Bioassays to Assess the Safety of Potassium and Sodium Nitrates and Nitrites
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. In Vivo Assays
2.2.1. Toxicity
2.2.2. Antitoxicity
2.2.3. Genotoxicity
2.2.4. Antigenotoxicity
2.2.5. Longevity
2.3. In Vitro Assays
2.3.1. Cytotoxicity
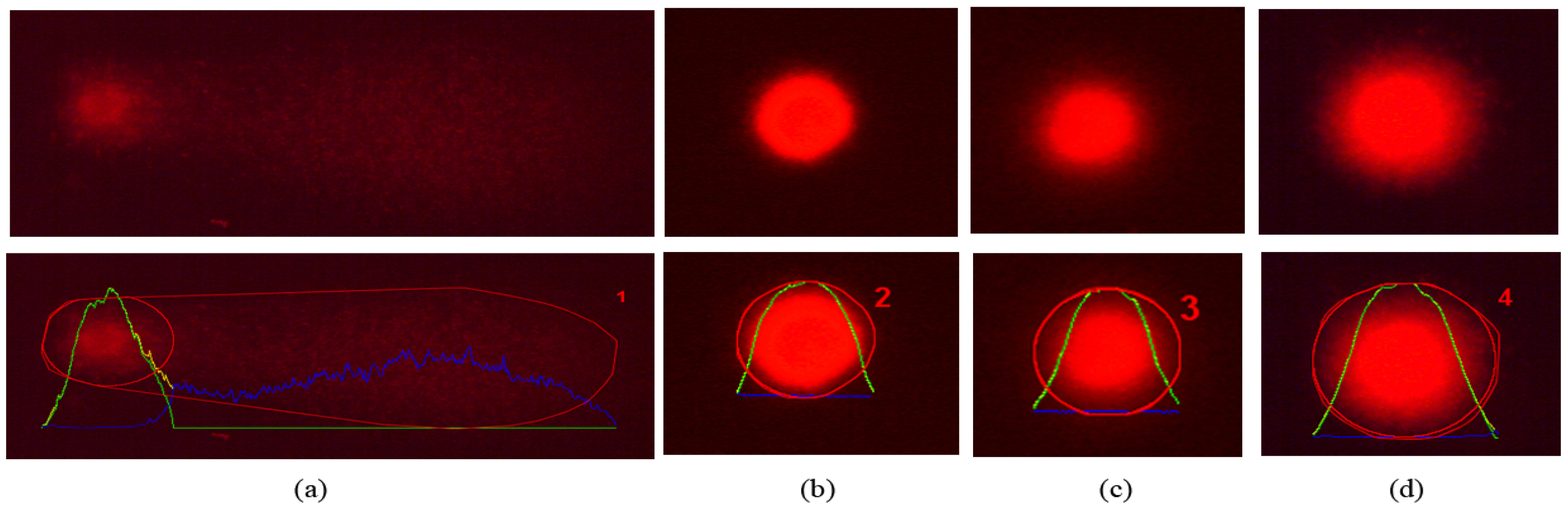
2.3.2. Internucleosomal DNA Fragmentation
2.3.3. Comet Assay
2.3.4. Methylation Status
3. Results
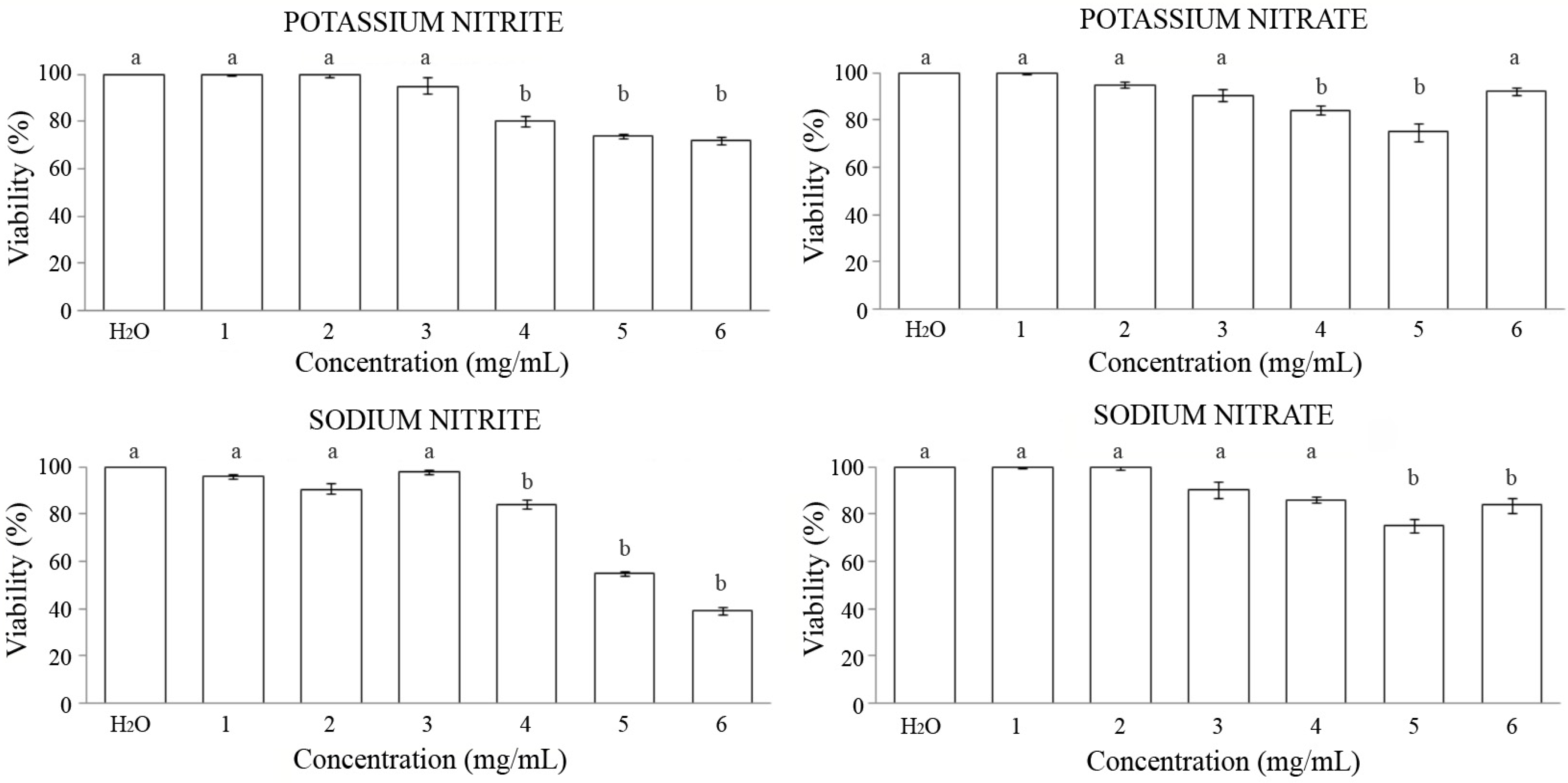
3.1. Toxicity
- Potassium nitrite: It presents a dose-dependent effect on toxicity, reaching slightly harmful results for Drosophila at the three highest concentrations studied. The viability range was modified between 80% and 72% compared to its control.
- Potassium nitrate: It showed a significant decrease in the survival of individuals treated at concentrations 4 and 5, seeing their viability decrease to 84% and 75%, respectively, compared to the control.
- Sodium nitrite: It exhibited a dose-dependent toxic effect, with a significant decrease in survival at the three highest concentrations studied, the highest concentration being the only one that shows a significantly toxic effect for Drosophila with respect to the control (under the LD50). The viability range decreased between 84% and 39% with respect to its control.
- Sodium nitrate: The results show a dose-dependent toxic effect, with the two highest concentrations studied showing a significant decrease in survival of individuals, although none of them reached LD50.The viability percentage decreased to 75% and 84%, respectively, compared to the control.
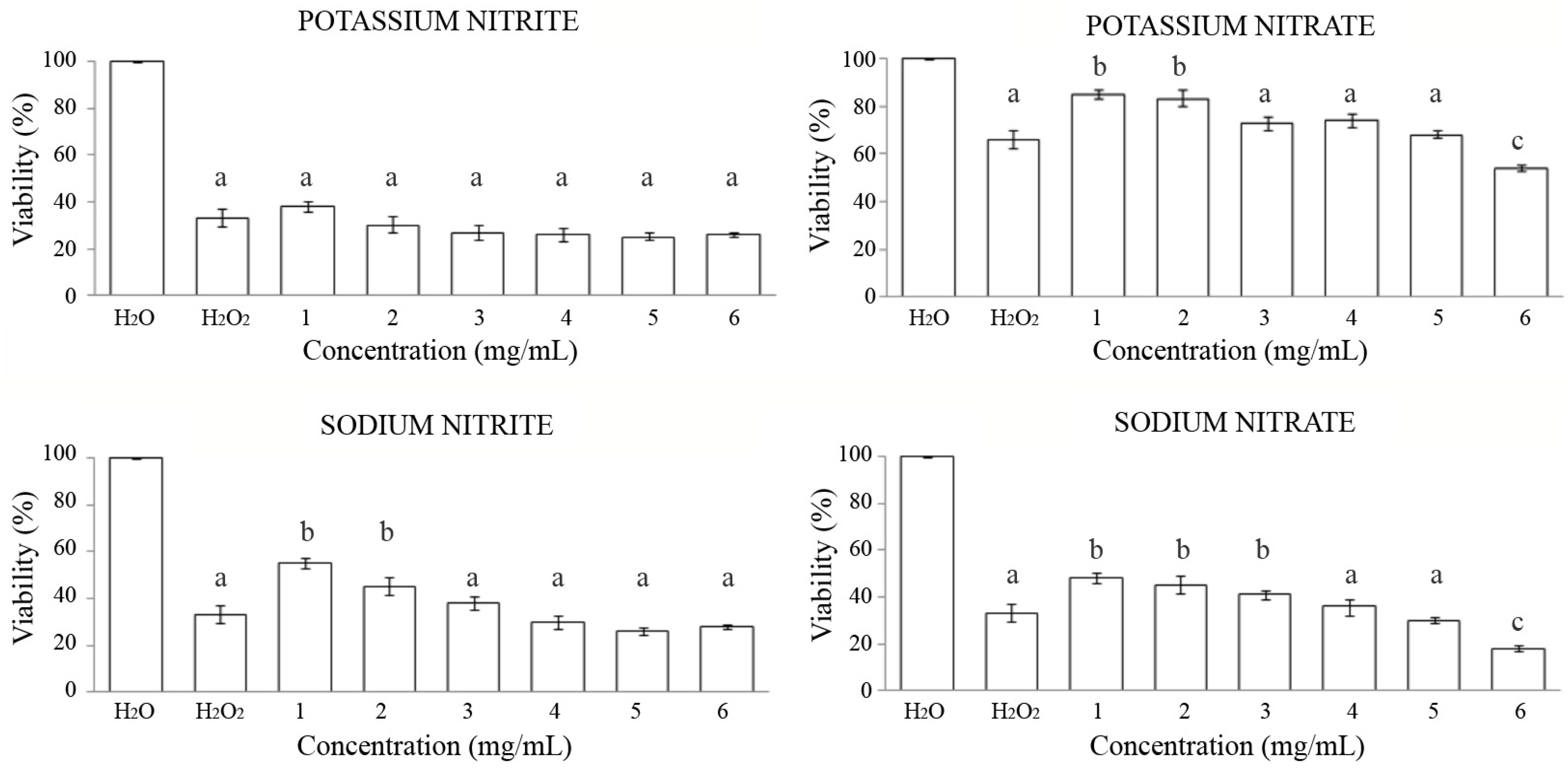
3.2. Antitoxicity
- Potassium nitrite: It did not show significant capacity for the protection of this additive against a stressor when combined in the model organism D. melanogaster.
- Potassium nitrate: The two minimum concentrations tested showed a significant protective effect with an increase in the survival of individuals of 19% and 17%, respectively, with respect to the positive control when combined treatments were carried out with a genotoxic agent. However, the highest concentration studied showed a significant negative antitoxic effect on Drosophila, with a decrease in the survival of individuals of 12% compared to the positive control.
- Sodium nitrite: It exerted a significant antitoxic effect for the two lowest concentrations studied in D. melanogaster, with an increase in viability of 22% and 12%, respectively compared to the positive control. The rest of the concentrations tested did not show significant effects on the survival of the flies.
- Sodium nitrate: The results exhibited a significant antitoxic effect at the three lowest concentrations studied in Drosophila, with an increase in the percentage of viability between 8% and 15% compared to the positive control and a significant negative effect on the survival of individuals in combined treatments at a high concentration of this additive, with a decrease in viability of 15% compared to the positive control.
3.3. Genotoxicity
3.4. Antigenotoxicity
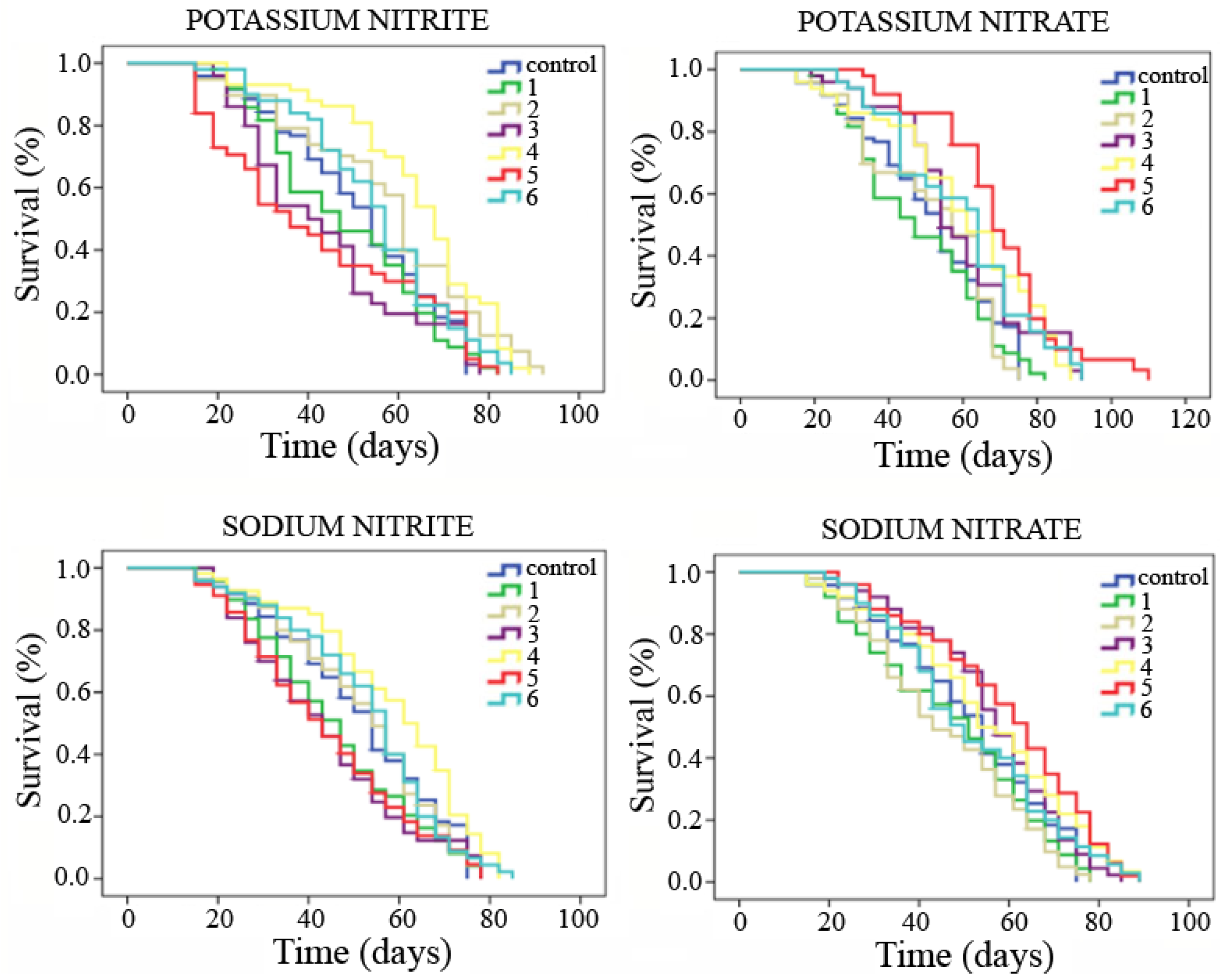
3.5. Longevity
- Potassium nitrite: The longevity results indicated that concentrations 2 and 4 studied for this additive induce a significant improvement between 6 and 13 days of Drosophila lifespan extension when it is chronically fed. In contrast, at concentrations 4 and 6, the quality of life of these individuals is significantly reduced between 4 and 5 days compared to their control.
- Potassium nitrate: The highest concentrations tested (4, 5 and 6) indicated a significant improvement in the longevity and healthspan of Drosophila with respect to their controls between 8 and 16 days, and between 5 and 15 days, respectively.
- Sodium nitrite: The results only showed a significant improvement in 7 days of Drosophila life expansion, with respect to its control, when the chronic treatment was carried out at concentration 4.
- Sodium nitrate: The longevity of treated flies increased significantly by 8 days, compared to their control, for treatments with the concentration numbered as 5. Moreover, healthspan of Drosophila was also significantly improved in 5 days, compared to its control, when the treatments were carried out at concentrations numbered as 3 and 5.
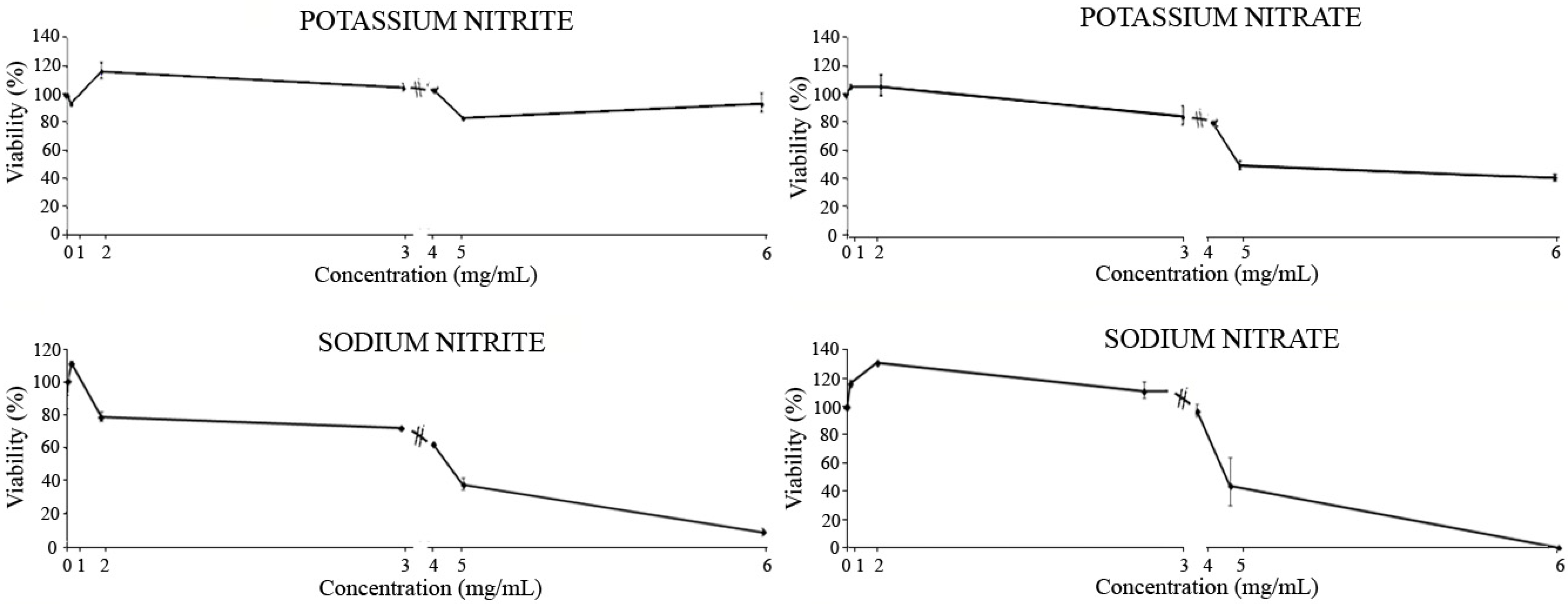
3.6. Cytotoxicity
- Potassium nitrite: The results showed little cytotoxic potential of this additive, with a cell viability range between 117% and 82% compared to the control. The ADI concentration induced a stimulation of leukemia cell growth by 10% compared to untreated cells. Furthermore, the IC50 was not reached, which would indicate that our additive had chemopreventive potential for any of the concentrations studied.
- Potassium nitrate: The results exhibited a dose-dependent effect on the inhibition of HL-60 cell growth. The percentage of cell viability for the different concentrations under study was between 105% and 49% with respect to the control. Concentration 3 (ADI concentration) showed an inhibition of cell growth of 18% compared to untreated cells, while the IC50 was reached at the concentration 5 studied.
- Sodium nitrite: The viability percentage for cells treated with different concentrations of this additive was between 112% and 8% with respect to the control. The cytotoxic effect induced at the ADI concentration (concentration 3) indicated a cell survival of 71% related to the control. The IC50 was reached between concentrations 4 and 5 under study.
- Sodium nitrate: The lowest concentrations studied showed stimulation of cell growth, reaching a viability percentage of 128% with respect to the control. Starting from concentration 3 (ADI concentration), whose viability exceeded the control growth by 13%, a dose-dependent decrease occurred until reaching a 100% cell death at the highest concentration studied. The IC50 was reached between concentrations 4 and 5 studied.
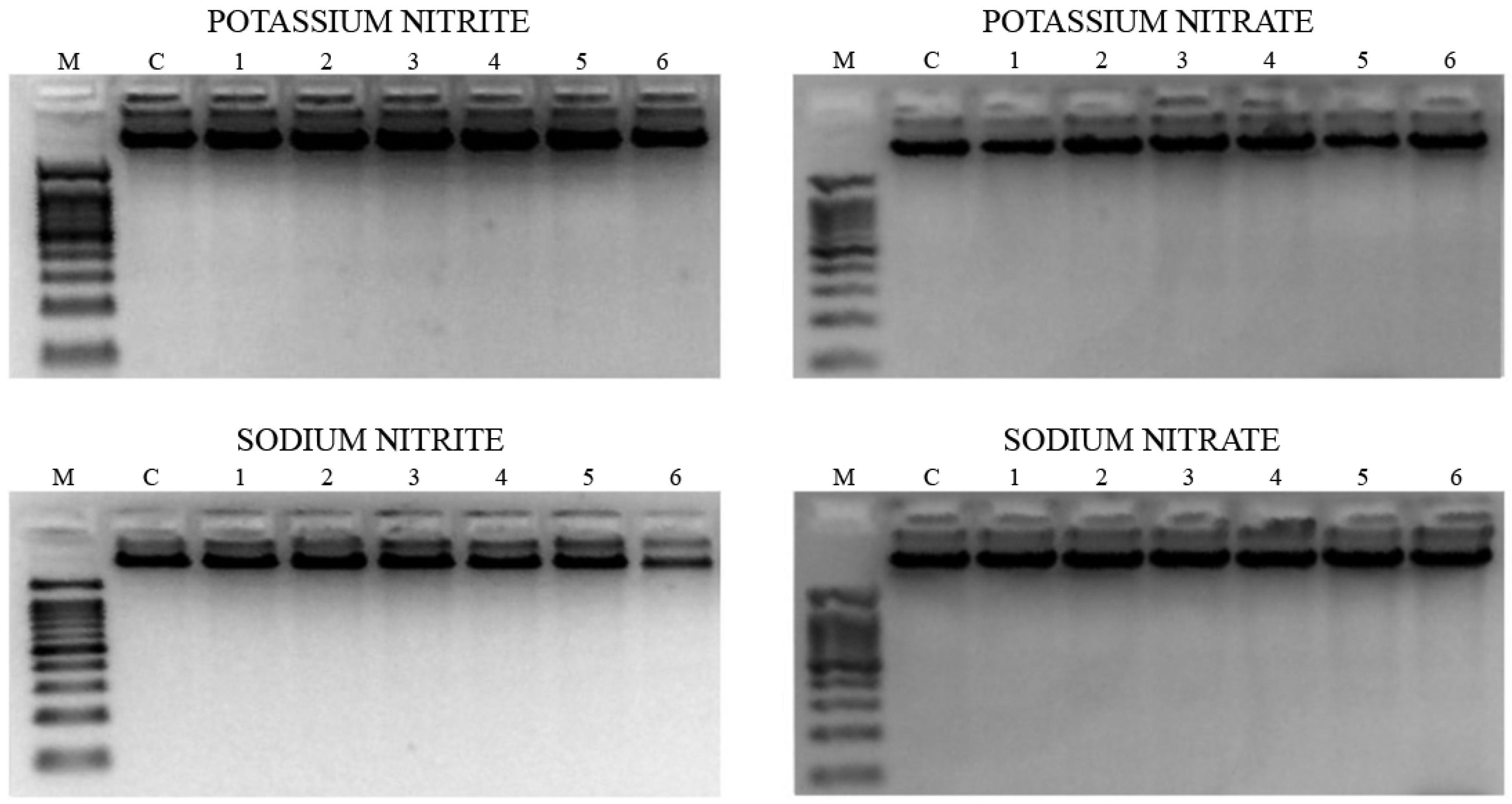
3.7. Internucleosomal DNA Fragmentation
3.8. Comet Assay
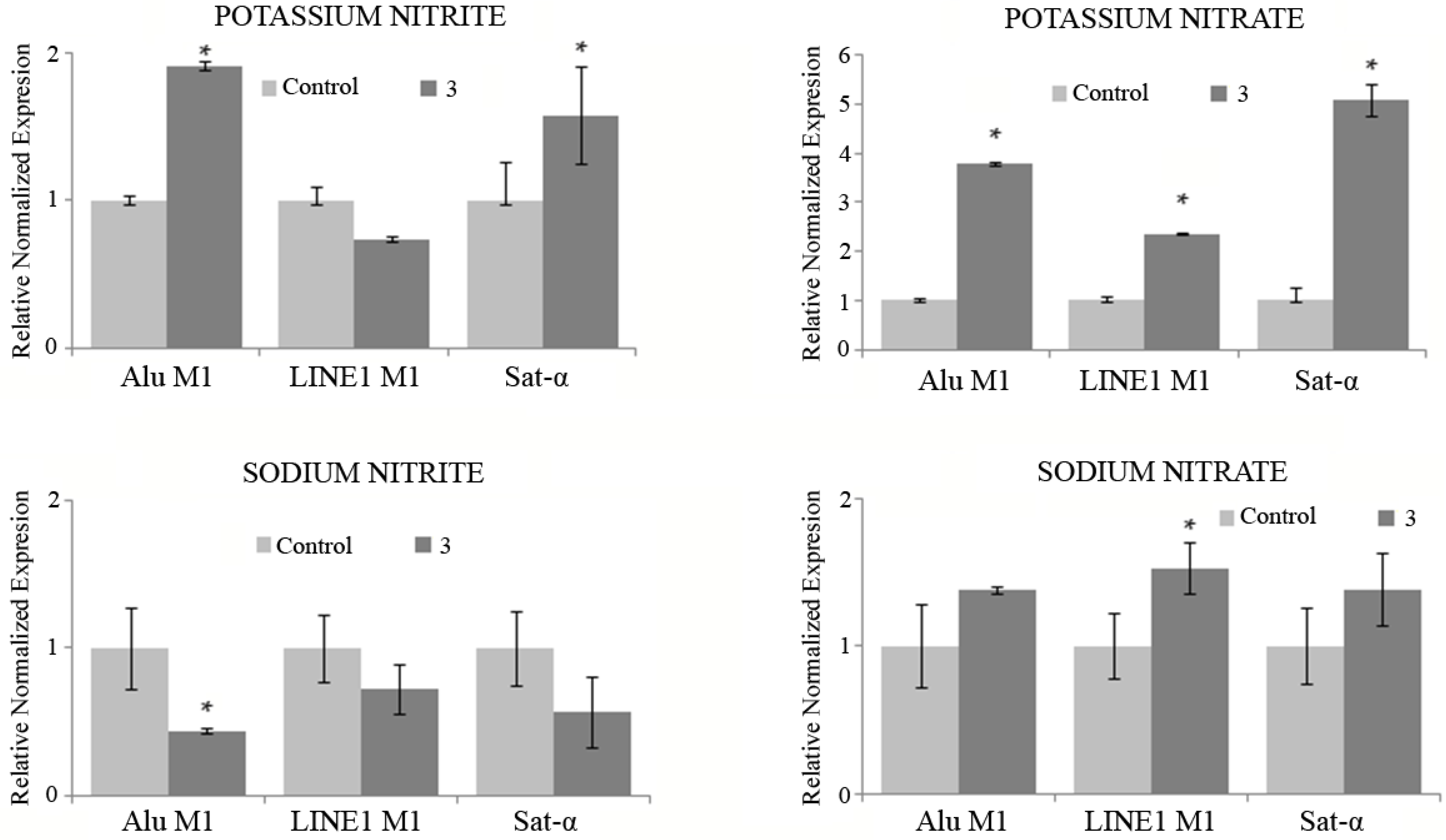
3.9. Methylation Status
- Potassium nitrite: The results showed significant hypermethylation for Alu M1 and Sat- α repetitive sequences relative to the control.
- Potassium nitrate: All the repetitive sequences were significantly hypermethylated in treated cells, related to the control.
- Sodium nitrite: A significant desmethylation was induced in Alu M1 sequence, related to the control.
- Sodium nitrate: A significant increase of methylation in LINE M1 sequence was induced in the treated HL-60 tumor cells in relation to the untreated ones.
- Methylation of repetitive sequences is regarded as a mechanism of genomic protection by silencing key genes in the onset and progression of cancer [71,72], Potassium nitrite, potassium nitrate and sodium nitrate can be considered promising chemopreventive compounds as they have been shown to partially or completely inhibit the effects of tumor cells at the studied sequences and concentrations.
4. Discussion
- (1)
- Nitrites
- (2)
- Nitrates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stein, L.Y.; Klotz, M.G. The nitrogen cycle. Curr. Biol. 2016, 26, R94–R98. [Google Scholar] [CrossRef]
- Borawska, M.; Omieljaniuk, N.; Rostkowski, J.; Otłog, T.; Hamid, F. Value of nitrates and nitrites in selected vegetables and potatoes sold in the marketplace of Białystok in the years 1991–1992. Rocz. Panstw. Zakl. Hig. 1994, 45, 89–96. [Google Scholar]
- Hunt, J.; Turner, M. A survey of nitrite concentrations in retail fresh vegetables. Food Addit. Contam. 1994, 11, 327–332. [Google Scholar] [CrossRef]
- Nabrzyski, M.; Gajewska, R. The content of nitrates and nitrites in fruits, vegetables and other foodstuffs. Rocz. Panstw. Zakl. Hig. 1994, 45, 167–180. [Google Scholar]
- National Research Council, Committee on Long-Range Soil & Water Conservation Policy. Soil and Water Quality: An Agenda for Agriculture; National Academies Press: Washington, DC, USA, 1993. [Google Scholar]
- Pennington, J.A. Dietary exposure models for nitrates and nitrites. Food Control 1998, 9, 385–395. [Google Scholar] [CrossRef]
- Rostkowski, J.; Borawska, M.; Omieljaniuk, N.; Otłog, K. Content of nitrates and nitrites in early vegetables and potatoes sold in the marketplace of Białystok in the year 1992. Rocz. Panstw. Zakl. Hig. 1994, 45, 81–87. [Google Scholar]
- Speijers, G. Nitrite. Toxicological Evaluation of Certain Food Additives and Contaminants in Food; World Health Organization: Geneva, Switzerland, 1996; pp. 269–324. [Google Scholar]
- Lundberg, J.O.; Weitzberg, E.; Cole, J.A.; Benjamin, N. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2004, 2, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- EFSA. Panel on Food Additives Nutrient Sources added to Food Mortensen. Re-evaluation of sodium nitrate (E 251) and potassium nitrate (E 252) as food additives. Efsa J. 2017, 15, e04787. [Google Scholar]
- Cornforth, D.P. Role of nitric oxide in treatment of foods. In Nitric Oxide Princ. Actions; Academic Press: Cambridge, MA, USA, 1996; pp. 259–287. [Google Scholar]
- Ibáñez, F.; Torre, P.; Irigoyen, A. Aditivos alimentarios. In Área de Nutrición y Bromatología; Universidad Pública de Navarra: Pampeluna, Spain, 2003; pp. 3–5. [Google Scholar]
- Igoe, R.S. Dictionary of Food Ingredients; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Lugo, E.B. Nitritos y Nitratos: Su uso, control y alternativas en embutidos cárnicos. Nacameh 2008, 2, 160–187. [Google Scholar]
- Sigler, W.A.; Bauder, J. Nitrato y Nitrito. Universidad Estatal de Montana Programa de Extensión en Calidad del Agua Departamento de Recursos de la Tierra y Ciencias Ambientales 2012. Available online: https://goo.gl/rjwCCB (accessed on 25 December 2024).
- EFSA. Panel on Food Additives Nutrient Sources added to Food Mortensen. Re-evaluation of potassium nitrite (E 249) and sodium nitrite (E 250) as food additives. Efsa J. 2017, 15, e04786. [Google Scholar]
- Chamandoost, S.; Fateh Moradi, M.; Hosseini, M.-J. A review of nitrate and nitrite toxicity in foods. J. Hum. Environ. Health Promot. 2016, 1, 80–86. [Google Scholar] [CrossRef]
- Laue, W.; Thiemann, M.; Scheibler, E.; Wiegand, K.W. Nitrates and nitrites. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Milkowski, A.; Garg, H.K.; Coughlin, J.R.; Bryan, N.S. Nutritional epidemiology in the context of nitric oxide biology: A risk–benefit evaluation for dietary nitrite and nitrate. Nitric Oxide 2010, 22, 110–119. [Google Scholar] [CrossRef]
- Bryan, N.S.; Fernandez, B.O.; Bauer, S.M.; Garcia-Saura, M.F.; Milsom, A.B.; Rassaf, T.; Maloney, R.E.; Bharti, A.; Rodriguez, J.; Feelisch, M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat. Chem. Biol. 2005, 1, 290–297. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Kennedy, D. Leafy Vegetable and Nitrate. In Nitrate and Nitrite in Food and Water; Horwood Publishers: London, UK, 2003; p. 195. [Google Scholar]
- Liubertas, T.; Poderys, J.; Vilma, Z.; Capkauskiene, S.; Viskelis, P. Impact of Dietary Potassium Nitrate on the Life Span of Drosoph. Melanogaster. Process. 2021, 9, 1270. [Google Scholar] [CrossRef]
- Moretti, C.H.; Schiffer, T.A.; Montenegro, M.F.; Larsen, F.J.; Tsarouhas, V.; Carlström, M.; Samakovlis, C.; Weitzberg, E.; Lundberg, J.O. Dietary nitrite extends lifespan and prevents age-related locomotor decline in the fruit fly. Free Radic. Biol. Med. 2020, 160, 860–870. [Google Scholar] [CrossRef]
- Graf, U.; Frei, H.; Kägi, A.; Katz, A.; Würgler, F. Thirty compounds tested in the Drosophila wing spot test. Mutat. Res. Genet. Toxicol. 1989, 222, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Sarıkaya, R.; Çakır, Ş. Genotoxicity testing of four food preservatives and their combinations in the Drosophila wing spot test. Environ. Toxicol. Pharmacol. 2005, 20, 424–430. [Google Scholar] [CrossRef]
- Barrett, J.H.; Parslow, R.C.; McKinney, P.A.; Law, G.R.; Forman, D. Nitrate in drinking water and the incidence of gastric, esophageal, and brain cancer in Yorkshire, England. Cancer Causes Control 1998, 9, 153–159. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Wu, D.-C.; Chang, C.-C. Nitrate in drinking water and risk of death from colon cancer in Taiwan. Environ. Int. 2007, 33, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Ishidate, M., Jr.; Sofuni, T.; Yoshikawa, K.; Hayashi, M.; Nohmi, T.; Sawada, M.; Matsuoka, A. Primary mutagenicity screening of food additives currently used in Japan. Food Chem. Toxicol. 1984, 22, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Ja, W.W.; Carvalho, G.B.; Mak, E.M.; de la Rosa, N.N.; Fang, A.Y.; Liong, J.C.; Brummel, T.; Benzer, S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. USA 2007, 104, 8253–8256. [Google Scholar] [CrossRef]
- Bier, E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 2005, 6, 9–23. [Google Scholar] [CrossRef]
- Lloyd, T.E.; Taylor, J.P. Flightless flies: Drosophila models of neuromuscular disease. Ann. New York Acad. Sci. 2010, 1184, E1–E20. [Google Scholar] [CrossRef]
- Bhargav, D.; Singh, M.P.; Murthy, R.C.; Mathur, N.; Misra, D.; Saxena, D.K.; Chowdhuri, D.K. Toxic potential of municipal solid waste leachates in transgenic Drosophila melanogaster (hsp70-lacZ): hsp70 as a marker of cellular damage. Ecotoxicol. Environ. Saf. 2008, 69, 233–245. [Google Scholar] [CrossRef]
- Coulom, H.; Birman, S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosoph. Melanogaster. J. Neurosci. 2004, 24, 10993–10998. [Google Scholar] [CrossRef]
- Hosamani, R.; Muralidhara. Acute exposure of Drosophila melanogaster to paraquat causes oxidative stress and mitochondrial dysfunction. Arch. Insect Biochem. Physiol. 2013, 83, 25–40. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Fatima, A.; Jyoti, S.; Naz, F.; Rahul; Khan, W.; Singh, B.R.; Naqvi, A.H. Evaluation of the toxic potential of graphene copper nanocomposite (GCNC) in the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ) Bg9. PLoS ONE 2013, 8, e80944. [Google Scholar] [CrossRef]
- Lindsley, D.L.; Zimm, G.G. The Genome of Drosophila melanogaster; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Ren, N.; Charlton, J.; Adler, P.N. The flare gene, which encodes the AIP1 protein of Drosophila, functions to regulate F-actin disassembly in pupal epidermal cells. Genetics 2007, 176, 2223–2234. [Google Scholar] [CrossRef]
- Yan, J.; Huen, D.; Morely, T.; Johnson, G.; Gubb, D.; Roote, J.; Adler, P.N. The multiple-wing-hairs gene encodes a novel GBD–FH3 domain-containing protein that functions both prior to and after wing hair initiation. Genetics 2008, 180, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Merinas-Amo, T.; Tasset-Cuevas, I.; Díaz-Carretero, A.M.; Alonso-Moraga, A.; Calahorro, F. Role of choline in the modulation of degenerative processes: In vivo and in vitro studies. J. Med. Food 2017, 20, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Anter, J.; Romero-Jiménez, M.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Analla, M.; Alonso-Moraga, A.; Muñoz-Serrano, A. Antigenotoxicity, cytotoxicity, and apoptosis induction by apigenin, bisabolol, and protocatechuic acid. J. Med. Food 2011, 14, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Graf, U.; Würgler, F.; Katz, A.; Frei, H.; Juon, H.; Hall, C.; Kale, P. Somatic mutation and recombination test in Drosophila melanogaster. Environ. Mutagen. 1984, 6, 153–188. [Google Scholar] [CrossRef]
- Frei, H.; Würgler, F. Statistical methods to decide whether mutagenicity test data from Drosophila assays indicate a positive, negative, or inconclusive result. Mutat. Res. Environ. Mutagen. Relat. Subj. 1988, 203, 297–308. [Google Scholar] [CrossRef]
- Frei, H.; Würgler, F.E. Optimal experimental design and sample size for the statistical evaluation of data from somatic mutation and recombination tests (SMART) in Drosophila. Mutat. Res. Environ. Mutagen. Relat. Subj. 1995, 334, 247–258. [Google Scholar] [CrossRef]
- Graf, U.; Abraham, S.K.; Guzmán-Rincón, J.; Würgler, F.E. Antigenotoxicity studies in Drosophila melanogaster. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1998, 402, 203–209. [Google Scholar] [CrossRef]
- Abraham, S.K. Antigenotoxicity of coffee in the Drosophila assay for somatic mutation and recombination. Mutagenesis 1994, 9, 383–386. [Google Scholar] [CrossRef]
- Tasset-Cuevas, I.; Fernández-Bedmar, Z.; Lozano-Baena, M.D.; Campos-Sánchez, J.; de Haro-Bailón, A.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Protective effect of borage seed oil and gamma linolenic acid on DNA: In vivo and in vitro studies. PLoS ONE 2013, 8, e56986. [Google Scholar] [CrossRef]
- Soh, J.W.; Hotic, S.; Arking, R. Dietary restriction in Drosophila is dependent on mitochondrial efficiency and constrained by pre-existing extended longevity. Mech. Ageing Dev. 2007, 128, 581–593. [Google Scholar] [CrossRef]
- Gallagher, R.; Collins, S.; Trujillo, J.; McCredie, K.; Ahearn, M.; Tsai, S.; Metzgar, R.; Aulakh, G.; Ting, R.; Ruscetti, F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood 1979, 54, 713–733. [Google Scholar] [CrossRef] [PubMed]
- Merinas-Amo, T.; Tasset-Cuevas, I.; Díaz-Carretero, A.M.; Alonso-Moraga, Á.; Calahorro, F. In vivo and in vitro studies of the role of lyophilised blond lager beer and some bioactive components in the modulation of degenerative processes. J. Funct. Foods 2016, 27, 274–294. [Google Scholar] [CrossRef]
- Mateo-Fernández, M.; Merinas-Amo, T.; Moreno-Millán, M.; Alonso-Moraga, Á.; Demyda-Peyrás, S. In vivo and in vitro genotoxic and epigenetic effects of two types of cola beverages and caffeine: A multiassay approach. BioMed Res. Int. 2016, 2016, 7574843. [Google Scholar] [CrossRef]
- Merinas-Amo, T.; Merinas-Amo, R.; Alonso-Moraga, A. A clinical pilot assay of beer consumption: Modulation in the methylation status patterns of repetitive sequences. Sylwan 2017, 161, 134–156. [Google Scholar]
- Deininger, P.L.; Moran, J.V.; Batzer, M.A.; Kazazian, H.H., Jr. Mobile elements and mammalian genome evolution. Curr. Opin. Genet. Dev. 2003, 13, 651–658. [Google Scholar] [CrossRef]
- Ehrlich, M. DNA hypomethylation, cancer, the immunodeficiency, centromeric region instability, facial anomalies syndrome and chromosomal rearrangements. J. Nutr. 2002, 132, 2424S–2429S. [Google Scholar] [CrossRef]
- Lee, C.; Wevrick, R.; Fisher, R.; Ferguson-Smith, M.; Lin, C. Human centromeric dnas. Hum. Genet. 1997, 100, 291–304. [Google Scholar] [CrossRef]
- Weiner, A.M. SINEs and LINEs: The art of biting the hand that feeds you. Curr. Opin. Cell Biol. 2002, 14, 343–350. [Google Scholar] [CrossRef]
- Weisenberger, D.J.; Campan, M.; Long, T.I.; Kim, M.; Woods, C.; Fiala, E.; Ehrlich, M.; Laird, P.W. Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005, 33, 6823–6836. [Google Scholar] [CrossRef]
- Nikolaidis, G.; Raji, O.Y.; Markopoulou, S.; Gosney, J.R.; Bryan, J.; Warburton, C.; Walshaw, M.; Sheard, J.; Field, J.K.; Liloglou, T. DNA methylation biomarkers offer improved diagnostic efficiency in lung cancer. Cancer Res. 2012, 72, 5692–5701. [Google Scholar] [CrossRef]
- Liloglou, T.; Bediaga, N.G.; Brown, B.R.; Field, J.K.; Davies, M.P. Epigenetic biomarkers in lung cancer. Cancer Lett. 2014, 342, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Joint Expert Committee on Food Additives (JECFA); World Health Organization. Evaluation of Certain Food Additives: Seventy-First Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2010; Volume 71. [Google Scholar]
- Scotter, M.; Castle, L. Chemical interactions between additives in foodstuffs: A review. Food Addit. Contam. 2004, 21, 93–124. [Google Scholar] [CrossRef] [PubMed]
- Romero-Jiménez, M.; Campos-Sánchez, J.; Analla, M.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Genotoxicity and anti-genotoxicity of some traditional medicinal herbs. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005, 585, 147–155. [Google Scholar] [CrossRef]
- Villatoro-Pulido, M.; Font, R.; De Haro-Bravo, M.I.; Romero-Jiménez, M.; Anter, J.; De Haro Bailon, A.; Alonso-Moraga, A.; Del Rio-Celestino, M. Modulation of genotoxicity and cytotoxicity by radish grown in metal-contaminated soils. Mutagenesis 2008, 24, 51–57. [Google Scholar] [CrossRef]
- Joint Expert Committee on Food Additives (JECFA). Toxicological evaluation of certain food additives. In Proceedings of the Forty-Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives and Contaminants, Rome, Italy, 14–23 February 1996; p. 35. [Google Scholar]
- Fabiani, R.; Rosignoli, P.; De Bartolomeo, A.; Fuccelli, R.; Morozzi, G. Genotoxicity of alkene epoxides in human peripheral blood mononuclear cells and HL60 leukaemia cells evaluated with the comet assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 747, 1–6. [Google Scholar] [CrossRef]
- Almeida-Lima, J.; Costa, L.S.; Silva, N.B.; Melo-Silveira, R.F.; Silva, F.V.; Cansancao Felipe, M.B.M.; Batistuzzo Medeiros, S.R.; Leite, E.L.; Rocha, H.A.O. Evaluating the possible genotoxic, mutagenic and tumor cell proliferation-inhibition effects of a non-anticoagulant, but antithrombotic algal heterofucan. J. Appl. Toxicol. 2010, 30, 708–715. [Google Scholar] [CrossRef]
- Poe, B.S.; O’Neill, K.L. Caffeine modulates heat shock induced apoptosis in the human promyelocytic leukemia cell line HL-60. Cancer Lett. 1997, 121, 1–6. [Google Scholar] [CrossRef]
- Fairbairn, D.W.; O’Neill, K.L. Necrotic DNA degradation mimics apoptotic nucleosomal fragmentation comet tail length. Vitr. Cell. Dev. Biol. Anim. 1995, 31, 171–173. [Google Scholar] [CrossRef]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef]
- Roman-Gomez, J.; Jimenez-Velasco, A.; Agirre, X.; Castillejo, J.A.; Navarro, G.; San Jose-Eneriz, E.; Garate, L.; Cordeu, L.; Cervantes, F.; Prosper, F. Repetitive DNA hypomethylation in the advanced phase of chronic myeloid leukemia. Leuk. Res. 2008, 32, 487–490. [Google Scholar] [CrossRef]
- Tajkarimi, M.; Ibrahim, S.A.; Cliver, D. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Gøtterup, J.; Olsen, K.; Knöchel, S.; Tjener, K.; Stahnke, L.H.; Møller, J.K. Relationship between nitrate/nitrite reductase activities in meat associated staphylococci and nitrosylmyoglobin formation in a cured meat model system. Int. J. Food Microbiol. 2007, 120, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Honikel, K.-O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Iammarino, M.; Di Taranto, A.; Cristino, M. Endogenous levels of nitrites and nitrates in wide consumption foodstuffs: Results of five years of official controls and monitoring. Food Chem. 2013, 140, 763–771. [Google Scholar] [CrossRef]
- Sebranek, J.G.; Bacus, J.N. Cured meat products without direct addition of nitrate or nitrite: What are the issues? Meat Sci. 2007, 77, 136–147. [Google Scholar] [CrossRef]
- Sindelar, J.J.; Milkowski, A.L. Human safety controversies surrounding nitrate and nitrite in the diet. Nitric Oxide 2012, 26, 259–266. [Google Scholar] [CrossRef]
- Watson, R.R.; Preedy, V.R. Bioactive Foods in Promoting Health: Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Fraga, C.G.; Trostchansky, A.; Rocha, B.S.; Laranjinha, J.; Rubbo, H.; Galleano, M. (Poly) phenols and nitrolipids: Relevant participants in nitric oxide metabolism. Mol. Asp. Med. 2023, 89, 101158. [Google Scholar] [CrossRef]
- Piesche, M.; Roos, J.; Kühn, B.; Fettel, J.; Hellmuth, N.; Brat, C.; Maucher, I.V.; Awad, O.; Matrone, C.; Comerma Steffensen, S.G. The emerging therapeutic potential of nitro fatty acids and other Michael acceptor-containing drugs for the treatment of inflammation and cancer. Front. Pharmacol. 2020, 11, 1297. [Google Scholar] [CrossRef]
- Ni, H.; Tan, X.; Du, J.; Wang, Y. Nitro-fatty acids: Mechanisms of action, roles in metabolic diseases, and therapeutics. Curr. Med. 2024, 3, 3. [Google Scholar] [CrossRef]
- Nujić, M.; Habuda-Stanić, M. Nitrates and nitrites, metabolism and toxicity. Food Health Dis. Sci.-Prof. J. Nutr. Diet. 2017, 6, 48–89. [Google Scholar]
- Butler, A.R.; Feelisch, M. Therapeutic uses of inorganic nitrite and nitrate: From the past to the future. Circulation 2008, 117, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.A.; Schultz, D.S.; Deen, W.M.; Young, V.R.; Tannenbaum, S.R. Metabolic fate of an oral dose of 15 N-labeled nitrate in humans: Effect of diet supplementation with ascorbic acid. Cancer Res. 1983, 43, 1921–1925. [Google Scholar]
- Bartholomew, B.; Hill, M. The pharmacology of dietary nitrate and the origin of urinary nitrate. Food Chem. Toxicol. 1984, 22, 789–795. [Google Scholar] [CrossRef]
- Woessner, M.; Smoliga, J.M.; Tarzia, B.; Stabler, T.; Van Bruggen, M.; Allen, J.D. A stepwise reduction in plasma and salivary nitrite with increasing strengths of mouthwash following a dietary nitrate load. Nitric Oxide 2016, 54, 1–7. [Google Scholar] [CrossRef]
- Hohensinn, B.; Haselgrübler, R.; Müller, U.; Stadlbauer, V.; Lanzerstorfer, P.; Lirk, G.; Höglinger, O.; Weghuber, J. Sustaining elevated levels of nitrite in the oral cavity through consumption of nitrate-rich beetroot juice in young healthy adults reduces salivary pH. Nitric Oxide 2016, 60, 10–15. [Google Scholar] [CrossRef]
- Montenegro, M.F.; Sundqvist, M.L.; Larsen, F.J.; Zhuge, Z.; Carlström, M.; Weitzberg, E.; Lundberg, J.O. Blood pressure–lowering effect of orally ingested nitrite is abolished by a proton pump inhibitor. Hypertension 2017, 69, 23–31. [Google Scholar] [CrossRef]
- Boink, A.; Vleeming, W.; Dormans, J.; Speijers, G. Effects of nitrates and nitrites in experimental animals. In Managing Risks of Nitrates to Humans and the Environment; Elsevier: Amsterdam, The Netherlands, 1999; pp. 317–326. [Google Scholar]
- Schiffer, T.A.; Larsen, F.J.; Lundberg, J.O.; Weitzberg, E.; Lindholm, P. Effects of dietary inorganic nitrate on static and dynamic breath-holding in humans. Respir. Physiol. Neurobiol. 2013, 185, 339–348. [Google Scholar] [CrossRef]
- Cammack, R.; Joannou, C.; Cui, X.-Y.; Martinez, C.T.; Maraj, S.R.; Hughes, M.N. Nitrite and nitrosyl compounds in food preservation. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1999, 1411, 475–488. [Google Scholar] [CrossRef]
- Gowans, W. Fatal methaemoglobinaemia in a dental nurse. A case of sodium nitrite poisoning. Br. J. Gen. Pract. 1990, 40, 470–471. [Google Scholar]
- Kodama, F.; Umeda, M.; Tsutsui, T. Mutagenic effect of sodium nitrite on cultured mouse cells. Mutat. Res. Genet. Toxicol. 1976, 40, 119–124. [Google Scholar] [CrossRef]
- Tsuda, H.; Kato, K. High rate of endoreduplications and chromosomal aberrations in hamster cells treated with sodium nitrite in vitro. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1977, 56, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Luca, D.; Luca, V.; Cotor, F.; Răileanu, L. In vivo and in vitro cytogenetic damage induced by sodium nitrite. Mutat. Res. Genet. Toxicol. 1987, 189, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Görsdorf, S.; Appel, K.E.; Engeholm, C.; Obe, G. Niltrogen dioxide induces DNA single-strand breaks in cultured Chinese hamster cells. Carcinogenesis 1990, 11, 37–41. [Google Scholar] [CrossRef] [PubMed]
- SCF. Reports of the Scientific Committee for Food; Office for Official Publications of the European Communities: Luxembourg, 1988. [Google Scholar]
- Joint Expert Committee on Food Additives (JECFA); World Health Organization. Evaluation of Certain Food Additives and Contaminants: Sixty-Eighth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Thurston, R.V.; Russo, R.C.; Smith, C.E. Acute toxicity of ammonia and nitrite to cutthroat trout fry. Trans. Am. Fish. Soc. 1978, 107, 361–368. [Google Scholar] [CrossRef]
- Poersch, L.H.; Santos, M.H.S.; Miranda Filho, K.C.; Wasielesky, W., Jr. Efeito agudo do nitrato sobre alevinos da tainha Mugil platanus (Pisces: Mugilidae). Bol. Inst. Pesca 2007, 33, 247–252. [Google Scholar]
- Hernández, A.; Schiffer, T.A.; Ivarsson, N.; Cheng, A.J.; Bruton, J.D.; Lundberg, J.O.; Weitzberg, E.; Westerblad, H. Dietary nitrate increases tetanic [Ca2+] i and contractile force in mouse fast-twitch muscle. J. Physiol. 2012, 590, 3575–3583. [Google Scholar] [CrossRef]
- Ertuğrul, N. Food Additives Regulations and Health Problems about Upper Limit of some Food Additives. Ph.D. Thesis, İstanbul University, İstanbul, Turkey, 1998. [Google Scholar]
- Simon, R.A. Adverse reactions to food additives. Curr. Allergy Asthma Rep. 2003, 3, 62–66. [Google Scholar] [CrossRef]
- Leips, J.; Mackay, T.F. Quantitative trait loci for life span in Drosophila melanogaster: Interactions with genetic background and larval density. Genetics 2000, 155, 1773–1788. [Google Scholar] [CrossRef]
- Kenyon, C. The plasticity of aging: Insights from long-lived mutants. Cell 2005, 120, 449–460. [Google Scholar] [CrossRef]
- Bell, R.; Hubbard, A.; Chettier, R.; Chen, D.; Miller, J.P.; Kapahi, P.; Tarnopolsky, M.; Sahasrabuhde, S.; Melov, S.; Hughes, R.E. A human protein interaction network shows conservation of aging processes between human and invertebrate species. PLoS Genet. 2009, 5, e1000414. [Google Scholar] [CrossRef]
- Van Loon, A.; Botterweck, A.; Goldbohm, R.; Brants, H.; Van Klaveren, J.; Van den Brandt, P. Intake of nitrate and nitrite and the risk of gastric cancer: A prospective cohort study. Br. J. Cancer 1998, 78, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Gulis, G.; Czompolyova, M.; Cerhan, J.R. An ecologic study of nitrate in municipal drinking water and cancer incidence in Trnava District, Slovakia. Environ. Res. 2002, 88, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Kilfoy, B.A.; Weyer, P.J.; Anderson, K.E.; Folsom, A.R.; Cerhan, J.R. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology 2010, 21, 389–395. [Google Scholar] [CrossRef] [PubMed]
| Compound | Concentrations * | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| E-249 | Potassium Nitrite (Cat. 12654) | 0.0000001 | 0.000001 | 0.00001 | 0.0001 | 0.001 | 0.01 |
| E-250 | Sodium Nitrite (Cat. 67398) | 0.0000001 | 0.000001 | 0.00001 | 0.0001 | 0.001 | 0.01 |
| E-251 | Sodium Nitrate (Cat. 15736) | 0.0000185 | 0.000185 | 0.00185 | 0.0185 | 0.185 | 1.85 |
| E-252 | Potassium Nitrate (Cat. 542020) | 0.0000185 | 0.000185 | 0.00185 | 0.0185 | 0.185 | 1.85 |
| Reaction ID | Gene Bank Access | Amplicon | Sequence 5′ to 3′ First Forward | Sequence 3′ to 5′ First Reverse | GC-Content | ||
| Start | End | Forward | Reverse | ||||
| GGTTAGGTA | ATTAACTAAA | ||||||
| Alu C4 | Sequence | 1 | 98 | TAGTGGTTTA | CTAATCTTAA | 25 | 27.3 |
| Consensus | TATTTGTAAT | ACTCCTAACC | |||||
| TTTAGTA | TCA | ||||||
| ATTATGTTAG | CAATCGACC | ||||||
| Alu M1 | Y07755 | 5059 | 5164 | TTAGGATGG | GAACGCGA | 27.6 | 58.8 |
| TTTCGATTTT | |||||||
| GGACGTATT | AATCTCGCGA | ||||||
| LINE-1 | X52235 | 251 | 331 | TGGAAAATC | TACGCCGTT | 47.6 | 52.6 |
| GGG | |||||||
| TGATGGAGT | AATTCTAAAA | ||||||
| ATTTTTAAAA | ATATTCCTCT | 23.5 | 21.2 | ||||
| Sat-α | M38468 | 139 | 260 | TATACGTTTT | TCAATTACGT | ||
| GTAGT | AAA | ||||||
| Clones Per Wings (Number of Spots) (1) | ||||||
|---|---|---|---|---|---|---|
| Compound | Wings Number | Small Single Spots (1–2 Cells) m = 2 | Large Single Spots (>2 Cells) m = 5 | Twin Spots m = 5 | Total Spots m = 2 | Mann–Whitney U-Test (2) |
| H2O | 38 | 0.105 (4) | 0.053 (2) | 0 | 0.158 (6) | |
| H2O2 | 40 | 0.200 (8) | 0.200 (8) | 0 | 0.400 (16) + | |
| Potassium Nitrite | 40 | 0.150 (6) | 0.050 (2) | 0 | 0.200 (8) i | Δ |
| Potassium Nitrate | 36 | 0.166 (6) | 0.000 (0) | 0 | 0.166 (6) i | Δ |
| Sodium Nitrite | 40 | 0.225 (9) | 0.100 (4) | 0 | 0.325 (13) i | Δ |
| Sodium Nitrate | 40 | 0.175 (7) | 0.025 (1) | 0 | 0.200 (8) i | Δ |
| Clones Per Wings (Number of Spots) (1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Wings Number | Small Single Spots (1–2 Cells) m = 2 | Large Single Spots (>2 cells) m = 5 | Twin Spots m = 5 | Total Spots m = 2 a b | Mann–Whitney U-Test (2) a b | IP (3) | ||
| H2O | 38 | 0.105 (4) | 0.053 (2) | 0 | 0.158 (6) | ||||
| H2O2 | 40 | 0.200 (8) | 0.200 (8) | 0 | 0.400 (16) | + | |||
| Potassium Nitrite | 39 | 0.282 (11) | 0.077 (3) | 0.026 (1) | 0.385 (15) i | + | Δ | 3.75 | |
| Potassium Nitrate | 31 | 0.322 (10) | 0.033 (1) | 0 | 0.355 (11) i | i | Δ | Δ | 11.25 |
| Sodium Nitrite | 12 | 0.416 (5) | 0 | 0 | 0.416 (5) i | + | Δ | −4.00 | |
| Sodium Nitrate | 26 | 0.154 (5) | 0.038 (1) | 0 | 0.192 (5) i | i | Δ | Δ | 52.00 |
| Compound | Concentration (mg/mL) | Longevity (1) (Days) | Healthspan (1) (Days) | ||
|---|---|---|---|---|---|
| Potassium Nitrite | Control | 51.355 | 26.569 | ||
| 0.0000001 | 48.212 | ns | 26.933 | ns | |
| 0.000001 | 57.613 | * | 28.266 | ns | |
| 0.00001 | 43.736 | ns | 27.846 | ns | |
| 0.0001 | 64.084 | * | 21.900 | * | |
| 0.001 | 42.179 | ns | 28.421 | ns | |
| 0.01 | 55.109 | ns | 21.421 | * | |
| Potassium Nitrate | Control | 51.355 | 26.569 | ||
| 0.0000185 | 48.212 | ns | 24.625 | ns | |
| 0.000185 | 51.583 | ns | 30.077 | ns | |
| 0.00185 | 59.178 | ns | 23.900 | ns | |
| 0.0185 | 60.310 | * | 33.583 | * | |
| 0.185 | 69.333 | * | 31.875 | * | |
| 1.85 | 59.712 | * | 41.271 | * | |
| Sodium Nitrite | Control | 51.355 | 25.250 | ||
| 0.0000001 | 46.184 | ns | 25.769 | ns | |
| 0.000001 | 51.879 | ns | 27.167 | ns | |
| 0.00001 | 43.804 | ns | 25.789 | ns | |
| 0.0001 | 58.476 | * | 26.125 | ns | |
| 0.001 | 43.950 | ns | 24.000 | ns | |
| 0.01 | 53.113 | ns | 25.333 | ns | |
| Sodium Nitrate | Control | 51.355 | 25.250 | ||
| 0.0000185 | 47.596 | ns | 24.067 | ns | |
| 0.000185 | 46.205 | ns | 25.500 | ns | |
| 0.00185 | 56.347 | ns | 30.000 | * | |
| 0.0185 | 55.468 | ns | 25.400 | ns | |
| 0.185 | 59.677 | * | 30.900 | * | |
| 1.85 | 52.026 | ns | 27.900 | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merinas-Amo, T.; Merinas-Amo, R.; Márquez Prados, L.; Font, R.; Celestino, M.D.R.; Alonso-Moraga, Á. Bioassays to Assess the Safety of Potassium and Sodium Nitrates and Nitrites. Processes 2025, 13, 325. https://doi.org/10.3390/pr13020325
Merinas-Amo T, Merinas-Amo R, Márquez Prados L, Font R, Celestino MDR, Alonso-Moraga Á. Bioassays to Assess the Safety of Potassium and Sodium Nitrates and Nitrites. Processes. 2025; 13(2):325. https://doi.org/10.3390/pr13020325
Chicago/Turabian StyleMerinas-Amo, Tania, Rocío Merinas-Amo, Laura Márquez Prados, Rafael Font, Mercedes Del Río Celestino, and Ángeles Alonso-Moraga. 2025. "Bioassays to Assess the Safety of Potassium and Sodium Nitrates and Nitrites" Processes 13, no. 2: 325. https://doi.org/10.3390/pr13020325
APA StyleMerinas-Amo, T., Merinas-Amo, R., Márquez Prados, L., Font, R., Celestino, M. D. R., & Alonso-Moraga, Á. (2025). Bioassays to Assess the Safety of Potassium and Sodium Nitrates and Nitrites. Processes, 13(2), 325. https://doi.org/10.3390/pr13020325







