm H2S at 100 °C is 13.50, while the response to 100 ppm ethylene glycol, ethanol, methanol, and acetone gas is 3.33, 1.67, 1.65, and 1.64, respectively. Both sensors exhibit the highest selectivity and response to H2S.
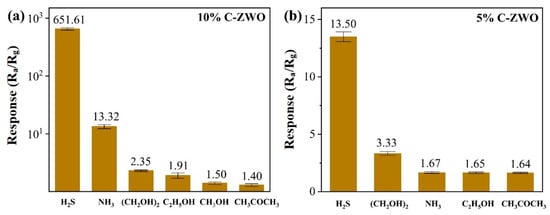
Figure 13.
The selectivity of (a) the 10% C-ZWO sensor at RT and (b) the 5% C-ZWO sensors at 100 °C, to 25 ppm H2S gas and 100 ppm other gases.
3.3. H2S-Sensing Mechanism
3.3.1. H2S-Sensing Process of the C-ZWO Sensors
In n-type MOSs, the potential barrier depends on the depletion layer formed due to the dissociation and adsorption of oxygen molecules and electron transport [37,53,54]. That is, electrons are released back into the conduction band (CB) and the conductance of the ZnWO4 increases. However, it has been found that the sensing performance of the ZnWO4 sample is not outstanding, i.e., a single sensing mechanism has little influence on the resistance of the C-ZWO composite sensing materials. More details are shown in the Supplementary Materials (Figure S3). When CuO was loaded onto ZnWO4, the gas-sensing properties of the composites were substantially enhanced, and the proposed H2S-sensing response mechanism diagram is shown in Figure S4.
Regarding the H2S gas composite surface, in addition to reacting with adsorbed On− (O2−, O2−, and O−), it has been demonstrated that when the sensor is exposed to H2S, a CuS layer may form on the CuO surface [55], with a spontaneous chemical reaction as in Equation (S6). When H2S is removed by introducing air, CuS is unstable and can be slowly oxidized by O2 in air (Equation (S7)). Therefore, recovery of the resistance will occur.
The chemical molecular change from CuO to metallic CuS affects the depletion region at the CuO/ZnWO4 boundary, smothering the conductive channel within the particles [56]. The fracture of p/n junctions in the nanostructures after exposure to H2S effectively broadened the conducting portion, leading to a significant increase in conductivity [56]. ZnWO4 loaded with CuO improves the sensitivity and also enhances the selectivity of the material to H2S gas.
3.3.2. Oxygen Reduction Reaction Analysis
The ORR test explored the effect of CuO loading on the oxygen reduction capacity of ZnWO4, and the oxygen reduction curves obtained are shown in Figure S5. The oxygen reduction ability of the material was investigated by exploring the change in the initial potential of the material before and after the recombination of ZnWO4 and CuO. The starting potential is an important indicator to characterize the strength of the material’s oxygen reduction ability; that is, the higher the initial potential, the stronger the oxygen reduction ability of the C-ZWO composites, and the lower the initial potential, the weaker the oxygen reduction ability of the C-ZWO composites.
The starting potentials of 0%, 5%, 10%, and 15% C-ZWO nanocomposites are 0.728, 0.736, 0.746, and 0.752 eV, respectively, and the initial potentials increase gradually. The results indicate that the recombination of CuO enhances the oxygen reduction ability of ZnWO4, i.e., the ability of the material to turn the O2 adsorbed on its surface into On− (O2−, O2−, and O−) is enhanced. It can consume more e− in the CB of the C-ZWO nanocomposites and improve the change in the resistance of the materials. Therefore, the oxygen reduction performance of the material is improved after the composite CuO, which means that the reaction between H2S and On− on the surface of the material is enhanced, which improves their H2S sensitivities.
3.3.3. Bandgap Analysis and H2S-Sensing Mechanism
To explore the influence of heterojunction nanostructure on H2S-sensing performance, UPS measurements were carried out on ZnWO4 and CuO, as shown in Figure 14. The cut-off edge energies (Ecut-off) of ZnWO4 and CuO are 18.39 and 17.01 eV, respectively; the Fermi edge energy values (EF) are 2.40 and 0.53 eV, respectively; the work functions (Φ) of ZnWO4 and CuO are 5.23 and 4.74 eV, respectively; and the band gaps of ZnWO4 (inset in Figure 13b) and CuO (inset in Figure 14d) are 3.20 and 1.20 eV, respectively. The obtained band structure of ZnWO4 and CuO is shown in Figure 15.
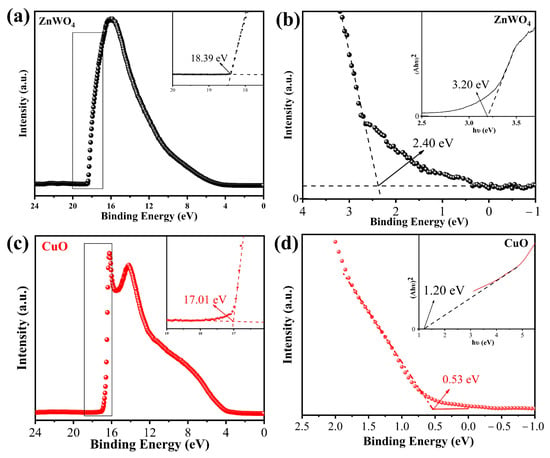
Figure 14.
The UPS spectra of ZnWO4 (a,b) and CuO (c,d) (the insets in (b) and (d) are the band gap spectra of ZnWO4 and CuO, respectively).
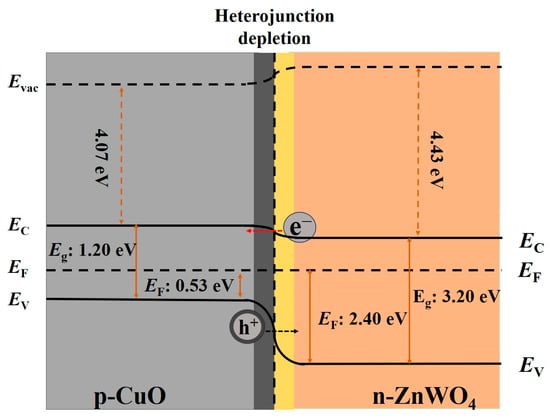
Figure 15.
Energy band structure diagram of the CuO-ZnWO4 nanocomposites.
As shown in Figure 15, the electrons in ZnWO4 and holes in CuO flow in opposite directions due to the large inhomogeneous gradient in carrier concentration, leading to the formation of an internal electric field between the ZnWO4 and CuO interface [57]. When two materials are combined, the energy bands of the ZnWO4 and CuO are bent, and the electrons are transferred until the Fermi level reaches equilibrium, and the material directly forms a large depletion region. The formation of the p–n heterojunction hinders the transfer of electrons, leading to a higher electrical resistance of the C-ZWO nanocomposite [57]. That is, before the C-ZWO nanocomposite sample explodes into the H2S gas, the material possesses high resistance (Ra) (Figure S4a).
When the CuO-ZnWO4 composites are exposed to H2S, the H2S molecules react with the oxygen anion ions on the surface. Firstly, the H2S molecules are oxidized by O2− following the reaction of 2H2S (g) + 3O2− (ads) → 2H2O (g) + 2SO2 (g) + 3e−. Then, the forming electrons are released into the C-ZWO nanocomposites, which narrows the depletion region mentioned above, leading to a reduction in the resistance (Rg) [58].
In addition, the p–n heterojunction between CuO and ZnWO4 (Figure S4a) will also be destroyed, as some of the CuO reacts with H2S to form CuS (Figure S4b), following the reaction of CuO (s) + H2S (g) → CuS (s) + H2O (g) [55] (Figure S4b). As a result of this phase transition, the p–n junction barrier of the CuO and ZnWO4 (Figure S4a) transforms into a metal-n-type semiconductor contact with a lower barrier between CuS and ZnWO4 (Figure S4b), making the resistance of the C-ZWO material (Rg) even smaller, i.e., exhibiting a high response value (S = Ra/Rg) to H2S gas.
Meanwhile, with the increase in the CuO contents, the response time of the C-ZWO sensors decreases gradually both at RT and 100 °C, as shown in Table S3. The short response time of the C-ZWO sensors with higher CuO contents may be due to the reaction of CuO (s) + H2S (g) → CuS (s) + H2O (g), which occurs fast and exhibits a shorter response time. However, when H2S is removed by introducing air, CuS is unstable and can be slowly oxidized by oxygen in the air, following the reaction 2CuS (s) + 3O2 (g) → 2CuO (s) + 2SO2 (g). At 100 °C, the 0% C-ZWO and 5% C-ZWO sensing recovered 90% in 180 and 138 s (Figure 8a,b). However, the recovery of the resistance of the other C-ZWO samples occurs with difficulty, especially for the high-CuO-content C-ZWO samples. Notably, by applying a 4.58 V short voltage pulse, i.e., heating it at 300 °C, the reaction 2CuS (s) + 3O2 (g) → 2CuO (s) + 2SO2 (g) can occur much more easily, which helps to recover the other sensors quickly in potential applications.
Furthermore, the composite CuO/ZnWO4 material has a larger SBET and more active sites, which can promote the surface reaction and increase its gas-sensing responses. XPS analysis further verified the increase in the proportion of OV. The presence of abundant OV on the surface of 10% C-ZWO can provide abundant unpaired electrons as electron donors, providing active sites for materials sensitive to the chemoadsorption of oxygen, thereby enhancing the ability of materials to ionize oxygen [35]. Meanwhile, the increase in the starting potential of the composite material in the ORR test also supports this conclusion. In conclusion, the formation of the p–n heterojunction, the increased specific surface area and active sites, and the enhanced oxygen reduction ability significantly improve the gas sensitivity of the C-ZWO sensors.
4. Conclusions
In conclusion, C-ZWO composites were synthesized by a two-step hydrothermal process, which led to a substantial improvement in H2S gas-sensing performance at RT and low temperatures (≤100 °C). The 10% C-ZWO sensor at RT exhibits a balanced response value of 651.6 and a response time of 78 s, while the 5% C-ZWO sensor at 100 °C shows potential with a response value of 13.5 and response and recovery times of 78 and 138 s, respectively. Meanwhile, the sensors show the advantages of good selectivity and stability. The H2S-sensing mechanism was studied by systematic characterization. The enhancement of the gas-sensitive properties of composite C-ZWO sensors mainly depends on the construction of p–n heterojunctions and the enhancement of surface adsorption and oxygen reduction capabilities of the materials. This study applies ZnWO4 in the direction of H2S gas detection; the construction of CuO/ZnWO4 heterojunction materials shows excellent potential for further study and practical applications.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pr13092727/s1. Figure S1: SEM images of the (a) 0%, (b) 5%, (c) 10%, and (d) 15% C-ZWO samples; Figure S2: (a) Micro area electron image, (b) EDS layered image, (c–f) EDS elemental mapping (O, Cu, Zn, and W), and (g) EDS map sum spectrum of the 10% C-ZWO sample; Figure S3: Schematic diagram of the gas-sensing mechanism of 0% C-ZWO to H2S gas; Figure S4: H2S-sensing response mechanism diagram (a) in air and (b) in H2S gas; Figure S5: The oxygen reduction curves of the C-ZWO composites. References [39,59,60] are cited in the supplementary materials; Table S1: The amount of Cu(C2H3O2)2·H2O for different C-ZWO samples; Table S2: The EDS element information of the 10% C-ZWO samples; Table S3: The response values, response and recovery times of different C-ZWO samples to 25 ppm H2S at RT and 100 °C.
Author Contributions
Conceptualization, Y.Z. and L.L.; methodology, Y.Z. and L.L.; software, Y.Z. and L.L.; validation, Y.Z. and L.L.; formal analysis, Y.Z. and L.L.; resources, Y.Z. and L.L.; data curation, Y.Z. and L.L.; writing—original draft preparation, Y.Z. and L.L.; writing—review and editing, J.F.; visualization, Y.Z. and L.L.; supervision, J.F.; project administration, J.F.; funding acquisition, J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Science and Technology of Henan Province: Science and Technology Vice President Program (12522180050).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed toward the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RT | Room temperature |
| C-ZWO | CuO/ZnWO4 |
| MOSs | Metal oxide semiconductors |
| Eg | Bandgap |
| S | Sensor response value |
| Ra | Resistance of the sensor in air |
| Rg | Resistance of the sensor in the target gas |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| EDS | Energy-dispersive X-ray spectroscopy |
| TEM | Transmission electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| UPS | Ultraviolet photoelectron spectroscopy |
| Uout | Output voltage |
| Uh | Heating voltage |
| ORR | Oxygen reduction reaction |
| OL | Lattice oxygen |
| OV | Oxygen vacancies |
| OC | Chemisorbed oxygen species |
| SBET | Specific surface areas |
| On− | Oxygen anion ions (O2−, O2−, and O−) |
| e− | Electron |
| Ecut-off | Cut-off edge energies |
References
- Shukla, P.; Khodade, V.S.; SharathChandra, M.; Chauhan, P.; Mishra, S.; Siddaramappa, S.; Pradeep, B.E.; Singh, A.; Chakrapani, H. “On demand” redox buffering by H2S contributes to antibiotic resistance revealed by a bacteria-specific H2S donor. Chem. Sci. 2017, 8, 4967–4972. [Google Scholar] [CrossRef]
- Adeniyi, K.I.; Wan, H.H.; Deering, C.E.; Bernard, F.; Chisholm, M.A.; Marriott, R.A. High-pressure hydrogen sulfide experiments: How did our safety measures and hazard control work during a failure event? Safety 2020, 6, 15. [Google Scholar] [CrossRef]
- Haouzi, P.; Sonobe, T.; Judenherc-Haouzi, A. Developing effective countermeasures against acute hydrogen sulfide intoxication: Challenges and limitations. Ann. N. Y. Acad. Sci. 2016, 1374, 29–40. [Google Scholar] [CrossRef]
- Elwood, M. The scientific basis for occupational exposure limits for hydrogen sulphide-A critical commentary. Int. J. Environ. Res. Public Health 2021, 18, 2866. [Google Scholar] [CrossRef]
- Hosseini-Shokouh, S.H.; Zhou, J.; Berger, E.; Lv, Z.P.; Hong, X.; Virtanen, V.; Kordas, K.; Komsa, H.P. Highly selective H2S gas snsor based on Ti3C2Tx MXene-organic composites. ACS Appl. Mater. Interfaces 2023, 15, 7063–7073. [Google Scholar] [CrossRef]
- El Brahmi, A.; Abderafi, S. Hydrogen sulfide removal from wastewater using hydrogen peroxide in-situ treatment: Case study of moroccan urban sewers. Mater. Today. Proc. 2021, 45, 7424–7427. [Google Scholar] [CrossRef]
- Kim, K.R.; Oh, J.; Hong, J.I. Highly selective electrochemiluminescence chemosensor for sulfide enabled by hierarchical reactivity. Anal. Chem. 2022, 94, 5091–5098. [Google Scholar] [CrossRef]
- Wu, M.Y.; Wang, S.W.; Hai, W.X.; Lu, X.M.; Li, P.Y. Development of a H2S-responsive NIR fluorescent probe for H2S detection and H2S releasing monitoring from prodrug. J. Fluoresc. 2023, 33, 1853–1860. [Google Scholar] [CrossRef]
- Engel, L.; Benito-Altamirano, I.; Tarantik, K.R.; Pannek, C.; Dold, M.; Prades, J.D.; Wöllenstein, J. Printed sensor labels for colorimetric detection of ammonia, formaldehyde and hydrogen sulfide from the ambient air. Sensors Actuators B Chem. 2021, 330, 129281. [Google Scholar] [CrossRef]
- Li, S.H.; Xie, L.L.; He, M.; Hu, X.B.; Luo, G.F.; Chen, C.; Zhu, Z.G. Metal-organic frameworks-derived bamboo-like CuO/In2O3 heterostructure for high-performance H2S gas sensor with low operating temperature. Sensors Actuators B Chem. 2020, 310, 127828. [Google Scholar] [CrossRef]
- Du, L.Y.; Zhang, H.P.; Zhu, M.M.; Zhang, M.Z. Construction of flower-like ZnSnO3/Zn2SnO4 hybrids for enhanced phenylamine sensing performance. Inorg. Chem. Front. 2019, 6, 2311–2317. [Google Scholar] [CrossRef]
- Yin, Y.Y.; Shen, Y.B.; Zhou, P.F.; Lu, R.; Li, A.; Zhao, S.K.; Liu, W.G.; Wei, D.Z.; Wei, K.F. Fabrication, characterization and n-propanol sensing properties of perovskite-type ZnSnO3 nanospheres based gas sensor. Appl. Surf. Sci. 2020, 509, 145335. [Google Scholar] [CrossRef]
- Guo, J.Y.; Zhang, D.Z.; Li, T.T.; Zhang, J.H.; Yu, L.D. Green light-driven acetone gas sensor based on electrospinned CdS nanospheres/Co3O4 nanofibers hybrid for the detection of exhaled diabetes biomarker. J. Colloid. Interface Sci. 2020, 606, 261–271. [Google Scholar] [CrossRef]
- Ochoa-Munoz, Y.H.; Mejia de Gutierrez, R.; Rodriguez-Paez, J.E. Metal oxide gas sensors to study acetone detection considering their potential in the diagnosis of diabetes: A review. Molecules 2023, 28, 1150. [Google Scholar] [CrossRef]
- Uma, S.; Shobana, M.K. Metal oxide semiconductor gas sensors in clinical diagnosis and environmental monitoring. Sensors Actuators B-Phys. 2023, 349, 114044. [Google Scholar] [CrossRef]
- Lee, K.; Cho, I.; Kang, M.; Jeong, J.; Choi, M.; Woo, K.Y.; Yoon, K.J.; Cho, Y.H.; Park, I. Ultra-low-power E-nose system based on multi-micro-LED-integrated, nanostructured gas sensors and deep learning. ACS Nano 2023, 17, 539–551. [Google Scholar] [CrossRef]
- Yuan, C.Y.; Ma, J.H.; Zou, Y.D.; Li, G.S.; Xu, H.L.; Sysoev, V.V.; Cheng, X.W.; Deng, Y.H. Modeling interfacial interaction between gas molecules and semiconductor metal oxides: A new view angle on gas sensing. Adv. Sci. 2002, 9, 2203594. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Yang, Z.M.; Yu, S.J.; Mi, Q.; Pan, Q.N. Diversiform metal oxide-based hybrid nanostructures for gas sensing with versatile prospects. Coord. Chem. Rev. 2000, 413, 213272. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, W.J.; Wang, X.Y.; Wang, Q.; Huang, B.Y.; Li, Y.; Hua, X.H.; Liu, G.; Li, B.S.; Zhou, J.Y.; et al. Binder-free CuO nanoneedle arrays based tube-type sensor for H2S gas sensing. Sensors Actuators B Chem. 2021, 326, 128993. [Google Scholar] [CrossRef]
- Li, D.J.; Tang, Y.L.; Ao, D.Y.; Xiang, X.; Wang, S.Y.; Zu, X.T. Ultra-highly sensitive and selective H2S gas sensor based on CuO with sub-ppb detection limit. Int. J. Hydrog. Energy 2019, 44, 3985–3992. [Google Scholar] [CrossRef]
- Jung, D.; Hwang, S.; Kim, H.J.; Han, J.H.; Lee, H.N. Characterization of porous CuO films for H2S gas sensors. Materials 2022, 15, 7270. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, S.; Wang, M.Y.; Liu, S.W.; Liu, G.W.; Meng, X.F.; Xu, Z.W.; Wang, M.S.; Qiao, G.J. Electrospun Cu-doped In2O3 hollow nanofibers with enhanced H2S gas sensing performance. J. Adv. Ceram. 2022, 11, 427–442. [Google Scholar] [CrossRef]
- Lv, L.X.; Zhang, X.L.; Wang, J.R.; Yuan, L.H.; Fan, J.J. Construction of Li0.5La0.5TiO3 (LLTO)-In2O3 n-n step scheme heterostructure nanorods for drastically heightening the sensing behavior in H2S gas. Mater. Chem. Phys. 2023, 295, 127085. [Google Scholar] [CrossRef]
- Yin, Y.Y.; Li, F.; Zhang, N.; Ruan, S.P.; Zhang, H.F.; Chen, Y. Improved gas sensing properties of silver-functionalized ZnSnO3 hollow nanocubes. Inorg. Chem. Front. 2018, 5, 2123–2131. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.Y.; Xiao, D.K.; Wang, S.J.; Zhang, T.; Yang, X.; Heng, S.Q.; Sun, M.J. CuO/WO3 hollow microsphere P-N heterojunction sensor for continuous cycle detection of H2S gas. Sensors Actuators B Chem. 2023, 374, 132823. [Google Scholar] [CrossRef]
- Feng, W.C.; Li, J.; Lei, Z.M.; Liu, Y.; Shen, Y.Q.; Chen, Z.X. The improved photocatalytic activity of CaWO4 nanorods by loading Bi on the surface. J. Mater. Sci. Mater. Electron. 2019, 30, 16049–16055. [Google Scholar] [CrossRef]
- Tasyurek, L.B.; Kilinc, N. Hydrogen detection and electrical properties of titanium silicate Schottky diode fabricated by RF-magnetron sputtering method. Int. J. Hydrogen Energy 2025, 144, 738–745. [Google Scholar] [CrossRef]
- He, G.P.; Mi, Y.M.; Wang, D.; Zhang, B.; Zheng, D.H.; Bai, Y.X.; Shi, Z.M. Synthesis methods and applications of semiconductor material ZnWO4 with multifunctions and multiconstructions. Energy Technol. 2021, 9, 2100733. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Shi, Y.B.; Niu, T.J.; He, J.J.; She, H.D.; Su, B.T. Preparing ZnWO4–CdS composite with excellent visible light photocatalytic activity under mild conditions. J. Sol. Gel Sci. Technol. 2017, 83, 555–566. [Google Scholar] [CrossRef]
- Yin, M.L.; Wang, Y.T.; Liu, S.Z. Synthesis of Fe2O3-ZnWO4 nanocomposites and their enhanced acetone sensing performance. J. Alloys Compd. 2020, 831, 154713. [Google Scholar] [CrossRef]
- Tian, L.Y.; Rui, Y.L.; Sun, K.L.; Cui, W.Q.; An, W.J. Surface decoration of ZnWO4 nanorods with Cu2O nanoparticles to build heterostructure with enhanced photocatalysis. Nanomaterials 2018, 8, 33. [Google Scholar] [CrossRef]
- Fu, S.R.; Hu, H.Y.; Feng, C.C.; Zhang, Y.J.; Bi, Y.P. Epitaxial growth of ZnWO4 hole-storage nanolayers on ZnO photoanodes for efficient solar water splitting. J. Mater. Chem. A 2019, 7, 2513–2517. [Google Scholar] [CrossRef]
- Yesuraj, J.; Suthanthiraraj, S.A. Bio-molecule templated hydrothermal synthesis of ZnWO4 nanomaterial for high-performance supercapacitor electrode application. J. Mol. Struct. 2019, 1181, 131–141. [Google Scholar] [CrossRef]
- Brijesh, K.; Nagaraja, H.S. ZnWO4/r-GO nanocomposite as high capacity anode for lithium-ion battery. Ionics 2020, 26, 2813–2823. [Google Scholar] [CrossRef]
- Sun, D.; Wang, Q.L.; Wang, W.; Chen, Y.; Ruan, S.P. Hollow ZnWO4/In2O3 Nanotubes for ultrasensitive and rapid trace detection of triethylamine. ACS Appl. Nano Mater. 2023, 6, 10581–10589. [Google Scholar] [CrossRef]
- Cai, L.X.; Chen, L.; Sun, X.Q.; Geng, J.; Liu, C.C.; Wang, Y.; Guo, Z. Ultra-sensitive triethylamine gas sensors based on polyoxometalate-assisted synthesis of ZnWO4/ZnO hetero-structured nanofibers. Sensors Actuators B Chem. 2022, 370, 132422. [Google Scholar] [CrossRef]
- Zang, D.M.; Wei, X.Y.; Liu, Q.Y.; Li, Y.; You, R. In-situ growth of ZnO@ZnWO4 heterojunction with flower-like structure by chemical vapor deposition for H2S gas sensor. Appl. Surf. Sci. 2025, 679, 161149. [Google Scholar] [CrossRef]
- Hu, P.F.; Chen, J.T.; Ma, Q.R.; Yin, J.Q.; Zhou, D.; Kou, C.H.; Xu, J.; Xu, J.Q. One-step thermal compensation decomposition synthesis of ZnWO4/WO3 composite with synergy of multiple structural effects for efficient trace H2S detection. Sensors Actuators B Chem. 2023, 381, 133388. [Google Scholar] [CrossRef]
- Zheng, N.C.; Li, X.F.; Yan, S.; Wang, Q.; Qiao, R.; Hu, J.H.; Fan, J.J.; Cao, G.Q.; Shao, G.S. Nano-porous hollow Li0.5La0.5TiO3 spheres and electronic structure modulation for ultra-fast H2S detection. J. Mater. Chem. A 2020, 8, 2376. [Google Scholar] [CrossRef]
- de Araújo, L.N.M.; Sousa, B.S.; de Araújo, A.G.F.; Monção, R.M.; Feitor, M.C.; Sczancoski, J.C.; Almeida, M.A.P.; Santos, F.E.P.; de Sousa, R.R.M.; Cavalcante, L.S. ZnWO4 nanocrystals prepared by thermal plasma processing. J. Mater. Sci. 2023, 58, 6944–6971. [Google Scholar] [CrossRef]
- Pereira, P.F.S.; Gouveia, A.F.; Assis, M.; de Oliveira, R.C.; Pinatti, I.M.; Penha, M.; Gonçalves, R.F.; Gracia, L.; Andrés, J.; Longo, E. ZnWO4 nanocrystals: Synthesis, morphology, photoluminescence and photocatalytic properties. Phys. Chem. Chem. Phys. 2018, 20, 1923–1937. [Google Scholar] [CrossRef]
- Li, Z.J.; Yan, S.N.; Zhang, S.C.; Wang, J.Q.; Shen, W.Z.; Wang, Z.G.; Fu, Y.Q. Ultra-sensitive UV and H2S dual functional sensors based on porous In2O3 nanoparticles operated at room temperature. J. Alloys Compd. 2019, 770, 721–731. [Google Scholar] [CrossRef]
- Chaloeipote, G.; Prathumwan, R.; Subannajui, K.; Wisitsoraat, A.; Wongchoosuk, C. 3D printed CuO semiconducting gas sensor for ammonia detection at room temperature. Mater. Sci. Semicon. Proc. 2021, 123, 105546. [Google Scholar] [CrossRef]
- Zhang, C.; Huan, Y.C.; Sun, D.J.; Lu, Y.L. Synthesis and NO2 sensing performances of CuO nanoparticles loaded In2O3 hollow spheres. J. Alloys Compd. 2020, 842, 155857. [Google Scholar] [CrossRef]
- Hu, J.; Sun, Y.J.; Xue, Y.; Zhang, M.; Li, P.W.; Lian, K.; Zhuiykov, S.; Zhang, W.D.; Chen, Y. Highly sensitive and ultra-fast gas sensor based on CeO2-loaded In2O3 hollow spheres for ppb-level hydrogen detection. Sensors Actuators B Chem. 2018, 257, 124–135. [Google Scholar] [CrossRef]
- Somdee, A.; Wannapop, S. Enhanced photocatalytic behavior of ZnO nanorods decorated with a Au, ZnWO4, and Au/ZnWO4 composite: Synthesis and characterization. Colloid Interface Sci. Commun. 2022, 47, 100591. [Google Scholar] [CrossRef]
- Bosigo, R.; Lepodise, L.M.; Kuvarega, A.; Muiva, C. Hydrothermal synthesis of CuO and CeO2/CuO nanostructures: Spectroscopic and temperature dependent electrical properties. J. Mater. Sci. Mater. El. 2021, 32, 7136–7152. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.H.; Xie, L.L.; Li, X.; Lin, D.H.; Zhu, Z.G. Low-temperature and highly sensitivity H2S gas sensor based on ZnO/CuO composite derived from bimetal metal-organic frameworks. Ceram. Int. 2020, 46, 15858–15866. [Google Scholar] [CrossRef]
- Jin, X.H.; Li, Y.W.; Zhang, B.; Xu, X.T.; Sun, G.; Wang, Y. Temperature-dependent dual selectivity of hierarchical porous In2O3 nanospheres for sensing ethanol and TEA. Sensors Actuators B Chem. 2021, 330, 129271. [Google Scholar] [CrossRef]
- Wang, W.X.; Zhen, Y.H.; Zhang, J.Y.; Li, Y.D.; Zhong, H.; Jia, Z.L.; Xiong, Y.; Xue, Q.Z.; Yan, Y.G.; Alharbi, N.S.; et al. SnO2 nanoparticles-modified 3D-multilayer MoS2 nanosheets for ammonia gas sensing at room temperature. Sensors Actuators B Chem. 2020, 321, 128471. [Google Scholar] [CrossRef]
- Dao, D.V.; Nguyen, T.T.D.; Kim, D.S.; Yoon, J.W.; Yu, Y.T.; Lee, I.H. Core and dopant effects toward hydrogen gas sensing activity using Pd@N-CeO2 core–shell nanoflatforms. J. Ind. Eng. Chem. 2021, 95, 325–332. [Google Scholar] [CrossRef]
- Hussain, S.; Amu-Darko, J.N.O.; Wang, M.S.; Alothman, A.A.; Ouladsmane, M.; Aldossari, S.A.; Khan, M.S.; Qiao, G.J.; Liu, G.W. CuO-decorated MOF derived ZnO polyhedral nanostructures for exceptional H2S gas detection. Chemosphere 2023, 317, 137827. [Google Scholar] [CrossRef]
- Zhao, C.H.; Zhang, G.Z.; Han, W.H.; Fu, J.C.; He, Y.M.; Zhang, Z.X.; Xie, E.Q. Electrospun In2O3/α-Fe2O3 heterostructure nanotubes for highly sensitive gas sensor applications. CrystEngComm 2013, 15, 6491. [Google Scholar] [CrossRef]
- Yin, X.T.; Zhou, W.D.; Li, J.; Wang, Q.; Wu, F.Y.; Dastan, D.; Wang, D.; Garmestani, H.; Wang, X.M.; Ţălu, Ş. A highly sensitivity and selectivity Pt-SnO2 nanoparticles for sensing applications at extremely low level hydrogen gas detection. J. Alloys Compd. 2019, 805, 229–236. [Google Scholar] [CrossRef]
- Li, Z.J.; Wang, N.N.; Lin, Z.J.; Wang, J.Q.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z.G. Room-temperature high-performance H2S sensor based on porous CuO nanosheets prepared by hydrothermal method. ACS Appl. Mater. Interfaces 2016, 8, 20962–20968. [Google Scholar] [CrossRef]
- Kumar, A.; Shringi, A.K.; Kumar, M. RF sputtered CuO anchored SnO2 for H2S gas sensor. Sensors Actuators B Chem. 2022, 370, 132417. [Google Scholar] [CrossRef]
- Yin, X.T.; Li, J.; Dastan, D.; Zhou, W.D.; Garmestani, H.; Alamgir, F.M. Ultra-high selectivity of H2 over CO with a p-n nanojunction based gas sensors and its mechanism. Sensors Actuators B Chem. 2020, 319, 28330. [Google Scholar] [CrossRef]
- Zhang, J.L.; Ma, S.Y.; Wang, B.J.; Pei, S.T. Hydrothermal synthesis of SnO2-CuO composite nanoparticles as a fast-response ethanol gas sensor. J. Alloys Compd. 2021, 886, 161299. [Google Scholar] [CrossRef]
- Végh, J. The Shirley background revised. J. Electron Spectrosc. 2006, 151, 159–164. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).