Heterogeneous UV–Fenton Process by Maize-Straw-Templated TiO2/Fe3O4 for the Degradation of Tetracycline: Optimization Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Synthesis and Characterization of MST-TiO2/Fe3O4
2.3. Experimental Procedure and Analysis
3. Result and Discussion
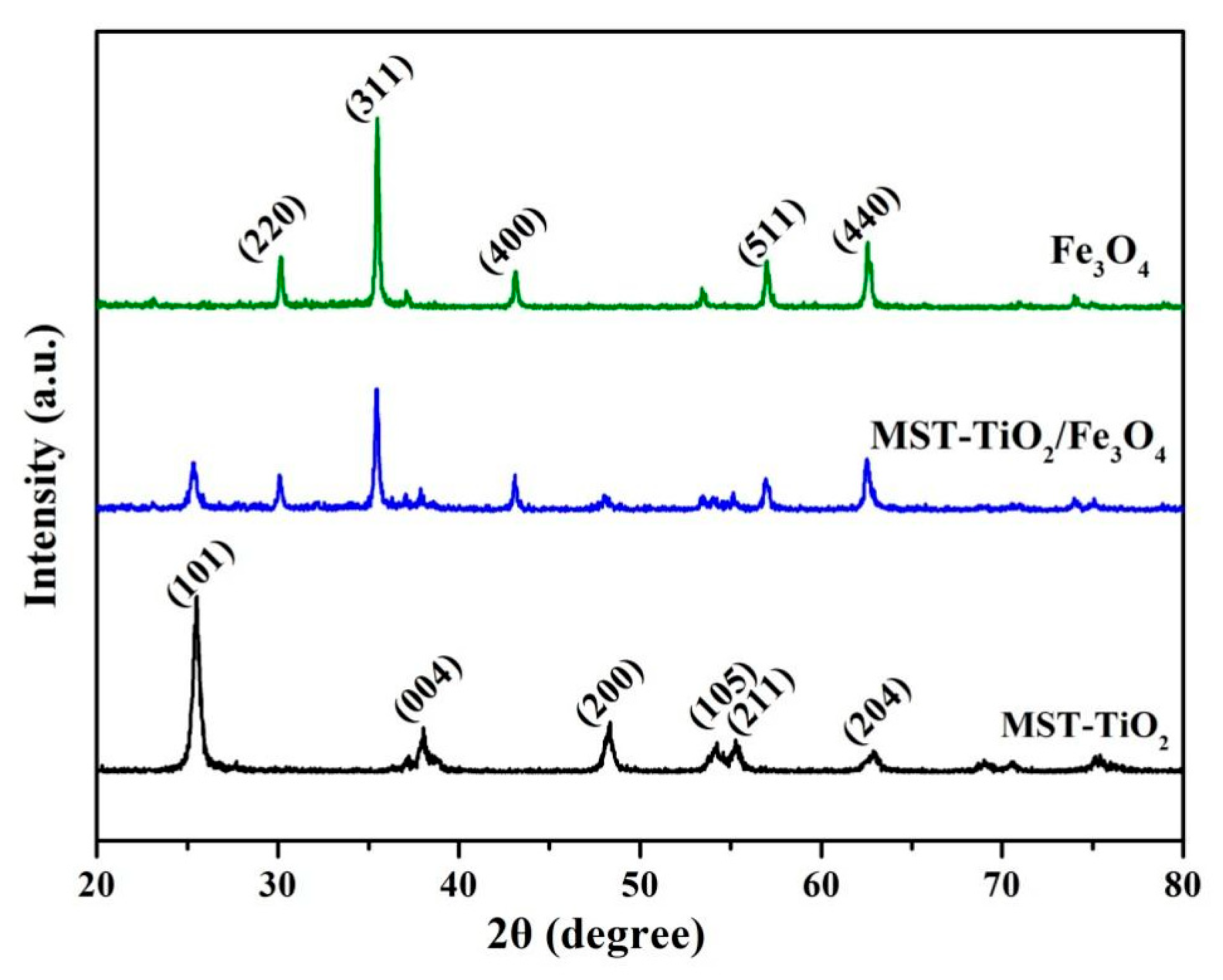
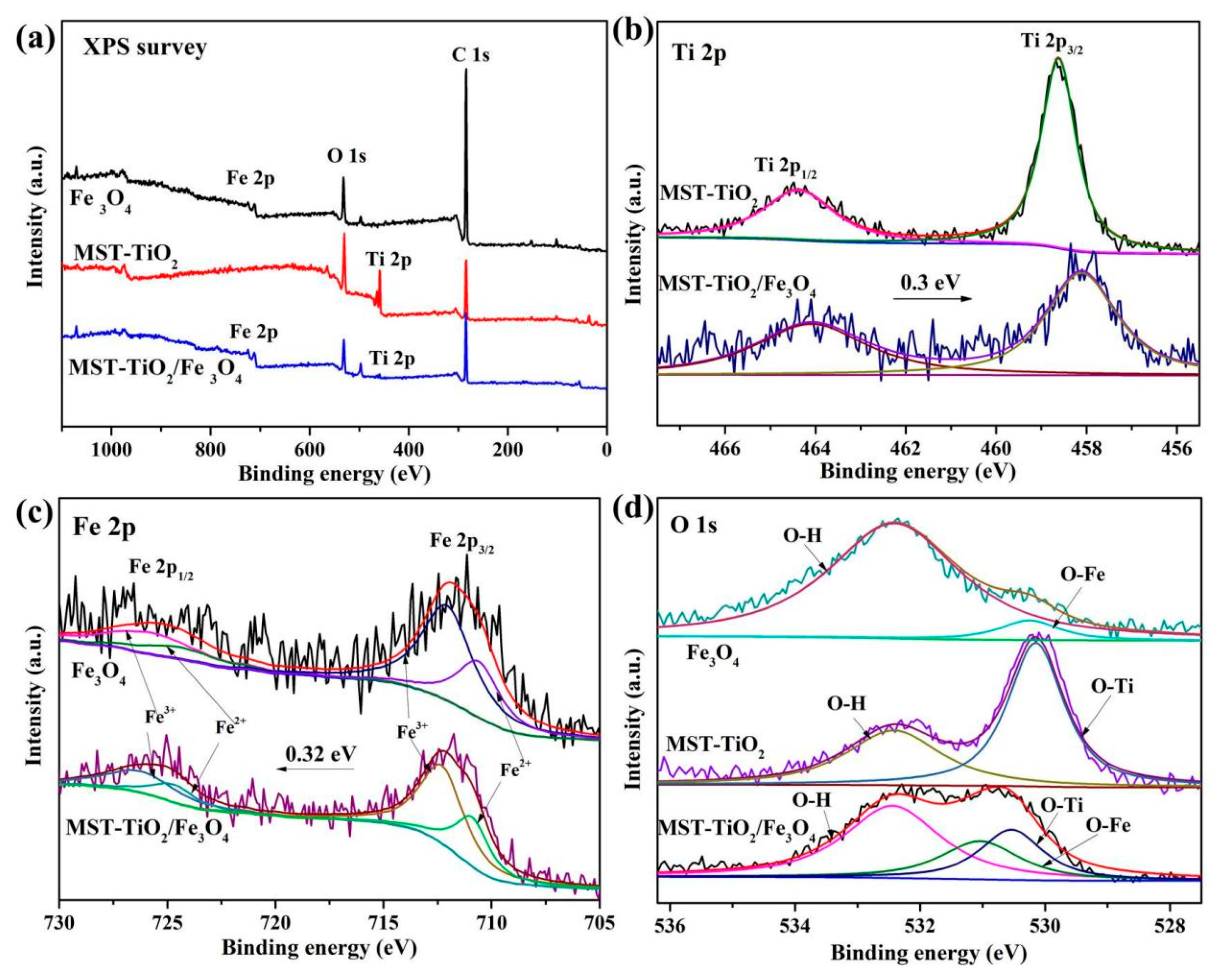
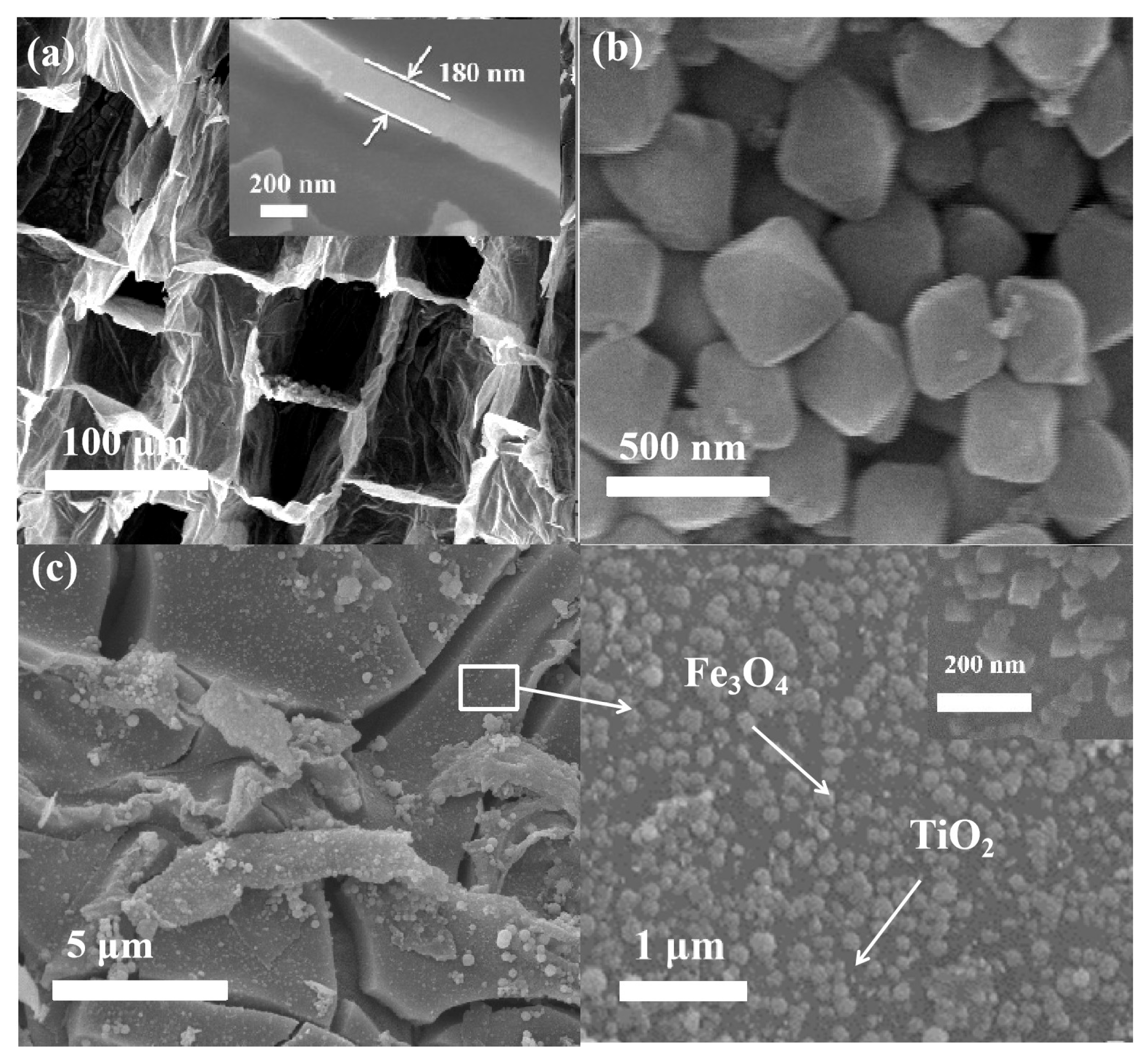
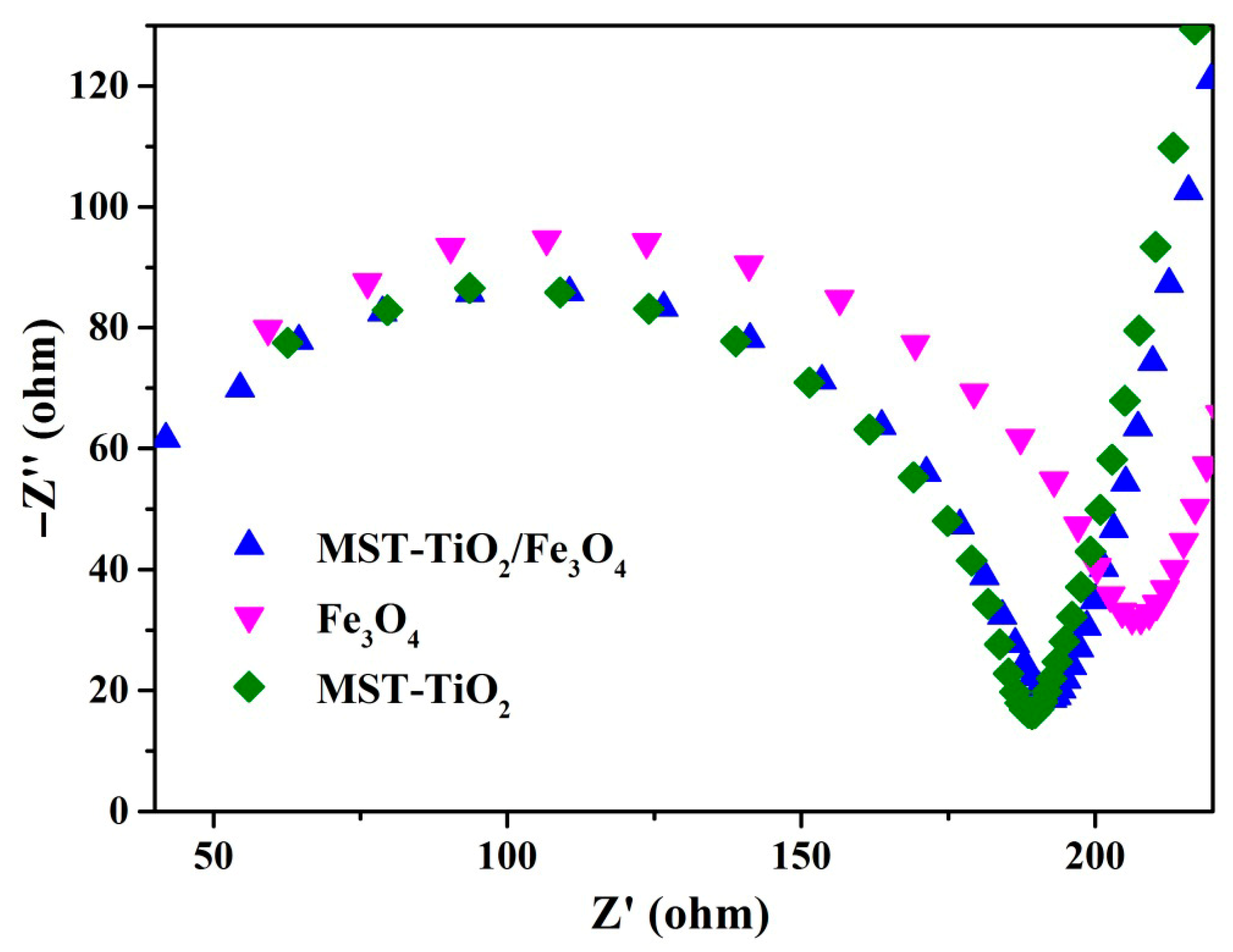
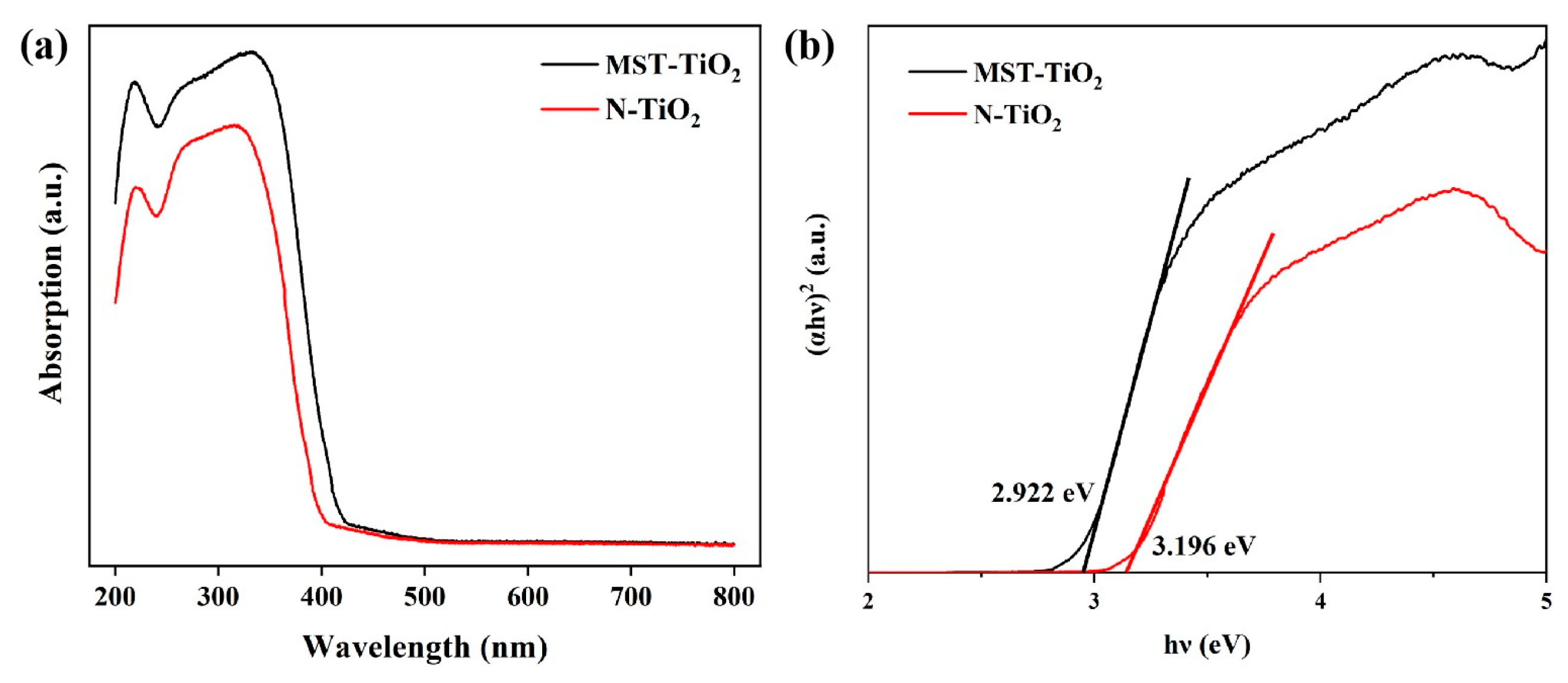
3.1. Characterization of MST-TiO2/Fe3O4
3.2. UV–Fenton Catalytic Activity for the Degradation of TC
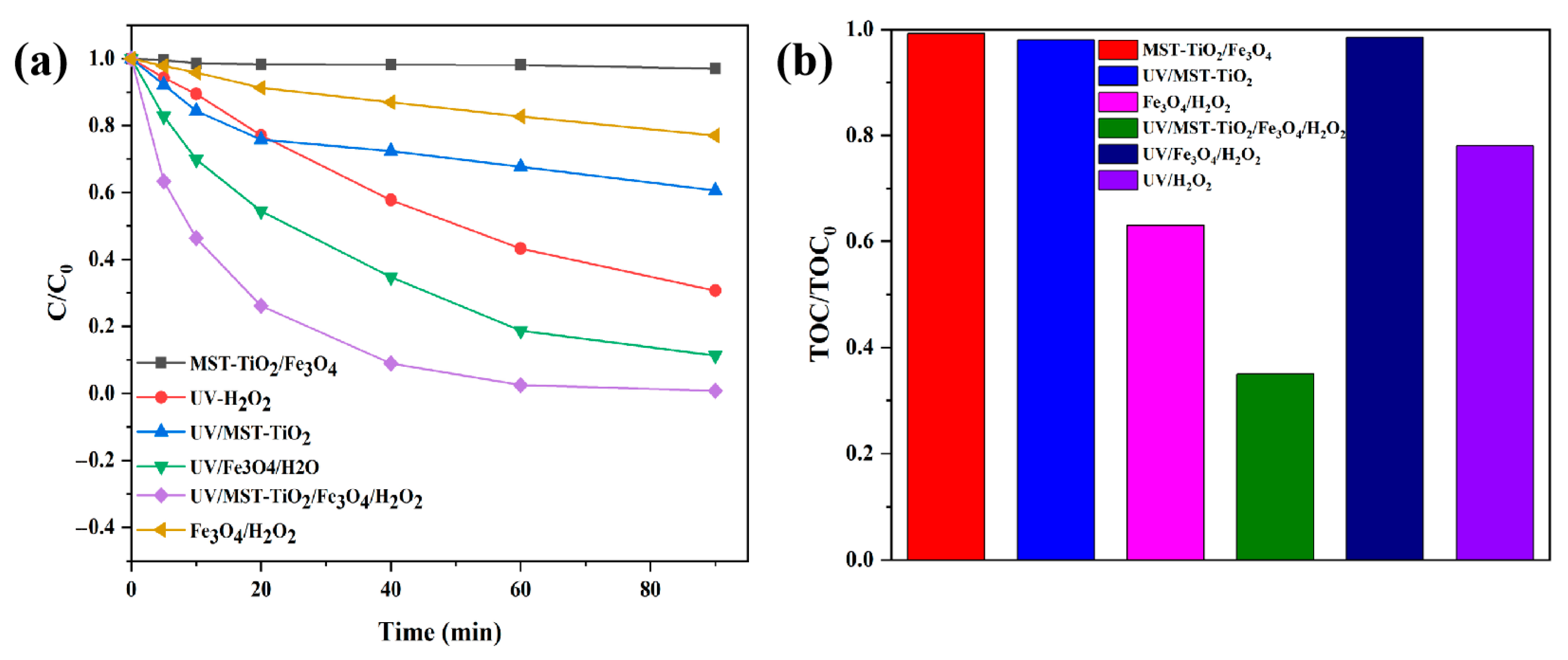
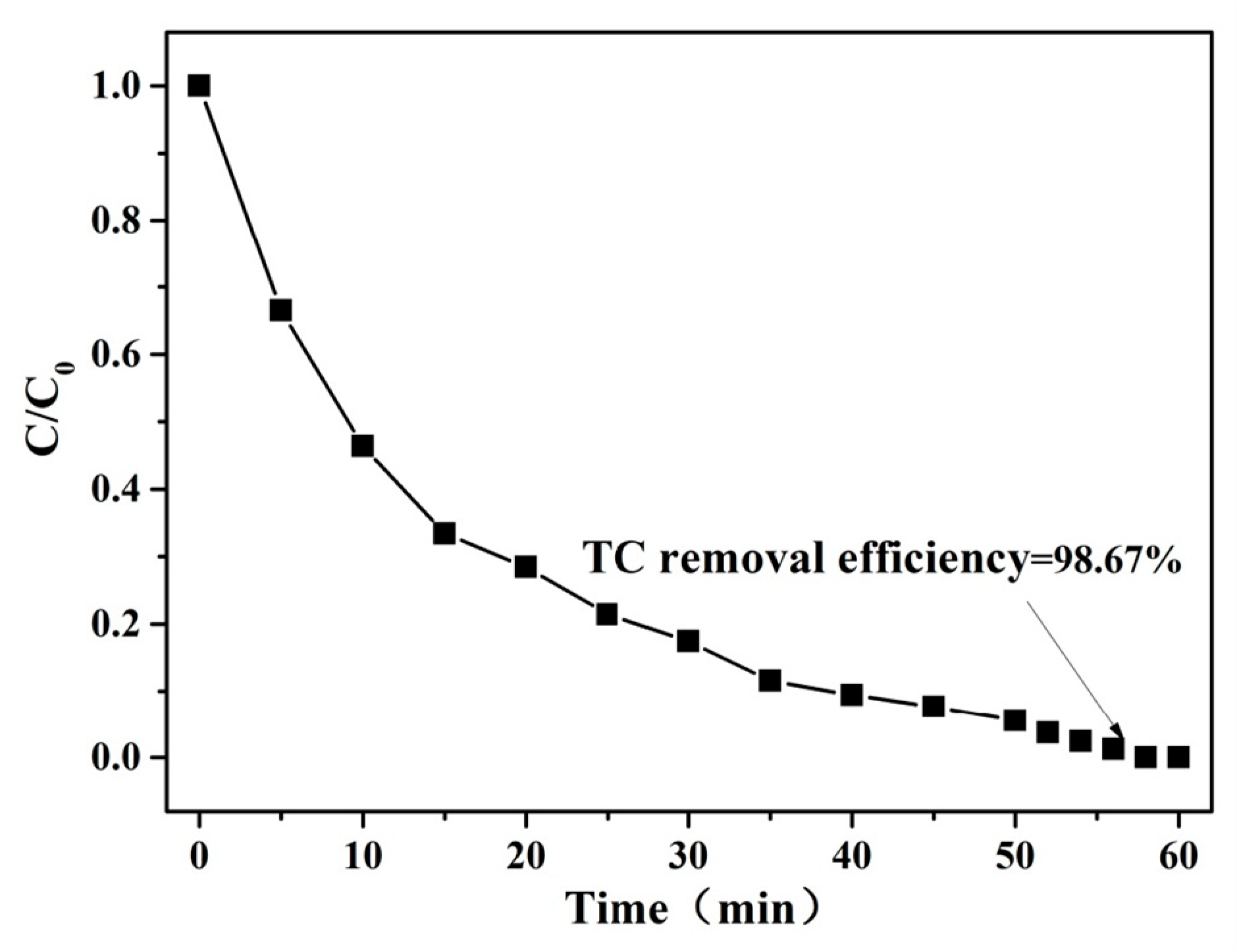
3.2.1. The Degradation Performance of TC in Different Reaction Systems
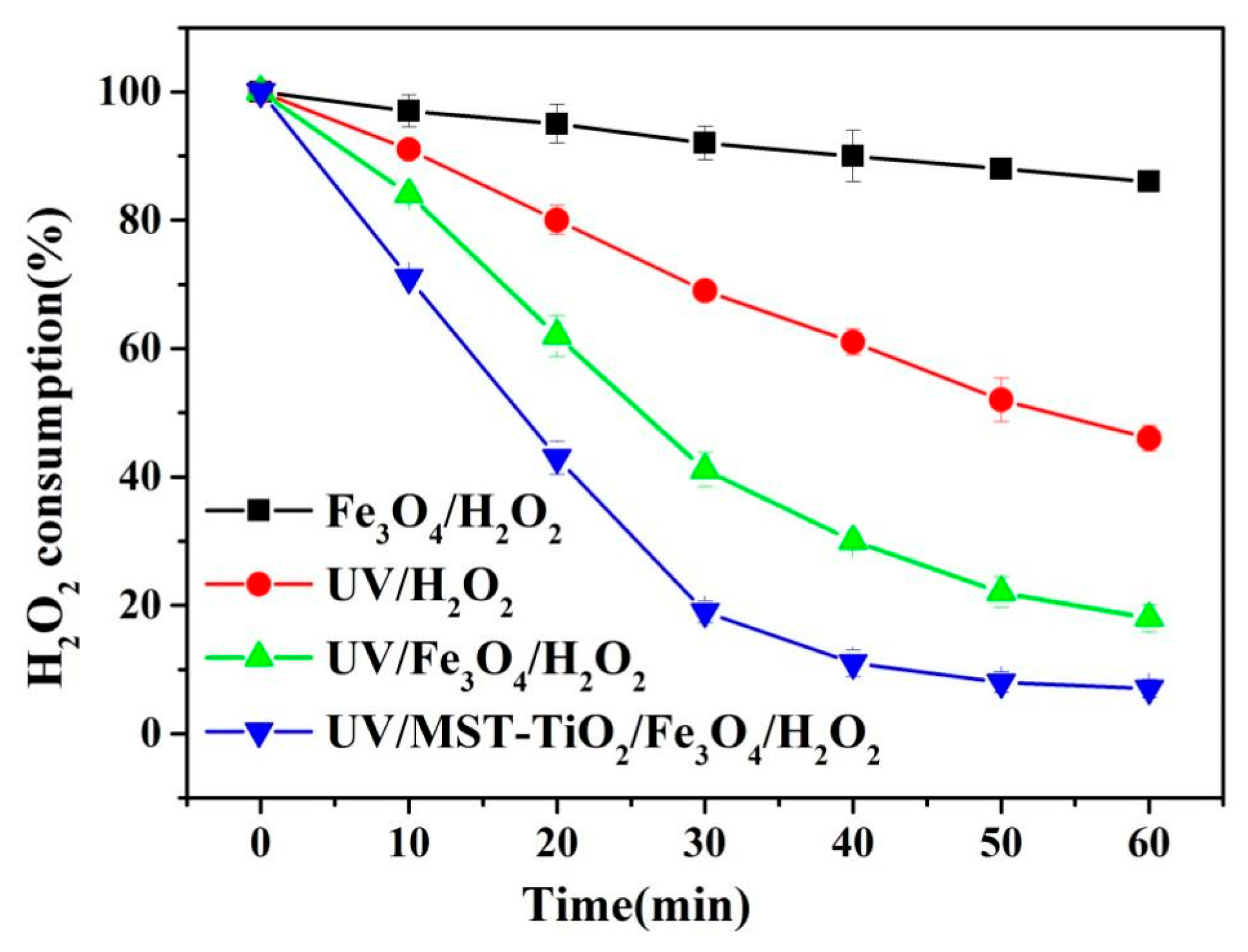
3.2.2. H2O2 Consumption
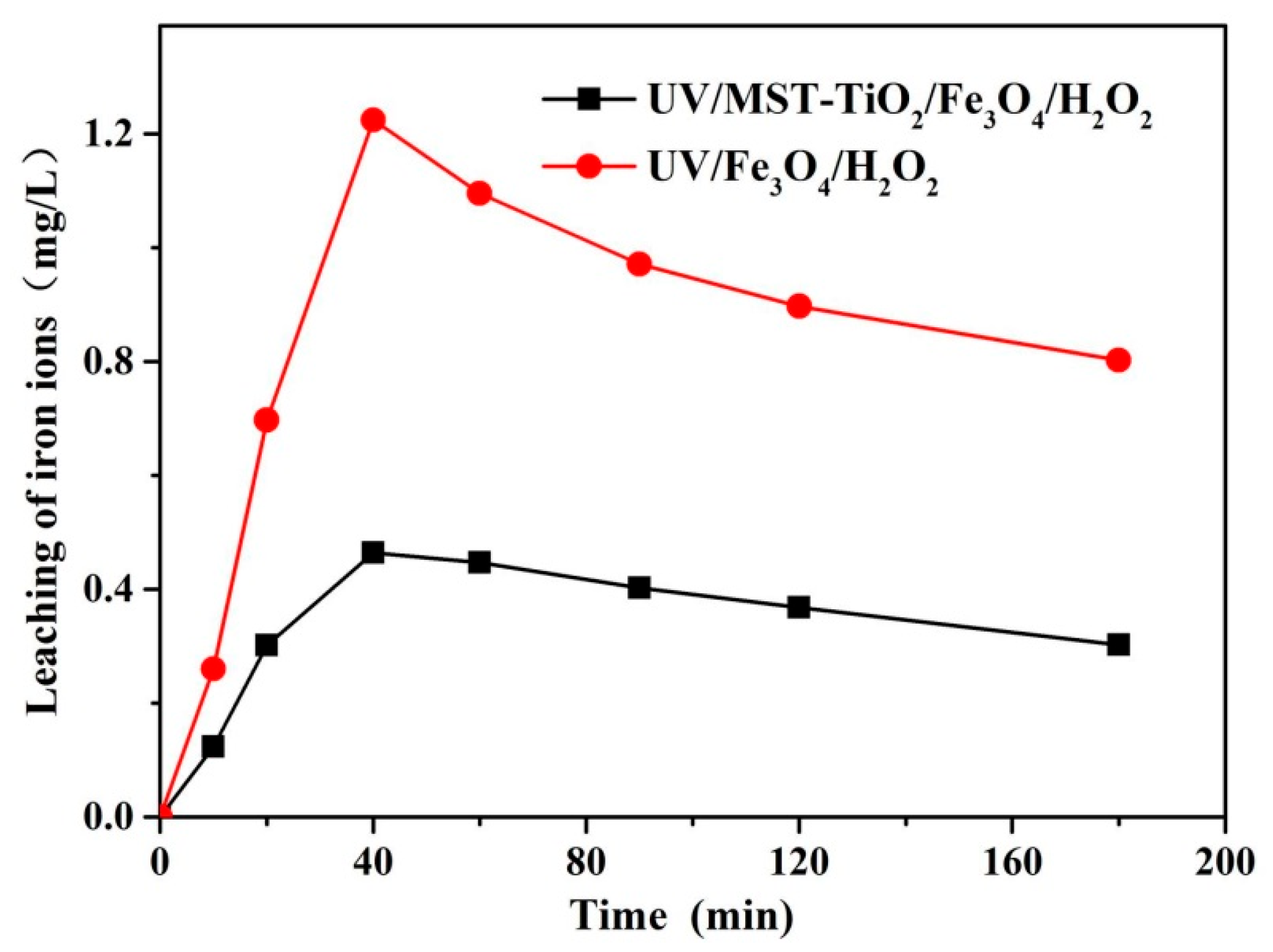
3.2.3. Leaching of Fe Ions
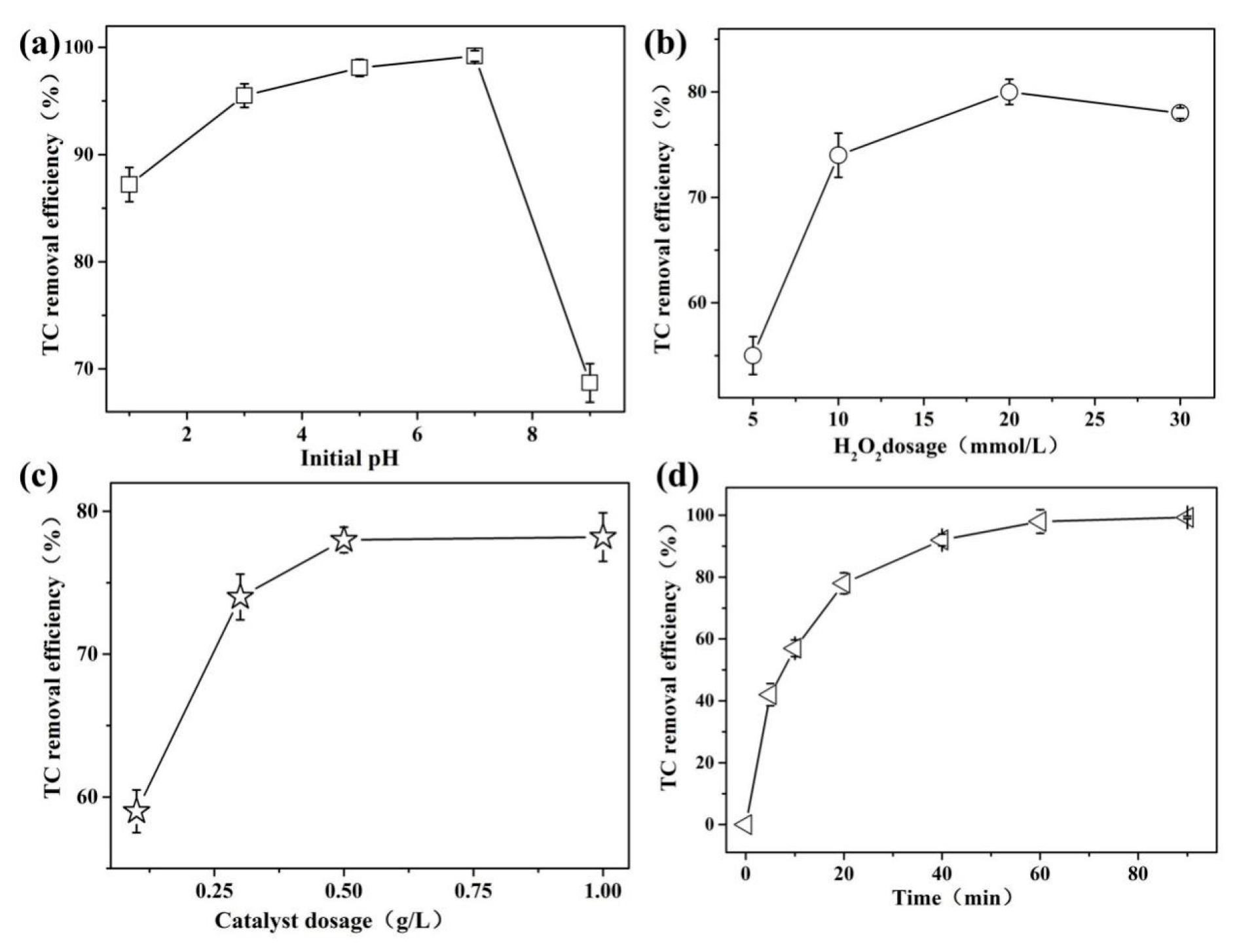
3.2.4. Effect of Single-Factor on TC Oxidation in MST-TiO2/Fe3O4-Catalyzing UV–Fenton
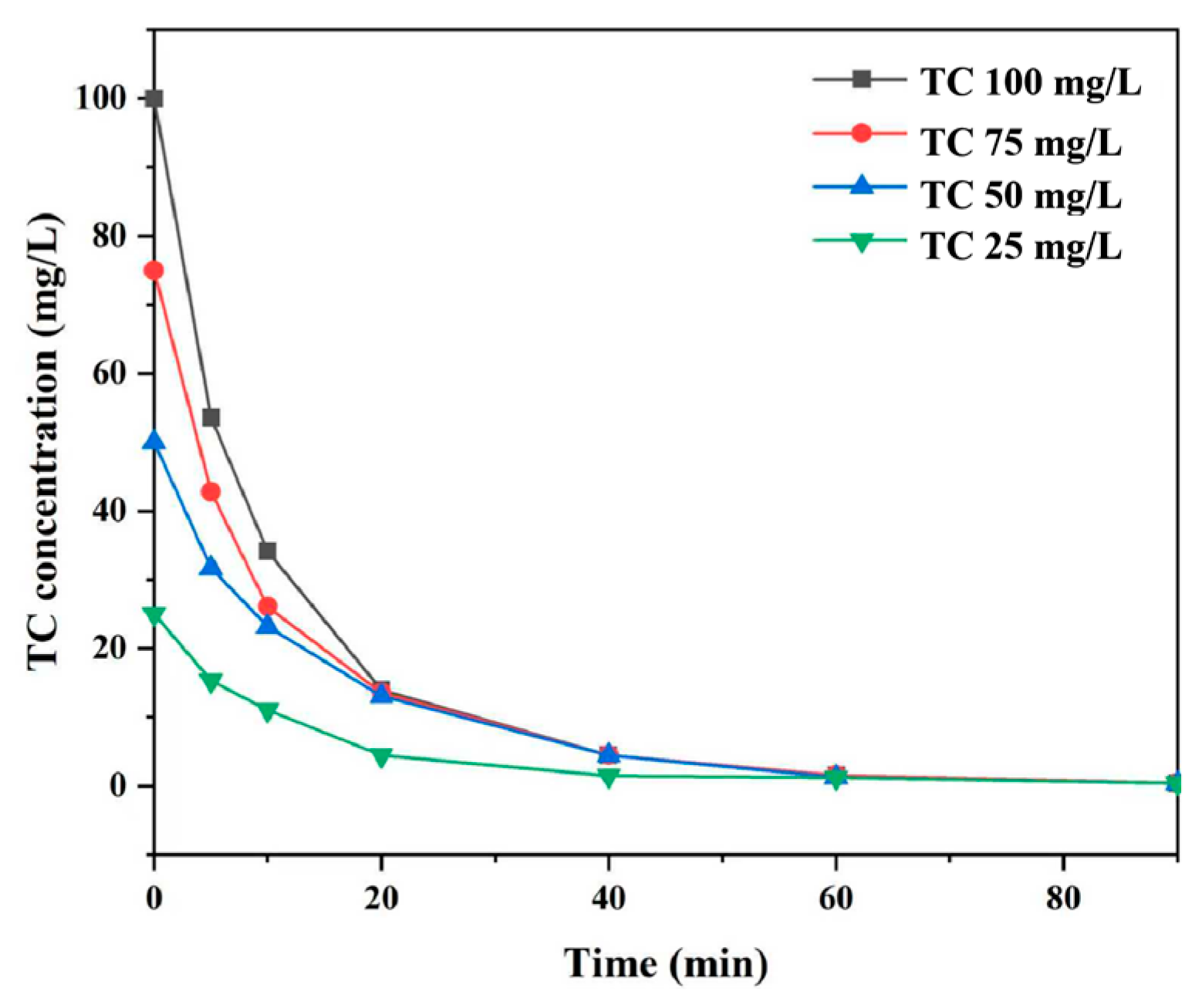
3.2.5. Effect of Initial TC Concentration
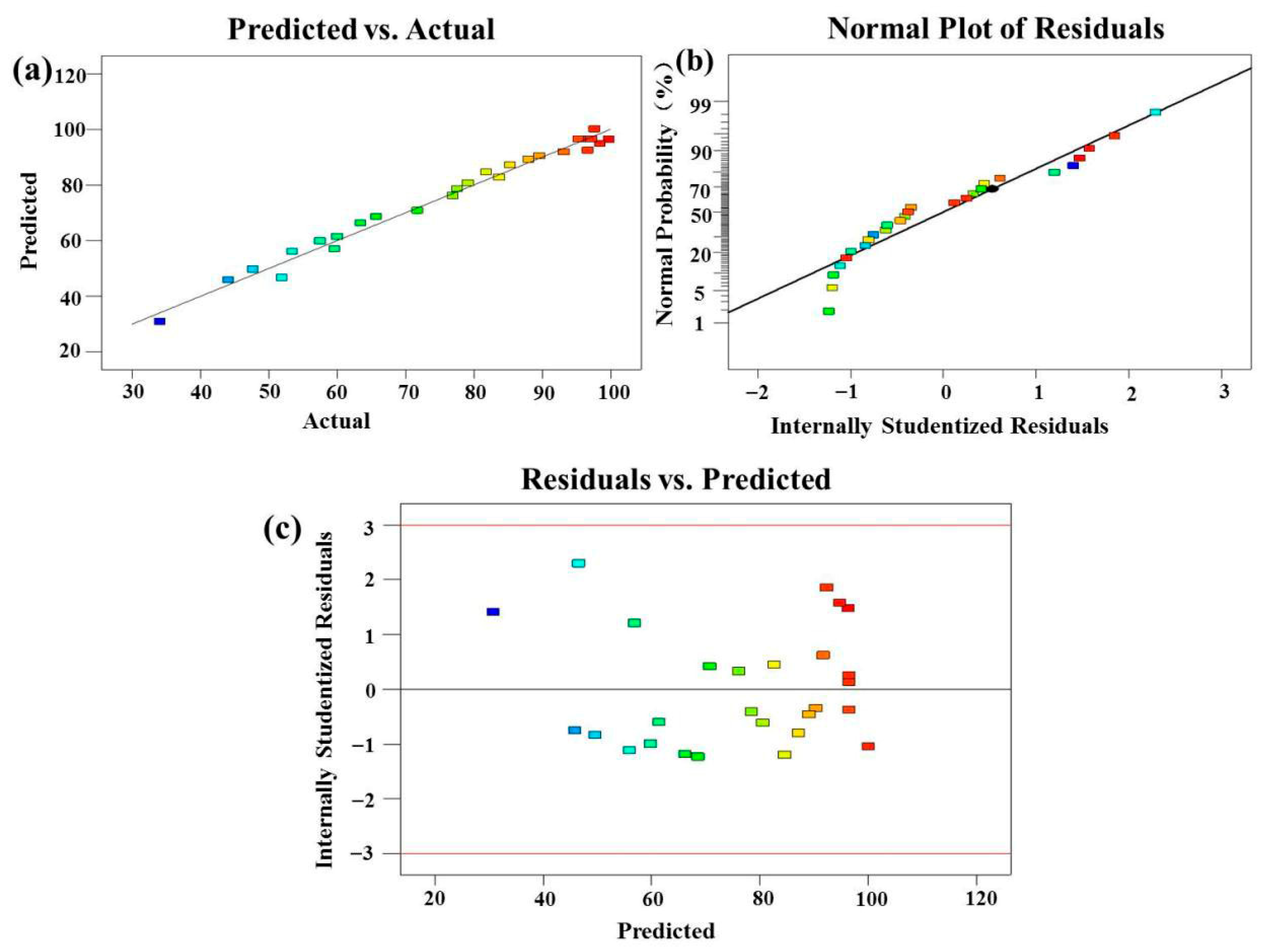
3.2.6. Optimization of the TC Degradation Using RSM
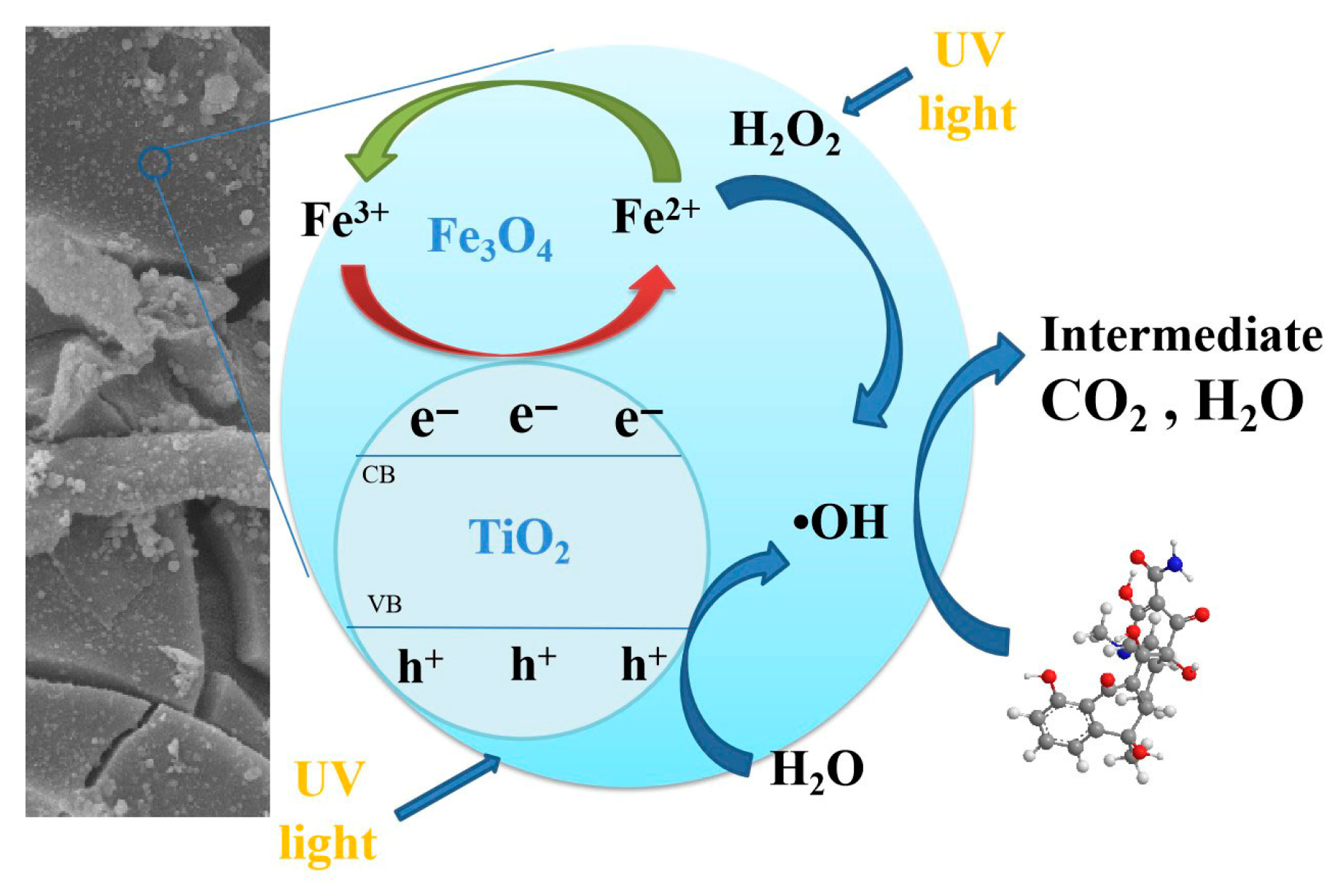
3.2.7. Catalytic Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akhter, S.; Bhat, M.A.; Ahmed, S.; Siddiqui, W.A. Antibiotic residue contamination in the aquatic environment, sources and associated potential health risks. Environ. Geochem. Health 2024, 46, 387. [Google Scholar] [CrossRef]
- Matheou, A.; Abousetta, A.; Pascoe, A.P.; Papakostopoulos, D.; Charalambous, L.; Panagi, S.; Panagiotou, S.; Yiallouris, A.; Filippou, C.; Johnson, E.O. Antibiotic Use in Livestock Farming: A Driver of Multidrug Resistance? Microorganisms 2025, 13, 779. [Google Scholar] [CrossRef]
- Wei, X.; Yi, H.; Lai, C.; Huo, X.; Ma, D.; Du, C. Synergistic effect of flower-like MnFe2O4/MoS2 on photo-Fenton oxidation remediation of tetracycline polluted water. J. Colloid Interface Sci. 2022, 608, 942–953. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Wu, L.; Wan, D.; Shi, Y.; He, Q.; Chen, J. Synergetic adsorption and Fenton-like degradation of tetracycline hydrochloride by magnetic spent bleaching earth carbon: Insights into performance and reaction mechanism. Sci. Total Environ. 2021, 761, 143956. [Google Scholar] [CrossRef]
- Xin, S.; Liu, G.; Ma, X.; Gong, J.; Ma, B.; Yan, Q.; Chen, Q.; Ma, D.; Zhang, G.; Gao, M.; et al. High efficiency heterogeneous Fenton-like catalyst biochar modified CuFeO2 for the degradation of tetracycline: Economical synthesis, catalytic performance and mechanism. Appl. Catal. B Environ. 2021, 280, 119386. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yuan, S.; Liu, J.; Zhang, Y.; Du, H.; Wu, C.; Zhao, P.; Chen, H.; Pei, Y. Synergistic effect and mechanism of mass transfer and catalytic oxidation of octane degradation in yolk-shell Fe3O4@C/Fenton system. Chem. Eng. J. 2020, 379, 122262. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Park, D.; Pham, T.N.; An, Y.; Choi, J.S.; Lee, H.U.; Kwon, O.-H.; Moon, J.-Y.; Kim, K.-T.; Lee, Y.-C. Synthesis of MgAC-Fe3O4/TiO2 hybrid nanocomposites via sol-gel chemistry for water treatment by photo-Fenton and photocatalytic reactions. Sci. Rep. 2019, 9, 11855. [Google Scholar] [CrossRef]
- Sun, C.; Yang, S.-T.; Gao, Z.; Yang, S.; Yilihamu, A.; Ma, Q.; Zhao, R.-S.; Xue, F. Fe3O4/TiO2/reduced graphene oxide composites as highly efficient Fenton-like catalyst for the decoloration of methylene blue. Mater. Chem. Phys. 2019, 223, 751–757. [Google Scholar] [CrossRef]
- Dong, D.; Wang, K.; Yi, M.; Liang, Y.; Muhammad, Y.; Wei, E.; Wei, Y.; Fujita, T. Preparation of TiO2 photocatalyst microspheres by geopolymer technology for the degradation of tetracycline. J. Clean. Prod. 2022, 339, 130734. [Google Scholar] [CrossRef]
- Belachew, N.; Fekadu, R.; Ayalew Abebe, A. RSM-BBD Optimization of Fenton-Like Degradation of 4-Nitrophenol Using Magnetite Impregnated Kaolin. Air Soil Water Res. 2020, 13, 117862212093212. [Google Scholar] [CrossRef]
- Babajani, N.; Jamshidi, S. Investigation of photocatalytic malachite green degradation by iridium doped zinc oxide nanoparticles: Application of response surface methodology. J. Alloys Compd. 2019, 782, 533–544. [Google Scholar] [CrossRef]
- Zhang, Y.; He, T.; Ding, S.; Li, H.; Song, W.; Ding, J.; Lu, J. Photo-fenton degradation of RhB via transition metal oxides composite catalyst Fe3O4/CuO under visible light optimized using response surface methodology. Mater. Technol. 2022, 37, 2347–2359. [Google Scholar] [CrossRef]
- Yu, X.; Lin, X.; Feng, W.; Li, W. Effective Removal of Tetracycline by Using Bio-Templated Synthesis of TiO2/Fe3O4 Heterojunctions as a UV–Fenton Catalyst. Catal. Lett. 2018, 149, 552–560. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Z.; Liu, J.; Shan, N.; Zhang, H.; Dionysiou, D.D. Visible light-assisted heterogeneous Fenton with ZnFe2O4 for the degradation of Orange II in water. Appl. Catal. B: Environ. 2016, 182, 456–468. [Google Scholar] [CrossRef]
- Song, T.; Gao, Y.; Hu, R.; Li, G.; Yu, X. Degradation of Methyl Orange in Aqueous Solution via Magnetic TiO2/Fe3O4 Conjugated with Persulfate. Water Air Soil Pollut. 2023, 234, 508. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Yu, B.; Wu, R.; Mai, J.; Wang, R.; Chen, L.; Yang, S.-T. Preparation of Fe3O4/TiO2/C Nanocomposites and Their Application in Fenton-Like Catalysis for Dye Decoloration. Catalysts 2016, 6, 146. [Google Scholar] [CrossRef]
- Tan, Y.; Shu, Z.; Zhou, J.; Li, T.; Wang, W.; Zhao, Z. One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl. Catal. B: Environ. 2018, 230, 260–268. [Google Scholar] [CrossRef]
- Song, T.; Gao, Y.; Ye, J.; Ohnuki, T.; Li, J.; Yu, X. Fabrication, characterization, and performance evaluation of Bi2WO6/TiO2/Fe3O4 photocatalyst responding to visible light for enhancing bisphenol A degradation. Environ. Sci. Pollut. Res. 2023, 30, 49917–49929. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Yao, X.; Zhang, Y.; Li, Z.; Pan, S.; Han, J.; Xu, L.; Qiao, W.; Li, J.; et al. A comprehensive insight into plasma-catalytic removal of antibiotic oxytetracycline based on graphene-TiO2-Fe3O4 nanocomposites. Chem. Eng. J. 2021, 425, 130614. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Z.; Liu, J.; Deng, Y.; Zhang, D.; Du, P.; Zhang, S.; Lu, X. Novel Fe-Mn-O nanosheets/wood carbon hybrid with tunable surface properties as a superior catalyst for Fenton-like oxidation. Appl. Catal. B Environ. 2019, 259, 118058. [Google Scholar] [CrossRef]
- Sun, J.; Zhen, W.; Xue, C. Magnetic template-assisted construction of 2D PCN/TiO2 heterostructures for efficient photocatalytic hydrogen generation. Appl. Surf. Sci. 2023, 623, 157131. [Google Scholar] [CrossRef]
- Tambosi, J.L.; Domenico, M.D.; Schirmer, W.N.; Jos, H.J.; Moreira, R.D.F. Treatment of paper and pulp wastewater and removal of odorous compounds by a Fenton-like process at the pilot scale. J. Chem. Technol. Biotechnol. 2010, 81, 1426–1432. [Google Scholar] [CrossRef]
- Martinez, N.S.S.; Fernández, J.F.; Segura, X.F.; Ferrer, A.S. Pre-oxidation of an extremely polluted industrial wastewater by the Fenton’s reagent. J. Hazard. Mater. 2003, 101, 315–322. [Google Scholar]
- Park, Y.; Kim, S.; Kim, J.; Khan, S.; Han, C. UV/TiO2 Photocatalysis as an Efficient Livestock Wastewater Quaternary Treatment for Antibiotics Removal. Water 2022, 14, 958. [Google Scholar] [CrossRef]
- Zhao, B.; Li, X.; Li, W.; Yang, L.; Li, J.; Xia, W.; Zhou, L.; Wang, F.; Zhao, C. Degradation of trichloroacetic acid by an efficient Fenton/UV/TiO2 hybrid process and investigation of synergetic effect. Chem. Eng. J. 2015, 273, 527–533. [Google Scholar] [CrossRef]
- Ge, M.; Hu, Z.; Wei, J.; He, Q.; He, Z. Recent advances in persulfate-assisted TiO2-based photocatalysis for wastewater treatment: Performances, mechanism and perspectives. J. Alloys Compd. 2021, 888, 161625. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ. Sci. Technol. 2012, 46, 10145–10153. [Google Scholar] [CrossRef]
- Alani, O.A.; Alani, S.O.; Ari, H.A.; Offiong, N.-A.O.; Ugya, A.Y.; Feng, W. Tetracycline degradation by efficient synergistic bio-templated CuO photocatalysis and Fenton hybrid process irradiated by visible light: Influential parameters, and mechanisms. J. Mater. Sci. Mater. Electron. 2022, 33, 25603–25618. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Modelling and optimization of photocatalytic degradation of phenol via TiO2 nanoparticles: An insight into response surface methodology and artificial neural network. J. Photochem. Photobiol. A Chem. 2019, 384, 112039. [Google Scholar] [CrossRef]
- Galedari, M.; Mehdipour Ghazi, M.; Rashid Mirmasoomi, S. Photocatalytic process for the tetracycline removal under visible light: Presenting a degradation model and optimization using response surface methodology (RSM). Chem. Eng. Res. Des. 2019, 145, 323–333. [Google Scholar] [CrossRef]
| Levels | Factors | |||
|---|---|---|---|---|
| Initial pH | H2O2 Dosage (mmol/L) | Catalyst Dosage (g/L) | Reaction Time (min) | |
| −1 | 5 | 5 | 0.1 | 45 |
| 0 | 7 | 10 | 0.3 | 60 |
| 1 | 9 | 15 | 0.5 | 75 |
| Run | A | B | C | D | Degradation Rate (%) |
|---|---|---|---|---|---|
| 1 | −1 (5) | 0 (10) | 0 (0.3) | 1 (75) | 88.05 |
| 2 | 0 (7) | 0 (10) | −1 (0.1) | −1 (45) | 77.57 |
| 3 | −1 (5) | 1 (15) | 0 (0.3) | 0 (60) | 89.62 |
| 4 | 1 (9) | 0 (10) | 1 (0.5) | 0 (60) | 63.47 |
| 5 | 1 (9) | 1 (15) | 0 (0.3) | 0 (60) | 65.76 |
| 6 | 0 (7) | 1 (15) | 0 (0.3) | 1 (75) | 97.67 |
| 7 | 0 (7) | 0 (10) | 1 (0.5) | 1 (75) | 99.79 |
| 8 | 1 (9) | −1 (5) | 0 (0.3) | 0 (60) | 34.07 |
| 9 | 0 (7) | −1 (5) | 1 (0.5) | 0 (60) | 53.43 |
| 10 | 0 (7) | −1 (5) | −1 (0.1) | 0 (60) | 47.68 |
| 11 | 0 (7) | 0 (10) | 0 (0.3) | 0 (60) | 97.25 |
| 12 | 1 (9) | 0 (10) | 0 (0.3) | −1 (45) | 59.66 |
| 13 | 0 (7) | 1 (15) | −1 (0.1) | 0 (60) | 96.71 |
| 14 | −1 (5) | −1 (5) | 0 (0.3) | 0 (60) | 51.91 |
| 15 | −1 (5) | 0 (10) | −1 (0.1) | 0 (60) | 79.18 |
| 16 | −1 (5) | 0 (10) | 1 (0.5) | 0 (60) | 81.86 |
| 17 | 0 (7) | 0 (10) | 0 (0.3) | 0 (60) | 95.41 |
| 18 | 0 (7) | −1 (5) | 0 (0.3) | 1 (75) | 57.53 |
| 19 | 0 (7) | 1 (15) | 0 (0.3) | −1 (45) | 85.34 |
| 20 | 0 (7) | −1 (5) | 0 (0.3) | −1 (45) | 44.08 |
| 21 | 1 (9) | 0 (10) | −1 (0.1) | 0 (60) | 60.04 |
| 22 | 0 (7) | 0 (10) | 1 (0.5) | −1 (45) | 83.75 |
| 23 | 1 (9) | 0 (10) | 0 (0.3) | 1 (75) | 71.77 |
| 24 | 0 (7) | 1 (15) | 1 (0.5) | 0 (60) | 98.49 |
| 25 | −1 (5) | 0 (10) | 0 (0.3) | −1 (45) | 76.92 |
| 26 | 0 (7) | 0 (10) | −1 (0.1) | 1 (75) | 93.2 |
| 27 | 0 (7) | 0 (10) | 0 (0.3) | 0 (60) | 96.87 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 9711.56 | 14 | 693.68 | 53.83 | <0.0001 | Significant |
| A | 1059.76 | 1 | 1059.76 | 82.23 | <0.0001 | |
| B | 4997.59 | 1 | 4997.59 | 387.79 | <0.0001 | |
| C | 58.12 | 1 | 58.12 | 4.51 | 0.0452 | |
| D | 542.57 | 1 | 542.57 | 42.1 | <0.0001 | |
| AB | 9.06 | 1 | 9.06 | 0.7 | 0.4182 | |
| AC | 0.14 | 1 | 0.14 | 0.011 | 0.9185 | |
| AD | 0.24 | 1 | 0.24 | 0.019 | 0.8937 | |
| BC | 3.94 | 1 | 3.94 | 0.31 | 0.5905 | |
| BD | 0.31 | 1 | 0.31 | 0.024 | 0.8786 | |
| CD | 0.042 | 1 | 0.042 | 0.00326 | 0.9554 | |
| A2 | 1864.51 | 1 | 1864.51 | 144.68 | <0.0001 | |
| B2 | 1865.01 | 1 | 1865.01 | 144.72 | <0.0001 | |
| C2 | 112.36 | 1 | 112.36 | 8.72 | 0.0121 | |
| D2 | 111.39 | 1 | 111.39 | 8.64 | 0.0124 | |
| Residual | 154.65 | 12 | 12.89 | |||
| Lack of fit | 152.76 | 10 | 15.28 | 16.19 | 0.0595 | Not significant |
| Pure error | 1.89 | 2 | 0.94 | |||
| Core total | 9866.21 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Ji, Q.; Cui, Y. Heterogeneous UV–Fenton Process by Maize-Straw-Templated TiO2/Fe3O4 for the Degradation of Tetracycline: Optimization Using Response Surface Methodology. Processes 2025, 13, 3701. https://doi.org/10.3390/pr13113701
Yu X, Ji Q, Cui Y. Heterogeneous UV–Fenton Process by Maize-Straw-Templated TiO2/Fe3O4 for the Degradation of Tetracycline: Optimization Using Response Surface Methodology. Processes. 2025; 13(11):3701. https://doi.org/10.3390/pr13113701
Chicago/Turabian StyleYu, Xiaodan, Qiancheng Ji, and Yang Cui. 2025. "Heterogeneous UV–Fenton Process by Maize-Straw-Templated TiO2/Fe3O4 for the Degradation of Tetracycline: Optimization Using Response Surface Methodology" Processes 13, no. 11: 3701. https://doi.org/10.3390/pr13113701
APA StyleYu, X., Ji, Q., & Cui, Y. (2025). Heterogeneous UV–Fenton Process by Maize-Straw-Templated TiO2/Fe3O4 for the Degradation of Tetracycline: Optimization Using Response Surface Methodology. Processes, 13(11), 3701. https://doi.org/10.3390/pr13113701





